Abstract
The high incidence of secondary hip fractures and the associated markedly increased mortality call for preventive actions that could help to avoid these injuries. By providing immediate strengthening and not relying on patient compliance, internal prophylactic augmentation of the osteoporotic proximal femur may overcome the main limitations of systemic bone drugs and wearable protective pads. However, such a method would have to provide sufficient and reliable strengthening effect with minimal risks and side effects to justify the need of an invasive treatment. The requirements for an internal reinforcement approach are thus strict and include mechanical, biological, clinical, ethical and financial criteria. Here we first attempt to describe the properties of an ideal augmentation method. Previously published methodologies and techniques developed at our research institute, including approaches using cements, metals, other materials or combined approaches, are then reviewed and evaluated according to these aspects. We conclude that none of the discussed methodologies appears to be able to deliver a sufficiently high gain-versus-risk ratio that could justify the clinical application and thus augmentation of the osteoporotic proximal femur remains a challenge. Finally, we provide suggestions for the development and evaluation of future strategies.
Introduction
Osteoporosis-related hip fractures are associated with severe decrease in the quality of life1,2 and 20–35% mortality in the following year.2,3 With closely 1.6 million patients suffering a hip fracture every year,1,4 the related financial load on the healthcare system is substantial and the severity of the problems is expected to increase with the growth and aging of the population.5,6 Already 1 year after the first injury, the risk of subsequent hip fractures is doubled and their incidence is 2–9%, increasing to 9–20% after 5 years.7,8,9 Mortality can be up to three times higher following a contralateral hip fracture.7 Prevention of these injuries is therefore of high interest.10
Most osteoporotic hip fractures occur in sideways falls.11 Although fall prevention is an important field, the focus of this review is on the event of fall, when fracture risk is, in a simplified view, the ratio of the acting loads and the resistance of the femur.12 Preventive strategies aim either at improving the reduced bone strength, or at decreasing the impact load.
The state-of-the-art therapy option to improve bone competence is the use of bone drugs. However, the treatment is often started too late and has a delayed effect, which can be further compromised by low patience compliance.13,14,15 Wearable protectors attenuate the force that reaches the hip, but only if they are worn.16 The associated discomfort and the relevant comorbidities, for example, incontinence, limit patient compliance and cause inconsistency in the data on effectiveness.17,18,19,20 Protectors could not be demonstrated to reduce the incidence of secondary hip fractures.16 Internal prophylactic augmentation of the osteoporotic femur may overcome these limitations by offering an immediate strengthening effect and excluding issues of patient compliance. However, an invasive method would raise further medical, ethical and financial questions.
Here we attempt to formulate the requirements for the ideal prophylactic augmentation method, review and compare previously evaluated approaches, and provide an outlook into future possibilities.
The Ideal Internal Prophylactic Augmentation Method
The ideal approach could offer an attractive gain-versus-risk ratio. In particular, it would provide immediate, significant and reliable mechanical strengthening of the osteoporotic femur with minimal invasiveness and low risk of side effects; it would be clinically feasible and both ethically and financially acceptable.
Mechanical effects
The most fundamental expectation is a definite positive strengthening effect and a consequent, significant decrease in fracture risk, such that the injury could be avoided in the most common low-energy fall situations.11,21,22 Quantification of the exact requirements remains challenging. Using a biomechanical approach, the subject-specific risk of fracture, that is, load-to-strength ratio of the proximal femur can be estimated.12,23 Bone strength can be predicted by means of validated, computed tomography image-based finite element analyses24,25 and accidental loads can be estimated from patient body characteristics with dynamic models and empirical relationships.22,26,27 Augmentation should aim at decreasing this ratio below 1.0. Fracture load of 2000 N or below was found to imply high risk of fracture, with the load-to-strength ratio being as high as 2.5,23,28 or even 4.0, for the most fragile individuals.29 Even if considering the more conservative estimate of 2.5, up to 150% increase in the fracture load should be reached. Nevertheless, spontaneous hip fractures can occur in stance30 and these injuries should also be avoided. Moreover, augmentation should withstand the low-magnitude fatigue loading of everyday physiological activities. A general solution for the various loading modes would be preferential. Besides the magnitude of strengthening, its reliability is essential: a highly reproducible outcome should be achieved, which could ensure predictable outcome for any patient at risk. An immediate, but sustainable effect would be desirable.
Risks and side effects
Augmentation should be achieved without weakening other relevant regions of the femur, or extensively increasing the local mechanical properties with abrupt transitions between stiff and soft regions, which may act as stress risers. These, analogous to the problem of periprosthetic fractures,31 may shift the location of the fracture, but not prevent its occurrence. With osteoporosis being a systemic disease, local over-strengthening of a single skeletal unit may increase the risk of fracturing adjacent structures. Alteration of the internal stress distribution could cause stress shielding, that is, bone resorption adjacent to the reinforced domain, which should be minimized.
Any deleterious chemical, thermal or biological consequence should be avoided, such that viability of bone tissue could be sustained. Invasiveness should be minimal, so that the risk of infection and other potential complications, for example, fat embolism, or the level and duration of pain, could be decreased. Quick recovery following the surgery should help to minimize the duration of hospital stay and the associated negative physical (for example, sarcopenia) and psychological consequences.
Clinical, ethical and financial considerations
Prophylactic augmentation should be accomplished using a fast, straightforward and reproducible surgical technique. Reasonable revision surgery options of a reinforced, but still fractured bone should be available. A removable solution would thus be advantageous.
Assuming that the optimal approach is found, it would still not be straightforward to decide whom to treat. Identification of patients at high risk of secondary hip fractures remains challenging10 and the decision about the treatment would depend also on other factors related to general health condition.
Invasive treatment of a not-yet fractured bone may raise ethical concerns. Acceptance would rely on the effectiveness, reliability and level of invasiveness of the method, as well as on the probability and severity of side effects. Not only the patients, but also the healthcare system and the insurance companies would demand a clear justification of the prophylactic procedure, such that the financial coverage could be secured. Cost-effectiveness of the intervention should be demonstrated, with the expenses being the used materials and surgery time, as well as the treatment of any potential side effects; although the gain would be the treatment expenses, hospitalization and consequential costs saved by avoiding the fracture.
Some of these restrictions could be eliminated or mitigated if prophylactic augmentation of the contralateral femur would be applied directly after the first fragility hip fracture: the patients would be aware of the consequences of the injury and thus be more compliant to receive the treatment; the intervention could be completed within the frame of the osteosynthesis surgery; the required health conditions would be available; and the additional costs, efforts and load on the patient (for example, anesthesia) could be minimized.
Where Do We Stand?
In the past decade, several groups presented various prophylactic augmentation approaches for the osteoporotic proximal femur and evaluated these by means of experimental biomechanical testing. The previously published methods are reviewed below and complemented with the non-published techniques developed and assessed at our research institute. The design and outcomes of the most relevant studies are summarized in Table 1.
Table 1. Summary of the design, sample set and outcome of previous experimental prophylactic augmentation studies aiming at strengthening the osteoporotic proximal femur, grouped according to the used materials.
| Study | Samples | Loading |
Augmentation |
Control group |
Strengthening |
||||
|---|---|---|---|---|---|---|---|---|---|
| N pairs | Mode and velocity (mm s−1) | Type (manufacturer) | Cement amount/implant size, mean±s.d. [range] | Location within the proximal femur | Fracture force (N), mean±s.d. [range] | Fracture energy (J), mean±s.d. [range] | Change in fracture load (%), mean±s.d. (DoM) [range] | Change in fracture energy (%), mean±s.d. (DoM) [range] | |
| First-generation femoroplasty studies | |||||||||
| Heini et al.34 | 10 | Stance (0.033) | PMMA (Palacos, Essex Chemie) | 34 ml [28 to 40 ml] | Neck and intertrochanter | 5764±1394 | 35 | 20±10 (21)* [8 to 40] | 42±32 (48)* [11 to 120] |
| 10 | Fall (2) | 38 ml [28 to 40 ml] | 2499±695 | 17 | 87±47 (82)* [24 to 178] | 249±152 (188)* [50 to 576] | |||
| de Bakker35 | 3 | Fall (100) | PMMA (Antibiotic Simplex, Howmedica International) | 50 ml | Neck and intertrochanter | 5257±2176 | 17±11 | 32±12 (24) [10 to 64] | 109±79 (84) [28 to 185] |
| Beckmann et al.36 | 9 | Fall (2) | Composite (Cortoss, Orthovita) | 40 ml | Neck and intertrochanter | 4430±1550 | 30±11 | 40±22 (43)* [11 to 86] | 211±167 (187)* [52 to 578] |
| Sutter et al.37 | 10 | Fall (100) | PMMA (Spineplex, Stryker) | [40 to 50 ml] | Head, neck, trochanter and subtrochanter | 2870±484 | 16±8 | (37)* | (154)* |
| Second-generation femoroplasty studies | |||||||||
| Zwicky38 | 6 | Fall (100) | PMMA (Vertecem, Synthes) | [9 to 10 ml] | Superior neck cortex (Figure 1a) | 2200±360 | 16.9±4.3 | (23)* | (−27)* |
| Sutter et al.40 | 9 | Fall (100) | PMMA (Spineplex, Stryker) | 15.2±1.5 ml | Neck | 1762 [1530 to 1994] | 10.2 [6.0 to 14.3] | (12) | (14) |
| 9 | 15.1±1.6 ml | Trochanter | 1912 [1470 to 2353] | 5.2 [3.6 to 6.8] | (3) | (129) | |||
| Beckmann et al.41 | 9 | Fall (2) | PMMA (Palamed, Heraeus Medical) | 12 ml [9 to 18 ml] | Single central | 3096±327 | 20.4±4.6 | (23)* | (160)* |
| 6 | Single centrodorsal | 2738±466 | 17.9±5.6 | (35)* | (164)* | ||||
| 6 | Double ventrodorsal | 2743±432 | 29.2±3.1 | (12)* | (−15) | ||||
| 7 | Double craniocaudal | 3696±601 | 44.2±10.4 | (−26)* | (−20) | ||||
| Fliri et al.42 | 5 | Fall (free fall) | PMMA (Vertecem, Synthes) | 11 ml [8 to 14 ml] | Sup and inf neck cortices, V-shaped | 3370±937 [2499 to 4726] | 10.0±5.8 [4.8 to 18.0] | −9±24 (−11) [−41 to 23] | 124±198 (102)* [17 to 477] |
| Springorum et al.43 | 10 | Fall (2) | PMMA (Palamed, Heraeus Medical) | [8 to 15 ml] | Single dorsal and caudal | 2763±1336 [879 to 5113] | 21.4±16.0 [5.1 to 55.0] | 45±33 (34)* [3 to 90] | 214±203 (118)* [1 to 636] |
| Basafa et al.44 | 8 | Fall (100) | PMMA (Spineplex, Stryker) | 9.5±1.7 ml | Subject-specific | 2576±1273 [1360 to 5410] | 9.2±5.2 [2.9 to 16.8] | 40±29 (30)* [−17 to 74] | 103±28 (94)* [47 to 139] |
| Raas et al.48 | 6 | Stance (2) | Traumacem (Synthes) | 13 ml [11 to 15 ml] | Sup and inf neck cortices, V-shaped | 6619 median [5278 to 10512] | 24 median [18 to 31] | (10 median) | (−1 median) |
| Other non-metallic materials and combinations | |||||||||
| de Bakker35 | 1 | Fall (100) | Carbon sleeve and PMMA (Antibiotic Simplex) | 12.7 mm diameter sleeve, 6 ml cement | Oblique in neck, greater trochanter | 2308 | 5.17 | −8 | 14 |
| 4 | Neck contouring composite | 19 mm diameter carbon sleevel, [9 to 15 ml] cement | Neck axis | 4120±2253 [1654 to 6996] | 12.7±7.6 [5.1 to 23.3] | 21±15 (18)* [8 to 42] | 71±44 (71)* [17 to 117] | ||
| Steenhoven et al.54 | 10 | Fall (2) | Silicon rubber (Polydimethylsiloxane, via Zym BV) | 35 ml [28 to 42 ml] | Head, neck and intertrochanter | 3097±1030 [1407 to 4710] | NA | −4±37 (−10) [−30 to 92] | NA |
| Szpalski et al.53 | 3 fresh 3 fixed | Fall (100) | Y-Strut: PEEK cylinders and PMMA (Cortoss, Stryker) | Two interconnected cylinders filled with 8 ml cement | Neck axis, intertrochanter and shaft | 3627±507 [3056 to 4515] | 19.3±5.5 [13 to 26] | 19±10 (18)* [7 to 34] | 38±38 (31)* [−19 to 81] |
| Hananouchi et al.57 | 7 plastic bones | Fall (100) | Bioactive screw (hydroxyapatite-containing) | Two screws | Neck axis | 2391±270 [1990 to 2704] | NA | (12)* | NA |
| Metal implants | |||||||||
| de Bakker35 | 1 | Fall (100) | Trochanteric gamma nail (Stryker) | NA | Blade: inferior neck cortex, nail: shaft axis | 5113 | 16.9 | 1 | 163 |
| Zwicky38 | 6 | Fall (100) | Dynamic hip screw (Synthes) | 7.3 mm diameter, 95 mm length, 3 ml cement augmentation | Inferior neck cortex (Figure 1b) | 3340±1490 | 22.6±14.1 | (−8) | (−6) |
| Springorum et al.43 | 10 | Fall (2) | Steel spiral (custom) | NA | Neck axis | 2459±1099 [1066 to 4395] | 35.0±20.7 [10.0 to 80.0] | 47±65 (32)* [13 to 224] | 94±131 (57)* [8 to 457] |
| Widmer et al (unpublished study) | 5 | Fall (free) | V-shape implant system (custom) | Two 5 mm diameter cannulated screws, lateral plate, 1 ml Traumacem V+ (Synthes) per screw | Sup and inf neck cortices, V-shaped (Figure 1d) | 2693±1080 | 5.0±2.0 | 27±29 (21) [−6 to 57] | 38±39 (30)* [−3 to 85] |
| Raas et al.48 | 6 | Stance (2) | PFNA blade (Synthes) | NA | Neck axis | 6958 median [5170 to 10224] | 31 median [21 to 42] | (−7 median)* | (−10 median) |
Abbreviation: NA, not available.
Strengthening is expressed with respect to the properties of the contralateral non-augmented bone. The mean and s.d. were computed by averaging the pair-wise strengthening of all samples, whereas difference of the means (DoM) was computed from the mean properties of the control and augmented groups within the given study. Stars (*) indicate significant differences compared with control (P<0.05). The median value is shown if the mean was not provided.
Femoroplasty—cement augmentation
In analogy to the previous successful percutaneous injection of poly(methyl methacrylate) (PMMA) cement into fractured or osteoporotic vertebral bodies, that is, vertebroplasty,32,33 Heini et al.34 were the first ones to demonstrate the feasibility of bone cement augmentation of osteoporotic proximal femora and introduced the term ‘femoroplasty'.
Mechanical effects
The first femoroplasty study filled the proximal femur with 28–40 ml PMMA and achieved a significant increase in both fracture load (Ffx: 82%) and energy (Efx: 188%) compared with the non-augmented controls.34 Note that the quantities provided here are only average values; more details can be found in Table 1. De Bakker found 24% and 84% increases in fracture load and energy, respectively, using 50 ml cement.35 To avoid the excessive temperature increase during the polymerization of PMMA, a composite cement that generated less heat was used by Beckmann et al.36 They reported significant strengthening (Ffx: 43%, Efx: 187%) using 40 ml cement and demonstrated the feasibility of a revision surgery. Similar augmentation effect (Ffx: 37%, Efx: 154%) was observed by Sutter et al.37
However, the biological risks related to the large cement volumes were realized. Thus, following the previously described ‘first-generation' studies, in ‘second-generation' work, the volume of the cement was largely reduced and its optimal location within the proximal femur was sought. Reinforcing the superior femoral neck cortex using 9–10 ml PMMA lead to statistically significant increase in strength (23%), but decreased energy to failure (−27%)38 and thus had an adverse effect (Figure 1a). Low modulus cement reduced bone stiffness, but not strength, compared with conventional cement when augmenting the inferior neck cortex39 (Figure 1c). Sutter et al.40 found no significant mechanical effect of 15 ml PMMA injected either in the femoral neck or intertrochanteric region. However, significant strengthening (Ffx: 35%, Efx: 165%) could be reached in another study using 12 ml PMMA.41 Moreover, cement location was demonstrated to be highly influential: the most successful locations were ‘single central' and ‘single centrodorsal', whereas ‘double craniocaudal' caused slight weakening, probably due to the required additional drill hole. Notably, for the best location, the magnitude of strengthening was similar to what the same authors found in their previous study using three times as much cement.36 Injecting two PMMA clouds in a diverging V-shape adjacent to the inferior and superior femoral neck cortices provided significant increase in fracture energy (124%), but not load.42 The single insertion hole and the different loading rates may explain the contrast to the outcome of the similar ‘double craniocaudal' approach of Beckmann et al.41 Springorum et al.43 could reproduce the strengthening results of the ‘single centrodorsal' technique. In contrast to the ‘first-generation' femoroplasty studies, none of the ‘second-generation' works reported significant changes in the fracture pattern or location due to cement augmentation. Bone stiffness was not affected significantly, either.
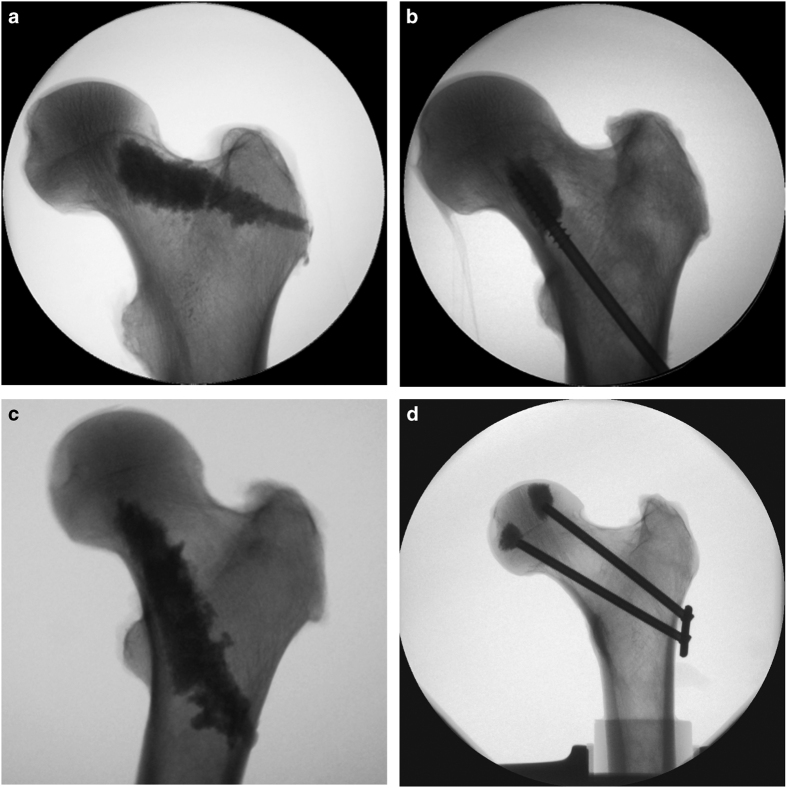
Figure 1.
X-ray based illustration of the unpublished augmentation approaches co-developed and evaluated at our research institute. (a) Cement augmentation of the superior neck cortex using 9–10 ml PMMA (Vertecem, Synthes, Switzerland) and (b) cannulated dynamic hip screw (diameter of 7.3 mm, length of 95 mm) with 3 ml cement at the tip, augmenting the inferior neck cortex; both strategies presented by Zwicky.38 (c) PMMA augmentation of the inferior neck cortex using 9–10 ml bone cement by Roffler,39 comparing conventional (Vertecem, Synthes) and N-methyl-pyrrolidone (NMP)-modified (low modulus) cements (N=5) in a free fall test setup. (d) V-shape metal implant consisting of two, 5 mm diameter cannulated screws aligned along the superior and inferior neck cortices, respectively, connected laterally with a custom 2-hole plate and augmented at the tip with 1 ml cement (Traumacem V+, Synthes) per screw; developed by Widmer et al. (unpublished study).
Already, Sutter et al.40 suggested to use computer models to improve femoroplasty. Basafa et al.44 utilized a computational pre-planning method to identify the subject-specific optimal cement locations,45 which, in line with previous experimental observation of others,46 was close to the neck cortex. By injecting 9.5 ml cement with a robotic device to the planned areas, they achieved 30% and 94% increases in maximum load and energy, respectively. Using a numerical algorithm based on bone remodeling, Varga et al.47 identified the optimal augmentation strategy as the establishment of a compression bridge, spanning between the femoral head and the greater trochanter, integrating the superior neck cortex.
Only a few studies have investigated the effect of femoroplasty on bone strength in stance. Heini et al.34 reported significant increase in fracture load (21%) and energy (48%) with ∼35 ml cement. Using the V-shape cementing technique introduced by Fliri et al.42 and 11–15 ml PMMA, Raas et al.48 found no significant improvement in either fracture load or energy. Strauss et al.49 showed that calcium phosphate cement augmentation of the hip screw hole can increase bone strength compared to the non-filled defect.
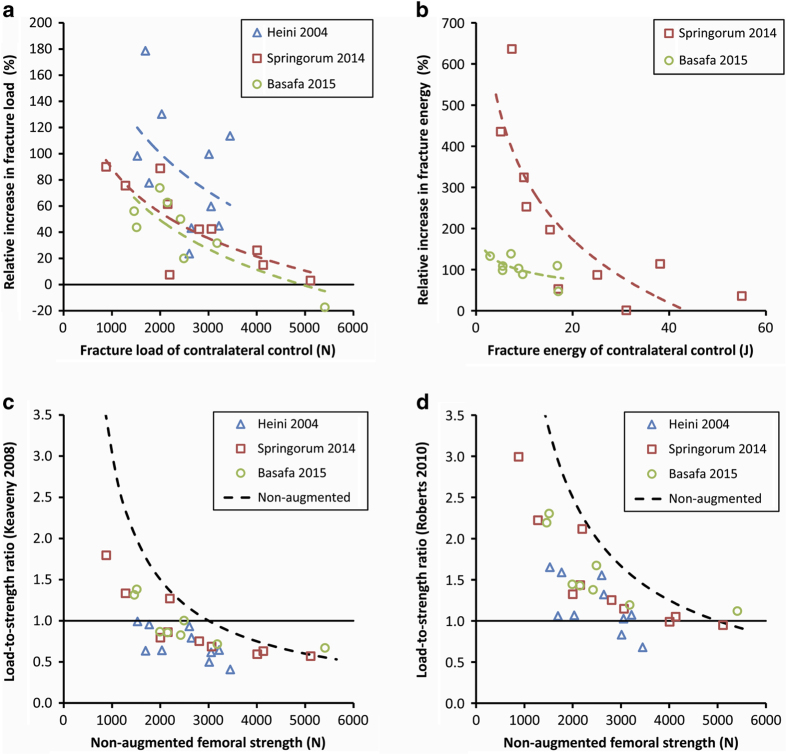
In summary, these studies have demonstrated that injection of 10–15 ml cement can provide, on average, 30–40% increase in fracture load and 120–150% increase in fracture energy in sideways fall test setups. The gain in stance is smaller. Further analysis of the data43,44 suggests that the effect of reinforcement is related to the non-augmented bone properties: weaker femora, that is, the real target group, experience larger strengthening than stronger ones (Figures 2a and b). Nevertheless, the biggest obstacle on the path to clinical application must probably be seen in the considerable scatter of the data. This indicates that predictability and reliability of the strengthening effect are rather moderate; however, these are critical requirements. The difficulty of controlling cement location in very osteoporotic bones may contribute to these results. Finite element simulation was found to moderately predict the augmented yield load.44
Figure 2.
Summary of the results of one ‘first-generation'34 and two ‘second-generation'43,44 femoroplasty studies. The increase in fracture load (a) and energy (b) depends on the properties of the contralateral non-augmented (control) femur, with weaker bones experiencing larger strengthening. Fracture risk of the control femora, estimated using the approximated load-to-strength ratio versus strength relationships published by Keaveny & Bouxsein23 (c), or by Roberts et al.29 (d), is shown as broken black lines. From this ratio, the magnitude of the accidental load can be estimated and used to compute the approximate load-to-strength ratio of the contralateral augmented bone (data points). A value larger than 1.0 indicates that strengthening was not sufficient and the augmented bone would probably still fracture in a low-energy sideways fall accident. Note that Heini et al. used quasi-static loading, the dynamic strength values are expected to be higher.
Risks and side effects
Polymerization of PMMA is an exothermic reaction. The generated heat depends on the used volume.34,48 Injection of 40–50 ml cement can cause 15–30 °C temperature increase on the bone surface34,35 and could induce bone necrosis, occurring already when exposing the tissue to 45–50 °C longer than 10 min.50 However, it has not been shown clinically if thermal damage would indeed occur due to the injection of this large amount of cement into the proximal femur. Reducing the cement volume to 11–15 ml can decrease the temperature change below 10 °C, which may rather be acceptable.40,48
Different cement compositions can lead to similarly moderate temperatures.36 Furthermore, cell necrosis may be caused by the interruption of the blood supply (avascular), the induced pressure (mechanical) or the unreacted monomer (chemical). The injection process can increase the risk of fat embolism.51 Although being a minimally invasive technique, femoroplasty still carries a relevant risk of infection. An allergic reaction due to the applied bone cement material cannot be excluded. Nevertheless, a recent study demonstrated the clinical feasibility of femoroplasty treatment of metastases patients using 5–10 ml cement, leading to few complications and significantly improved life quality.52
Strengthening the femur has not yet been demonstrated to affect the risk of acetabular fractures. Increase in stiffness may change the function of the hip joint; induce pain, arthrosis or other complications. The mechanobiological effects of cement augmentation under physiological conditions are unknown, stress shielding or altered remodeling cannot be excluded. The extent and relevance of these effects for the target population needs to be examined.
Clinical, ethical and financial considerations
Femoroplasty requires a minimally invasive, relatively straightforward and fast surgical procedure that has an immediate effect. However, as cement location affects the resulting strengthening,41 careful surgical planning and execution would be needed. The individualized design and robotic execution presented by Basafa et al.44,45 may ensure optimized, predictable and reproducible results; however, may not be generally available. Rather straightforward instructions on the injection location may be advantageous.41 Revision surgery of a cemented, but still fractured proximal femur may be challenging, in particular, drilling of the cement and the related heat generation.36
Other non-metallic materials and combinations
Composites of a carbon sleeve and bone cement were investigated by de Bakker.35 A simple combination slightly decreased fracture load and increased fracture energy on a single femur pair. With the sleeve-cement compound pushed towards the neck cortex using an inflatable balloon, non-significant increases in fracture load (21%) and energy (71%) were reached.35
Y-Strut, consisting of two perforated, interlocking peek rods connected to each other and to the surrounding bone via PMMA cement injection led to significant improvement in fracture load (19%) and energy (38%), and was claimed to reduce fracture displacement.53
Elastomer augmentation was shown to reposition and stabilize the bone fragments following a proximal femur fracture, with the potential of avoiding surgical fixation and using conservative treatment.54,55 Although the used injectable silicon rubber cures without heat generation and ensures straightforward revision surgery,56 it weakened the femur, potentially also due to the lateral entrance hole.54
Bioactive screw fixation, that is, a hydroxyapatite-containing bioresorbable implant, was evaluated on composite femora and provided significant increase (12%) in fracture load, which was however significantly lower than the augmentation effect of cement.57
Metal-based augmentation approaches
Taking the still limited mechanical competence of the available injectable polymeric biomaterials into account, it appears reasonable to utilize the strength of metals or combine both materials. Indeed, femoral nails have been used as prophylactic devices in specific conditions, where bone strength is severely diminished, for example, following bone graft harvesting by means of reaming, for the contralateral side of atypically fractured femora and for metastatic bones.58,59
Preliminary results on trochanteric gamma nail augmentation of a single femur loaded in sideways fall configuration showed large increase in fracture energy (163%, single sample), but not in the force, and shifted the fracture towards the head.35 This outcome was surprising, as a finite element model in the same study predicted much larger (84%) increase in strength. These indicate that, although prophylactic nailing may be predicted by finite element analysis to deliver large strengthening effect in sideways fall,60 experimental justification is required. Here the point when the bone fails would have to be identified and separated from the failure of the entire structure including the metal implant. In metastatic femora and stance loading, the addition of a nail implant did not increase the strengthening effect significantly compared with cement only.46 Nevertheless, the acceptance of prophylactic nailing of osteoporosis patients is improbable due to its high invasiveness.
Several other, less invasive techniques have been evaluated. A cannulated dynamic hip screw inserted close to the inferior neck cortex and augmented with 3 ml PMMA cement at the tip caused non-significant decrease in all mechanical properties38 (Figure 1b). Widmer et al. developed a V-shape metal implant system consisting of two cannulated locking screws augmented with 1 ml cement and connected laterally with a custom 2-hole lateral plate (Figure 1d). Using this construct, non-significant increase in the fracture load (27%) and significant improvement in fracture energy (38%) could be achieved, but the caused fractures were more severe (unpublished study). A steel spiral significantly increased fracture load (32%) and energy (57%), that were not different from the results of cement injection, but reduced fracture displacement.43 The authors emphasized further advantages of this method, such as minimal invasiveness, avoidance of the cement-related complications; simplicity, reproducibility of the procedure and the ease of revision surgery. Application of a proximal femoral blade augmentation led to significant reduction in strength (7%) and a 10% decrease in fracture energy in single leg stance loading.48 The missing lateral anchorage was suggested to be the reason for these results. However, based on the observations explained above, adding the nail component of the implant is not expected to improve the results, probably due to local stress rising effects.
Clinically applied methods
Femoroplasty has been used to treat bone metastases.52 With respect to prophylactic augmentation of osteoporotic femora, to the knowledge of the authors, there are two approaches already in the human clinical trial phase. The ‘prevention nail system' is a hydroxyapatite coated, cannulated titanium screw, introduced by Giannini et al.61 A prospective randomized clinical trial involving 67 patients reported no second hip fractures in either the treated or in the control group; however, controls had fewer falls. Although the clinical feasibility of the technique was demonstrated, the study was not conclusive about the biomechanical effectiveness. The other method is the above described Y-Strut, which was briefly stated to be in a clinical trial; however, no further details were provided.53
Summary and Conclusions
With the expected increase of osteoporotic hip fractures and the accompanying potentially devastating consequences, a clear need to avoid these injuries is apparent. Internal prophylactic reinforcement of femora at risk is appealing as it may offer an immediate and permanent effect. On the other hand, surgical intervention on an intact bone with the known side effects of operative treatment is questionable both medically and ethically. A first step to evaluate the feasibility of such an approach is an in vitro validation of benefits and risks to draw a concise picture of the concept.
When evaluating mechanical effectiveness, previous femoroplasty studies focused on the average increase in fracture load and energy. However, in most studies, these results depended on the properties of the non-augmented (control) femora (Figures 2a and b). Moreover, the aimed effect was usually not exactly defined. Using the estimated load-to-strength ratio, which also exhibits strength dependence,23,29 the individual fracture risk of augmented bones can be approximated (Figures 2c and d). As a result, it becomes apparent that the ‘second-generation' femoroplasty approaches may not be able to sufficiently strengthen the most osteoporotic femora. The strength of conventional PMMA bone cement appears insufficient to provide the wished augmentation effect when using reasonable amounts that may not cause biological harm. However, a critical limit has not been quantified yet.
The other extreme is the application of stiff and strong metal implants such as nails and screws. With the considerable mismatch in the mechanical properties of bone and metals, these devices can act as stress risers and may even decrease bone strength, increase severity of the fracture or only shift its location. There may be a relevant risk of cut-out under repetitive physiological loading. A metal spiral is an example of using a stiff material in a compliant construct and, while offering other advantages, allowed bone tissue to deform till failure and thus could not ensure larger strengthening than cements. The same was true for alternative solutions utilizing other injectable materials or combinations.
However, not only the magnitude of strengthening may be problematic. Previous studies are consistent in showing considerable variation of the results that can be unrelated to the original bone strength (Figures 2a and b), but may depend on surgical execution, implant placement, or other factors. Reliability and reproducibility of the augmentation effect are hence questionable. Further, none of the previous studies has investigated if strengthening of the proximal femur would lead to distal femoral or acetabular fractures. Although medical, ethical and financial criteria would also have to be met, these data indicate that not even the mechanical, that is, most fundamental criterion may be satisfied at the moment. Even though some prophylactic augmentation approaches have been reported to be already in a clinical trial phase, we believe that, to date, no sufficiently advantageous method exists, clinical use of which could be justified.
Outlook
Given the expected increase of the clinical problem and the limitations of previous methods, there is a clear need for novel approaches. New materials or combinations of the advantageous properties of different materials may be utilized. A change in the overall strategy may be made, that is, prophylactic augmentation can be seen as a temporary solution that can supplement medical therapy by bridging the time until another, for example, drug treatment takes effect.48 Injected materials could carry drugs for targeted local release.
Whatever the solution will be, the way to clinical application and acceptance is still long and thorough investigations are needed before this step. The previously demonstrated potential of computer simulations24 should be utilized to better understand the fracture mechanism,62,63 and, by allowing parametric analysis of geometries and material properties, assist and expedite the development process of new augmentation approaches.44,46,47 Realistic64 and standardized experimental evaluation techniques should be used to confirm the predicted biomechanical effectiveness, reproducibility and reliability. The concept of load-to-strength ratio should be used to evaluate the subject-specific efficacy of the different augmentation strategies (Figures 2c and d). However, the concept would require further refinement such that its accuracy could be increased. Especially, the estimation of the direction and magnitude of the loads reaching the femur should be improved. Computer models are expected to provide further insights also in this respect.65 Investigation of multiple loading cases may be required to find the worst case scenario. Population-based analyses may be needed to assess the general effectiveness. The probability of causing acetabulum of shaft fractures must be investigated. Biological effects of the mechanically most promising approaches should be evaluated first in appropriate animal models.66 Finally, all these being successful and an approach entering the clinical phase, the subject-specific gain-versus-risk ratio should be assessed. There may be no generally applicable method that would work for all patients of the target group.
Acknowledgments
This work was performed with the assistance of the AO Foundation via the AOTRAUMA Network (grant no.: AR2013_07).
Footnotes
The authors declare no conflict of interest.
References
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17: 1726–1733. [DOI] [PubMed] [Google Scholar]
- Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ 1993; 307: 1248–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Tosteson ANA, Gabriel SE, Ransom JE, Melton LJ. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc 2002; 50: 1644–1650. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002; 359: 1761–1767. [DOI] [PubMed] [Google Scholar]
- Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporos Int 1992; 2: 285–289. [DOI] [PubMed] [Google Scholar]
- Melton LJ 3rd. Epidemiology of hip fractures: implications of the exponential increase with age. Bone 1996; 18: 121S–125SS. [DOI] [PubMed] [Google Scholar]
- Ryg J, Rejnmark L, Overgaard S, Brixen K, Vestergaard P. Hip fracture patients at risk of second hip fracture: a nationwide population-based cohort study of 169,145 cases during 1977-2001. J Bone Miner Res 2009; 24: 1299–1307. [DOI] [PubMed] [Google Scholar]
- Sobolev B, Sheehan KJ, Kuramoto L, Guy P. Risk of second hip fracture persists for years after initial trauma. Bone 2015; 75: 72–76. [DOI] [PubMed] [Google Scholar]
- Berry SD, Samelson EJ, Hannan MT, McLean RR, Lu M, Cupples LA et al. Second hip fracture in older men and women: The framingham study. Arch Intern Med 2007; 167: 1971–1976. [DOI] [PubMed] [Google Scholar]
- Moll MA, Bachmann LM, Joeris A, Goldhahn J, Blauth M. Parameters pointing at an increased risk for contralateral hip fractures: systematic review. Geriatr Orthop Surg Rehabil 2016; 7: 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkari J, Kannus P, Palvanen M, Natri A, Vainio J, Aho H et al. Majority of hip fractures occur as a result of a fall and impact on the greater trochanter of the femur: a prospective controlled hip fracture study with 206 consecutive patients. Calcif Tissue Int 1999; 65: 183–187. [DOI] [PubMed] [Google Scholar]
- Hayes W, Piazza S, Zysset P. Biomechanics of fracture risk prediction of the hip and spine by quantitative computed tomography. Radiol Clin North Am 1991; 29: 1–18. [PubMed] [Google Scholar]
- Rabenda V, Mertens R, Fabri V, Vanoverloop J, Sumkay F, Vannecke C et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int 2008; 19: 811–818. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Brookhart MA, Tsao P, Sundaresan D, Andrade SE, Mazor K et al. Predictors of very low adherence with medications for osteoporosis: towards development of a clinical prediction rule. Osteoporos Int 2011; 22: 1737–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-K, Nho J-H, Ha Y-C, Koo K-H. Persistence with intravenous zoledronate in elderly patients with osteoporosis. Osteoporos Int 2012; 23: 2329–2333. [DOI] [PubMed] [Google Scholar]
- Birks YF, Hildreth R, Campbell P, Sharpe C, Torgerson DJ, Watt I. Randomised controlled trial of hip protectors for the prevention of second hip fractures. Age Ageing 2003; 32: 442–444. [DOI] [PubMed] [Google Scholar]
- Harada A, Mizuno M, Takemura M, Tokuda H, Okuizumi H, Niino N. Hip fracture prevention trial using hip protectors in japanese nursing homes. Osteoporos Int 2001; 12: 215–221. [DOI] [PubMed] [Google Scholar]
- Hubacher M, Wettstein A. Acceptance of hip protectors for hip fracture prevention in nursing homes. Osteoporos Int 2001; 12: 794–799. [DOI] [PubMed] [Google Scholar]
- Sawka AM, Boulos P, Beattie K, Thabane L, Papaioannou A, Gafni A et al. Do hip protectors decrease the risk of hip fracture in institutional and community-dwelling elderly? A systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 2005; 16: 1461–1474. [DOI] [PubMed] [Google Scholar]
- Parker MJ, Gillespie WJ, Gillespie LD. Effectiveness of hip protectors for preventing hip fractures in elderly people: systematic review. BMJ 2006; 332: 571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes WC, Myers ER, Morris JN, Gerhart TN, Yett HS, Lipsitz LA. Impact near the hip dominates fracture risk in elderly nursing home residents who fall. Calcif Tissue Int 1993; 52: 192–198. [DOI] [PubMed] [Google Scholar]
- van den Kroonenberg AJ, Hayes WC, McMahon TA. Hip impact velocities and body configurations for voluntary falls from standing height. J Biomech 1996; 29: 807–811. [DOI] [PubMed] [Google Scholar]
- Keaveny TM, Bouxsein ML. Theoretical implications of the biomechanical fracture threshold. J Bone Miner Res 2008; 23: 1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset PK, Dall'ara E, Varga P, Pahr DH. Finite element analysis for prediction of bone strength. Bonekey Rep 2013; 2: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga P, Schwiedrzik J, Zysset PK, Fliri-Hofmann L, Widmer D, Gueorguiev B et al. Nonlinear quasi-static finite element simulations predict in vitro strength of human proximal femora assessed in a dynamic sideways fall setup. J Mech Behav Biomed Mater 2016; 57: 116–127. [DOI] [PubMed] [Google Scholar]
- Robinovitch SN, Hayes WC, McMahon TA. Prediction of Femoral Impact Forces in Falls on the Hip. J Biomech Eng 1991; 113: 366–374. [DOI] [PubMed] [Google Scholar]
- Robinovitch SN, McMahon TA, Hayes WC. Force attenuation in trochanteric soft tissues during impact from a fall. J Orthop Res 1995; 13: 956–962. [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Marshall LM, Nielson CM, Cummings SR, Lapidus J, Cauley JA et al. Finite element analysis of the proximal femur and hip fracture risk in older men. J Bone Miner Res 2009; 24: 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BJ, Thrall E, Muller JA, Bouxsein ML. Comparison of hip fracture risk prediction by femoral aBMD to experimentally measured factor of risk. Bone 2010; 46: 742–746. [DOI] [PubMed] [Google Scholar]
- Viceconti M, Taddei F, Cristofolini L, Martelli S, Falcinelli C, Schileo E. Are spontaneous fractures possible? An example of clinical application for personalised, multiscale neuro-musculo-skeletal modelling. J Biomech 2012; 45: 421–426. [DOI] [PubMed] [Google Scholar]
- Lindahl H, Garellick G, Regnér H, Herberts P, Malchau H. Three hundred and twenty-one periprosthetic femoral fractures. J Bone Joint Surg Am 2006; 88: 1215–1222. [DOI] [PubMed] [Google Scholar]
- Heini PF, Wälchli B, Berlemann U. Percutaneous transpedicular vertebroplasty with PMMA: operative technique and early results: A prospective study for the treatment of osteoporotic compression fractures. Eur Spine J 2000; 9: 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heini PF, Berlemann U, Kaufmann M, Lippuner K, Fankhauser C, van Landuyt P. Augmentation of mechanical properties in osteoporotic vertebral bones – a biomechanical investigation of vertebroplasty efficacy with different bone cements. Eur Spine J 2001; 10: 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heini PF, Franz T, Fankhauser C, Gasser B, Ganz R. Femoroplasty-augmentation of mechanical properties in the osteoporotic proximal femur: a biomechanical investigation of PMMA reinforcement in cadaver bones. Clin Biomech 2004; 19: 506–512. [DOI] [PubMed] [Google Scholar]
- de Bakker P. Hip fractures: understanding the mechanism and seeking prevention through prophylactic augmentation of the proximal femur. MSc thesis, University of British Columbia, Vancouver, BC, Canada, 2006.
- Beckmann J, Ferguson SJ, Gebauer M, Luering C, Gasser B, Heini P. Femoroplasty--augmentation of the proximal femur with a composite bone cement--feasibility, biomechanical properties and osteosynthesis potential. Med Eng Phys 2007; 29: 755–764. [DOI] [PubMed] [Google Scholar]
- Sutter EG, Mears SC, Belkoff SM. A biomechanical evaluation of femoroplasty under simulated fall conditions. J Orthop Trauma 2010; 24: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicky L. Prophylactic stabilization of osteoporotic femora for fracture prevention (in German). MSc thesis, ETH Zürich, Davos and Zurich, Switzerland, 2008.
- Roffler E. Prophylactic augmentation of osteoporotic femora for fracture prevention (in German). MSc thesis, ETH Zürich, Davos and Zurich, Switzerland, 2009.
- Sutter EG, Wall SJ, Mears SC, Belkoff SM. The effect of cement placement on augmentation of the osteoporotic proximal femur. Geriatr Orthop Surg Rehabil 2010; 1: 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann J, Springorum R, Vettorazzi E, Bachmeier S, Luring C, Tingart M et al. Fracture prevention by femoroplasty--cement augmentation of the proximal femur. J Orthop Res 2011; 29: 1753–1758. [DOI] [PubMed] [Google Scholar]
- Fliri L, Sermon A, Wahnert D, Schmoelz W, Blauth M, Windolf M. Limited V-shaped cement augmentation of the proximal femur to prevent secondary hip fractures. J Biomater Appl 2013; 28: 136–143. [DOI] [PubMed] [Google Scholar]
- Springorum HR, Gebauer M, Mehrl A, Stark O, Craiovan B, Puschel K et al. Fracture prevention by prophylactic femoroplasty of the proximal femur--metallic compared with cemented augmentation. J Orthop Trauma 2014; 28: 403–409. [DOI] [PubMed] [Google Scholar]
- Basafa E, Murphy RJ, Otake Y, Kutzer MD, Belkoff SM, Mears SC et al. Subject-specific planning of femoroplasty: an experimental verification study. J Biomech 2015; 48: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basafa E, Armand M. Subject-specific planning of femoroplasty: a combined evolutionary optimization and particle diffusion model approach. J Biomech 2014; 47: 2237–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo BT, Nalley C, Gaskins RB III, Gutierrez S, Alexander GE III, Anijar L et al. Biomechanical analysis of impending femoral neck fractures: the role of percutaneous cement augmentation for osteolytic lesions. Clin Biomech 29: 289–295. [DOI] [PubMed] [Google Scholar]
- Varga P, Schwiedrzik J, Zysset PK, Gueorguiev B, Blauth M, Windolf M. How to augment the osteoporotic proximal femur? – Ask the bone!. Twenty First Congress of the European Society of Biomechanics: Prague, 2015. https://de.scribd.com/document/284553117/ESB2015-Abstract-Book.
- Raas C, Hofmann-Fliri L, Hörmann R, Schmoelz W. Prophylactic augmentation of the proximal femur: an investigation of two techniques. Arch Orthop Trauma Surg 2016; 136: 345–351. [DOI] [PubMed] [Google Scholar]
- Strauss EJ, Pahk B, Kummer FJ, Egol K. Calcium phosphate cement augmentation of the femoral neck defect created after dynamic hip screw removal. J Orthop Trauma 2007; 21: 295–300. [DOI] [PubMed] [Google Scholar]
- Li S, Chien S, Brånemark P-I. Heat shock-induced necrosis and apoptosis in osteoblasts. J Orthop Res 1999; 17: 891–899. [DOI] [PubMed] [Google Scholar]
- Peter RE, Schopfer A, Le Coultre B, Hoffmeyer P. Fat embolism and death during prophylactic osteosynthesis of a metastatic femur using an unreamed femoral nail. J Orthop Trauma 1997; 11: 233–234. [DOI] [PubMed] [Google Scholar]
- Feng H, Feng J, Li Z, Feng Q, Zhang Q, Qin D et al. Percutaneous femoroplasty for the treatment of proximal femoral metastases. Eur J Surg Oncol 2014; 40: 402–405. [DOI] [PubMed] [Google Scholar]
- Szpalski M, Gunzburg R, Aebi M, Delimoge C, Graf N, Eberle S et al. A new approach to prevent contralateral hip fracture: Evaluation of the effectiveness of a fracture preventing implant. Clin Biomech 2015; 30: 713–719. [DOI] [PubMed] [Google Scholar]
- van der Steenhoven TJ, Schaasberg W, de Vries AC, Valstar ER, Nelissen RG. Augmentation with silicone stabilizes proximal femur fractures: an in vitro biomechanical study. Clin Biomech 2009; 24: 286–290. [DOI] [PubMed] [Google Scholar]
- van der Steenhoven TJ, Schaasberg W, de Vries AC, Valstar ER, Nelissen RG. Elastomer femoroplasty prevents hip fracture displacement In vitro biomechanical study comparing two minimal invasive femoroplasty techniques. Clin Biomech 2011; 26: 464–469. [DOI] [PubMed] [Google Scholar]
- Schaasberg W, van der Steenhoven TJ, van de Velde SK, Nelissen RG, Valstar ER. Feasibility of osteosynthesis of fractured cadaveric hips following preventive elastomer femoroplasty. Clin Biomech 2014; 29: 742–746. [DOI] [PubMed] [Google Scholar]
- Hananouchi T. Prophylactic bioactive screw fixation as an alternative augmentation for femoroplasty. Biomed Tech 2015; 60: 165–169. [DOI] [PubMed] [Google Scholar]
- Scholl BM, Jaffe KA. Oncologic uses of the retrograde femoral nail. Clin Orthop Relat Res 2002; 394: 219–226. [DOI] [PubMed] [Google Scholar]
- Piccioli A, Rossi B, Scaramuzzo L, Spinelli MS, Yang Z, Maccauro G. Intramedullary nailing for treatment of pathologic femoral fractures due to metastases. Injury 2014; 45: 412–417. [DOI] [PubMed] [Google Scholar]
- Jenni D. Finite element modelling of an implant-based prophylactic augmentation approach in the proximal femur. MSc thesis, ETH Zürich, Davos, Switzerland, 2016.
- Giannini S, Luciani D, Chiarello E, Cadossi M, Tedesco G, Hoque M et al. Osteosynthetic improvement of osteoporotic bone: prevention surgery. Clin Cases Miner Bone Metab 2011; 8: 51–54. [PMC free article] [PubMed] [Google Scholar]
- Nawathe S, Akhlaghpour H, Bouxsein ML, Keaveny TM. Microstructural failure mechanisms in the human proximal femur for sideways fall loading. J Bone Miner Res 2014; 29: 507–515. [DOI] [PubMed] [Google Scholar]
- Zysset PK. Where does hip fracture initiate[quest]. Bonekey Rep 2014; 3: 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist S, Guy P, Cripton PA. Development of an Inertia-Driven Model of Sideways Fall for Detailed Study of Femur Fracture Mechanics. J Biomech Eng 2013; 135: 121001–121001. [DOI] [PubMed] [Google Scholar]
- Fleps I, Enns-Bray W, Ferguson SJ, Helgason B. Importance of soft tissues for load estimates and fracture prediction during a sideways fall. Twenty Second Congress of the European Society of Biomechanics: Lyon, 2016. https://esbiomech.org/conference/index.php/congress/lyon2016/paper/viewFile/867/246.
- Luo Q, Lu WW, Lau T-W, Leung F. Development of an animal fracture model for evaluation of cement augmentation femoroplasty. Biores Open Access 2014; 3: 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]