Abstract
Identifying lipids that experience coordinated metabolism during heat stress would provide information regarding lipid dynamics under stress conditions and assist in developing heat-tolerant wheat varieties. We hypothesized that co-occurring lipids, which are up-or-down-regulated together through time during heat stress, represent groups that can be explained by coordinated metabolism. Wheat plants (Triticum aestivum L.) were subjected to 12 days of high day and/or night temperature stress, followed by a 4-day recovery period. Leaves were sampled at four time points, and 165 lipids were measured by electrospray ionization-tandem mass spectrometry. Correlation analysis of lipid levels in 160 leaf samples from each of two wheat genotypes revealed 13 groups of lipids. Lipids within each group co-occurred through the high day and night temperature stress treatments. The lipid groups can be broadly classified as groups containing: extraplastidic phospholipids, plastidic glycerolipids, oxidized glycerolipids, triacylglycerols, acylated sterol glycosides, and sterol glycosides. Current knowledge of lipid metabolism suggests that the lipids in each group co-occur because they are regulated by the same enzyme(s). The results suggest that increases in activities of desaturating, oxidizing, glycosylating, and acylating enzymes lead to simultaneous changes in levels of multiple lipid species during high day and night temperature stress in wheat.
Keywords: wheat, lipid groups, high day temperature, high night temperature, lipid co-occurrence, coordinated metabolism, correlation analysis, direct infusion automated electrospray ionization-tandem mass spectrometry, dendrogram, lipidomics
INTRODUCTION
Global mean surface air temperature has increased by 0.5°C in the 20th century (Intergovernmental Panel on Climate Change, 2013). This increase is brought about by increases in day-maximum and night-minimum temperatures (Harvey, 1995; Easterling et al., 1997, 2000). High day and night temperatures for seven days during flowering decrease seed set, grain number, and grain yield in wheat by 13-18% (Narayanan et al., 2015) and thresholds for temperature and duration are quantified (Prasad and Djanaguiraman, 2014). In the companion paper (Narayanan et al., 2015), we found that high day and night temperatures cause major lipid alterations in wheat that might have associated with heat tolerance or susceptibility. These lipid alterations include changes in the amounts of galactolipids, phospholipids, triacylglycerols, sterol lipids, and lipids with oxidized acyl chains. Identifying lipids that experience coordinated metabolism would provide further details regarding lipid dynamics under high temperature stress conditions and assist in developing heat-tolerant wheat varieties.
Application of stress to a plant activates or suppresses multiple pathways involving various lipid metabolizing enzymes. For example, low temperature activates phospholipase A and phospholipase D in Arabidopsis (Arabidopsis thaliana) plants (Welti et al., 2002). Phospholipase A cleaves acyl side chains of phospholipids and releases lysophospholipids and free fatty acids, while phospholipase D hydrolyzes phospholipids to phosphatidicacid (PA) and free head groups. The increased level of PA and lysophospholipids may lead to destabilizing membrane bilayers that result in membrane fusion and cell death (Cullis and Dekruijff, 1979; Welti et al., 2002). High temperature leads to reduced activity of digalactosyldiacylglycerol synthase 1, which catalyzes the conversion of monogalactosyldiacylglycerol (MGDG) to digalactosyldiacylglycerol (DGDG), in susceptible plants (reviewed by Welti et al., 2007). The resulting increase in the ratio of MGDG to DGDG could lead to loss of membrane integrity. Wounding stress causes activation of phospholipase D and phospholipase A and oxidation of fatty acids on galactolipids (Narvaez-Vasquez et al., 1999; Ryu, 2004; Buseman et al., 2006; Zien et al., 2001). The amount of oxidized galactolipids might reflect the degree of oxidative stress a plant is experiencing.
Many lipid-metabolizing enzymes act on multiple, related substrates, as many lipid species contain similar component acyl chains or head group (e.g., phospholipase D acts on many phospholipids such as phosphatidylcholine [PC], phosphatidylethanolamine [PE] and phosphatidylglycerol [PG] [Welti et al., 2002]). This is confirmed by gene knock-out or suppression studies in which suppression of individual lipid-metabolizing enzymes affected multiple lipid molecular species (Welti et al., 2002; Peters et al., 2010). Taken together, these reports suggest that, under stress conditions, plant lipid composition will change due to activation of multiple enzymes, and activation of most of these enzymes will result in simultaneous changes in a group of lipids acted on by the same enzyme.
In the companion paper, we found that high day and night temperature stress caused significant changes in the lipid profile of wheat (Triticum aestivum L.) genotypes, and plants responded to high temperature stress by lipid remodeling and decreasing the level of lipid unsaturation (Narayanan et al., 2015). In the current work, we hypothesize that co-occurring lipids, which are up-or-down-regulated together through time under high day and night temperature conditions, represent groups that can be explained by co-metabolism. We employ correlation analyses (on lipid data collected from the experiment described in the companion paper, Narayanan et al., 2015) to detect co-occurring lipid groups, as has recently been done by Vu et al. (2014a), who analyzed plants undergoing wounding stress in Arabidopsis.
MATERIALS AND METHODS
The experimental design, plant material and growth conditions, and the lipid extraction and ESI-MS/MS lipid profiling procedures were described in the companion paper; these papers describe two aspects of the same experiment. A summary of the experimental design is given in Supporting Information Fig. S1. Please see ‘Materials and Methods’ section of the companion paper (Narayanan et al., 2015) for details. However, the present paper describes lipid data collected on days 1, 6, and 12 during the stress period and day 4 during the recovery period (referred as day 16 as stress was imposed for 12 days), whereas, the companion paper describes only data collected on day 12.
Data analyses
The lipid data of each genotype (Supporting Information Table S4 of the companion paper, Narayanan et al., 2015) were uploaded to Cluster 3.0 (Eisen et al., 1998) for determining lipid clusters. Cluster 3.0 generated lipid clusters for each genotype using a single-linkage hierarchical algorithm based on Spearman’s correlation coefficient, ρ. The clustering outputs (.gtr and .cdt files) were converted to NEWICK format (.nwk) using a Python script written by Haibao Tang (J. Craig Venter Institute, Rockville, MD, USA). The script can be obtained from the following link: https://github.com/tanghaibao/treecut/blob/master/scripts/eisen_to_newick.py. The NEWICK files of each genotype were exported to Dendroscope (Huson et al., 2007; Huson and Scornavacca, 2012) to produce the dendrograms (Figs. 1 and 2), which were modified in color. These dendrograms include clusters of lipids in which every lipid is correlated with another lipid with ρ ≥ 0.80. Of the 165 lipids analyzed in our study, 79 were included in the clusters of one or both genotypes. Lipid groups were assigned (Fig. 3) using the lipids included in clusters of one or both genotype. A combined dendrogram (Supporting Information Fig. S2) was produced using the data on the 79 lipids, pooled across genotypes.
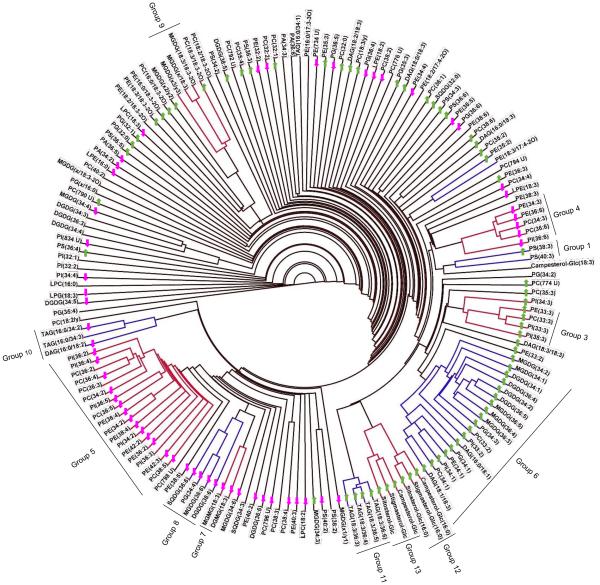
Figure 1.
Lipid dendrogram of wheat genotype Ventnor. One hundred sixty-five lipid analytes were clustered using a single-linkage hierarchical algorithm based on Spearman’s correlation coefficient, ρ (Supporting Information Table S2). Twelve clusters with ρ ≥ 0.80 are indicated by red and blue bars on the dendrogram. Co-occurring lipid groups, which are composed of whole clusters or parts of clusters, are marked on the dendrogram. The arrows on the dendrogram indicate the directionality of significant differences in levels of each lipid under high day and night temperature stress conditions compared to optimum temperature conditions on day 12; lipids that increased in amount are indicated by green-colored upward arrows, and lipids that decreased in amount are indicated by pink-colored downward arrows. PG(x/16:0) indicates PG(18:4-O/16:0) or PG(19:3/16:0). MGDG(x/18:3) indicates MGDG(18:4-O/18:3) or MGDG(19:3/18:3). MGDG(x1/y1) indicates MGDG(18:4-O/17:3) or MGDG(19:3/16:4-O). MGDG(x2/y2) indicates MGDG(18:3-2O/16:3), MGDG(18:4-O/17:1), or MGDG(19:3/17:1). MGDG(x3/y3) indicates MGDG(18:4-O/18:1), MGDG(19:3/16:3-2O), MGDG(18:3-2O/17:3), or MGDG(18:3-2O/16:4-O). MGDG(x/18:3-2O) indicates MGDG(18:4-O/18:3-2O) or MGDG(19:3/18:3-2O). PC(18:3/y) indicates PC(18:3/18:2-O) or PC(18:3/17:3-2O). PC(18:2/y) indicates PC(18:2/18:2-O) or PC(18:2/17:3-2O).
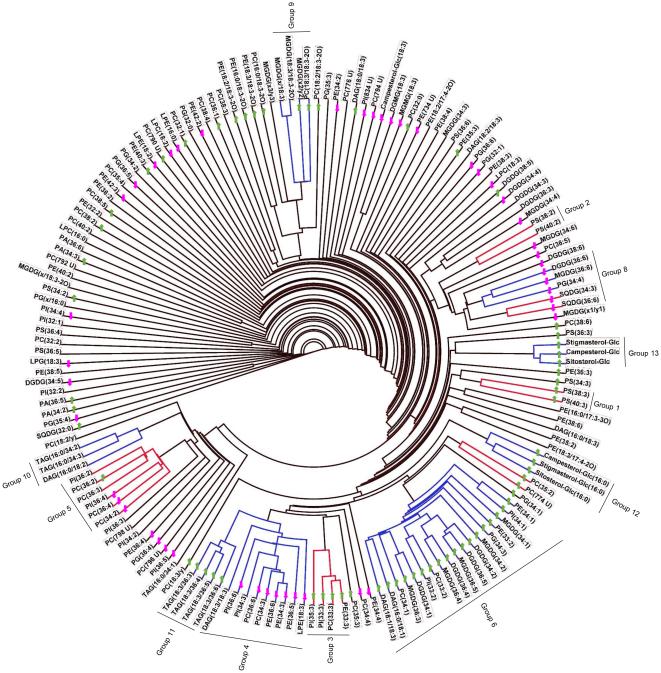
Figure 2.
Lipid dendrogram of wheat genotype Karl 92. One hundred sixty-five lipid analytes were clustered using a single-linkage hierarchical algorithm based on Spearman’s correlation coefficient, ρ (Supporting Information Table S3). Thirteen clusters with ρ ≥ 0.80 are indicated by red and blue bars on the dendrogram. Co-occurring lipid groups, which are composed of whole clusters, parts of clusters, or a combination of two adjacent clusters, are marked on the dendrogram. The arrows on the dendrograms indicate the directionality of significant differences in levels of each lipid under high day and night temperature stress conditions compared to optimum temperature conditions on day 12; lipids that increased in amount are indicated by green-colored upward arrows and lipids that decreased in amount are indicated by pink-colored downward arrows. PG(x/16:0) indicates PG(18:4-O/16:0) or PG(19:3/16:0). MGDG(x/18:3) indicates MGDG(18:4-O/18:3) or MGDG(19:3/18:3). MGDG(x1/y1) indicates MGDG(18:4-O/17:3) or MGDG(19:3/16:4-O). MGDG(x2/y2) indicates MGDG(18:3-2O/16:3), MGDG(18:4-O/17:1), or MGDG(19:3/17:1). MGDG(x3/y3) indicates MGDG(18:4-O/18:1), MGDG(19:3/16:3-2O), MGDG(18:3-2O/17:3), or MGDG(18:3-2O/16:4-O). MGDG(x/18:3-2O) indicates MGDG(18:4-O/18:3-2O) or MGDG(19:3/18:3-2O). PC(18:3/y) indicates PC(18:3/18:2-O) or PC(18:3/17:3-2O). PC(18:2/y) indicates PC(18:2/18:2-O) or PC(18:2/17:3-2O).
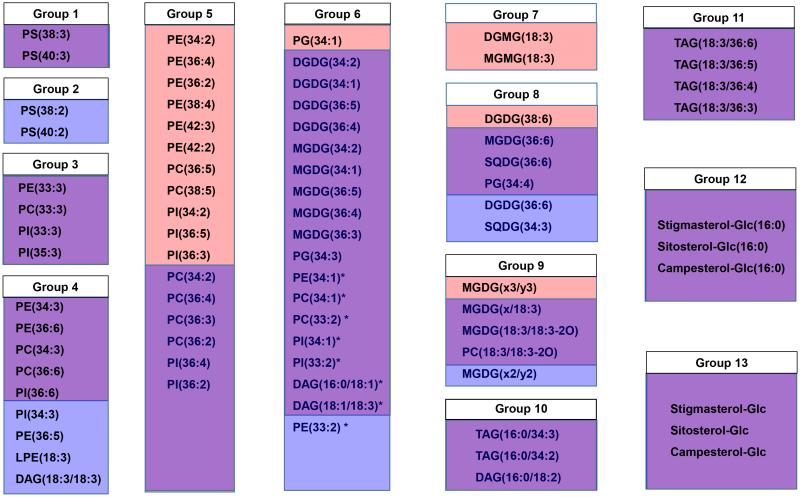
Figure 3.
Comparison of lipid groups between genotypes Ventnor and Karl 92. Red boxes indicate lipids that were included in a group only in Ventnor, blue boxes indicate lipids that were included in a group only in Karl 92, and lavender boxes indicate lipids that were included in a group in both genotypes. Group 6 is divided into extraplastidic (PE, PC, PI, and DAG; Group 6a, marked with asterisks) and plastidic (DGDG, MGDG, and PG; Group 6b) groups. In Group 9, MGDG(x/18:3) indicates MGDG(18:4-O/18:3) or MGDG(19:3/18:3); MGDG(x2/y2) indicates MGDG(18:3-2O/16:3), MGDG(18:4-O/17:1), or MGDG(19:3/17:1); and MGDG(x3/y3) indicates MGDG(18:4-O/18:1), MGDG(19:3/16:3-2O), MGDG(18:3-2O/17:3), or MGDG(18:3-2O/16:4-O).
Utilities of the MetaboAnalyst web server were used to perform the auto scaling of lipid data (Supporting Information Table S1) and to produce the heat map (Fig. 4a) and correlation tables (Supporting Information Tables S2 and S3) (metabolanalyst.ca; Xia et al., 2009, 2012). Auto scaling allows for easy comparison of lipid levels in different samples. The autoscaled value of a lipid in a sample is calculated as: [(the amount of lipid in that sample) – (the average amount of that lipid among all samples)] divided by (the standard deviation for the amount of that lipid among all samples).
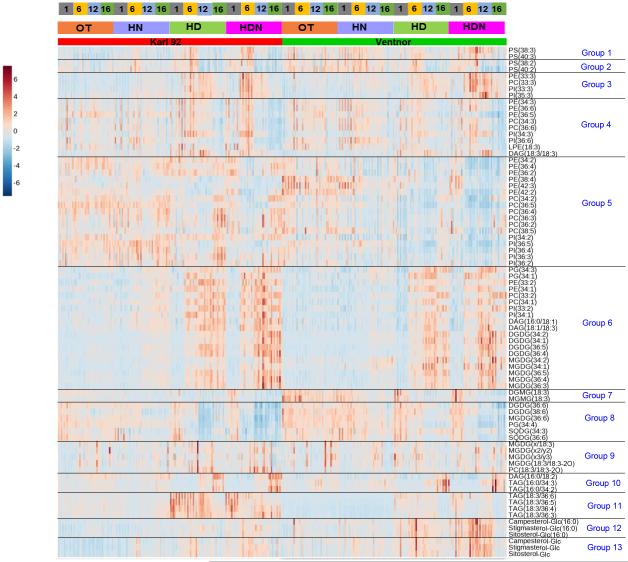
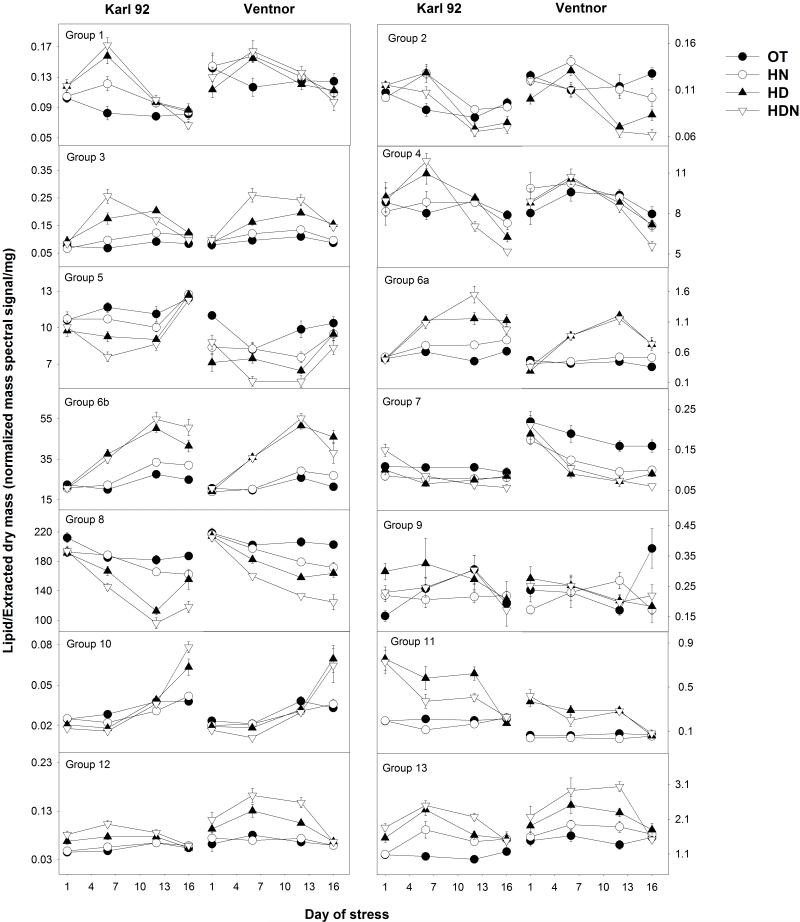
Figure 4a.
Heat map of lipid groups. Genotypes (Ventnor and Karl 92), temperature regimes (optimum temperature [OT; 25/15°C, day-maximum/night-minimum], high night temperature [HN; 25/24°C], high day temperature [HD; 35/15°C], and high day and night temperature [HDN; 35/24°C]), and days of measurement (days 1, 6, 12, and 16, with day 1 indicating the 1st day of stress and day 16 indicating the recovery period, as stress was imposed for 12 days) are indicated at the top. Auto scaled data from 320 plant samples (two genotypes, four temperature regimes, four days of measurement, five replications [plants] and two experiments) are shown in the figure. Data corresponding to day 12 in the above figure are the same as those presented in Figures 4, 5, 7, 8, 9, and 10 in the companion paper (Narayanan et al., 2015). Group 1- PS with an 18:3 acyl chain and a long acyl chain, Group 2- PS with an 18:2 acyl chain and a long acyl chain, Group 3- Extraplastidic lipids with a 15:0 or 17:0 acyl chain combined with an 18:3 acyl chain, Group 4- Extraplastidic lipids with an 18:3 acyl chain and an 18:3, 18:2, or 16:0 acyl chain, Group 5- Extraplastidic lipids with an 18:2 acyl chain, Group 6- Polar lipids with 18:1 and/or 18:2 acyl chains, Group 7- Plastidic monoacyl lipids with 18:3 acyl chains, Group 8- Plastidic diacyl lipids with 18:3 acyl chains, Group 9- Ox-lipids, Group 10- TAGs and DAGs with a 16:0 acyl chain(s), Group 11- TAGs with an 18:3 acyl chain(s), Group 12- ASGs, Group 13- SGs. In Group 9, MGDG(x/18:3) indicates MGDG(18:4-O/18:3) or MGDG(19:3/18:3); MGDG(x2/y2) indicates MGDG(18:3-2O/16:3), MGDG(18:4-O/17:1), or MGDG(19:3/17:1); and MGDG(x3/y3) indicates MGDG(18:4-O/18:1), MGDG(19:3/16:3-2O), MGDG(18:3-2O/17:3), or MGDG(18:3-2O/16:4-O).
RESULTS AND DISCUSSION
Wheat genotypes (Ventnor, heat-tolerant and Karl 92, heat-susceptible) were grown at optimum temperature conditions (OT; 25/15°C, maximum/minimum) until the onset of flowering. Thereafter, plants were exposed to HN (25/24°C), HD (35/15°C), HDN (35/24°C), or OT for 12 days. Plants were allowed to recover under OT from day 12 to 16. For lipid extraction, leaf samples were collected from five plants per genotype from each temperature regime on days 1, 6, 12, and 16, with day 1 indicating the 1st day of stress and day 16 indicating the recovery period (Supporting Information Fig. S1). A direct infusion automated electrospray ionization-tandem mass spectrometry (ESI-MS/MS) approach was used to quantitatively profile the lipid molecular species.
Variation in lipid analytes among samples under varied heat treatments can be used to group lipids
Autoscaled levels of lipids in individual samples are presented in Supporting Information Table S1. Within each temperature regime, the amounts of different lipids varied on days 1, 6, 12, and 16. To test the hypothesis that co-occurring lipids under high day and night temperatures represent groups that can be explained by co-metabolism, Spearman’s correlation coefficient (ρ) was calculated among lipid analytes across all 160 individual plant samples of each genotype (2 trials of 5 replicates each under 4 temperature regimes over 4 sampling days) (Supporting Information Tables S2 and S3). Spearman’s correlation coefficient ranges from -1 (perfect negative correlation) to 1 (perfect positive correlation), and a value of zero indicates no correlation.
By matching each lipid analyte with the one to which it was most highly correlated, dendrograms were created for genotypes Ventnor and Karl 92 (Figs. 1 and 2, respectively; see “Materials and Methods”). These dendrograms include clusters of lipids in which every lipid is correlated with at least one other lipid within the group with ρ ≥ 0.80. The arrows on the dendrograms indicate the directionality of significant differences in levels of each lipid under HDN compared to OT on day 12. The dendrograms of Ventnor and Karl 92 included 12 (Fig. 1) and 13 clusters (Fig. 2), respectively, which are indicated by the red and blue bars on each dendrogram. Of the 165 lipids analyzed in our study, 48% were included in the clusters. Vu et al. (2014a) reported that high analytical precision helps in detection of clusters, whereas lack of detected correlation could be due to either poor analytical precision or true lack of co-occurrence. Only one negative correlation with ρ ≤ −0.80 (between 36:6-SQDG and 34:2-DGDG in Ventnor) was found in either genotype (Supporting Information Tables S2 and S3).
Clusters in Ventnor and Karl 92 (Figs. 1 and 2) were similar. Comparison of the two dendrograms and construction of a combined dendrogram (Supporting Information Fig. S2) allowed us to define 13 lipid groups (Fig. 3; see “Materials and Methods”). The relationships between the groups and the clusters detected in the individual dendrograms are shown in Fig. 3, where lavender indicates lipids found in the same cluster in both genotypes. Lipids included in clusters of either genotype are retained in lipid groups, so each lipid group includes lipids that are up-or-down-regulated together through time under various temperature treatments in at least one genotype (Fig. 3).
Autoscaled levels of 13 lipid species (representing the 13 lipid groups) in individual plant samples at various temperature regimes and time points are shown in Supporting Information Fig. S3. The 13 lipid groups can be broadly classified into five categories: groups containing extraplastidic phospholipids (Groups 1-6a), groups containing plastidic glycerolipids (Groups 6b-8), a group containing oxidized glycerolipids (Group 9), groups containing TAGs (Groups 10-11), and groups containing ASGs and SGs (Groups 12-13).
The kinetic changes in individual lipids in each of the 13 groups are presented as a heat map in Fig. 4a and as time courses of the treatments in Supporting Information Fig. S4. Fig. 4b shows the kinetic changes for the total lipid level for each lipid group. The kinetic patterns of changes in individual lipids varied among the groups, both during heat stress and during the recovery period (Fig. 4a and Supporting Information Fig. S4). In general, the effects of HD were generally greater than or equal to that of HN on various lipids groups (Fig. 4b) and on individual lipids (Fig. S4).
Figure 4b.
Kinetic changes in the total lipid level for each lipid group under optimum temperature (OT; 25/15°C, day-maximum/night-minimum), high night temperature (HN; 25/24°C), high day temperature (HD; 35/15°C), and high day and night temperature (HDN; 35/24°C). Days of measurement (days 1, 6, 12, and 16) are indicated on the x-axis with day 1 indicating the 1st day of stress and day 16 indicating the recovery period, as stress was imposed for 12 days. Each data point indicates mean value (n=10; two experiments and five replications [plants] of each genotype), and vertical bars denote standard errors. Group 1- PS with an 18:3 acyl chain and a long acyl chain, Group 2- PS with an 18:2 acyl chain and a long acyl chain, Group 3- Extraplastidic lipids with a 15:0 or 17:0 acyl chain combined with an 18:3 acyl chain, Group 4- Extraplastidic lipids with an 18:3 acyl chain and an 18:3, 18:2, or 16:0 acyl chain, Group 5- Extraplastidic lipids with an 18:2 acyl chain, Group 6- Polar lipids with 18:1 and/or 18:2 acyl chains, Group 7- Plastidic monoacyl lipids with 18:3 acyl chains, Group 8- Plastidic diacyl lipids with 18:3 acyl chains, Group 9- Ox-lipids, Group 10- TAGs and DAGs with a 16:0 acyl chain(s), Group 11- TAGs with an 18:3 acyl chain(s), Group 12- ASGs, Group 13- SGs.
It is well established that many lipid-metabolizing enzymes act on multiple, related lipid substrates that contain the same component acyl chain or head group. Camacho et al. (2005) evaluated the origin of correlations in metabolomics data using metabolic control analysis and computer simulation of biochemical networks, and attributed strong metabolite correlations to strong mutual control by a single enzyme. Vu et al. (2014a) analyzed the lipid changes after leaf wounding in Arabidopsis and found that lipids that showed high correlations (ρ > 0.96) were the products of the same rate-limiting enzyme or were downstream of the rate-limiting enzyme(s) in a pathway. For example, SGs with ρ > 0.96 were likely the products of UDP-Glc:sterol glycosyltransferases. In the sections below, we discuss how the lipid clustering relates to metabolic pathways (for the lipids that are included in groups in both genotypes, i.e., lipids that are highlighted by lavender in Fig. 3).
Groups containing extraplastidic phospholipids (Groups 1-6a)
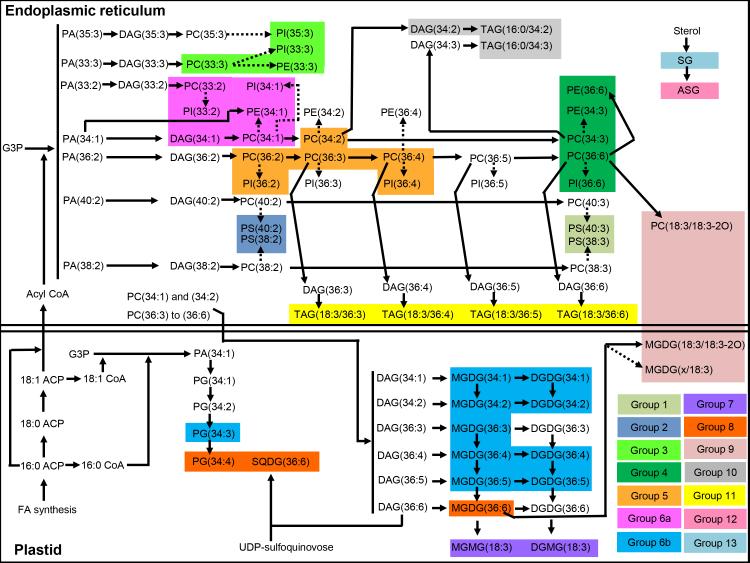
As illustrated in Fig. 5, fatty acids that are synthesized de novo in the plastid can be transferred to the endoplasmic reticulum where the activated fatty acids are present as acyl CoAs (Ohlrogge and Browse, 1995). In the endoplasmic reticulum, these activated fatty acids can be transferred sequentially to glycerol-3-phosphate (G3P) to form PAs, which are dephosphorylated to diacylglycerols (DAG) and are used to synthesize PCs. The PC species can then be desaturated by FATTY ACID DESATURASES (FAD), converting PC(34:1) to PC(34:3) in steps; PC(36:2) to PC(36:6) in steps; PC(38:2) to PC(38:3); and PC(40:2) to PC(40:3). PC species are also formed and modified by acyl editing, which is a deacylation-reacylation cycle of PC that exchanges the fatty acids on PC with fatty acids in the acyl CoA pool (Bates et al., 2007, 2009). Thus, the PC acyl editing cycle involves deacylation of PC, releasing a fatty acid or acyl CoA and generation of LPC. Reacylation of LPC by a different acyl CoA from the pool completes the cycle. The incorporation into PA of fatty acids released from PC can lead to the formation of PE, PI, and PS containing acyl chains with various degrees of unsaturation (Bates et al., 2009).
Figure 5.
Metabolic pathway map showing the biosynthesis of lipids that are included in groups in wheat genotypes Ventnor and Karl 92. The map is based on Ongun and Mudd (1970), Ohlrogge and Browse (1995), Bates et al. (2007, 2009), Benning (2009), DeBolt et al. (2009), Shimojima (2011), and Li-Beisson et al., 2013. The dashed arrows indicate that those conversions involve multiple steps in terms of metabolism. ACP – acyl carrier protein, CoA - Coenzyme A, FA - fatty acid, G3P - glycerol-3-phosphate, and UDP - uridine diphosphate. MGDG(x/18:3) in Group 9 indicates MGDG(18:4-O/18:3) or MGDG(19:3/18:3).
In the present study, Group 1 includes phosphatidylserine (PS) with an 18:3 acyl chain combined with a long acyl chain, whereas Group 2 includes PS with an 18:2 acyl chain combined with a long acyl chain (Figs. 1, 2, 3, and 4a) [Component fatty acid chains associated with groups were identified based on Devaiah et al. (2006)]. Group 4 primarily includes extraplastidic diacyl lipids with an 18:3 acyl chain combined with an 18:3, 18:2, or 16:0 acyl chain. These include normal-chain species of PE, PC, PI, and DAG. Group 5 includes extraplastidic phospholipids (PE, PC, and PI) with an 18:2 acyl chain. Group 6a includes extraplastidic diacyl species, i.e., PE, PC, PI, and DAG species, with limited desaturation, i.e., with 18:1 or 18:2 acyl chains.
Group 5 includes PC species that are acted on by FAD2 (which converts 18:1 to 18:2) or FAD3 (which converts 18:2 to 18:3), and PE and PI species that are produced from desaturated PC species (Fig. 5). Group 4 includes PC species that are acted on by FAD3 and PE and PI species that have the same fatty acyl chains as the PC. Previous reports suggest that PEs are likely formed from PCs with the same acyl chains (Bates et al., 2007). The ratios among acyl-identical PC, PE, and PI species that are included in the same group (Groups 4, 5, or 6a) did not show much variability among different plants (CoV < 30%), compared to the ratios among PC, PE, and PI species included in different groups (CoV > 50%) (Supporting Information Table S4). Taken together, these results suggest that PC-PE or PC-PI conversions within the same groups were not the rate-limiting processes in the formation of PE or PI molecular species under high day and night temperature stress. Instead, the desaturase activities of FAD2 that lead to formation of 18:2-containing PCs in Group 5 from 18:1-containing PCs in Group 6, and the desaturase activities of FAD3 that lead to the formation of 18:3-containing PCs in Group 4 from 18:2-containing PCs in Group 5, were the rate-limiting steps.
Group 3 includes extraplastidic phospholipids (PE, PC, and PI) with a 15:0 or 17:0 acyl chain combined with an 18:3 acyl chain. The biosynthesis of phospholipids containing odd-numbered acyl chains included in Groups 3 and 6 are not clearly understood. Wendel (1989) reported that propionyl-CoA acts as a primer in the biosynthesis of odd-chain fatty acids. Jenkins et al. (2015) suggested that endogenous production of odd-chain fatty acids occurs through α-oxidation of fatty acids, involving hydroxylation of the α-carbon in relation to the terminal carboxylic acid, followed by the removal of the terminal carboxyl group. However, the lipids in this group are produced synchronously and are likely the products of the same pathway.
When comparing the amount of lipids under HDN with the amount at OT on days 6 and 12, Groups 1, 3, and 6a include lipids that are found at higher levels under high temperature stress and Group 5 includes lipids that are found at lower levels under high temperature stress (Fig. 4a and Supporting Information Fig. S4). While patterns of lipid levels among samples are more complex for Group 2 (PS with 18:2 and a long chain) and Group 4 (18:3-containing molecular species), it is clear that the lipids within these groups, as well as the other groups, are altered in similar ways through heat treatment and time (Fig. 4a and Supporting Information Fig. S4).
Groups containing plastidic glycerolipids (Groups 6b-8)
Group 6b includes plastidic diacyl species with 18:1 and/or 18:2 acyl chains (Figs. 1, 2, 3, and 4a). These include DGDG, MGDG, and PG species with limited desaturation. Groups 7 and 8 include plastidic monoacyl lipids and diacyl lipids, respectively, with 18:3 acyl chains. These are normal-chain species of DGMG and MGMG (Group 7) and DGDG, MGDG, PG, and SQDG (Group 8). Lipids in Groups 7 and 8 were at lower levels under high temperatures stress, whereas, lipids in Group 6b was at higher levels under high temperatures stress, compared to OT (Fig. 4a & b).
MGMG and DGMG could be produced by lipases (acyl hydrolases) acting on MGDG or DGDG. DGMG could also be produced by transacylation producing acylated MGDG (MGDG + DGDG → acylated MGDG + DGMG). The pathways responsible for MGMG and DGMG metabolism in wheat under heat stress are not clear. However, Vu et al. (2014b) showed that acylated MGDG is indeed formed in wheat under wounding stress. Groups 7 and 8 include the completely desaturated galactolipids (18:3- DGMG and MGMG, 36:6- DGDG, MGDG, and SQDG, and 38:6-DGDG), while Group 6b includes the incompletely desaturated galactolipids (34:2-, 34:1-, 36:5-, and 36:4-DGDG and MGDG and 36:3-MGDG).
In the plastid, fatty acids that are synthesized de novo are sequentially transferred from acyl carrier protein (ACP) to G3P to form primarily PA(34:1) (18:1/16:0; reviewed by Ohlrogge and Browse, 1995). PA(34:1) can be used to form PG (34:1) (18:1/16:0), which can be desaturated sequentially to PG(34:3) through the action of FAD4 (converts 16:0 to 16:1) and FAD6 (converts 18:1 to 18:2) (Fig. 5). Plastidic lipids MGDG and DGDG are formed from the acyl components of PC that are imported into the plastid (reviewed by Benning, 2009). Synthesis of MGDG involves the action of monogalactosyldiacylglycerol transferase (MGDGS), which transfers a galactose moiety from UDP-galactose to DAG, whereas synthesis of DGDG involves the action of digalactoslydiacylglycerol synthase (DGDGS), which transfers a galactose moiety from UDP-galactose to MGDG (reviewed by Li-Beisson et al., 2013). Fatty acyl chains can undergo desaturation on either MGDG or DGDG. Fatty acid desaturation of MGDG and DGDG species in Group 6b involves the action of FAD5 (converts 16:0 to 16:1), FAD6 (converts 18:1 to 18:2), and FAD7 and FAD8 (convert 18:2 to 18:3), whereas fatty acid desaturation of MGDG and PG species in Group 8 involves the action of FAD7 and FAD8. The ratios among acyl-identical MGDG and DGDG species in Group 6b did not show much variability among different plants (CoV < 50%), compared to the ratios among MGDG and DGDG species included in different groups (Groups 6b and 8) (CoV > 50%) (Supporting Information Table S4). Taken together, these results suggest that MGDG-DGDG conversions within the same groups were not rate-limiting under high day and night temperature stress. Instead, the desaturase activities of FAD7 and FAD8 that lead to the formation of the completely desaturated MGDG and DGDG species of Group 8 (18:3 acyl chain-containing) from the incompletely desaturated (18:2 acyl chain-containing) MGDG and DGDG species of Group 6b were rate-limiting.
Group containing oxidized glycerolipids (Group 9)
Group 9 includes glycerolipids with oxidized acyl chains (ox-lipids) (Figs. 1, 2, 3, and 4a). These include MGDG and PC containing a normal acyl chain and an oxidized acyl chain (e.g., 18:3-2O; three double bond equivalents and two oxygen atoms beyond the carbonyl group). The ox-lipids in Group 9 can be produced non-enzymatically through the action of reactive oxygen species (Zoeller et al., 2012) or enzymatically through the action of lipoxygenase. Nilsson et al. (2012) found that oxidized acyl chains are formed in lipids without release of the acyl chains from the lipids. The enzymatic formation of ox-lipids might involve oxidation and cyclization of the esterified fatty acyl chains, as suggested by Nilsson et al. (2012). Again, the lipids in this group are produced under the same conditions, with similar timing, and are likely the products of the same pathway.
Groups containing TAGs and DAGs (Groups 10-11)
Group 10 includes TAGs and DAGs with a 16:0 acyl chain(s), whereas Group 11 includes TAGs with an 18:3 acyl chain(s) (Figs. 1, 2, 3, and 4a). Interestingly, the patterns of TAG accumulation in Groups 10 and 11 were distinctly different. Lipids in Group 11 (18:3-containing TAGs) were higher under HD and HDN stress conditions than under OT, decreasing to levels similar to OT in the recovery period (Fig. 4a and Supporting Information Fig. S4). These lipids were at higher levels even on the first day under high temperature stress. While plants were under heat stress, the 16:0-containing TAGs in Group 10 were at levels similar to those from plants at OT, but their levels were higher during the recovery period (day 16) (Fig. 4a and Supporting Information Fig. S4). Taken together, the changes in Groups 10 and 11 under high temperature stress are consistent with a role of TAGs in sequestering 18:3 (during the stress period, as described in the companion paper, Narayanan et al., 2015) and 16:0 (during the recovery period) from membrane lipids in order to adjust the degree of unsaturation in the membrane lipids.
Triacylglycerols in Groups 10 and 11 might be formed through the action of phospholipid:diacylglycerol acyltransferase (PDAT) and/or acyl CoA:diacylglycerol acyltransferase (DGAT) (Zhang et al., 2009; Fan et al., 2013). Higashi et al. (2015) reported that the expression of PDAT and DGAT is increased under heat stress. Bates et al. (2012) reported that PC acyl editing and phosphocholine head group exchange between PC and DAG are the major mechanisms responsible for directing polyunsaturated fatty acids into TAGs. The PC acyl editing is catalyzed by lysophosphatidylcholine acyltransferases LPCAT1 and LPCAT2 (Bates et al., 2012), while transfer of the phosphocholine head group from PC to DAG is catalyzed by phosphatidylcholine:diacylglycerol cholinephosphotransferase (PDCT) (Lu et al., 2009). Our data also show that PC species with an 18:3 acyl chain(s) (Group 4) decreased under high temperature stress. This might be partly explained by PC acyl editing that involves removal of 18:3 acyl chains from PC, followed by incorporation into TAGs.
Groups containing SGs and ASGs (Groups 12-13)
Groups 12 and 13 include ASGs and SGs, respectively (Figs. 1, 2, 3, and 4a). All lipids in Groups 12 and 13 were present at higher amounts in heat stressed samples than in samples at OT (Fig. 4a and Supporting Information Fig. S4). Lipids in these groups increased under high temperature stress even on the first day of stress, continued to increase through time under stress, and returned to baseline during the recovery period (day 16). These results suggest a possible role of SGs and ASGs in heat tolerance in wheat. This is consistent with the suggestion in the companion paper that sterol lipids were associated with heat tolerance in Ventnor (Narayanan et al., 2015).
Sterol glycosides in Group 13 are likely produced by the glycosylation of sterols by UDP-Glucose:sterol glycosyltransferase(s) (DeBolt et al., 2009) (Fig. 5). Acyl sterol glycosides in Group 12 are likely produced by the acylation of SGs, although the acylating enzymes acting on SGs have not been identified (Fig. 5). The high correlation of SGs in Group 13 (ρ, 0.90; Supporting Information Fig. S2) indicate that these species might have been produced through parallel glycosylation of stigmasterol, sitosterol, and campesterol. Further, it is possible that the three ASG species in Group 12 were formed by parallel acylation of SGs, with the same acyl chain. Our data showed that Groups 12 and 13 exhibited a clear pattern of response to high temperature stress, as they increased under high temperature stress even on the first day of stress, continued to increase through time under stress, and returned to baseline during the recovery period (day 16) (Fig. 4a & b and Supporting Information Fig. S4).
The current work identifies lipid groups that include co-occuring lipids that are regulated by the same enzyme(s). Further studies are required to determine whether the activities of these enzymes vary between the heat-tolerant-and-susceptible genotypes. Even though the groups included lipids that experience coordinated metabolism, some lipids in certain groups exhibited a different pattern/rate of change under high temperature stress conditions (e.g., Group 5). This is not surprising as these are highly complex data and some lipids, even within a single group, were acted upon in a different way by the lipid metabolizing enzyme(s).
CONCLUSIONS
Correlation analysis of levels of the lipid molecular species revealed 13 groups of lipids. Lipids within each group co-occurred (were up-or-down-regulated together through time) across samples, through the high day and night temperature stress treatments. While temporal patterns of changes in levels of lipid molecular species within each group were similar, the temporal patterns of lipid level changes varied among the groups. The lipid groups can be broadly classified as groups containing: (1) extraplastidic phospholipids, (2) plastidic glycerolipids, (3) oxidized glycerolipids, (4) triacylglycerols, (5) acylated sterol glycosides, and (6) sterol glycosides. Current knowledge of lipid metabolism suggests that the lipids in each group co-occur because they are regulated by the same enzyme(s). The results suggest that increases in activities of desaturating, oxidizing, glycosylating, and acylating enzymes lead to simultaneous changes in levels of multiple lipid molecular species during high day and night temperature stress in wheat. Application of co-occurrence analysis to additional lipids, species, and plants subjected to other stresses will provide further information on coordinated metabolism of plant lipids under stress conditions.
Supplementary Material
SUMMARY.
Identifying lipids that experience coordinated metabolism during heat stress would provide information regarding lipid dynamics under stress conditions and assist in developing heat-tolerant wheat varieties. We found that co-occurring lipids, which are up-or-down-regulated together through time during high day and night temperature stress, represent groups that can be explained by coordinated metabolism. Current knowledge of lipid metabolism suggests that the lipids in each group co-occur because they are regulated by the same enzyme(s). The results indicate that increases in activities of desaturating, oxidizing, glycosylating, and acylating enzymes lead to simultaneous changes in levels of multiple lipid molecular species during high day and night temperature stress in wheat.
ACKNOWLEDGMENTS
We thank Triticeae Coordinated Agricultural Project Grant no. 2011–68002–30029 (Triticeae-CAP) from the USDA NIFA, United States Agency for International Development (USAID) Feed the Future Innovation Lab for Climate Resilient Wheat (Grant no. AID-OAA-A-13-00008), and Kansas Wheat Alliance for financial support. We thank Prakarsh Tiwari and Predeesh Chandran for help in data collection. We are grateful to Mary Roth for excellent technical assistance with the analysis and to Danny Vu for assistance in production of the dendrograms. We thank Pamela Tamura for critical editing of the manuscript. The lipid analyses described in this work were performed at the Kansas Lipidomics Research Center Analytical Laboratory. Instrument acquisition and lipidomics method development was supported by National Science Foundation (EPS 0236913, MCB 0920663, MCB 1413036, DBI 0521587, DBI1228622), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20GM103418), and Kansas State University. Contribution 15-429-J from the Kansas Agricultural Experiment Station.
REFERENCES
- Bates PD, Ohlrogge JB, Pollard M. Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. The Journal of Biological Chemistry. 2007;282:31206–31216. doi: 10.1074/jbc.M705447200. [DOI] [PubMed] [Google Scholar]
- Bates PD, Durrett TP, Ohlrogge JB, Pollard M. Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiology. 2009;150:55–72. doi: 10.1104/pp.109.137737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Fatihi A, Snapp AR, Carlsson AS, Browse J, Lu C. Acyl editing and head group exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiology. 2012;160:1530–1539. doi: 10.1104/pp.112.204438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning C. Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annual Review of Cell and Developmental Biology. 2009;25:71–91. doi: 10.1146/annurev.cellbio.042308.113414. [DOI] [PubMed] [Google Scholar]
- Buseman CM, Tamura P, Sparks AA, Baughman EJ, Maatta S, Zhao J, Welti R. Wounding stimulates the accumulation of glycerolipids containing oxophytodienoic acid and dinor-oxophytodienoic acid in Arabidopsis leaves. Plant Physiology. 2006;142:28–39. doi: 10.1104/pp.106.082115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho D, de la Fuente A, Mendes P. The origin of correlations in metabolomics data. Metabolomics. 2005;1:53–63. [Google Scholar]
- Cullis PR, Dekruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochimica et Biophysica Acta. 1979;559:399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- DeBolt S, Scheible WR, Schrick K, Auer M, Beisson F, Bischoff V, Somerville C. Mutations in UDP-Glucose:sterol glucosyltransferase in Arabidopsis cause transparent testa phenotype and suberization defect in seeds. Plant Physiology. 2009;151:78–87. doi: 10.1104/pp.109.140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah SP, Roth MR, Baughman E, Li M, Tamura P, Jeannotte R, Wang X. Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a PHOSPHOLIPASE Dα1 knockout mutant. Phytochemistry. 2006;67:1907–1924. doi: 10.1016/j.phytochem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling DR, Horton B, Jones PD, Peterson TC, Karl TR, Parker DE, Folland CK. Maximum and minimum temperature trends for the globe. Science. 1997;277:364–367. [Google Scholar]
- Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. Climate extremes: observations, modeling, and impacts. Science. 2000;289:2068–2074. doi: 10.1126/science.289.5487.2068. [DOI] [PubMed] [Google Scholar]
- Fan J, Yan C, Xu C. Phospholipid: diacylglycerol acyltransferase-mediated triacylglycerol biosynthesis is crucial for protection against fatty acid-induced cell death in growing tissues of Arabidopsis. The Plant Journal. 2013;76:930–942. doi: 10.1111/tpj.12343. [DOI] [PubMed] [Google Scholar]
- Harvey LD. Warm days, hot nights. Nature. 1995;377:15–16. [Google Scholar]
- Higashi Y, Okazaki Y, Myouga F, Shinozaki K, Saito K. Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Scientific reports. 2015;5 doi: 10.1038/srep10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, Rupp R. Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinformatics. 2007;8:460. doi: 10.1186/1471-2105-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Scornavacca C. Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Systematic Biology. 2012;61:1061–1067. doi: 10.1093/sysbio/sys062. [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change . Intergovernmental Panel on Climate Change fifth assessment report: Climate change 2013. World Meteorological Organization: Geneva, Switzerland: 2013. [Google Scholar]
- Jenkins B, West JA, Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) in health and disease. Molecules. 2015;20:2425–2444. doi: 10.3390/molecules20022425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Ohlrogge J. Acyl-lipid metabolism. Arabidopsis Book. 2013;11:e0161. doi: 10.1199/tab.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Xin Z, Ren Z, Miquel M, Browse J. An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18837–18842. doi: 10.1073/pnas.0908848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Prasad PVV, Fritz AK, Boyle DL, Gill BS. Impact of high night-time and high daytime temperature stress on winter wheat. Journal of Agronomy and Crop Science. 2015;201:206–218. [Google Scholar]
- Narayanan S, Tamura PJ, Roth MR, Prasad PVV, Welti R. Wheat leaf lipids during heat stress: I. High day and night temperatures results in major lipid alternations. Plant Cell and Environment. 2015 doi: 10.1111/pce.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez-Vasquez J, Florin-Christensen J, Ryan CA. Positional specificity of a phospholipase A activity induced by wounding, systemin, and oligosaccharide elicitors in tomato leaves. The Plant Cell. 1999;11:2249–2260. doi: 10.1105/tpc.11.11.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson AK, Fahlberg P, Ellerström M, Andersson MX. Oxo-phytodienoic acid (OPDA) is formed on fatty acids esterified to galactolipids after tissue disruption in Arabidopsis thaliana. FEBS Letters. 2012;586:2483–2487. doi: 10.1016/j.febslet.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J, Browse J. Lipid biosynthesis. The Plant Cell. 1995;7:957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongun A, Mudd JB. The biosynthesis of steryl glucosides in plants. Plant Physiology. 1970;45:255–262. doi: 10.1104/pp.45.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Li M, Narasimhan R, Roth M, Welti R, Wang X. Nonspecific phospholipase C NPC4 promotes responses to abscisic acid and tolerance to hyperosmotic stress in Arabidopsis. The Plant Cell. 2010;22:2642–2659. doi: 10.1105/tpc.109.071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad PVV, Djanaguiraman M. Response of floret fertility and individual grain weight of wheat to high temperature stress: sensitive stages, and thesholds for temperature and duration. Functional Plant Biology. 2014;41:1261–1269. doi: 10.1071/FP14061. [DOI] [PubMed] [Google Scholar]
- Ryu SB. Phospholipid-derived signaling mediated by phospholipase A in plants. Trends in Plant Science. 2004;9:229–235. doi: 10.1016/j.tplants.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Shimojima M. Biosynthesis and functions of the plant sulfolipid. Progress in Lipid Research. 2011;50:234–239. doi: 10.1016/j.plipres.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Vu HS, Shiva S, Roth MR, Tamura P, Zheng L, Li M, Welti R. Lipid changes after leaf wounding in Arabidopsis thaliana: expanded lipidomic data form the basis for lipid co-occurrence analysis. The Plant Journal. 2014a;80:728–743. doi: 10.1111/tpj.12659. [DOI] [PubMed] [Google Scholar]
- Vu HS, Roth MR, Tamura P, Samarakoon T, Shiva S, Honey S, Welti R. Head-group acylation of monogalactosyldiacylglycerol is a common stress response, and the acyl-galactose acyl composition varies with the plant species and applied stress. Physiologia Plantarum. 2014b;150:517–528. doi: 10.1111/ppl.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Wang X. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. The Journal of Biological Chemistry. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- Welti R, Shah J, Li W, Li M, Chen J, Burke JJ, Wang X. Plant lipidomics: discerning biological function by profiling plant complex lipids using mass spectrometry. Frontiers in Bioscience. 2007;12:2494–2506. doi: 10.2741/2250. [DOI] [PubMed] [Google Scholar]
- Wendel U. Abnormality of odd-numbered long-chain fatty acids in erythrocyte membrane lipids from patients with disorders of propionate metabolism. Pediatric Research. 1989;25:147–150. doi: 10.1203/00006450-198902000-00014. [DOI] [PubMed] [Google Scholar]
- Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Research. 2009;37:W652–60. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2.0-a comprehensive server for metabolomic data analysis. Nucleic Acids Research. 2012;40:W127–33. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Fan J, Taylor DC, Ohlrogge JB. DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. The Plant Cell. 2009;21:3885–3901. doi: 10.1105/tpc.109.071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zien CA, Wang C, Wang X, Welti R. In vivo substrates and the contribution of the common phospholipase D, PLDα, to wound-induced metabolism of lipids in Arabidopsis. Biochimica et Biophysica Acta. 2001;1530:236–248. doi: 10.1016/s1388-1981(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Zoeller M, Stingl N, Krischke M, Fekete A, Waller F, Berger S, Mueller MJ. Lipid profiling of the Arabidopsis hypersensitive response reveals specific lipid peroxidation and fragmentation processes: biogenesis of pimelic and azelaic acid. Plant Physiology. 2012;160:365–378. doi: 10.1104/pp.112.202846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.