Abstract
The majority of patients with chronic-phase (CP) chronic myeloid leukemia (CML) who are treated with Bcr-Abl tyrosine kinase inhibitors such as imatinib and dasatinib achieve cytogenetic disease remission (ie, Philadelphia chromosome-positive cells undetectable by cytogenetic evaluation). However, more sensitive methods are required for monitoring residual disease (ie, molecular monitoring of BCR-ABL transcript levels). It is generally accepted that molecular responses have prognostic significance. Patients with CP CML who achieve early molecular responses are more likely to achieve durable cytogenetic responses and are less likely to experience disease progression. Rising BCR-ABL transcript levels also indicate loss of response, often as a consequence of developing BCR-ABL mutations. However, some studies have suggested that patients who achieve complete cytogenetic disease remission may not derive an additional prognostic benefit from achieving a major molecular response. Practical issues also exist for molecular monitoring with respect to restricted access and variability in methodologies and data reporting. Although molecular monitoring has a clear role in assessing residual disease and determining the risk of disease progression in patients with CML, the importance of cytogenetic monitoring should not be ignored.
Keywords: chronic myeloid leukemia, polymerase chain reaction, cytogenetic analysis, tyrosine kinase inhibitors
Chronic myeloid leukemia (CML) is characterized by a consistent cytogenetic abnormality known as the Philadelphia (Ph) chromosome. The Ph chromosome is generated by a reciprocal translocation between chromosomes 9 and 22, causing the ABL gene (encoding a nonreceptor tyrosine kinase) to fuse to the BCR gene. The resulting BCR-ABL oncogene encodes a protein with constitutive and aberrant Abl tyrosine kinase activity, which has been shown to play a causal role in CML.1,2 Bcr-Abl mediates the development and maintenance of CML through interaction with multiple downstream signaling partners, resulting in altered cellular adhesion, activation of mitogenic signaling, and inhibition of apoptosis, leading to the transformation of hematopoietic stem cells. Bcr-Abl signaling is also associated with defective DNA repair, which results in additional chromosomal alterations and mutations, and may partly explain the aggressive nature of advanced CML.3
Targeted inhibition of Bcr-Abl tyrosine kinase activity inhibits proliferation and induces apoptosis in Bcr-Abl–expressing human cells in vitro.4,5 Current drug treatments for CML, such as imatinib (Glivec [US: Gleevec]; Novartis, Basel, Switzerland), dasatinib (SPRYCEL; Bristol-Myers Squibb, New York, NY), and nilotinib (Tasinga; Novartis), aim to control disease by inhibiting Bcr-Abl activity and decreasing the number of Bcr-Abl–positive cells. Continuous monitoring of disease levels in individual patients is required to determine the effectiveness of specific therapies so that timely and appropriate decisions can be made regarding treatment strategy. Achieving defined levels of response (reductions in residual disease) within specified timeframes has prognostic significance, both in terms of the durability of treatment responses and progression-free survival (PFS).6 Molecular assessment of BCR-ABL transcript levels is widely used for monitoring CML disease status, and there are accumulating reports of molecular responses achieved with available treatments and associated prognostic benefits. However, there are conflicting data regarding the role of molecular monitoring compared with conventional assessments.
The aim of this review was to briefly summarize current recommendations for CML disease monitoring, to discuss studies reporting molecular treatment responses in CML patients, and to debate the prognostic value and potential limitations of molecular monitoring using available data in patients in chronic phase (CP).
Disease Monitoring in CML
Understanding the cellular and molecular basis of CML has allowed the development of disease monitoring methods that detect responses to therapy and disease recurrences at an early stage. Although treatment responses can be observed using hematologic evaluations, the most sensitive methods for assessing CML disease status involve the cytogenetic measurement of the frequency of Ph-positive cells and the molecular measurement of BCR-ABL transcript levels.6,7
Cytogenetic assessment is the most widely used method for disease monitoring in patients with CML. Ph-positive bone marrow cells in metaphase are quantified in a sample of ≥20 cells to determine cytogenetic response (CyR). Fluorescent in situ hybridization (FISH), which analyses of a higher number of cells (up to 200), can be used instead of conventional cytogenetic assessment for quantifying Ph positivity.7–9 However, a background level of false-positive results limits the use of FISH and prevents full correlation with conventional assessment. Recommendations state that cytogenetic assessments should be performed at least every 3 to 6 months until a complete cytogenetic response (CCyR: 0% Ph-positive cells) has been achieved and confirmed (Table 1).6,7,10 Current definitions of suboptimal response published by the European LeukemiaNet include failure to achieve a major CyR (MCyR: ≤35% Ph-positive cells) within 6 months of diagnosis or failure to achieve a CCyR within 12 months.6
TABLE 1.
European LeukemiaNet Response Definitions and Monitoring Recommendations in Patients With Chronic Myeloid Leukemia
| Cytogenetic response (% Ph-positive metaphases) | Molecular response (BCR-ABL transcript level) | |
|---|---|---|
| Definitions | Complete: 0% | Complete: transcript is nonquantifiable and nondetectable |
| Partial: 1–35% | ||
| Major: ≤35% | ||
| Minor: 36–65% | ||
| Minimal: 66–95% | Major: ≤0.10 ratio for BCR-ABL: standardized control gene | |
| None: >95% | ||
| Monitoring | Check at least every 6 mo until complete response achieved and confirmed, then at least every 12 mo | Check every 3 mo; mutational analysis in case of failure, suboptimal response, or transcript level increase |
Ph indicates Philadelphia chromosome. Adapted from Baccarani 2006.6
Once a patient has achieved a CCyR, cytogenetic evaluation is less useful for monitoring residual disease. Because of the presence of the leukemia-specific BCR-ABL gene, CML disease status can be monitored using real-time quantitative polymerase chain reaction (RT-qPCR) techniques to quantify levels of BCR-ABL mRNA in peripheral blood.6,7,11 Molecular monitoring is often reserved for patients who have achieved a CCyR and to our knowledge represents the most sensitive method available for monitoring disease status and residual disease. Some investigators have suggested that BCR-ABL monitoring should become the method of choice for monitoring patients receiving imatinib,12 although this has been debated.13 It is recommended that molecular evaluation is performed every 3 months in patients with CML.6,7,11 In a patient in stable CCyR, less frequent molecular monitoring (eg, every 6 months) may be reasonable. A recent internet-based analysis of treatment practice among hematologists/oncologists in the U.S. and Europe who treat patients with CML found that 39% and 53%, respectively, perform RT-qPCR every 3 months, with 38% and 31%, respectively, performing 6-month assessments.14
A major molecular response (MMR) has been defined as a ratio of BCR-ABL transcripts compared with a standardized control gene (normally ABL) of ≤0.1% (equivalent to a 3-log lower level),6,11 although other slight variations are classified as MMR within the published literature. According to LeukemiaNet guidelines, failure to achieve a MMR within 18 months of diagnosis and treatment represents a suboptimal treatment response.6 Achieving an undetectable or nonquantifiable level of BCR-ABL transcripts is classified as a complete molecular response (CMR).
There is broad correlation between levels of molecular and cytogenetic response.6,15 A BCR-ABL: ABL ratio of 10% (1-log reduction) approximates an MCyR and a 1% ratio (2-log reduction) approximates a CCyR. Significant differences in median BCR-ABL transcript levels have been demonstrated among patients with different levels of CyR.15
Molecular Responses Achieved in CML
Targeted tyrosine kinase inhibitors have produced significant and durable treatment responses in patients with CML, leading to a change in the natural course of the disease. Because a MMR represents a low level of residual disease, it could be argued that the subset of patients achieving this response derive the greatest clinical benefit from treatment. It should be noted that because of methodologic differences, molecular response rates cannot necessarily be directly compared between studies.
Imatinib is the current first-line treatment for all phases of CML.6,7 In the pivotal phase 3 International Randomized Study of Interferon (IFN) and STI571 (IRIS) trial conducted in 1106 patients with newly diagnosed CP CML, an estimated 87% of patients who received imatinib at a dose of 400 mg/day as initial therapy had achieved a CCyR at some point within 5 years of follow-up.16 The MMR rate at 12 months in the total group was estimated to be 37%. High response rates have also been observed in a single-arm study of patients with newly diagnosed CP CML who were treated with high-dose imatinib (800 mg/day).17 Over a median follow-up period of 15 months, 90% of 114 patients attained a CCyR, 63% attained a MMR (BCR-ABL:ABL ratio <0.05%), and 28% achieved a CMR.
Molecular responses to imatinib have also been reported in patients with CP CML treated after IFN failure. In a noncomparative study from a single center (median follow-up of 45 months; n = 261 patients), the CCyR rate was 63%, the MMR rate (BCR-ABL:ABL ratio <0.05%) was 31%, and the CMR rate was 15%.18 In a study conducted by the GIMEMA Working Party in CML of patients treated with imatinib for 2 years (n = 191), a CCyR was achieved in 44% of patients, and among patients in continuous cytogenetic disease remission, approximately half achieved a CMR.19 In a small study of patients receiving high-dose imatinib after IFN failure (n = 36), a CCyR was reported in 89%, with 56% and 41% of patients achieving a MMR (BCR-ABL:ABL ratio <0.045%) or CMR, respectively.20
To better understand why molecular responses are achieved in some imatinib recipients but not others, Cortes et al. performed a multivariate analysis to identify factors predicting molecular response.21 The study group included patients with CP CML who achieved a CCyR with imatinib administered at a dose of 400 or 800 mg/day after IFN failure (n = 117) or patients with newly diagnosed disease (n = 163) who were followed for a median of 31 months. During this period, the MMR (BCR-ABL:ABL ratio <0.05%) rate across the entire group was 62%, and the CMR rate was 34%. Using multivariate analyses, only high-dose imatinib treatment (800 mg/day) was found to be independently associated with a higher probability of achieving a MMR at any time (P =.02). In terms of achieving a CMR, only Ph-positive metaphases <90% at the onset of imatinib treatment were found to be significantly predictive (P =.01), with high-dose imatinib found to be marginally significant (P =.08). In patients with newly diagnosed disease, high-dose imatinib treatment was found to be predictive of MMR (P =.01) or CMR (P =.02) within 12 months.
Dasatinib is approved for patients with any phase of CML who are resistant or intolerant to imatinib.7 A randomized phase 2 study was performed comparing dasatinib treatment with high-dose imatinib (800 mg/day) in 150 patients who had failed prior therapy with imatinib administered at a dose of 400 to 600 mg/day.22 With a median follow-up of 15 months, CCyRs were detected in 40% of the dasatinib group compared with 16% of the patients treated with high-dose imatinib (P =.004). In addition, MMRs were detected in 16% of dasatinib recipients compared with 4% of imatinib recipients (P =.038). In a single-arm phase 2 study of dasatinib in patients with CP CML after imatinib failure (n = 186), the median BCR-ABL:ABL ratio decreased from 66% at baseline to 2.6% after 9 months.23 Preliminary data have been presented from a study of first-line dasatinib therapy in patients with CP CML. Among 21 evaluable patients assessed after 12 months of treatment, 33% achieved a MMR (BCR-ABL:ABL ratio <0.05%) and 5% achieved a CMR.24
Prognostic Implications of Molecular Responses
There is an increasing body of evidence indicating that achieving a molecular response has prognostic significance, both in terms of durability of treatment responses and PFS. Molecular monitoring, therefore, may be particularly useful for identifying patients at greatest risk for disease recurrence or progression.
To our knowledge, 2 studies to date have demonstrated that early reductions in BCR-ABL transcript levels predict the subsequent achievement of an MCyR. Merx et al. reported that among patients receiving imatinib after IFN failure, an early reduction in the BCR-ABL:ABL ratio to <20% of baseline at 2 months was correlated with a significantly higher probability of MCyR at 6 months (P =.007).25 In a similar study by Wang et al., decreases in the BCR-ABL:ABL ratio to <50% of baseline after 4 weeks or to <10% after 3 months were associated with a significantly higher probability of achieving a MCyR at 6 months (P <.001).26
Molecular responses also are predictive of the duration of CCyR. In a study by the Mannheim group, mean levels of the best BCR-ABL:ABL ratio reduction achieved were significantly lower in patients with continuous cytogenetic disease remissions compared with those who developed disease recurrence (P =.0011).27 In addition, no patient who achieved a MMR subsequently experienced cytogenetic disease recurrence, compared with 46% of patients who did not achieve a MMR and subsequently developed recurrence (P =.0036; median follow-up of 13 months). Similarly, investigators at the University of Texas M. D. Anderson Cancer Center reported that a significantly lower proportion of patients achieving a molecular response (BCR-ABL:ABL ratio <0.05%; 5%) or CMR (4%) subsequently lost their CCyR compared with patients who did not achieve a MMR (37%; P =.0001).21 Furthermore, patients who achieved a molecular response within 6 months or 12 months were found to have a significantly longer duration of CCyR compared with those without a molecular response. A comparable trend was reported by the GIMEMA group; patients who received imatinib after IFN failure and who attained a MMR at the time of first achieving a CCyR, or patients who attained a MMR within 12 months, had a significantly longer duration of cytogenetic disease remission than comparator groups (P <.05 and P =.021, respectively).28
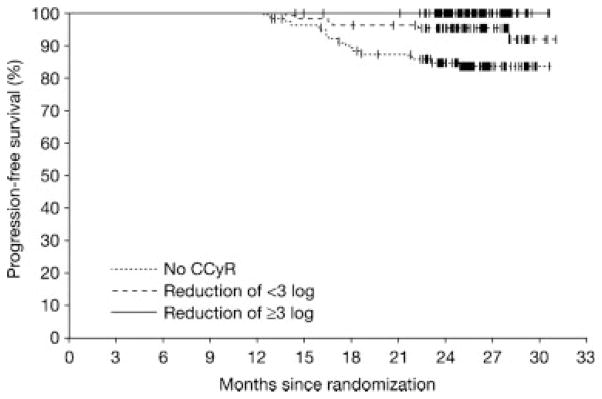
Importantly, early molecular response also may predict lack of disease progression. In the IRIS trial, patients who had a CCyR and MMR (3-log reduction) at 12 months had a 100% probability of remaining free from disease progression at 24 months, compared with a 95% probability in patients who had a CCyR but no MMR (P =.007) (Fig. 1).29 In the study by Wang et al., decreases in the BCR-ABL:ABL ratio with imatinib therapy to <50% after 4 weeks or <10% after 3 months were associated with a superior PFS (P ≤ .01; median follow-up of 16.5 months).26 Similarly, a recently reported study found that rates of disease progression were significantly lower in patients who had achieved BCR-ABL transcript ratios of <10% at 3 months (P =.04) or <1% at 6 months (P =.005).30 Achieving a 2-log molecular response at the time of CCyR also was found to be predictive of a significantly longer duration of PFS (P =.005).31
FIGURE 1.
Probability of progression-free survival in the International Randomized Study of Interferon (IFN) and STI571 (IRIS) trial among imatinib-treated patients without a complete cytogenetic response (CCyR) at 12 months or patients who achieved a CCyR with or without a concurrent major molecular response (MMR) (BCR-ABL reduction of at least 3 log). P was <.001 for the overall comparison, .013 for the comparison of patients without a CCyR with those with a CCyR plus MMR, and .007 for the comparison of patients with a CCyR with or without a MMR.29
In addition to predicting the duration of CyR or PFS, BCR-ABL transcript levels may also provide an early indication of loss of response. A 2-fold or serial rise in BCR-ABL levels has been associated with BCR-ABL mutation and the development of acquired imatinib resistance (P < .0001).32,33 Among 183 patients with CP CML (median follow-up of 20 months) with simultaneous RT-qPCR and bone marrow cytogenetic measurements, no patient experienced cytogenetic disease progression without an indication from molecular monitoring (20 of 24 patients had a simultaneous or preceding BCR-ABL level increase of at least 2-fold).15
Overall, various data indicate that achieving a molecular response, and particularly an early molecular response, predicts the subsequent achievement and duration of cytogenetic responses, as well as a significantly lower likelihood of disease progression. However, some questions still remain regarding the use of molecular monitoring.
Potential Issues With Molecular Monitoring
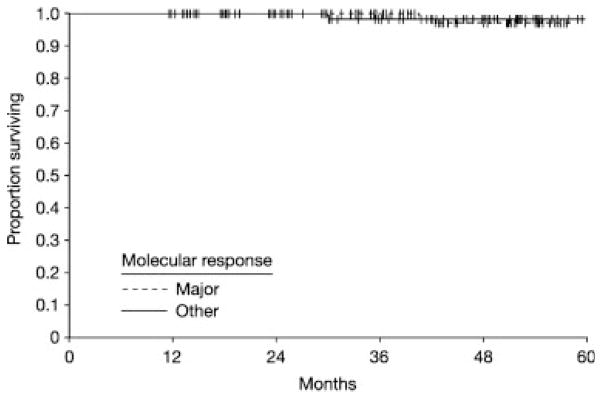
Although the prognostic significance of molecular responses in CML is widely stated, to our knowledge no studies to date have reported an advantage of greater overall survival associated with achieving a molecular response, although this may reflect the duration of follow-up. One study to date has reported that in patients with newly diagnosed CML who achieved a CCyR with imatinib treatment, the overall survival and PFS were similar regardless of whether a molecular response had been achieved (Fig. 2).34 In this study, the survival benefit for imatinib treatment was associated with an improvement of CyR. In a study of patients with CP or accelerated-phase CML, cytogenetic, but not molecular, response at 3 months was identified by multivariate analysis as the only independent parameter predictive of PFS at 2 years.13 It should also be noted that in the IRIS study 5-year update, freedom from disease progression to advanced disease was found to be similar in patients in cytogenetic disease remission at 18 months with or without a molecular response (100% vs 98%; P = .11), but was significantly lower in patients who did not achieve cytogenetic disease remission (87%; P <.001).16 These data may support a hypothesis that for some endpoints, achieving a CCyR has greater prognostic significance than achieving a molecular response in CML. The predictive value of achieving a molecular response within a particular timeframe after cytogenetic disease remission also has been questioned. In the study by Press et al., once CCyR had been achieved with imatinib treatment, the PFS benefit associated with achieving a subsequent MMR existed regardless of when the MMR occurred.31
FIGURE 2.
Survival with imatinib therapy by molecular response at 12 months in patients with newly diagnosed chronic myeloid leukemia in the chronic phase who achieved a complete cytogenetic response with (n = 91) or without (n = 96) a concurrent major molecular response (P =.87). One patient in each group died.34
There are practical issues associated with measuring molecular responses. Molecular monitoring of CML is performed in a restricted number of centers worldwide. Techniques for BCR-ABL measurement vary with respect to choice of control gene, quality of RNA extract, and assay sensitivity. The apparent differences between MMR and CMR rates reported in separate studies often reflect variability in testing methods, making accurate comparisons impossible, and a “negative” test (CMR) may simply indicate inadequate test sensitivity.35 Precise standardization is required before data from different laboratories can be compared.11,36 Confusion surrounding terminology also may contribute to difficulties in reporting and understanding molecular monitoring. The term “log reduction” may be erroneously interpreted to mean that BCR-ABL levels are decreased relative to baseline in a particular individual, rather than relative to an established control level.11 To address some of these issues, an international scale for molecular responses has been proposed, in which BCR-ABL levels are reported as a percentage ratio of a control gene that has been validated using reference standards.
A note of caution regarding molecular monitoring terminology has also been voiced. The term “complete molecular response” may be interpreted as an absolute lack of leukemia, which is misleading.12 Although imatinib treatment is highly effective, the most primitive leukemic stem cells are resistant to imatinib and persist despite continuous treatment.37,38 Data from several reports indicate that discontinuing imatinib treatment after CMR results in disease recurrence in approximately 50% of patients, usually occurring rapidly after discontinuation.39–43
In summary, some questions may still remain regarding the significance of molecular monitoring and reporting in patients with CML.
Conclusions
The value of measuring molecular response in CML is solidified by its usefulness as a sensitive predictor of cytogenetic disease remission and lack of disease progression. Whether this supersedes or adds to the cytogenetic response assessment requires further evaluation. Several studies have demonstrated that early molecular responses of different levels are associated with significantly improved long-term outcomes. Rising BCR-ABL levels may provide the earliest indication that a patient has become resistant to imatinib and/or whether BCR-ABL mutation has occurred. Therefore, molecular monitoring enables timely decisions to be made with regard to therapeutic strategy. Standardization of testing procedures may make molecular monitoring more widespread, and as experience increases, potential benefits should become clearer. Although available evidence clearly demonstrates that molecular monitoring has an important role in CML, the prognostic significance of achieving a CCyR should not be forgotten.
Acknowledgments
Dr. Kantarjian has received research grants from Novartis and Bristol-Myers Squibb.
Dr. Cortes has received research grants from Novartis, Bristol-Myers Squibb, and Wyeth.
References
- 1.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- 2.Kantarjian HM, Talpaz M, Giles F, O’Brien S, Cortes J. New insights into the pathophysiology of chronic myeloid leukemia and imatinib resistance. Ann Intern Med. 2006;145:913–923. doi: 10.7326/0003-4819-145-12-200612190-00008. [DOI] [PubMed] [Google Scholar]
- 3.Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004;103:4010–4022. doi: 10.1182/blood-2003-12-4111. [DOI] [PubMed] [Google Scholar]
- 4.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 5.Gambacorti-Passerini C, le Coutre P, Mologni L, et al. Inhibition of the ABL kinase activity blocks the proliferation of BCR/ABL+ leukemic cells and induces apoptosis. Blood Cells Mol Dis. 1997;23:380–394. doi: 10.1006/bcmd.1997.0155. [DOI] [PubMed] [Google Scholar]
- 6.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: chronic myelogenous leukemia, version 2.2008. Fort Washington, PA: National Comprehensive Cancer Network; 2007. [Accessed October 2007]. Available at: http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf. [DOI] [PubMed] [Google Scholar]
- 8.Lesser ML, Dewald GW, Sison CP, Silver RT. Correlation of 3 methods of measuring cytogenetic response in chronic myelocytic leukemia. Cancer Genet Cytogenet. 2002;137:79–84. doi: 10.1016/s0165-4608(02)00558-7. [DOI] [PubMed] [Google Scholar]
- 9.Schoch C, Schnittger S, Bursch S, et al. Comparison of chromosome banding analysis, interphase- and hypermetaphase-FISH, qualitative and quantitative PCR for diagnosis and for follow-up in chronic myeloid leukemia: a study on 350 cases. Leukemia. 2002;16:53–59. doi: 10.1038/sj.leu.2402329. [DOI] [PubMed] [Google Scholar]
- 10.Simonsson B on behalf of the ESMO Guidelines Working Group. Chronic myelogenous leukemia: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2007;18(suppl 2):ii51–ii52. doi: 10.1093/annonc/mdm035. [DOI] [PubMed] [Google Scholar]
- 11.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes T, Branford S. Molecular monitoring of BCR-ABL as a guide to clinical management in chronic myeloid leukaemia. Blood Rev. 2006;20:29–41. doi: 10.1016/j.blre.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Lange T, Bumm T, Otto S, et al. Quantitative reverse transcription polymerase chain reaction should not replace conventional cytogenetics for monitoring patients with chronic myeloid leukemia during early phase of imatinib therapy. Haematologica. 2004;89:49–57. [PubMed] [Google Scholar]
- 14.Kantarjian HM, Cortes J, Guilhot F, Hochhaus A, Baccarani M, Lokey L. Diagnosis and management of chronic myeloid leukemia: a survey of American and European practice patterns. Cancer. 2007;109:1365–1375. doi: 10.1002/cncr.22523. [DOI] [PubMed] [Google Scholar]
- 15.Ross DM, Branford S, Moore S, Hughes TP. Limited clinical value of regular bone marrow cytogenetic analysis in imatinib-treated chronic phase CML patients monitored by RQ-PCR for BCR-ABL. Leukemia. 2006;20:664–670. doi: 10.1038/sj.leu.2404139. [DOI] [PubMed] [Google Scholar]
- 16.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian H, Talpaz M, O’Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian HM, Cortes JE, O’Brien S, et al. Long-term survival benefit and improved complete cytogenetic and molecular response rates with imatinib mesylate in Philadelphia chromosome-positive chronic-phase chronic myeloid leukemia after failure of interferon-alpha. Blood. 2004;104:1979–1988. doi: 10.1182/blood-2004-02-0711. [DOI] [PubMed] [Google Scholar]
- 19.Rosti G, Martinelli G, Bassi S, et al. Molecular response to imatinib in late chronic-phase chronic myeloid leukemia. Blood. 2004;103:2284–2290. doi: 10.1182/blood-2003-07-2575. [DOI] [PubMed] [Google Scholar]
- 20.Cortes J, Giles F, O’Brien S, et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-alpha. Blood. 2003;102:83–86. doi: 10.1182/blood-2003-01-0025. [DOI] [PubMed] [Google Scholar]
- 21.Cortes J, Talpaz M, O’Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 22.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 23.Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 24.Atallah EL, Kantarjian H, O’Brien S, et al. Use of dasatinib in patients with previously untreated chronic myelogenous leukemia in chronic phase. J Clin Oncol ASCO Annu Meet Proc Pt I. 2007;25(18 suppl):358s. Abstract 7005. [Google Scholar]
- 25.Merx K, Muller MC, Kreil S, et al. Early reduction of BCR-ABL mRNA transcript levels predicts cytogenetic response in chronic phase CML patients treated with imatinib after failure of interferon alpha. Leukemia. 2002;16:1579–1583. doi: 10.1038/sj.leu.2402680. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Pearson K, Ferguson JE, Clark RE. The early molecular response to imatinib predicts cytogenetic and clinical outcome in chronic myeloid leukaemia. Br J Haematol. 2003;120:990–999. doi: 10.1046/j.1365-2141.2003.04200.x. [DOI] [PubMed] [Google Scholar]
- 27.Paschka P, Muller MC, Merx K, et al. Molecular monitoring of response to imatinib (Glivec) in CML patients pretreated with interferon alpha. Low levels of residual disease are associated with continuous remission. Leukemia. 2003;17:1687–1694. doi: 10.1038/sj.leu.2403033. [DOI] [PubMed] [Google Scholar]
- 28.Iacobucci I, Saglio G, Rosti G, et al. Achieving a major molecular response at the time of a complete cytogenetic response (CCgR) predicts a better duration of CCgR in imatinib-treated chronic myeloid leukemia patients. Clin Cancer Res. 2006;12:3037–3042. doi: 10.1158/1078-0432.CCR-05-2574. [DOI] [PubMed] [Google Scholar]
- 29.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 30.Quintas-Cardama A, Kantarjian HM, Jones D, et al. Delayed achievement of molecular responses is associated with increased risk of progression among patients with chronic myelogenous leukemia in chronic phase treated with imatinib. Blood ASH Annu Meet Proc. 2006;108:613a. Abstract 432. [Google Scholar]
- 31.Press RD, Love Z, Tronnes AA, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response (CCR) predict the duration of CCR in imatinib mesylate-treated patients with CML. Blood. 2006;107:4250–4256. doi: 10.1182/blood-2005-11-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Branford S, Rudzki Z, Parkinson I, et al. Real-time quantitative PCR analysis can be used as a primary screen to identify patients with CML treated with imatinib who have BCR-ABL kinase domain mutations. Blood. 2004;104:2926–2932. doi: 10.1182/blood-2004-03-1134. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Knight K, Lucas C, Clark RE. The role of serial BCR-ABL transcript monitoring in predicting the emergence of BCR-ABL kinase mutations in imatinib-treated patients with chronic myeloid leukemia. Haematologica. 2006;91:235–239. [PubMed] [Google Scholar]
- 34.Kantarjian HM, Talpaz M, O’Brien S, et al. Survival benefit with imatinib mesylate versus interferon alpha-based regimens in newly diagnosed chronic phase chronic myelogenous leukemia. Blood. 2006;108:1835–1840. doi: 10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- 35.Oehler V, Radich JP. Monitoring patients with chronic myeloid leukemia receiving Abl tyrosine kinase inhibitor therapy. Clin Lymphoma Myeloma. 2007;7(suppl 2):S58–S63. doi: 10.3816/clm.2007.s.003. [DOI] [PubMed] [Google Scholar]
- 36.Branford S, Cross NC, Hochhaus A, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia. 2006;20:1925–1930. doi: 10.1038/sj.leu.2404388. [DOI] [PubMed] [Google Scholar]
- 37.Bhatia R, Holtz M, Niu N, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 38.Michor F, Hughes TP, Iwasa Y, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–1270. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- 39.Cortes J, O’Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood. 2004;104:2204–2205. doi: 10.1182/blood-2004-04-1335. [DOI] [PubMed] [Google Scholar]
- 40.Mauro MJ, Druker BJ, Maziarz RT. Divergent clinical outcome in 2 CML patients who discontinued imatinib therapy after achieving a molecular remission. Leuk Res. 2004;28(suppl 1):S71–S73. doi: 10.1016/j.leukres.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Hess G, Bunjes D, Siegert W, et al. Sustained complete molecular remissions after treatment with imatinib-mesylate in patients with failure after allogeneic stem cell transplantation for chronic myelogenous leukemia: results of a prospective phase II open-label multicenter study. J Clin Oncol. 2005;23:7583–7593. doi: 10.1200/JCO.2005.01.3110. [DOI] [PubMed] [Google Scholar]
- 42.Merante S, Orlandi E, Bernasconi P, Calatroni S, Boni M, Lazzarino M. Outcome of 4 patients with chronic myeloid leukemia after imatinib mesylate discontinuation. Haematologica. 2005;90:979–981. [PubMed] [Google Scholar]
- 43.Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]