Abstract
Different mechanisms have been hypothesized to explain the increase in prevalence and severity of periodontitis in older adults, including shifts in the periodontal microbiota. However, the actual impact of aging in the composition of subgingival biofilms remains unclear. In the present article, we provide an overview of the composition of the subgingival biofilm in older adults and the potential effects of age on the oral microbiome. In particular, this review covers the following topics: (i) the oral microbiota of an aging mouth, (ii) the effects of age and time on the human oral microbiome, (iii) the potential impact of inflammaging and immunosenescence in the host-oral microbiota interactions, and (iv) the relationship of the aging oral microbiota and Alzheimer’s disease. Finally, in order to explore in greater breadth the potential effects of aging on the periodontal microbiota, we present analyses of data compiled from large clinical studies that evaluated the subgingival microbiota of periodontally healthy subjects and periodontitis patients from a wide age spectrum (20–83 years old). Those studies were conducted at Guarulhos University (São Paulo, SP, Brazil) and at The Forsyth Institute (Cambridge, USA), from 1999 to 2014.
Keywords: microbiology, periodontal diseases, periodontitis, aging, older adults, biofilm
The sharp increase in average life expectancy achieved during the 20th century is certainly one of society’s greatest accomplishments. Most children born just over 100 years ago did not live past age 50, while currently life expectancy reaches 80 years old in several countries (67). The latest figures from the World Health Organization revealed that in 2010, approximately 524 million people worldwide were aged 65 or older, representing almost 8% of the world’s population. This is an unprecedented large population of older individuals, which is expected to triple by 2050, reaching approximately 1.5 billion people (117). One of the major factors contributing to this achievement was the control of infectious and parasitic diseases. While the widespread increase in lifespan is a welcome indicator of socio-economic progress, it does not come without challenges. Now the leading causes of deaths and illnesses are non-communicable diseases and chronic conditions (67), since there is a wide range of health problems that initiate or worsen with advancing age. Most of these are chronic conditions, which are typically seen with highest frequency in older individuals.
Periodontal diseases, including gingivitis and periodontitis, are the most common chronic infections of adults. In a recent study, published on the journal Lancet, Vos et al. (115) quantified the global burden of non-fatal health outcomes. Periodontal diseases were ranked the #31 among the leading causes of years lived with disability worldwide. Since the prevalence and severity of periodontitis are known to increase with age (3, 57), efforts to optimize the treatment and prevention of those diseases should be a priority both for the scientific community as well as public health policymakers. In the US, the most recent population-based dental survey conducted by the National Health and Nutrition Examination Survey (NHANES) (22) showed that 46% of adults had periodontitis, representing 64.7 million people. Further, the survey revealed that 8.9% of the population presented severe periodontitis, a figure that increased to 11% in subjects aged 65 years and older.
Collectively, the growing world population coupled with worldwide rising life expectancy and the significant decrease in the prevalence of tooth loss throughout the world from 1990 to 2010 (53) are expected to significantly increase the burden posed by periodontal diseases on the individuals and on the healthcare system. Furthermore, the increasing prevalence of several chronic systemic conditions with age, such as cardiovascular and respiratory diseases, as well as diabetes and Alzheimer’s disease along with their proposed association with periodontal diseases (5, 37, 70, 83) strongly indicate that the impact of periodontal diseases as a public health problem cannot be overlooked.
Different mechanisms have been hypothesized to explain the increase in prevalence and severity of periodontitis in older adults, including shifts in the periodontal microbiota. However, the actual impact of aging in the composition of subgingival biofilms remains unclear. In the present paper, we will provide a ‘state of the art’ overview on the composition of the subgingival biofilm of older adults and the potential effects of age on the oral microbiome, based on the most recent literature on the topic. In fact, a review on the topic reveals that most of the previous studies either report on a small number of subjects or utilize low-throughput microbial diagnostic techniques. Thus, in order to explore in greater breadth the potential effects of aging on the periodontal microbiota, in this article we will present analyses of data compiled from large clinical studies that evaluated the subgingival microbiota of periodontally healthy subjects and periodontitis patients from a wide age spectrum (20–83 years old). Those studies were conducted at Guarulhos University (São Paulo, SP, Brazil) and The Forsyth Institute (Cambridge, USA) from 1999 to 2014, and followed very similar protocols for selection of participants, sample collection and microbial analysis.
The oral microbiota of an aging mouth - Current evidence
There is very limited information available in the literature about the periodontal microbiota of older adults (Table 1). The first study to describe the composition of the subgingival microbiota of older adults was published by Newman et al. in 1978 (68). The authors analyzed subgingival microbial samples from seven periodontally healthy older adults presenting a mean age of 63 years old. They observed a predominance of Gram-positive, aerobic bacteria and relatively small numbers of Gram-negative anaerobes. In addition, Percival et al. (72) studied the effects of the ageing process on the composition of the oral microbiota in a group of 79 dentate periodontally healthy individuals who were subset into four age groups: 20–39, 40–59, 60–79, and >79 years of age. Samples of supragingival biofilm and saliva were cultured on selective and non-selective media. The authors reported no differences among age groups in relation to total counts of bacteria in saliva, as well as for the prevalence of Streptococcus mutans and Spirochaetes species. On the other hand, Actinomyces species, especially Actinomyces naeslundii and Actinomyces oris, were in statistically significantly higher proportions in the supragingival biofilm of subjects over 60 years of age. Similar results have also been observed by Marsh et al. (59). More recently, Preza et al. (75) described the bacterial diversity of different oral niches from older adults without root caries or periodontitis. Biofilm samples from the tongue dorsum, mucosa of the buccal fold, hard palate, supragingival biofilm from sound root surfaces and subgingival biofilm from the same roots were collected from thirty elderly subjects, with a mean age of 83.9 years (range 73–93), and analyzed using microarrays. The most commonly detected species when all sites were evaluated together were Streptococcus oralis, Veillonella atypica, Streptococcus parasanguinis and Fusobacterium nucleatum. The authors suggested that the bacterial profiles of periodontally healthy elders might be more diverse than those of young and middle aged adults.
Table 1.
Studies evaluating the oral/periodontal microbiota of older adults or that have compared the oral/periodontal microbiota of different age groups
| Reference | Number of subjects | Age groups (years old) | Microbiological technique | Sample | Summary of main results | |
|---|---|---|---|---|---|---|
|
| ||||||
| Drake et al. (20) | Test 1: 375 PH (black) Test 2: 300 PH (white) |
Mean Age: Test 1: 72.9 Test 2: 73.4 |
Immunofluorescence and BANA test Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia |
Subgingival biofilm | Older black adults had higher proportions of Porphyromonas gingivalis, Prevotella intermedia and BANA scores in the subgingival plaque than older white adults (p<0.05). | |
|
| ||||||
| Marsh et al. (59) | 120 PH (41 with partial dentures) | Four categories: 20–39 40–59 60–79 ≥ 80 |
Microbial culture selective and non-selective media. |
Supragingival biofilm and saliva | The proportions, mean log10 viable counts and the frequency of detection of yeasts and lactobacilli in saliva and plaque were consistently higher in partial-denture wearers (p<0.05). The proportions of Staphylococcus species and Streptococcus mutans were elevated in denture wearers, but these differences were not statistically significant. | |
|
| ||||||
| Newman et al. (68) | 7 PH | Mean: 63.0 | Microbial culture selective and non-selective media. |
Subgingival biofilm | Gram-positive organisms accounted for 50% of the predominant cultivable microbiota. Streptococcus species were the most frequently isolated organisms and were found in 83.8% of the samples. | |
|
| ||||||
| Percival et al. (72) | 79 PH | Four categories: 20–39 40–59 60–79 ≥ 80 |
Microbial culture selective and non-selective media. |
Supragingival biofilm and saliva | The total counts of viable bacteria in saliva, and the prevalence of Streptococcus mutans in supragingival plaque and saliva did not differ among age groups (p>0.05). Mean proportions, mean viable counts and the isolation frequency of Lactobacillus species were higher in the saliva samples of subjects ≥ 70 years old compared to those ≤ 39 years old (p<0.05). Actinomyces naeslundii was found in higher proportions in the younger age groups (20–39 and 40–59 years old), when compared with the older age groups and Actinomyces oralis was found in higher proportions in the oldest age groups 60–79, ≥ 80 years old (p<0.05). | |
|
| ||||||
| Preza et al. (75) | Control: 20 PH Test: 21 PH with root caries |
Mean: Control: 84.3 Test: 88.7 |
16S rRNA-based microarray for 300 species | Supragingival biofilm | Lactobacillus casei/paracasei/rhamnosus and Pseudoramibacter alactolyticus were associated with root caries biofilm samples. Actinomyces species were found more frequently in subjects from the control group (p<0.05). | |
|
| ||||||
| Rodenburg et al. (78) | Test 1: 38 (severe periodontites) Test 2: 104 (refractory periodontites) |
Test 1 and Test 2 groups presented the following age categories: 14–20 21–10 31–40 41–70 |
Microbial culture Porphyromonas gingivalis, Prevotella intermedia and Aggregatibacter actinomycetemcomitans |
Subgingival biofilm | In Test 1 group the prevalence of Aggregatibacter actinomycetemcomitans-positive subjects and the number of those solely infected with A. Actinomycetemcomitans decreased with increasing age (p<0.05), whereas the prevalence of P. gingivalis-infected subjects increased with increasing age (p<0.05). However, these trends were not observed in the refractory periodontitis patients | |
|
| ||||||
| Savitt et al. (82) | 1,492 | Test 1 and Test 2 groups presented the following age categories: 10–19 20–29 30–39 40–49 50–59 60–69 |
DNA probes Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis |
Subgingival biofilm | Aggregatibacter actinomycetemcomitans was correlated with subjects in the youngest age group (10 to 19 years old) with decreasing prevalence and levels in older age groups in comparison with the younger groups (p<0.05). In contrast, Porphyromonas gingivalis showed an inverse association with subject age, showing highest prevalence and levels in older groups (>30 years old) compared to subjects in younger age groups (10–29, 20–29 years old) (p<0.05). | |
|
| ||||||
| Slots et al. (86) | 1624 periodontitis subjects | Range: 15 to 89 Six categories: 15–24 25–34 35–44 45–54 55–64 >64 |
Microbial culture Prevotella intermedia and Aggregatibacter actinomycetemcomitans |
Subgingival biofilm | Aggregatibacter actinomycetemcomitans was in higher prevalence (74%) in subjects < 25years old than in adults and geriatric subjects (31%). Prevotella intermedia was present in 45% of the subjects and no predilection for a specific age group was detected. | |
|
| ||||||
| Slots et al (87) | 3075 “refractory” periodontitis | Range: 12 to 93 Fifteen categories: |
Microbial culture selective and non-selective media. |
Subgingival biofilm | Older females (15.9%) and males (15.3%) had higher prevalence of enteric rods and pseudomonads than younger subjects (10.9%) (p<0.05). Older infected females yielded significantly higher viable counts of these microorganisms than younger infected females (p<0.05). | |
| 10–14 20–24 30–34 40–44 50–54 60–64 70–74 >80 |
15–19 25–29 35–39 45–49 55–59 65–69 75–79 |
|||||
PH, periodontal health
Only a few studies published in the 1990’s evaluated the periodontal microbiota of older adults with periodontitis (11, 20, 78, 82, 86). Rodenburg et al. (78) studied the occurrence of Porphyromonas gingivalis, Prevotella intermedia and Aggregatibacter actinomycetemcomitans in 242 subjects, including 138 individuals with untreated severe periodontitis and 104 with refractory periodontal disease. Pooled subgingival samples were cultured anaerobically. The authors’ subset these subjects into four age groups: 14–20, 21–30, 31–40 and 41–70 years of age. They found that the prevalence of subjects colonized by A. actinomycetemcomitans appeared to be age related, as it decreased with increasing age. On the other hand, the prevalence of subjects colonized by P. gingivalis increased with aging. Other authors have also observed similar correlations between A. actinomycetemcomitans and P. gingivalis with age (23, 82, 86, 103).
Slots et al. (87) have proposed that older subjects with refractory periodontitis may harbor superinfecting microorganisms. The authors studied the occurrence of subgingival enteric rods, yeasts, Pseudomonas and Staphylococcus species in 3075 subjects with refractory periodontitis with age ranging from 12 to 93 years old. Older subjects (15%) revealed significantly higher prevalence of enteric rods and Pseudomonas species than younger individuals (10.9%). Staphylococcus species, on the other hand, showed a higher prevalence in the youngsters compared with older adults. The authors concluded that high levels of subgingival enteric rods and Pseudomonas species in some individuals may be important in the pathogenesis of geriatric and other forms of periodontitis and may have therapeutic implications.
Several advances have been made in the microbial techniques used to study the periodontal microbiota since the studies listed above. The newer techniques allowed for increased sensitivity and specificity, as well as the ability to analyze a much greater number of patients and samples, which are important steps toward a better understanding of the periodontal microbiota, including that of older adults. That is crucial step for the development of better strategies for prevention and treatment of periodontitis in these individuals, which may significantly contribute to their oral and systemic health.
The Human Oral Microbiome: the Effects of Age and Time
Recent studies of oral and periodontal microbiology have used sequencing platforms, most commonly employing 16S rRNA –based taxonomic surveys (2, 38, 51). These platforms allow for an open-ended agnostic description of the composition of the microbiomes under study, since they do not target any taxa specifically. However, so far no studies have employed those platforms to specifically investigate the effects of aging in the oral microbiome. Yet, valuable information on the ecological aspects of the oral microbiome, which might provide valuable insights into the influence of aging in the oral microbiome, may be drawn from publications stemming from the Human Microbiome Project (HMP). The HMP is an initiative funded by the National Institutes of Health since 2008 to enable the comprehensive characterization of the human microbiome that shed new light in the importance of the analysis of its role in human health and disease (107).
Given that data regarding the human oral microbiome is lacking, one of the most relevant HMP publications for the topic in question is the study by Yatsunenko et al. (120). In that paper the authors report on the microbial composition of the human gut microbiome viewed across age and geography. The authors evaluated gut microbiome samples from 531 subjects, ranging in age from birth to 83 years old, originating from three distinct geographic locations (USA, Venezuela and Malawi). Their results demonstrated that the phylogenetic composition fluctuated considerably during the first 3 years of life. But, at that age, the bacterial communities evolved towards an adult-like configuration, regardless of the geographic location of the individuals.
Even though it is not known whether the same dynamic microbial volatility is true for the oral cavity, it would not be surprising. After all, major environmental pressures are shaping the developing microbiome as demonstrated by the impact of the mode of delivery (vaginal or cesarian section) (19), the type of feeding (breast or formula feeding (48) and a child’s co-habitants and environment (97) in their microbial profiles. It seems that, after an intense albeit brief period of microbial influx and acquisition, humans seem to reach a climax, health-compatible community that seems to be resistant to extrinsic disturbances. Although we do not have direct proof that the same events occur in the oral cavity, and that little changes happen in the oral microbiome from early infancy to old age, indirect evidence exists. For example, Stahringher et al. (97) studied the variability in the microbiome over a decade during adolescence. Eighty-two individuals were sampled more than once, approximately in 5-yr intervals at up to three time-points (12/13, 17/18, and 22/23/24 yr of age). They compared the salivary microbiome of the cohort population of the same age from age 12 to age 17, from age 17 to age 22, and from age 12 to 22, spanning a period of 5 and 10 yr. After both 5-yr spans, the oral microbiome of an individual resembles itself more closely than that of the population. After 10 yr (12–22), the oral microbiome has a trend toward self-similarity, but this trend was not statistically significant. Therefore, even in the human oral microbiome, where one may anticipate frequent environmental perturbations, there is remarkable stability over long time periods during development up to 5 yr.
Although not controlled for age, the long-term stability of the oral microbiome has been demonstrated in several recent studies. In a benchmark study, Caporaso et al. (12) analyzed tongue biofilms collected daily over a period of 15 months and 6 months, in one female and one male subject, respectively, as well as gut and skin (left and right palm) samples. The authors observed clear body site differentiation as well as individual microbial specificity, which were stable during the observation period. However, their data suggested a surprisingly small temporal ‘core human microbiota’ (defined as species-level taxa observed across all sampling events) within each individual’s body sites, including the oral cavity. When the size of this “core” was assessed, it decreased as follows: mouth > gut > right palm ≈ left palm > across body sites within an individual > across body sites and individuals. The authors hypothesized that this temporal variation may arise from extrinsic factors, including exposure to different types of foods, medications and physical environments (due to travel, for instance) or from intrinsic factors, such as the immune system. It is noteworthy, though, that the oral cavity, which is an open ecosystem exposed to all these factors, showed the greatest compositional temporal stability overtime, in comparison with other body sites.
The extrinsic factors proposed by Caporaso et al. (12) as modulators of the (oral) microbiome were explored in exquisite detail by David et al. (18). The authors addressed an important point: even though disturbances to human microbiota underlie several pathologies, there is currently limited understanding of how lifestyle affects the dynamics of human-associated microbial communities in health. In the most comprehensive time series analysis of the oral microbiome thus far, the authors collected saliva (and fecal) samples daily, from two individuals, during the course of one year. In addition, study participants were equipped with an IPhone and an app to record more than 10,000 longitudinal measurements of human wellness and action, such as fitness, diet, exercise, illnesses and travel, which were ultimately linked to the microbial data. The authors confirmed previous findings regarding the long term compositional stability of the oral microbiome. In agreement with Caporaso et al. (12), the authors also found a relatively small core salivary microbiome, as only a subset of species-level taxa were consistently present over time. Yet, that small consortium dominated the community and comprised 99.7% of the total counted bacteria. It is tempting to speculate that this small consortia comprised the oral microbial species consistently observed in the majority of the classical oral microbiology studies, particularly those known as the “checkerboard panel”. The authors also reported that, despite the overall evidence for microbiota stability, certain host actions and health changes led to broad disturbance of the microbial community. For instance, one of the subjects relocated from a major US metropolitan area to Southeast Asia, and thus was exposed to a novel diet and environment. During this period, clear disturbances in community structure were observed, up to the phylum level, revealing increases in Bacteroidetes and decreases in Firmicutes. Interestingly, the microbiota shifts observed during this subjects’ travel reversed upon his return home. One of the key findings in the paper by David et al. (18) is that the stability of the oral microbiome seems to be quite robust. After all, only a few of the myriad of host factors evaluated could lead to detectable changes in community structure and, in that regard, a change in geographic location and dietary habits was a major influence. It is noteworthy that the findings by David et al. (18) are in accord with those of Haffajee et al. (39). In that benchmark study, the authors demonstrated effect of geographic location on the oral microbiota, as there were some differences in the subgingival microbial profiles of subjects from the US, Sweden, Brazil and Chile.
While the findings of Caporaso et al. (12) and David et al. (18) regarding the stability of the oral microbiome were based on comprehensive time series of daily sample collection of tongue biofilms and saliva samples spanning from 6 to 12 months, respectively, they were obtained from a very small co-hort (1–2 subjects). Yet, they are corroborated by the results from a larger, although less detailed, longitudinal survey of the microbial biogeography of the human microbiome. In that study, Zhou et al. (125) determined the microbial composition of 22 habitats from 279 healthy humans. Those habitats included multiple oral niches (hard palate, keratinized gingiva, buccal mucosa, subgingival biofilm, supragingival biofilm, saliva, tongue dorsum, palatine tonsil, and throat), and other body sites including the anterior nares, skin and vaginal sites as well as stool samples. Samples were collected during 2 time points, and the mean time interval between the two time points was 212 days. The temporal variation analysis, performed using the community similarity between two visits using the Spearman correlation, revealed that, among all sites evaluated, the bacterial communities in oral habitats presented the strongest correlation (>0.6) between first and second visits, particularly tongue dorsum and saliva.
The recent “microbiome revolution” was brought about by democratization of sequencing technologies and has taught and revived many ecological concepts in oral microbiology. It reinforced the notions of microbial community stability, resistance, resilience and core, which are impressive, given the multitude of daily disturbances that human experience from infancy to old age. Yet, the current literature seems insufficient to draw a definitive conclusion about a possible impact of aging on the periodontal microbiota. Most of the reported studies focused on a small number of species and often in a limited number of samples. To the best of our knowledge, no studies to date have comprehensively studied or compared the composition of the subgingival microbiota of older adults with periodontal health or periodontitis. Thus, in order to contribute to this knowledge, the latter portion of this manuscript will present a thorough analysis of the microbial composition of the subgingival biofilm from subjects with periodontal health or disease in different age groups.
Host-microbiota interactions in the oral cavity: the potential impact of Inflammaging and immunosenescence
The oral microbiota is highly complex in organization, holds enormous diversity and have unique capacity to rapid phenotypic changes in response to a wide array of environmental pressures (92, 93, 104). As a whole, it represents a dynamic structure, since it constantly needs to adapt to an ever-changing environment, from birth to old age. Those changes can be elicited by the local milieu, the host immune system and the environment (12, 124). Factors such as oral hygiene (or lack thereof) (47, 79, 110, 116), smoking (51, 60) and geographic location (18, 39) are examples of local and environmental pressures that can modulate shifts in the oral microbiota, which may also exacerbate host inflammatory response. The balance between the host and microbiota ensures the homeostasis that ultimately leads to oral health. Disturbances in this equilibrium are the basis of the two of the most common infections of the human body, caries and periodontitis (15, 44, 84).
Clearly, the transition from periodontal health to disease is accompanied by a shift from a health-compatible microbiota to a pathogen-enriched community that initiates and maintains the clinical signs of periodontal inflammation and destruction. Interestingly, the benchmarks of the induction and resolution of this process seem to be quite reproducible, as demonstrated by experimental gingivitis studies in different populations and types of patients, including diabetes (80), smokers (81) and older individuals (31, 32). In addition, the microorganisms typically associated with periodontal health and with periodontitis also appear to be a consensus. In recent years, the development and accessibility of microarrays and open-ended, deep-coverage sequencing platforms allowed for the study of the periodontal microbiome in much greater breadth. Yet, along with the identification of new putative pathogens such as Filifactor alocis and members of the uncultivated segment of the microbiota, including Fretibacterium (Synergistetes) species (55, 56, 73), results from studies using those platforms also identified the “usual suspects”, showing members of the orange and red complexes in higher levels in diseases and Actinomyces sp and Streptcococcus sp, in greater abundance in periodontal health (2, 38, 56, 73, 104). However, despite decades of research in this field, the steps leading to these microbial shifts are not totally understood. Nevertheless, the acquisition or, more likely, the proliferation of periodontal pathogens, individually or in combination, along with changes in the local environment, appears to be essential. In fact, conditions that may interfere with the local or systemic environments, such as smoking, diabetes, genetic polymorphisms and advanced age, are considered risk factors/indicators for periodontal diseases.
The main theories that have been proposed to explain the increased prevalence and/or severity of periodontitis with aging are: i) the cumulative effect of periodontal loss over time (6, 35), ii) alterations in the innate immune and/or inflammatory status (45, 50), and iii) shifts in the composition of the subgingival microbiota (63). The hypotheses ii and iii are greatly interrelated, as changes in the local or systemic immune inflammatory status may influence the oral microbiota and vice-versa (93). It has been postulated that the aging process is associated with a low-grade systemic inflammatory status, even in the absence of clinical signs of infections. This phenomenon, named as inflammaging, increases morbidity and mortality in elders (29) and might interfere with the resident microbial population of the naturally infected areas of the human body, such as the gut and the oral cavity (45, 74). In addition, the decline in function of the immune system brought on by natural age advancement, generally referred to as “immunosenescence” (29, 45, 58, 99), may also contribute to the increased susceptibility of elderly individuals to microbial infections, which may further magnify the effects of inflammaging (30).
Inflammaging and immunosenescense might contribute to the basis of the observed responses of elderly individuals to the periodontal bacterial challenge. Early experimental gingivitis studies have shown that older subjects responded earlier and more pronounced to biofilm accumulation than younger subjects (49). Besides, they appear to accumulate more biofilm than their younger counterparts, even though the microscopic counts of various types of microorganisms did not reveal any differences between the two groups throughout the period of biofilm accumulation (49). Later studies of similar design (31, 32) confirmed that older individuals accumulated more biofilm and developed more severe gingivitis. They also showed that older individuals presented higher volume of GCF, higher GCF levels of α 2 macroglobulin and IgG3 and larger and more severe inflammatory infiltrate, as determined by gingival biopsies which harbored a higher proportion of B-cells and lower density of neutrophils. Those studies also failed to detect obvious differences between the groups in the composition of biofilm, based on total viable counts and proportions of A. actinomycetemcomitans, P. gingivalis, P. intermedia and F. nucleatum ssp. However, divergent observations were reported by others, in studies that did not observe clinical, immunological or microbial inflammatory differences between young adults and the elderly using the experimental gingivitis model (1, 111, 112, 114, 116).
The aging oral microbiota and its relationship to Alzheimer’s disease
The prevailing hypotheses linking oral diseases to systemic conditions posit that periodontal diseases contribute to a systemic inflammatory state and/or the systemic dissemination of oral microorganism, the so-called “mobile microbiome” (46), which may trigger disease processes elsewhere in the body (42). Aging is accompanied by several health problems, including the cognitive decline associated with Alzheimer’s disease. Recent epidemiological studies have indicated a possible association between oral health conditions, particularly tooth loss, and decline in cognitive function (34, 66, 71, 98). Research done in the 1990s have demonstrated the elevated presence of spirochetes (including oral treponemes) in the brain of Alzheimer’s patients compared to cognitively normal controls (62). Murine models of neurodegeneration have also indicated that mice raised in sterile conditions presented a delayed onset of declined cognitive function (13). It has also been demonstrated that elevated serum tumor necrosis factor-alpha (TNFα) and serum antibodies to A. actinomycetemcomitans, Tannerella forsythia and P. gingivalis were present in Alzheimer’s patients compared to controls (52). Interestingly, those authors showed that serum antibodies for these periodontal pathogens had an odds ratio of 6.1 for Alzheimer’s disease. After examining data from 2,355 participants in the Third National Health and Nutrition Examination Survey (NHANES-III) who were 60 years of age or older, Noble et al. (69) demonstrated that individuals with the highest serum levels of antibodies to P. gingivalis had low cognitive test outcomes. In another study, elevated baseline serum levels of antibodies for F. nucleatum and P. intermedia also correlated with declined cognitive function 10 years later (96). These studies have led some to propose a mechanism where systemic inflammation, characterized by prolonged exposure to circulating TNFα, would compromise the blood-brain barrier (BBB), allowing bacteria to spread into the brain. In addition, the low-grade systemic inflammation associated with periodontal diseases could contribute to the damage to the BBB. These mechanisms would be compounded by the “immunosenescence” that accompanies aging, potentially leading to an increase in levels of certain members of the oral microbiota and their systemic dissemination. While the adaptive immune system wanes, innate immunity takes over, contributing to the increased levels of circulating pro-inflammatory cytokines such as TNFα (83).
High-throughput 16S ribosomal RNA gene sequencing has been used to examine differences in the subgingival microbiota in a small group of individuals with and without dementia. Due to the small sample size the results were not statistically significant but the authors reported higher relative abundance of Fusobacteriaceae and lower relative abundance of Prevotellaceae in subjects without dementia (14). Interestingly, Fusobaterium species have been reported to be among the most commonly identified taxa in elderly patients (75, 76). The authors postulated that species from the genera Fusobacterium could provide a certain level of protection against cognitive decline by occupying habitats that could be populated by more inflammation-inducing taxa. These recent findings implicating the subgingival microbiota and “immunosenescence” in the cognitive decline that follows Alzheimer’s disease highlight the importance of additional studies examining changes in the oral microbiota that occur with aging.
The periodontal microbiota of an aging mouth: a comprehensive evaluation
Study design and subject population
These analyses included data from various cross-sectional and longitudinal studies conducted at the Center for Clinical Research at Guarulhos University (São Paulo, Brazil) and at The Forsyth Institute (Cambridge, USA), from 2002 to 2014 and 1999 to 2014, respectively. Data from 1330 subjects, being 1084 with periodontitis and 246 with periodontal health, were evaluated. The clinical data included probing depth (PD) and clinical attachment level (CAL), which were recorded at six sites per tooth, from all teeth. Subgingival biofilm samples were taken from nine sites per subject at each experimental time point, 3 in each of the following PD categories: ≤ 3 mm (shallow), 4–6 mm (intermediate) and ≥ 7 mm (deep). The samples were analyzed for their content of 40 bacterial species using checkerboard DNA–DNA hybridization (61, 94). A total of 10.568 biofilm samples and 422.720 data points were evaluated.
Included subjects were at least 20 years old and were in good general health. Subjects with periodontitis had a minimum of 15 natural teeth and four teeth with at least one site each with PD and CAL ≥5 mm. Periodontally healthy individuals had a minimum of 20 teeth, no sites with PD or CAL >3 mm, and <20% of the sites with bleeding on probing. Exclusion criteria were pregnancy, nursing, periodontal therapy and antibiotic administration within the previous 3 months, any systemic condition that might have affected the progression of periodontitis and need of antibiotic coverage for routine periodontal procedures. No subjects with localized aggressive periodontitis were included in the study.
Table 2 presents the baseline demographic and clinical characteristics of the population, discriminated by center. The significance of differences between the two databases in each clinical group was assessed using the Student T test. Subjects ranged in age from 20 to 83 years. Subjects with periodontitis and periodontal health from Guarulhos University presented higher mean full-mouth PD and CAL than those from the Forsyth Institute (p<0.000). In addition, the Forsyth database had more smokers in the periodontally healthy group, and their periodontitis group had a higher mean age than that of Guarulhos University (p<0.000). No differences were observed between the two databases for gender.
Table 2.
Demographic characteristics and mean (± SD) full-mouth probing depth (PD) and clinical attachment level (CAL) of subjects in each center
| Variables | Experimental groups | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Periodontitis
|
Periodontal health
|
|||||
| Forsyth Institute n= 594 |
Guarulhos University n= 490 |
p-value | Forsyth Institute n= 193 |
Guarulhos University n= 53 |
p-value | |
| Age (years) | 49.6 ± 11.6 | 44.8 ± 12.0 | 0.000 | 37.8 ± 9.8 | 37.3 ± 15.8 | 0.756 |
| Gender (% of Male) | 54% | 40% | 0.126 | 37% | 45% | 0.091 |
| Smokers (%) | 29% | 20% | 0.056 | 12% | 0% | 0.000 |
| PD baseline (mm) | 3.4 ± 0.8 | 3.9 ± 0.7 | 0.000 | 2.5 ± 0.3 | 2.8 ± 0.1 | 0.000 |
| CAL baseline (mm) | 3.5 ± 1.1 | 4.4 ± 0.9 | 0.000 | 2.2 ± 0.7 | 2.5 ± 0.1 | 0.000 |
The significance of differences between the two databases was assessed using the Student T test. SD, standard deviation
Subjects with periodontitis and periodontal health from both centers were grouped and categorized according to three age groups as follows: <35 (young), 35–64 (adults) and >64 (older adults) years old. Although the population included in this study was not rigorously categorized in Chronic and Aggressive Periodontitis, according to the standard periodontal disease classification of the AAP (4), most of the individuals in the young group presented Aggressive Periodontitis, while those in the other two groups (adults and older adults) had exclusively Chronic Periodontitis. The demographical and clinical characteristics of the full database, stratified according to the three age groups are presented in Table 3. The significance of differences among groups was assessed using analysis of covariance (ANCOVA) adjusted for smoking, gender, baseline PD and geographic population. The significance of differences between pairs of comparisons was determined using Tukey’s multiple comparison test. As expected, the mean age differed significantly among the three groups, as well as the percentage of smokers, which was lower in the young group with periodontitis (p<0.05). Mean PD did not differ among age groups, but older adults with periodontal health or disease had a statistically significantly higher mean CAL in comparison with the young and adult groups.
Table 3.
Demographic characteristics and mean (± SD) full-mouth probing depth (PD) and clinical attachment level (CAL) of the three age groups
| Periodontitis | Periodontal health | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Variables | Age groups | p-value | Age groups | p-value | ||||
|
|
|
|||||||
| <35 years (n=152) |
35–64 years (n=833) |
>64 years (n=99) |
<35 years (n=119) |
35–64 years (n=112) |
>64 years (n=15) |
|||
| Age (years) | 28.5 ± 4.7 A | 48.2 ± 7.6 B | 69.6 ± 4.2 C | 0.000 | 29.24± 6.2 | 44.1 ± 6.7 | 67.8 ± 9.8 | 0.000 |
| Gender (% of Male) | 47% | 48% | 49% | 0.944 | 26% | 32% | 25% | 0.630 |
| Smokers (%) | 16% A | 21% B | 19% B | 0.013 | 26% | 32% | 25% | 0.632 |
| PD baseline (mm) | 3.7 ± 0.8 | 3.6 ± 0.8 | 3.7 ± 0.8 | 0.080 | 2.6 ± 0.3 | 2.6 ± 0.3 | 2.7 ± 0.4 | 0.356 |
| CAL baseline (mm) | 3.8 ± 1.0 A | 3.9 ± 1.1 A | 4.2 ± 1.0B | 0.031 | 2.2 ± 0.6 A | 2.3 ± 0.6 A | 2.9 ± 0.2 B | 0.000 |
| % subjects from UnG | 55% | 43% | 42% | 0.054 | 26% | 13% | 50% | 0.000 |
The significance of differences among age groups was assessed using the ANCOVA adjusted for smoking, gender, baseline PD and geographic population. The significance of differences between pairs of comparisons was determined using Tukey’s multiple comparison test and are represented by different capital letters. SD, standard deviation, UnG, Guarulhos University.
In order to test the hypothesis that “older adults with chronic periodontitis present a subgingival microbiota distinct of that observed in younger individuals” we have formulated a series of questions that will be discussed in the following sections.
Does aging influence the subgingival microbiota associated with periodontal health or disease?
With the aim of addressing the influence of aging in the subgingival microbiota, we evaluated the mean counts and proportions of 40 bacterial species in the subgingival biofilm samples of subjects with periodontal health or periodontitis in each age group and the results are depicted in Figs 1 and 2. Figure 1 presents the results for the periodontally healthy individuals. No statistically significant differences were observed in the levels or proportions of the bacterial species evaluated between the older adults and the other group of adults or the youngsters. Although not statistically significant, it is worth mentioning that older adults showed a trend towards higher levels of the three F. nucleatum subspecies. This finding is in accord with the results from Preza et al. (75, 76), who described the high prevalence of F. nucleatum subsp. polymorphum oral taxon 202 in a group of elderly subjects (age range 73–93), associated particularly with healthy root surfaces (75). The presence of gingival recession and root surface exposure in the older group of our study might help justify this finding. Interestingly, using 16S bacterial ribosomal RNA gene sequencing, Cockburn et al. (14) found higher relative proportions of Fusobacterium species in subgingival biofilm samples from subjects without dementia compared to individuals with declined cognitive function. They proposed that species form the genus Fusobacterium could provide a certain level of protection against dementia by filling the subgingival habitat, warding off the colonization of more inflammation-inducing bacterial species.
Figure 1.
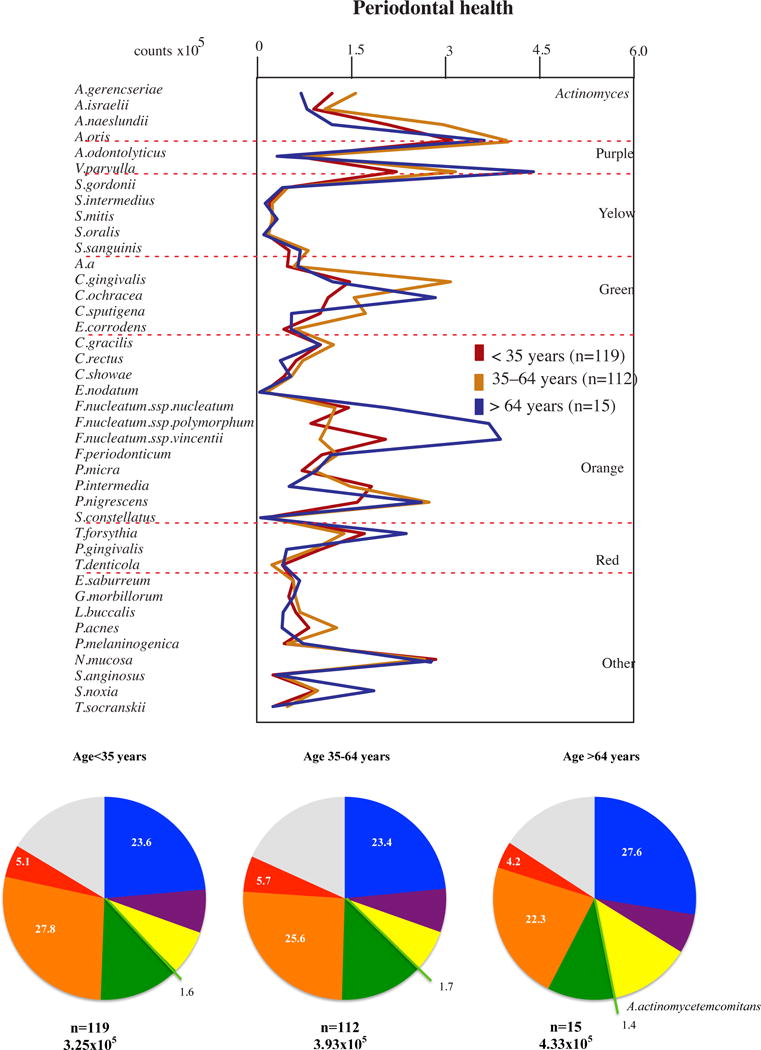
Profiles of the mean counts (×105) and of the mean proportions of 40 taxa in subgingival biofilm samples taken from 246 subjects with periodontal health subset into three age groups (<35, 35–64 and >64 years of old). Nine subgingival biofilm samples were taken from each subject and were analyzed separately to determine their content of the 40 species of bacteria. The species were ordered and grouped according to the microbial complexes described by Socransky et al. [89]. The mean values for each species were averaged within a subject and then across subjects in each age group. The colors in the pie charts represent the different complexes. Actinomyces spp. are represented in blue and A. actinomycetemcomitans in light green. The grey color represents species that did not fall into any complex. The significance of differences among groups was determined using analysis of covariance (ANCOVA), adjusted for smoking, gender, baseline probing depth and clinical attachment level and geographic population. The significance of differences between pairs of comparisons was determined using Tukey’s multiple comparison test (different letters indicate significant differences between age groups).
Figure 2.
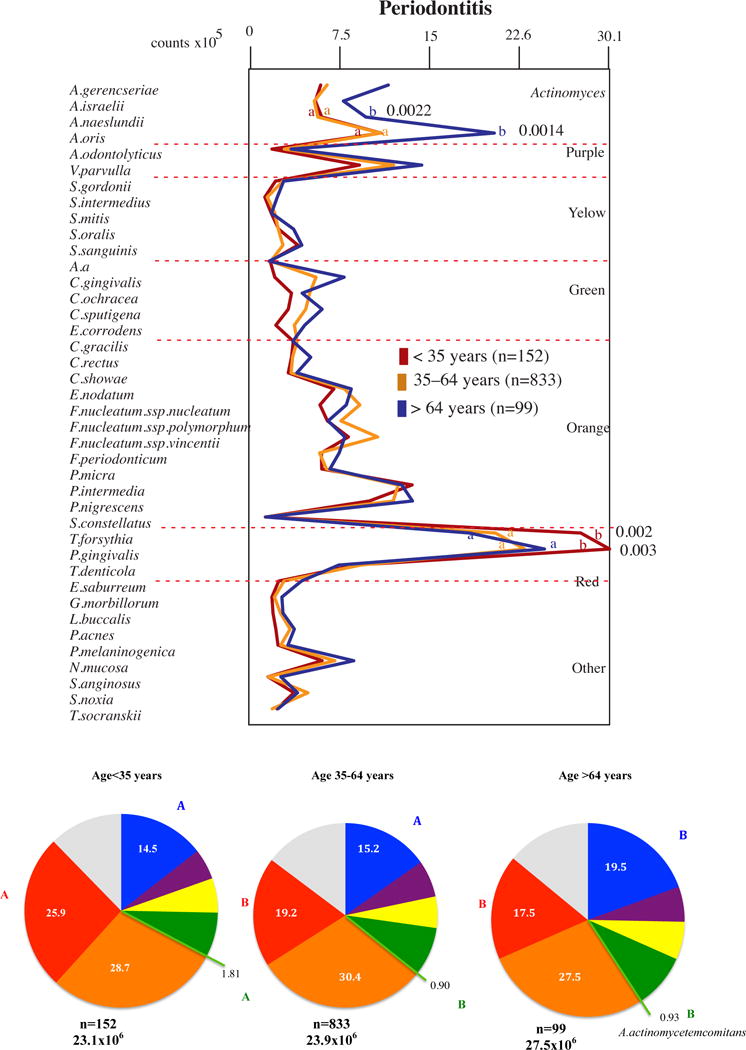
Profiles of the mean counts (×105) of 40 taxa in subgingival biofilm samples taken from 1084 subjects with periodontitis subset into three age groups (<35, 35–64 and >64 years of old). Nine subgingival biofilm samples were taken from each subject and were analyzed separately to determine their content of the 40 species of bacteria. The species were ordered and grouped according to the microbial complexes described by Socransky et al. [89]. The mean values for each species were averaged within a subject and then across subjects in each age group. The colors in the pie charts represent the different complexes. Actinomyces spp. are represented in blue and A. actinomycetemcomitans in light green. The grey color represents species that did not fall into any complex. The significance of differences among age groups was determined using analysis of covariance (ANCOVA), adjusted for smoking, gender, baseline probing depth and clinical attachment level and geographic population. The significance of differences between pairs of comparisons was determined using Tukey’s multiple comparison test (different letters indicate significant differences between age groups).
Figure 2 display the results for subjects with periodontal disease. It was noted that the levels of two red complex pathogens, P. gingivalis and T. forsythia, were elevated in the young group in comparison with the other two groups (p<0.05), and two species normally associated with periodontal health, A. naeslundii and A. oris, were elevated in the older adults in comparison with youngsters and adults (p<0.05). This same trend was observed when the proportions of the microbial complexes were evaluated. The young group had higher proportions of the red complex pathogens in comparison with the other two groups (p<0.05). Red complex pathogens accounted for 25.9% of the 40 species evaluated in the youngsters, 19.2% in the adults and 17.5% in older adults. On the other hand, older adults harbored the highest mean proportions of the microbial group comprised of four Actinomyces species: Actinomyces gerencseriae, Actinomyces israelii, A. naeslundii and A. oris (p<0.05). Also, the youngsters presented higher proportions A. actinomycetemcomitans in comparison with the other two groups (p<0.05).
The next analysis provides a comparison of the subgingival microbial composition of subjects with periodontal health and disease within each age group (Fig. 3). Overall, the differences between health and periodontitis for the proportions of the 40 bacterial species evaluated were very similar among the three age groups. The proportions of the three red complex pathogens, Eubacterium nodatum and P. intermedia were statistically significantly elevated in the periodontitis subjects of the older adults, adults and young groups, while A. naeslundii and A. oris were elevated in health. A few other species compatible with periodontal health were also elevated in the samples from periodontally healthy individuals in comparison with those with periodontitis, such as Actinomyces odontolyticus and Veillonella parvula in the young group, Capnocytophaga gingivalis in the young and adult groups, and Streptococcus sanguinis in the older adults group.
Figure 3.
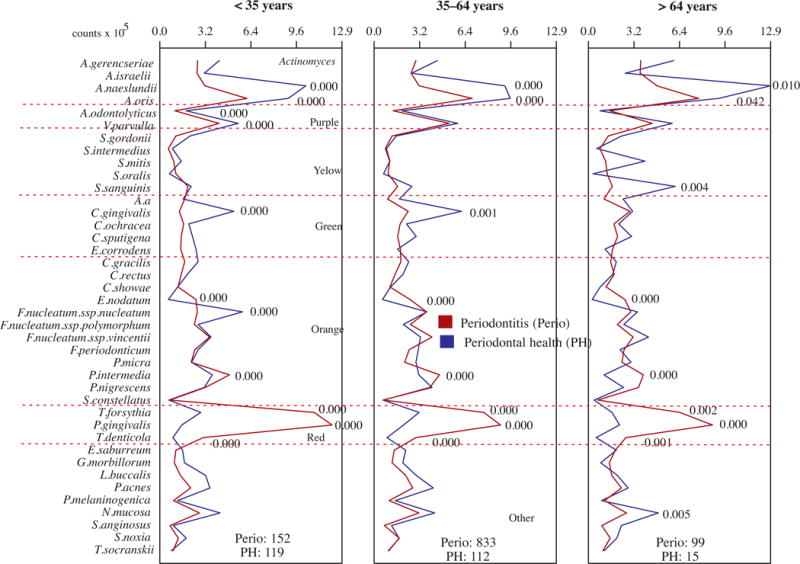
Profiles of the mean counts (×105) of 40 taxa in subgingival biofilm samples taken from 1084 subjects with periodontitis (Perio) and 246 periodontally healthy subjects subset into three age groups (<35, 35–64 and >64 years of old). Nine subgingival biofilm samples were taken from each subject and were analyzed separately to determine their content of the 40 species of bacteria. The species were ordered and grouped according to the microbial complexes described by Socransky et al. [89]. The mean values for each species were averaged within a subject and then across subjects in the two clinical groups for each age category. The significance of differences between clinical groups was determined using analysis of covariance (ANCOVA), adjusted for smoking, gender, baseline probing depth and clinical attachment level and geographic population.
The overall evaluation of the results described above suggest that the main difference observed between the composition of the subgingival microbiota of the older adults and the two other age groups was higher levels and proportions of the Actinomyces species in the group of older individuals (p<0.05). The actual significance of this information is not totally understood, since it seems contradictory to the higher prevalence and severity of periodontitis observed in these subjects, as Actinomyces are healthy associated species (2, 89, 90, 104, 106). It could be speculated that the higher diversity of surfaces found in the oral cavity of older individuals, in comparison to younger individuals, could favor the overgrowth of Actinomyces, which are considered important biofilm formers due to their good adherence properties (100, 113). Therefore, this species might have a selective advantage, over other species, in colonizing the wide variety of surfaces found in the oral cavity of older adults. Previous studies have pointed out Actinomyces as a main genus of the predominant cultivable microbiota of dentures from patients with a healthy mucosa or stomatitis (108, 109). In addition, it has been shown that biofilms on tooth surfaces may harbor higher proportions of Actinomyces compared to soft tissue surfaces (93). Older subjects normally present numerous exposed root surfaces, increasing the area for the development of tooth-associated biofilms. This might have been the case of the older adults group in the present study, which presented greater mean full-mouth CAL than PD, indicating a high number of recessions. Percival et al. (72) reported that Actinomyces species were in statistically significantly higher proportions in the supragingival biofilm of subjects older than 60 years of age. In accord with these findings, Preza et al. (75, 76) reported that Actinomyces species were commonly found in supragingival biofilms adjacent to healthy root surfaces (75). Further, Actinomyces have also been demonstrated to be associated with healthy and carious root surfaces in previous studies (122).
Another interesting observation in this evaluation was the higher levels and proportions of A. actinomycetemcomitans and red complex species in the young group (mostly composed of aggressive periodontitis) in comparison with the other two groups of adults with periodontitis (chronic periodontitis). The finding of higher proportions of A. actinomycetemcomitans in the youngsters is in accord with previous studies in the literature that suggested an important role of this microorganism in the etiology of aggressive periodontitis in young individuals (23, 28, 36, 64, 123). On the other hand, this species seems to have a lower influence on the onset and progression of periodontitis in adults, as previously suggested (23, 78), or in older adults, as shown in the present study. The highest counts and proportions of red complex pathogens in the young group is also in agreement with previous studies that have indicated a possible role of red and orange complex pathogens in the etiology of aggressive periodontitis (16, 23, 33, 36, 54, 101, 119).
Does aging influence the microbial composition of sites with different PDs or of subjects with different extent and severity of disease?
The next question asked was whether aging would influence the microbiota of specific sites or subjects with different severity of disease. Since we did not find main differences between the subgingival microbial profile of the older adults and the other two age groups, we wanted to assess if there were any differences that could be related to a specific category of sites, as the depth of the pocket may influence the local environment and consequently the microbiota. For this analysis we compared the microbial profiles of the three age groups according to different PD categories: shallow, intermediate and deep. These results (Fig. 4) were very similar to those observed when all sites were analyzed together (Fig. 2), namely higher levels of Actinomyces species in the older adults group. Interestingly, these species seemed to be quite well distributed in the oral cavity of the older subjects, as they were statistically significantly elevated in the shallow, intermediate and deep pockets, in comparison with the youngsters and the adults. On the other hand, the red complex species and A. actinomycetemcomitans were statistically significantly elevated in the young group only in the shallow sites. This information might have ecological implications, as it might indicate an important role of these pathogens in initiating the disease process, and not only as merely accessory pathogens that grow in levels and proportions favored by the inflammatory environment associated with periodontitis.
Figure 4.
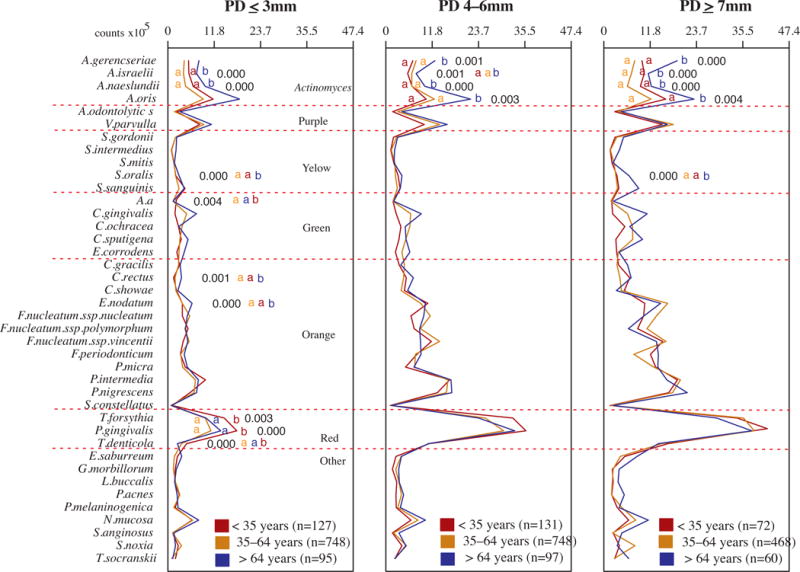
Profiles of the mean counts (×105) of 40 taxa in subgingival biofilm samples taken from 1084 subjects with periodontitis subset into shallow (probing depth (PD) ≤ 3mm), intermediate (PD 4–6mm) and deep (PD ≥ 7mm) sites in different age categories (<35, 35–64 and >64 years of old). Three subgingival biofilm samples of each PD category were taken from each subject and were analyzed separately to determine their content of the 40 species of bacteria. The species were ordered and grouped according to the microbial complexes described by Socransky et al. [89]. The mean values for each species in each PD category were averaged within a subject and then across subjects in the three age groups. The significance of differences among age categories was determined using analysis of covariance (ANCOVA), adjusted for smoking, gender and geographic population. The significance of differences between pairs of comparisons was determined using Tukey’s multiple comparison test (different letters indicate significant differences between age groups).
We also wanted to access any possible difference between the microbial profiles of the different age groups that might have been hidden by the various degree of disease severity of the subjects from each age group. Thus, we subset the subjects according to their disease severity, as follows: i) “the least” severe subgroup: subjects presenting mean PD below the lower quartile of their age group, and ii) “the most” severe subgroup: subjects presenting mean PD above the upper quartile of their age group. The subgroups were compared for the levels of the 40 species evaluated and the results are presented in Fig. 5. Overall, the individuals with most severe disease in each age group had statistically significantly higher levels of red complex pathogens and of a few orange complex species. The young subjects with severe disease individuals also presented statistically significantly higher levels of two Actinomyces and three Streptococcus species in comparison with the youngsters from the least severe subgroup. Two Actinomyces species and two Capnocytophaga species were, respectively, elevated in the subgroups of adults and older adults with severe disease. We also compared the three age groups according to the two disease severity categories (Fig. 6). The comparison among the most severe subgroups showed the same trend observed previously, when all subjects from each age group were compared (Fig. 2), which was higher levels of Actinomyces in the older adults group and higher levels of red complex species in the young group, but these differences were not statistically significant. The smaller sample size in the subgroups might have influenced the lack of statistical significance in the present analysis.
Figure 5.
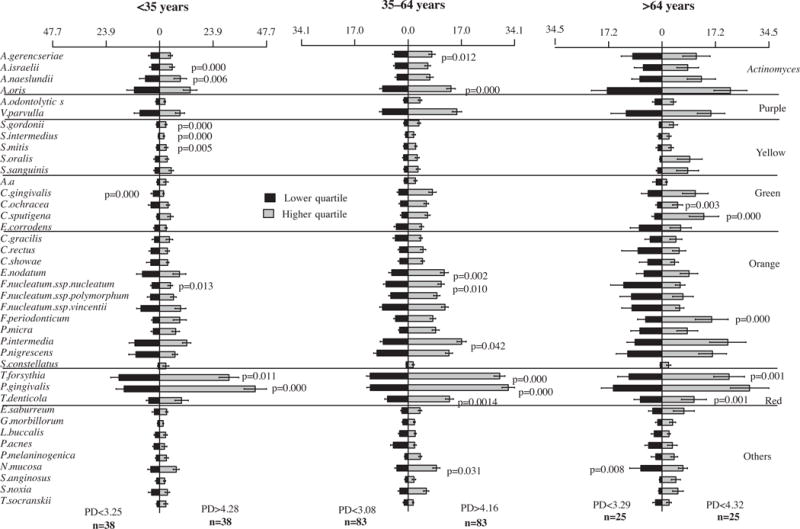
Bars charts (± standard error of the mean) of the mean counts (×105) of 40 taxa in subgingival biofilm samples taken from 292 subjects with periodontitis subset according to two severity categories (“the least” severe and “the most” severe) in different age groups (<35, 35–64 and >64 years old). Nine subgingival biofilm samples were taken from each subject and were analyzed separately to determine their content of 40 species of bacteria. The species were ordered and grouped according to the microbial complexes described by Socransky et al. [89]. The mean values for each species were averaged within a subject and then across subjects. The significance of differences between severity category in each age group was determined using analysis of covariance (ANCOVA), adjusted for smoking, gender and geographic population.
Figure 6.
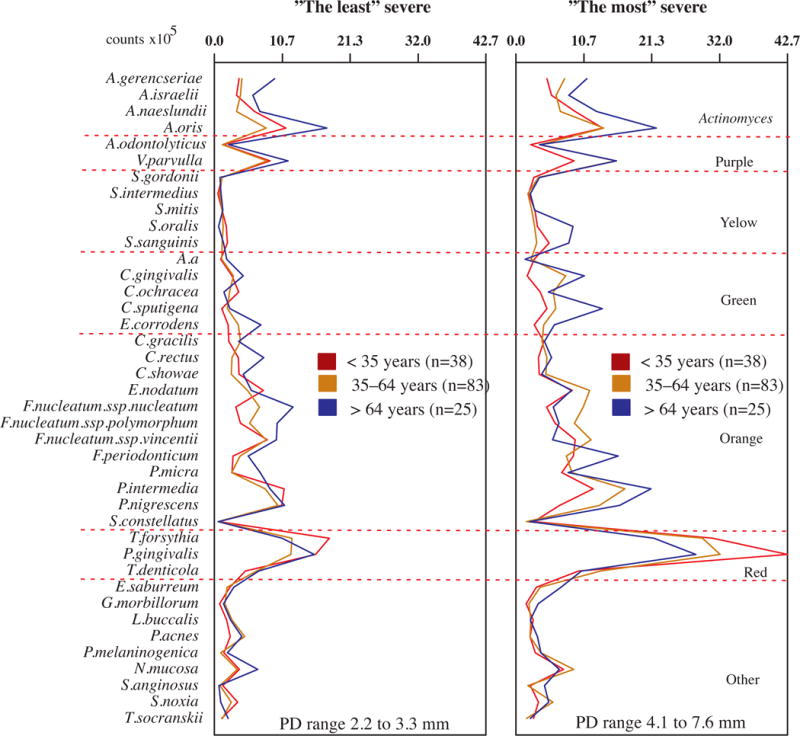
Profiles of the mean counts (×105) of 40 taxa in subgingival biofilm samples taken from 292 subjects with periodontitis subset into three age groups (<35, 35–64 and >64 years old) in different severity categories (“the least” severe and “the most” severe). Nine subgingival biofilm samples were taken from each subject and were analyzed separately to determine their content of 40 species of bacteria. The species were ordered and grouped according to the microbial complexes described by Socransky et al. [89]. The mean values for each species were averaged within a subject and then across subjects in each severity categories. The significance of differences among age groups into each severity category was determined using analysis of covariance (ANCOVA), adjusted for smoking, gender, baseline probing depth and geographic population. The significance of differences between pairs of comparisons was determined using Tukey’s multiple comparison test (different letters indicate significant differences between age groups).
Concluding remarks
The proportion of older adults has increased substantially worldwide in the past decades, and the demands of this growing segment of the population have been influencing science, technology and the health services. The prevention and treatment of oral and systemic health problems in older adults represent a new challenge for researchers and clinicians. Periodontology is one of the specialties of dentistry mostly affected by this new reality, as novel theories suggest the existence of a complex interplay between the immune system of older adults and the microbiota of naturally infected areas of the body, such as the gut and the oral cavity (8, 45, 120). There is growing evidence that the low-grade, chronic, systemic inflammation observed in aging (inflammaging) is an important risk factor for both morbidity and mortality in the elderly people (30), and inflammaging may be potentiated by products produced by the microbial communities existent in the human body, such as the periodontal or gut microbiota, which can reach the circulation (7, 8, 85). Indeed, there is also evidence that periodontitis is associated with elevated levels of systemic pro-inflammatory biomarkers (65, 102) especially in older adults (10). Thus, a deep understanding about the onset, progression and treatment of periodontitis in older adults is crucial for improving the oral and systemic health of these individuals.
One of the theories that has been considered to explain the higher prevalence and/or severity of periodontitis in older adults is a different subgingival microbial profile. However, it is quite surprising to observe that no studies to date have comprehensively evaluated the periodontal microbiota of older adults, and no association studies have directly compared the subgingival microbial profile of older adults with that of periodontal health or disease. Although the majority of the association studies have included older adults in the periodontitis groups, most of the periodontally healthy subjects were younger. Thus, healthy and periodontitis subjects are normally not matched by age in these studies, hampering definitive conclusions about the microbiota associated with periodontal health or disease in older individuals (63).
The analyses presented in this article aimed to provide information about the composition of the subgingival biofilm of older adults, aged 64 years or more, by evaluating data from 1330 subjects with periodontal health or periodontitis from different age groups. It should be underlined that the present study has limitations. First, as most studies that investigated the impact of aging in the local microbiome, it is not a true longitudinal study. Rather it presents a comparison of the snapshots of three different groups of individuals, subset according to randomly determined age brackets. An ideal comparison of the effects of aging in the periodontal microbiome should follow a cohort of individuals over time, where each individual is its own control. However, one can appreciate the huge logistical challenges imposed by such design. Yet, the relevance of these types of analyses highlight the need to create large, curated and standardized biorepository of oral (and other types of) samples from birth cohorts.
Second, the combination of clinical and microbiological data from different research centers or from different studies within each center may present certain constrains. The inclusion criteria may have varied slightly among the various studies, the examiners were not calibrated and there were statistically significant differences between the two centers in terms of the baseline clinical and demographic features. The analyses were adjusted for these differences by using age, gender, mean PD, mean CAL, geographic location and smoking status as covariates.
Third, regarding the microbiological evaluation, this study presents results for the 40 bacterial species proposed by Socransky et al. (89, 92) as the main microorganisms associated with either periodontal health or disease. It is well recognized that the periodontal microbiome encompasses considerably more taxa than those included in this “small” group of bacterial species (21). Nevertheless, this panel of species has been successfully used as a biological marker for studies of periodontal disease risk and treatment in both research centers (23, 25, 26, 27, 40, 41, 88, 91, 95, 104, 106) and by other groups of investigators worldwide for over 20 years (16, 17, 118, 119). Furthermore, many recent studies that have used more comprehensive techniques, such as microarrays (104) and 16S rRNA sequencing (2, 38, 125) to study the oral microbiome have, in general, supported many of the results obtained in earlier studies using checkerboard DNA-DNA hybridization.
In spite of the limitations described above, the present study provides the first comprehensive evaluation of the periodontal microbiota associated with periodontal health or periodontitis in older adults. Overall, the data does not support a substantial influence of aging in the composition of the subgingival microbiota, which is corroborated by a number of earlier studies using the experimental gingivitis model and indirectly supported by recent studies demonstrating the long-term stability of the oral microbiome (1, 12, 18, 31, 49, 97, 116).
The data of the present study suggested that the composition of the subgingival biofilms of older adults with periodontal health and periodontitis is very similar to that of youngsters and adults. In addition, the association study comparing the subgingival biofilm of healthy and periodontitis subjects in the different age groups supported the notion that the already known periodontal pathogens were elevated in disease, in the three groups. The only difference observed in all the analyses, between the microbial composition of older adults and the other two age groups, was higher levels and proportions of Actinomyces in the older subjects. This might be explained by the higher diversity of surfaces and a higher prevalence of exposed roots in these subjects, as Actinomyces species may present a selective advantage over other oral species to colonize the supragingival plaque and prosthesis surfaces. Nonetheless, Actinomyces are considered host-compatible species, and their elevated levels and proportion in older patients might not have major impact in treatment response. Also, when the groups were subset into different severities of disease, the subgingival microbial composition of the older adults showed a similar pattern of that observed in youngsters or adults, which was more strict anaerobe pathogens in subjects with the highest disease severity. Likewise, aging did not seem to influence the microbiological profile of sites with different PDs.
Although the data of this study did not indicate major differences between the subgingival biofilm composition between older adults and the other age groups for the 40 bacterial species evaluated, it is possible that other species of bacteria that were not detected by these DNA probes could have an important role in the disease process in a specific age group. However, in agreement with our data, the results of recent studies using open-ended approaches, which in theory can identify any taxon present in the sample under study also failed to indicate a major role for aging as a determinant of the oral microbiome (12, 18). What is possible though, and has never been investigated, is that the activity of the oral microbiome might be influenced by age. The potential importance of function analysis in the study of the impact of aging in the oral microbiome is supported by two recent benchmark studies evaluating the metatranscriptomics of subgingival biofilm in periodontal health and disease (21) and during periodontal disease progression (121). Those types of studies have opened new venues for the understanding of the mechanisms used by oral bacteria to induce periodontal breakdown and might do the same for the aging process. Thus the oral microbiome transcriptome should be further explored during the process of aging in order to expand the knowledge in this field. For instance, using metagenome function analysis of the gut microbiome, Rampelli et al. (77) recently were able to distinguish groups of young adults, elderly and centenarians, presenting mean age of 30, 70 and 100 years old. It should also be highlighted that their peculiar study population (including individuals over 100 years of age) might have contributed to their ability of find such differences, because the majority of the previous studies did not include a group of extreme aging. Likewise, studying young adults, elderly and centenarians, recently, Biagi et al. (9) showed that the (extreme) ageing process affects the structure of the human gut microbiota, as well as its homeostasis with the host’s immune system. The above-mentioned studies indicate that the centenarians might offer valuable insights that can only be observed in the extremes of the lifespan, and this would be an important group of subjects for future studies.
Finally, future studies should also focus on the interplay between periodontal pathogens/periodontal destruction and the host/immune-response of older individuals, as well as in the most effective preventive and treatment protocols for these subjects. It must be bear in mind that the same infectious burden in an older adult may have worse consequences than in a younger individual. As mentioned before, periodontitis may influence inflammaging, inflammaging may influence periodontitis, or still, the two processes may reinforce and potentiate each other (45). This may have harmful consequences for the patient general health, since inflammaging may increase mortality in older individuals. Indeed, some studies have associated specific segments of the oral microbiota with systemic problems, including dementia and Alzheimer’s disease (14, 83, 124). Therefore, the knowledge of how aging as well as inflammaging influence the microbiomes of older adults could greatly benefit the overall health of these individuals.
Acknowledgments
This work was supported, in part, by research grants 5 R03 TW006269-02 and 1 R01 DE024767-01 from the National Institute of Dental and Craniofacial Research (NIH/NIDCR, USA); by research grants 2007/56413-0, 2007/55291-9, 2009/17677-8, 2010/10384-2 and 2011/23034-2 from São Paulo Research Foundation (FAPESP, Brazil); and research grants 304887/2013-7 309015/2012-0 and 308124/2013-8 from The National Council for Scientific and Technological Development (CNPq, Brazil).
The authors would like to thank Sigmund Socransky, Anne Haffajee and Max Goodson from The Forsyth Institute for designing and guiding the conduction of several of the studies included in the present analyses. The authors would also like to thank Geisla M. Soares and Belén R. Valdes, from Guarulhos University, for their support with the formatting of this manuscript.
References
- 1.Abbas F, van der Velden U, Moorer WR, Everts V, Vroom TM, Scholte G. Experimental gingivitis in relation to susceptibility to periodontal disease. II. Phase-contrast microbiological features and some host-response observations. J Clin Periodontol. 1986;13:551–557. doi: 10.1111/j.1600-051x.1986.tb00846.x. [DOI] [PubMed] [Google Scholar]
- 2.Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–1025. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aimetti M, Perotto S, Castiglione A, Mariani GM, Ferrarotti F, Romano F. Prevalence of periodontitis in an adult population from an urban area in North Italy: findings from a cross-sectional population-based epidemiological survey. J Clin Periodontol. 2015 doi: 10.1111/jcpe.12420. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Awano S, Ansai T, Takata Y, Soh I, Akifusa S, Hamasaki T, Yoshida A, Sonoki K, Fujisawa K, Takehara T. Oral health and mortality risk from pneumonia in the elderly. J Dent Res. 2008;87:334–339. doi: 10.1177/154405910808700418. [DOI] [PubMed] [Google Scholar]
- 6.Bertoldi C, Lalla M, Pradelli JM, Cortellini P, Lucchi A, Zaffe D. Risk factors and socioeconomic condition effects on periodontal and dental health: A pilot study among adults over fifty years of age. Eur J Dent. 2013;7:336–346. doi: 10.4103/1305-7456.115418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biagi E, Candela M, Franceschi C, Brigidi P. The aging gut microbiota: new perspectives. Ageing Res Rev. 2011;10:428–429. doi: 10.1016/j.arr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res. 2013;69:11–20. doi: 10.1016/j.phrs.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bretz WA, Weyant RJ, Corby PM, Ren D, Weissfeld L, Kritchevsky SB, Harris T, Kurella M, Satterfield S, Visser M, Newman AB. Systemic inflammatory markers, periodontal diseases, and periodontal infections in an elderly population. J Am Geriatr Soc. 2005;53:1532–1537. doi: 10.1111/j.1532-5415.2005.53468.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown LF, Beck JD, Rozier RG. Incidence of attachment loss in community-dwelling older adults. J Periodontol. 1994;65:316–323. doi: 10.1902/jop.1994.65.4.316. [DOI] [PubMed] [Google Scholar]
- 12.Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombaugh J, Knights D, Gajer P, Ravel J, Fierer N, Gordon JI, Knight R. Moving pictures of the human microbiome. Genome Biol. 2011;12:R50. doi: 10.1186/gb-2011-12-5-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capsoni S, Carucci NM, Cattaneo A. Pathogen free conditions slow the onset of neurodegeneration in a mouse model of nerve growth factor deprivation. J Alzheimers Dis. 2012;31:1–6. doi: 10.3233/JAD-2012-120427. [DOI] [PubMed] [Google Scholar]
- 14.Cockburn AF, Dehlin JM, Ngan T, Crout R, Boskovic G, Denvir J, Primerano D, Plassman BL, Wu B, Cuff CF. High throughput DNA sequencing to detect differences in the subgingival plaque microbiome in elderly subjects with and without dementia. Investig Genet. 2012;21:19. doi: 10.1186/2041-2223-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 2014;162:22–38. doi: 10.1016/j.imlet.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva-Boghossian CM, do Souto RM, Luiz RR, Colombo AP. Association of red complex, A actinomycetemcomitans and non-oral bacteria with periodontal diseases. Arch Oral Biol. 2011;56:899–906. doi: 10.1016/j.archoralbio.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Dahlen G, Preus HR, Baelum V. Methodological issues in the quantification of subgingival microorganisms using the checkerboard technique. J Microbiol Methods. 2015;110:68–77. doi: 10.1016/j.mimet.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 18.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, Erdman SE, Alm EJ. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15:R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drake CW, Hunt RJ, Beck JD, Zambon JJ. The distribution and interrelationship of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, and BANA scores among older adults. J Periodontol. 1993;64:89–94. doi: 10.1902/jop.1993.64.2.89. [DOI] [PubMed] [Google Scholar]
- 21.Duran-Pinedo AE, Paster B, Teles R, Frias-Lopez J. Correlation network analysis applied to complex biofilm communities. PLoS One. 2011;6:e28438. doi: 10.1371/journal.pone.0028438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faveri M, Figueiredo LC, Duarte PM, Mestnik MJ, Mayer MP, Feres M. Microbiological profile of untreated subjects with localized aggressive periodontitis. J Clin Periodontol. 2009;36:739–749. doi: 10.1111/j.1600-051X.2009.01449.x. [DOI] [PubMed] [Google Scholar]
- 24.Faveri M, Mayer MP, Feres M, de Figueiredo LC, Dewhirst FE, Paster BJ. Microbiological diversity of generalized aggressive periodontitis by 16S rRNA clonal analysis. Oral Microbiol Immunol. 2008;23:112–118. doi: 10.1111/j.1399-302X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 25.Faveri M, Rebello A, de Oliveira Dias R, Borges-Junior I, Duarte PM, Figueiredo LC, Feres M. Clinical and microbiologic effects of adjunctive metronidazole plus amoxicillin in the treatment of generalized chronic periodontitis: smokers versus non-smokers. J Periodontol. 2014;85:581–91. doi: 10.1902/jop.2013.130278. [DOI] [PubMed] [Google Scholar]
- 26.Feres M, Figueiredo LC, Soares GM, Faveri M. Systemic antibiotics in the treatment of periodontitis. Periodontol 2000. 2015;67:131–186. doi: 10.1111/prd.12075. [DOI] [PubMed] [Google Scholar]
- 27.Feres M, Haffajee AD, Allard K, Som S, Socransky SS. Change in subgingival microbial profiles in adult periodontitis subjects receiving either systemically-administered amoxicillin or metronidazole. J Clin Periodontol. 2001;28:597–609. doi: 10.1034/j.1600-051x.2001.028007597.x. [DOI] [PubMed] [Google Scholar]
- 28.Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, McKiernan M, Gunsolley J. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J Clin Microbiol. 2007;45:3859–3869. doi: 10.1128/JCM.00653-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 30.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 31.Fransson C, Berglundh T, Lindhe J. The effect of age on the development of gingivitis. Clinical, microbiological and histological findings. J Clin Periodontol. 1996;23:379–85. doi: 10.1111/j.1600-051x.1996.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 32.Fransson C, Mooney J, Kinane DF, Berglundh T. Differences in the inflammatory response in young and old human subjects during the course of experimental gingivitis. J Clin Periodontol. 1999;26:453–60. doi: 10.1034/j.1600-051x.1999.260707.x. [DOI] [PubMed] [Google Scholar]
- 33.Fritschi BZ, Albert-Kiszely A, Persson GR. Staphylococcus aureus and other bacteria in untreated periodontitis. J Dent Res. 2008;87:589–593. doi: 10.1177/154405910808700605. [DOI] [PubMed] [Google Scholar]
- 34.Gatz M, Mortimer JA, Fratiglioni L, Johansson B, Berg S, Reynolds CA, Pedersen NL. Potentially modifiable risk factors for dementia in identical twins. Alzheimers Dement. 2006;2:110–117. doi: 10.1016/j.jalz.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–1049. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- 36.Gonçalves LFH, Fermiano D, Feres M, Figueiredo LC, Teles FRP, Mayer MPA, Faveri M. Levels of Selenomonas species in genereralized aggressive periodontitis. J Periodontal Res. 2012;47:711–718. doi: 10.1111/j.1600-0765.2012.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graves DT, Jiang Y, Genco C. Periodontal disease: bacterial virulence factors, host response and impact on systemic health. Curr Opin Infect Dis. 2000;13:227–232. doi: 10.1097/00001432-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, Podar M, Leys EJ. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176–1185. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haffajee AD, Bogren A, Hasturk H, Feres M, Lopez NJ, Socransky SS. Subgingival microbiota of chronic periodontitis subjects from different geographic locations. J Clin Periodontol. 2004;31:996–1002. doi: 10.1111/j.1600-051X.2004.00597.x. [DOI] [PubMed] [Google Scholar]
- 40.Haffajee AD, Teles RP, Socransky SS. Association of Eubacterium nodatum and Treponema denticola with human periodontitis lesions. Oral Microbiol Immunol. 2006;21:269–282. doi: 10.1111/j.1399-302X.2006.00287.x. [DOI] [PubMed] [Google Scholar]
- 41.Haffajee AD, Teles RP, Socransky SS. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontol 2000. 2006;42:219–258. doi: 10.1111/j.1600-0757.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 42.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hajishengallis G. Aging and its Impact on Innate Immunity and Inflammation: Implications for Periodontitis. J Oral Biosci. 2014;56:30–37. doi: 10.1016/j.job.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92:485–491. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hillam DG, Hull PS. The influence of experimental gingivitis on plaque formation. J Clin Periodontol. 1977;4:56–61. doi: 10.1111/j.1600-051x.1977.tb01883.x. [DOI] [PubMed] [Google Scholar]
- 48.Holgerson PL, Vestman NR, Claesson R, Ohman C, Domellof M, Tanner AC, et al. Oral microbial profile discriminates breast-fed from formula-fed infants. J Pediatr Gastroenterol Nutr. 2013;56:127–136. doi: 10.1097/MPG.0b013e31826f2bc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holm-Pedersen P, Agerbaek N, Theilade E. Experimental gingivitis in young and elderly individuals. J Clin Periodontol. 1975;2:14–24. doi: 10.1111/j.1600-051x.1975.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 50.Huttner EA, Machado DC, de Oliveira RB, Antunes AG, Hebling E. Effects of human aging on periodontal tissues. Spec Care Dentist. 2009;29:149–155. doi: 10.1111/j.1754-4505.2009.00082.x. [DOI] [PubMed] [Google Scholar]
- 51.Joshi V, Matthews C, Aspiras M, de Jager M, Ward M, Kumar P. Smoking decreases structural and functional resilience in the subgingival ecosystem. J Clin Periodontol. 2014;41:1037–47. doi: 10.1111/jcpe.12300. [DOI] [PubMed] [Google Scholar]
- 52.Kamer AR, Craig RG, Pirraglia E, Dasanayake AP, Norman RG, Boylan RJ, Nehorayoff A, Glodzik L, Brys M, de Leon MJ. TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol. 2009;216:92–97. doi: 10.1016/j.jneuroim.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of tooth loss: a systematic review and metaanalysis. J Dent Res. 2014;93:20S–28S. doi: 10.1177/0022034514537828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Könönen E, Müller HP. Microbiology of aggressive periodontitis. Periodontol 2000. 2014;65:46–78. doi: 10.1111/prd.12016. [DOI] [PubMed] [Google Scholar]
- 55.Kumar PS, Griffen AL, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82:338–344. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- 56.Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43:3944–3955. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Locker D, Slade GD, Murray H. Epidemiology of periodontal disease among older adults: a review. Periodontol 2000. 1998;16:16–33. doi: 10.1111/j.1600-0757.1998.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 58.Makinodan T, Hahn TJ, McDougall S, Yamaguchi DT, Fang M, Iida-Klein A. Cellular immunosenescence: an overview. Exp Gerontol. 1991;26:281–288. doi: 10.1016/0531-5565(91)90021-d. [DOI] [PubMed] [Google Scholar]
- 59.Marsh PD, Percival RS, Challacombe SJ. The influence of denture-wearing and age on the oral microflora. J Dent Res. 1992;71:1374–1381. doi: 10.1177/00220345920710070501. [DOI] [PubMed] [Google Scholar]
- 60.Mason MR, Preshaw PM, Nagaraja HN, Dabdoub SM, Rahman A, Kumar PS. The subgingival microbiome of clinically healthy current and never smokers. ISME J. 2015;9:268–72. doi: 10.1038/ismej.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mestnik MJ, Feres M, Figueiredo LC, Duarte PM, Lira EA, Faveri M. Short-term benefits of the adjunctive use of metronidazole plus amoxicillin in the microbial profile and in the clinical parameters of subjects with generalized aggressive periodontitis. J Clin Periodontol. 2010;37:353–365. doi: 10.1111/j.1600-051X.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- 62.Miklossy J. Alzheimer’s disease - a neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J Neuroinflammation. 2011;8:90. doi: 10.1186/1742-2094-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mombelli A. Aging and the periodontal and peri-implant microbiota. Periodontol 2000. 1998;16:44–52. doi: 10.1111/j.1600-0757.1998.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 64.Moore WE, Holdeman LV, Cato EP, Smibert RM, Burmeister JA, Palcanis KG, Ranney RR. Comparative bacteriology of juvenile periodontitis. Infect Immun. 1985;48:507–519. doi: 10.1128/iai.48.2.507-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann N Y Acad Sci. 2006;1088:251–264. doi: 10.1196/annals.1366.032. [DOI] [PubMed] [Google Scholar]
- 66.Naorungroj S, Slade GD, Beck JD, Mosley TH, Gottesman RF, Alonso A, Heiss G. Cognitive decline and oral health in middle-aged adults in the ARIC study. J Dent Res. 2013;92:795–801. doi: 10.1177/0022034513497960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.National institute on aging. 2015 https://www.nia.nih.gov/ (June 30th 2015)
- 68.Newman MG, Grinenco V, Weiner M, Angel I, Karge H, Nisengard R. Predominant microbiota associated with periodontal health in the aged. J Periodontol. 1978;49:553–559. doi: 10.1902/jop.1978.49.11.553. [DOI] [PubMed] [Google Scholar]
- 69.Noble JM, Borrell LN, Papapanou PN, Elkind MS, Scarmeas N, Wright CB. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J Neurol Neurosurg Psychiatry. 2009;80:1206–1211. doi: 10.1136/jnnp.2009.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Novak MJ, Potter RM, Blodgett J, Ebersole JL. Periodontal disease in Hispanic Americans with type 2 diabetes. J Periodontol. 2008;79:629–636. doi: 10.1902/jop.2008.070442. [DOI] [PubMed] [Google Scholar]
- 71.Paganini-Hill A, White SC, Atchison KA. Dentition, dental health habits, and dementia: The LeisureWorld Cohort Study. J Am Geriatr Soc. 2012;60:1556–1563. doi: 10.1111/j.1532-5415.2012.04064.x. [DOI] [PubMed] [Google Scholar]
- 72.Percival RS, Challacombe SJ, Marsh PD. Age-related microbiological changes in the salivary and plaque microflora of healthy adults. J Med Microbiol. 1991;35:5–11. doi: 10.1099/00222615-35-1-5. [DOI] [PubMed] [Google Scholar]
- 73.Pérez-Chaparro PJ, Gonçalves C, Figueiredo LC, Faveri M, Lobão E, Tamashiro N, Duarte P, Feres M. Newly identified pathogens associated with periodontitis: a systematic review. J Dent Res. 2014;93:846–858. doi: 10.1177/0022034514542468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peterson CT, Sharma V, Elmén L, Peterson SN. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol. 2015;179:363–377. doi: 10.1111/cei.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Preza D, Olsen I, Willumsen T, Boches SK, Cotton SL, Grinde B, Paster BJ. Microarray analysis of the microflora of root caries in elderly. Eur J Clin Microbiol Infect Dis. 2009;28:509–517. doi: 10.1007/s10096-008-0662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Preza D, Olsen I, Willumsen T, Grinde B, Paster BJ. Diversity and sitespecificity of the oral microflora in the elderly. Eur J Clin Microbiol Infect Dis. 2009;28:1033–1040. doi: 10.1007/s10096-009-0743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rampelli S, Candela M, Turroni S, Biagi E, Collino S, Franceschi C, O’Toole PW, Brigidi P. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging (Albany NY) 2013;5:902–912. doi: 10.18632/aging.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodenburg JP, van Winkelhoff AJ, Winkel EG, Goené RJ, Abbas F, de Graff J. Occurrence of Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in severe periodontitis in relation to age and treatment history. J Clin Periodontol. 1990;17:392–399. doi: 10.1111/j.1600-051x.1990.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 79.Rüdiger Sg, Carlén A, Meurman JH, Kari K, Olsson J. Dental biofilms at healthy and inflamed gingival margins. J Clin Periodontol. 2002;29:524–530. doi: 10.1034/j.1600-051x.2002.290609.x. [DOI] [PubMed] [Google Scholar]
- 80.Salvi GE, Kandylaki M, Troendle A, Persson GR, Lang NP. Experimental gingivitis in type 1 diabetics: a controlled clinical and microbiological study. J Clin Periodontol. 2005a;32:310–316. doi: 10.1111/j.1600-051X.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 81.Salvi GE, Ramseier CA, Kandylaki M, Sigrist L, Awedowa E, Lang NP. Experimental gingivitis in cigarette smokers: a clinical and microbiological study. J Clin Periodontol. 2005b;32:441–447. doi: 10.1111/j.1600-051X.2005.00691.x. [DOI] [PubMed] [Google Scholar]
- 82.Savitt ED, Kent RL. Distribution of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis by subject age. J Periodontol. 1991;62:490–494. doi: 10.1902/jop.1991.62.8.490. [DOI] [PubMed] [Google Scholar]
- 83.Shoemark DK, Allen SJ. The microbiome and disease: reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. J Alzheimers Dis. 2015;43:725–738. doi: 10.3233/JAD-141170. [DOI] [PubMed] [Google Scholar]
- 84.Simón-Soro A, Mira A. Solving the etiology of dental caries. Trends Microbiol. 2015;23:76–82. doi: 10.1016/j.tim.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 85.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Slots J, Feik D, Rams TE. Actinobacillus actinomycetemcornitans and Bacteroides intermedius in human periodontitis: age relationship and mutual association. J Clin Periodontol. 1990a;17:659–662. [PubMed] [Google Scholar]
- 87.Slots J, Feik D, Rams TE. Age and sex relationships of superinfecting microorganisms in periodontitis patients. Oral Microbiol Immunol. 1990b;5:305–308. doi: 10.1111/j.1399-302x.1990.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 88.Soares GM, Mendes JA, Silva MP, Faveri M, Teles R, Socransky SS, Wang X, Figueiredo LC, Feres M. Metronidazole alone or with amoxicillin as adjuncts to non-surgical treatment of chronic periodontitis: a secondary analysis of microbiological results from a randomized clinical trial. J Clin Periodontol. 2014;41:366–376. doi: 10.1111/jcpe.12217. [DOI] [PubMed] [Google Scholar]
- 89.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 90.Socransky SS, Haffajee AD, Dzink JL. Relationship of subgingival microbial complexes to clinical features at the sampled sites. J Clin Periodontol. 1988;15:440–444. doi: 10.1111/j.1600-051x.1988.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 91.Socransky SS, Haffajee AD, Teles R, Wennstrom JL, Lindhe J, Bogren A, Hasturk H, van Dyke T, Wang X, Goodson JM. Effect of periodontal therapy on the subgingival microbiota over a 2-year monitoring period. I. Overall effect and kinetics of change. J Clin Periodontol. 2013;40:771–780. doi: 10.1111/jcpe.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 93.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 94.Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. “Checkerboard” DNA-DNA hybridization. Biotechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- 95.Socransky SS, Haffajee AD, Smith C, Martin L, Haffajee JA, Uzel NG, Goodson JM. Use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral Microbiol Immunol. 2004;19:352–362. doi: 10.1111/j.1399-302x.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 96.Sparks Stein P, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E, Dawson D., 3rd Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement. 2012;8:196–203. doi: 10.1016/j.jalz.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stahringer SS, Clemente JC, Corley RP, Hewitt J, Knights D, Walters WA, et al. Nurture trumps nature in a longitudinal survey of salivary bacterial communities in twins from early adolescence to early adulthood. Genome Res. 2012;22:2146–2152. doi: 10.1101/gr.140608.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun study. J Am Dent Assoc. 2007;138:1314–1322. doi: 10.14219/jada.archive.2007.0046. [DOI] [PubMed] [Google Scholar]
- 99.Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takahashi N, Washio J, Mayanagi G. Metabolomics of supragingival plaque and oral bacteria. J Dent Res. 2010;89:1383–1388. doi: 10.1177/0022034510377792. [DOI] [PubMed] [Google Scholar]
- 101.Takeuchi Y, Umeda M, Ishizuka M, Huang Y, Ishikawa I. Prevalence of periodontopathic bacteria in aggressive periodontitis patients in a Japanese population. J Periodontol. 2003;74:1460–1469. doi: 10.1902/jop.2003.74.10.1460. [DOI] [PubMed] [Google Scholar]
- 102.Teeuw WJ, Slot DE, Susanto H, Gerdes VE, Abbas F, D’Aiuto F, Kastelein JJ, Loos BG. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. 2014;41:70–79. doi: 10.1111/jcpe.12171. [DOI] [PubMed] [Google Scholar]
- 103.Teixeira SR, Mattarazo F, Feres M, Figueiredo LC, de Faveri M, Simionato MR, Mayer MP. Quantification of Porphyromonas gingivalis and fimA genotypes in smoker chronic periodontitis. J Clin Periodontol. 2009;36:482–487. doi: 10.1111/j.1600-051X.2009.01411.x. [DOI] [PubMed] [Google Scholar]
- 104.Teles R, Teles F, Frias-Lopez J, Paster B, Haffajee A. Lessons learned and unlearned in periodontal microbiology. Periodontol 2000. 2013;62:95–162. doi: 10.1111/prd.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Teles RP, Gursky LC, Faveri M, Rosa EA, Teles FRF, Feres M, Socransky SS, Haffajee AD. Relationships between subgingival microbiota and GCF biomarkers in generalized aggressive periodontitis. J Clin Periodontol. 2010;37:313–323. doi: 10.1111/j.1600-051X.2010.01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teles RP, Haffajee AD, Socransky SS. Microbiological goals of periodontal therapy. Periodontol 2000. 2006;42:180–218. doi: 10.1111/j.1600-0757.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 107.The Human Microbiome. 2015 http://hmpdacc.org/overview/about.php (June 30th 2015)
- 108.Theilade E, Budtz-Jorgensen E, Theilade J. Predominant cultivable microflora of plaque on removable dentures in patients with healthy oral mucosa. Arch Oral Biol. 1983;28:675–680. doi: 10.1016/0003-9969(83)90101-2. [DOI] [PubMed] [Google Scholar]
- 109.Theilade E, Budtz-Jorgensen E. Predominant cultivable microflora of plaque on removable dentures in patients with denture-induced stomatitis. Oral Microbiol Immunol. 1988;3:8–13. doi: 10.1111/j.1399-302x.1988.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 110.Theilade E, Wright WH, Jensen SB, Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–13. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]
- 111.Trombelli L, Scapoli C, Orlandini E, Tosi M, Bottega S, Tatakis DN. Modulation of clinical expression of plaque-induced gingivitis. III. Response of “high responders” and “low responders” to therapy. J Clin Periodontol. 2004;31:253–9. doi: 10.1111/j.1600-051X.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 112.Trombelli L, Scapoli C, Tatakis DN, Grassi L. Modulation of clinical expression of plaque-induced gingivitis: effects of personality traits, social support and stress. J Clin Periodontol. 2005;32:1143–1150. doi: 10.1111/j.1600-051X.2005.00835.x. [DOI] [PubMed] [Google Scholar]
- 113.Uzel NG, Teles FR, Teles RP, Song XQ, Torresyap G, Socransky SS, Haffajee AD. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J Clin Periodontol. 2011;38:612–620. doi: 10.1111/j.1600-051X.2011.01730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van der Velden U, Abbas F, Hart AA. Experimental gingivitis in relation to susceptibility to periodontal disease. (I.) Clinical observations. J Clin Periodontol. 1985;12:61–68. doi: 10.1111/j.1600-051x.1985.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 115.Vos T, Flaxman AD, Naghavi M, Lozano R, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Winkel EG, Abbas F, Van der Velden U, Vroom TM, Scholte G, Hart AA. Experimental gingivitis in relation to age in individuals not susceptible to periodontal destruction. J Clin Periodontol. 1987;14:499–507. doi: 10.1111/j.1600-051x.1987.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 117.World Health Organization; US National Institute of Aging. Global health and ageing. 2011 Oct;:32. http://www.who.int/ageing/publications/global_health.pdf?ua=1.
- 118.Xajigeorgiou C, Sakellari D, Slini T, Baka A, Konstantinidis A. Clinical and microbiological effects of different antimicrobials on generalized aggressive periodontitis. J Clin Periodontol. 2006;33:254–264. doi: 10.1111/j.1600-051X.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 119.Ximenez-Fyvie LA, Almaguer-Flores A, Jacobo-Soto V, Lara-Cordoba M, Moreno-Borjas JY, Alcantara-Maruri E. Subgingival microbiota of periodontally untreated Mexican subjects with generalized aggressive periodontitis. J Clin Periodontol. 2006;33:869–877. doi: 10.1111/j.1600-051X.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- 120.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yost S, Duran-Pinedo AE, Teles R, Krishnan K, Frias-Lopez J. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. 2015;7:27. doi: 10.1186/s13073-015-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zambon JJ, Kasprzak SA. The microbiology and histopathology of human root caries. Am J Dent. 1995;8:323–328. [PubMed] [Google Scholar]
- 123.Zambon JJ, Slots J, Genco RJ. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect Immun. 1983;41:19–27. doi: 10.1128/iai.41.1.19-27.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zapata HJ, Quagliarello VJ. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc. 2015;63:776–781. doi: 10.1111/jgs.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou Y, Gao H, Mihindukulasuriya KA, LaRosa PS, Wylie KM, Vishnivetskaya T, et al. Biogeography of the ecosystems of the healthy human body. Genome Biol. 2013;14:R1. doi: 10.1186/gb-2013-14-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]


