Neurodegeneration with brain iron accumulation (NBIA) is a group of genetic diseases characterized by progressive extrapyramidal symptoms and focal iron accumulation in the basal ganglia. β-Propeller protein-associated neurodegeneration (BPAN), also known as static encephalopathy of childhood with neurodegeneration in adulthood or NBIA 5, is an X-linked dominant subtype of NBIA.1 Brain MRI studies consistently demonstrate iron accumulation in the globus pallidus and substantia nigra with a subset of patients also demonstrating a halo of hyperintense signal surrounding a thin region of hypointense signal in the substantia nigra on T1-weighted imaging.2 The majority of patients with BPAN are female, but several affected males with identical phenotypes have been described, most likely harboring postzygotic mutations leading to somatic mosaicism.3 BPAN has been shown to be caused by heterozygous mutations in WDR45 at Xp11.23. To date, all mutations have been de novo, with no affected relatives.1,3,4 We report here on a patient with BPAN with a novel c.597_598 deletion mutation in WDR45.
Case history.
Our patient presented at 2 years of age with global developmental delay. She crawled at 9 months and walked at 17 months. At baseline, she is described as moving slow and is clumsy with poor fine motor control. Although nonverbal at baseline, she completed special education schooling up to age 20 and did very well with social interactions. She has never been able to perform activities of daily living and has been dependent upon her family for support and care. Her condition was stable up until the age of 32 when her family noted that she was having difficulty stepping with her left foot, especially when climbing stairs. She also began holding her left arm at her side. At the age of 33, she began having bowel and urinary incontinence. A careful family history revealed 1 paternal uncle with Down syndrome, but no known neurologic disease.
At the age of 34, she was evaluated in a neurology clinic and noted to be nonverbal with limited comprehension, but otherwise alert and cooperative. She had features consistent with a dystonic smile as well as left upper and lower extremity dystonia. Her gait was slow and purposeful, but there was no shuffling or other signs concerning for parkinsonism appreciated.
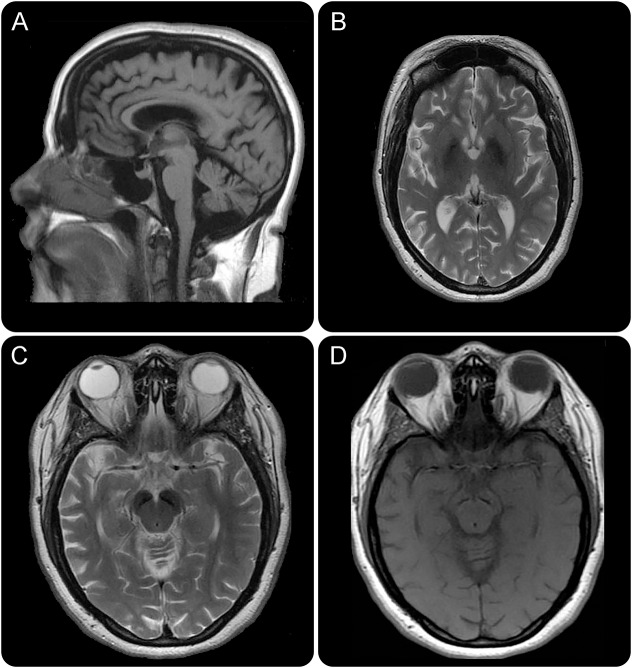
Laboratory workup included a basic metabolic panel including electrolytes and glucose levels, cell blood count, ceruloplasmin, and serum copper—all of which were within normal limits. Brain MRI revealed cerebral and cerebellar atrophy (figure, A). T2-weighted imaging revealed low signal intensities in the globus pallidus and substantia nigra indicating iron accumulation (figure, B and C). T1-weighted images revealed similar hypointensities in the substantia nigra, with a thin halo of hyperintensity around it (figure, D).
Figure. MRI demonstrating features unique to β-propeller protein-associated neurodegeneration.
(A) T1 fluid-attenuated inversion recovery MRI sagittal image demonstrating both cerebral and cerebellar atrophy. (B) T2 MRI axial image with hypointensity in the globus pallidus and (C) in the substantia nigra corresponding with iron deposition. (D) T1 MRI axial image demonstrating a thin line of hypointensity in the substantia nigra with a surrounding halo of hyperintensity.
Serum samples were sent to Knight Diagnostic Laboratories at Oregon Health and Science University for molecular genetic analysis using an NBIA gene panel consisting of 17 genes.5 The genetic analysis revealed a novel heterozygous frameshift mutation (c.597_598 deletion) in WDR45, resulting in a premature truncation of the protein at amino acid position 21 (p.Leuo201Lysfs*21). Per laboratory report, this mutation was deemed a likely pathogenic variant. The patient's parents were tested for this same mutation, which was absent in both.
Discussion.
BPAN is a very rare disease, and to this point the largest case series available describes only 19 different mutations.1 Subsequent publications include a small number of single case reports as well as small case series describing 5 or 7 mutations.4,6,7 The phenotype is characterized by a static, global encephalopathy in early childhood with later development of progressive cognitive decline in adolescence and early adulthood. Onset of neurodegeneration is usually heralded by parkinsonism, but dystonia, sleep abnormalities, Rett-like syndrome, and epileptic encephalopathies have also been described.1,6
The WDR45 gene encodes for a β-propeller scaffolding protein that is part of the family of WD40 repeat proteins which are key components of many essential biological functions. These proteins regulate the assembly of multiprotein complexes and are thought to be involved in autophagy.1 An autophagic pathogenesis is further supported by an immunofluorescence study that demonstrated the accumulation of autophagic material in BPAN patient cells.4 It is likely that WDR45 serves different functions during development and later during neuronal homeostasis associated with aging.
Previously described mutations in WDR45 are usually frameshift mutations that result in a premature truncation of the protein, although several missense mutations affecting highly conserved regions have also been described.1,3,4,7 We report herein a novel frameshifting mutation (c.597_598delGT, p.Leu201Lysfs*21) resulting in a premature truncation. Our patient's clinical course is somewhat unusual in that she did not have any features of parkinsonism, and the onset of her neurodegenerative symptoms was later than most previously described cases of BPAN.7 Her brain MRI results were, however, classical for BPAN. This single case adds to the existing literature on a very rare neurodegenerative disease.
Footnotes
Author contributions: DonRaphael P. Wynn: study concept and design and acquisition of data. Stefan Pulst: acquisition of data, analysis and interpretation of data, and critical revision of manuscript for intellectual content.
Study funding: No targeted funding reported.
Disclosure: DonRaphael P. Wynn reports no disclosures. Stefan M. Pulst has served on the editorial boards of the Journal of Cerebellum, Neuromuscular Medicine, CONTINUUM, Experimental Neurology, Neurogenetics, Nature Clinical Practice Neurology, and Current Genomics; holds patents for the following: Nucleic acids encoding ataxin-2 binding proteins, Nucleic acid encoding Schwannomin-binding-proteins and products related thereto, Transgenic mouse expressing a polynucleotide encoding a human ataxin-2 polypeptide, Methods of detecting spinocerebellar ataxia-2 nucleic acids, Nucleic acid encoding spinocerebellar ataxia-2 and products related thereto, Shwannomin-binding-proteins, and Compositions and methods for spinocerebellar ataxia; receives royalties from AAN, CSHS, Churchill Livingston, Academic Press, and Oxford University Press; has been a consultant to Ataxion Therapeutics; has served on the speaker's bureau of Athena Diagnostics Inc.; has received research support from NIH, the National Ataxia Foundation, Target ALS, and Utah DoH; has received license fee payments from Cedars-Sinai Medical Center; has given expert testimony in legal proceedings involving Hall & Evans LLC; and is the Editor of Neurology® Genetics. Go to Neurology.org/ng for full disclosure forms. The Article Processing charge was paid by the Department of Neurology, University of Utah.
References
- 1.Hayflick SJ, Kruer MC, Gregory A, et al. Beta-propeller protein-associated neurodegeneration: a new X-linked dominant disorder with brain iron accumulation. Brain 2013;136:1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruer MC, Boddaert N, Schneider SA, et al. Neuroimaging features of neurodegeneration with brain iron accumulation. Am J Neuroradiol 2012;22:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haack TB, Hogarth P, Kruer MC, et al. Exome sequencing reveals de novo WDR45 mutations causing phenotypically distinct, X-linked dominant form of NBIA. Am J Hum Genet 2012;91:1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saitsu H, Nishimura T, Muramatsu K, et al. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat Genet 2013;45:445–449. [DOI] [PubMed] [Google Scholar]
- 5.Knight Diagnostic Laboratories. Neurodegeneration with Brain Iron Accumulation (NBIA) Sequencing. Available at: ohsu.edu/custom/knight-diagnostic-labs/home/test-details?id=Neurodegeneration+with+Brain+Iron+Accumulation+(NBIA)+Sequencing. Accessed September 26, 2016. [Google Scholar]
- 6.Hoffjan S, Ibisler A, Tschentscher A, et al. WDR45 mutation in Rett (-like) syndrome and developmental delay: case report and an appraisal of the literature. Mol Cell Probes 2016:30:44–49. [DOI] [PubMed] [Google Scholar]
- 7.Nishioka K, Oyama G, Yoshino H, et al. High frequency of beta-propeller protein-associated neurodegeneration (BPAN) among patients with intellectual disability and young-onset parkinsonism. Neurobiol Aging 2015;36:2004.e9–2004.e15. [DOI] [PubMed] [Google Scholar]