Abstract
Background: Nonadherence to prescribed medications is an important consideration in the clinical management of patients and in clinical research and drug development. The ID-Cap System is a novel technology that provides an objective measure of medication ingestion and enables real-time reporting of verified medication adherence data at the dose level. The ID-Cap System consists of an ingestible microsensor that is embedded in an oral dosage form and, once activated by stomach fluid, communicates digital messages to an external wearable reader to confirm ingestion.
Objective: The objective of this exploratory study was to evaluate the performance, reliability, usability, and safety of the ID-Cap System for remote monitoring of 20 ingestion events over four weeks in 20 healthy volunteers.
Design: This study was an open-label, single-arm, exploratory study of the ID-Cap System. The study design included the following three phases: 1) screening phase, 2) treatment phase consisting of 20 daily capsule ingestion events over a four-week period, and 3) follow-up phase consisting of a follow-up study visit that included an abdominal X-ray and a follow-up phone call. The initial use of the ID-Cap Reader and ingestion of the first study capsule were directly observed by the investigator during the first study visit. Subsequent study capsule ingestions were completed outside the research facility at the study participant’s home or other location of his or her choice with ingestion assessed using the ID-Cap System.
Setting: The study was conducted at a single clinical research site in Gainesville, Florida.
Participants: Twenty healthy volunteers were enrolled in this four-week pilot study that was conducted between September and November 2014.
Measurements: Study measurements included ID-Tag detection indicating capsule ingestion, utilization of the ID-Cap System consistent with instructions for use, adverse event reports, discontinuations of the System during the study, and safety assessments related to excretion of the ID-Tags through abdominal X-ray evaluations.
Results: Positive detection accuracy was 100 percent for the 20 directly observed ingestions of study capsules that occurred during the initial study visits. Of the 384 ingestion events that were self-administered by the study participants without direct observation, 371 were accurately detected using the ID-Cap System. Overall adherence to the prescribed study capsules as measured by the ID-Cap System was 97.75 percent (391 detections/400 expected ingestion events). Significant intra-individual and inter-individual variability in the timing of self-administered doses was observed in this study. No adverse events were reported, and no study participants discontinued use of the ID-Cap System for any reason during the study. There was no evidence indicating retention of ID-Tags based on abdominal X-ray evaluations.
Conclusion: The ID-Cap System enables accurate measurement of medication adherence for oral drug therapy at the dose level. This study supports the clinical validation of the technology and feasibility in using the system for the collection and real-time reporting of medication adherence in the clinical management of patients and in clinical research and drug development.
Keywords: Medication adherence, treatment compliance, ingestible event monitor, ingestible sensor, drug development tool, telemedicine
INTRODUCTION
Nonadherence to prescribed medications is an important consideration in the clinical management of patients and in clinical research and drug development. Considerable research has been conducted to elucidate the reasons for nonadherence, which are often unique to the individual patient and the specific medication of interest.1 Across different disease states and medications, nonadherence directly impacts drug exposure and therapeutic outcomes. An estimated 50 percent of patients in developed countries are not adherent to prescribed medications for chronic health conditions, and the magnitude and impact of the problem are even greater in developing countries.2 Poor adherence is associated with increased healthcare costs and contributes over $100 billion in avoidable medical costs in just the United States alone.3 The accurate detection and reporting of medication nonadherence are critical for healthcare providers, payers, and clinical researchers to evaluate and optimize therapeutic interventions. Current methods that are commonly used for monitoring medication adherence, such as patient self-reports, pill counts, and prescription refill histories, comprise indirect measures that are generally incomplete and inaccurate and cannot be effectively utilized for understanding and tracking actual medication use.4 Reliable methods for objectively measuring medication adherence behaviors that can be easily implemented for remote health monitoring are needed.
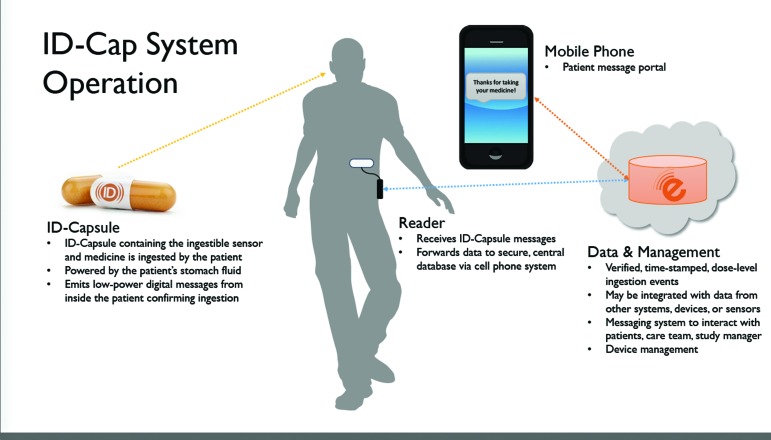
The ID-Cap System is a novel technology that provides an objective measure of medication ingestion and enables real-time reporting of verified medication adherence data at the dose level. The system is classified as an ingestible event monitoring system and used for detecting the presence of an ingested solid oral dosage form inside the gastrointestinal (GI) tract. Ingestible medical devices have been well-characterized in the scientific literature and regulatory approval processes.5 The ID-Cap System consists of an ingestible microsensor that is embedded in an oral dosage form and, once activated by stomach fluid, communicates digital messages to an external wearable reader to confirm ingestion. The adherence data, including time-stamped messages verifying ingestion, are transmitted to a secure, centralized database via the mobile phone network(Figure 1ߝFigure 4). The collection and real-time reporting of accurate medication adherence data has the potential to strengthen health information datasets and significantly improve medication use and therapeutic outcomes(Figure 4). The objective of this exploratory study was to evaluate the performance, reliability, usability, and safety of the ID-Cap System for remote monitoring of 20 ingestion events over four weeks in 20 healthy volunteers.
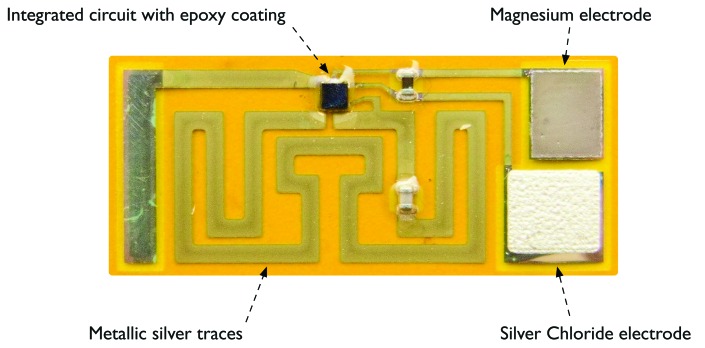
FIGURE 1.
The ID-Tag
FIGURE 4.
Overview of ID-Cap System
METHODS
Study participants. Twenty healthy volunteers were screened and enrolled in this four-week pilot study that was conducted between September and November 2014 at a single study site in Gainesville, Florida. Individuals aged 18 to 45 years who were deemed healthy by the study physician as determined by past medical and surgical history, physical examination, and vital signs at screening were eligible for enrollment in the study. To be eligible, individuals had to weigh at least 50kg and have a body mass index (BMI) within the range of 18 to 35. Exclusion criteria for the study included the following:
Any known hypersensitivity to silver or magnesium
Difficulty swallowing solids and/or capsules
Any implanted electronic device
A significant illness within two weeks of entering the study
A history of drug or alcohol abuse within the six months prior to screening
Participation in another study at the time of screening or those who have taken an investigational drug within 30 days of enrollment
A significant medical and/or mental condition that would compromise the study participant’s welfare or confound the results of this study in the opinion of the investigator
Pregnancy.
Before enrollment, each healthy volunteer had to review and sign a written informed consent form with signature witnessed by a third party.
Materials. The ID-Cap System (etectRx, Inc., Newberry, Florida) is an ingestible sensor system for use in the real-time monitoring and reliable reporting of patient adherence to medication protocols. The ID-Cap System provides an in-vivo communications platform that enables verification of oral medication ingestion through detection of the presence of an ingested solid oral dosage form inside the gastrointestinal (GI) tract. The ID-Cap System comprises three key components: 1) the ID-Tag, an ingestible component that sends information from within the body after it has been ingested; 2) the ID-Cap Reader, an external receiver that records the data transmitted by the ID-Tag; and 3) an information system used to monitor ingestion events, manage the data, and send messages to study participants.
The ID-Tag is a small, biocompatible ingestible device that is composed of an integrated circuit on a flexible film (23mm x 10.5mm x 300microns)(Figure 1). The ID-Tag has an exposed sensor that is intended to safely come into contact with the Gl fluids(Figure 2A). The exposed metal elements of the sensor (i.e., magnesium and silver chloride) serve as a battery, which powers the ID-Tag to generate the in-body communication signal. Within a few minutes after ingestion of the ID-Tag, an electrolyte in the stomach, chloride, powers up the tiny battery on the surface of the ID-Tag. This process is independent of the pH of the stomach. The ID-Tag generates a radio frequency signal that propagates through the body and can be detected by the ID-Cap Reader, an external receiver(Figure 2B). Once ingested and activated, the ID-Tag will transmit the in-body communication signal for approximately 30 minutes on average. After sending the digital messages to the reader, the ID-Tag becomes inactive and is excreted intact in the feces. Each ID-Tag can be assigned a distinct identifier, enabling multiple ID-Tags to be detected and differentiated when they are ingested at or around the same time. All materials in the ID-Tag are biocompatible and are either known to be safe or have been safely utilized in other approved ingestible medical devices.
FIGURE 2A-C.


Components of the ID-Cap System. A: ID-Capsule—A standard capsul with an embedded ingestible wireless sensor; B: ID-Cap Reader—Receives the digital messages from the ID-Capsule and sends them to a central database via the mobile phone network; C: Data Management System—Repository for real time ingestion event information and patient reminder system
The ID-Tag can be incorporated into a capsule for the purpose of providing adherence data for the medication dose that is delivered in the capsule. In this study, the ID-Tag was encapsulated within a Size 00 clear hard gelatin capsule that also contained a Size 1 hard gelatin capsule filled with sucrose. While the capsules administered in this study represented placebo capsules, the Size 00 capsule shell containing the ID-Tag provides a well-characterized and convenient vehicle for delivery of active pharmaceutical ingredients, oral dosage forms, or other ingestible products for which ingestion events will be monitored.
The ID-Cap Reader is a wearable, battery-powered, digital radio transceiver that detects the signal generated by the ID-Tag following its ingestion(Figure 2B). For this study, the ID-Cap Reader (10cm x 6cm x 2.5cm) was clipped onto a belt or waistband, and the accompanying patch antenna (16cm x 5.7cm) was affixed to the skin of the abdomen and connected to the reader with a thin coaxial cable(Figure 3). Study participants were instructed to apply the patch and connect the reader before taking their study capsule and to leave it on for one hour following capsule ingestion. Study participants were also instructed to keep the ID-Cap Reader plugged into an AC power outlet to charge it when they were not wearing the device. A unique ID-Cap Reader was assigned to each study participant.
FIGURE 3.
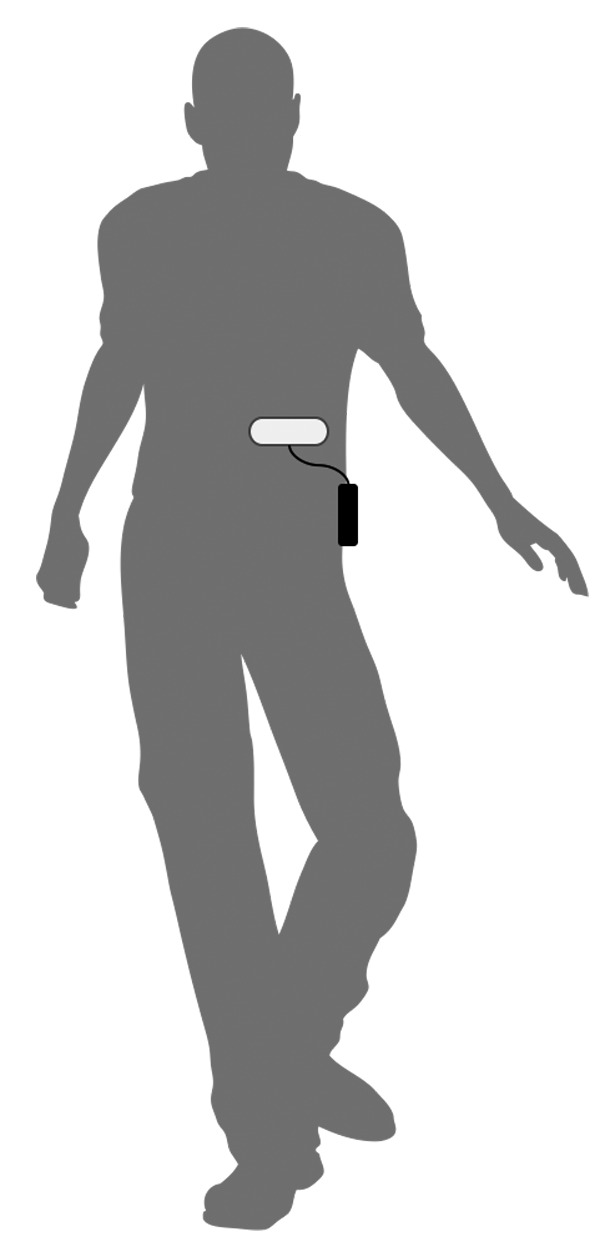
The receiver and patch antenna (ID-Cap Reader)
The wearable reader has three options for uploading data to the software system: cellular, Bluetooth, and USB. The cellular and Bluetooth capabilities allow real-time communication of ingestion events to enable real-time monitoring of medication adherence events and automated, context-sensitive messaging. The stored, encrypted data are securely and privately transmitted from the ID-Cap Reader to the central database(Figure 2C). The data transmitted by the ID-Cap Reader include the ID-Tag information, unique identifier for the ID-Cap Reader, antenna connection status, on-charger status, reader battery status, and device status messages(Figure 4). No personally identifiable information is stored on the ID-Cap Reader or otherwise transmitted throughout the process. The data remain stored on the ID-Cap Reader and can be downloaded directly from the reader as necessary.
Study design. This open-label, single-arm exploratory study evaluated the performance, reliability, usability, and safety of the ID-Cap System for remote monitoring of 20 ingestion events over four weeks in 20 healthy volunteers. The study endpoints included ID-Tag detection indicating capsule ingestion, utilization of the ID-Cap System consistent with instructions for use, adverse event reports, discontinuations of the System during the study, and safety assessments related to excretion of the ID-Tags through abdominal X-ray evaluations. The study was reviewed and approved by the Western Institutional Review Board (WIRB, Puyallup, Washington).
The study design included the following three phases: 1) screening phase, 2) treatment phase consisting of 20 daily capsule ingestion events over a four-week period, and 3) follow-up phase consisting of a follow-up study visit that included an abdominal X-ray and a follow-up phone call.
Screening phase. Healthy volunteers were screened to determine eligibility for enrollment in the study. Past medical and surgical history, medication history for the prior 30 days, physical examination, body height, body mass index, and vital signs were recorded during the screening visit. After receiving information about the study, each eligible study participant signed the informed consent form. Female participants were given a urine pregnancy test before the treatment phase, at the mid-point of the treatment phase, and before the abdominal X-ray conducted at the follow-up visit. All current medications, as well as their doses and routes of administration, were recorded at the screening visit and at each subsequent study visit.
All study participants were enrolled in a mobile phone message system used to send reminder messages to their personal mobile phones. At a predetermined time each day, the participant received a text message reminding him or her to put on the ID-Cap Reader as directed and to ingest the study capsule if the appropriate ingestion event had not already been recorded. Participants were instructed to call the study staff if any unusual or adverse events were noted between study visits. The study participant also received a follow-up text message one hour after the initial text message if an anticipated ingestion event was not detected with the ID-Cap System within that time window.
Treatment phase. During the first study visit, participants were reminded of the study plan, provided instructions for taking study capsules, and trained to use the ID-Cap System. Study participants were instructed to take one study capsule every day with sufficient water or fluid to comfortably swallow the capsule. They received direction to put on the fully-charged ID-Cap Reader before taking the study capsule and wear it continuously for one hour following capsule ingestion. They were instructed to clip the reader onto a belt or waistband and affix the patch antenna to the skin of the abdomen. They were instructed to replace the patch antenna after each use and to dispose of the used patch antenna. Study participants received instructions on how to charge the reader when not in use. The study capsule consisted of a Size 00 hard gelatin capsule that encapsulated both an ID-Tag and a smaller Size 1 hard gelatin capsule filled with sucrose.
At the first study visit, the initial use of the ID-Cap Reader and ingestion of the first study capsule were directly observed by the investigator. The investigator recorded the date and time of capsule ingestion, monitored the participant for one hour following ingestion, and reported any adverse events. Subsequent ingestions (i.e., Study Capsules 2–20) were completed outside the research facility at the study participant’s home or other location of their choice with ingestion assessed using the ID-Cap System. The participants were instructed to record in writing the capsule identification code and the date and time when they took each study capsule on a log that was attached to the back of the ID-Cap Reader. The reader was returned to the research facility each week to download the data that were securely stored on it. Study capsules were dispensed to the participant each week. Each study participant received five study capsules to take over the course of a week for four consecutive weeks. Weekly study visits were scheduled with each participant for return of the reader, dispensing of capsules, and collection of any adverse events or usability issues.
Study participants were instructed to call the investigator if they experienced any unusual or adverse events during the treatment phase. If study personnel observed that an assigned device was not functioning as intended or if they were notified of the same, the device was repaired or replaced as needed. If the study participant did not properly recharge the reader prior to its use before taking a study capsule, a replacement capsule was provided for inclusion in the evaluation and the study participant was reminded to use the system as instructed. The data collected from the ID-Cap System included presence of the signal from the ingested ID-Tag, other electrical information such as the strength and frequency of the received signal, and information about the status of the reader.
Follow-up phase. A follow-up study visit conducted at a radiology facility was scheduled within 10 to 14 days after the last study capsule ingestion for assessment of the safety and tolerability of the ID-Cap System. This follow-up visit was scheduled with each study participant who ingested at least one study capsule. During the follow-up visit, each study participant met with the investigator for the documentation of any adverse events and received an abdominal X-ray to assess retention of ID-Tags. Female participants were given a urine pregnancy test before the X-ray. Each study participant received a follow-up phone call from the investigator within 30 days after the last study capsule ingestion to assess for any adverse events and to document any changes noted during the study. Changes to medications were also recorded at the follow-up visit and follow-up phone call.
Statistical analysis plan. All statistical analyses were descriptive. Summary data for the performance, reliability, and usability of the ID-Cap System included all study participants who ingested at least one study capsule. Summary data for safety and tolerability included all study participants who ingested at least one study capsule.
RESULTS
Twenty-eight individuals were screened to determine eligibility for participation, and 20 healthy volunteers were enrolled in the study. The study population included 12 men and eight women with a range of ages from 20 to 46 years. Study participants included 15 Caucasian, three Hispanic, and two African-American individuals with a mean body mass index of 27.8 (range of 20.0–34.5). Table 1 provides the demographic information for the 20 enrolled participants.
TABLE 1.
Study demographics and results
| SUBJECT ID | AGE (YEARS) | SEX | RACE | HEIGHT | WEIGHT (POUNDS) | BMI | DETECTION OF STUDY CAPSULE INGESTION | ADDITIONAL STUDY CAPSULE TAKEN* |
|---|---|---|---|---|---|---|---|---|
| 20-20-001 | 28 | F | Caucasian | 5' | 111 | 21.7 | 20/20 (100%) | 1 |
| 20-20-002 | 24 | F | Caucasian | 5'5" | 167 | 27.8 | 20/20 (100%) | 0 |
| 20-20-003 | 35 | F | Caucasian | 4'11" | 128 | 25.9 | 20/20 (100%) | 0 |
| 20-20-004 | 23 | M | Caucasian | 6'0.5" | 147 | 19.7 | 18/20 (90%) | 0 |
| 20-20-005 | 28 | M | Caucasian | 6'4" | 235 | 28.6 | 19/20 (95%) | 0 |
| 20-20-006 | 29 | M | Caucasian | 6'2" | 214 | 27.5 | 19/20 (95%) | 0 |
| 20-20-007 | 41 | M | Caucasian | 5'9" | 206 | 30.4 | 20/20 (100%) | 1 |
| 20-20-008 | 27 | M | Hispanic | 5'6" | 176 | 28.4 | 20/20 (100%) | 0 |
| 20-20-009 | 20 | M | Hispanic | 6' | 225 | 30.5 | 19/20 (95%) | 1 |
| 20-20-010 | 26 | F | Hispanic | 5'6" | 140 | 22.6 | 19/20 (95%) | 0 |
| 20-20-011 | 30 | F | Caucasian | 5'3" | 189 | 33.5 | 20/20 (100%) | 0 |
| 20-20-012 | 36 | M | African American | 6'6" | 231 | 26.7 | 19/20 (95%) | 0 |
| 20-20-013 | 25 | M | Caucasian | 6'1" | 211 | 27.8 | 20/20 (100%) | 0 |
| 20-20-014 | 25 | M | African American | 5'6" | 178 | 28.7 | 20/20 (100%) | 0 |
| 20-20-015 | 28 | F | Caucasian | 5'5.75" | 212 | 34.5 | 20/20 (100%) | 1 |
| 20-20-018 | 21 | M | Caucasian | 5'11.75" | 239.5 | 32.7 | 20/20 (100%) | 0 |
| 20-20-025 | 30 | F | Caucasian | 5"8.25" | 154 | 23.2 | 20/20 (100%) | 0 |
| 20-20-026 | 31 | M | Caucasian | 6'0.5" | 190 | 25.8 | 20/20 (100%) | 0 |
| 20-20-027 | 46 | F | Caucasian | 5'5" | 154 | 25.6 | 19/20 (95%) | 0 |
| 20-20-028 | 34 | M | Caucasian | 5'10" | 240 | 34.4 | 20/20 (100%) | 0 |
Additional study capsule taken due to operator error in fully charging the ID-Cap Reader before use of the ID-Cap System
BMI: body mass index
Each study participant used the ID-Cap System and took one study capsule once daily for 20 days as directed over a four-week period. No study participants prematurely discontinued use of the ID-Cap System or stopped taking study capsules prior to the end of the treatment period.
Performance, reliability, and usability for ingestion event monitoring. A total of 20 study capsules were dispensed to each study participant during the study with instructions to take one capsule once daily. Four study participants were asked to take one additional study capsule as a replacement for an ingestion event that was not recorded due to operator error. In these situations, the study participant did not follow the instructions for recharging the reader prior to its use. A total of 404 study capsules were dispensed to study participants throughout the study, and, thus, 404 ingestion events were remotely monitored using the ID-Cap System.
Positive detection accuracy was 100 percent for the 20 directly observed ingestions of study capsules that occurred during the initial study visits for all participants. Of the 384 ingestion events that were self-administered by the study participants without direct observation, 371 were accurately detected using the ID-Cap System. Overall adherence to the prescribed study capsules as measured by the ID-Cap System was 97.75 percent (391 detections/400 expected ingestion events) when the reader was used by the study participants according to the instructions for use (excluding the four ingestion events that were not recorded due to operator error for which replacement capsules were tracked). Nine (2.25%) ingestion events were not detected by the system within the one-hour detection window of reader use following self-reported ingestion. The ID-Cap Readers collected and reliably stored data for all 391 events that were detected using the ID-Cap System. Data for 385 of the 391 (98.47%) recorded ingestion events were remotely transmitted from the reader to the secure server in real time. Data for the remaining six ingestion events were downloaded to the server when the reader was returned to the research facility by the study participant. Text notifications of successfully reported ingestion events were automatically sent to each study participant within two to four minutes of the reader detecting a positive signal.
The time that each ingestible sensor was detected in the gastrointestinal tract, indicating when the ID-Tag generated a signal, was also reported using the ID-Cap System. Significant intra-individual and inter-individual variability in the timing of self-administered doses was observed in this study.
Safety and tolerability. No adverse events were reported throughout the study, either through self-reporting by study participants or assessments by study personnel at pre-specified data collection points. No study participants discontinued use of the ID-Cap System for any reason during the study. There was no evidence indicating retention of ID-Tags in any of the 20 study participants based on abdominal X-ray evaluations conducted during follow-up study visits.
DISCUSSION
This exploratory study of the ID-Cap System, a novel ingestible sensor system to directly measure medication adherence, provides new information on the performance and potential use of this technology in patient management and clinical trials. The system demonstrated 97.75 percent accuracy in detecting oral medication use at the level of each dose. In this study, the high level of adherence to self-administered therapy may have been influenced by the daily reminder text messages, weekly study visits allowing reinforcement of medication taking, and, most likely, the well-documented Hawthorne effect.6 With regard to the latter, the ID-Cap System allows verification and active oversight of medication taking, and study participants were made aware of this functionality and goal of the system through the informed consent process. While the study evaluated the technical feasibility of automated text message reminders as a means for engaging patients related to actual medication use, the study was not designed to modify patient behavior, but rather to report on actual behavior related to medication ingestion. No specific adherence interventions were planned in this study beyond automated reminder text messages and reminder calls from the study investigator, and this presents an area for future study.
For the nine ingestion events that were not detected by the system within the one-hour detection window (9 of 400 events, or 2.25%), either the ID-Tag within the study capsule failed to power up properly or the reader failed to detect the signal generated by the activated ID-Tag. The study participants utilized the ID-Cap System over four weeks and a total of 20 doses, demonstrating consistent performance throughout the study and potential applications in monitoring adherence and persistence for chronic medications.
The reliability of the verified adherence data generated using this innovative system and valuable information regarding timing of dose ingestion and inter-dose intervals enable meaningful use of this information to transform healthcare and efficiently advance clinical trials. This technology offers unique and valuable insights into actual medication use and objective evidence of adherence, or lack thereof, to strengthen patient treatment plans and clinical research protocols.
This study demonstrated that the ID-Cap System can be successfully used by study participants after simple instruction. User error that impacted system performance occurred in only four instances out of the 404 ingestion events that were monitored throughout this study. Several factors were identified during this study that could contribute to potential failures of the system, including the limited reader battery life, durability of the reader when dropped or exposed to fluids, and design elements that impact patient acceptability and use (e.g., an adhesive patch on the skin). The manufacturer of the ID-Cap System has developed strategies to mitigate the identified risks and has designed a new reader to enhance performance and usability. The system was well tolerated across study participants with no adverse events reported and no discontinuations of the system throughout the 20 days of use. Of note, participants kept the adhesive patch antenna on their abdomen for a limited duration of one hour following capsule ingestion. Published research involving other ingestible sensor systems have reported significant skin irritation issues with adhesive patches that are used as components of these systems for longer durations.7 The ID-Cap System will evolve toward external wearable readers that do not require skin contact or adhesive patches, modules that allow integration into widely used wearable and patient monitoring devices, and other patient-centered form factors, all of which are enabled by the unique in-body communications platform of the ID-Cap System. This study was not designed to specifically address the acceptability or usability of the system, and future research supporting human factors evaluations and usability will provide important insights regarding use of this system. While information about data capture and transmission through the mobile phone network was collected in this study, the study was not designed for validation of the software and hardware for data management.
CONCLUSION
The ID-Cap System enables accurate measurement of medication adherence for oral drug therapy at the dose level. This study supports the clinical validation of the technology and feasibility in using the system for the collection and real-time reporting of medication adherence in the clinical management of patients and in clinical research and drug development. Further studies will be needed to evaluate usability of the system, use in specific patient populations and for specific clinical applications, and the impact of the system on the outcomes and economics of therapeutic interventions and clinical trials. This technology joins other connected health devices that can be used independently or in an integrated manner to bolster the data available for precision medicine. The ID-Cap System holds significant promise to transform clinical research and drug development, enhance healthcare delivery, and improve health outcomes by providing meaningful adherence data, informing key decisions, and enabling new insights into medication-taking behaviors.
ACKNOWLEDGMENTS
The authors thank Elizabeth Babcock, MD, PA, for participating in the performance of the clinical research. The authors also thank Zora DeGrandpre, MS, ND, for her contributions to the data analysis and editorial review of the article.
Footnotes
FUNDING:Research reported in this paper was supported by the National Institute on Drug Abuse (NIDA) of the National Institutes of Health under award number 4R44DA036277-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
FINANCIAL DISCLOSURES:All authors (except Drs. Baumgartner and Smith) are employees of or consultants to etectRX, Inc., the owner of this patent-pending technology. All authors (except Drs. Baumgartner and Smith) are shareholders of etectRx, Inc. Dr. Baumgartner was a consultant to etectRx, Inc. at the time of this study.
REFERENCES
- 1.Brown M, Bussell J. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabaté E, editor. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: The World Health Organization; 2013. [Google Scholar]
- 3.IMS Institute for Healthcare Informatics. Avoidable costs in U.S. healthcare. 2013. [Accessed November 5, 2015]. http://www.imshealth.com/deployedfiles/imshealth/Global/Content/Corporate/IMS%20Institute/RUOM-2013/IHII_Responsible_Use_Medicines_2013.pdf Available at:
- 4.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 5.Kiourti A, Psathas K, Nikita K. Implantable and ingestible medical devices with wireless telemetry functionalities: a review of current status and challenges. Bioelectromagnetics. 2014;35:1–15. doi: 10.1002/bem.21813. [DOI] [PubMed] [Google Scholar]
- 6.Davis S, Feldman S. Using Hawthorne effects to improve adherence in clinical practice: lessons from clinical trials. JAMA Dermatol. 2013;149(4):490–491. doi: 10.1001/jamadermatol.2013.2843. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberger U, Wüthrich R, Bock A, et al. Medication adherence assessment: high accuracy of the new ingestible sensor system in kidney transplants. Transplantation. 2013;96:245–250. doi: 10.1097/TP.0b013e31829b7571. [DOI] [PMC free article] [PubMed] [Google Scholar]