Abstract
Flammulina velutipes (enoki, velvet shank, golden needle mushroom or winter mushroom), one of the main edible mushrooms on the market, has long been recognized for its nutritional value and delicious taste. In recent decades, research has expanded beyond detailing its nutritional composition and delved into the biological activities and potential health benefits of its constituents. Many bioactive constituents from a range of families have been isolated from different parts of the mushroom, including carbohydrates, protein, lipids, glycoproteins, phenols, and sesquiterpenes. These compounds have been demonstrated to exhibit various biological activities, such as antitumour and anticancer activities, anti-atherosclerotic and thrombosis inhibition activity, antihypertensive and cholesterol lowering effects, anti-aging and antioxidant properties, ability to aid with restoring memory and overcoming learning deficits, anti-inflammatory, immunomodulatory, anti-bacterial, ribosome inactivation and melanosis inhibition. This review aims to consolidate the information concerning the phytochemistry and biological activities of various compounds isolated from F. velutipes to demonstrate that this mushroom is not only a great source of nutrients but also possesses tremendous potential in pharmaceutical drug development.
Keywords: Flammulina velutipes, enoki, mushroom, nutritional value, biological activity
Introduction
In addition to their nutritional value, folk medicine has long recognized mushrooms for their wide spectrum of therapeutic and prophylactic uses. Many medicinal mushrooms are important ingredients in Traditional Chinese Medicine, such as Flammulina velutipes (enokitake), Lentinus edodes (shiitake), and Grifola frondosa (maitake) (Sullivan et al., 2006). While their properties remained unknown to the scientific community for a long time, in recent decades, there has been significant research focused on the sources, medicinal properties and applications of mushrooms (Encarnacion et al., 2012; Kalač, 2013; Soares et al., 2013).
From a nutritional stand point, these health promoting mushrooms have high nutritional value. They contain dietary fiber, are low in calories, have a high content of protein consisting of all the essential amino acids, minerals and vitamins and are free of cholesterol (Karaman et al., 2010). Beyond their nutritional value, mushrooms have great potential for production of useful metabolites, making them a prolific resource for drug isolation and development (Leung et al., 1997). Mushrooms are now gaining worldwide recognition as a functional food as well as a potential source of nutraceuticals which may reduce severity of, prevent or treat illnesses. The current research on these medicinal mushrooms has, in fact, now progressed beyond validating their traditional medical uses and into the isolation and production of bioactive compounds against specific illnesses (Wang et al., 2012c; Liu et al., 2014).
F. velutipes is also commonly known as enokitake, velvet shank or golden needle mushroom winter mushroom. The synonyms for F. velutipes are Agaricus nigripes, Agaricus velutipes, Collybia eriocephala, Collybia veluticeps, Collybia velutipes, Collybidium velutipes, Gymnopus velutipes, Myxocollybia velutipes, Panaeolus veluticeps, Paxillus veluticeps, Phylloporus veluticeps, and Pleurotus velutipes (Information was retrieved from MycoBank website http://www.mycobank.org, 1st August 2016). The cultivated F. velutipes has a pure white bean sprout look with a velvety stem topped with a tiny snowy-white cap; while the wild varieties appear in different colors ranging from orange to brown, and have a larger, shiny cap. The significant difference in the appearance of wild and the cultivated F. velutipes is attributed to the cultivation of F. velutipes without the exposure to light which leads to its white color while the wild ones are brown. F. velutipes normally grows on dead elm trees and has been found abundantly on diseased elm trees caused by Dutch elm infection (Ingold, 1980). The species of Flammulina have also been reported to occur ubiquitously on a wide variety of deciduous trees such as poplar, plum, maple and birch (Sharma et al., 2009).
F. velutipes (Curtis) Singer is one of the most popular edible mushrooms that possesses a wide spectrum of interesting biological activities. It is found ubiquitously throughout the north-temperate regions including North America, Europe and Asia (Ingold, 1980). Historically, it has been has been cultivated for consumption and medicinal use in China since 800 AD. Currently, F. velutipes is among the four most widely cultivated mushrooms globally due to its desirable taste, aroma and high nutritional value. It is commonly available in the market or groceries stores sold in vacuum packages. This mushroom is also well known for its curative properties for liver diseases and gastroenteric ulcers (Ingold, 1980).
Research on F. velutipes has clearly shown it possesses various pharmacological properties including anticancer, antimicrobial, antioxidant, and immunomodulatory properties, demonstrating that F. velutipes has great potential for successful bioprospecting. We aim to give an overview of the present knowledge regarding the bioactive chemical constituents and pharmacological potential of F. velutipes.
Taxonomic classification of F. velutipes
| Taxonomic level | Taxonomic name |
|---|---|
| Kingdom | Fungi |
| Phylum | Basidiomycota |
| Class | Agaricomycetes |
| Order | Agaricales |
| Family | Physalacriaceae |
| Genus | Flammulina |
| Species | F. velutipes |
Chemical composition and nutritional value of F. velutipes
Similar to many other edible mushrooms, F. velutipes is consumed as a delicacy, noted for the pleasant aroma and texture it gives to a dish. More importantly, consuming F. velutipes can provide key nutrients such as proteins, vitamins, minerals, unsaturated fatty acids and fiber. Additionally, consumption of F. velutipes can confer health promoting effects including immunity enhancement, blood cholesterol and blood pressure lowering effects as well as chemopreventive effects by virtue of the bioactive constituents contained in the mushroom which are ingested during consumption. However, it is important to note that the composition of the beneficial compounds present in F. velutipes can be highly influenced by the growing site, types of substrate, maturity of the mushroom at the harvesting stage and also the post-harvest handling including the processing and storage conditions. All these factors could account for the variability in composition data published by different studies examining the same mushroom, in addition to the intraspecific genetic variability of mushrooms from different provenance and producers (Reis et al., 2012). We have consolidated the data available in the current literature reporting the nutrient analysis of F. velutipes collected from several regions in order to provide further insight into the nutritional benefits of consuming this mushroom.
Based on the findings of Kalač (2013), the dry matter of both wild and cultivated mushrooms is relatively low, usually within the range of 80–140 g/kg. For F. velutipes, the dry matter of both wild and cultivated F. velutipes are within the normally reported range for mushrooms, measuring between 93 and 114 g/kg (Dikeman et al., 2005; Ko et al., 2007; Beluhan and Ranogajec, 2011; Reis et al., 2012). The low dry matter of the mushrooms accounts for their high water content that leads to shorter shelf life of the fruiting bodies. However, in a comparison among cultivated mushrooms, Dikeman et al. (2005) found that the dry matter of cultivated raw F. velutipes was the highest among the other cultivated mushrooms sampled for the study. However, this was not the case for the studies that examined the wild F. velutipes collected from European regions (Beluhan and Ranogajec, 2011; Pereira et al., 2012). Furthermore, the dry matter content of F. velutipes was reduced after cooking while the dry matter of the other mushrooms was increased after cooking, suggesting that cooking can result in losses for F. velutipes.
The data on approximate composition and energy value of both wild and cultivated F. velutipes are summarized in Table 1. All the studies reviewed showed that carbohydrates and proteins are the two major constituents contained in the dry matter of F. velutipes. No obvious difference is seen in carbohydrate and protein content between the wild and cultivated F. velutipes. Given the fact that, similar to other mushrooms, F. velutipes has low dry matter and fats content, it is thus a low energy delicacy. Furthermore, the calculated energy value is suggested to be somewhat overestimated as some of the carbohydrates are only partially digestible or indigestible, such as chitin and mannitol (Kalač, 2013). It is interesting to note that a slight difference can be observed between the energy value of wild and the cultivated F. velutipes, where the energy value of cultivated F. velutipes appears higher than the wild F. velutipes, ranging from 410.1 to 459.3 kcal/kg fresh weight and from 342.0 to 398.2 kcal/kg fresh weight, respectively. The energy value was computed according to the following equation: Energy (kcal) = 4 × (g carbohydrate) + 3.75 × (g protein) + 9 × (g fat) (Reis et al., 2012).
Table 1.
The proximate composition of F. velutipes in dry weight basis.
| Proximate composition (g/kg dry matter) | Origin of growing site | References | |
|---|---|---|---|
| Wild | Cultivated | ||
| Dry matter | 93.2–119.5 | 103.0–114.0 | Dikeman et al., 2005; Ko et al., 2007; Beluhan and Ranogajec, 2011; Pereira et al., 2012; Reis et al., 2012 |
| Carbohydrates | 426.0–708.5 | 580.0–871.4 | Ko et al., 2007; Beluhan and Ranogajec, 2011; Akata et al., 2012; Pereira et al., 2012; Reis et al., 2012 |
| Proteins | 232.0–275.0 | 178.9–279.5 | Dikeman et al., 2005; Ko et al., 2007; Beluhan and Ranogajec, 2011; Akata et al., 2012; Pereira et al., 2012 |
| Fats | 17.3–70.0 | 18.4–73.3 | Dikeman et al., 2005; Ko et al., 2007; Beluhan and Ranogajec, 2011; Akata et al., 2012; Pereira et al., 2012; Reis et al., 2012 |
| Ash | 72.5–74.0 | 73.9–104.0 | Ko et al., 2007; Beluhan and Ranogajec, 2011; Akata et al., 2012; Pereira et al., 2012; Reis et al., 2012 |
| Energy (kcal/kg fresh weight) | 342.0–398.2 | 410.1–459.3 | Ko et al., 2007; Beluhan and Ranogajec, 2011; Pereira et al., 2012; Reis et al., 2012 |
*Energy (kcal) = 4 × (g carbohydrate) + 3.75 × (g protein) + 9 × (g fat) (Reis et al., 2012).
Protein and amino acids
Protein represents the most critical component contributing to the nutritional value of a particular food, due to the fact that fats and carbohydrates are rarely lacking in a diet. As one of the major components in the dry matter of F. velutipes, protein accounts for approximately 178.9 g/kg dry weight to 279.5 g/kg dry weight of F. velutipes (Dikeman et al., 2005; Ko et al., 2007; Beluhan and Ranogajec, 2011; Akata et al., 2012; Pereira et al., 2012). The large range in the protein concentrations could be explained by the fact that protein concentration in this mushroom been shown to vary depending on the growth substrate, size of pileus, time of harvest and the availability of nitrogen sources in the growth substrate. The protein content of F. velutipes is comparable to many green leafy vegetables— in the range of 200–300 g/kg dry weight (Singh et al., 2001; Gupta et al., 2005)—demonstrating that F. velutipes is a good source of protein. Several unique studies even quantified the concentration of amino acids from F. velutipes. Amino acids are the important monomeric building blocks of proteins and can further classified into two classes, the essential and nonessential amino acids. The essential amino acids can only be obtained through dietary intake while the non-essential amino acids are those that can be synthesized by the body. Varying concentrations of free amino acids contained in F. velutipes were demonstrated in the studies reviewed (Table 2) (Smiderle et al., 2008; Kim et al., 2009; Beluhan and Ranogajec, 2011). Based on these studies, the major amino acids found in F. velutipes were L-glutamic acid, L-alanine, glycine and L-lysine, which contributed 2.6–3.0% of dry weight. Furthermore, essential amino acids such as methionine, valine, isoleucine, leucine, lysine, phenylalanine and threonine were detected in F. velutipes (Smiderle et al., 2008; Kim et al., 2009; Beluhan and Ranogajec, 2011). Amino acids also play an important role in contributing to the pleasant taste of mushrooms. For instance, aspartic and glutamic acids are the two amino acids that contribute the monosodium glutamate-like or palatable taste while the alanine, glycine, threonine and serine give a sweet taste to the mushrooms. Beluhan and Ranogajec (2011) found that among the amino acids in F. velutipes, amino acids that give MSG-like taste made up the highest composition, followed by sweet, bitter and tasteless amino acids. The study also showed that among a variety of mushrooms, the total content of amino acids that determined the taste characteristics of F. velutipes was the lowest at 27.87 mg/g dry weight, accounting for the delicate and very mild in taste of F. velutipes unlike other mushroom species which have stronger flavor and taste (Beluhan and Ranogajec, 2011).
Table 2.
Concentration of amino acids of F. velutipes.
| Amino acids (mg/g dry weight) | Source of F. velutipes | ||
|---|---|---|---|
| Local supermarket, Korea (Kim et al., 2009) | Municipal market, Brazil (Smiderle et al., 2008) | Forests, Croatia regions (Beluhan and Ranogajec, 2011) | |
| D,L-O-Phosphoserine | 1.31 | nt | nt |
| Taurine | 1.74 | nt | nt |
| L-Aspartic acid | 2.81 | nt | 2.59 |
| L-Threonine | 6.41 | 10.047 | 5.21 |
| L-Serine | 6.83 | 7.686 | 0.21 |
| L-Glutamic Acid | 31.54 | 9.975 | 29.98 |
| D,L-α-Aminoadipic Acid | 0.64 | nt | nt |
| Glycine | 6.13 | 28.482 | 0.15 |
| L-Alanine | 26.86 | 7.591 | 1.95 |
| L-Valine | 1.76 | 6.539 | 1.54 |
| L-Cysteine | 6.32 | 8.76 | 1.39 |
| L-Methionine | 0.06 | 3.108 | 0.03 |
| L-Cystathionine | 1.89 | nt | nt |
| L-Isoleucine | 0.37 | 5.09 | 0.44 |
| L-Tyrosine | 0.99 | 3.471 | 0.04 |
| L-Phenylalanine | 0.19 | 5.654 | 1.23 |
| D,L-β-Amino-i-Butyric Acid | 2.16 | nt | nt |
| γ-AminoButyric Acid | 11.63 | nt | nt |
| Ethanolamine | 1.68 | nt | nt |
| D,L & allo-Hydroxylysine | 0.3 | nt | nt |
| L-Ornithine | 12.55 | nt | nt |
| L-Lysine | 6.21 | 30.896 | 5.68 |
| L-Histidine | 2.44 | 1.456 | 2.54 |
| L-Carnosine | 0.56 | nt | nt |
| L-Arginine | 1.27 | 3.88 | 0.49 |
| L-Leucine | 0.49 | 5.404 | 0.73 |
| Proline | nt | 4.947 | nt |
nt, not tested.
Besides that, various bioactive proteins also have been isolated from F. velutipes. Fungal immunomodulatory protein, FIP-fve, mainly extracted from fruiting body of F. velutipes has been studied extensively and explored for its diverse bioactivities including immunomodulatory, anticancer and anti-inflammatory properties (Chang et al., 2013, 2014; Lee et al., 2013). Ribosome inactivating proteins such as flammin, velin, velutin and flammulin are present in the fruiting bodies and extract of the mushroom (Wang and Ng, 2000, 2001; Ng and Wang, 2004). Studies have also reported isolation of proteins such as hemagglutanin which has mitogenic and antiproliferative functions, ice binding proteins and also the flammutoxin as a cytolysin (Tadjibaeva et al., 2000; Ng et al., 2006; Raymond and Janech, 2009). In addition, proflamin, a glycoprotein enzyme with anticancer activity, and asparaginase were also discovered in the mycelium of the mushroom (Maruyama and Ikekawa, 2007; Eisele et al., 2011; Kotake et al., 2011).
Carbohydrates
Based on the available data, carbohydrates generally constitute roughly one-half of the total dry weight of F. velutipes. Cultivated F. velutipes have a slightly higher median value of carbohydrate content compared to the wild F. velutipes (Ko et al., 2007; Beluhan and Ranogajec, 2011; Akata et al., 2012; Pereira et al., 2012; Reis et al., 2012). Carbohydrates in F. velutipes can be categorized into three main groups: monosaccharides such as ribose, mannose, glucose, xylose, fucose, galactose, and fructose; disaccharides such as sucrose and trehalose, and polysaccharides such as chitin, β-glucan and starch (Dikeman et al., 2005; Kim et al., 2009; Beluhan and Ranogajec, 2011; Pereira et al., 2012; Reis et al., 2012). The concentration of each individual group of carbohydrates contained in F. velutipes is tabulated in Table 3. The observed differences in sugar content may be due to geographical factors such as soil conditions, as well as the cultivation method and also the analytical method used. Glucose and trehalose were the two major sugar components detected in both wild and cultivated F. velutipes (Dikeman et al., 2005; Kim et al., 2009; Beluhan and Ranogajec, 2011; Pereira et al., 2012; Reis et al., 2012). It was indicated that sugars participate in cellular energy metabolism and also the synthesis of structural polysaccharides of the mushroom (Barros et al., 2008). Moreover, sugars are only a small portion of the total carbohydrate content while the remaining portion consists of other polysaccharides such as starch, chitin and β-glucan.
Table 3.
Concentration of monosaccharides, disaccharides and polysaccharides of F. velutipes.
| Carbohydrates (mg/g dry weight) | Provenance | References | ||
|---|---|---|---|---|
| Wild | Cultivated | |||
| Monosaccharides | Fucose | nt | 12 | Dikeman et al., 2005 |
| Mannose | 72.3 | 7.51–61 | Dikeman et al., 2005; Kim et al., 2009; Beluhan and Ranogajec, 2011 | |
| Glucose | 120.1 | 11.34–575 | Dikeman et al., 2005; Kim et al., 2009; Beluhan and Ranogajec, 2011 | |
| Ribose | nt | 11.47 | Kim et al., 2009 | |
| Xylose | nt | 28–51.56 | Dikeman et al., 2005; Kim et al., 2009 | |
| Fructose | nd | 379.23 | Kim et al., 2009; Reis et al., 2012 | |
| Mannitol | 59.8–79.0 | 79.97 | Beluhan and Ranogajec, 2011; Pereira et al., 2012; Reis et al., 2012 | |
| Galactose | nt | 27 | Dikeman et al., 2005 | |
| Disaccharides | Trehalose | 29.7–150.8 | 2.33–216.82 | Kim et al., 2009; Beluhan and Ranogajec, 2011; Pereira et al., 2012; Reis et al., 2012 |
| Sucrose | nt | nd, 7.41 | Kim et al., 2009; Reis et al., 2012 | |
| Polysaccharides | Chitin | nt | 97 | Dikeman et al., 2005 |
| β-glucan | nt | 2 | Dikeman et al., 2005 | |
| Starch | nt | 150 | Dikeman et al., 2005 | |
nd, not detected; nt, not tested.
Recently, there have been increased investigations into the bioactivities of polysaccharides extracted from both the fruiting body and mycelium of F. velutipes. F. velutipes derived polysaccharides were found to possess many health promoting properties, such as antioxidant and anticancer activity, immunomodulation, hepatocyte protection and even the ability to treat learning and memory impairment (Pang et al., 2007; He and Zhang, 2013; Wu et al., 2014; Yang et al., 2015) (Zhang et al., 2013). Beta-glucan is one of the many interesting polysaccharides found in F. velutipes, having demonstrated anticancer properties (Smiderle et al., 2006).
Carbohydrate content also includes dietary fiber, which is known to be high in mushrooms. According to Kalač (2013), both insoluble and soluble dietary fiber content were shown to make up between 4.2–9.2% and 22.4–31.2% of dry weight in mushrooms. Dikeman et al. (2005) revealed that approximately 29.3% of the dry weight of raw F. velutipes consists of dietary fibers; with 90% of the total dietary fiber being insoluble while the remaining 10% is soluble. Furthermore, it was found that the total dietary fiber content increased as a result of cooking for F. velutipes (Dikeman et al., 2005). A previous study also demonstrated that the high dietary fiber content of F. velutipes extract conferred hypolipidemic effect, lowering total cholesterol levels in animals on a high fat diet (Yeh et al., 2014).
Lipids and fatty acids
Generally, mushrooms are a low energy delicacy with a caloric value of approximately 350 kcal per kg owing to its low dry matter and lipid content (Kalač, 2013). The lipid content of F. velutipes falls in the range of 17.3–73.3 g/kg dry weight and is composed of sterols, sphingolipids and fats (Ko et al., 2007; Beluhan and Ranogajec, 2011; Akata et al., 2012; Pereira et al., 2012). The total fatty acid content (in terms of dry weight basis) of F. velutipes consists predominantly of monounsaturated and polyunsaturated fatty acids (79.23%) while saturated fatty acids make up the remaining 20.67% (Günç Ergönül et al., 2013). Table 4 summarizes the percentages of the individual fatty acids detected in F. velutipes. Linoleic acid is the major fatty acid contained in F. velutipes, making up 40.93–56.33% of the total fatty acid content. Linoleic acid is an essential fatty acid for mammals and is the precursor for biosynthesis of many important inflammatory mediators such as arachidonic acid and prostaglandins in mammals (Salem et al., 1999). Thus, F. velutipes may represent an important food source for humans or other animals to obtain sufficient amounts of the essential fatty acids our body requires but which cannot be synthesized. Many studies also demonstrated that sterols extracted from F. velutipes possess antiproliferative activity against several cancer cell lines and have the potential to be developed as chemotherapeutic agents (Yi et al., 2012, 2013a); while mycosterol, derived from the extract of F. velutipes, exhibits potent hypolipidemic activity (Yeh et al., 2014).
Table 4.
Main fatty acids (percentage) found in F. velutipes.
| Main fatty acids | Percentage (%) | References |
|---|---|---|
| Stearic acid | 1.38–3.56 | Pereira et al., 2012; Günç Ergönül et al., 2013 |
| Palmitic acid | 10.31–14.56 | Pereira et al., 2012; Günç Ergönül et al., 2013 |
| Oleic acid | 15.08–16.43 | Pereira et al., 2012; Günç Ergönül et al., 2013 |
| Linoleic acid | 40.93–56.33 | Pereira et al., 2012; Günç Ergönül et al., 2013 |
Micronutrients
The ash content in mushrooms generally ranges within 5–12% of dry matter and 72.5–104.0 g/kg of ash was reported in F. velutipes (Ko et al., 2007; Beluhan and Ranogajec, 2011; Akata et al., 2012; Pereira et al., 2012). The concentration of elements contained in F. velutipes are tabulated in Table 5. Similar to other mushrooms (Manzi et al., 1999; Smiderle et al., 2008; Zeng et al., 2012), potassium is the most abundant mineral element contained in F. velutipes (28.00–28.98mg/g dry weight), followed by phosphorus (8.80–9.40 mg/g dry weight). This indicates that a 100g portion of F. velutipes can contribute to around 9% of the recommended daily intake of potassium which is 3100 mg/day according to FDA (Akhter et al., 2003). The high potassium and low sodium content may also make F. velutipes of potential benefit in a salt restricted diet for suitable for those with hypertension or heart disease. In fact, studies also suggested that potassium from fruit and vegetables can reduce blood pressure (John et al., 2002; He et al., 2006). The other minerals present in minor amounts include copper, iron, zinc and sulfur, which are also important supplementary elements in our diet (Smiderle et al., 2008). It is also interesting to note that the wild Australian F. velutipes contain higher copper and potassium levels as compared to other Australian mushrooms species (Zeng et al., 2012).
Table 5.
Content of major mineral elements in F. velutipes.
| Minerals | Concentration (mg/g dry weight) | References |
|---|---|---|
| Calcium | 0.36–1.18 | Smiderle et al., 2008; Zeng et al., 2012 |
| Potassium | 28.00–28.98 | Smiderle et al., 2008; Zeng et al., 2012 |
| Sodium | 0.657–0.755 | Smiderle et al., 2008; Zeng et al., 2012 |
| Magnesium | 0.68–1.43 | Smiderle et al., 2008; Zeng et al., 2012 |
| Zinc | 0.048–0.068 | Smiderle et al., 2008; Zeng et al., 2012 |
| Selenium | <0.00050 | Smiderle et al., 2008 |
| Lithium | <0.00020 | Smiderle et al., 2008 |
| Copper | 0.057 | Zeng et al., 2012 |
| Manganese | 0.0096 | Smiderle et al., 2008 |
| Iron | 0.0963 | Smiderle et al., 2008 |
| Sulfur | 6.06 | Zeng et al., 2012 |
| Phosphorus | 8.80–9.40 | Smiderle et al., 2008 |
It has been demonstrated that the vitamin content in mushrooms are species and source-dependent (Pereira et al., 2012; Nakalembe et al., 2015). According to Pereira et al. (2012), F. velutipes was shown to contain tocopherols (α-tocopherol, β-tocopherol and δ-tocopherol, but not γ-tocopherol) (0.6 μg/g dry weight), ascorbic acid (238 μg/g dry weight), β-carotene (3.4 μg/g dry weight) and lycopene (0.2 μg/g dry weight). These vitamins or micronutrients may play an important role in contributing to the mushroom's antioxidant activity (Breene, 1990; Pereira et al., 2012).
Health promoting constituents/non-nutrients
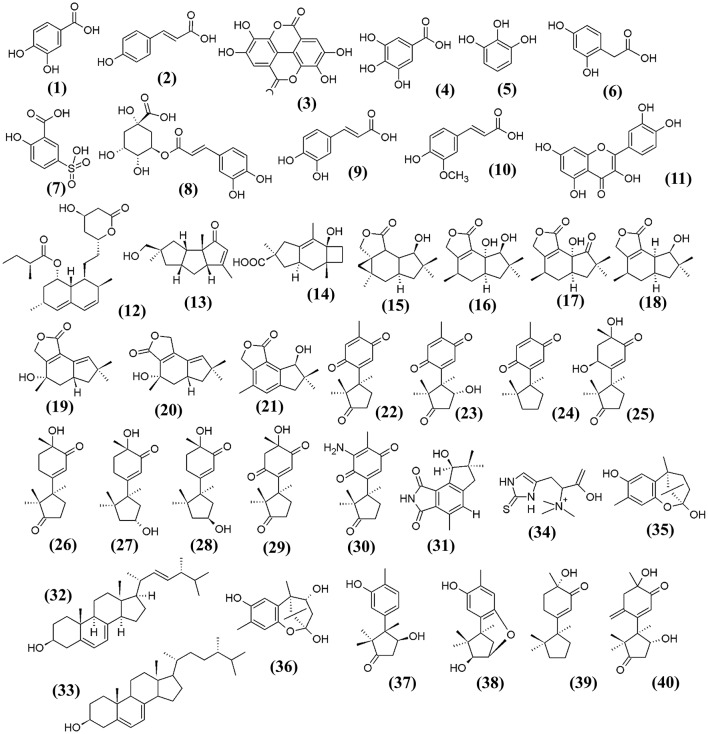
Besides the macronutrients and micronutrients present in F. velutipes, phenolics, especially the phenolic acids which are known to be the main antioxidants in the mushroom, have been detected (Kim et al., 2008; Rahman et al., 2015). Phenolics are heterocyclic compounds and well-recognized for their potent antioxidant activity; they are particularly effective in protecting the body against diseases associated with oxidative stresses such as cancer, cardiovascular disease and diabetes. Rahman et al. (2015) identified several polyphenolic antioxidants present in the methanol:dichloromethane fraction of F. velutipes, including the protocatechuic acid (1), p-coumaric (2), and ellagic acid (3). These bioactive phenolic and polyphenolic compounds confer the antioxidative effect of F. velutipes in preventing the progression of atherosclerosis (Rahman et al., 2015). Gallic acid (4), pyrogallol (5), homogentisic acid (6), 5-sulfosalicylic acid (7), chlorogenic acid (8), caffeic acid (9), ferulic acid (10), and quercetin (11) are other examples of phenolic compounds detected in F. velutipes, ranging from 9.0 to 26.0 μg/g dry weight (Kim et al., 2008). The chemical structures of these bioactive compounds are illustrated in Figure 1. Interestingly, an interspecies comparison study by Zeng et al. (2012) showed that F. velutipes had the highest phenolic content of 2.823 ± 0.007 mg gallic acid equivalent (GAE)/g extract among other mushrooms harvested from Australia. The study also suggested the variation in the phenolic content in those mushrooms may be ascribed to the different geographical locations and also dependent on the ability of a particular sub-species in the synthesis of phenolic compounds (Zeng et al., 2012).
Figure 1.
The chemical structures of bioactive constituents isolated from F. velutipes.
In addition, various novel sesquiterpenes and norsequiterpenes were also identified from the extract of F. velutipes. These compounds exhibited several bioactivities such as anticancer, antibacterial and antioxidant activity (Wang et al., 2012a,d). Lovastatin, (12) which is effective in lowering cholesterol levels, was detected in the fruiting bodies of F. velutipes, estimated at 90.8 ± 2.0 mg/kg dry weight (Chen et al., 2012).
In summary, F. velutipes has excellent nutritional content—high protein and carbohydrate content, low fat content and also high polyunsaturated fatty acid content, making it an excellent food suitable for low calorie diet. However, as noted, there are differences in the chemical composition between the cultivated and the wild samples of F. velutipes, including carbohydrates, proteins and fatty acid profiles. Based on the current literature, it should be noted that data on digestibility and bioavailability of F. velutipes or indeed any other mushrooms are still lacking. In future, more studies could explore the bioavailability of specific nutrients or bioactive constituents contained in F. velutipes as well as investigate the changes in the individual constituents during different preservation methods, storage conditions and different cooking processes.
Biological activities of F. velutipes
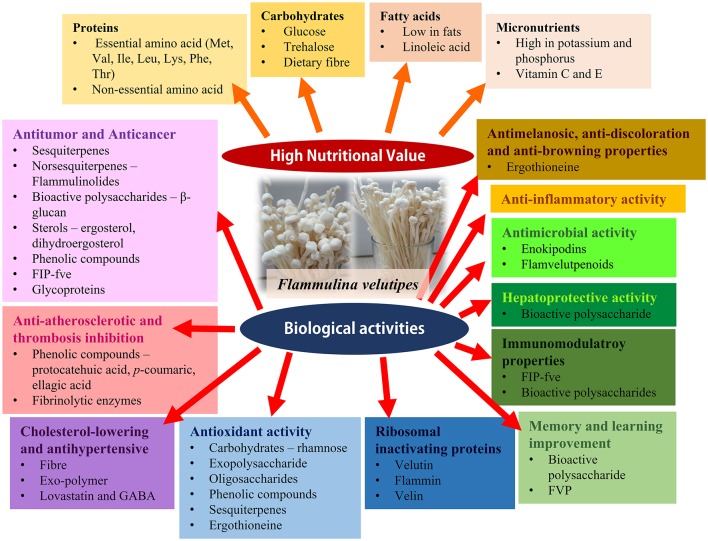
F. velutipes has been reported to have multiple beneficial effects on human health. They include antitumour, anticancer and anti-atherosclerotic activity, thrombosis inhibition, antihypertensive and cholesterol lowering effects, anti-aging and antioxidant properties, ability to restore neurotransmitters associated with memory and learning, anti-inflammatory, immunomodulatory and anti-bacterial activities (Gu and Leonard, 2006; Karaman et al., 2010; Lee et al., 2013; Wu et al., 2014; Rahman et al., 2015; Yang et al., 2015). These biological activities of F. velutipes are summarized in Table 6; a summary of the biological activities of F. velutipes in a graphical from is depicted in Figure 2.
Table 6.
The bioactivities of extracts and constituents isolated from F. velutipes.
| Bioactivities | Tested substance | Experimental design | Dosage and results | References |
|---|---|---|---|---|
| Antitumor and anticancer | Water extract of F. velutipes | In vitro cell culture, BT-20, MCF-7 and MDA-MB-231 cells | Growth inhibition against BT-20, MCF-7 and MDA-MB-231 at IC50 = 30, 150, and 75 μg/mL, respectively | Gu and Leonard, 2006 |
| Flammulinolide A (15) Flammulinolide B (16) Flammulinolide C (17) Flammulinolide F (20) |
In vitro cell culture, Hela, HepG2 and KB cells | Flammulinolide A (15), B (16) and F (20)—cytotoxicity against KB cell at IC50 = 3.9, 3.6 and 4.7 μM Flammulinolide C (17)—cytotoxicity against Hela at IC50 = 3.0 μM |
Wang et al., 2012c | |
| Enokipodin B (22) Enokipodin D (23) Enokipodin J (30) 2,5-cuparadiene-1,4-dione (24) |
In vitro cell culture, HepG2, MCF-7, A549 and SGC7901 cells | Moderate cytotoxicity against all the tested cancer cell lines with IC50 within 20 to 100 μg /mL | Wang et al., 2012d | |
| A novel norsequiterpene alkaloid (31) | In vitro cell culture, KB cell | Cytotoxicity against KB cells at IC50 = 16.6 μM | Xu et al., 2013 | |
| Alkaline-soluble polysaccharide from cell wall of F. velutipes | SC-180 mouse model | Significant inhibition (94.1–97.8%) on the growing of SC-180 tumor at dose of 15 mg/kg | Leung et al., 1997 | |
| Hot-water extract of polysaccharides from F. velutipes fruiting body | S-180 mice tumor model and SMMC-7721 human hepatoma cells (in vitro) | Inhibition (54.7–58.1%) on proliferation of S-180 implanted tumor in vivo at dose of 24 mg/kg 59.5% inhibition on proliferation of SMMC-7721 cells at 50 mg/F. velutipes polysaccharide in vitro |
Jiang et al., 1999 | |
| Polysaccharide extracts from F. velutipes mycelium | In vitro cell culture, BEL-7402 cell | FvP-2 and FvP 3 showed inhibition against BEL-7402 for 45% and 40% at 20 μg/mL and 40 μg/mL, respectively | Zhao et al., 2013 | |
| Triple helical polysaccharide | In vitro cell culture, BCG-823 and A549 | FVP-1 showed inhibitory rate of 32.3% against A549 at 100 μg/mL; 78% against BGC-823 at 200 μg/mL FVP-2 showed inhibitory rate of 27.9% against A549 at 200 μg/mL; 95% against BGC-823 at 200 μg/mL |
Yang et al., 2012 | |
| Sterols (ergosterol (32) (54.8%) and 22, 23-dihydroergosterol (33) (27.9%)) extracted from F. velutipes | In vitro cell culture, SGC, HepG2, A549 and U251 | Cytotoxicity against SGC cell with IC50 at 11.99 μg/mL; HepG-2 cell with IC50 at 9.3 μg/mL; A549 lung cancer cell with IC50 at 20.4 μg/mL and U251 glioblastoma with IC50 at 23.42 μg/mL | Yi et al., 2012, 2013a,b | |
| FIP-fve, an immunomodulatory protein | In vitro cell culture, A549 cell BNL hepatoma-bearing female BALB/c mice | Reduced expression of RACGAP1 gene responsible for metastasis Reduced tumor size of BNL hepatoma in mice (10 mg/kg oral administration) by expression of IFN-γ through ERK/MAPK signaling pathway; inhibited angiogenesis through CD4+T-cell-derived IFN-γ Inhibited the proliferation of A549 mediated through the increased p53 and p21 expression Acted as adjuvant with HPV-16 E7, increased lifespan of tumor-bearing mice through increased HPV-16 E7-specific antibodies and T cells |
Chang et al., 2010, 2013 | |
| Proflamin, a glycoprotein extracted from mycelium of F. velutipes | Tumor bearing mice model with B-16 melanoma and Ca755 adenocarcinoma | Increased mean survival time of mice with B-16 and Ca-755 for 86 and 84%, respectively, at 10 mg/kg administrated orally | Ikekawa et al., 1985 | |
| Anti-atherosclerotic activity | Methanol:dichloromethane fraction of F. velutipes (contained protocatechuic acid (1), p-coumaric (2) acid and ellagic acid (3) | In vitro lag time of conjugated diene formation in LDL molecule and TBARS inhibition assay | Prolonged the lag time of CD formation up to 120 min at 1 μg/mL concentration Inhibited 48.71% of TBARS formation at 1 mg/mL concentration | Rahman et al., 2015 |
| Thrombosis inhibitory activity | Fermented extract of F. velutipes | Coagulability test, thrombin time Fibrinolytic activity test | Prolonged thrombin clotting time for 358.6 ± 0.4 s (2.2 × control) Demonstrated fibrinolytic activity on fibrin plate with clear zone of 20 ± 0.5 mm2 |
Okamura et al., 2001 |
| Fibrinolytic enzyme isolated from culture supernatant of F. velutipes | Fibrinolytic and fibrinogenolytic assays (SDS-PAGE analysis) | Hydrolyzed fibrin α-chain followed by the γ-γ chain and β-chain (similar hydrolysis pattern as of plasmin) Hydrolyzed Aα- and Bβ- chains of fibrinogen | Park et al., 2007 | |
| Cholesterol-lowering effect and antihypertensive activity | F. velutipes powder (FVP) and water extract (FVE) | Male Syrian hamsters under high cholesterol diet (lipoproteins determination) | Reduced levels of serum TC (28.7%), TG (33.6%), LDL (54.5%) and LDL/HDL ration (61.8%) and also liver TC (37.6%) and TG (46.1%) of high cholesterol diet hamsters supplemented with 3% of FVP Reduced levels of serum TC (27.0%), TG (28.6%), LDL (48.5%) and LDL/HDL ration (57.9%) and also liver TC and TG of high cholesterol diet hamsters supplemented with 3% of FVE | Yeh et al., 2014 |
| Exopolymer extracted from mycelium culture | Sprague-Dawley male rat under hyperlipidemic diet | Reduced the plasma triglyceride (20.2%), TC (21.7%), LDL (27.6%) and liver TC (21.4%) in hyperlipidemic rat at 100 g/kg body weight for 4 weeks | Yang et al., 2002 | |
| F. velutipes fiber | Male F344/DuCrj rats | Reduced levels of serum TC (9.93%), and VLDL+IDL+LDL (20.6%) when fed with 50 g/kg for 4 weeks as compared to control fed with cellulose diet only | Fukushima et al., 2001 | |
| GABA (6–7%) produced by F. velutipes | Spontaneously hypertensive rats | Lowered the systolic blood pressure by ~30 mmHg at 0.9 mg GABA/kg | Harada et al., 2011 | |
| Mycelium culture of F. velutipes | In vitro ACE inhibitory assay | Showed strong ACE inhibitory effect from 40.7 to 52.8% with IC50 from 7.4 to 22.6 mg/mL compared to other basidiomycetes cultures | Kim et al., 2002 | |
| Memory and learning improvement | Polysaccharides extracted from F. velutipes | Scopolamine-induced memory and learning impaired rat model | Increased SOD and GSH-Px activities in the brain; inhibited TBARS formation in the brain from 100 to 400 μg/kg Restored the neurotransmitters (DA, NE, 5-HT and Ach) level in the brain of scopolamine treated rat Increased the expression of p-CaMK II and connexin 36 in the brain |
Yang et al., 2015 |
| Water extract of F. velutipes and polysaccharides extracted from the water extract with ultrasonic | In vitro AChE inhibitory activity | Water extract demonstrated ~ 20% AChE inhibitory effect The FVP extracted using ultrasonic displayed AChE inhibitory rate of 18.51% at 0.6mg/ml |
Yang et al., 2011 | |
| Ribosome inactivating protein | Velutin | ELISA kit—glycohydrolase inhibitory activity assay Cell-free rabbit reticulocyte lysate system |
At 5 mg/mL, velutin showed inhibition against HIV-1 reverse transcriptase, α-glucosidase, β-glucosidase and β-glucuronidase for 102.3, 8.3, 62.3 and 64.7%, respectively Cell-free translation-inhibitory activity (prevented protein synthesis of 3H-leucine at IC50 of 0.29 nM) |
Wang and Ng, 2001 |
| Flammin | Cell-free rabbit reticulocyte lysate system | Inhibited translation at IC50 of 1.4 nM | Ng and Wang, 2004 | |
| Velin | Cell-free rabbit reticulocyte lysate system | Inhibited translation at IC50 of 2.5 nM | Ng and Wang, 2004 | |
| Flammulina | Cell-free rabbit reticulocyte lysate system | Inhibited 3H-leucine incorporation into protein at IC50 of 0.25 nM | Wang and Ng, 2000 | |
| Antioxidant activity | Intracellular polysaccharides from F. velutipes mycelium SF-08 |
In vitro antioxidant assays–DPPH, superoxide, hydroxyl and reducing power assays In vivo antioxidant status–SOD, GSH-Px and LPO assays |
DPPH scavenging activity reached up to 66.38% at 1 μg/mL Superoxide scavenging activity reached up to 84.29% at 1 μg/mL Hydroxyl radical scavenging activity reached up to 84.42% at 1 μg/mL Reducing power reached up to 1.56 at 1 μg/mL Increased SOD and GSH-Px activities and decreased LPO level in all heart, kidney and blood at 800 mg/kg body weight of mice administrated orally |
Ma et al., 2015b |
| Exopolysaccharide extracted from culture of F. velutipes SF-06 |
In vitro antioxidant assays—DPPH, superoxide, hydroxyl and reducing power assays In vivo antioxidant status—catalase and TBARS assays |
DPPH scavenging activity reached up to 64.53% at 1 μg/mL Superoxide scavenging activity reached up to 67.64% at 1 μg/mL Hydroxyl radical scavenging activity reached up to ~80.0% at 1 μg/mL Reducing power reached up to 1.45 at 1 μg/mL Increased catalase activity and decreased TBARS formation in all heart, kidney and blood at 800 mg/kg body weight of mice administrated orally |
Ma et al., 2015a | |
| Oligosaccharides from F. velutipes | In vitro antioxidant assays—hydroxyl scavenging and reducing power assays | Hydroxyl scavenging activity reached up to 80.24% at 100 μg/mL Reducing power reached up to 0.856 at 100 μg/mL | Xia, 2015 | |
| Water-soluble polysaccharide | In vitro antioxidant assays—superoxide radical scavenging, hydroxyl radical scavenging and reducing power assays | Superoxide radical scavenging activity with an IC50 of ~10 mg/mL Hydroxyl scavenging activity with an IC50 of ~12 mg/mL Demonstrated reducing power of 1.04 at 5 mg/mL | Wu et al., 2014 | |
| Polysaccharide extracted from mycelia of F. velutipes | In vitro antioxidant assays—DPPH scavenging and hydroxyl radical scavenging activities | DPPH scavenging activity reached up to 65.58% at 200 μg/mL Hydroxyl scavenging activity reached up to 71.24% at 71.24 μg/mL | Zhao et al., 2013 | |
| Methanol extract of F. velutipes | Total phenolic content, in vitro antioxidant assays—Trolox equivalent antioxidant capacity, ferric reducing power and ferrous ion chelating activities | Contained 2.823 mg GAE/g extract, highest among the three other mushrooms species Exhibited 221 μmol TE/g extract, 138 μmol Fe[II]-E/g extract and 524 μmol EDTA-E/g extract | Zeng et al., 2012 | |
| Water soluble nucleotide extract from F. velutipes | In vitro antioxidant assays—ABTS scavenging and total reducing ability | Total reducing power of ~0.3 at 20 mg/mL Exhibited ABTS scavenging rate of ~40% at 20 mg/mL | Cheng et al., 2012 | |
| Enokipodin B (22) Enokipodin D (23) Enokipodin J (30) 2,5-cuparadiene-1,4-dione (24) |
In vitro antioxidant assay—DPPH scavenging activity | Enokipodin J (30) with IC50 at 78.6 μM; 2,5-cuparadiene-1,4-dione (24) with IC50 at 80.7 μM; Enokipodin B (22) with IC50 at 154.2 μM and Enokipodin D (23) with IC50 at 116.5 μM | Wang et al., 2012d | |
| Immunomodulatory properties | FIP-fve purified from extract of F. velutipes |
In vitro experiment—cell proliferation assay murine splenocytes; cytokine profiling (ELISA) In vivo experiment—food allergy murine model (oral sensitization by ovalbumin) |
Induced proliferation of total murine splenocytes and only the T cells that is APC-dependent (co-cultured with irradiated splenocytes) (10–40 μg/mL) Resulted Th1-skewed immune response (prominent IFN-γ; littler or no IL-4 production in the supernatant) (10–40 μg/mL) Resulted increased serum IFN-γ in mice fed with 100–400 μg per mouse every day for 8 days Reduced systemic anaphylaxis-like symptom score and plasma histamine level in OVA challenged mice treated with FIP-fve |
Hsieh et al., 2003 |
| FIP-fve purified from extract of F. velutipes | In vitro experiment—cell cycle analysis, cytokine analysis and Western blot | Induced G1/G0 to S phase proliferation in PBMC at 100 μg after 72 h Increased IFN-γ production dose-dependently in PBMC until reached plateau at 100 μg/mL Induced ICAM-1 expression dose-dependently; activated p38 MAP kinase (increased expression of phospho-p38) |
Wang et al., 2004 | |
| FIP-fve purified from extract of F. velutipes | In vitro experiment—Ca2+ analysis, Western blot and RT-PCR | Caused elevation of intracellular Ca2+ concentration release in PBMC (increase transiently at 30 s after treated with FIP-fve and reached maximum at 70 s) Maximum activation of PKC-α at 3 h incubation with 7.69 μM concentration Increased expression of IFN-γ mRNA upon treatment with FIP-fve at7.69 μM for 48 h |
Ou et al., 2009 | |
| FIP-fve | In vitro experiment—eosinophil survival under IL-5 (apoptosis analysis), apoptotic protein expression (flow cytometry), RT-PCR | Reduced the protective effect of IL-5 for eosinophils from apoptosis (at 10 and 25 μg/mL), increased in early apoptotic and late apoptotic eosinophils Diminished the anti-apoptotic Bcl-2 in eosinophil stimulated with IL-5 when treated with FIP-fve Downregulated IL-5Rα proteins and mRNA expression on eosinophils |
Hsieh et al., 2007 | |
| FIP-fve purified from extract of F. velutipes | In vivo experiment—mouse model of allergic asthma (sensitization to OVA intraperitoneally 1st to 14th day, followed by intranasal challenge 3% OVA) | Both pre-treatment and post-treatment of FIP-fve suppressed airway hyperactivity (assessed with whole-body barometric plethysmography) Reduced IgE (54.7–58.6%) and increased IgG2a (83.2–144.5%) in the serum Decreased Th2 cytokines (IL-4, IL-5 and IL-13 and IL-10), increased Th1 and Treg cytokines (IFN-γ and TGF-β) in the serum Reduced inflammation and epithelial cell thickness (histological examination) |
Lee et al., 2013 | |
| FIP-fve purified from extract of F. velutipes | In vivo experiment—mouse models of allergic asthma (sensitization to HDM or DM intraperitoneally 1st to 14th day, followed by intranasal challenge 50 μg HDM/DM) | Reduced airway hyperresponsiveness (assessed with whole-body barometric plethysmography) Reduced infiltrating cells (eosinophils and lymphocytes) in mice challenged by HDM or DM upon treatment with FIP-fve At humoral level, increased IgG2a while decreased IgE in the serum Reduced Th2 cytokines while increased Th1 and Treg cytokines in serum and BALF Reduced inflammation and epithelial cell thickness (histological examination) |
Chang et al., 2015; Chu et al., 2015 | |
| FIP-fve purified from extract of F. velutipes | Female BALB/c mice with RSV intranasal challenge (2 × 105PFU) In vitro experiment—plaque formation assay (HEp-2 cell), RT-PCR | FIP-fve administrated orally (2 days before to 6 days after RSV infection)—decreased airway hyperreactivity Reduced IL-6 while increased IFN-γ Reduced RSV infection rate in lung tissues Inhibited plaque formation (from 111.0 plaque count to 17.3 and 0 at 7.5 μM and 30 Mm, respectively Decreased IL-6 mRNA expression |
Chang et al., 2014 | |
| FIP-fve purified from extract of F. velutipes | In vivo experiment—neutropenia mouse model induced by docetaxel | 10 mg/kg mouse for 3 days (oral gavage) before docetaxel injection—restored docetaxal-induced myelotoxicity (elevated hemoglobin level), protected the bone marrow and haematopoietic cells from damages by docetaxel, prevented damages on the bone microenvironment Partial protection for intestinal villi against docetaxel-induced interstinal injuries |
Ou et al., 2015 | |
| Polysaccharide purified from F. velutipes mycelium | In vitro experiment—NO production assay, TNF-α production assay and IL-1 ELISA | Enhanced NO, TNF-α, IL-1 production by macrophages in a dose dependent manner | Yin et al., 2010 | |
| Polysaccharide derived from F. velutipes mycorrhizae (PFVM) | In vivo experiment—oral gavage of PFVM twice a day for 60 days on female Wister mice, weight ratio of thymus and spleen, flow cytometry, cytokine profile In vitro experiment—isolation of mice spleen lymphocytes, MTT | Increased the weight ratio of thymus and spleen at doses of 2 g/kg and 4 g/kg, respectively Increased the percentage of CD3+ and CD4+thymocytes dose-dependently in the peripheral blood, thymus, spleen of the mice Increased IL-2 and TNF-α in the serum dose-dependently Increased the percentage of CD3+, CD4+ and ratio of CD4+ /CD8+ in spleen lymphocytes |
Yan et al., 2014 | |
| Water-soluble polysaccharide from F. velutipes | In vitro experiment—NO production assay, IL-1, IL-6 and TNF-α ELISA and lymphocyte proliferation assay | Increased the NO production from macrophages dose-dependently from 5 to 160 μg/mL Increased production of IL-1β at 20 μg/mL while increased production of IL-6 and TNF-α at 5 μg/mL Promoted lymphocytes proliferation from 50 to 500 μg/mL in dose-dependent manner |
Wu et al., 2014 | |
| Water extract of F. velutipes | In vitro experiment—proliferation assay, cytokine profile | Increased proliferation of splenocytes at concentrations ranging from 10 to 1000 μg/mL Increased Th1 cytokine productions |
Ryu et al., 2014 | |
| Boiling water extract of F. velutipes | In vitro experiment—cytokine profile (ELISA), cytotoxicity assay | Stimulated the production of IFN-γ from large intestinal lamina propria leukocytes Promoted the cytotoxicity of large intestinal lamina propria leukocytes isolated from rat against YAC-1 cells | Lee et al., 2011 | |
| Melanosis inhibitory activity | Ergothioneine (34) containing hot water extract of F. velutipes | In vitro experiment—mushroom polyphenol oxidase inhibition assay, RT-PCR In vivo experiment—Immersion treatment, postharvest melanosis | Inhibited mushroom polyphenol oxidase activity by 58% after 300 s at 0.38 mg ergothioneine/mL extract, and in a dose-dependent manner Lowered expression of proPO gene in group fed with F. velutipes extract Suppressed the polyphenol oxidase activity in the hemolymphs of the organisms supplemented with F. velutipes extract diet (25% in wet diet each day for 7 days) 0.5% F. velutipes extract in immersing solution prevented melanosis in the carapace during ice storage |
Encarnacion et al., 2010, 2011a,b; Encarnacion et al., 2012 |
| Ergothioneine (34) containing hot water extract of F. velutipes | In vitro experiment—total lipid hydroperoxides analysis and metmyoglobin formation | Suppressed metMB formation and HPO accumulation by 1 mL of extract (10 g fresh material to 100 g of minced tuna meat) Showed anti-discoloration activity with 1 mL of extract (10 g fresh material to 100 g of minced tuna meat) |
Bao et al., 2010b | |
| Ethyl acetate of F. velutipes mycelium grown in 2% glucose | In vitro experiment—anti-tyrosinase activity assay | Demonstrated inhibitory activity of 58.8% against tyrosinase at 0.5 mg/mL of extract | Kim et al., 2011 | |
| Antimicrobial activity | Methanol and chloroform extracts of F. velutipes | In vitro experiment—plate diffusion | Methanol extract exhibited strongest antibacterial activity against S. aureus from horse wound infection with 19.75 mm zone inhibition Chloroform extract exhibited strongest antibacterial activity against Bacillus sp. from animal skin with 15.75 mm zone inhibition | Karaman et al., 2010 |
| Methanol extract of F. velutipes | In vitro experiment—microdilution method | Displayed antibacterial activity against B. subtilis ATCC6633, B. pumilus NCTC8241, S. aureus ATCC6538 and P. aeruginosa ATCC9027 with MIC measured at 12.5, 3.125, 50 and 50 mg/mL, respectively | Nedelkoska et al., 2013 | |
| F. velutipes colony | In vitro experiment—duel culture technique | Showed complete replacement against Ophiostoma ulmi Showed partial replacement against F. oxysporum, P. funereal and F. culmirum LM2091 |
Borhani et al., 2011 | |
| Enokipodin A (35) Enokipodin C (36) |
In vitro experiment—plate diffusion and broth dilution | Enokipodin A (35)—against B. subtilis LMA0011 (21–30 mm), B. subtilis IFO12734 (21–30) mm and S. aureus AHU1142 (16–20 mm) at 50 μg Enokipodin A (35) showed antibacterial activity against B. subtilis LMA0011 with MID of 3.12 μg Enokipodin C (36)—against B. subtilis LMA0011 (16–20 mm), B. subtilis IFO12734 (16–20 mm) and S. aureus AHU1142 (16–20 mm) at 50 μg Enokipodin C (36) showed antibacterial activity against B. subtilis LMA0011 with MID of 6.25 μg |
Ishikawa et al., 2001, 2005 | |
| Flamvelutpenoid A–D (37 to 40) | In vitro experiment—broth dilution | Displayed weak activity against E. coli, B. subtilis and methicillin-resistant S. aerues with MIC of >100 μM | Wang et al., 2012b | |
| Enokipodin F (26) Emokipodin G (27) Enokipodin I (29) Enokipodin J (30) 2,5-cuparadiene-1,4-dione (24) Enokipodin B (22) Enokipodin D (23) |
In vitro experiment—microdilution technique | Enokipodins I (29), J (30), B (22), D (23) and 2,5-cuparadiene-1,4-dione (24) showed activity against B. subtilis with IC50 measured at 164.3, 151.2, 140.5, 167.6 and 154.6, respectively Enokipodin F (26), G (27) and I (29) showed activity against A. fumigatus with IC50 measured at 229.1, 233.4 and 235.1, respectively |
Wang et al., 2012d | |
| Anti-inflammatory activity | F. velutipes in dried powder form | In vitro experiment—Griess assay and ELISA (TNF-α) | Exhibited NO inhibitory activity of IC50 at 24 μg/mL Exhibited TNF-α inhibitory activity of IC50 at 99 μg/mL |
Gunawardena et al., 2014 |
| Water extract of F. velutipes | In vitro experiment—NO inhibition assay and Western blot | Inhibited NO production Inhibited LPS-induced iNOS and COX-2 expression in macrophages | Kang, 2012 | |
| Hepatoprotective activity | Water-soluble polysaccharide of F. velutipes (FVP2) | In vitro experiment—viability test, ALT analysis | Promoted proliferation of primary culture of mouse hepatocytes at concentrations ranging from 25 to 200 μg/mL Reduced the intracellular release of ALT from hepatocytes induced by CCl4 intoxication |
Pang et al., 2007 |
Figure 2.
The graphical abstract of the nutritional values and biological activities of F. velutipes.
Antitumor and anticancer
With the increasing rate of life-threatening neoplastic diseases in recent years, development of more effective antitumor drugs is a research area of great relevance (Ajith and Janardhanan, 2007). Currently, chemotherapeutic agents used in cancer treatment are able to slow down the progress of the disease; however, they are also toxic toward healthy, non-neoplastic cells (Tan et al., 2016b). An alternative approach currently being explored intensively is pursuing anticancer agents from natural food products to inhibit the onset of cancer (Chung et al., 2010; Chan et al., 2012; Goh et al., 2014; Tan et al., 2016a). Over the years, bioactive compounds derived from Inonotus obliquus (chaga mushroom) and L. edodes (shiitake) have been shown to exhibit anticancer effects against certain cell lines such as human leukemic U937 cells, stomach adenocarcinoma AGS cells, lung carcinoma A549 cells, sarcoma S-180 cells and human colorectal adenocarcinoma HT-29 cells. (Ou et al., 2005; Chung et al., 2010; Jeff et al., 2013). F. velutipes has also been shown to contain bioactive compounds with antitumor and anticancer properties (Gu and Leonard, 2006; Smiderle et al., 2006; Wang et al., 2012b; Yi et al., 2013b).
In 2003, Ikekawa (2001) presented an epidemiological study spanning 15 years (1972–1986) which showed that the cancer related death rates of farmers who grew F. velutipes mushroom—assuming that they would have eaten some of the mushrooms they farmed—were lower by 39% when compared to comparable populations not involved in mushroom farming (Monro, 2003). Experimental evidence also demonstrated that F. velutipes extract possesses anticancer properties, and several anticancer compounds have been isolated from F. velutipes in recent decades. Gu and Leonard (2006), among others, revealed the anticancer potential of fruiting bodies extract from F. velutipes, which was particularly effective against breast cancer cell lines. In the study, F. velutipes extract was shown to inhibit the growth of both estrogen-receptor positive (ER+) MCF-7 and estrogen-receptor negative (ER−) MDA-MB-231 human breast cancer cell lines. Furthermore, the extract induced apoptosis in the breast cancer cells and also caused 99% inhibition of colony formation of MCF-7 (Gu and Leonard, 2006).
Recently, several novel bioactive compounds with anticancer activities were isolated from the cultures of F. velutipes. A research group from China discovered a sesquiterpene with a novel carbon skeleton, known as flammulinol A (13), together with sterpuric acid (14), an isolactarane sesquiterpene and six isolactarane-related norsesquiterpenes (flammulinolides A–G) (15 to 21) from the solid culture of F. velutipes (Wang et al., 2012c). These isolactarane-related norsesquiterpenes (15 to 17, 19 to 21) extracted from the solid culture of F. velutipes were found to possess cytotoxic effect against several cancer cell lines. Flammulinolide A (15) showed cytotoxic effect against KB cells (human nasopharyngeal carcinoma) and HepG2 (human hepatocellular liver carcinoma) with an IC50 of 3.6 and 34.7 μM, respectively, whereas flammulinolide C (17) exhibited strong cytotoxicity against HeLa cells (human cervical adenocarcinoma) with an IC50 of 3.0 μM (Wang et al., 2012c). Other groups have reported the isolation of sesquiterpenes (22 to 30) from solid culture of F. velutipes grown on cooked rice, sesquiterpenes (22 to 24, 30) were reported to have moderate cytotoxicity against human tumor cell lines: HepG2 (liver cancer cells), MCF-7 (breast cancer cells), SGC7901 (stomach cancer cells), and A549 (lung cancer cells) with IC50 within 20 to 100 μg /mL (Wang et al., 2012d). A new nonsesquiterpene alkaloid (31) derived from fermented rice substrate of F. velutipes was also discovered to exhibit inhibitory effect against human KB cells in vitro (Xu et al., 2013).
Aside from the sesquiterpenes, bioactive polysaccharides from F. velutipes are also potential anticancer agents. The polysaccharides of F. velutipes demonstrated antitumor and anticancer properties against sarcoma SC-180 mouse model and S-180 mice tumor model in vivo and hepatoma SMMC-7721 cells in vitro (Leung et al., 1997; Jiang et al., 1999). Recently, crude polysaccharide from mycelia of the mushroom was also found to reduce the proliferation of human BEL-7402 lung cancer cells by 45% at 640 μg/mL (Zhao et al., 2013). Triple helix structured polysaccharides extracted by ultrasonic wave from F. velutipes exhibited high inhibitory effect against BGC-82 gastric cancer cells, suggesting a potential role in prevention of gastric carcinoma (Yang et al., 2012). Beta-glucan, which is well known for its antitumor activity (Zhang et al., 2007; Mantovani et al., 2008), was also isolated from F. velutipes (Smiderle et al., 2006).
Sterols extracted from F. velutipes, consisting of mainly ergosterol (32) (54.8%) and 22, 23-dihydroergosterol (33) (27.9%), were found to be potential therapeutic agents against stomach, liver, lung cancer and gliomas. It showed potent antiproliferative activity against human SGC cells (stomach cancer cells) with an IC50 of 11.99 μg/mL, HepG-2 cells with IC50 at 9.3 μg/mL, A549 lung cancer cell with IC50 at 20.4 μg/mL and U251 glioblastoma with IC50 at 23.42 μg/mL (Yi et al., 2012, 2013a,b). Studies have also been done to improve the bioavailability, biodistribution and solubility of sterols from F. velutipes through encapsulation in liposomes, mixed micelles nanoformulation and microemulsion (Yi et al., 2012, 2013a,b).
F. velutipes extract was found to contain phenolic compounds such as protocatechuic acid (1), p-coumaric (2), and ellagic acid (3) (Rahman et al., 2015) which have anticancer effects (Ferguson et al., 2005; Seeram et al., 2005). Both p-coumaric (2) and ellagic acid (3) have potent antioxidative effect on human colon cells, HT-29 and HCT 16 cell lines, respectively (Ferguson et al., 2005; Seeram et al., 2005). Ellagic acid was shown to be able to reduce hepatic P450 level and also its catalytic activities in vitro, thus decreasing the metabolism of carcinogens that can cause chemically induced cancer (Ahn et al., 1996).
The biological activities of FIP-fve, a bioactive protein isolated from the mushroom F. velutipes, have also been investigated extensively. FIP-fve belongs to a fungal immunomodulatory protein (FIP) family that modulates immune responses, including antitumor activity (Chang et al., 2013). Chang et al. (2010) suggested that FIP-fve is able to reduce the expression of RACGAP1 gene and also reduce its reporter activity (Chang et al., 2013). The RACGAP1 gene is responsible for the survival and metastatic activity of lung cancer cells, thus, silencing of this gene reduces the migration of cancer cells (Wang et al., 2011). Besides that, FIP-fve also increased tumor suppressor gene p53 expression and also its downstream gene, p21, thus attenuating the proliferation of A549 lung cancer cells (Chang et al., 2013). In another study, Chang et al. (2010) demonstrated that the oral administration of FIP-fve reduced the tumor size of BNL hepatoma-bearing mice and suggested that the antitumor effect was mediated by IFN-γ-induced tumor growth inhibition effect involving both innate and specific immunity and ERK/MAPK signaling pathway. The study also showed that the antitumor effect of FIP-fve was mediated through the inhibition of angiogenesis by CD4+ T-cell-derived IFN-γ. In addition, the study showed that the expression of MHC class I and II and co-stimulatory molecule CD80 on peripheral blood mononuclear cell was also increased, suggesting the oral administration of FIP-fve exerted antitumor effect through upregulation of presenting ability of APCs (Chang et al., 2010). These findings are in concordance with another in vivo study whereby FIP-fve protein activated the maturation of splenic dendritic cells, an APC and stimulated antigen-specific CD8+ T-cell immune responses. Administration of FIP-fve as adjuvant therapy along with HPV-16 E7 vaccine to tumor bearing mice resulted in increased production of HPV-16 E7-specific antibodies and increased expansion of HPV-16 E7-specific interferon (IFN)-γ-producing CD4+ and CD8+ T cells compared with HPV-16 E7 vaccine alone, resulting prolonged lifespan of the mice (Ding et al., 2009). Overall, the evidence is strongly suggestive that FIP-fve is a potential agent for the development of novel adjuvants for cancer immunotherapy.
Glycoproteins found in the fruiting body and mycelium of F. velutipes also exhibit anticancer effect (Zhang et al., 2007). Proflamin, an acidic glycoprotein isolated from the mycelium enhanced several immunosuppression processes and exhibited antiproliferative effects against various cancer cells. It was demonstrated to be able to prolong the lifespan of mice bearing B-16 melanoma and adenocarcinoma 755 tumor cells (Ikekawa et al., 1985). When given as combination therapy along with vaccines or surgery, it also inhibited the growth of sarcoma S-180, L1210 leukemia ascite cells and Meth-A fibrosarcoma. The study also revealed another protein-bound polysaccharide, EA6 significantly inhibited the proliferation of Meth-A fibrosarcoma. Similar to FIP-fve, the antitumor effects of EA6 which are manifested by virtue of strengthening the specific and innate immunity, were shown to be mediated by CD4+ T cells (Maruyama and Ikekawa, 2007). To date, it is obvious that many bioactive compounds with effective anticancer and antitumor properties have been isolated from different parts of F. velutipes, indicating that this mushroom is a good source for future development of chemotherapeutic agents. Nevertheless, studies are still required to further study the bioavailability of these bioactive compounds derived from F. velutipes and also their exact mechanisms of action.
Vascular diseases: anti-atherosclerotic and thrombosis inhibition
Oxidized low density lipoprotein (ox-LDL) has long been known to be the key player in the early events of the atherosclerosis cascade. Lipid hydroperoxides are formed when polyunsaturated fatty acid is oxidized by free radicals, and the continuous oxidation and reduction of the hydroperoxides will further augment the peroxidation process. Recently, Rahman et al. (2015) demonstrated the polyphenolic compounds present in methanol:dichloromethane (M:DCM) fraction of F. velutipes are able to retard LDL oxidation, thus possibly being effective in impeding the progression of atherosclerosis. Protocatechuic acid (1), p-coumaric (2) and ellagic acid (3) were identified from the M:DCM fraction of F. velutipes that showed the longest lag time of conjugated diene formation and inhibition of TBARS formation (Rahman et al., 2015). The study suggested that the inhibition of the peroxidation processes may be attributed to the chain breaking actions of the phenolic compounds which are able to reduce the alkoxyl or peroxyl radicals to alkoxides or hydroperoxides, respectively, interfering with the peroxidation process (Rice-Evans et al., 1997). In addition, the study also proposed that the antiatherosclerotic effect demonstrated by the M:DCM fraction of F. velutipes may be mediated through anti-inflammatory effects. Protocatechuic acid (1) is known to prevent the adhesion of monocytes to tumor necrosis factor-alpha(TNF-α)activated endothelial cells, leading to a reduction of the expression of vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1) and also nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) binding activity, thus reducing the formation of atheroma (Kakkar and Bais, 2014). Another phenolic constituent of the fraction, p-coumaric acid (2) is able to inhibit ADP-induced platelet aggregation, interrupt the arachidonic acid cascade and decrease thromboxane B2 and lipopolysaccharide-induced prostaglandin E2 production, hence inhibiting the formation of plaque and inflammation process (Luceri et al., 2007). In conclusion, the phenolic components in F. velutipes, which have high ability toward withstanding the oxidation of LDL as well as their anti-inflammatory activity, may be the agents contributing to the overall anti-atherosclerotic properties of the extract.
Thrombosis is one of the important initial events that takes place during accelerated atherosclerosis (Walters et al., 1994). One study revealed that the use of F. velutipes in fermentation may produce fermented products which possess preventive activity against thrombosis. It has been demonstrated that the addition of F. velutipes in fermentation process resulted in end-product with a prolonged thrombin clotting time (358.6 ± 0.4 sec) of 2.2-fold than that of the control. Further experimentation has also demonstrated a strong fibrinolytic activity on a fibrin plate (Okamura et al., 2001). A more recent study successfully purified and characterized a fibrinolytic enzyme from the culture supernatant of F. velutipes mycelium (Park et al., 2007). The fibrinolytic enzyme (FVP-I), a protease from F. velutipes was shown to be a direct-acting fibrinolytic and fibrinogenolytic agent which elicits direct cleavage of fibrin and fibrinogen without the need of plasminogen activator, thus demonstrating potential as thrombolytic therapy (Park et al., 2007). Collectively, the evidence suggests that F. velutipes is a potential source of bioactive substances for drug development in vascular disease prevention.
Antihypertensive and cholesterol-lowering effect
Almost 17 million deaths, approximately 1/3 of the total deaths in a year are due to cardiovascular diseases; and among these, complications of hypertension account for approximately 9.4 million deaths. Hypertension, together with other health risk factors, is associated with highly detrimental complications including increased probability of heart attack, stroke and kidney disease as well as other chronic diseases. These risk factors include tobacco use, diabetes and hypercholesterolemia (World Health Organization, 2013). The consumption of herbs and mushrooms as dietary supplement has been known to be beneficial to people with cardiovascular diseases (Tan et al., 2016c). Owing to the rich content of dietary fiber, mushrooms can help in cholesterol metabolism and absorption, thereby lowering the risk of cardiovascular diseases. There is also a growing interest in F. velutipes as an attractive source of various biologically active components including dietary fiber, polysaccharides and mycosterol that have been long known to possess cholesterol and blood pressure lowering effects (Yeh et al., 2014). In fact, F. velutipes was shown to contain the highest fiber content as compared to other mushrooms such as L. edodes, oyster cap fungi and cap fungi (Yang et al., 2001).
Yeh et al. (2014) investigated the effect of the active components of both F. velutipes powder and F. velutipes extract on the lipid metabolism of male hamsters on a high fat diet. The study revealed that both F. velutipes extract and powder at dose of 3% are capable of reducing the level of TC (total cholesterol), TG (triacylglycerol), LDL (low density lipoprotein cholesterol), and LDL/HDL (high density lipoprotein cholesterol) in the serum and liver of the hamsters significantly (Yeh et al., 2014). This is in agreement with the findings of another study whereby the exo-polymer of F. velutipes exerted hypolipidemic effect on diet-induced hyperlipidemic rats. Significant reduction in plasma triglyceride, plasma TC, LDL and liver TC levels were observed from the animals administered F. velutipes exo-polymer at 100g/kg body weight for 4 weeks (Yang et al., 2002). It was also demonstrated that the F. velutipes fiber diet resulted in reduction of plasma TC and increased fecal cholesterol excretion and liver LDL receptor mRNA level in rats (Fukushima et al., 2001).
Mushrooms are known to elicit hypocholesterolemia and also to possess anti-hypertensive properties. Chen et al. (2012) reported the detection of both lovastatin (12) and γ-aminobutyric acid (GABA) in the fruiting body of F. velutipes. Lovastatin (12) is used clinically for its inhibitory effect on cholesterol production, thereby reducing the risk of coronary heart disease. Many studies have shown that food products containing GABA are able to lower the blood pressure of hypertensive subjects (Aoki et al., 2003; Inoue et al., 2003; Hayakawa et al., 2004). Another study reported that the administration of GABA enriched F. velutipes powder (0.9mg GABA /kg) was successful in reducing the systolic blood pressure by 30 mmHg in spontaneously hypertensive rats. Of particular note, the study also reported that those with normal blood pressure were not affected by the powder (Harada et al., 2011). These results were suggested to be due to GABA's effects on inhibiting noradrenaline release from the sympathetic nervous system, ameliorating the rise in blood pressure (Hayakawa et al., 2002).
Furthermore, an optimized culture broth used for the growth of F. velutipes mycelium was shown to display prominent inhibition of angiotensin converting enzyme with IC50 of 22.6 mg/mL (Kim et al., 2002). The authors also highlighted that the use of the culture broth of F. velutipes as a source for ACE inhibitor provides many benefits for the future development of anti-hypertensive agents that are important for the treatment for cardiovascular diseases (Kim et al., 2002).
Memory and learning improvement
Alzheimer's disease is a progressive neurodegenerative disorder characterized by the deterioration of cognition and memory. Studies have indicated that loss of basal forebrain cholinergic neurons involved in learning and memory processes constitutes a pathological hallmark of Alzheimer's disease (Martorana et al., 2010). Besides the cholinergic hypothesis, neurodegenerative disease, including Alzheimer's disease, are also associated with oxidative damage in the brain resulting from an imbalance between reactive oxygen species generation and antioxidant enzyme activity (Wong et al., 2012; Sayyad et al., 2016; Ser et al., 2016). Previous reports revealed that several mushrooms have been demonstrated to exhibit cognitive enhancing activity including Hericium erinaceus (Yamabushitake) (Mori et al., 2009), Inonotus obliquus (Chaga) (Giridharan et al., 2011) and Cordyceps militaris (Tsai et al., 2015). Several recent studies have also sought to investigate the beneficial effects of F. velutipes in cognitive function improvement (Yang et al., 2011, 2015).
An in vivo study showed that polysaccharides from F. velutipes (FVP) were effective against the progression of scopolamine induced learning and memory deficits in rats (Yang et al., 2015). The study revealed that the administration of FVP prevented the reduction of the antioxidant defense enzymes activities and elevation of TBARS levels caused by scopolamine in the rats, showing that FVP improved the memory deficits in the rats through amelioration of oxidative stress (Gao et al., 2012; Yang et al., 2015). FVP was also found to restore the level of the neurotransmitter acetylcholine (ACh) in the hippocampus and cerebral cortex by modulating the activities of its synthetic enzyme, choline acetyltransferase (ChAT) and its hydrolysing enzyme, acetylcholinesterase (AChE). It also normalized the levels of other neurotransmitters including the serotonin, dopamine, and norepinephrine, thereby reversing the effect of scopolamine on the reduction of the neurotransmitters (Yang et al., 2015). These neurotransmitters are known to be involved in both memory and learning functions (Wang et al., 2013). In addition, FVP was also shown to prevent learning and memory impairment by regulating the expression of protein kinases, CaMK II and connexin 36 which plays a role in the synthesis and secretion of neurotransmitters (Yang et al., 2015). Another study also demonstrated that the FVP extracted using ultrasonic methods displayed AChE inhibitory rate of 18.51% at 0.6 mg/ml, suggesting FVP has potential in improvement of learning and cognitive ability (Yang et al., 2011).
Ribosome inactivating protein
Ribosome inactivating protein (RIP) is well known for exhibiting diverse bioactivities including antitumour, immunomodulatory, abortifacient, and anti-human immunodeficiency (anti –HIV) virus actions (Ng et al., 1992). Wang and Ng (2001) isolated an RIP designated as velutin from F. velutipes. Velutin was shown to possess N-terminal sequence which resembles most other plant RIPs to a certain extent, however, its 10kDA molecular weight is much lower compared to the others, which mostly range from 25 to 30 kDA (Ng et al., 1992; Wang and Ng, 2001). Velutin was reported to inhibit the activity of HIV virus reverse transcriptase and also glycohydrolase, mainly α- and β-glucosidases which play a part in HIV infection (Wang and Ng, 2001). Besides that, velutin was also non-teratogenic when tested on mouse embryos (Ng et al., 2010).
Flammin and velin were the other two RIPs discovered from F. velutipes (Ng and Wang, 2004). Both of these RIPs do not show much resemblance in terms of N-terminal sequence to the published mushrooms RIPs, instead they show more similarities with angiosperm RIPs. Flammin and velin do not exhibit any RNase and protease activities. This lack of ribonuclease activity further confirms that the cell free translation-inhibitory activity is attributed to ribosome inactivation and not the result of hydrolysis of RNA and protein (Ng and Wang, 2004). Flammulina was another RIP found in the mushroom, and similar to flammin and velin, this protein is also found to be devoid of ribonuclease activity (Wang and Ng, 2000).
Antioxidant activities
Reactive oxygen species (ROS) refers to free radicals derived from oxygen, including superoxide anion, nitric oxide, hydroxyl radical and hydroxyl peroxide. ROS have important functions such as signaling and regulating the fundamental cellular processes of development such as cell death, oogenesis, spermatogenesis, angiogenesis and redox regulation of cells (Chan et al., 2015). However, excessive accumulation of ROS leads to conditions of oxidative stress which causes damage to lipid, DNA and protein, resulting in detrimental effects to the body (Covarrubias et al., 2008). Antioxidants are substances which can delay, prevent or reverse oxidative damage and are known to prevent several chronic diseases such as cancer and diabetes (Karaman et al., 2010; Lau et al., 2015). Butylated hydroxyanisole and butylated hydroxytoluene are examples of potent synthetic antioxidants, however, some of them are found to be toxic and carcinogenic to human body, thus efforts are now focusing on discovering natural antioxidant products (Moghadamtousi et al., 2014; Ma et al., 2015b; Tan et al., 2015a; Ser et al., 2016). Due to its various biological activities including the antioxidant properties, F. velutipes, as one of the most popular edible mushrooms, has attracted a considerable amount of attention in different fields including biochemistry and pharmacology (He and Zhang, 2013).
Over the years, many studies have reported that carbohydrate content, mainly the polysaccharides of F. velutipes exhibit antioxidant activity (Ma et al., 2015b; Xia, 2015). In recent decades, studies have reported high antioxidant activities shown by the different carbohydrate products derived from F. velutipes by using various extraction methods. Ma et al. (2015b) investigated the antioxidant capacity of the intracellular polysaccharides (IPS) extracted from F. velutipes mycelia. The study indicated rhamnose was the major component in IPS responsible for the strong antioxidant activity in vitro including the ability to scavenge hydroxyl and DPPH radicals (Ma et al., 2015b). Besides, the study also showed IPS exhibited anti-aging potential in which the anti-aging enzyme, superoxide dismutase (SOD) in the blood, heart and kidney was increased following treatment with increasing concentrations of IPS (Ma et al., 2015b). SOD protects cells from being damaged by superoxide anion radicals by converting them to hydrogen peroxide, a less active free radical (Siu et al., 2013). In another study, exopolysaccharides (EPS) from F. velutipes were purified and characterized and it was found that the purified fractions were mainly composed of rhamnose (Ma et al., 2015a). Similarly, these EPS fractions were shown to exhibit potent antioxidant activity in vitro such as reducing power and the scavenging capability of hydroxyl, DPPH and superoxide anions (Ma et al., 2015a). This study also demonstrated the purified EPS fractions stimulated anti-aging activity in mice as evidenced by the increased catalase level and decreased malondialdehye content in the organs of the mice. At 800 mg/kg weight, which was the highest dosage of EPS used in the experiment, catalase activity was the highest in heart with 10.12 ± 0.05 U/mg protein. At 800 mg/kg dosage of EPS, the MDA content was the least in liver, 0.62 ± 0.03 nmol/mg protein which is 110% lower than the model control group treated with saline and d-galactose.
Oligosaccharides derived from F. velutipes by hydrolysis using hydrogen peroxide also showed a strong hydroxyl radical scavenging of 80.24% at the concentration of 100 μg/mL (Xia, 2015). Wu et al. (2014) extracted polysaccharide, FVP 1-A from F. velutipes, which exhibited superoxide radical scavenging ability with an IC50 value of about 10 mg/mL, hydroxyl radical scavenging ability with an IC50 value of about 12 mg/mL and reducing power of 1.04, showing a high antioxidant capacity. At 200 μg/mL, polysaccharide from liquid culture mycelia extracted using double distilled water, displayed DPPH scavenging rate of 65.85% and hydroxyl radical scavenging rate of 71.24% (Zhao et al., 2013).
Variation in extraction methods may also influence the antioxidant properties of the polysaccharides derived from F. velutipes. A study showed that the polysaccharides of F. velutipes extracted using various extraction methods (conventional solvent extraction, ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), enzymatic aqueous extraction (EAE)) were found to exhibit differential antioxidant activities in different in vitro assays. Crude polysaccharides (CFP) demonstrated the highest antioxidant activity in terms of reducing power, EAE polysaccharides (EFP) had the highest hydroxyl radical scavenging and metal chelating activity, whereas UAE polysaccharides (UFP) had the highest DPPH scavenging activity. The researchers deduced that the molecular weight and chemical structure of the polysaccharides obtained from different extraction methods played a role in determining their antioxidant properties (Zhang et al., 2013). UFP and MFP exhibited very much greater DPPH scavenging ability compared to EFP and CFP; the high antioxidant capacity observed may be due to the further alteration of chemical structures and decomposition of polysaccharides caused by the ultrasonic and microwave treatments (Yang et al., 2008).
Aside from polysaccharides, the phenolic compounds are also major naturally occurring antioxidant compounds in mushrooms (Barros et al., 2007; Kim et al., 2008). The total phenolic content in the mushrooms has a positive correlation with its antioxidant property measured by in vitro assays such as DPPH assay, hydroxyl assay and lipid peroxidation assay (Karaman et al., 2010). Zeng et al. (2012) demonstrated that F. velutipes possesses the highest phenolic content based on it having the highest antioxidant activities in terms of ferric reducing antioxidant power and ferrous ion chelating activity among three other Australian mushrooms. In contrast, Karaman et al. (2009) found that F. velutipes has poorer phenolic content compared to other lignicolous fungi, suggesting that the prominent antioxidant activity may due to other secondary biomolecules which had yet to be identified at the time of the study. Besides the fruiting body of F. velutipes, the spent culture medium of the mushroom was also shown to contain high amount of phenolic acids and show potent antioxidative action against lipid oxidation, demonstrating its role as a potential antioxidative agent (Bao et al., 2010a). For instance, p-coumaric acid (2), one of the hydroxycinnamic acids found in the mushroom, was shown to increase the activity of SOD and inhibited oxidative stress, resulting in reduction of cardiac apoptosis in isoprenol-induced myocardial infarction in rats (Stanely Mainzen Prince and Roy, 2013). Ellagic acid (3), which was detected in the fruiting bodies of F. velutipes, and is also present in other fruits and nuts such as pomegranate and walnuts, was shown to be a potent antioxidant exhibiting DPPH radical and hydroxyl radical scavenging, reducing capacity and metal chelating activities (Kilic et al., 2014).
Besides phenols and carbohydrates, a few other bioactive compounds present in F. velutipes were shown to exhibit antioxidant activity. Nucleotides derived from F. velutipes were reported to possess mild antioxidant activity. They showed reducing power value of 0.5 at 40 mg/mL and ABTS radical scavenging ability of 60% at 50 mg/mL (Cheng et al., 2012). F. velutipes was reported to contain 46 mg/100g dry matter of vitamin C which is a well-known antioxidant (Breene, 1990; Fu et al., 2002). Sesquiterpenes (22 to 24, 30) extracted from solid culture F. velutipes grown on cooked rice were demonstrated to have DPPH radical scavenging ability (Wang et al., 2012d).
Recently, a study investigated the effect of selenium on the antioxidant activity of F. velutipes (Milovanovic et al., 2015). Selenium participates in the process of selenoproteins and selenoenzyme synthesis which work to protect cells from free radicals. The study demonstrated that selenium supplemented mycelium had enhanced total phenol content and also enhanced DPPH radicals scavenging ability of the mushroom extract (Milovanovic et al., 2015).
Interestingly, a study conducted by Zhang et al. (2013) demonstrated that the different varieties of F. velutipes possess different degrees of antioxidant activities. The investigation included four F. velutipes varieties: Fxuexiu (snowy white), FD (off-white), F3415 (yellow) and FYehuang (snuff color). Fxuexiu and F3415 had higher total phenolic and ergothioneine (34) (ESH) content compared to the other two. In term of their antioxidant activities, F3415 exhibited the strongest DPPH radical scavenging ability and metal chelating ability, Fxuexiu showed the greatest reducing capability and FD had the most potent hydroxyl radical scavenging ability. Meanwhile, Fyehuang displayed the weakest antioxidant activity among the three other varieties (Zhang et al., 2013). A linear relationship was observed between the phenolic acid and ESH content of the samples and their DPPH radical scavenging ability and reducing power. These findings were concordant with those of Bao et al. (2009) which stated that high DPPH radical scavenging activity of the mushroom was mainly attributed to its ESH content (Bao et al., 2009; Zhang et al., 2013). Furthermore, greater ESH level was associated with a stronger delay in autoxidation of oxymyoglobin (Bao et al., 2010a; Chen et al., 2012).
Based on these studies, it can be concluded that the strong antioxidant activity of F. velutipes is attributed to its various bioactive components, namely polysaccharides, phenols, rhamnose sugar, ESH, vitamin C and nucleotides. Different parts of F. velutipes including fruiting body, mycelium and even its spent culture medium are sources of potential antioxidants. Different extraction methods of polysaccharides yield extracts with varying antioxidant properties. Antioxidant ability of the mushroom extract also can be increased with the addition of selenium in the medium. Lastly, antioxidant capacity of F. velutipes varies between different varieties.
Immunomodulatory properties: anti-allergy, anti-viral and anti-complement activity
Recently, many studies have been done on medicinal plants in search of compounds that exhibit immunomodulatory properties as it has been discovered that the modulation of the immune system helps to prevent diseases. The currently available chemical drugs used as immunomodulators have shown to possess a higher risk profile when compared to natural immunomodulators (El Enshasy and Hatti-Kaul, 2013; Shukla et al., 2014). Mushrooms have been known for their medicinal value for decades, and many of the compounds extracted from mushrooms have been demonstrated to have modulatory effects on the immune system (Lee et al., 2011). F. velutipes is one of the medicinal mushrooms that exhibits immunomodulatory activities. Fungal Immunomodulating Protein (FIP) is one of the main compounds exhibiting immunomodulatory properties in F. velutipes. There was also evidence in a recombinant study expressing the recombinant FIP-fve cloned in the expression cassette vector pQE-30 in E. coli M15. The recombinant FIP-fve was shown to modulate different cytokine gene expression in mouse spleen cells, including the increased expression of IL-2, IL-4, IFN-γ, TNF-α, LT, and IL-2R (Li et al., 2011).
One of the earliest studies showed that oral administration of FIP extracted from F. velutipes (FIP-fve) induced Th-1 predominant allergen-specific immune response and protected the mice from anaphylaxis-like symptoms induced by oral challenge with ovalbumin (OVA) (Hsieh et al., 2003). Therefore, Hsieh et al. (2003) proposed that FIP-fve can be developed as an immunoprophylactic agent against allergic diseases, showing potential to be used clinically in children for food allergy prevention. In the following year, it was found that FIP-fve exerted mitogenic effect on human peripheral blood lymphocytes, acting as a potent activator for lymphocyte proliferation (Wang et al., 2004). The study demonstrated that the activated lymphocytes showed enhanced secretion of interferon-γ (IFN-γ) associated with ICAM-1 expression, both in vitro and in vivo. Furthermore, the study showed that the expression of IFN-γ in Th1 cells was regulated by p38 MAP kinase pathway in response to FIP-fve (Wang et al., 2004). In addition, Ou et al. (2009) revealed the IFN-γ production induced by FIP-fve in human peripheral mononuclear cells was also mediated by the Ca2+ release and PKC-α activation.
These findings were then followed by several studies investigating the anti-allergic effect of FIP-fve against allergen-induced airway diseases (Hsieh et al., 2007; Lee et al., 2013). Hsieh et al. (2007) investigated the effect of FIP-fve on the survival of eosinophils isolated and purified from allergic asthmatic patients in the presence of IL-5. The study indicated that FIP-fve induced apoptosis of eosinophils in the presence of IL-5 (which is a survival factor of eosinophils), thereby preventing eosinophils from undergoing necrosis. Eosinophils are known to be related to allergic diseases, and apoptosis of eosinophils, without the release of their contents, is an important feature in the resolution of inflammation. Furthermore, the study further clarified that the inhibition of the IL-5-mediated survival of eosinophils by FIP-fve was mediated through the upregulation of CD95 expression and the downregulation of BcL-xL and pro-caspase 3 expression (Hsieh et al., 2007). Lee et al. (2013) conducted an in vivo study that utilized an OVA-induced chronic airway inflammation murine model to evaluate the effect of FIP-fve against allergic airway diseases. The study indicated that both pre-treatment and post-treatment with FIP-fve successfully suppressed airway inflammation and hyperresponsiveness. FIP-fve was shown to inhibit inflammatory cell infiltration and Th2 cytokines, further strengthening the view that FIP-fve possesses potential as a therapeutic agent for allergy related diseases (Lee et al., 2013). Recent studies also demonstrated the anti-allergic effect of FIP-fve against different allergen-induced airway inflammation in mice (Chang et al., 2015; Chu et al., 2015). Chu et al. (2015) showed oral administration of FIP-fve inhibited house dust mite (HDM)-induced asthma inflammation in mouse model via the modulation of Th1 cytokine production. Meanwhile, Chang et al. (2015) showed that intranasal application of FIP-fve reduced Dermatophagoides microceras (DM)-induced airway hyper-responsiveness, airway inflammation and cytokine expression in mice.
Aside from the immunomodulatory properties against allergic diseases, Chang et al. (2014) showed that FIP-fve can be potentially used for viral prevention and therapy. The study revealed that FIP-fve suppressed airway hyperresponsiveness and inflammation as the result of the downregulated IL-6 expression in mice infected by respiratory syncytial virus. Chang et al. (2014) also indicated that pre-treatment of FIP-fve did not prevent RSV infection but inhibited the replication of RSV through reduction in NF-κB translocation and increased IFN-γ expression.
Furthermore, FIP-fve was shown to have the potential to serve as a novel therapeutic protective agent in preventing adverse effects of certain drugs (Ou et al., 2015). This study demonstrated that FIP-fve reversed several side effects of docetaxel—an anticancer drug—against non-small cell lung cancer, without affecting the potency of docetaxel (Ou et al., 2015). FIP-fve was shown to significantly reduce the adverse effects caused by docetaxel with fewer empty vacuoles in bone marrow, less small intestinal mucosa damage and decreased reduction of white blood cell counts in mice. Therefore, the study indicated that FIP-fve showed protective effects against docetaxel-induced bone and intestinal damages and also enhanced WBC counts via induction of G-CSF and IL-20 gene expressions (Ou et al., 2015).
Apart from fungal immunomodulating protein, the polysaccharides extracted from F. velutipes are also known to exhibit immunomodulatory properties. Yin et al. (2010) isolated polysaccharides from F. velutipes mycelium which were shown to increase nitric oxide (NO) production, IL-1 production and TNF-α production from macrophages in a dose-dependent manner. Another study showed that polysaccharides from F. velutipes mycorrhizae (PFVM) increased the body weight of mice and the weight ratio of the thymus and spleen (Yan et al., 2014). According to the study, the T cell subpopulation of thymocytes and splenocytes were modulated by the administration of PFVM; CD3+, CD4+ and CD4+/CD8+ counts were increased while CD8+ counts decreased in a dose dependent manner. Increasing dosage of PFVM resulted in increased levels of IL-2 and TNF-α. However, IL-2 levels were highest when a medium dose was administered while TNF-α was found to be highest when a high dose was administered. A separate study revealed that a water-soluble polysaccharide from F. velutipes (FVP l-A) resulted in increased NO and TNF-α production. IL-1β and IL-6 were also increased and lymphocyte proliferation was promoted (Wu et al., 2014). Similarly, the water extract of F. velutipes was also demonstrated to enhance splenocyte proliferation and Th1 cytokine production in mice (Ryu et al., 2014). Another interesting study by Lee et al. (2011) showed that the boiling water mushroom F. velutipes extract potentiated the production of IFNγ and cytotoxic activity against YAC-1 (lymphoma cell) of large intestinal lamina propria leukocytes but not small intestinal lamina propria leukocytes (Lee et al., 2011).
The human complement system also participates in the host defense system in both innate and adaptive immunity to protect the body from foreign invading agents such as bacteria, fungi and viruses (Carroll, 2004). Activation of the complement system can initiate a series of processes including opsonisation/phagocytosis, release of inflammatory mediators and the formation of the membrane attack complex, which subsequently leads to cell lysis. Under the normal physiological state, the effects of the complement system activation are beneficial to the host, but the excessive activation of the system may cause undesirable adverse effects which then contribute to the pathogenesis of autoimmune and inflammatory diseases. Therefore, the modulation of complement activity can be an important and useful tool in the effort to treat inflammatory diseases such as rheumatism and arthritis. Shin et al. (2007) investigated the anti-complement activity of seven different basidiomycetes extracts. The investigation revealed that both the hot water extracts and ethanol soluble fractions of F. veluitipes had the strongest anti-complement activity among the tested basidiomycetes extracts, with IC50 of inhibitory activity toward total hemolytic complement at 47.1% and 57.5%, respectively.
Antimelanosis, anti-discoloration and anti-browning properties
There has been increased interest directed toward natural products with melanosis inhibiting properties because post-harvest melanosis occurring in seafood like crabs and shrimps reduces the potential value of the seafood thus affecting the economy (Encarnacion et al., 2011b). In addition, this work also has potential for developing natural whitening cosmetic products or natural food anti-browning product (Kim et al., 2011). Several experiments showed that ergothioneine (34) isolated from F. velutipes extract decreased polyphenoloxidase (PPO) activity which causes melanosis in different species of shrimps and crabs in a dose-dependent manner (Encarnacion et al., 2010, 2011a,b, 2012). Those studies also showed reduction in expression of prophenoloxidase (proPO) gene in the hemocytes of shrimps and crabs which had been submerged in ergothioneine-rich mushroom extract. Furthermore, the melanosis inhibition activity of the ergothioneine (34) from mushrooms was also demonstrated by the absence or reduction in blackening of the carapace of the treated shrimps and crabs compared to the untreated group. Encarnacion et al. (2012) also suggested that ergothioneine (34) may be a non-competitive inhibitor which possibly interacts directly with Cu2+ at the putative binding sites of polyphenol oxidase enzyme.
The extracts of several mushrooms containing ergothioneine (34) were also shown to prevent brown discoloration in processed fish meat (Bao et al., 2010b). Brown discoloration occurs when both deoxymyoglobin and oxymyoglobin are oxidized into metmyoglobin (metMb). The study suggested that F. velutipes extract is able to suppress the formation of metMB. Moreover, tyrosinase activity, which is involved in the production of melanin, was shown to be inhibited by ethyl acetate extract of F. velutipes mycelia grown in a specific medium containing 2% glucose (Kim et al., 2011). These studies have demonstrated the potential application of F. velutipes extract as an effective natural alternative to synthetic antimelanosis, anti-discoloration and anti-browning agents for the food industry.
Antimicrobial properties: antibacterial and antifungal activities
Antimicrobial resistance is a significant global public health concern, particularly the emergence of multi-drugs resistant strains of pathogens which have developed resistance toward almost all the available antibiotics (Letchumanan et al., 2015a,b; Tan et al., 2016d). As a result, there is an increasing push to search for bioactive compounds from natural sources to serve as alternative antimicrobials (Tan et al., 2015b, 2016a; Azman et al., 2016; Chan et al., 2016) Reports on the occurrence of antimicrobials in F. velutipes mushroom are also well documented. The antimicrobial activities of extracts from different parts of F. velutipes have been the focus of several studies. Karaman et al. (2010) showed that both methanol and chloroform extracts from mature fruiting bodies of F. velutipes exhibited strong antibacterial activities, particularly against Staphylococcus aureus and Bacillus subtilis. Similarly, the methanol extract of Macedonian wild F. velutipes fruiting body was demonstrated to show antibacterial activity against both Gram-positive and Gram-negative bacteria, including B. subtilis, Bacillus pumilus, S. aureus, and Pseudomonas aeruginosa (Nedelkoska et al., 2013). Besides that, the antagonistic activity of F. velutipes against plant pathogenic fungi was also examined by evaluating the competitive interactions of the mushroom and the pathogens in a dual culture in vitro experiment (Borhani et al., 2011). The study showed that after an initial deadlock, F. velutipes was able to completely replace Ophiostoma ulmi by F. velutipes. F. velutipes also partially replaced the other three plant pathogenic fungi tested namely Fusarium oxysporum, Pestalotiopsis funerea and Fusarium culmurum LM2091 (Borhani et al., 2011). Recently, a study demonstrated that the extract from F. velutipes exhibited inhibitory activity toward the adhesion of pathogenic fungi (Sporothrix schenckii and Candida albicans) to epithelial cells (L929 cell line) (Kashina et al., 2016).
Enokipodins are a group of α-cuparene type sesquiterpenoids that have been isolated from F. velutipes and are known to be major constituents responsible for F. velutipes antimicrobial activities. Four enokipodins A (35), B (22), C (36) and D (23) with known chemical structures have been isolated and purified from the mycelial culture of F. velutipes by a group of researchers (Ishikawa et al., 2000, 2001). This group of compounds, enokipodins A–D (22, 23, 35, 36) were also successfully synthesized chemically (Srikrishna and Rao, 2004; Saito and Kuwahara, 2005; Secci et al., 2007). It was reported that enokipodins A-D (22, 23, 35, 36) exhibited antibacterial activity mainly against the Gram-positive bacteria such as B. subtilis and S. aureus (Ishikawa et al., 2001, 2005; Saito and Kuwahara, 2005). Moreover, both enokipodins A (35) and C (36) demonstrated minimum inhibitory doses against B. subtilis LMA0011 comparable to those of penicillin G (Ishikawa et al., 2005). A more recent study investigated the effect of culture conditions on the production and antimicrobial activity of the antimicrobial metabolites of F. velutipes (De Melo et al., 2009). The study revealed that dextrose potato broth best supported mycelia growth while complete Pontecorvo's culture medium resulted in greater antimicrobial metabolite production by F. velutipes (De Melo et al., 2009).
In addition to the discovery of enokipodins A–D (22, 23, 35, 36), another research group from China isolated new cuparene-type sesquiterpenes from the solid culture of F. velutipes which also demonstrated antibacterial activities (Wang et al., 2012b). The chemical structures of these sesquiterpenes were elucidated and named as flamvelutpenoids A–D (37 to 40). They were shown to exhibit antibacterial activity against E. coli, B. subtilis and methicillin-resistant S. aureus with MIC measuring more than 100 μM (Wang et al., 2012b). Furthermore, Wang et al. (2012d) also revealed the isolation of six new cuparene sesquiterpenes, enokipodins E–J (25 to 30) with antibacterial and antifungal activities. Enokipodins I (29) and J (30) were shown to exhibit antibacterial activity against B. subtilis with MICs of 164.3 ± 6.2 and 151.2 ± 4.5 μM, respectively. Meanwhile, enokipodins F, G and I (26, 27, 29) exhibited antifungal activity against Aspergillus fumigatus with MICs ranging between 229.1 and 235.1 μM. Interestingly, the antifungal potency of F. velutipes was found to be enhanced in selenium (Se)-enhanced cultivation medium (Milovanovic et al., 2015). The antifungal activity of the ethanol extract of F. velutipes mycelia supplemented with Se was shown to be enhanced, as evidenced by 8-fold lower MIC against Candida parapsilosis (Milovanovic et al., 2015).
Although numerous studies have demonstrated the antimicrobial potential of F. velutipes as well as the bioactive compounds responsible for the ascribed activity, additional work to understand their mechanisms of action would need to be undertaken before proceeding to practical implementation of any of these compounds as nutraceuticals or drugs in the food and pharmaceutical industries.
Anti-inflammatory
Inflammation is known to be a complex biological response to infection and tissue injury that ultimately leads to recovery of tissue structure and function. However, prolonged inflammation contributes to the development of many inflammatory diseases (Supriady et al., 2015). Although many steroidal and nonsteroidal anti-inflammatory drugs have been introduced for anti-inflammatory therapy, their prolonged use has been reported to pose serious adverse effects including significant gastrointestinal upset, gastritis, renal problems, and even myocardial infarction and strokes (Hyllested et al., 2002; Basu and Hazra, 2006). Therefore, much interest has been shown toward alternative anti-inflammmatory agents of plant origin as they appear to be natural and safe drugs which pose minimal, if any, adverse effects (Supriady et al., 2015). A well-known medicinal mushroom, F. velutipes has been shown to possess anti-inflammatory activities. Gunawardena et al. (2014) revealed that unprocessed F. velutipes mushrooms possess anti-inflammatory properties, inhibiting the production of NO (IC50 = 0.024 ± 0.01 mg/mL) and TNF-α (IC50 = 0.099 ± 0.012 mg/mL) from murine macrophage RAW264.7 activated by lipopolysaccharides and IFN-γ. Additionally, the study showed that mushrooms that had undergone food processing steps such as boiling and heating showed less potent anti-inflammatory property, suggesting that the anti-inflammatory bioactive factors may have been degraded in the processed mushroom. Another study showed that the water and ethanol extracts of F. velutipes exhibited strong nitric oxide inhibitory activity and also inhibitory effect on iNOS and COX-2 expression in macrophages (Kang, 2012).
Hepatoprotective activity
Recently, a considerable number of studies have focused on the characterization of polysaccharides of mushrooms due to their protective action against hepatotoxicity (Wu et al., 2011; Gan et al., 2012; Soares et al., 2013; Liu et al., 2014). F. velutipes is among the mushrooms documented to have potential in exerting protective effect toward hepatocytes. Pang et al. (2007), isolated an α-(1 → 4)-d-glucan, a water soluble polysaccharide (FVP2) with hepatoprotective activitiy from F. velutipes. The study indicated that FVP2 enhanced the growth of primary hepatocytes from mice in vitro significantly at concentrations ranging from 25 to 200 μg/mL. Pang et al. (2007) demonstrated the hepatoprotective effect of FVP2 was mediated by the inhibition of the release of intracellular alanine aminotransferase (ALT) from the intoxicated hepatocytes induced by carbon tetrachloride (CCl4). The authors also suggested that FVP2 may have protected the hepatocytes by preventing the production of CCl3• radical caused by CCl4, subsequently inhibiting lipid peroxidation and intracellular release of ALT (Pang et al., 2007).
Limitations and future works
The collective evidence presented in this review strongly suggests that F. velutipes should be exploited as a great source for development of functional foods, nutraceuticals and even pharmaceutical drugs. Despite that, there are still many challenges that need to be faced in order to facilitate the development of these natural products before they enter the pharmaceutical markets (Wasser, 2011). One of them is that considerable effort is required to perform precise identification of specific bioactive molecules responsible for the bioactivity of the mushroom extracts. This provides a better understanding of the mechanism of action of each particular compound on the ascribed bioactivity. Furthermore, this also would address the issue of whether the bioactive effects are caused by a single component or are the result of a synergistic effect from several components in the extracts.
There is also insufficient quality control and regulatory protocols to guarantee a standardized extraction process and the eventual quality and efficacy of mushroom-derived natural products. For instance, some compounds such as polysaccharides, are highly diversified in structure and molecular weight (Jing et al., 2014); hence, it is challenging to maintain batch to batch quality. Without consistency in the quality of the mushroom-derived natural products, the composition and the effectiveness of any commercially available preparation of mushroom products would be highly variable and different. Hence, only proper standards and protocols can ensure the product quality. Moreover, it is also important to establish simple and reliable analytic techniques to assess the authenticity or detect adulteration in mushrooms; preventing adverse effects attributed to adulteration. Apart from the use of gene markers to authenticate F. velutipes (Su et al., 2008; Zhang et al., 2010), a recent effort has shown that the analysis of IR spectroscopic fingerprints using principal component analysis is a reliable identification and qualification technique suitable for quality control of the polysaccharides extracted from F. velutipes (Jing et al., 2014).
Even with the current knowledge on F. velutipes, there is still limited work assessing the bioavailability, pharmacokinetics and pharmacodynamics of the bioactive compounds isolated from F. velutipes. A full understanding of the pharmacokinetic profiles of the natural products from these mushrooms is required as it impacts their bioactivity after metabolism. For instance, information regarding the ability of a bioactive compound to be absorbed from the site of administration and to pass through several biological barriers are crucial for the compound to exert its effect at the target site (Chen et al., 2011). To date, there have been abundant scientific investigations involving extracts or compounds of this mushroom and their potential health promoting benefits toward mankind, mainly on the basis of in vitro and in vivo animal trials. However, clinical studies exploring the therapeutic potential of F. velutipes are unfortunately very few in number. Therefore, clinical trials are highly needed to ascertain the dosage, efficacy and safety of these compounds as adequate alternatives to currently available drugs.
Conclusion
An increasing awareness of the potential side effects of synthetic therapeutic agents and nutraceuticals has led to increased efforts to seek out natural products with beneficial effects on health. Studies have shown that F. velutipes, an easily available mushroom, has excellent nutritional value and also remarkable potential applications for medicinal purposes. Nutritionally, F. velutipes is a good source for carbohydrates, proteins, unsaturated fatty acids, many valuable micronutrients and dietary fiber which are highly comparable to vegetables. Although the nutritional values and culinary uses of F. velutipes are well documented, its medicinal qualities have yet to be. Bioactive polysaccharides extracted from F. velutipes have been demonstrated to possess a wide variety of bioactivities, particularly anticancer, immunomodulatory and anti-neurodegenerative effects. However, the exact mode of action of these carbohydrates is still elusive and deserves special attention in future study. Extensive research has also focused on a fungal immunomodulatory protein, FIP-fve isolated from F. velutipes, which exhibits both promising anticancer and immunomodulatory effects. The majority of the studies showed congruent findings that the FIP-fve elicits its immunomodulatory effects by shifting toward Th1 immune response. Apart from that, extracts of F. velutipes are also found to possess anticancer, anti-atherosclerotic, anti-thrombotic, cholesterol lowering, anti-hypertensive, memory and learning enhancement, antioxidant, antiaging, immunomodulatory, melanosis inhibition, anti-inflammatory, anti-complement, antimicrobial and hepatoprotective activities. A number of chemically defined bioactive molecules from diverse chemical groups have also been isolated from F. velutipes extracts that show invaluable prospects for future applications in the form of nutraceuticals/dietary supplements. In conclusion, F. velutipes has great potential as a nutraceutical and functional food, as well as potentially representing a valuable source for bioactive compounds therapeutic use and pharmaceutical application. The information presented in this review may also form the basis of providing adequate knowledge for future studies and developments as well as commercial exploitation of this fascinating mushroom.
Author contributions
CT, PH, and LT contributed to the literature database search, data collection, data extraction and writing of the manuscript. LL, KC, PP, TK, and BG contributed vital insights and proofread the writing. The research topic was conceptualized by BG.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by PVC Award Grant (Project No. PVC-ECR-2016), External Industry Grant (Biotek Abadi—Vote No. GBA-808813), MOSTI eScience funds (Project No. 02-02-10-SF0215 and 06-02-10-SF0300), Fundamental Research Grant Scheme (FRGS/1/2014/SKK01/MUSM/03/2), and University of Malaya for High Impact Research Grant (UM-MOHE HIR Nature Microbiome Grant No. H-50001-A000027 and No. A000001-50001) and PG136-2016A.
References
- Ahn D., Putt D., Kresty L., Stoner G. D., Fromm D., Hollenberg P. F. (1996). The effects of dietary ellagic acid on rat hepatic and esophageal mucosal cytochromes P450 and phase II enzymes. Carcinogenesis 17, 821–828. 10.1093/carcin/17.4.821 [DOI] [PubMed] [Google Scholar]
- Ajith T. A., Janardhanan K. K. (2007). Indian medicinal mushrooms as a source of antioxidant and antitumor agents. J. Clin. Biochem. Nutr. 40:157. 10.3164/jcbn.40.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akata I., Ergonul B., Kalyoncu F. (2012). Chemical compositions and antioxidant activities of 16 wild edible mushroom species grown in Anatolia. Int. J. Pharm. 8, 134–138. 10.3923/ijp.2012.134.138 [DOI] [Google Scholar]
- Akhter P., Ashraf N., Mohammad D., Orfi S. D., Ahmad N. (2003). Nutritional and radiological impact of dietary potassium on the Pakistani population. Food Chem. Toxicol. 41, 531–534. 10.1016/s0278-6915(02)00283-1 [DOI] [PubMed] [Google Scholar]
- Aoki H., Furuya Y., Endo Y., Fujimoto K. (2003). Effect of γ-aminobutyric acid-enriched tempeh-like fermented soybean (GABA-Tempeh) on the blood pressure of spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 67, 1806–1808. 10.1271/bbb.67.1806 [DOI] [PubMed] [Google Scholar]
- Azman A.-S., Othman I., Fang C.-M., Chan K.-G., Goh B.-H., Lee L.-H. (2016). Antibacterial, anticancer and neuroprotective activities of rare Actinobacteria from mangrove forest soils. Indian J. Microbiol. 10.1007/s12088-016-0627-z. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H. N. D., Shinomiya Y., Ikeda H., Ohshima T. (2009). Preventing discoloration and lipid oxidation in dark muscle of yellowtail by feeding an extract prepared from mushroom (Flammulina velutipes) cultured medium. Aquaculture 295, 243–249. 10.1016/j.aquaculture.2009.06.042 [DOI] [Google Scholar]
- Bao H. N., Ochiai Y., Ohshima T. (2010a). Antioxidative activities of hydrophilic extracts prepared from the fruiting body and spent culture medium of Flammulina velutipes. Bioresour. Technol. 101, 6248–6255. 10.1016/j.biortech.2010.03.026 [DOI] [PubMed] [Google Scholar]
- Bao H. N., Osako K., Ohshima T. (2010b). Value-added use of mushroom ergothioneine as a colour stabilizer in processed fish meats. J. Sci. Food Agr. 90, 1634–1641. 10.1002/jsfa.3992 [DOI] [PubMed] [Google Scholar]
- Barros L., Ferreira M.-J., Queiros B., Ferreira I. C., Baptista P. (2007). Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 103, 413–419. 10.1016/j.foodchem.2006.07.038 [DOI] [Google Scholar]
- Barros L., Venturini B. A., Baptista P., Estevinho L. M., Ferreira I. C. (2008). Chemical composition and biological properties of portuguese wild mushrooms: a comprehensive study. J. Agric. Food Chem. 56, 3856–3862. 10.1021/jf8003114 [DOI] [PubMed] [Google Scholar]
- Basu S., Hazra B. (2006). Evaluation of nitric oxide scavenging activity, in vitro and ex vivo, of selected medicinal plants traditionally used in inflammatory diseases. Phytother. Res. 20, 896–900. 10.1002/ptr.1971 [DOI] [PubMed] [Google Scholar]
- Beluhan S., Ranogajec A. (2011). Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 124, 1076–1082. 10.1016/j.foodchem.2010.07.081 [DOI] [Google Scholar]
- Borhani A., Badalyan S. M., Garibyan N. N., Mosazadeh S. A., Yasari E. (2011). Flammulina velutipes (Curt.: Fr.) Singer: an edible mushroom in northern forest of Iran and its antagonistic activity against selected plant pathogenic fungi. Int. J. Biol. 3:162 10.5539/ijb.v3n2p162 [DOI] [Google Scholar]
- Breene W. M. (1990). Nutritional and medicinal value of specialty mushrooms. J. Food Prot. 53, 883–899. [DOI] [PubMed] [Google Scholar]
- Carroll M. C. (2004). The complement system in regulation of adaptive immunity. Nat. Immunol. 5, 981–986. 10.1038/ni1113 [DOI] [PubMed] [Google Scholar]
- Chan C. K., Goh B. H., Kamarudin M. N., Kadir H. A. (2012). Aqueous fraction of Nephelium ramboutan-ake rind induces mitochondrial-mediated apoptosis in HT-29 human colorectal adenocarcinoma cells. Molecules 17, 6633–6657. 10.3390/molecules17066633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. K., Supriady H., Goh B. H., Kadir H. A. (2015). Elephantopus scaber induces apoptosis through ROS-dependent mitochondrial signaling pathway in HCT116 human colorectal carcinoma cells. J. Ethnopharmacol. 168, 291–304. 10.1016/j.jep.2015.03.072 [DOI] [PubMed] [Google Scholar]
- Chan W.-K., Tan L. T.-H., Chan K.-G., Lee L.-H., Goh B.-H. (2016). Nerolidol: a sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 21, 529. 10.3390/molecules21050529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. H., Hsieh K. Y., Yeh C. H., Tu Y. P., Sheu F. (2010). Oral administration of an Enoki mushroom protein FVE activates innate and adaptive immunity and induces anti-tumor activity against murine hepatocellular carcinoma. Int. Immunopharmacol. 10, 239–246. 10.1016/j.intimp.2009.10.017 [DOI] [PubMed] [Google Scholar]
- Chang Y. C., Chow Y. H., Sun H. L., Liu Y. F., Lee Y. T., Lue K. H., et al. (2014). Alleviation of respiratory syncytial virus replication and inflammation by fungal immunomodulatory protein FIP-fve from Flammulina velutipes. Antiviral Res. 110, 124–131. 10.1016/j.antiviral.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Chang Y. C., Hsiao Y. M., Hung S. C., Chen Y. W., Ou C. C., Chang W. T., et al. (2015). Alleviation of Dermatophagoides microceras-induced allergy by an immunomodulatory protein, FIP-fve, from Flammulina velutipes in mice. Biosci. Biotechnol. Biochem. 79, 88–96. 10.1080/09168451.2014.956682 [DOI] [PubMed] [Google Scholar]
- Chang Y. C., Hsiao Y. M., Wu M. F., Ou C. C., Lin Y. W., Lue K. H., et al. (2013). Interruption of lung cancer cell migration and proliferation by fungal immunomodulatory protein FIP-fve from Flammulina velutipes. J. Agric. Food Chem. 61, 12044–12052. 10.1021/jf4030272 [DOI] [PubMed] [Google Scholar]
- Chen S.-Y., Ho K.-J., Hsieh Y.-J., Wang L.-T., Mau J.-L. (2012). Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT Food Sci. Technol. 47, 274–278. 10.1016/j.lwt.2012.01.019 [DOI] [Google Scholar]
- Chen X.-W. B, Sneed K. B., Zhou S.-F. (2011). Pharmacokinetic profiles of anticancer herbal medicines in humans and the clinical implications. Curr. Med. Chem. 18, 3190–3210. 10.2174/092986711796391624 [DOI] [PubMed] [Google Scholar]
- Cheng C. L., Wang Z. Y., Cheng L., Zhao H. T., Yang X., Liu J. R., et al. (2012). In vitro antioxidant activities of water-soluble nucleotide-extract from edible fungi. Food Sci. Technol. Res. 18, 405–412. 10.3136/fstr.18.405 [DOI] [Google Scholar]
- Chu P.-Y., Sun H.-L., Ko J.-L., Ku M.-S., Lin L.-J., Lee Y.-T., et al. (2015). Oral fungal immunomodulatory protein-Flammulina velutipes has influence on pulmonary inflammatory process and potential treatment for allergic airway disease: a mouse model. J. Microbiol. Immunol. Infect. 10.1016/j.jmii.2015.07.013. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Chung M. J., Chung C. K., Jeong Y., Ham S. S. (2010). Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in BALBC/c mice bearing Sarcoma-180 cells. Nutr. Res. Pract. 4, 177–182. 10.4162/nrp.2010.4.3.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L., Hernandez-Garcia D., Schnabel D., Salas-Vidal E., Castro-Obregon S. (2008). Function of reactive oxygen species during animal development: passive or active? Dev. Biol. 320, 1–11. 10.1016/j.ydbio.2008.04.041 [DOI] [PubMed] [Google Scholar]
- De Melo M. R., Paccola-Meirelles L. D., De Jesus Faria T., Ishikawa N. K. (2009). Influence of Flammulina velutipes mycelia culture conditions on antimicrobial metabolite production. Mycoscience 50, 78–81. 10.1007/s10267-008-0447-z [DOI] [Google Scholar]
- Dikeman C. L., Bauer L. L., Flickinger E. A., Fahey G. C., Jr. (2005). Effects of stage of maturity and cooking on the chemical composition of select mushroom varieties. J. Agric. Food Chem. 53, 1130–1138. 10.1021/jf048541l [DOI] [PubMed] [Google Scholar]
- Ding Y., Seow S. V., Huang C. H., Liew L. M., Lim Y. C., Kuo I. C., et al. (2009). Coadministration of the fungal immunomodulatory protein FIP-Fve and a tumour-associated antigen enhanced antitumour immunity. Immunology 128, e881–894. 10.1111/j.1365-2567.2009.03099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele N., Linke D., Bitzer K., Na'amnieh S., Nimtz M., Berger R. G. (2011). The first characterized asparaginase from a basidiomycete, Flammulina velutipes. Bioresour. Technol. 102, 3316–3321. 10.1016/j.biortech.2010.10.098 [DOI] [PubMed] [Google Scholar]
- El Enshasy H. A., Hatti-Kaul R. (2013). Mushroom immunomodulators: unique molecules with unlimited applications. Trends Biotechnol. 31, 668–677. 10.1016/j.tibtech.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Encarnacion A. B., Fagutao F., Hirayama J., Terayama M., Hirono I., Ohshima T. (2011a). Edible mushroom (Flammulina velutipes) extract inhibits melanosis in Kuruma shrimp (Marsupenaeus japonicus). J. Food Sci. 76, C52–C58. 10.1111/j.1750-3841.2010.01890.x [DOI] [PubMed] [Google Scholar]
- Encarnacion A. B., Fagutao F., Hirono I., Ushio H., Ohshima T. (2010). Effects of ergothioneine from mushrooms (Flammulina velutipes) on melanosis and lipid oxidation of kuruma shrimp (Marsupenaeus japonicus). J. Agric. Food Chem. 58, 2577–2585. 10.1021/jf903944y [DOI] [PubMed] [Google Scholar]
- Encarnacion A. B., Fagutao F., Jintasataporn O., Worawattanamateekul W., Hirono I., Ohshima T. (2012). Application of ergothioneine-rich extract from an edible mushroom Flammulina velutipes for melanosis prevention in shrimp, Penaeus monodon and Litopenaeus vannamei. Food Res. Int. 45, 232–237. 10.1016/j.foodres.2011.10.030 [DOI] [Google Scholar]
- Encarnacion A. B., Fagutao F., Shozen K.-I., Hirono I., Ohshima T. (2011b). Biochemical intervention of ergothioneine-rich edible mushroom (Flammulina velutipes) extract inhibits melanosis in crab (Chionoecetes japonicus). Food Chem. 127, 1594–1599. 10.1016/j.foodchem.2011.02.023 [DOI] [Google Scholar]
- Ferguson L. R., Zhu S. T., Harris P. J. (2005). Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutr. Food Res. 49, 585–593. 10.1002/mnfr.200500014 [DOI] [PubMed] [Google Scholar]
- Fu H. Y., Shieh D. E., Ho C. T. (2002). Antioxidant and free radical scavenging activities of edible mushrooms. J. Food Lipis 9, 35–43. 10.1111/j.1745-4522.2002.tb00206.x [DOI] [Google Scholar]
- Fukushima M., Ohashi T., Fujiwara Y., Sonoyama K., Nakano M. (2001). Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. Exp. Biol. Med. (Maywood) 226, 758–765. Available online at: http://ebm.sagepub.com/content/226/8/758.long [DOI] [PubMed] [Google Scholar]
- Gan D., Ma L., Jiang C., Wang M., Zeng X. (2012). Medium optimization and potential hepatoprotective effect of mycelial polysaccharides from Pholiota dinghuensis Bi against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 50, 2681–2688. 10.1016/j.fct.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Gao Y., Li C., Yin J., Shen J., Wang H., Wu Y., et al. (2012). Fucoidan, a sulfated polysaccharide from brown algae, improves cognitive impairment induced by infusion of Aβ peptide in rats. Environ. Toxicol. Pharmcol. 33, 304–311. 10.1016/j.etap.2011.12.022 [DOI] [PubMed] [Google Scholar]
- Giridharan V. V., Thandavarayan R. A., Konishi T. (2011). Amelioration of scopolamine induced cognitive dysfunction and oxidative stress by Inonotus obliquus–a medicinal mushroom. Food Funct. 2, 320–327. 10.1039/c1fo10037h [DOI] [PubMed] [Google Scholar]
- Goh B. H., Chan C. K., Kamarudin M. N., Kadir H. A. (2014). Swietenia macrophylla King induces mitochondrial-mediated apoptosis through p53 upregulation in HCT116 colorectal carcinoma cells. J. Ethnopharmacol. 153, 375–385. 10.1016/j.jep.2014.02.036 [DOI] [PubMed] [Google Scholar]
- Gu Y. H., Leonard J. (2006). In vitro effects on proliferation, apoptosis and colony inhibition in ER-dependent and ER-independent human breast cancer cells by selected mushroom species. Oncol. Rep. 15, 417–423. 10.3892/or.15.2.417 [DOI] [PubMed] [Google Scholar]
- Gunawardena D., Bennett L., Shanmugam K., King K., Williams R., Zabaras D., et al. (2014). Anti-inflammatory effects of five commercially available mushroom species determined in lipopolysaccharide and interferon-gamma activated murine macrophages. Food Chem. 148, 92–96. 10.1016/j.foodchem.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Günç Ergönül P., Akata I., Kalyoncu F., Ergönül B. (2013). Fatty acid compositions of six wild edible mushroom species. ScientificWorldJournal. 2013:163964. 10.1155/2013/163964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Lakshmi A. J., Manjunath M., Prakash J. (2005). Analysis of nutrient and antinutrient content of underutilized green leafy vegetables. LWT Food Sci. Technol. 38, 339–345. 10.1016/j.lwt.2004.06.012 [DOI] [Google Scholar]
- Harada A., Nagai T., Yamamoto M. (2011). Production of GABA-enriched powder by a brown variety of Flammulina velutipes (Enokitake) and its antihypertensive effects in spontaneously hypertensive rats. J. Jpn. Soc. Food Sci. 58, 446–450. 10.3136/nskkk.58.446 [DOI] [Google Scholar]
- Hayakawa K., Kimura M., Kamata K. (2002). Mechanism underlying γ-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. Eur. J. Pharmacol. 438, 107–113. 10.1016/s0014-2999(02)01294-3 [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Kimura M., Kasaha K., Matsumoto K., Sansawa H., Yamori Y. (2004). Effect of a γ-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br. J. Nutr. 92, 411–417. 10.1079/bjn20041221 [DOI] [PubMed] [Google Scholar]
- He F. J., Nowson C. A., Macgregor G. A. (2006). Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet 367, 320–326. 10.1016/S0140-6736(06)68069-0 [DOI] [PubMed] [Google Scholar]
- He W. Q., Zhang Z. F. (2013). Antioxidant activities of polysaccharides of Flammulina velutipes obtained by ultra filtration. Agro. Food Indust. Hi-Tech 24, 53–56. Available online at: http://www.teknoscienze.com/articles/agro-food-industry-hi-tech-antioxidant-activities-of-polysaccharides-of-flammulina-velutipes.aspx#.V7LSe5h942w [Google Scholar]
- Hsieh C.-W., Lan J.-L., Meng Q., Cheng Y.-W., Huang H.-M., Tsai J.-J. (2007). Eosinophil apoptosis induced by Fungal Immunomodulatory Peptide-fve via reducing IL-5α receptor. J. Formos. Med. Assoc. 106, 36–43. 10.1016/s0929-6646(09)60214-x [DOI] [PubMed] [Google Scholar]
- Hsieh K. Y., Hsu C. I., Lin J. Y., Tsai C. C., Lin R. H. (2003). Oral administration of an edible-mushroom-derived protein inhibits the development of food-allergic reactions in mice. Clin. Exp. Allergy 33, 1595–1602. 10.1046/j.1365-2222.2003.01790.x [DOI] [PubMed] [Google Scholar]
- Hyllested M., Jones S., Pedersen J. L., Kehlet H. (2002). Comparative effect of paracetamol, NSAIDs or their combination in postoperative pain management: a qualitative review. Br. J. Anaesth. 88, 199–214. 10.1093/bja/88.2.199 [DOI] [PubMed] [Google Scholar]
- Ikekawa T. (2001). Beneficial effects of edible and medicinal mushrooms on health care. Int. J. Med. Mushrooms 3, 291–298. 10.1615/IntJMedMushr.v3.i4.20 [DOI] [Google Scholar]
- Ikekawa T., Maruyama H., Miyano T., Okura A., Sawasaki Y., Naito K., et al. (1985). Proflamin, a new antitumor agent: preparation, physicochemical properties and antitumor activity. Jpn. J. Cancer Res. 76, 142–148. [PubMed] [Google Scholar]
- Ingold C. (1980). Flammulina velutipes. Bull. Br. Mycol. Soc. 14, 112–118. 10.1016/s0007-1528(80)80006-x [DOI] [Google Scholar]
- Inoue K., Shirai T., Ochiai H., Kasao M., Hayakawa K., Kimura M., et al. (2003). Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 57, 490–495. 10.1038/sj.ejcn.1601555 [DOI] [PubMed] [Google Scholar]
- Ishikawa N. K., Fukushi Y., Yamaji K., Tahara S., Takahashi K. (2001). Antimicrobial cuparene-type sesquiterpenes, Enokipodins C and D, from a mycelial culture of Flammulina velutipes. J. Nat. Prod. 64, 932–934. 10.1021/np000593r [DOI] [PubMed] [Google Scholar]
- Ishikawa N. K., Yamaji K., Ishimoto H., Miura K., Fukushi Y., Takahashi K., et al. (2005). Production of enokipodins A, B, C, and D: a new group of antimicrobial metabolites from mycelial culture of Flammulina velutipes. Mycoscience 46, 39–45. 10.1007/s10267-004-0208-6 [DOI] [Google Scholar]
- Ishikawa N. K., Yamaji K., Tahara S., Fukushi Y., Takahashi K. (2000). Highly oxidized cuparene-type sesquiterpenes from a mycelial culture of Flammulina velutipes. Phytochemistry 54, 777–782. 10.1016/s0031-9422(00)00189-8 [DOI] [PubMed] [Google Scholar]
- Jeff I. B., Yuan X., Sun L., Kassim R. M., Foday A. D., Zhou Y. (2013). Purification and in vitro anti-proliferative effect of novel neutral polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 52, 99–106. 10.1016/j.ijbiomac.2012.10.007 [DOI] [PubMed] [Google Scholar]
- Jiang S. M., Xiao Z. M., Xu Z. H. (1999). Inhibitory activity of polysaccharide extracts from three kinds of edible fungi on proliferation of human hepatoma SMMC-7721 cell and mouse implanted S180 tumor. World J. Gastroenterol. 5, 404–407. 10.3748/wjg.v5.i5.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing P., Zhao S.-J., Lu M.-M., Cai Z., Pang J., Song L.-H. (2014). Multiple-fingerprint analysis for investigating quality control of Flammulina velutipes fruiting body polysaccharides. J. Agr. Food Chem. 62, 12128–12133. 10.1021/jf504349r [DOI] [PubMed] [Google Scholar]
- John J. H., Ziebland S., Yudkin P., Roe L. S., Neil H. A., Oxford F., et al. (2002). Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet 359, 1969–1974. 10.1016/s0140-6736(02)98858-6 [DOI] [PubMed] [Google Scholar]
- Kakkar S., Bais S. (2014). A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014:952943. 10.1155/2014/952943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalač P. (2013). A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agr. 93, 209–218. 10.1002/jsfa.5960 [DOI] [PubMed] [Google Scholar]
- Kang H.-W. (2012). Antioxidant and anti-inflammatory effect of extracts from Flammulina velutipes (Curtis) Singer. J. Korean Soc. Food Sci. Nutr. 41, 1072–1078. 10.3746/jkfn.2012.41.8.1072 [DOI] [Google Scholar]
- Karaman M., Jovin E., Malbasa R., Matavuly M., Popovic M. (2010). Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phytother. Res. 24, 1473–1481. 10.1002/ptr.2969 [DOI] [PubMed] [Google Scholar]
- Karaman M., Mimica-Dukic N., Matavuly M. (2009). Lignicolous fungi from northern Serbia as natural sources of antioxidants. Open Life Sci. 4, 387–396. 10.2478/s11535-009-0017-1 [DOI] [Google Scholar]
- Kashina S., Flores Villavicencio L. L., Balleza M., Sabanero G. B., Tsutsumi V., López M. S. (2016). Extracts from Flammulina velutipes inhibit the adhesion of pathogenic fungi to epithelial cells. Pharmacognosy Res. 8:S56. 10.4103/0974-8490.178648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic I., Yesiloglu Y., Bayrak Y. (2014). Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 130, 447–452. 10.1016/j.saa.2014.04.052 [DOI] [PubMed] [Google Scholar]
- Kim J. M., Ra K. S., Noh D. O., Suh H. J. (2002). Optimization of submerged culture conditions for the production of angiotensin converting enzyme inhibitor from Flammulina velutipes. J. Ind. Microbiol. Biotechnol. 29, 292–295. 10.1038/sj.jim.7000306 [DOI] [PubMed] [Google Scholar]
- Kim M.-Y., Chung L.-M., Lee S.-J., Ahn J.-K., Kim E.-H., Kim M.-J., et al. (2009). Comparison of free amino acid, carbohydrates concentrations in Korean edible and medicinal mushrooms. Food Chem. 113, 386–393. 10.1016/j.foodchem.2008.07.045 [DOI] [Google Scholar]
- Kim M. Y., Seguin P., Ahn J. K., Kim J. J., Chun S. C., Kim E. H., et al. (2008). Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 56, 7265–7270. 10.1021/jf8008553 [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Kong W. S., Cho J. Y. (2011). Identification of differentially expressed genes in Flammulina velutipes with anti-tyrosinase activity. Curr. Microbiol. 62, 452–457. 10.1007/s00284-010-9728-9 [DOI] [PubMed] [Google Scholar]
- Ko W. C., Liu W. C., Tsang Y. T., Hsieh C. W. (2007). Kinetics of winter mushrooms (Flammulina velutipes) microstructure and quality changes during thermal processing. J. Food Eng. 81, 587–598. 10.1016/j.jfoodeng.2006.12.009 [DOI] [Google Scholar]
- Kotake T., Hirata N., Degi Y., Ishiguro M., Kitazawa K., Takata R., et al. (2011). Endo-beta-1,3-galactanase from winter mushroom Flammulina velutipes. J. Biol. Chem. 286, 27848–27854. 10.1074/jbc.M111.251736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau W. K., Goh B. H., Kadir H. A., Shu-Chien A. C., Tengku Muhammad T. S. (2015). Potent PPARγ ligands from Swietenia macrophylla are capable of stimulating glucose uptake in muscle cells. Molecules 20, 22301–22314. 10.3390/molecules201219847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-S., Oka K., Watanabe O., Hara H., Ishizuka S. (2011). Immunomodulatory effect of mushrooms on cytotoxic activity and cytokine production of intestinal lamina propria leukocytes does not necessarily depend on β-glucan contents. Food Chem. 126, 1521–1526. 10.1016/j.foodchem.2010.12.063 [DOI] [PubMed] [Google Scholar]
- Lee Y. T., Lee S. S., Sun H. L., Lu K. H., Ku M. S., Sheu J. N., et al. (2013). Effect of the fungal immunomodulatory protein FIP-fve on airway inflammation and cytokine production in mouse asthma model. Cytokine 61, 237–244. 10.1016/j.cyto.2012.09.024 [DOI] [PubMed] [Google Scholar]
- Letchumanan V., Pusparajah P., Tan L. T., Yin W. F., Lee L. H., Chan K. G. (2015a). Occurrence and antibiotic resistance of Vibrio parahaemolyticus from shellfish in Selangor, Malaysia. Front. Microbiol. 6:1417. 10.3389/fmicb.2015.01417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchumanan V., Yin W. F., Lee L. H., Chan K. G. (2015b). Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front. Microbiol. 6:33. 10.3389/fmicb.2015.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung M. Y., Fung K. P., Choy Y. M. (1997). The isolation and characterization of an immunomodulatory and anti-tumor polysaccharide preparation from Flammulina velutipes. Immunopharmacology 35, 255–263. 10.1016/s0162-3109(96)00157-9 [DOI] [PubMed] [Google Scholar]
- Li Q., Huang L., Wang X., Li X., Wu S., Zhou X. (2011). Fungal immunomodulatory protein from Flammulina velutipes induces cytokine gene expression in mouse spleen cells. Curr. Top. Nutroceutical Res. 9, 111–118. Available online at: http://search.proquest.com/openview/690c431ef4d13997c893304693378183/1?pq-origsite=gscholar [Google Scholar]
- Liu Q., Tian G., Yan H., Geng X., Cao Q., Wang H., et al. (2014). Characterization of polysaccharides with antioxidant and hepatoprotective activities from the wild edible mushroom Russula vinosa Lindblad. J. Agric. Food Chem. 62, 8858–8866. 10.1021/jf502632c [DOI] [PubMed] [Google Scholar]
- Luceri C., Giannini L., Lodovici M., Antonucci E., Abbate R., Masini E., et al. (2007). p-Coumaric acid, a common dietary phenol, inhibits platelet activity in vitro and in vivo. Br. J. Nutr. 97, 458–463. 10.1017/S0007114507657882 [DOI] [PubMed] [Google Scholar]
- Ma Z., Cui F., Gao X., Zhang J., Zheng L., Jia L. (2015a). Purification, characterization, antioxidant activity and anti-aging of exopolysaccharides by Flammulina velutipes SF-06. Antonie Van Leeuwenhoek 107, 73–82. 10.1007/s10482-014-0305-2 [DOI] [PubMed] [Google Scholar]
- Ma Z., Zhang C., Gao X., Cui F., Zhang J., Jia M., et al. (2015b). Enzymatic and acidic degradation effect on intracellular polysaccharide of Flammulina velutipes SF-08. Int. J. Biol. Macromol. 73, 236–244. 10.1016/j.ijbiomac.2014.11.028 [DOI] [PubMed] [Google Scholar]
- Mantovani M. S., Bellini M. F., Angeli J. P. F., Oliveira R. J., Silva A. F., Ribeiro L. R. (2008). β-Glucans in promoting health: Prevention against mutation and cancer. Mutat. Res. Rev. Mutat. 658, 154–161. 10.1016/j.mrrev.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Manzi P., Gambelli L., Marconi S., Vivanti V., Pizzoferrato L. (1999). Nutrients in edible mushrooms: an inter-species comparative study. Food Chem. 65, 477–482. 10.1016/s0308-8146(98)00212-x [DOI] [Google Scholar]
- Martorana A., Esposito Z., Koch G. (2010). Beyond the cholinergic hypothesis: do current drugs work in Alzheimer's disease? CNS Neurosci. Ther. 16, 235–245. 10.1111/j.1755-5949.2010.00175.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H., Ikekawa T. (2007). Immunomodulation and antitumor activity of a mushroom product, Proflamin, isolated from Flammulina velutipes (W. Curt.: Fr.) Singer (Agaricomycetideae). Int. J. Med. Mushrooms 9, 109–122. 10.1615/IntJMedMushr.v9.i2.20 [DOI] [Google Scholar]
- Milovanovic I., Stanojkovic T., Stajic M., Vukojevic J., Knezevic A. (2015). Se effect on biological activity of Flammulina velutipes. Ital. J. Food Sci. 27, 1–7. Available online at: www.chiriottieditori.it/ojs/index.php/ijfs/article/download/74/12 [Google Scholar]
- Moghadamtousi S. Z., Kamarudin M. N. A., Chan C. K., Goh B. H., Kadir H. A. (2014). Phytochemistry and biology of Loranthus parasiticus Merr, a commonly used herbal medicine. Am. J. Chin. Med. 42, 23–35. 10.1142/s0192415x14500025 [DOI] [PubMed] [Google Scholar]
- Monro J. A. (2003). Treatment of cancer with mushroom products. Arch. Environ. Health 58, 533–537. 10.3200/AEOH.58.8.533-537 [DOI] [PubMed] [Google Scholar]
- Mori K., Inatomi S., Ouchi K., Azumi Y., Tuchida T. (2009). Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytother. Res. 23, 367–372. 10.1002/ptr.2634 [DOI] [PubMed] [Google Scholar]
- Nakalembe I., Kabasa J. D., Olila D. (2015). Comparative nutrient composition of selected wild edible mushrooms from two agro-ecological zones, Uganda. Springerplus 4:433. 10.1186/s40064-015-1188-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelkoska D. N., Pančevska N. A., Amedi H., Veleska D., Ivanova E., Karadelev M., et al. (2013). Screening of antibacterial and antifungal activities of selected Macedonian wild mushrooms. Matica Srpska J. Nat. Sci. 124, 333–340. 10.2298/zmspn1324333n [DOI] [Google Scholar]
- Ng T. B., Chan W. Y., Yeung H. W. (1992). Proteins with abortifacient, ribosome inactivating, immunomodulatory, antitumor and anti-AIDS activities from Cucurbitaceae plants. Gen. Pharmacol. 23, 579–590. 10.1016/0306-3623(92)90131-3 [DOI] [PubMed] [Google Scholar]
- Ng T. B., Lam J. S., Wong J. H., Lam S. K., Ngai P. H., Wang H. X., et al. (2010). Differential abilities of the mushroom ribosome-inactivating proteins hypsin and velutin to perturb normal development of cultured mouse embryos. Toxicol. In vitro 24, 1250–1257. 10.1016/j.tiv.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Ng T. B., Ngai P. H., Xia L. (2006). An agglutinin with mitogenic and antiproliferative activities from the mushroom Flammulina velutipes. Mycologia 98, 167–171. 10.3852/mycologia.98.2.167 [DOI] [PubMed] [Google Scholar]
- Ng T. B., Wang H. X. (2004). Flammin and velin: new ribosome inactivating polypeptides from the mushroom Flammulina velutipes. Peptides 25, 929–933. 10.1016/j.peptides.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Okamura T., Ogata T., Minamimoto N., Takeno T., Noda H., Fukuda S., et al. (2001). Characteristics of wine produced by mushroom fermentation. Biosci. Biotechnol. Biochem. 65, 1596–1600. 10.1271/bbb.65.1596 [DOI] [PubMed] [Google Scholar]
- Ou C.-C., Hsiao Y.-M., Hou T.-Y., Wu M.-F., Ko J.-L. (2015). Fungal immunomodulatory proteins alleviate docetaxel-induced adverse effects. J. Funct. Foods 19, 451–463. 10.1016/j.jff.2015.09.042 [DOI] [Google Scholar]
- Ou C. C., Hsiao Y. M., Wu W. J., Tasy G. J., Ko J. L., Lin M. Y. (2009). FIP-fve stimulates interferon-gamma production via modulation of calcium release and PKC-alpha activation. J. Agric. Food Chem. 57, 11008–11013. 10.1021/jf902725s [DOI] [PubMed] [Google Scholar]
- Ou H. T., Shieh C. J., Chen J. Y., Chang H. M. (2005). The antiproliferative and differentiating effects of human leukemic U937 cells are mediated by cytokines from activated mononuclear cells by dietary mushrooms. J. Agric. Food Chem. 53, 300–305. 10.1021/jf0493425 [DOI] [PubMed] [Google Scholar]
- Pang X., Yao W., Yang X., Xie C., Liu D., Zhang J., et al. (2007). Purification, characterization and biological activity on hepatocytes of a polysaccharide from Flammulina velutipes mycelium. Carbohydr. Polym. 70, 291–297. 10.1016/j.carbpol.2007.04.010 [DOI] [Google Scholar]
- Park S. E., Li M. H., Kim J. S., Sapkota K., Kim J. E., Choi B. S., et al. (2007). Purification and characterization of a fibrinolytic protease from a culture supernatant of Flammulina velutipes mycelia. Biosci. Biotechnol. Biochem. 71, 2214–2222. 10.1271/bbb.70193 [DOI] [PubMed] [Google Scholar]
- Pereira E., Barros L., Martins A., Ferreira I. C. F. R. (2012). Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chem. 130, 394–403. 10.1016/j.foodchem.2011.07.057 [DOI] [Google Scholar]
- Rahman M. A., Abdullah N., Aminudin N. (2015). Antioxidative effects and inhibition of human low density lipoprotein oxidation In vitro of polyphenolic compounds in Flammulina velutipes (Golden Needle Mushroom). Oxid. Med. Cell. Longev. 2015:403023. 10.1155/2015/403023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J. A., Janech M. G. (2009). Ice-binding proteins from enoki and shiitake mushrooms. Cryobiology 58, 151–156. 10.1016/j.cryobiol.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Reis F. S., Barros L., Martins A., Ferreira I. C. (2012). Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an inter-species comparative study. Food Chem. Toxicol. 50, 191–197. 10.1016/j.fct.2011.10.056 [DOI] [PubMed] [Google Scholar]
- Rice-Evans C., Miller N., Paganga G. (1997). Antioxidant properties of phenolic compounds. Tremds Plant Sci. 2, 152–159. 10.1016/s1360-1385(97)01018-2 [DOI] [Google Scholar]
- Ryu H.-S., Kim K.-O., Liu Y., Yoon L., Kim H.-S. (2014). Effects of edible mushrooms (Pleurotus ostreatus (Jacq.) P. Kumm., Pleurotus eryngil, Flammulina velutipes) extracts on immune cell activation in mice (830.17). FASEB J. 28:830.817. Available online at: http://www.fasebj.org/content/28/1_Supplement/830.17.short
- Saito M., Kuwahara S. (2005). Enantioselective total synthesis of enokipodins A-D, antimicrobial sesquiterpenes produced by the mushroom, Flammulina velutipes. Biosci. Biotechnol. Biochem. 69, 374–381. 10.1271/bbb.69.374 [DOI] [PubMed] [Google Scholar]
- Salem N., Jr., Pawlosky R., Wegher B., Hibbeln J. (1999). In vivo conversion of linoleic acid to arachidonic acid in human adults. Prostaglandins Leukot. Essent. Fatty Acids 60, 407–410. 10.1016/s0952-3278(99)80021-0 [DOI] [PubMed] [Google Scholar]
- Sayyad M., Ning T., Kumari Y., Hing G. B., Jaiswal Y., Rosli R., et al. (2016). Acute toxicity profiling of the ethyl acetate fraction of Swietenia macrophylla seeds and in-vitro neuroprotection studies. Saudi Pharm. J. [Epub ahead of print]. 10.1016/j.jsps.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secci F., Frongia A., Ollivier J., Piras P. P. (2007). Convenient formal synthesis of (±)-cuparene,(±)-enokipodins A and B, and (±)-cuparene-1, 4-quinone. Synthesis 2007, 999–1002. 10.1002/chin.200732190 [DOI] [Google Scholar]
- Seeram N. P., Adams L. S., Henning S. M., Niu Y., Zhang Y., Nair M. G., et al. (2005). In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 16, 360–367. 10.1016/j.jnutbio.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Ser H.-L., Tan L. T.-H., Palanisamy U. D., Malek S. N. A., Yin W.-F., Chan K.-G., et al. (2016). Streptomyces antioxidans sp. nov., a novel mangrove soil actinobacterium with antioxidative and neuroprotective potentials. Front. Microbiol. 7:899. 10.3389/fmicb.2016.00899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Kumar S., Tewari R. (2009). Flammulina velutipes, the Culinary Medicinal Winter Mushroom. Directorate of Mushroom Research, Indian Council of Agricultural Research.
- Shin K.-S., Yu K.-W., Lee H.-K., Lee H., Cho W.-D., Suh H.-J. (2007). Production of anti-complementary exopolysaccharides from submerged culture of Flammulina velutipes. Food Technol. Biotech. 45, 319–326. Available online at: https://koreauniv.pure.elsevier.com/en/publications/production-of-anti-complementary-exopolysaccharides-from-submerge [Google Scholar]
- Shukla S., Bajpai V. K., Kim M. (2014). Plants as potential sources of natural immunomodulators. Rev. Eviron. Sci. Biotechnol. 13, 17–33. 10.1007/s11157-012-9303-x [DOI] [Google Scholar]
- Singh G., Kawatra A., Sehgal S. (2001). Nutritional composition of selected green leafy vegetables, herbs and carrots. Plant Foods Hum. Nutr. 56, 359–364. 10.1023/A:1011873119620 [DOI] [PubMed] [Google Scholar]
- Siu M. T., Shapiro A. M., Wiley M. J., Wells P. G. (2013). A role for glutathione, independent of oxidative stress, in the developmental toxicity of methanol. Toxicol. Appl. Pharmacol. 273, 508–515. 10.1016/j.taap.2013.09.020 [DOI] [PubMed] [Google Scholar]
- Smiderle F. R., Carbonero E. R., Mellinger C. G., Sassaki G. L., Gorin P. A., Iacomini M. (2006). Structural characterization of a polysaccharide and a beta-glucan isolated from the edible mushroom Flammulina velutipes. Phytochemistry 67, 2189–2196. 10.1016/j.phytochem.2006.06.022 [DOI] [PubMed] [Google Scholar]
- Smiderle F. R., Carbonero E. R., Sassaki G. L., Gorin P. A. J., Iacomini M. (2008). Characterization of a heterogalactan: Some nutritional values of the edible mushroom Flammulina velutipes. Food Chem. 108, 329–333. 10.1016/j.foodchem.2007.10.029 [DOI] [Google Scholar]
- Soares A. A., de Sa-Nakanishi A. B., Bracht A., da Costa S. M., Koehnlein E. A., De Souza C. G., et al. (2013). Hepatoprotective effects of mushrooms. Molecules 18, 7609–7630. 10.3390/molecules18077609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikrishna A., Rao M. S. (2004). The first total synthesis of the antimicrobial sesquiterpenes (±)-enokipodins A and B. Synlett 2004, 0374–0376. 10.1055/s-2003-45003 [DOI] [Google Scholar]
- Stanely Mainzen Prince P., Roy A. J. (2013). p-Coumaric acid attenuates apoptosis in isoproterenol-induced myocardial infarcted rats by inhibiting oxidative stress. Int. J. Cardiol. 168, 3259–3266. 10.1016/j.ijcard.2013.04.138 [DOI] [PubMed] [Google Scholar]
- Su H., Wang L., Liu L., Chi X., Zhang Y. (2008). Use of inter-simple sequence repeat markers to develop strain-specific SCAR markers for Flammulina velutipes. J. Appl. Genet. 49, 233–235. 10.1007/bf03195619 [DOI] [PubMed] [Google Scholar]
- Sullivan R., Smith J. E., Rowan N. J. (2006). Medicinal mushrooms and cancer therapy: translating a traditional practice into Western medicine. Perspect. Biol. Med. 49, 159–170. 10.1353/pbm.2006.0034 [DOI] [PubMed] [Google Scholar]
- Supriady H., Kamarudin M. N. A., Chan C. K., Goh B. H., Kadir H. A. (2015). SMEAF attenuates the production of pro-inflammatory mediators through the inactivation of Akt-dependent NF-κB, p38 and ERK1/2 pathways in LPS-stimulated BV-2 microglial cells. J. Funct. Foods 17, 434–448. 10.1016/j.jff.2015.05.042 [DOI] [Google Scholar]
- Tadjibaeva G., Sabirov R., Tomita T. (2000). Flammutoxin, a cytolysin from the edible mushroom Flammulina velutipes, forms two different types of voltage-gated channels in lipid bilayer membranes. Biochim. Biophys. Acta 1467, 431–443. 10.1016/S0005-2736(00)00240-6 [DOI] [PubMed] [Google Scholar]
- Tan H.-L., Chan K.-G., Pusparajah P., Duangjai A., Saokaew S., Khan T. M., et al. (2016c). Rhizoma coptidis: a potential cardiovascular protective agent. Front. Pharmacol. 7:362. 10.3389/fphar.2016.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H.-L., Chan K.-G., Pusparajah P., Lee L.-H., Goh B.-H. (2016a). Gynura procumbens: an overview of the biological activities. Front. Pharmacol. 7:52. 10.3389/fphar.2016.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H.-L., Chan K.-G., Pusparajah P., Saokaew S., Duangjai A., Lee L.-H., et al. (2016b). Anti-cancer properties of the naturally occurring aphrodisiacs: icariin and its derivatives. Front. Pharmacol. 7:191. 10.3389/fphar.2016.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L. T., Chan K.-G., Lee L.-H., Goh B.-H. (2016d). Streptomyces bacteria as potential probiotics in aquaculture. Front. Microbiol. 7:79. 10.3389/fmicb.2016.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L. T., Lee L. H., Yin W. F., Chan C. K., Abdul Kadir H., Chan K. G., et al. (2015b). Traditional uses, phytochemistry, and bioactivities of Cananga odorata (Ylang-Ylang). Evid. Based Complement. Alternat. Med. 2015:896314. 10.1155/2015/896314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L. T., Ser H. L., Yin W. F., Chan K. G., Lee L. H., Goh B. H. (2015a). Investigation of antioxidative and anticancer potentials of Streptomyces sp. MUM256 isolated from Malaysia mangrove soil. Front. Microbiol. 6:1316. 10.3389/fmicb.2015.01316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.-H., Yen Y.-H., Yang J. P.-W. (2015). Finding of polysaccharide-peptide complexes in Cordyceps militaris and evaluation of its acetylcholinesterase inhibition activity. J. Food Dryg Anal. 23, 63–70. 10.1016/j.jfda.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters T., Gorog D., Wood R. (1994). Thrombin generation following arterial injury is a critical initiating event in the pathogenesis of the proliferative stages of the atherosclerotic process. J. Vasc. Res. 31, 173–177. 10.1159/000319584 [DOI] [PubMed] [Google Scholar]
- Wang H., Khor T. O., Shu L., Su Z., Fuentes F., Lee J.-H., et al. (2012a). Plants against cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med. Chem. 12, 1281. 10.2174/187152012803833026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. M., Wang L. W., Liu X. M., Li C. L., Xu S. P., Farooq A. D. (2013). Neuroprotective effects of forsythiaside on learning and memory deficits in senescence-accelerated mouse prone (SAMP8) mice. Pharmacol. Biochem. Behav. 105, 134–141. 10.1016/j.pbb.2012.12.016 [DOI] [PubMed] [Google Scholar]
- Wang H., Ng T. B. (2001). Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci. 68, 2151–2158. 10.1016/s0024-3205(01)01023-2 [DOI] [PubMed] [Google Scholar]
- Wang H. X., Ng T. B. (2000). Flammulin: a novel ribosome-inactivating protein from fruiting bodies of the winter mushroom Flammulina velutipes. Biochem. Cell Biol. 78, 699–702. 10.1139/bcb-78-6-699 [DOI] [PubMed] [Google Scholar]
- Wang P.-H., Hsu C.-I., Tang S.-C., Huang Y.-L., Lin J.-Y., Ko J.-L. (2004). Fungal immunomodulatory protein from Flammulina velutipes induces interferon-γ production through p38 mitogen-activated protein kinase signaling pathway. J. Agric. Food Chem. 52, 2721–2725. 10.1021/jf034556s [DOI] [PubMed] [Google Scholar]
- Wang S. M., Ooi L. L. P., Hui K. M. (2011). Upregulation of Rac GTPase-activating protein 1 is significantly associated with the early recurrence of human hepatocellular carcinoma. Clin. Cancer Res. 17, 6040–6051. 10.1158/1078-0432.ccr-11-0557 [DOI] [PubMed] [Google Scholar]
- Wang Y., Bao L., Liu D., Yang X., Li S., Gao H., et al. (2012c). Two new sesquiterpenes and six norsesquiterpenes from the solid culture of the edible mushroom Flammulina velutipes. Tetrahedron 68, 3012–3018. 10.1016/j.tet.2012.02.021 [DOI] [Google Scholar]
- Wang Y., Bao L., Yang X., Li L., Li S., Gao H., et al. (2012d). Bioactive sesquiterpenoids from the solid culture of the edible mushroom Flammulina velutipes growing on cooked rice. Food Chem. 132, 1346–1353. 10.1016/j.foodchem.2011.11.117 [DOI] [PubMed] [Google Scholar]
- Wang Y.-Q., Bao L., Yang X.-L., Dai H.-Q., Guo H., Yao X.-S., et al. (2012b). Four new cuparene-type sesquiterpenes from Flammulina velutipes. Helvetica Chimica Acta 95, 261–267. 10.1002/hlca.201100289 [DOI] [Google Scholar]
- Wasser S. P. (2011). Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 89, 1323–1332. 10.1007/s00253-010-3067-4 [DOI] [PubMed] [Google Scholar]
- Wong D. Z., Kadir H. A., Lee C. L., Goh B. H. (2012). Neuroprotective properties of Loranthus parasiticus aqueous fraction against oxidative stress-induced damage in NG108-15 cells. J. Nat. Med. 66, 544–551. 10.1007/s11418-011-0622-y [DOI] [PubMed] [Google Scholar]
- World Health Organization (2013). A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis. Available online at: http://ish-world.com/downloads/pdf/global_brief_hypertension.pdf (accessed July 16, 2016).
- Wu M. F., Hsu Y. M., Tang M. C., Chen H. C., Chung J. G., Lu H. F., et al. (2011). Agaricus blazei Murill extract abrogates CCl4-induced liver injury in rats. In Vivo 25, 35–40. Available online at: http://iv.iiarjournals.org/content/25/1/35.long [PubMed] [Google Scholar]
- Wu M., Luo X., Xu X., Wei W., Yu M., Jiang N., et al. (2014). Antioxidant and immunomodulatory activities of a polysaccharide from Flammulina velutipes. J. Tradit. Chin. Med. 34, 733–740. 10.1016/s0254-6272(15)30089-3 [DOI] [PubMed] [Google Scholar]
- Xia Z. (2015). Preparation of the oligosaccharides derived from Flammulina velutipes and their antioxidant activities. Carbohydr. Polym. 118, 41–43. 10.1016/j.carbpol.2014.10.074 [DOI] [PubMed] [Google Scholar]
- Xu Z.-Y., Wu Z.-A., Bi K.-S. (2013). A novel norsesquiterpene alkaloid from the mushroom-forming fungus Flammulina velutipes. Chin. Chem. Lett. 24, 57–58. 10.1016/j.cclet.2012.11.012 [DOI] [Google Scholar]
- Yan Z. F., Liu N. X., Mao X. X., Li Y., Li C. T. (2014). Activation effects of polysaccharides of Flammulina velutipes mycorrhizae on the T lymphocyte immune function. J. Immunol. Res. 2014:285421. 10.1155/2014/285421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B. K., Park J. B., Song C. H. (2002). Hypolipidemic effect of exo-polymer produced in submerged mycelial culture of five different mushrooms. J. Microbiol. Biotechnol. 12, 957–961. Available online at: http://www.jmb.or.kr/submission/Journal/012/JMB012-06-14.pdf [Google Scholar]
- Yang B., Zhao M., Shi J., Yang N., Jiang Y. (2008). Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp. Food Chem. 106, 685–690. 10.1016/j.foodchem.2007.06.031 [DOI] [Google Scholar]
- Yang J.-H., Lin H.-C., Mau J.-L. (2001). Non-volatile taste components of several commercial mushrooms. Food Chem. 72, 465–471. 10.1016/s0308-8146(00)00262-4 [DOI] [Google Scholar]
- Yang W., Fang Y., Liang J., Hu Q. (2011). Optimization of ultrasonic extraction of Flammulina velutipes polysaccharides and evaluation of its acetylcholinesterase inhibitory activity. Food Res. Int. 44, 1269–1275. 10.1016/j.foodres.2010.11.027 [DOI] [Google Scholar]
- Yang W., Pei F., Shi Y., Zhao L., Fang Y., Hu Q. (2012). Purification, characterization and anti-proliferation activity of polysaccharides from Flammulina velutipes. Carbohydr. Polym. 88, 474–480. 10.1016/j.carbpol.2011.12.018 [DOI] [Google Scholar]
- Yang W., Yu J., Zhao L., Ma N., Fang Y., Pei F., et al. (2015). Polysaccharides from Flammulina velutipes improve scopolamine-induced impairment of learning and memory of rats. J. Funct. Foods 18, 411–422. 10.1016/j.jff.2015.08.003 [DOI] [Google Scholar]
- Yeh M. Y., Ko W. C., Lin L. Y. (2014). Hypolipidemic and antioxidant activity of enoki mushrooms (Flammulina velutipes). Biomed. Res. Int. 2014, 352385. 10.1155/2014/352385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C., Fu M., Cao X., Tong S., Zheng Q., Firempong C. K., et al. (2013a). Enhanced oral bioavailability and tissue distribution of a new potential anticancer agent, Flammulina velutipes sterols, through liposomal encapsulation. J. Agric. Food Chem. 61, 5961–5971. 10.1021/jf3055278 [DOI] [PubMed] [Google Scholar]
- Yi C., Sun C., Tong S., Cao X., Feng Y., Firempong C. K., et al. (2013b). Cytotoxic effect of novel Flammulina velutipes sterols and its oral bioavailability via mixed micellar nanoformulation. Int. J. Pharm. 448, 44–50. 10.1016/j.ijpharm.2013.03.020 [DOI] [PubMed] [Google Scholar]
- Yi C., Zhong H., Tong S., Cao X., Firempong C. K., Liu H., et al. (2012). Enhanced oral bioavailability of a sterol-loaded microemulsion formulation of Flammulina velutipes, a potential antitumor drug. Int. J. Nanomed. 7, 5067–5078. 10.2147/IJN.S34612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Wang Y., Wang Y., Chen T., Tang H., Wang M. (2010). Purification, characterization and immuno-modulating properties of polysaccharides isolated from Flammulina velutipes mycelium. Am. J. Chin. Med. 38, 191–204. 10.1142/S0192415X10007750 [DOI] [PubMed] [Google Scholar]
- Zeng X., Suwandi J., Fuller J., Doronila A., Ng K. (2012). Antioxidant capacity and mineral contents of edible wild Australian mushrooms. Food Sci. Technol. Int. 18, 367–379. 10.1177/1082013211427993 [DOI] [PubMed] [Google Scholar]
- Zhang M., Cui S., Cheung P., Wang Q. (2007). Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Tech. 18, 4–19. 10.1016/j.tifs.2006.07.013 [DOI] [Google Scholar]
- Zhang R., Hu D., Zhang J., Zuo X., Jiang R., Wang H., et al. (2010). Development and characterization of simple sequence repeat (SSR) markers for the mushroom Flammulina velutipes. J. Biosci. Bioeng. 110, 273–275. 10.1016/j.jbiosc.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Jin Q., Lv G., Fan L., Pan H., Fan L. (2013). Comparative study on antioxidant activity of four varieties of Flammulina velutipes with different colour. Int. J. Food Sci. Technol. 48, 1057–1064. 10.1111/ijfs.12062 [DOI] [Google Scholar]
- Zhao C., Zhao K., Liu X. Y., Huang Y. F., Liu B. (2013). In vitro antioxidant and antitumor activities of polysaccharides extracted from the mycelia of liquid-cultured Flammulina velutipes. Food Sci. Technol. Res. 19, 661–667. 10.3136/fstr.19.661 [DOI] [Google Scholar]