Abstract
Introduction
Contemporary state-of-the-art management of cancer is increasingly defined by individualized treatment strategies. For very rare tumors, like hepatoblastoma, the development of biologic markers, and the identification of reliable prognostic risk factors for tailoring treatment, remains very challenging. The Children's Hepatic tumors International Collaboration (CHIC) is a novel international response to this challenge.
Methods
Four multicenter trial groups in the world, who have performed prospective controlled studies of hepatoblastoma over the past two decades (COG; SIOPEL; GPOH; and JPLT), joined forces to form the CHIC consortium. With the support of the data management group CINECA, CHIC developed a centralized online platform where data from eight completed hepatoblastoma trials were merged to form a database of 1605 hepatoblastoma cases treated between 1988 and 2008. The resulting dataset is described and the relationships between selected patient and tumor characteristics, and risk for adverse disease outcome (event-free survival; EFS) are examined.
Results
Significantly increased risk for EFS-event was noted for advanced PRETEXT group, macrovascular venous or portal involvement, contiguous extrahepatic disease, primary tumor multifocality and tumor rupture at enrollment. Higher age (≥8 years), low AFP (<100 ng/ml) and metastatic disease were associated with the worst outcome.
Conclusion
We have identified novel prognostic factors for hepatoblastoma, as well as confirmed established factors, that will be used to develop a future common global risk stratification system. The mechanics of developing the globally accessible web-based portal, building and refining the database, and performing this first statistical analysis has laid the foundation for future collaborative efforts. This is an important step for refining of the risk based grouping and approach to future treatment stratification, thus we think our collaboration offers a template for others to follow in the study of rare tumors and diseases.
Keywords: Hepatoblastoma, Pathology, Risk stratification, Chemotherapy
1. Introduction
Hepatoblastoma is a rare pediatric tumor, and even though it remains the most common liver tumor in children, with an increasing annual incidence of 1.2–1.5/million population/year, even major tertiary children's cancer centers may encounter only 1 or 2 newly diagnosed patients a year [1] Historically, four separate cooperative multicenter trial groups have undertaken the systematic treatment studies of this disease: the International Childhood Liver Tumour Strategy Group (SIOPEL); the Children's Oncology Group (COG), and its legacy groups the Children's Cancer Group (CCG) and the Pediatric Oncology Group (POG); the German Society for Pediatric Oncology and Haematology (GPOH); and the Japanese Study Group for Pediatric Liver Tumors (JPLT) [2]. Although each of these groups has made significant contributions to improving the treatment and outcomes for patients with hepatoblastoma, they have developed and used disparate, yet variably overlapping, staging systems. This fact has made any outcome and prognostic factor comparisons between groups challenging and unreliable.
Each of the four cooperative groups have collected data that have been used to relate patient characteristics to disease outcome. Common poor prognostic factors, such as metastatic disease at diagnosis, have been identified across groups [3–6]. Some risk factors achieved statistical significance in certain cooperative group studies, while remaining non-significant in others [3–6]. Other potential risk factors were postulated, but due to low patient numbers, never achieved statistical significance [5, 6]. The Children's Hepatic tumors International Collaboration (CHIC) was pursued specifically to address this challenge by combining the data of published clinical trials that were started on or after 1988 to reflect the results of prospective clinical trials that employ modern therapeutic approaches to the treatment of hepatoblastoma from which prognostic factors, related to either disease, host or treatment, could be established.
Efforts to identify such prognostic factors in the setting of a rare tumor have been hampered by the extensive fragmentation of relatively small cohorts of patients. Classical rare cancers definitions are based on their prevalence, which does not properly reflect their true health burden: the Italian TREP project defined pediatric rare tumors as “any malignancy characterized by an annual incidence less than 2/million” [7]. Moreover, these definitions do not reflect the added complexity of studying rare tumors in children, where individual treatment centers often treat limited numbers of patients annually. One potential response to this challenge is the organization of global networks of researchers sharing data collaboratively.
2. Methods
2.1. Strategy for worldwide collaboration and definitions used
As the result of collaborative decisions four groups (SIOPEL, COG, GPOH and JPLT) embarked on an effort to merge the data collected in the conduct of eight clinical trials into one database for analysis. Trials, conducted between 1989 and 2008 and included in this database, are presented in Table 1 [8–12], [13–15,16,17]. All data on 1605 subjects were obtained through the studies which were IRBs approved. Ongoing trials, where results were not yet published at the time the database was constructed, were not included. A memorandum of understanding was signed in March 2011. This memorandum provides the formal framework for the organization and work of CHIC, which is governed by the CHIC Steering Committee (CHIC-SC). This consisted of four multidisciplinary representatives – one each oncologist, surgeon, pathologist, and statistician – from each of the four participating multicenter trial groups. The steering committee reviewed the data from the groups' published clinical trials, as described below, which were and will be included in the common database (`CHIC database').
Table 1.
Multicenter trials included in CHIC database.
| Study | Reference | Number of patients | Enrollment (mm/yyyy) |
Event status |
Median follow upa (Range; years) | Number alive at last contact | |
|---|---|---|---|---|---|---|---|
| Start | End | No event | |||||
| HB 89 | VonSchweinitz 1995 [14] | 72 | 3/1988 | 10/1993 | 53 | 4.7 (1.6–5.7) | 56 |
| HB 99 | Haeberle 2003 [15] | 141 | 1/1999 | 12/2008 | 103 | 5.4 (1.5–10.6) | 110 |
| INT0098 | Ortega 2000 [11] | 170 | 8/1989 | 12/1992 | 108 | 10.3 (0.9–19.2) | 120 |
| JPLT 1 | Sasaki 2002 [16] | 106 | 12/1990 | 11/1997 | 72 | 5.7 (0.9–16.8) | 79 |
| JPLT 2 | Hishiki 2011 [17] | 298 | 4/1999 | 12/2010 | 212 | 4.0 (0.2–12.5) | 243 |
| P9645 | Malogolowkin 2006 [18]; Katzenstein 2009 [13] | 277 | 4/1999 | 11/2006 | 190 | 7.9 (0–11.7) | 219 |
| SIOPEL 2 | Perilongo 2004 [8] | 135 | 11/1995 | 5/1998 | 97 | 7.4 (0.2–9.4) | 100 |
| SIOPEL 3 | Perilongo 2009 [9] | 406 | 7/1998 | 12/2006 | 319 | 5.0 (0.2–10.9) | 334 |
| Overall | 1605 | 3/1988 | 12/2010 | 1154 | 5.9 (0–19.2) | 1271 | |
For patients without an EFS-event.
2.2. Data compilation and central data portal
Key clinical variables were selected based on availability, relevance, and ability to be re-coded into a common format. CHIC contracted with CINECA (supercomputing center run by a non-profit consortium of Italian Universities and the Italian Ministry of Universities and Research; www.cineca.it) for hosting and managing access to the integrated database. Once the data were uploaded, the CHIC database was populated with the merged datasets of all the data variables selected by the CHIC steering committee.
PRETEXT definition was based on the one used in the SIOPEL group publications [6,8–10,19]. The vessel involvement was defined in all studies as involvement (tumor in the vessel) but not only compression of the main vena cava or all three liver veins, in case of the portal vein it concerned either both branches or the main portal vein. Tumor rupture was not specifically defined in any of the included studies, however its recognition was based upon imaging and clinical symptoms.
Although the SIOPEL PRETEXT groups and PRETEXT annotations (VPERF) were not used by the German, American, and Japanese trial groups until recently, we have chosen to use the PRETEXT system to define the extent of tumor burden at diagnosis for the cohesiveness and cross study comparability of the all of the data. Because, this terminology has been adopted gradually across the different study groups over the past fifteen years, some studies (SIOPEL 2,3,4 and GPOH HB 99) have used this terminology for staging at the outset of their study and some studies have not (INT 0098, COG 9645, GPOH 94, JPLT 1 and JPLT 2). Fortunately, all of these studies required on-study prospective data collection of ALL of the data elements needed to retrospectively assign a PRETEXT group and the PRETEXT annotation factors (VPERF). Specifically, there were data fields asking for the presence or absence of tumor in the right posterior section; right anterior section; left medial section; left lateral section. By knowing how many contiguous liver sections were reported as tumor free the PRETEXT group can by assigned retrospectively. Moreover, the CRF for COG contained a diagram asking the institution to draw the extent of tumor involvement in the liver at diagnosis and this diagram was able to be cross referenced retrospectively with the report of which sections were involved for data consistency. Finally, radiology reports were submitted and could be cross referenced with both the on-study report of sections involved and with the diagram, again to insure data consistency and accuracy. To assign the PRETEXT annotation factors (V,P,E,R,F) we were able to reference a specific “yes” or “no” checklist for tumor involvement of the diaphragm, kidney, adrenal, colon, hepatic vein, inferior vena cava, porta hepatis. There was a specific question “yes” or “no” for tumor around the vena cava or portal vein. The drawing required the surgeon to draw the tumor at diagnosis, if multiple nodules were drawn this was multifocal, but for the data inclusion in the CHIC database it was considered multifocal, only if the drawing and the on study radiographic report listed multiple primary tumors in the liver. The data sheets also included a “yes” or “no” for metastasis to the lungs; or other. Some studies were more inclusive of the “other” non-lung metastatic sites than others and because this data was not uniformly reported in all of the studies we were not able to include this in the collective database as a variable. Several other data fields were also included on the on study CRF's of the different studies but those detailed here were the ones needed to assign the PRETEXT group and PRETEXT annotation factors. The information was reviewed centrally with the original MRI or CT report to be certain it was reported with consistency. If on careful data review there were datapoints reported with any discrepancies, it was rejected for entry into the CHIC database and listed as “missing”.
Data quality standards were developed by the CHIC-SC and applied to the aggregated database. Inconsistencies were identified and brought to the attention of the data management teams from each of the respective coordinating groups. Any inconsistencies that could not be resolved to the satisfaction of the CHIC-SC were considered missing in the final dataset.
2.3. Analysis of prognostic factors and statistical methods
The analysis reported herein focuses specifically on patient and tumor characteristics present at the time of diagnosis. The list of candidate variables was chosen based on the experience of the four participating multi center trial groups and their prior individual efforts at risk stratification. Table 2 contains the list of potential prognostic factors and separates them into factors present at diagnosis and those present only as a response to treatment. The steering committee chose to focus as a first step on those variables present at diagnosis. The factors representing quality of response to treatment in the second column will be the target of a future phase of this collaboration.
Table 2.
Potential prognostic factors at diagnosis in hepatoblastoma.
| Pre-treatment at diagnosisc |
|---|
| PRETEXT (Ia, IIa, III, IVb) |
| Metastasis at diagnosisb |
| Unresectable vessel involvement (+V, +P) |
| Extrahepatic tumor extension (+E) |
| Multifocal tumor (+F) |
| Tumor rupture at enrollment (+R) |
| Initial AFP level |
| Pathologic subtypec (Pure fetal, Small cell undifferentiated) |
| Age |
| Birth weight |
| Co-morbidity (Beckwith–Wiedemann, prematurity, low birth weight) |
Event-free survival (EFS) was chosen as the outcome endpoint, and defined as the interval from enrollment date as used by the respective trial protocol to the date of first occurrence of progression, relapse, diagnosis of a second malignant neoplasm, death from any cause or last patient contact whichever occurred first. Patients who were alive, progression free and SMN free at the time of last contact were considered censored; all other patients were considered to have experienced an EFS-event. The definitions of progression or relapse differed slightly between groups, and were not altered for this analysis.
In 214 patients (13%), the PRETEXT group was either not assigned at the time of patient enrollment or was unavailable for analysis due to insufficient documentation for the assignment. In SIOPEL 2, SIOPEL 3, HB 99, and JPLT 2 PRETEXT group was assigned at enrollment, whereas in HB 89, INT 0098, P9645, and JPLT 1 the PRETEXT group was assigned retrospectively. Retro PRETEXT was assigned to all patients in these studies based upon retrospective review of both the diagnostic imaging reports and the surgeon's report form completed at the time of study enrollment in which the liver sections involved and extent of extrahepatic spread were identified (the PRETEXT annotations V,P,E,M,C,F,R). Retro PRETEXT was assigned if both the surgeon's report form and the radiographic imaging were present, sufficiently detailed to assign a group, and agreed with each other. Table 3 shows the definitions of the PRETEXT groups and annotations for extent of extrahepatic spread [19].
Table 3.
Definitions of PRETEXT/POST-TEXT group (I, II, II, IV) and PRETEXT grouping system annotations V,P,E,M,C,F,N,R.
| PRETEXT/POST-text group | Definition |
|---|---|
| I | One liver section involved |
| Three adjoining sections are tumor free | |
| II | One or two liver sections involved |
| Two adjoining sections are tumor free | |
| III | Two or three liver sections involved |
| One adjoining section is tumor free | |
| IV | Four liver sections involved |
| Annotation: | |
| V | Venous involvement, V, denotes vascular involvement of the retrohepatic vena cava or involvement of ALL THREE major hepatic veins (right, middle, and left) |
| P | Portal involvement, P, denotes vascular involvement of the main portal vein and/or BOTH right and left portal veins |
| E | Extrahepatic involvement of a contiguous structure such as the diaphragm, abdominal wall, stomach, colon, etc. |
| M | Distant metastatic disease (usually lungs, occasionally bone or brain) |
| C | Caudate lobe |
| F | Multifocal tumor nodules |
| N | Lymph node involvement |
| R | Tumor rupture |
Because, the presence or absence of macroscopic venous involvement was not centrally reviewed, the exact definitions may have varied slightly over time and across individual treatment centers and trials. Vascular and extrahepatic involvement (the PRETEXT annotations V,P,E,M,C,F,R) at diagnosis were based upon radiographic evidence. “V″ denotes tumor involvement of the retrohepatic inferior vena cava and/or major hepatic veins. “P″ denotes tumor involvement of either the main portal venous bifurcation and/or both right and left portal veins. “E″ denotes contiguous extrahepatic spread to diaphragm, stomach, bowel, etc. “M″ stands for distant metastasis, “C” – for caudate tumor, “F” – for primary tumor with multifocal sites within the liver, and “R” – for tumor rupture at enrollment.
EFS as a function of time since enrollment was estimated by the method of Kaplan and Meier [19]. Equality of the EFS hazard rate was compared across groups defined by the particular patient characteristic using the log rank statistic [20] Relative hazard rates (RHRs) were estimated using proportional hazards regression with the variable of interest as the only component of the model [20] Since, this was an exploratory analysis, two-sided p-values were used. A p-value of 0.05 or less was considered indicative of a significant result.
2.4. Web portal
A designated internet portal (www.siopel.org) was developed in order to establish a Pediatric Liver Cancer Web Community Area used to work online on the CHIC project, as well as to access and to share information pertaining to meetings, conferences, documents and research proposals.
3. Results
The spectrum of patients' demographics and univariate survival analysis of the prognostic variables at diagnosis included in the CHIC dataset is presented in Table 4. The Kaplan-Meier plots for factors considered significantly related to prognosis are presented in Figs. 1–9.
Table 4.
Prognostic variables with univariate survival analysis.
| Patient feature | Characteristic | Value | Percent of Total (Percent of Non-Missing Values) | Relative risk | P-Value |
|---|---|---|---|---|---|
| Age at start treatment (Years) | Mean | 2.1 | |||
| Median | 1.4 | ||||
| Range | 0–15.5 | ||||
| ≤2 Year | 1318 | 82 | 1.0 | <0.0001 | |
| 3–7 | 281 | 14 | 1.6 | ||
| ≥8 | 69 | 4.0.3 | 3.3 | ||
| Patient sex | Female | 639 | 40 | 1.0 | 0.41 |
| Male | 966 | 60 | 1.1 | ||
| Prematurity | No | 818 | 79 | 1.0 | 0.46 |
| Yes | 217 | 21 | 1.1 | ||
| Missing | 570 | – | |||
| Birth weight | 1500 g or more | 719 | 97 | 1.0 | 0.83 |
| Less than 1500 g | 25 | 3.4 | 1.1 | ||
| Missing | 861 | – | |||
| Beckwith–Wiedemann syndrome | No | 999 | 97 | 1.0 | |
| Yes | 29 | 2.8 | 0.97 | 0.93 | |
| Missing | 577 | ||||
| Metastatic disease at diagnosis (M) | No | 1320 | 83 | 1.0 | |
| Yes | 277 | 17 | 3.8 | <0.0001 | |
| Missing | 8 | ||||
| AFP (ng/nl) | 1000–1 × 106 | 971 | 77 | 1.0 | <0.0001 |
| >1 × 106 | 120 | 9.5 | 1.4 | ||
| 100–999 | 110 | 8.7 | 1.7 | ||
| −99 | 65 | 5.1 | 4.3 | ||
| Missing | 319 | ||||
| PRETEXT staging | I | 97 | 6 | 1.0 | <0.0001 |
| II | 529 | 34 | 1.4 | ||
| III | 621 | 40 | 2.4 | ||
| IV | 297 | 19 | 4.8 | ||
| Missing | 61 | – | |||
| Hepatic venous involvement (V) | No | 1386 | 90 | 1.0 | <0.0001 |
| Yes | 147 | 9.5 | 2.2 | ||
| Missing | 72 | ||||
| Portal venous involvement (P) | No | 1387 | 90 | 1.0 | <0.001 |
| Yes | 146 | 10 | 2.3 | ||
| Missing | 72 | ||||
| Extrahepatic tumor extension (E) | No | 1529 | 96 | 1.0 | 0.0013 |
| Yes | 71 | 4 | 1.9 | ||
| Missing | 5 | ||||
| Multifocal primary tumor (F) | No | 1295 | 18 | 1.0 | <0.0001 |
| Yes | 280 | 82 | 2.3 | ||
| Missing | 30 | ||||
| Tumor rupture at enrollment (R) | No | 1440 | 95 | 1.0 | <0.0001 |
| Yes | 69 | 5 | 2.1 | ||
| Missing | 96 |
Fig. 1.
Outcome by age.
Fig. 9.
Outcome by rupture at enrollment.
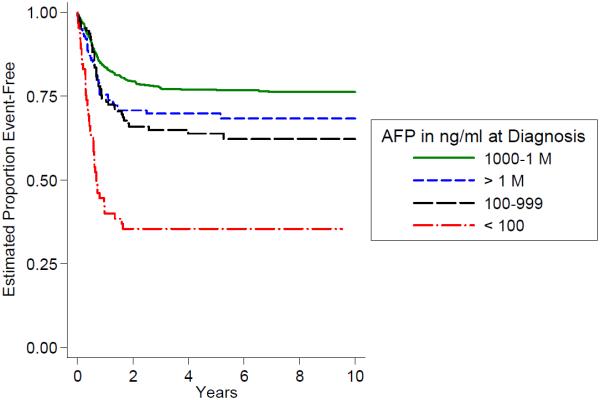
In our cohort less than 20% of patients presented with metastatic disease which was strongly associated with poor outcome (Fig. 2). Low AFP serum levels at diagnosis (<100 ng/ml) were even less frequently encountered (below 5%), however they carried similarly poor prognosis (Fig. 3). High serum AFP levels in excess of 1 million ng/ml were also associated with elevated risk (Fig. 3).
Fig. 2.
Outcome by metastasis AT diagnosis.
Fig. 3.
Outcome by serum AFP AT diagnosis.
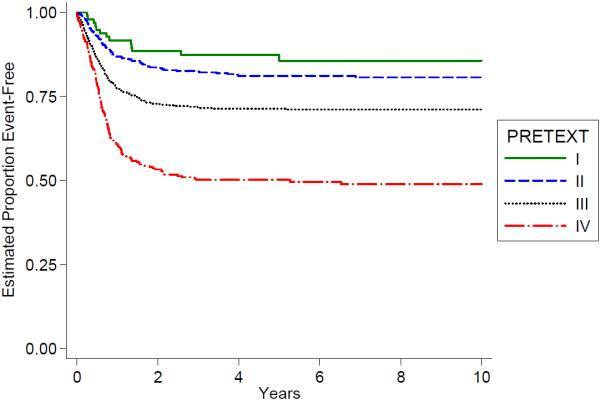
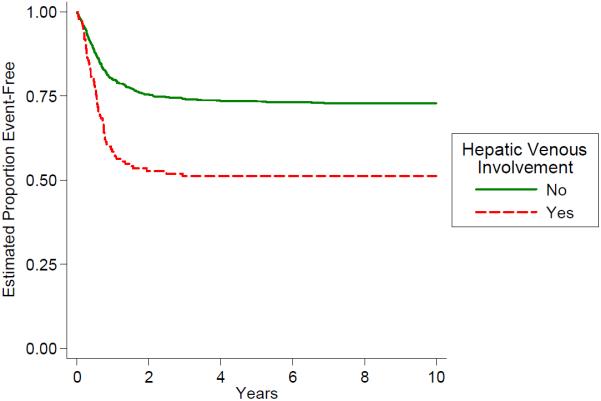
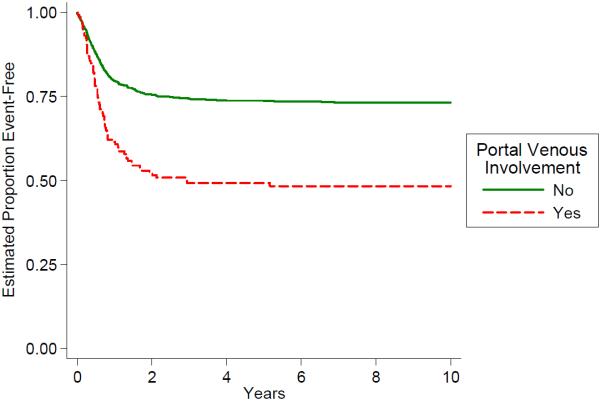
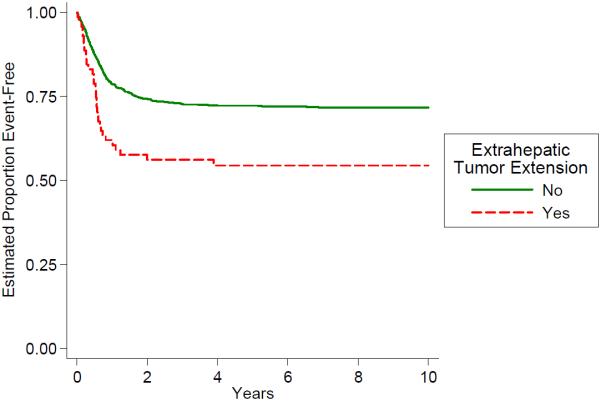
Distribution of the PRETEXT groups and the PRETEXT annotations V,P,E,F,R is shown in Table 4. The PRETEXT annotation “M” for metastatic disease is discussed above. The PRETEXT annotation “C” was not independently analyzed because a lack of consistent reporting. We found that PRETEXT grouping itself and all of the PRETEXT annotations analyzed for prognostic significance were very predictive of outcome as shown in Figs. 4–9 and Table 4.
Fig. 4.
Outcome by PRETEXT.
There were additional potential risk factors, postulated by prior smaller single group analyses, which did not achieve statistical significance following univariate analysis in this large retrospective database. These included: sex, prematurity, extremely low birth weight (<1500 g), and the presence of Beckwith–Wiedemann syndrome. Conversely, the impact of patient age on outcome, with several different age groups analyzed (data not shown), proved to be important with a common pattern of worsening prognosis with age in excess of 2 years.
4. Discussion
The clinical research in rare pediatric tumors constitutes a significant challenge. This effort requires the creation of large common databases, and the development of a uniform scientific language which leads to the standardization of risk criteria and patient stratification. However, this alone may be insufficient as global cooperation is limited by financial constraints and busy agendas of researchers. Thus, novel e-environments need to be constructed to facilitate cooperation at the global level and enable e-learning and web-based consultation services in order to meet increasing expectations of patients and their families for access to high levels of medical expertise [21].
The reported collaboration was promoted first by the open exchange of information and ideas from the leaderships of all study groups involved. This was greatly facilitated by holding the formal and smaller informal meetings whose agendas were entirely devoted to liver tumors. This helped to build a mutual trust that provided an environment which fostered the identification of common problems and key questions in risk stratification and treatment approaches. At the same time, we increasingly realized the limitations for further progress in a rare tumor setting if we did not pursue a common agenda. Based on this attitude, the framework on collaboration was developed that included a structured approach to data aggregation, analyses and future usage.
The key component of this research endeavor was the development of a collaborative environment focused on pediatric liver tumors. Our ability to make analytic progress was predicated by acknowledgment of key questions and organization of several face-to-face meetings and brainstorming sessions followed by thorough effort planning. Modern communication tools significantly augmented but did not replace these traditional face-to-face meetings.
Previous cooperative group studies have identified such variables as PRETEXT, presence of metastatic disease, and very low serum AFP (<100 ng/ml) as poor prognostic factors [21,4–6]. Other variables such as spontaneous tumor rupture at enrollment, tumor multifocality, macroscopic vascular tumor invasion, contiguous extrahepatic tumor extension, age at diagnosis, very high or borderline low AFP levels (100–999 ng/ml), have been suggested as poor prognostic factors but their prognostic significance has not been consistently shown [3,5,21]. We hypothesized that this was largely due to the relative decreased statistical power in prior analyses, compared to the statistical power of this large database.
We made a conscious decision to exclude pathologic subtypes from this initial risk factor analysis which may be viewed as a limitation. This decision was based on the fact, that historically the different trial groups have used disparate definitions and reporting categories to describe the histology of hepatoblastoma. For example, completely resected at diagnosis “pure fetal histology” HB tumors were shown in COG studies INT0098 and P9645 to convey decreased risk for death EFS-event [12]. However, this histologic subtype is recognized only within the COG studies and not within the other multicenter trial groups. Hence, before considering histology in a global risk analysis, the four major international study groups had to agree upon the categories and common definitions of the histologic subtypes. This parallel project of the CHIC group was recently published [22]. Now that common globally agreed upon definitions and reporting categories are in place, the CHIC group is currently engaged in the retrospective review of all available pathologic slides for patients in the CHIC database. This first required the ambitious effort of digitalizing and uploading thousands of slides to the central web-based interactive pathology review platform developed in collaboration with CINECA. As results of the central CHIC pathology review become available, they will be added to the CHIC database and interrogated for prognostic significance.
We have to admit that a certain weakness of the reported analysis is its retrospective nature. An inherent problem of the retrospective approach taken is the fact that it was based on data collection and data definitions used internally throughout different studies at various points in time. We have been cognizant of the challenge of data accuracy from the outset and, hence, have gone to great lengths to retroactively recode the on study information into common terminology with as much accuracy as possible. Nevertheless, the current findings and hypotheses generated need to be ultimately verified by a prospective approach within a new international multi-group study.
Future multivariate analyses are planned with the goal to create an internationally accepted risk stratification scheme for hepatoblastoma at diagnosis. A primary goal of this effort is directed to better define prognostic factors in hepatoblastoma with a goal of personalization of therapy, in part through the future development of a common risk stratification scheme. The CHIC Steering Committee recognizes that this effort constitutes only an initial step. The participating groups have committing to adding to the CHIC database the results of more recent trials, such as SIOPEL 4 and 6, and COG-AHEP0731 once those studies are published. These additional data will provide a platform to validate the results reported herein. Other new possibilities emerged in parallel with this effort include creation of the web platform for collaborative review of pathology and histologic tumor subtypes, as well as future development of a real-time international collaborative web-based consultation service for difficult cases.
Finally, we consider the CHIC database a valuable resource to be used by all researchers interested in pediatric liver tumors. Thus, we are providing a platform to access the CHIC database for the testing of novel hypotheses. This will be accomplished through web portal application process supported by CINECA. Thus, we believe that this effort represents a template for others to follow in the research of rare tumors, both pediatric and adult, in an era of ever increasing goals of personalized medicine.
Fig. 5.
Outcome by primary tumor multifocality.
Fig. 6.
Outcome by hepatic venous involvement.
Fig. 7.
Outcome by portal venous involvement.
Fig. 8.
Outcome by extrahepatic tumor extension.
Acknowledgments
We are greatly indebted to the significant contributions from all of the co-authors who, as a group, comprise the CHIC steering committee. The dedicated time of statistical analysis from our statisticians, data management from our colleagues at CINECA, and clinical guidance from our oncologists, surgeons, and pathologists has fostered a true collaborative spirit of collective effort. B Haeberle and E Hiyama, in particular, should be recognized as co-first authors for their extensive contributions to the preparation of the manuscript, and leadership of the German and Japanese data contributions respectively.
The research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7/2007–2013) under the project ENCCA, grant agreement n° 261474, from the COG Foundation through a COG Curesearch grant contributed by the Hepatoblastoma Foundation, from the Madeleine Schickedanz Children's Cancer Foundation, and by the Japanese Grant in Aids for Scientific Research (A) (22390328 and 20406028) from the Ministry of Education, Culture, Sports, Science of Japan.
Footnotes
Conflict of interest statement To be confirmed.
References
- [1].Darbari A, Sabin KM, Shapiro CN, Schwarz KB. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology. 2003;38:560–6. doi: 10.1053/jhep.2003.50375. [DOI] [PubMed] [Google Scholar]
- [2].Perilongo G, Malogolowkin M, Feusner J. Hepatoblastoma clinical research: lessons learned and future challenges. Pediatr Blood Cancer. 2012;59:818–21. doi: 10.1002/pbc.24217. [DOI] [PubMed] [Google Scholar]
- [3].Fuchs J, Rydzynski J, von Schweinitz D, Bode U, Hecker H, Weinel P, et al. Pretreatment prognostic factors and treatment results in children with hepatoblastoma. A report of the German Cooperative Paediatric Liver Tumor Study HB-94. Cancer. 2002;95:172–82. doi: 10.1002/cncr.10632. [DOI] [PubMed] [Google Scholar]
- [4].Aronson DC, Schnater JM, Staalman CR, Weverling GJ, Plaschkes J, Perilongo G, et al. Predictive value of the Pretreatment extent of disease system in hepatoblastoma: results from the international society of pediatric oncology liver tumor study group SIOPEL-1 study. J Clin Oncol. 2005;23:1245–52. doi: 10.1200/JCO.2005.07.145. [DOI] [PubMed] [Google Scholar]
- [5].Meyers RL, Rowland JR, Krailo M, Chen Z, Katzenstein HM, Malogolowkin MH. Pretreatment prognostic factors in hepatoblastoma: a report of the Children's Oncology Group. Pediatr Blood Cancer. 2009;53:1016–22. doi: 10.1002/pbc.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maibach R, Roebuck D, Brugieres L, Capra M, Brock P, Dall'Igna P, et al. Prognostic stratification for children with hepatoblastoma: the SIOPEL experience. Eur J Cancer. 2012;48:1543–9. doi: 10.1016/j.ejca.2011.12.011. [DOI] [PubMed] [Google Scholar]
- [7].Ferrari A, Bisogno G, De Salvo GL, Indolfi P, Perilongo G, Cecchetto G, Italian Study on Rare Tumours in Paediatric Age (TREP) Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) The challenge of very rare tumours in childhood: the Italian TREP project. Eur J Cancer. 2007;43:654–9. doi: 10.1016/j.ejca.2006.08.028. [DOI] [PubMed] [Google Scholar]
- [8].Perilongo G, Shafford E, Maibach R, Aronson D, Brugières L, Brock P, et al. Risk adapted treatment for childhood hepatoblastoma; final report of the second study of the International Society of Paediatric Oncology – SIOPEL 2. Eur J Cancer. 2004;40:411–21. doi: 10.1016/j.ejca.2003.06.003. [DOI] [PubMed] [Google Scholar]
- [9].Perilongo G, Maibach R, Shafford E, Brugieres L, Brock P, Morland B, et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med. 2009;361:1662–70. doi: 10.1056/NEJMoa0810613. [DOI] [PubMed] [Google Scholar]
- [10].Zsiros J, Maibach R, Shafford E, Brugieres L, Brock P, Czauderna P, et al. Successful treatment of childhood high-risk hepatoblatoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol. 2010;28:2584–90. doi: 10.1200/JCO.2009.22.4857. [DOI] [PubMed] [Google Scholar]
- [11].Ortega JA, Douglass EC, Feusner JH, Reynolds M, Quinn JJ, Finegold MJ, et al. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: a report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol. 2000;18:2665–75. doi: 10.1200/JCO.2000.18.14.2665. [DOI] [PubMed] [Google Scholar]
- [12].Malogolowkin MH, Katzenstein HM, Meyers RL, Krailo MD, Rowland JM, Haas J, et al. Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children's Oncology Group. J Clin Oncol. 2011;29:3301–6. doi: 10.1200/JCO.2010.29.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Katzenstein HM, Chang KW, Krailo M, Chen Z, Finegold MJ, Rowland J, et al. Amifostine does not prevent platinum-induced hearing loss associated with the treatment of children with hepatoblastoma: a report of the Intergroup Hepatoblastoma Study P9645 as a part of the Children's Oncology Group. Cancer. 2009;115:5828–35. doi: 10.1002/cncr.24667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Von Schweinitz D, Hecker H, Harms D, Bode U, Weinel P, Bürger D, et al. Complete resection before development of drug resistance is essential for survival from advanced hepatoblastoma — a report from the German cooperative pediatric liver tumor study HB-89. J Pediatr Surg. 1995;30:845–52. doi: 10.1016/0022-3468(95)90762-9. [DOI] [PubMed] [Google Scholar]
- [15].Haeberle B, Bode U, von Schweinitz D. Differentiated treatment protocols for high- and standard-risk hepatoblastoma e an interim report of the German liver tumor study HB99. Klin Padiatr. 2003;215:159–65. doi: 10.1055/s-2003-39375. [DOI] [PubMed] [Google Scholar]
- [16].Sasaki F, Matsunaga T, Iwafuchi M, Hayashi Y, Ohkawa H, Ohira M, et al. Outcome of hepatoblastoma treatment with JPLT-1 protocol-1: a report from the Japanese study group for pediatric liver tumor. J Pediatr Surg. 2002;37:851–6. doi: 10.1053/jpsu.2002.32886. [DOI] [PubMed] [Google Scholar]
- [17].Hishiki T, Matsunaga T, Sasaki F, Yano M, Ida K, Horie H, et al. Outcome of hepatoblastoma treated using the Japanese Study Group for Pediatric Liver Tumor (JPLT) protocol- 2: report from the JPLT. Pediatr Surg Int. 2011;27:1–8. doi: 10.1007/s00383-010-2708-0. [DOI] [PubMed] [Google Scholar]
- [18].Malogolowkin MH, Katzenstein HM, Krailo M, Chen Z, Bowman L, Reynolds M, et al. Intensified platinum therapy is an ineffective strategy for improving outcome in pediatric patients with advanced hepatoblastoma. J Clin Oncol. 2006;24:2879–84. doi: 10.1200/JCO.2005.02.6013. [DOI] [PubMed] [Google Scholar]
- [19].Roebuck DJ, Aronson D, Clapuyt P, Czauderna P, de Ville de Goyet J, Gauthier F. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol. 2007;37:123–32. 1096–1100. doi: 10.1007/s00247-006-0361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. John Wiley and Sons; New York: 2002. [Google Scholar]
- [21].Brown J, Perilongo G, Shafford E, Keeling J, Pritchard J, Brock P, et al. Pretreatment prognostic factors for children with hepatoblastoma – results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer. 2000;36:1418–25. doi: 10.1016/s0959-8049(00)00074-5. [DOI] [PubMed] [Google Scholar]
- [22].Lopez-Terrada D, Alaggio R, DeDavila MT, Czauderna P, Hiyama E, Katzenstein H, et al. Towards an international pediatric liver tumor consensus classification: Proceedings of the Los Angeles COG International Pathology Pediatric Liver Tumors Symposium. Modern Pathology. 2014;26:19–22. doi: 10.1038/modpathol.2013.80. [DOI] [PubMed] [Google Scholar]
- [23]. [last accessed August 2014];SIOPEL website. www.siopel.org.
- [24]. [last accessed August 2014];CINECA website. www.cineca.it.
- [25].Surveillance of Rare Cancers in Europe (RARECARE) www.rarecarenet.eu.