Abstract
Liver ischemia reperfusion activates innate immune system to drive the full development of inflammatory hepatocellular injury. Damage-associated molecular patterns (DAMPs) stimulate myeloid and dendritic cells via pattern recognition receptors (PRRs) to initiate the immune response. Complex intracellular signaling network transduces inflammatory signaling to regulate both innate immune cell activation and parenchymal cell death. Recent studies have revealed that DAMPs may trigger not only proinflammatory, but also immune regulatory responses by activating different PRRs or distinctive intracellular signaling pathways or in special cell populations. Additionally, tissue injury milieu activates PRR-independent receptors which also regulate inflammatory disease processes. Thus, the innate immune mechanism of liver IRI involves diverse molecular and cellular interactions, subjected to both endogenous and exogenous regulation in different cells. A better understanding of these complicated regulatory pathways/network is imperative for us in designing safe and effective therapeutic strategy to ameliorate liver IRI in patients.
Introduction
Ischemia reperfusion injury (IRI) causes organ dysfunction and failure after liver surgery and represents a major risk factor in the development of both acute and chronic graft rejection in liver transplantation. Importantly, it is the limiting factor in the utility of marginal or extended criteria donor organs, which are highly susceptible to IRI, contributing to severe organ shortages. Thus, effective therapeutics targeting liver IRI will not only improve patient’s outcome, but also expand donor pool for liver transplantation. Despite its apparent clinical significance, studies of liver IRI remains insufficient and mechanisms are only partially understood. No clinical applicable therapeutics are currently available.
Based on ischemia conditions, IRI can be categorized into 2 types. The “warm” IRI develops during liver surgery and various forms of shock and trauma. The “cold” ischemia occurs during liver graft preservation and is accompanied with additional warm ischemia during liver transplant operation. Although susceptible cellular targets of the 2 IRI types may differ, they share a common mechanism in the disease pathophysiology, ie, inflammatory immune activation. Although the initial organ damage due to ischemia results from oxygen and nutrient deprivations, the subsequent and more extensive injury during reperfusion is driven by tissue inflammation. It has become clear that vertebrates utilize the same sentinel innate immune receptor systems, pattern recognition receptors (PRRs), in response to both microbial invasion and tissue damages via pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), respectively. Liver is a unique organ in our body with the highest content of tissue resident macrophages, ie, Kupffer cells, dendritic cells (DCs) and NK/NKT/T cells. They function together with infiltrating myeloid and lymphoid cells to respond to DAMP stimulations. The resulting inflammatory milieu further recruits and activates innate as well as adaptive immune cells to amplify the response. Interestingly, hepatocytes represent not only immune effector targets, but also active participants of tissue inflammatory response during liver IRI. This review aims to summarize recent progress in our understanding of the innate immune mechanism of liver IRI, with emphasis on its activation, effector mechanisms, and regulation. Cell types discussed include KCs, infiltrating macrophages, DCs, hepatocytes, hepatic stellate cells (HSC), γδT and iNK T cells.
Innate Immune Activation
Six different classes of PPRs have been identified thus far. Toll-like receptors (TLRs) and C-type lectin receptors (CLRs) are membrane-bound receptors; Retinoic acid-inducible gene (RIG)-I-like receptors (RLRs), NOD-like receptors (NLRs), absent in melanoma 2 (AIM2)-like receptors (ALRs) and relatively new oligoadenylate synthetase (OAS)-like receptors (OLRs) are cytoplasmic receptors 1. PRRs recognize PAMPs, conserved molecular structures that are shared by a large number of pathogens, as well as host-derived DAMPs, which include cell wall components, nucleic acids, and cell metabolites. In liver IRI, high mobility group box 1 (HMGB1) and histone/DNA complex are best characterized DAMPs, which activate TLR4, TLR9 and NLRP3. Although these PRRs are expressed predominantly in myeloid and DCs, parts of their functions may also be executed by tissue parenchymal cells.
(i) HMGB1-TLR4 and beyond
TLR4 was the first PRR identified as the trigger of liver immune activation against IR by studying its gene knock-out (KO) animal models 2,3. Furthermore, enhanced TLR4 activation was implicated to be responsible for the increased susceptibilities of steatotic livers to IRI in both animal models and clinical patients 4,5. Recently, the utility of TLR4 targeted therapy was demonstrated in an animal model in which TLR4 antagonist eritoran tetrasodium effectively attenuated liver IRI 6. Although initial study indicated that TLR4 signaling in liver non-parenchyma (NPCs) rather than parenchyma cells was more important in the innate immune activation by IR 7, analysis of cell-type specific TLR4 gene knock-out (KO) mice has found that hepatocyte TLR4 facilitated the release of HMGB1 from hypoxic hepatocytes, which constituted the major source of circulating HMGB1 after liver IR 8. HMGB1 was the key DAMP in liver inflammation and injury in response to IR 9. As a chromatin protein, HMGB1 acts as an DAMP only when passively released from necrotic cells 10 or actively secreted from immune-competent cells upon stimulations 11. Hypoxic hepatocytes actively secreted HMGB1 via TLR4-dependent reactive oxygen species (ROS) generation12. In cell-type specific HMGB1 gene KO mice, it was demonstrated that hepatocyte-, but not bone marrow-derived HMGB1 is required for sterile inflammation following injury 13. Additionally, HMGB1 seemed to play a role in hepatocyte homeostasis during IRI. Hepatocyte-specific HMGB1 gene deletion exacerbated liver injury by increasing hepatocyte death, ROS production, and release of histones14, which then promoted liver inflammation via TLR9 activation. HMGB1 activated multiple types of cells via different receptors. TLR4 on KCs/macrophages was shown to be the receptor for HMGB1 in liver IRI at the early stage (6h post reperfusion)9,12. A more recent study revealed that HMGB1 also triggered recruitment of neutrophils, but not macrophages, through receptors for advanced glycation end product (RAGE), toward necrosis, at the late (24h), but not early (6h), stage of liver IRI 13.
HMGB1 is a redox-sensitive protein. It contains 3 conserved cysteine residues (at position 23, 45, and 106) and their redox status dictates its extracellular chemokine- or cytokine-inducing properties. It has been shown that fully reduced (all thiol) HMGB1 binds to CXCL12 and stimulates immune cell infiltration via the CXCR4 receptor; and that only partially oxidized (disulfide) HMGB1, with a Cys23-Cys45 disulfide bond and a reduced Cys106, activates immune cells to produce cytokines/chemokines via the TLR4 receptor. Oxidized HMGB1 (sulfonyl HMGB1) is devoid of chemotactic and cytokine activities 15,16. The disulfide HMGB1 was the dominant extracellular form in liver IRI or APAP overdose 13. Studies have shown that myeloid differentiation factor 2 (MD-2), an extracellular TLR4 adaptor, was required for TLR4 recognition of HMGB1 by binding specifically to the cytokine-inducing disulfide isoform of HMGB1 (Fig. 1). MD-2 specific antagonist was able to disrupt the HMGB1-MD2, but preserve the LPS-MD2, interaction and ameliorate APAP-mediated toxicity, liver IRI, and sepsis mortality in vivo 17.
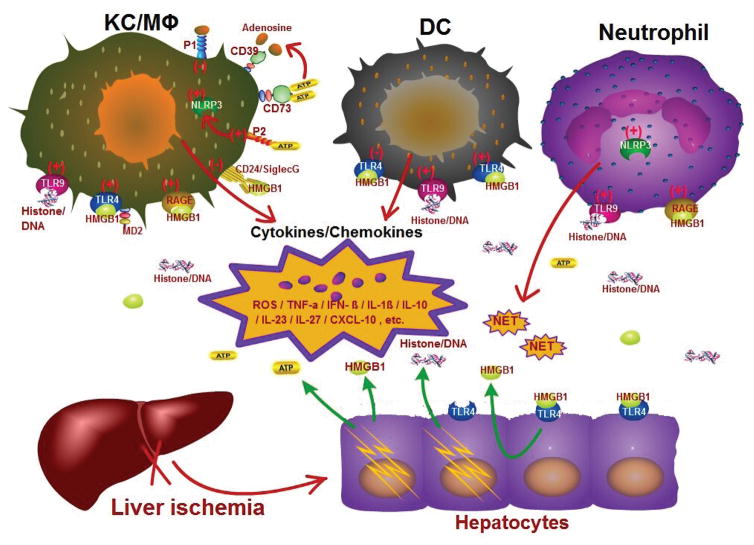
Fig. 1.
Innate immune activation and regulation - A multi-cellular and -molecular interaction network. Liver innate immune activation involves multiple cells, including Kupffer cells/macrophages, neutrophils, dendritic cells, as well as hepatocytes. Initial hepatocellular damages due to ischemia result in releases of extracellular HMGB1, histone/DNA and ATP. These DAMPs stimulate different cells via TLR4/MD2, RAGE (by HMGB1) and TLR9, NLRP3 (by histone/DNA, ATP) to activate (+) or inhibit (−) liver inflammatory immune responses, leading to productions of cytokines/chemokines, ROSs, DAMPs (HMGB1, ATP) and cytotoxic molecules, including NETs by neutrophils. The proinflammatory milieu recruits and activate more innate and adaptive immune cells into inflamed livers and causes further hepatocellular damages; while immune regulatory milieu, such as IL-10, inhibits inflammation. Hepatocytes actively secrete HMGB1 upon TLR4 stimulation or IR stress. Resident and infiltrating DCs/macrophages respond to DAMPs differently. Rather than proinflammatory activation, resident DCs/macrophages produce IL-10 to inhibit tissue inflammation. Cells also upregulate CD24/Siglec, purinergic receptors (P1/P2) and CD39/CD73 upon inflammatory stimulation, which play immune regulatory roles in liver inflammation. CD39/CD73 converts ATP into adenosine, which binds to P1 receptors to facilitate M2 macrophage activation.
HMGB1 signals through multiple receptors with distinctive functional effects. In addition to TLR4, RAGE, CD24/Siglec-10, and T cell immunoglobulin and mucin-domain containing-3 (TIM-3) have also been shown to bind to this particular DAMP 16. In liver IRI, the involvement of RAGE has been documented in both partial warm (above) 13 and total ischemia models 18,19. Blockade of RAGE signaling by using soluble RAGE (extracellular ligand-binding domain of RAGE)18 or RAGE gene deletion19 alleviated organ damages and promoted liver regeneration. Two functional mechanisms downstream of RAGE activation were demonstrated important for the disease pathogenesis, Egr-1-mediated MIP2 expression, and Egr-1-independent TNF-a production and apoptosis. We have found in a liver partial ischemia model that RAGE antagonist peptides or shRNA protected livers from early stage IRI only in hyperglycemic, but not normal, hosts20. Although functional roles of CD24/Siglec in liver IRI remain to be determined, CD24 deficient mice suffered significantly higher levels of tissue damage in an acetaminophen (AAP)-induced liver injury model by increasing inflammatory cytokine/chemokine production, indicating its negative regulatory roles in liver innate immune responses. The study further showed that HMGB1 released from necrotic hepatocytes ligated CD24 and transduces a signaling to Src-homology 2 domain (SH2)-containing protein tyrosine phosphatase 1 (SHP-1), a negative regulator of NF-kB, via Siglec G (or 10 in human) 21. Most interestingly, CD24 was shown to bind to only DAMPs, but not PAMPs, which acted as a discriminating mechanism to downregulate immune response to non-infectious self, but not infectious non-self. Roles of TIM-3 in liver IRI have been documented in both partial warm ischemia and transplantation models 22–24. The blockade of TIM-3 signaling with Abs exacerbated liver inflammation and injury; while TIM-3 over expression in its transgenic animals protected livers from IRI. However, mechanistic focus in these studies was on its function to downregulate CD4 T cell activation. The question of whether the HMGB1-TIM-3 interaction dampens liver innate immune response has yet to be addressed.
(ii) NLRP, ALR and RLRs
As critical innate immune system receptors and sensors, inflammasomes are intracellular multi-protein complexes, consisting of NLRs, ALRs and the adaptor protein apoptosis-associated specklike protein (ASC), which regulate the activation of caspase-1. Caspase 1 is required for the production of interleukin (IL)-1β, IL-18 and IL-33 25, all of which have been implicated in the pathogenesis of liver IRI 26,27,28. Although functional roles of nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) in sterile liver inflammatory injury have been documented, the underlying mechanism remains controversial. It was shown initially that NLRP3 deficient mice developed similar levels of liver inflammation and injury induced by hemorrhagic shock and trauma. Caspase-1 deficient mice, in fact, suffered more severe liver damage, with higher levels of inflammatory cytokine production and neutrophil influx, as compared with WT mice 29. In contrast, in a murine partial ischemia model, short hairpin (sh) RNA-mediated NLRP3 gene knock-down inhibited proinflammatory cytokine productions and HMGB1 release, and protected livers from IRI 30. More recent studies showed that NLRP3 and caspase-1 knockout mice were protected from liver IRI, and that extracellular histones activated the NLRP3 inflammasome through TLR9-dependent generation of ROS 31. Furthermore, ASC deficiency and IL-1β neutralization were also shown to ameliorate liver damages against IR by downregulating HMGB1 activities 32. Biochemical evidence of inflammasome activation in livers by IR was provided by the result of immunoprecipitation of inflammasome components 33. IR induced not only upregulations of protein levels of NLRP3 and ASC, but more importantly their physical association. Pannexin-1 and cathepsin B were shown to be required for inflammasome activation following liver IR, as well as AIM2. AIM2 is a cytosolic protein which has a C-terminal DNA binding domain and an N-terminal pyrin domain, capable of recognizing dsDNA from both hosts and pathogens. Increases of serum and cytosolic levels of dsDNA were detected after liver IR 33. Thus, multiple pathways may lead to liver inflammasome activation after IR, triggered by diverse alarmins, including ROS, extracellular ATP, and dsDNA. However, inflammasome activation may not be the only functional mechanism downstream of NLRP3 in the disease process. It was shown that NLRP3 promoted liver inflammation and injury independently of inflammasomes by regulating chemokine-mediated functions and recruitment of neutrophils. Livers were not protected in ASC KO or caspase-1 KO, but only in NLRP3 KO mice 34. Similar roles of NLRP3, independent of inflammasomes, were also found in renal IRI 35. Thus, NLRP3 activation may facilitate liver inflammation via multitude of molecular and cellular mechanisms, and inflammasome activation by liver IR may involve both NLRP3 and AIM2 signaling pathways.
Functional evidence of RLRs in liver IRI was demonstrated recently. Adenosine deaminase acting on RNA 1 (ADAR1) is a RNA-binding/editing protein and plays an anti-inflammatory role by suppressing cytosolic innate immune sensing of dsRNA by RLRs. ADAR1 has been found to be essential in maintaining liver homeostasis by preventing hepatocyte inflammation and cell death 36. In liver IRI, ADAR1 expression was induced. In vivo transfection with adenoviral ADAR1 shRNA resulted in significant increases of tissue damage with simultaneous upregulation of type I IFN productions in livers; while transfection with ADAR1 cDNA alleviated liver injury 37. ADAR1 regulation of RIG-1 signaling was demonstrated in vitro. ADAR1 knock-down triggered type I IFN induction in hepatocytes, which was prevented by simultaneous knock-down of RIG-1 and its adaptor IFN-β promoter stimulator 1 (IPS-1).
Thus, liver innate immune activation by IR involves interactions of multiple types of PRRs with variety of DAMPs. Additionally, both proinflammatory and immune regulatory signaling can be triggered downstream of PRRs. One challenging question we are facing now is how these different pathways are integrated into the disease process. As many types of innate immune cells are involved in liver immune response to IR, 1 obvious answer may be related to cell-type specific activation of different PRRs. Additionally, functions of PRR activation may evolve kinetically in disease-stage-specific (activation vs. resolution) manners. Thus, the spacial (cellular) and temporal complexity of the disease process dictates the engagement of multiple PRRs with distinctive functional outcomes in liver IRI.
Innate Immune Effector Mechanisms
PRR-mediated innate immune activation by IR results in the production of inflammatory cytokines/chemokine and generation of ROS and other cytotoxic molecules. They exert either direct cytotoxicity against hepatocytes or indirect effects via recruiting/activating both innate and adaptive immune cells. We will summarize recent findings in the function and regulation of TNF-α and IFN pathways, 2 major effector mechanisms in liver inflammatory tissue injury after IR, and discuss several novel innate immune effector mechanisms.
(i) The TNF-a pathway
TNF-α, rather than Fas, was initially found to be the key effector molecule of inflammatory hepatocellular damages in liver IRI 38. As TNF-α triggers both pro- and anti-apoptotic pathways in cells, the fate of hepatocytes against TNF-α in liver IR is determined by the activation of NF-kB 39–41. Oxidative stress, in particular ROS, inhibited hepatocyte NF-kB activation induced by IR and facilitated TNF-α mediated cell death and liver IRI 42,43. Additionally, endoplasmic reticulum (ER) stress was shown to also inhibit NF-kB activation in hepatocytes to modulate their response against TNF-α and promote liver IRI 44,45. Downstream of NF-κB, the up-regulation of CCAAT/enhancer-binding protein β (C/EBPβ) was documented to mediate hepatocyte protection against TNF-α cytotoxicity in vivo in LPS or TNFα-treated mice 46. Roles of other tumor necrosis factor receptor superfamily (TNFRSF) members and their ligands in liver IRI have been explored. Receptor activator of nuclear factor kappa-B (RANK) was constitutively expressed in hepatocytes, but not Kupffer cells. RANK ligand (RANKL) and its decoy receptor osteoprotegerin (OPG) were induced by liver IR with distinctive kinetics and cellular distribution 47. Interestingly, although anti-RANKL Abs did not affect the development of liver IRI due possibly to the endogenous neutralization of RANKL by OPG, recombinant RANKL did protected livers by upregulating NK-kB activation in hepatocytes. A novel regulatory role of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in liver IRI was also identified recently. Rather than facilitating inflammatory tissue damage, TRAIL downregulated NK cell cytotoxicities and IFN-g production and protected livers from IRI 48.
Tumor necrosis factor receptor (TNFR)-associated factor (TRAF) proteins are essential components of signaling pathways downstream of TNFR and TLR family members. There are 7 known TRAFs which can function either alone or in combination to control many biological processes, including cytokine production and cell survival, in cell type-specific and context-specific manner 49. TRAF1 is transcriptionally upregulated by TNF-α and has been shown to regulate TNF-α–induced cell death. Its role in liver IRI was investigated by using global TRAF1 gene KO mice and cell type-specific TRAF1 overexpressing transgenic mice 50. Results showed that TRAF1 was specifically required for hepatocyte death against IR by interacting with apoptosis signal-regulating kinase 1 (ASK1) to facilitate JNK activation. TRAF3 was found recently to have a similar role in promoting hepatocyte death. Using cell-type specific gene KO mice, it was shown that TRAF3 deficiency in hepatocyte, but not myeloid, protected livers from IRI by downregulating TGF-β-activated kinase 1(TAK1)-dependent JNK activation 51.
(ii) The type I interferon pathway
The activation of type I, but not type II, interferon pathway has been shown critical for liver inflammatory injury after IR 52 or in TNF-a-induced lethal shock 53. Liver plasmacytoid dendritic cells (pDCs) were the principal cellular source of type I interferon in response to IR 54. Receptors for type I IFN are ubiquitously expressed. Hepatocytes responded to IFN by upregulating expressions of multiple interferon regulatory factors (IRF). IRF1 was initially found to mediate IFN-γ induced cell cycle arrest and apoptosis in primary cultured hepatocytes 55. Studies using IRF1 KO mice documented that it promoted liver IRI in both partial warm ischemia model 56 and liver transplantation model 57. IRF1 deficiency in donors, but not recipients, protected livers by preventing hepatocyte inductions of death receptors and ligands, including TRAIL and Fas 57. Furthermore, IFN-α produced by liver pDCs was responsible for the activation of hepatocyte IRF-1 to promoted liver IRI 54,58. However, as Fas-FasL interaction was dispensable for hepatocyte death after partial warm ischemia 38, the exact mechanism downstream of the type I IFN-IRF1 signaling in hepatocellular damages during IR remains an open question. Tumor suppressor gene p53-mediated cell arrest, as well as ROS and ER stress has been implicated in IFN-γ-induced hepatocyte apoptosis 59. Additionally, IRF-1 was shown to regulate expressions and secretions of IL-15 and IL-15Rα in hepatocytes to facilitate infiltration and activation of NK, NKT, and CD8(+) T cells in livers 60, which constitutes potentially an indirect cytotoxic mechanism.
IRF-2 is structurally related to IRF-1 with 62% homology in their DNA binding domains (amino-terminal regions). It turns out that IRF-1 and IRF-2 bind to the same DNA element and function as a transcriptional activator or repressor, respectively 61. IRF-2 represses IRF-1-induced transcriptional activation. IRF2 heterozygote knockout (IRF2+/−) donor grafts that have reduced endogenous IRF2 levels suffered more severe IRI than their WT conterparts in liver transplantation model 62. IRF-9 was also involved in liver IRI 63. Hepatocellular damages were increased in IRF-9-overexpressing mice, but reduced in IRF-9 deficient mice after liver IR. Mechanistically, IRF9 promoted hepatocyte apoptosis by directly binding to silent mating type information regulation 2 homolog 1 (SIRT1) promoter to suppress its transcription. SIRT1 is a nicotinamide adenine dinucleotide (NAD)-dependent deacetylase, which physically interacted with p53 and attenuated p53-induced apoptosis through the deacetylation (inactivation) of p53 at its C-terminal Lys382. Indeed, IRF9-KO mice had reduced, but IRF-9-overexpressing mice had increased, levels of acetyl-p53 and p53 downstream factors Bax, Noxa, and Puma. SIRT1 deletion aggravated liver IRI and abolished liver protective effects of IRF9 deletion in IRF9/SIRT1 double KO mice 63.
The activation of type I IFN pathway in livers by IR results in the production of CXCL10, which functioned as a chemokine to recruit proinflammatory CXCR3+ T cells to facilitate liver immune activation against IR 64,65. More recent studies had revealed that CXCL10-CXCR3 interaction was also involved in macrophage accumulation in inflamed tissues 66. Furthermore, CXCL10 was shown to induce hepatocyte apoptosis via its noncognate receptor TLR4 67. Although relevance of the latter 2 mechanisms in liver IRI remains to be determined, they provide new directions in our analysis of the functional mechanism of the type 1 IFN pathway.
(iii) Novel mechanisms and pathways
We have found that IRF-3 was critical for inflammatory immune activation and the development of hepatocellular damages by activating the type I interferon pathway in livers in response to IR 3. A more recent study revealed that IRF-3 in fact played an anti-inflammatory role by downregulating IL23/IL-17-mediated tissue injury mechanism, which occurred late during reperfusion (24–48h post reperfusion) 68. At the cellular level, IRF-3 deficient KCs produced higher levels of IL-23, but lower levels of IL-27, as compared with their WT counterparts, which led to increased accumulation of IL-17A+ γδT and iNKT cells in livers after IR. Thus, IRF-3 differentially regulates 2 distinctive innate immune responses at different phases of liver IRI: an early cytotoxic phase involving a type I IFN-dependent inflammation and a late protective phase attenuating IL-23/IL-17-type innate immune response in an IL-27-dependent manner.
Neutrophils are classical inflammatory effector cells and their involvement in liver IRI has been well recognized. Functionally, neutrophils execute anti-microbial effects by phagocytosis and degranulation, and pro-inflammatory effects by cytokine/chemokine and ROS/proteases. Neutrophil extracellular traps (NETs) is a newly discovered effector mechanism of neutrophils. NETs contain de-condensed chromatin, histones and granule proteins and are generated via a specific form of neutrophil death, NETosis, or released from viable neutrophils upon activation 69. Liver IR resulted in the formation of NETs via TLR4- and TLR9-MyD88 signaling pathways. Treatment with peptidyl-arginine-deiminase 4 inhibitor or DNase I, which disrupted NETs, effectively protected hepatocytes and reduced inflammation after liver IR 70.
Thus, liver TRAF and IRF activation upon IR are critical regulators of inflammatory hepatocellular damages. They function to promote hepatocyte death directly via regulation of distinctive intracellular signaling pathways. Additionally, their activation/inactivation leads to the generation of unique cytokine profiles, which facilitate infiltration and activation of distinctive lymphocyte populations to amplify tissue inflammation/injury.
Regulation of innate immune activation
PRR-mediated innate immune activation triggers multiple intracellular signaling pathways, which function in synergy to activate transcriptional factors, including NF-kB, activator protein-1 (AP-1) and IRFs, leading to transcriptions of inflammatory cytokines/chemokine/type I IFN. Because PRR-induced inflammation is essential for host immune defense against infections and its over-activation is harmful to host own tissues, cells developed complex endogenous regulatory mechanisms to counteract activating signaling 71–73. Additionally, exogenous factors or the context of PRR activation, which consists of co-activating ligand-receptor, cell’s metabolic conditions, and cell-cell interactions may also determine the level and nature of inflammation. We will discuss those regulatory mechanisms that have been studied in vivo in liver IRI models.
(i) Endogenous pathways
In parallel with activations of proinflammatory signaling pathways, TLR ligation also triggers an immune regulatory pathway, the lipid kinase, class I phosphatidylinositol 3-kinase (PI3K) 74 and its downstream signaling mediators, including glycogen synthase kinase 3β (Gsk3β), mammalian target of rapamycin complexes (mTORC) 1/2 and forkhead box protein O1 (FoxO1) 72,75–77. The PI3K complex is recruited to the plasma membrane after TLR ligation, where it can access its substrate PI(4,5)P2 resulting in PI(3,4,5)P3 production. This results in the recruitment and activation of phosphoinositide-dependent kinase-1 (PDK-1) and protein kinase B (Akt) through the phosphatidylinositol binding pleckstrin homology domain. PDK1 phosphorylates Akt at Thr308. Full activation of Akt requires additionally mTORC2-mediated phosphorylation at Ser473. Akt regulates several key inflammatory signaling/transcription molecules. It promotesTORC1 activation by phosphorylating and inactivating its negative regulator proline-rich Akt substrate of 40 kDa (PRAS40) and tuberous sclerosis protein 2 (TSC2). It inactivates Gsk3β via inhibitory phosphorylation at Ser9, and FoxO1 via phosphorylation induced-nuclear export. Based on the finding that Gsk3b inhibition differentially regulated pro- and anti-inflammatory cytokine productions and protected mice from endotoxin shock 75, we tested its role in liver IRI. Results showed that Gsk3 inhibitor SB216763 effectively inhibited liver inflammatory immune activation and protected livers from IRI by an IL-10-mediated mechanism 78. As Gsk3β also plays critical roles in hepatocyte death, and chemical inhibitors target non-specifically in vivo, to determine innate immune regulatory roles of the PI3K-Akt-Gsk3β pathway, we created myeloid-specific phosphatase and tensin homolog (PTEN)-KO mice. PTEN is the endogenous antagonist of PI3K and negative regulator of Akt. We showed that KCs and BMMs, but not hepatocytes, from PTEN KO mice had constitutively higher levels of phosphorylated Akt (activation) and Gsk3β (inactivation). Myeloid PTEN deficiency resulted in the inhibition of liver immune activation and protection of livers from IRI 79. In fact, PTEN deficient macrophages were M2-like and responded to TLR stimulation by producing increasing amount of IL-10, but decreasing amounts of TNF-a, IL-6 and IL-12, as compared with their WT counterparts. In addition, in vivo administration of PTEN siRNA was shown to protect livers from IRI, which was associated with an increase in FoxO1phosphorylation 80. Furthermore, the β-catenin signaling pathway, a downstream target of Gsk3β, was demonstrated to play a critical role in the Stat3-mediated cytoprotective/anti-inflammatory mechanism subsequent to IL-10 or HO-1 activation in liver IRI 81.
The role of mTORC 1 in regulating TLR responses has been controversial. The initial rapamycin study found that mTORC1 inhibition promoted proinflammatory cytokine productions via NF-kB, but blocked interleukin-10 release via STAT3 82. The opposite effects were seen in TSC2 KO cells with elevated mTORC1 activities82,83. Gsk3β was found to be involved in mTORC1 regulation of innate immune response by the mTORC1-S6K-mediated Gsk3β S9 inhibitory phosphorylation 84. In contrary to the anti-inflammatory role, TSC1 deficiency, which also results in mTORC1 activation as TSC2 KO, was found to promote pro-inflammatory cytokine production in macrophages in response to multiple types of PRR stimulation, which could be partially reversed by rapamycin 85. Furthermore, mice with myeloid-specific deletion of TSC1 were shown to not only exhibit enhanced M1 response and spontaneously develop M1-related inflammatory disorders, but also be highly resistant to M2-polarized allergic asthma 86. Interestingly, rapamycin was only able to correct the defect in M2 polarization, but not the hypersensitive M1 response in TSC1-deficient macrophages. Reasons for these conflicting results remain to be determined. One possibility is derived from the observation that mTORC2, unlike mTORC1, was positively regulated by TSC2 87 that mTORC2 inhibition might be responsible for the hyper-inflammatory phenotype in TSC2 KO mice. The anti-inflammatory role of mTORC2 is mediated by its ability to activate Akt. Experimental evidence and mechanism were established recently by using Rictor deficient cells. It turned out that FoxO1 phosphorylation downstream of Akt activation resulted in FoxO1 export from nuclear and played a critical role in mTORC2-mediated downregulation of pro-inflammatory gene expression 88. Rictor deletion induced macrophage M1 polarization and potentiated their in vivo pro-inflammatory response to LPS 89; and enhanced myeloid dendritic cell allogeneic Th1 and Th17 stimulatory ability after TLR4 activation in vitro and in vivo 90. Although both mTORC1 inhibition and mTORC2 activation have been shown as cytoprotective in IRI, their direct roles in liver innate immune regulation in vivo remain to be determined. We failed to detect immune regulatory effects of rapamycin in vitro and in vivo in liver IRI, but rather cytoprotection via both autophagy and a mTORC2-dependent mechanism 91. Rictor KO/mTORC2 activation has been shown to play a pivotal role in cardioprotection via ribosomal protein S6 in a heart IRI model 92.
A20 is a major negative feed-back regulator of TNFR and TLR signaling. A20 KO mice develop severe inflammation in multiple organs and die prematurely 93. As a deubiquitinase, A20 cleaves K63-kinked ubiquitin chains from TRAF6 and RIP1 to interrupt TNFR/TLR-induced NF-kB activation 94. Its role in liver IRI, however, has been associated mainly with hepatocytes with controversies. Adenoviral-mediated A20 overexpression, in 1 study, protected mice from lethal liver IRI by increasing peroxisome proliferator-activated receptor-alpha expression, which inhibited oxidative stress-induced hepatocyte necrosis 95. In another study, A20 aggravated partial liver IRI due to NF-kB inhibition 96. The question of whether A20 regulates liver inflammatory immune response directly in myeloid cells remains open. As A20 is induced via NF-kB activation, it is possible that A20 may function in the resolution (late), rather than the activation, stage of liver inflammation against IRI. In hepatocytes, it may also differentially regulate early ischemia-induced, vs. inflammation-induced cell death after IR, which are mediated by intrinsic or extrinsic cell death pathways, respectively.
(ii). Purinergic Receptors
Innate immune response against IR is a sterile inflammation triggered by DAMPs. In addition to large molecules such as HMGB1/Histone/DNA, cellular metabolites, including ATP and adenosine, are also released from necrotic cells which can activate/regulate innate immune cells independent of PRRs. Extracellular ATP has been characterized as a potent danger signal. It binds to purinergic receptor 2 (P2), capable of facilitating macrophage inflammasome activation via pannexin-1, as well as neutrophil chemotaxis/activation 97,98. However, extracellular ATP undergoes rapidly catabolism by cell surface ectonucleotidases. CD39 converts ATP into AMP, and CD73 further dephosphorylates AMP into adenosine. Adenosine are recognized by P1 receptors, which exert potent regulatory function in inflammatory responses 98,99. Extracellular adenosine signaling is terminated by equilibrative nucleoside transporters (ENTs), which uptake adenosine from the extracellular towards the intracellular compartment. Adenosine has been shown to inhibit M1, or promote M2, macrophage activation via A2A or A2B receptor, respectively. Interestingly, macrophages actively release low levels of ATP upon stimulation. These extracellular ATP is converted to adenosine by CD39/CD73, which drives a switch of macrophage polarization 100. CD39 and CD79 are expressed by many types of innate and adaptive immune cells, and upregulated by hypoxia and inflammatory stimulations.
In liver IRI, extracellular ATP is generated from necrotic cells and cell activation. Its involvement in liver inflammatory immune activation is implicated by the finding that its receptor pannexin-1 was critical for the disease pathogenesis by activating inflammasomes 33. Additionally, ATP catabolism and adenosine have been shown to play critical regulatory roles in the pathogenesis of liver IRI. It was shown that activation of P1 receptor A2A by synthetic agonists on bone marrow-derived cells protected livers from IRI 101. Mechanistically, A2A receptor activation inhibited IFN-γ production in NKT cells upon stimulation 102. Additionally, the stimulation of A2A receptors induced a preconditioning effect in hepatocytes via the activation of HIF-1 103. More recently, study of different adenosine receptors revealed that A2B receptors (ADORA2B) were selectively upregulated in livers during IR and exerted a cytoprotective function in hepatocytes by attenuating NF-kB activation 104. As CD39/CD73 converts ATP from an alarmin into immune regulatory adenosine, the question of whether these ectonucleotidases played roles in regulating liver inflammatory immune response has also been addressed. CD73 deficiency or pharmacological inhibition attenuated extracellular adenosine production induced by liver ischemic pre-conditioning (IP), which abolished liver protection against subsequent IR 105. In a murine liver transplantation model, it was shown that CD39 deficient donor grafts suffered worse IRI, due to increased inflammatory activation of myeloid DCs 106. Furthermore, the inhibition of equilibrative nucleoside transporters by either pharmacological inhibitors or gene KO resulted in elevated adenosine levels in livers and protection from IRI in the A2B receptor signaling-dependent manner 107. However, effects of ATP/adenosine signaling in liver IRI could be complicated by its distinctive roles in different immune cells. Opposite to its pro-inflammatory role in macrophages/DCs, P2 receptor activation by ATP inhibited NK cell IFN-γ production upon activation. In a hepatic partial warm ischemia model, CD39 KO mice were in fact protected from IRI, due possibly to the enhanced P2 receptor-mediated inhibitory signaling in NK cells 108. Thus, ATP/adenosine signaling regulates liver inflammatory immune response against IR in cell-type specific manner with potentially diverse functional outcomes.
(iv). Cellular mechanisms of liver innate immune activation
Although KCs have been assumed as the dominant player in liver inflammatory immune response against IR, their precise responses and functions in the disease process remains incompletely understood. Majority of studies analyzed liver immune activation at the organ level without cell-specific analysis of liver macrophages. Accumulating evidence has suggested that both tissue resident KCs and infiltrating macrophages are involved in liver immune response against IR and play distinctive roles. KCs are CD11b negative and relatively radiation-resistant 109,110. Thus, experiments using bone marrow chimeras or CD11b- diphtheria toxin receptor (DTR) mice document functions of only infiltrating macrophages, but not KCs 7,111,112. Results from these experiments are generally supportive of a pro-inflammatory role of infiltrating macrophages. Clodronate-liposomes target mainly KCs. The impact of KC-depletion on liver IRI, however, has been controversial with either pro- or anti-inflammatory effects 113–115. The recent seminal finding in macrophage biology reveals that tissue resident and monocyte-derived macrophages (circulating) are distinctive in their lineages and functions 116–121. Tissue resident macrophages are embryonically derived from yolk sac and play fundamental roles in tissue homeostasis 117–119. Clearly, responses and functional mechanisms of KCs in liver IRI need to be redefined in this new context.
Although liver DCs are considered as poor APCs, they express PRRs and are capable of producing inflammatory cytokines/chemokines in response to DAMPs/PAMPs. Their roles in liver IRI have been addressed with interesting findings. Similar to macrophages, 2 populations of DCs, resident and infiltrating types, responded to liver IR. General depletion of CD11c+ DCs (in CD11c-DTR mice) resulted in exacerbated liver IRI. It was shown that these DCs responded to IR by producing IL-10 via TLR9 signaling 122. Using CD11c-Cre-TLR4-Loxp system, the anti-inflammatory role of DCs was confirmed by showing that DC-specific TLR4 KO resulted in aggravated liver IRI 8. However, in Flt3L KO mice, which were completely devoid of DCs, liver IRI was attenuated, as compared with WT mice, indicative of their proinflammatory roles. Further study demonstrated that adaptive transfer of splenic or hepatic DCs into Flt3L mice restored liver IRI, and Flt3L KO liver grafts (absence of resident DCs) suffered more severe IRI than WT counterparts in transplantation model, suggesting that infiltrating and resident DCs played distinctive roles in liver IRI 123. Hepatic stellate cells (HSCs), a key player in liver fibrosis, were shown recently to regulate liver IRI. In a unique mouse model 124 in which HSCs could be specifically depleted, both liver inflammatory immune activation and hepatocellular injury against IR were attenuated. Thus, multiple types of liver innate immune cells participate in inflammatory immune response against IR with distinctive functions.
An important aspect of the regulation of liver innate immune response is the conditioning effect of IR. Innate immune activation by IR is a continuum of tissue/cell stress response against oxygen and nutrient deprivation. Primary stress responses including oxidative (mitochondrial), endoplasmic reticulum, as well as autophagy are induced potentially in all types of cells in ischemia organs and directly regulate not only parenchymal cell death but also responses of tissue resident innate immune cells 125–128. Although detailed discussion on this subject is beyond the scope of this review, it is worth pointing out that not much have been done in vivo in liver IRI models. Majority of study have been focusing on dissecting mechanisms of stress responses on hepatocellular injuries, which have an indirect effect on innate immune activation. Whether and how these stress responses regulate activation of tissue resident macrophages/DCs remains an open question.
Summary
We have reviewed recent progresses in our understanding of the innate immune activation and regulation in liver IRI. It becomes evident that DAMP-initiated PRR activation triggers not only proinflammatory, but also immune regulatory signaling in myeloid and dendritic cells. The outcome of innate immune activation on tissue inflamation and injury is regulated at many levels: both molecular (TRAFs, IRFs) and cellular (KCs/macrophages, DCs, neutrophils, HSCs and hepatocytes), as well as cell intrinsic (PI3K) and extrinsic (adenosine). Roles of HMGB1 in liver inflammation highlighed this complexity. HMGB1 stimulates KCs/macrophages to initiate a proinflammatory response via TLR4/MD2 and RAGE, but potenially an immune regulatory response via CD24/SiglecG. The same HMGB1-TLR4 interaction results in anti-inflammatory IL-10 productions in resident DCs and possibly KCs, but pro-inflammatory response in infiltrating DCs/macrophages. Additionally, IR triggers the PI3K-Akt signaling pathway downstream of PRRs, as well as purinergic receptor activation, both of which play important regulatory roles in liver inflammatory immune activation. Figure 1 is a simpified summary of major innate immune cells, receptors and their ligands involved in liver IRI, as well as functional impact of these receptor activation on disease pathogenesis. Clearly, our comprehension of these complex immune regulatory network in livers is essential for us to design safe and effective therapeutic strategy to ameliorate liver IRI in clinical patients.
Abbreviations
- ALR
absent in melanoma 2 (AIM2)-like receptor
- CLR
C-type lectin receptor
- DAMP
damage-associated molecular pattern
- DC
dendritic cells
- ER stress
endoplasmic reticulum stress
- HMGB1
high mobility group box 1
- IFN
interferon
- IRF
interferon regulatory factor
- KO
knock-out
- LPS
lipopolysaccharide
- NETs
Neutrophil extracellular traps
- NLR
nucleotide-binding oligomerization domain-like receptor
- NPCs
non-parenchymal cells
- OLR
oligoadenylate synthetase (OAS)-like receptor
- PAMP
pathogen-associated molecular pattern
- PRR
pattern recognition receptor
- RAGE
advanced glycation end product
- RLR
retinoic acid-inducible gene-I-like receptor
- ROS
reactive oxygen species
- SIRT
silent mating type information regulation 2 homolog
- TLR
toll-like receptor
- TRAF
tumor necrosis factor receptor (TNFR)-associated factor
Footnotes
LL, HZ, MN and YZ draft, XW, RB, JK and YZ revise, YZ finalizes the manuscript.
The authors declare no conflicts of interest.
References
- 1.Thaiss CA, Levy M, Itav S, Elinav E. Integration of Innate Immune Signaling. Trends Immunol. 2016;37(2):84–101. doi: 10.1016/j.it.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Wu HS, Zhang JX, Wang L, Tian Y, Wang H, Rotstein O. Toll-like receptor 4 involvement in hepatic ischemia/reperfusion injury in mice. Hepatobiliary Pancreat Dis Int. 2004;3(2):250–253. [PubMed] [Google Scholar]
- 3.Zhai Y, Shen XD, O’Connell R, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173(12):7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 4.Ellett JD, Evans ZP, Atkinson C, Schmidt MG, Schnellmann RG, Chavin KD. Toll-like receptor 4 is a key mediator of murine steatotic liver warm ischemia/reperfusion injury. Liver Transpl. 2009;15(9):1101–1109. doi: 10.1002/lt.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gehrau RC, Mas VR, Dumur CI, et al. Donor Hepatic Steatosis Induce Exacerbated Ischemia-Reperfusion Injury Through Activation of Innate Immune Response Molecular Pathways. Transplantation. 2015;99(12):2523–2533. doi: 10.1097/TP.0000000000000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald KA, Huang H, Tohme S, et al. Toll-like receptor 4 (TLR4) antagonist eritoran tetrasodium attenuates liver ischemia and reperfusion injury through inhibition of high-mobility group box protein B1 (HMGB1) signaling. Molecular medicine. 2014;20:639–648. doi: 10.2119/molmed.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsung A, Hoffman RA, Izuishi K, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175(11):7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 8.Nace GW, Huang H, Klune JR, et al. Cellular-specific role of toll-like receptor 4 in hepatic ischemia-reperfusion injury in mice. Hepatology. 2013;58(1):374–387. doi: 10.1002/hep.26346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 11.Gardella S, Andrei C, Ferrera D, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3(10):995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsung A, Klune JR, Zhang X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204(12):2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huebener P, Pradere J-P, Hernandez C, et al. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest. 2015;125(2):539–550. doi: 10.1172/JCI76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H, Nace GW, McDonald K-A, et al. Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: a role for intracellular high-mobility group box 1 in cellular protection. Hepatology. 2014;59(5):1984–1997. doi: 10.1002/hep.26976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venereau E, Casalgrandi M, Schiraldi M, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209(9):1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang D, Billiar TR, Lotze MT. A Janus tale of two active high mobility group box 1 (HMGB1) redox states. Mol Med. 2012;18:1360–1362. doi: 10.2119/molmed.2012.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Wang H, Ju Z, et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J Exp Med. 2015;212(1):5–14. doi: 10.1084/jem.20141318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng S, Feirt N, Goldstein M, et al. Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatology. 2004;39(2):422–432. doi: 10.1002/hep.20045. [DOI] [PubMed] [Google Scholar]
- 19.Zeng S, Dun H, Ippagunta N, et al. Receptor for advanced glycation end product (RAGE)-dependent modulation of early growth response-1 in hepatic ischemia/reperfusion injury. J Hepatol. 2009;50(5):929–936. doi: 10.1016/j.jhep.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Yue S, Zhou HM, Zhu JJ, et al. Hyperglycemia and liver ischemia reperfusion injury: a role for the advanced glycation endproduct and its receptor pathway. Am J Transplant. 2015;15(11):2877–2887. doi: 10.1111/ajt.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G-Y, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323(5922):1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchida Y, Ke B, Freitas MC, et al. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology. 2010;139(6):2195–2206. doi: 10.1053/j.gastro.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Ji H, Zhang Y, et al. Negative CD4 + TIM-3 signaling confers resistance against cold preservation damage in mouse liver transplantation. Am J Transplant. 2015;15(4):954–964. doi: 10.1111/ajt.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Ji H, Zhang Y, et al. Recipient T cell TIM-3 and hepatocyte galectin-9 signalling protects mouse liver transplants against ischemia-reperfusion injury. J Hepatol. 2015;62(3):563–572. doi: 10.1016/j.jhep.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo H, Callaway JB, Ting JPY. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161(5):1797–1803. doi: 10.1016/S0002-9440(10)64456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeuchi D, Yoshidome H, Kato A, et al. Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice. Hepatology. 2004;39(3):699–710. doi: 10.1002/hep.20117. [DOI] [PubMed] [Google Scholar]
- 28.Sakai N, Van Sweringen HL, Quillin RC, et al. Interleukin-33 is hepatoprotective during liver ischemia/reperfusion in mice. Hepatology. 2012;56(4):1468–1478. doi: 10.1002/hep.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menzel CL, Sun Q, Loughran PA, Pape HC, Billiar TR, Scott MJ. Caspase-1 Is Hepatoprotective during Trauma and Hemorrhagic Shock by Reducing Liver Injury and Inflammation. Mol Med. 2011;17(9–10):1031–1038. doi: 10.2119/molmed.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu P, Duan L, Chen J, et al. Gene Silencing of NALP3 Protects Against Liver Ischemia-Reperfusion Injury in Mice. Hum Gene Ther. 2011 doi: 10.1089/hum.2010.145. [DOI] [PubMed] [Google Scholar]
- 31.Huang H, Chen HW, Evankovich J, et al. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J Immunol. 2013;191(5):2665–2679. doi: 10.4049/jimmunol.1202733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamo N, Ke B, Ghaffari AA, et al. ASC/caspase-1/IL-1β signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology. 2013;58(1):351–362. doi: 10.1002/hep.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H-Y, Kim S-J, Lee S-M. Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. The FEBS journal. 2015;282(2):259–270. doi: 10.1111/febs.13123. [DOI] [PubMed] [Google Scholar]
- 34.Inoue Y, Shirasuna K, Kimura H, et al. NLRP3 regulates neutrophil functions and contributes to hepatic ischemia-reperfusion injury independently of inflammasomes. J Immunol. 2014;192(9):4342–4351. doi: 10.4049/jimmunol.1302039. [DOI] [PubMed] [Google Scholar]
- 35.Shigeoka AA, Mueller JL, Kambo A, et al. An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol. 2010;185(10):6277–6285. doi: 10.4049/jimmunol.1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang G, Wang H, Singh S, et al. ADAR1 Prevents Liver Injury from Inflammation and Suppresses Interferon Production in Hepatocytes. Am J Pathol. 2015;185(12):3224–3237. doi: 10.1016/j.ajpath.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Wang G, Zhang L, et al. ADAR1 Suppresses the Activation of Cytosolic RNA-Sensing Signaling Pathways to Protect the Liver from Ischemia/Reperfusion Injury. Scientific reports. 2016;6:20248. doi: 10.1038/srep20248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122(1):202–210. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 39.Lentsch AB. Activation and function of hepatocyte NF-kappaB in postischemic liver injury. Hepatology. 2005;42(1):216–218. doi: 10.1002/hep.20779. [DOI] [PubMed] [Google Scholar]
- 40.Luedde T, Assmus U, Wustefeld T, et al. Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis but protects from ischemia/reperfusion injury. J Clin Invest. 2005;115(4):849–859. doi: 10.1172/JCI23493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuboki S, Okaya T, Schuster R, et al. Hepatocyte NF-kappaB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G201–207. doi: 10.1152/ajpgi.00186.2006. [DOI] [PubMed] [Google Scholar]
- 42.Imanishi H, Scales WE, Campbell DA. Tumor necrosis factor alpha alters the cytotoxic effect of hydrogen peroxide in cultured hepatocytes. Biochem Biophys Res Commun. 1997;230(1):120–124. doi: 10.1006/bbrc.1996.5901. [DOI] [PubMed] [Google Scholar]
- 43.Llacuna L, Mari M, Lluis JM, Garcia-Ruiz C, Fernandez-Checa JC, Morales A. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappaB inactivation in prolonged ischemia/reperfusion. Am J Pathol. 2009;174(5):1776–1785. doi: 10.2353/ajpath.2009.080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben Mosbah I, Alfany-Fernandez I, Martel C, et al. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell Death Dis. 2010;1:e52. doi: 10.1038/cddis.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Ren F, Cheng Q, et al. Endoplasmic reticulum stress modulates liver inflammatory immune response in the pathogenesis of liver ischemia and reperfusion injury. Transplantation. 2012;94(3):211–217. doi: 10.1097/TP.0b013e318259d38e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Singh R, Xiang Y, Greenbaum LE, Czaja MJ. Nuclear factor κB up-regulation of CCAAT/enhancer-binding protein β mediates hepatocyte resistance to tumor necrosis factor α toxicity. Hepatology. 2010;52(6):2118–2126. doi: 10.1002/hep.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakai N, Van Sweringen HL, Schuster R, et al. Receptor activator of nuclear factor-κB ligand (RANKL) protects against hepatic ischemia/reperfusion injury in mice. Hepatology. 2012;55(3):888–897. doi: 10.1002/hep.24756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fahrner R, Trochsler M, Corazza N, et al. Tumor necrosis factor-related apoptosis-inducing ligand on NK cells protects from hepatic ischemia-reperfusion injury. Transplantation. 2014;97(11):1102–1109. doi: 10.1097/TP.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 49.Häcker H, Tseng P-H, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev Immunol. 2011;11(7):457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X-F, Zhang R, Huang L, et al. TRAF1 is a key mediator for hepatic ischemia/reperfusion injury. Cell Death Dis. 2014;5:e1467. doi: 10.1038/cddis.2014.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu J, Zhu X-H, Zhang X-J, et al. Targeting TRAF3 signaling protects against hepatic ischemia/reperfusions injury. J Hepatol. 2016;64(1):146–159. doi: 10.1016/j.jhep.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Zhai Y, Qiao B, Gao F, et al. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47(1):199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
- 53.Huys L, Van Hauwermeiren F, Dejager L, et al. Type I interferon drives tumor necrosis factor-induced lethal shock. J Exp Med. 2009;206(9):1873–1882. doi: 10.1084/jem.20090213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castellaneta A, Yoshida O, Kimura S, et al. Plasmacytoid dendritic cell-derived IFN-α promotes murine liver ischemia/reperfusion injury by induction of hepatocyte IRF-1. Hepatology. 2014;60(1):267–277. doi: 10.1002/hep.27037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kano A, Haruyama T, Akaike T, Watanabe Y. IRF-1 is an essential mediator in IFN-gamma-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochem Biophys Res Commun. 1999;257(3):672–677. doi: 10.1006/bbrc.1999.0276. [DOI] [PubMed] [Google Scholar]
- 56.Tsung A, Stang MT, Ikeda A, et al. The transcription factor interferon regulatory factor-1 mediates liver damage during ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1261–1268. doi: 10.1152/ajpgi.00460.2005. [DOI] [PubMed] [Google Scholar]
- 57.Ueki S, Dhupar R, Cardinal J, et al. Critical role of interferon regulatory factor-1 in murine liver transplant ischemia reperfusion injury. Hepatology. 2010;51(5):1692–1701. doi: 10.1002/hep.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe Y, Suzuki O, Haruyama T, Akaike T. Interferon-gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J Cell Biochem. 2003;89(2):244–253. doi: 10.1002/jcb.10501. [DOI] [PubMed] [Google Scholar]
- 59.Kanki K, Kawamura T, Watanabe Y. Control of ER stress by a chemical chaperone counteracts apoptotic signals in IFN-gamma-treated murine hepatocytes. Apoptosis. 2009;14(3):309–319. doi: 10.1007/s10495-009-0318-x. [DOI] [PubMed] [Google Scholar]
- 60.Yokota S, Yoshida O, Dou L, et al. IRF-1 promotes liver transplant ischemia/reperfusion injury via hepatocyte IL-15/IL-15Rα production. J Immunol. 2015;194(12):6045–6056. doi: 10.4049/jimmunol.1402505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 62.Klune JR, Dhupar R, Kimura S, et al. Interferon regulatory factor-2 is protective against hepatic ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2012;303(5):G666–673. doi: 10.1152/ajpgi.00050.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang P-X, Zhang R, Huang L, et al. Interferon regulatory factor 9 is a key mediator of hepatic ischemia/reperfusion injury. J Hepatol. 2015;62(1):111–120. doi: 10.1016/j.jhep.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 64.Zhai Y, Shen XD, Gao F, et al. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology. 2008;47(1):207–214. doi: 10.1002/hep.21986. [DOI] [PubMed] [Google Scholar]
- 65.Zhai Y, Shen XD, Hancock WW, et al. CXCR3+CD4+ T cells mediate innate immune function in the pathophysiology of liver ischemia/reperfusion injury. J Immunol. 2006;176(10):6313–6322. doi: 10.4049/jimmunol.176.10.6313. [DOI] [PubMed] [Google Scholar]
- 66.Zhou J, Tang PCY, Qin L, et al. CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses. J Exp Med. 2010;207(9):1951–1966. doi: 10.1084/jem.20100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sahin H, Borkham-Kamphorst E, do ONT, et al. Proapoptotic effects of the chemokine, CXCL 10 are mediated by the noncognate receptor TLR4 in hepatocytes. Hepatology. 2013;57(2):797–805. doi: 10.1002/hep.26069. [DOI] [PubMed] [Google Scholar]
- 68.Loi P, Yuan Q, Torres D, et al. IRF3 deficiency leads to IL-17-mediated liver ischemia-reperfusion injury. Hepatology. 2012 doi: 10.1002/hep.26022. [DOI] [PubMed] [Google Scholar]
- 69.Kruger P, Saffarzadeh M, Weber ANR, et al. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11(3):e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang H, Tohme S, Al-Khafaji AB, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62(2):600–614. doi: 10.1002/hep.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33(9):449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Hamerman JA, Pottle J, Ni M, He Y, Zhang ZY, Buckner JH. Negative regulation of TLR signaling in myeloid cells-implications for autoimmune diseases. Immunol Rev. 2016;269(1):212–227. doi: 10.1111/imr.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 74.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24(7):358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 75.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6(8):777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fan W, Morinaga H, Kim JJ, et al. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. The EMBO journal. 2010;29(24):4223–4236. doi: 10.1038/emboj.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohtani M, Nagai S, Kondo S, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112(3):635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ren F, Duan Z, Cheng Q, et al. Inhibition of glycogen synthase kinase 3 beta ameliorates liver ischemia reperfusion injury by way of an interleukin-10-mediated immune regulatory mechanism. Hepatology. 2011;54(2):687–696. doi: 10.1002/hep.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yue S, Rao J, Zhu J, et al. Myeloid PTEN deficiency protects livers from ischemia reperfusion injury by facilitating M2 macrophage differentiation. J Immunol. 2014;192(11):5343–5353. doi: 10.4049/jimmunol.1400280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kamo N, Ke B, Busuttil RW, Kupiec-Weglinski JW. PTEN-mediated Akt/beta-catenin/Foxo1 signaling regulates innate immune responses in mouse liver ischemia/reperfusion injury. Hepatology. 2013;57(1):289–298. doi: 10.1002/hep.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ke B, Shen XD, Kamo N, et al. beta-catenin regulates innate and adaptive immunity in mouse liver ischemia-reperfusion injury. Hepatology. 2013;57(3):1203–1214. doi: 10.1002/hep.26100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weichhart T, Costantino G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29(4):565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 83.Haidinger M, Poglitsch M, Geyeregger R, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185(7):3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 84.Wang H, Brown J, Gu Z, et al. Convergence of the mammalian target of rapamycin complex 1- and glycogen synthase kinase 3-β-signaling pathways regulates the innate inflammatory response. J Immunol. 2011;186(9):5217–5226. doi: 10.4049/jimmunol.1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pan H, O’Brien TF, Zhang P, Zhong X-P. The role of tuberous sclerosis complex 1 in regulating innate immunity. J Immunol. 2012;188(8):3658–3666. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu L, Yang T, Li L, et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat Commun. 2014;5:4696. doi: 10.1038/ncomms5696. [DOI] [PubMed] [Google Scholar]
- 87.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28(12):4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown J, Wang H, Suttles J, Graves DT, Martin M. Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J Biol Chem. 2011;286(52):44295–44305. doi: 10.1074/jbc.M111.258053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Festuccia WT, Pouliot P, Bakan I, Sabatini DM, Laplante M. Myeloid-specific rictor deletion induces m1 macrophage polarization and potentiates in vivo pro-inflammatory response to lipopolysaccharide. PloS one. 2014;9(4):e95432. doi: 10.1371/journal.pone.0095432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raïch-Regué D, Rosborough BR, Watson AR, McGeachy MJ, Turnquist HR, Thomson AW. mTORC2 Deficiency in Myeloid Dendritic Cells Enhances Their Allogeneic Th1 and Th17 Stimulatory Ability after TLR4 Ligation In Vitro and In Vivo. J Immunol. 2015;194(10):4767–4776. doi: 10.4049/jimmunol.1402551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu J, Lu T, Yue S, et al. Rapamycin protection of livers from ischemia and reperfusion injury is dependent on both autophagy induction and mammalian target of rapamycin complex 2-Akt activation. Transplantation. 2015;99(1):48–55. doi: 10.1097/TP.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yano T, Ferlito M, Aponte A, et al. Pivotal role of mTORC2 and involvement of ribosomal protein S6 in cardioprotective signaling. Circ Res. 2014;114(8):1268–1280. doi: 10.1161/CIRCRESAHA.114.303562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee EG, Boone DL, Chai S, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289(5488):2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boone DL, Turer EE, Lee EG, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5(10):1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 95.Ramsey HE, Da Silva CG, Longo CR, et al. A20 protects mice from lethal liver ischemia/reperfusion injury by increasing peroxisome proliferator-activated receptor-alpha expression. Liver Transpl. 2009;15(11):1613–1621. doi: 10.1002/lt.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu J, Lee H-S, Lee S-M, et al. Aggravation of post-ischemic liver injury by overexpression of A20, an NF-κB suppressor. J Hepatol. 2011;55(2):328–336. doi: 10.1016/j.jhep.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 97.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509(7500):310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19(6):355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haskó G, Pacher P. Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):865–869. doi: 10.1161/ATVBAHA.111.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cohen HB, Briggs KT, Marino JP, Ravid K, Robson SC, Mosser DM. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood. 2013;122(11):1935–1945. doi: 10.1182/blood-2013-04-496216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Day Y-J, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J Immunol. 2005;174(8):5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- 102.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203(12):2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alchera E, Tacchini L, Imarisio C, et al. Adenosine-dependent activation of hypoxia-inducible factor-1 induces late preconditioning in liver cells. Hepatology. 2008;48(1):230–239. doi: 10.1002/hep.22249. [DOI] [PubMed] [Google Scholar]
- 104.Zimmerman MA, Grenz A, Tak E, et al. Signaling through hepatocellular A2B adenosine receptors dampens ischemia and reperfusion injury of the liver. Proc Natl Acad Sci U S A. 2013;110(29):12012–12017. doi: 10.1073/pnas.1221733110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Hart ML, Much C, Gorzolla IC, et al. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135(5):1739–1750. e1733. doi: 10.1053/j.gastro.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 106.Yoshida O, Kimura S, Jackson EK, et al. CD39 expression by hepatic myeloid dendritic cells attenuates inflammation in liver transplant ischemia-reperfusion injury in mice. Hepatology. 2013;58(6):2163–2175. doi: 10.1002/hep.26593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zimmerman MA, Tak E, Ehrentraut SF, et al. Equilibrative nucleoside transporter (ENT)-1-dependent elevation of extracellular adenosine protects the liver during ischemia and reperfusion. Hepatology. 2013;58(5):1766–1778. doi: 10.1002/hep.26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beldi G, Banz Y, Kroemer A, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51(5):1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klein I, Cornejo JC, Polakos NK, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110(12):4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ikarashi M, Nakashima H, Kinoshita M, et al. Distinct development and functions of resident and recruited liver Kupffer cells/macrophages. J Leukoc Biol. 2013;94(6):1325–1336. doi: 10.1189/jlb.0313144. [DOI] [PubMed] [Google Scholar]
- 111.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ji H, Liu Y, Zhang Y, et al. T-cell immunoglobulin and mucin domain 4 (TIM-4) signaling in innate immune-mediated liver ischemia-reperfusion injury. Hepatology. 2014;60(6):2052–2064. doi: 10.1002/hep.27334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Devey L, Ferenbach D, Mohr E, et al. Tissue-resident macrophages protect the liver from ischemia reperfusion injury via a heme oxygenase-1-dependent mechanism. Mol Ther. 2009;17(1):65–72. doi: 10.1038/mt.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ellett JD, Atkinson C, Evans ZP, et al. Murine Kupffer cells are protective in total hepatic ischemia/reperfusion injury with bowel congestion through IL-10. J Immunol. 2010;184(10):5849–5858. doi: 10.4049/jimmunol.0902024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raptis DA, Limani P, Jang JH, et al. GPR120 on Kupffer cells mediates hepatoprotective effects of ω3-fatty acids. J Hepatol. 2014;60(3):625–632. doi: 10.1016/j.jhep.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 116.Perdiguero EG, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2014 doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 118.Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Davies LC, Taylor PR. Tissue-resident macrophages: then and now. Immunology. 2015;144(4):541–548. doi: 10.1111/imm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120(2):559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang M, Ueki S, Kimura S, et al. Roles of dendritic cells in murine hepatic warm and liver transplantation-induced cold ischemia/reperfusion injury. Hepatology. 2013;57(4):1585–1596. doi: 10.1002/hep.26129. [DOI] [PubMed] [Google Scholar]
- 124.Stewart RK, Dangi A, Huang C, et al. A novel mouse model of depletion of stellate cells clarifies their role in ischemia/reperfusion- and endotoxin-induced acute liver injury. J Hepatol. 2014;60(2):298–305. doi: 10.1016/j.jhep.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48(9):1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Claudio N, Dalet A, Gatti E, Pierre P. Mapping the crossroads of immune activation and cellular stress response pathways. EMBO J. 2013;32(9):1214–1224. doi: 10.1038/emboj.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu Rev Pathol. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity. 2013;39(2):211–227. doi: 10.1016/j.immuni.2013.07.017. [DOI] [PubMed] [Google Scholar]