Summary
The complex machineries involved in replication and transcription translocate along the same DNA template, often in opposing directions and at different rates. These processes routinely interfere with each other in prokaryotes, and mounting evidence now suggests that RNA polymerase complexes also encounter replication forks in higher eukaryotes. Indeed, cells rely on numerous mechanisms to avoid, tolerate, and resolve such transcription-replication conflicts, and the absence of these mechanisms can lead to catastrophic effects on genome stability and cell viability. In this article, we review the cellular responses to transcription-replication conflicts and highlight how these inevitable encounters shape the genome and impact diverse cellular processes.
Introduction
With every entry into S-phase, our genomes are threatened by multiple intrinsic factors that can lead to DNA damage and genomic instability. For example, the protective chromatin structure is disrupted to allow replication fork progression, exposing individual nucleotides to mutagenic agents from both extrinsic and intrinsic sources. In addition, the replication machinery encounters numerous natural obstacles, such as non-B form DNA structures, tightly associated DNA-protein complexes, and DNA lesions that interfere with replication fork progression, challenging its mission to faithfully complete chromosome duplication.
Transcription complexes are one such endogenous impediment to replication, and a longstanding question has been how replication forks cope with the transcription machinery engaged on the same DNA template. In prokaryotic cells, a single origin is used to assemble two replisome complexes that duplicate the circular chromosome in opposite directions. Moreover, DNA replication and transcription initiate on the same DNA template without spatial or temporal separation (Figure 1A). Because bacterial replisomes move more than 10-fold faster than RNA polymerase (RNAP) (Helmrich et al., 2013), encounters between transcription and replication complexes are unavoidable. Indeed, transcription-replication conflicts (TRCs) are routine in bacteria, and several highly orchestrated processes minimize their negative impact on genome stability and cell viability (Merrikh et al., 2012).
Figure 1. Head-on and co-directional transcription-replication conflicts.
A) In prokaryotes, a single origin (oriC) is used to initiate replication in opposite directions along the single circular chromosome. B) In eukaryotes, multiple origins initiate DNA synthesis during S-phase along the linear chromosomes. In both prokaryotic and eukaryotic genomes, head-on conflicts occur when a gene is encoded on the lagging strand, whereas co-directional encounters occur when a gene is encoded on the leading strand. C) Schematic representations of the replisome and ternary RNAP complexes converging on the template DNA in head-on or co-directional orientations. Some key eukaryotic replisome components needed for processive DNA synthesis are indicated, including the replicative helicase (MCM2-7, or DnaB in prokaryotes), leading and lagging strand DNA polymerases (Pol ε/δ), DNA primase (Pol α-Primase), single-strand DNA binding protein (RPA) and clamp loader complex (PCNA).
Eukaryotic genomes are significantly larger than prokaryotic genomes, and several hundreds to thousands of replication origins fire along their multiple, linear chromosomes in a staggered fashion (Figure 1B). DNA replication is typically restricted to S-phase, allowing for unperturbed transcription in other cell cycle phases and potentially minimizing TRCs. In addition, transcription in S-phase is spatially separated from replication in distinct territories of the nucleus (Wei et al., 1998). This spatio-temporal segregation, coupled with comparable translocation rates for replication and transcription complexes (Helmrich et al., 2013), once raised the question of whether these complexes necessarily interfere in eukaryotic cells. However, recent studies described herein suggest that conflicts between eukaryotic replisomes and transcription machineries do arise in certain situations and at specific genomic regions which could lead to chromosome instability in eukaryotic cells. In addition, mounting evidence that the vast majority of the human genome is transcribed well beyond the boundaries of known genes increases the possibility that TRCs are more pervasive than previously anticipated (Hangauer et al., 2013).
Here, we review the different types of conflict that can occur between replication forks and transcription complexes. Although prokaryotic and eukaryotic cells employ different strategies to reduce the frequency and impact of TRCs, they are unavoidable. We will focus our discussion on the transcription- and replication-dependent mechanisms utilized by the cell to tolerate, coordinate and resolve these conflicts as well as discuss their potential physiological and pathological consequences.
Types of Transcription-Replication Conflicts
Transcription-replication encounters can occur in different ways depending on the directionality and the functional state in which both machineries meet. Although our understanding of these processes is still far from complete, it is becoming evident that the severity and impact of a collision on genome stability depends on the orientation and the type of transcriptional block.
DNA replication and transcription complexes initiate synthesis of complementary DNA or RNA strands from distinct genomic locations, termed origins and promoters, respectively. Both machineries are highly processive and exhibit strict 5’ to 3’ polarity. As a result, they can approach each other either in a head-on or co-directional orientation (Figure 1C). Studies in both bacteria and eukaryotic cells indicate that head-on collisions inhibit fork movement to a greater extent than co-directional collisions and are the more relevant cause of genomic alterations (Mirkin and Mirkin, 2005; Prado and Aguilera, 2005; Srivatsan et al., 2010). Several molecular mechanisms could underlie these findings. First, the initial replisome factors likely to encounter the RNAP complex are the replicative helicases, DnaB in prokaryotes and MCM2-7 in eukaryotes, which unwind the parental DNA strands ahead of the replisome. The opposing forces associated with a frontal clash may inactivate these helicases. Consistent with this idea, replication pauses only in transcribed regions of plasmids with head-on TRCs, suggesting that the replication fork is blocked upon head-on physical interaction with RNAP complexes (Mirkin and Mirkin, 2005; Prado and Aguilera, 2005; Srivatsan et al., 2010). A second possible explanation is that positive DNA supercoils which accumulate between converging transcription and replication machineries impede DNA unwinding between the two advancing complexes, thereby inhibiting both processes (Bermejo et al., 2012). In human cells, depletion of Topoisomerase I increases negative supercoiling in actively-transcribed DNA, resulting in replication fork stalling and DNA breaks at certain S-phase-transcribed genes (Tuduri et al., 2009). A co-directional encounter is not subject to either of the aforementioned challenges because the co-directional collision would push RNAP in the same direction as the replisome and the positive topological stress arising in front of the fork would neutralize the negative supercoiling behind RNAP. Indeed, in bacterial systems co-directional collisions can result in productive displacement of the RNAP complex, allowing DNA replication to proceed (French, 1992; Pomerantz and O’Donnell, 2008).
Although head-on collisions are typically more problematic for the cell, co-directional collisions can also be detrimental, particularly when the replication fork is challenged with more persistent transcriptional blocks than a normally elongating RNAP complex. Indeed, RNAP complexes exist in different states and are prone to spontaneous or regulated pausing at certain DNA sequences. Moreover, the rate of transcription is inherently heterogeneous, and RNAP can be persistently blocked by DNA lesions. Such “trapped” transcription complexes may have a different conformation or stability than actively transcribing RNAP molecules and may represent greater barriers for an approaching replication fork.
One type of immobilized transcriptional roadblock is backtracked RNAP. Paused or stalled transcription complexes can undergo a dramatic conformational change, allowing the enzyme to backtrack along the DNA template. This results in displacement of the 3′ end of the nascent RNA from the active site, trapping the enzyme in a highly stable but transcriptionally inactive state (Nudler, 2012). Importantly, the knockout of anti-backtracking factors in Escherichia (E.) coli leads to replication fork arrest (Mirkin et al., 2006) and can induce double strand breaks (DSBs) on a plasmid template in the co-directional orientation (Dutta et al., 2011). Thus, RNAP backtracking may promote TRCs with potentially deleterious consequences.
Backtracked or arrested transcription complexes may also block other RNAPs on the same DNA template, leading to the accumulation of these complexes especially in highly transcribed genes. Consistent with this view, highly transcribed ribosomal RNA (rRNA) genes in Bacillus (B.) subtilis require replication restart factors to allow passage of the replication fork, indicating that replication is perturbed upon collision (Merrikh et al., 2011). In yeast, it is not clear whether replication forks slow upon encounter with highly transcribed genes. One study provided evidence for fork slowing at highly transcribed RNAPII genes, independent of gene orientation (Azvolinsky et al., 2009), while another study using a distinct approach concluded that only a small number of forks pause in this scenario (Sekedat et al., 2010). In any case, it seems likely that clearing an array of RNAP complexes would pose a greater challenge for the cell than bypassing a single RNAP complex, even in the generally more permissive co-directional orientation.
R-loops are another type of transcriptional barrier to replication that form when the nascent RNA strand hybridizes with the template strand. These RNA:DNA hybrid-containing secondary structures arise at thousands of genes throughout the genomes of bacteria to humans (Chan et al., 2014; Gowrishankar et al., 2013; Sanz et al., 2016). Although physiological functions for R-loops have been described, their accumulation also inhibits fork progression and results in hyperrecombination, DNA damage, and genomic instability phenotypes (Gan et al., 2011; Stirling et al., 2012; Wahba et al., 2011). Whether the orientation of the approaching replication machinery (head-on versus co-directional) affects the outcome of R-loop mediated TRCs is unclear.
Despite clear evidence that R-loops cause TRCs in a variety of organisms, the molecular details underlying the mechanism by which these structures perturb DNA replication and lead to DNA breaks remain elusive. For example, whether the associated RNAP complex, the R-loop itself or a processed intermediate blocks the replication fork in vivo is unclear. A recent study showed that R-loops induced by the absence of diverse RNA processing factors are processed into DSBs by the transcription-coupled nucleotide excision repair (TC-NER) nucleases XPG and XPF (Sollier et al., 2014). Whether these R-loops are recognized as a canonical substrate of the TC-NER pathway or aberrantly processed by these flap-endonucleases is not clear. Such processing may lead directly to DSB formation, or to the formation of ssDNA breaks or gaps that ultimately cause fork collapse and DSBs during replication. Interestingly, recent studies indicate that R-loop forming regions also accumulate histone markers of condensed chromatin (Castellano-Pozo et al., 2013; Skourti-Stathaki et al., 2014). Thus, R-loop mediated chromatin compaction may contribute to the impairment of fork progression and aggravate TRCs at R-loop forming regions.
Coordinating Transcription and Replication
Although prokaryotes cannot avoid TRCs on their circular chromosome, bacterial genomes have evolved a simple strategy to minimize their impact: highly transcribed and essential genes are preferentially co-oriented with the replisome (Figure 2A). This bias is conserved in all known bacteria studied (Guy and Roten, 2004) and especially important at highly transcribed and essential genes, as reversing the orientation of ribosomal RNA (rRNA) clusters, for example, disrupted replication and resulted in genome instability, activation of the SOS response, and cell death (Srivatsan et al., 2010). Interestingly, such a co-orientation bias is not obvious in S. cerevisiae, whereas it may be present in the human genome (Petryk et al., 2016). This difference may be explained by the compact nature of the yeast genome, which increases the likelihood that a replication fork meets a transcription complex in either orientation (McGuffee et al., 2013). With the discovery of pervasive transcription, however, analyses focusing solely on canonical genes may present an incomplete picture.
Figure 2. Coordinating transcription and replication.
A) Schematic representation of the E. coli and B. subtilis circular chromosomes. Genes encoded on the (+) and (−) strand are shown in red and black, respectively, with a bias of ~55% or 75% of the genes encoded on the leading strand of replication, respectively. oriC, Replication origin; Ter, Termination region. The chromosome maps were generated with SnapGene.
B) Replication fork barriers prevent head-on collisions at the highly transcribed rRNA genes. The yeast rDNA locus consists of the RNAP I transcribed 35S rRNA gene as well as an intergenic spacer region that contains an autonomously replicating sequence (ARS). Although replication is initiated bidirectionally, a replication fork barrier (RFB) bound by the fork blocking protein 1 (Fob1) prevents forks from entering the 3’ end of the rRNA gene.
C) Spatial separation of replication and transcription sites throughout S phase. The images show nuclei of mouse 3T3 cells in early, mid or late S-phase pulsed simultaneously with digoxigenin-dUTP or BrUTP to mark active sites of DNA replication or transcription, respectively. The images were modified and reprinted from (Wei et al., 1998) with permission. The cartoon illustrates a possible interpretation of such clusters of high transcription (RNAP complexes in red) or replication activity (replisome complexes in green), supporting the view that the functional organization of the nucleus in space and time can reduce interference between the two machineries.
Although the orientation of replication and transcription is generally random in the yeast genome, certain regions may be protected from head-on TRCs. The multicopy rRNA gene cluster contains repeats of the heavily transcribed 35S rRNA gene, separated by an intergenic spacer encoding an autonomous replicating sequence (ARS). Although replication is initiated bidirectionally at the ARS, a polar replication fork barrier downstream of active 35S rRNA genes prevents frequent head-on collisions by blocking forks from progressing in the direction opposite to RNAP I transcription (Figure 2B) (Linskens and Huberman, 1988). Transcription-dependent replication fork pause sites have also been described at yeast transfer RNA (tRNA) genes and at the ribosomal gene arrays of other organisms (Labib and Hodgson, 2007). These conserved regulatory elements in genomic regions with exceptionally high transcriptional activity may help reinforce co-directionality and minimize the more deleterious consequences of head-on TRCs.
Another mechanism by which TRCs may be minimized involves the redistribution of replication initiation factors on chromatin prior to their activation. The genome-wide distribution of Mcm2–7 in Drosophila is shaped by active transcription (Powell et al., 2015). Similarly, in yeast, Mcm2-7 complexes loaded on DNA in G1-phase are repositioned by RNA polymerase outside of new transcription zones formed in transcription termination mutants (Gros et al., 2015). The ability of transcription to redistribute future replication origins in G1 could help minimize the interference between replication and transcription machineries in the subsequent S phase.
During S-phase, eukaryotes compartmentalize replication of their genomes so that DNA replication and transcription machineries occupy distinct nuclear territories and act at distinct times (Figure 2C) (Wei et al., 1998). Coordinated timing of genome replication and transcription is particularly apparent at the human rDNA gene clusters. Transcriptionally active rDNA genes are replicated early in S-phase, whereas silent rDNA copies replicate later (Dimitrova, 2011). More generally, a nascent RNA capture assay showed a global anti-correlation between transcription and replication timing (Meryet-Figuiere et al., 2014). Thus, higher eukaryotes coordinate transcription and replication in the nucleus spatially and temporally and thereby minimize interference between both processes.
Nevertheless, there appear to be certain places in the genome where these safeguards are difficult to implement. For example, conflicts are unavoidable at the longest human genes, which undergo transcription for longer than one cell cycle. Some of these genes overlap with common fragile sites (CFSs), loci that replicate late in S-phase and are hotspots for chromosomal instability. The highly recurrent association of CFSs with large genes suggests a functional relationship between transcription and fragility, but the mechanisms underlying this association are not understood (Le Tallec et al., 2014). R-loops may aggravate the fragility of a small subset of CFSs (Helmrich et al., 2011), but the majority of large genes are not fragile (Le Tallec et al., 2013). Interestingly, neural stem and progenitor cells have recurrent DSB clusters which are also found in long, transcribed and late-replicating genes (Wei et al., 2016), suggesting such conflicts may be more common than previously anticipated. Another class of fragile sites are known as early-replicating fragile sites (ERFSs). As these are found at actively transcribed genes replicated in early S phase, it is tempting to speculate that the interference of transcription and replication machineries at genes in proximity to these ERFSs may account for their fragility (Barlow et al., 2013). Interestingly, ERFS exhibit significant overlap with recurrent amplifications and deletions observed in B cell lymphomas, indicating that TRCs near early-firing origins may be relevant to the etiology of certain cancer types. Transcription-replication interference may also contribute to oncogene- and growth-factor induced replication stress, which is often associated with increased transcriptional activity (Jones et al., 2013). This deregulated transcription could drive cells into S-phase prematurely, leading to stronger transcriptional blocks and altering the timing of origin firing, both of which could augment the number of TRCs. Although it is currently unclear whether the high levels of oncogene expression in many cell models is comparable to the levels observed in vivo in cancer cells, the connections between replication, origin firing and transcription highlight the possibility that TRCs represent a potent source of genomic lesions that drives instability in different cancer types based on differential transcription of the regions involved (Barlow et al., 2013; Stork et al., 2016).
Co-transcriptional Mechanisms to Suppress Conflicts
Because transcription can lead to several different types of roadblocks along the path of the replisome (Figure 3), mechanisms that reactivate, destabilize, or remove these transcriptional blocks should suppress conflicts between the transcription and replication machineries. In this section, we review the mechanisms utilized by prokaryotic and eukaryotic cells to relieve different transcriptional blocks.
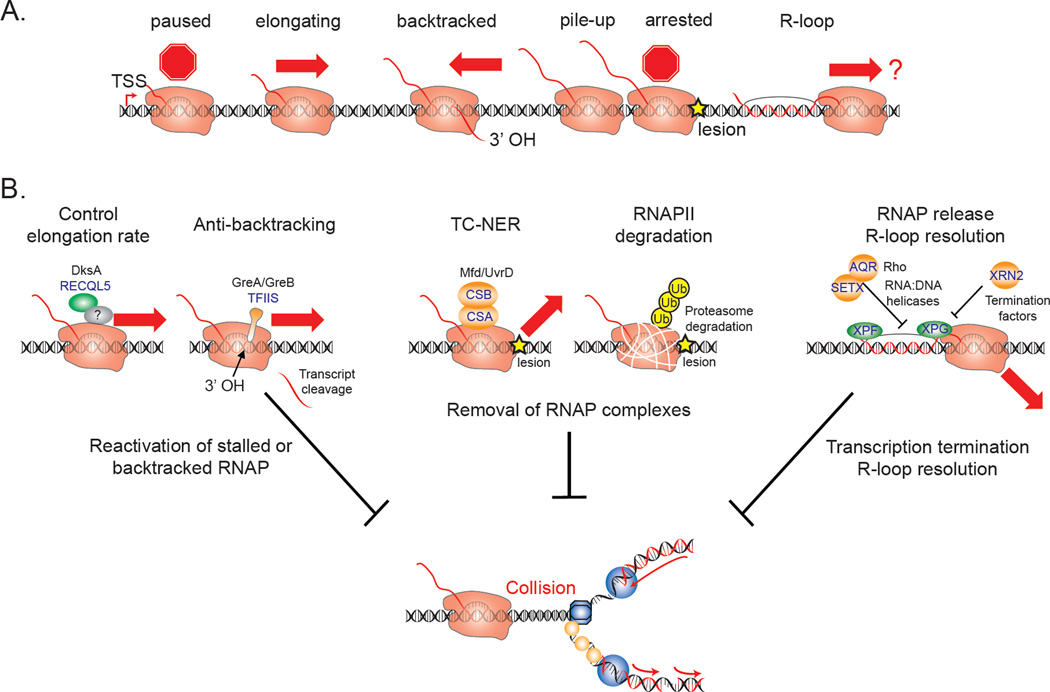
Figure 3. Co-transcriptional mechanisms to suppress TRCs.
A) During transcription, the ternary RNAP complex associates with the DNA template in different functional states and conformations that could lead to different types of transcription blocks. B) Transcription roadblocks may be resolved by distinct pathways. The prokaryotic (black) and eukaryotic (blue) proteins involved are shown. DksA in bacteria and the human RECQL5 helicase reduce stalling or pausing events by controlling the transcription elongation rate. The anti-backtracking factors GreA/GreB or TFIIS promote cleavage of the backtracked transcript and create a new 3’OH group in the active site to resume transcription. RNAP complexes stalled at a site of DNA damage can be removed from the DNA template by TC-NER or proteasome-mediated degradation via poly-ubiquitylation. Transcription termination and resolution of R-loops is mediated by the Rat1/XRN2 exonuclease and RNA:DNA helicases including Rho in prokaryotes and Sen1/SETX or AQR in eukaryotes. In addition, R-loops may be recognized and processed by the TC-NER endonucleases XPF/XPG. All of these co-transcriptional mechanisms help to remove the transcriptional blocks and thereby suppress collisions with replication forks. Yellow star represents the lesion in both panels.
Reactivation of Stalled or Backtracked RNAP Complexes
There are multiple mechanisms in place to promote RNAP elongation and reactivate backtracked polymerases. In prokaryotes, the absence of a nuclear membrane allows direct coupling of transcription and translation, and ribosomes are loaded onto the nascent RNA strand as it emerges from RNAP. This prevents spontaneous RNAP backtracking and effectively increases the rate of transcription elongation (Dutta et al., 2011; Proshkin et al., 2010). However, additional factors are required to suppress RNAP stalling along the bacterial chromosome. For example, GreA and GreB promote cleavage of the displaced transcript in a backtracked RNAP, producing a new 3’-OH end to resume transcription (Opalka et al., 2003). Moreover, the transcription factor DksA promotes elongation by reducing nucleotide misincorporation, which can result in pausing and backtracking, thereby limiting TRCs (Roghanian et al., 2015; Tehranchi et al., 2010). In eukaryotes, the stimulator of Pol II transcription elongation factor (TFIIS) has functions related to these bacterial factors. Although not conserved in sequence or structure to GreA or GreB, TFIIS stimulates transcript cleavage to restart arrested RNAP complexes using an analogous mechanism (Cheung and Cramer, 2011). Importantly, TFIIS also has RNA proofreading activity and promotes cleavage of misincorporated nucleotides (Thomas et al., 1998), thereby maintaining transcriptional fidelity and reducing RNAP arrest. Interestingly, the human RECQL5 helicase decreases the transcription elongation rate, so as to reduce stalling and/or backtracking (Saponaro et al., 2014). Moreover, human cells lacking RECQL5 show increased chromosomal rearrangements, a significant fraction of which are located at CFSs. Together, these findings suggest transcription rates must be carefully controlled, as both reduced and increased rates may contribute to transcription-associated genome instability. In addition, prokaryotic and eukaryotic cells have independently evolved multiple mechanisms to reactivate backtracked polymerases, indicating that the ability to resume productive transcription is crucial for limiting conflicts with replication forks.
Removal of RNAP Complexes
A variety of DNA damaging agents can cause lesions in the DNA template that efficiently block RNAP progression. In these cases, transcription can only proceed when the lesion is repaired by NER, a process that requires RNAP removal. Prokaryotes use two different NER factors to dislodge the blocked RNAP complex. The Mfd protein approaches RNAP from behind to displace the ternary complex (Ganesan et al., 2012), whereas a complex containing the translocase UvrD pulls RNAP back from the damaged site to displace it (Epshtein et al., 2014). In eukaryotes, the TC-NER pathway specifically repairs lesions in actively transcribed genes, and RNAP removal is also a crucial step in this process. The arrested RNAP complex recruits Cockayne Syndrome (CS)A and CSB, which are instrumental in recruiting the basal NER machinery and auxiliary proteins required for subsequent repair-dependent DNA synthesis. How RNAP is removed in the context of TC-NER is not clear. However, if TC-NER fails, permanently arrested RNAPII is poly-ubiquitylated and degraded in a proteasome-dependent pathway (Wilson et al., 2013). After RNAP degradation, other repair pathways like global genome nucleotide excision repair or base-excision repair may remove the transcription-blocking lesion.
Importantly, long-term arrest of RNAP complexes on the DNA template will ultimately lead to interference with replication forks. Indeed, a yeast RNAPII mutant with increased chromatin retention causes replication fork stalling, suggesting that RNAPII removal is an important step to facilitate fork progression upon a transcription-replication encounter (Felipe-Abrio et al., 2015). Intriguingly, TC-NER-deficient human fibroblasts also exhibit apoptosis during S-phase progression (McKay et al., 2002). This result suggests that an irreversibly arrested RNAP complex may trigger a lethal event in case of collision with the replisome. It also highlights the importance of the mechanisms described here to suppress TRCs and may be relevant to the phenotypes found in CS patients with TC-NER defects.
Transcription Termination and R-loop Resolution
Rapid removal of transcription complexes at the end of genes can also reduce potential interference between transcription and replication. In bacteria, read-through is prevented by transcription termination, one form of which involves Rho, a homohexameric helicase that triggers the dissociation of RNAP from RNA and the template (Peters et al., 2011). Inhibition of Rho-dependent transcription termination induces replication fork arrest and DNA DSBs, suggesting that a critical function of termination factors is to prevent RNAP complexes from blocking DNA replication (Washburn and Gottesman, 2011). Termination of eukaryotic protein-coding genes involves pausing of RNAPII and mRNA processing by components of the cleavage and polyadenylation machinery (CPM). Intriguingly, a screen for yeast mutants that increase the formation of Rad52 foci, a marker for recombination-mediated repair, identified seven subunits of the CPM, indicating that defects in transcription termination lead to recombination (Stirling et al., 2012). Termination of non-coding RNA genes in yeast is mediated by a complex containing the RNA:DNA helicase Sen1. After binding to the nascent RNA, Sen1 translocates to RNAPII and initiates termination. Mutation of the Sen1 helicase domain results in genome-wide transcription termination defects (Steinmetz et al., 2006), concurrent with transcription-associated genomic instability (Mischo et al., 2011). A similar function was described for the human homolog Senataxin (SETX), which promotes transcription termination by exonuclease-mediated (XRN2) degradation of the RNA at G-rich pause sites downstream of the polyadenylation signal (Skourti-Stathaki et al., 2011). Interestingly, numerous termination mutants, including CPM mutants and Sen1/SETX mutants, show increased R-loop levels, in the latter case particularly at transcription termination sites (Mischo et al., 2011; Skourti-Stathaki et al., 2011; Stirling et al., 2012). The strong link between transcription termination and R-loop formation may be explained by the notion that inefficient termination increases the presence of a transcript at the 3’ end of genes, allowing for unscheduled interactions with the DNA duplex. The formation of stable RNA:DNA hybrids and RNAP complexes in this scenario may pose a stronger transcriptional block, resulting in a higher chance of collisions with replication forks.
Intriguingly, some termination factors may also be used to release transcription complexes “trapped” by a TRC. Sen1 associates directly with replication forks that encounter highly expressed RNAPII genes, suggesting a role for this helicase in coordinating replication with transcription at these sites (Alzu et al., 2012). Importantly, the Sen1 function at replication forks may be conserved, as human SETX forms distinct foci that co-localize with DNA damage markers in S/G2 phase cells, particularly after the induction of replication stress, and these SETX foci are diminished after RNaseH overexpression. This could indicate a potential role for SETX in resolving R-loops specifically formed during TRCs (Yüce and West, 2013).
Interestingly, a recent genome-wide study in fission yeast indicates that Dcr1, but not other components of the RNA interference (RNAi) machinery, can promote the release of RNAPII at TRCs (Castel et al., 2014). How Dcr1 is specifically recruited to these sites and can distinguish them from other stalled RNAP complexes not involved in TRCs is an unresolved question. Importantly, this link to the RNAi machinery may also exist in human cells, as dicer and other components of this pathway are recruited to double-stranded RNA formed by antisense transcription over R-loops at certain G-rich terminator regions (Skourti-Stathaki et al., 2014). Future research will be needed to determine whether this mechanism is tied to TRCs in higher eukaryotes.
R-loops are prevalent, dynamic structures throughout the genome of prokaryotic and eukaryotic cells, and in addition to termination sites, they are enriched at telomeres, rDNA and highly transcribed genes. Moreover, numerous factors leading to the increased formation of these structures have been reported. The causes and consequences of increased R-loop formation have been extensively discussed in other reviews (Groh and Gromak, 2014; Santos-Pereira and Aguilera, 2015; Sollier and Cimprich, 2015). However, it is important to emphasize here that their accumulation can cause replication fork stalling. It follows that the mechanisms to suppress or remove RNA:DNA hybrids, including the use of topoisomerases, RNaseH enzymes, endonucleases and RNA:DNA helicases can reduce the frequency of TRCs in the genome. An open question is whether these different activities remove R-loops from overlapping or distinct sets of target genes. If this is the case, these factors may prevent TRCs at specific genomic loci, and their dysfunction could cause distinct patterns of genome instability.
Replication-Associated Mechanisms to Overcome Conflicts
Replication stress is defined as the slowing or stalling of replication fork progression and/or DNA synthesis and various types of natural DNA lesions can lead to replication stress. Strikingly, stalled transcription complexes or transcription-associated RNA:DNA hybrids can also induce the replication stress response. Thus, mechanisms that stabilize or reactivate stalled replication forks can help the cell overcome conflicts with transcription-associated obstacles.
Auxiliary Helicases
Auxiliary helicases traveling with replication forks are found in both prokaryotic and eukaryotic cells, and their activities may help the replisome dislodge transcription complexes (Figure 4A). Among these are E. coli Rep and UvrD and their B. subtilis homologue PcrA, all of which promote replication through transcribed genes. Notably, these SF1 superfamily helicases may act in distinct ways. Rep and PcrA may be fork-specific motor proteins that facilitate replication across RNAP bound DNA templates, whereas UvrD may directly interact with arrested RNAP complexes as part of the TCR pathway (Guy et al., 2009; Gwynn et al., 2013; Merrikh et al., 2015). Rrm3, a yeast Pif1 family helicase, is also associated with the replisome and facilitates progression through highly transcribed genes (Azvolinsky et al., 2009). Interestingly, RNase H overexpression significantly reduced the association of Rrm3 with these genes (Gómez-González et al., 2011), indicating that this helicase accumulates at RNA:DNA hybrids that impair replication fork progression. In addition, the fission yeast Pif1 homologue Pfh1 is enriched at highly transcribed genes and its absence leads to replication fork stalling (Sabouri et al., 2012). Importantly, other non-B DNA structures, like G-quadruplexes that can form co-transcriptionally, are also substrates of Pif1 family helicases (Paeschke et al., 2013). As most eukaryotes encode a Pif1 family helicase, the function of these enzymes to promote fork progression across highly transcribed or co-transcriptional non-B DNA structures may be conserved.
Figure 4. Replication-associated mechanisms to prevent and resolve transcription-replication conflicts.
A) Accessory helicases including Rep (E. coli), PcrA (B. subtilis), Rrm3, Sen1 (yeast) or SETX (human) can assist the replicative helicase by dislodging transcription complexes ahead of the replication fork.
B) The S-phase checkpoint can monitor and respond to replication forks stalled at transcription complexes in eukaryotic cells. The Mec1/ATR kinase may promote fork progression and stability at transcribed genes by phosphorylating the nucleoporin Mlp1, thereby releasing genes from the nuclear pore to reduce topological tension. The checkpoint also controls tRNA gene transcription mediated by the Maf1 repressor to reduce interference with replication. The osmostress-induced protein kinase Hog1 phosphorylates Mrc1, a downstream component of the Mec1/ATR pathway, thereby preventing early origin firing and fork progression to prevent TRCs during osmostress. In addition, Mec1/ATR, in cooperation with INO80 and PAF1C, can trigger the efficient removal of chromatin-bound RNAPII near early firing origins. NPC, Nuclear Pore Complex
C) Replication forks stalled at transcription complexes can resume DNA synthesis by different fork restart and DNA repair pathways. (i) A replication fork stalled at a transcription complex can be rescued by firing of an adjacent dormant origin. This back-up replication fork encounters the transcription complex from the opposite direction and may represent a second chance to remove the transcription block and resume DNA synthesis. Alternatively, replication forks stalled at TRCs may be stabilized by ATR, BRCA2, or the FA-pathway (ii). Prolonged stalling of the replication fork may also promote re-annealing of parental strands priming fork reversal. Removal of the transcription block by one or several of the pathways/factors listed can then promote fork restart (iii). If the transcription block persists, this may ultimately lead to fork breakage (iv). Break-induced replication (BIR) and/or homologous recombination (HR)-dependent repair mechanisms may then be used to overcome the obstacle.
S-phase Checkpoint
In prokaryotes, replication is largely regulated at the point of initiation, and there appears to be no checkpoint that monitors the progress of ongoing replication. In eukaryotes, however, the spatial and temporal coordination of hundreds to thousands of origins requires multi-faceted regulation. Replication origins are first recognized and licensed in G1, and origin firing only occurs upon entry into S-phase. This two-step process prevents re-replication by restricting origin licensing to G1. If replication forks stall in bacteria, replication restart factors can replace essential replisome components to resume DNA synthesis. Eukaryotic cells use two different strategies in this scenario. First, because only a subset of all licensed origins are activated in any one cell cycle, a backup set of origins can rescue and complete DNA synthesis in the vicinity of a defective replication fork. Second, the ataxia telangiectasia mutated and Rad3-related (ATR)-dependent replication checkpoint is activated to promote the completion of DNA synthesis by regulating origin firing, stabilizing replication forks and promoting fork repair and restart (Ciccia and Elledge, 2010; Cimprich and Cortez, 2008).
Growing evidence also implicates the ATR kinase pathway in coordinating replication with transcription (Figure 4B). In yeast, highly transcribed genes are anchored to the nuclear pore to couple mRNA synthesis with cytoplasmic export. Although such gene gating promotes rapid gene expression, it also creates topological stress and inhibits replication fork progression. In yeast, the ATR homolog, Mec1, and its downstream effector, Rad53, uncouple transcribed genes from nuclear pores by phosphorylating the nucleoporin Mlp1, thereby neutralizing the topological tension generated (Bermejo et al., 2011). Mec1 also responds to replication stress by phosphorylating Maf1, a conserved repressor of tRNA genes. This response inhibits tRNA gene transcription and may therefore prevent interference with replication (Nguyen et al., 2010). In addition, phosphoproteomic data suggest that Mec1-Rad53 regulates many other proteins involved in transcription and RNA processing in normal S phase, raising the possibility that Mec1 is needed for the replication of many transcriptional barriers (Bastos de Oliveira et al., 2015). Furthermore, Mec1 acts with the INO80 chromatin remodeling complex and the PAF1C transcription complex to degrade a subset of chromatin-bound RNAPII at genes transcribed near early firing origins (Poli et al., 2016). Finally, R-loop accumulating mutants in yeast exhibit synthetic growth defects with S-phase checkpoint mutants, suggesting that replication forks stalled at RNA:DNA hybrids rely on checkpoint-dependent mechanisms to overcome the transcription barrier (Gómez-González et al., 2009). In mammalian cells, the ATR kinase appears to play a related role as it regulates the stability of both ERFS and CFS regions, some of which correspond to R-loop accumulating or highly transcribed genes (Barlow et al., 2013; Casper et al., 2002). Together, these studies indicate the importance of a functional S-phase checkpoint in monitoring ongoing replication and negotiating TRCs.
Cells also have a distinct checkpoint pathway that may coordinate transcription and replication under conditions of induced transcription. The stress-activated protein kinase Hog1 promotes the activation of hundreds of genes under osmostress. Interestingly, Mrc1, a downstream effector of Mec1, is phosphorylated by Hog1. This phosphorylation is independent of the Mec1 pathway and delays origin firing to prevent TRCs and transcription-associated recombination during this transcriptional outburst (Duch et al., 2013). It will be interesting to determine if this general mechanism might be important in other physiological situations and species where a similar outburst of transcription occurs. For example, there are dramatic transcriptional changes that occur during the host cell response to viral infections, during hormone stimulation of breast cancer cells and upon activation of dormant hematopoietic stem cells, and many of these physiological stress conditions lead to DNA damage and genome instability (Stork et al., 2016; Walter et al., 2015). It is tempting to speculate that TRCs increase in these situations, and the mechanisms to avoid and resolve them become particularly important to minimize their effect on genome stability.
Replication Restart and DNA Repair
The fate of the replisome following TRCs remains an intriguing question that is the subject of intense research. How can a replication fork stalled at a transcription block overcome the conflict and restart DNA synthesis? Several fork restart and repair mechanisms have been implicated in this important task (Figure 4C). For example, an in vitro study with bacterial proteins showed that replication forks remain stable and displace RNAP when they meet in the co-directional orientation. Intriguingly, the RNA transcript was used as a primer to restart DNA synthesis after the collision (Pomerantz and O’Donnell, 2010). It is unclear, however, if such a mechanism exists in vivo, especially if the replisome is challenged with an array of multiple RNAPs. Indeed, in B. subtilis, the replication restart protein PriA and the helicase loaders DnaB and DnaD are required to resume replication after resolution of a TRC at the highly transcribed rDNA operon (Merrikh et al., 2011). Thus, if the replicative helicase is inactivated or disassembled at the conflict site, prokaryotic cells may use these factors to establish a new replication fork and complete DNA synthesis. This type of replication initiation allows for transient clearance of the replisome from the conflict region, giving NER or anti-backtracking factors access to remove the lesion or reactivate transcription.
In eukaryotes, alternative mechanisms to replication restart may be more important for completing DNA replication. As noted previously, eukaryotes only use a subset of licensed replication origins in any one cell cycle. Thus, a common mechanism utilized to complete DNA synthesis may involve the firing of a backup origin adjacent to a TRC. Nevertheless, a large body of evidence indicates that overcoming transcription-dependent replication blocks requires the activity of additional factors involved in DNA recombination, the activity of which appears to be particularly important during head-on confrontations (Gottipati et al., 2008; Prado and Aguilera, 2005; Wellinger et al., 2006). The molecular mechanism(s) by which a fork stalled at a transcription complex gives rise to the DSB or ssDNA gap needed to initiate recombination are still unresolved.
Interestingly, recent studies showing that R-loops accumulate in cells without the recombinational repair and tumor suppressor genes BRCA1 and BRCA2 provide a mechanistic connection between recombination and R-loops in higher eukaryotes (Bhatia et al., 2014; Hatchi et al., 2015). In particular, it was shown that BRCA1 interacts with SETX to prevent ssDNA break formation and mutation at certain R-loop prone transcription termination regions. This finding suggests that BRCA1 may suppress R-loop formation and subsequent processing. How BRCA2 acts at R-loops is less clear. Replication forks stalled at R-loops may be stabilized and restarted by BRCA2, or a BRCA2-dependent recombination event may be necessary to overcome this obstacle (Roy et al., 2012). Interestingly, BRCA2 is also a member of the Fanconi anemia (FA) DNA repair pathway of interstrand crosslinks, which was recently shown to be activated by R-loop-mediated conflicts between transcription and replication (García-Rubio et al., 2015; Schwab et al., 2015). In vitro experiments also raise the possibility that one member of the FA pathway, FANCM, directly resolves the fork-blocking R-loop using its translocase activity (Schwab et al., 2015).
As our understanding of the molecular mechanism(s) by which a fork stalled at a transcription complex can restart is nascent, additional mechanisms are likely to emerge. For example, topological stress or fork remodeling enzymes could promote fork reversal when forks stall at transcription barriers. This could stabilize the fork structure and allow more time to promote resolution of the transcriptional block. Once resolved, the reversed fork could be remodeled for direct restart. If the block persists, however, restart could be initiated by break formation and recombination-mediated processes like those that act on forks stalled by other means. Similarly, template switching and bypass pathways could be important for progression past TRCs (Gómez-González et al., 2009). Indeed, some TRCs could be “tolerated” by the cell using the template switching pathways that are utilized to tolerate some lesions during S phase. This could be one mechanism to manage the conflicts that inevitably arise at long genes, and it would also allow cells to maintain R-loop structures that may have physiological functions in the cell. The recently identified PrimPol activity could also facilitate fork restart by reinitiating DNA synthesis downstream of TRCs (García-Gómez et al., 2013). Interestingly, in the rDNA gene cluster of yeast, co-transcriptional R-loops can initiate DNA synthesis in a non-canonical manner using the transcript as a primer (Stuckey et al., 2015). Although speculative, this R-loop-mediated and origin-independent mechanism for reinitiation of DNA synthesis may help overcome R-loop mediated TRCs. More direct experiments focusing on the replication intermediates and the fork structure during TRCs will be necessary to determine if and how TRC-stalled forks differ from those blocked by other lesions.
Physiological and Pathological Consequences of Transcription-Replication Conflicts
As TRCs may represent a significant endogenous source of replication stress, a failure to avoid or resolve these conflicts could lead to genomic instability, a hallmark of cancer and other human diseases. Indeed, in prokaryotes a distinct mutational signature arises from TRCs (Sankar et al., 2016). Moreover, in eukaryotes, the previously discussed connections between transcription and fragile sites, as well as connections between TRCs and the FA pathway, support this idea. TRCs may also be relevant to mutations found in BRCA1 and BRCA2-associated cancers. Indeed, BRCA1 null breast tumors exhibit specific genomic alterations at R-loop transcription termination sites where BRCA1 normally associates (Hatchi et al., 2015), linking this protein and transcription more directly to mutation accumulation. In this context, it is interesting to note that gene expression patterns differ significantly between different cell and tissue types. Thus, the sites where TRCs arise may vary from one cell type to the next, based on differential gene expression patterns (Barlow et al., 2013; Stork et al., 2016). Although speculative, this may contribute to tissue- or cell type-specific DNA damage and mutation patterns that appear in cancer cells.
TRCs may also have pathological consequences in the context of trinucleotide repeat disorders. R-loop dependent TRCs may occur at the GAA and CGG repeats found in the FMR1 and FXN genes, expansion of which is associated with Friedreich ataxia and fragile X syndrome, respectively. These repeat regions form stable RNA:DNA hybrids, making it tempting to speculate that subsequent TRCs can induce DSBs at these regions. Aberrant slippage and recombinational repair of these breaks may ultimately lead to repeat expansion, increasing the severity of the disease (Groh et al., 2014; Loomis et al., 2014). Stable R-loops were also shown to form in vitro at GGGGCC-expanded regions in C9ORF72. Thus, TRCs may also contribute to repeat expansion at this locus, most common in the neurodegenerative disorders amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (Haeusler et al., 2014). Interestingly, a link to neurodegenerative disorders is associated with the mutation of some R-loop resolution factors (Groh and Gromak, 2014). For example, mutations in human SETX cause ataxia with oculomotor apraxia 2 (AOA2) and ALS4 (Chen et al., 2004; Moreira et al., 2004). It is unclear if and how the accumulation of R-loops and subsequent TRCs may contribute to these neurodegenerative phenotypes, since neurons are generally not dividing in mammalian brains. Clearly, further research is needed to determine whether there is a molecular link between TRCs, R-loop formation, and neurodegeneration phenotypes.
Although largely discussed in a pathological context, TRCs may have some beneficial roles as well. For example in bacteria, head-on TRCs were shown to significantly increase adaptive structural variation in coded proteins, allowing for accelerated gene evolution (Paul et al., 2013). In higher eukaryotes, TRCs may have a function at the midblastula transition (MBT) where dramatic changes in cell cycle progression and zygotic gene transcription occur and are necessary for morphogenesis. Intriguingly, the ATR-homolog in Drosophila is activated at this transition as a result of replication fork stalling at sites of de novo RNAPII transcription. This checkpoint activation leads to changes in the cell cycle necessary for the MBT. Thus, TRCs may play a crucial role in this developmental context (Blythe and Wieschaus, 2015). Finally, it is tempting to speculate that TRCs may be important in promoting diversification of neuronal function by changing the genomic sequence of individual neurons. The repair of DSBs is required for neural development, analogous to what is observed in the immune system, and somatic diversity in the brain has been observed. Interestingly, the recurrent DSB clusters in certain long, late replicating and active genes of neural stem and progenitor cells may have a functional link to these observations. These clusters occur predominantly in genes with roles in higher brain functions, thus raising the possibility that TRCs may, by design, govern the special behavior of brain cells, thereby affecting individual brain function and neuronal disease (Wei et al., 2016; Weissman and Gage, 2016). Future studies will be needed to explore this and other physiological processes in which these conflicts may have beneficial roles in development, for example during programmed cell death or cell removal.
Conclusions and Future Perspectives
Conflicts between the transcription and replication complexes represent a potent source of genome instability in both prokaryotes and eukaryotes, and cells have evolved multiple transcription- and replication-dependent mechanisms to control, minimize and overcome such encounters. Still, many questions remain. In the future, it will be important to determine the impact of unwanted encounters on the stability and fate of the replisome, the transcription machinery, and the underlying DNA template. Does the damage occur at the site of the transcription block and/or the replication fork? Which factors and repair pathways are required to resolve and overcome different types of conflicts? And, lastly, how does orientation affect each these events? Due to the inability to reliably predict the direction of such collisions on eukaryotic chromosomes, a future challenge will be to create new systems that allow one to study the effects of orientation and different transcriptional blocks on TRCs. Identifying the mutational consequences and molecular mechanisms by which these collisions are resolved is a necessary step in understanding the connections between TRCs and genome instability in cancer, neurodegenerative disease and other physiological processes, and may ultimately pave the way to exploit them for therapeutic purposes.
Acknowledgments
We apologize to all scientists whose work we could not cite due to space limitations. We are especially grateful to Dr. Caroline Stork and Dr. Madzia Crossley for critical reading and helpful comments on the manuscript. K.A.C. is supported by grants from the NIH (GM119334) and the Komen Foundation (IIR12222368). S.H. has been supported by a fellowship from the German Research Foundation (DFG HA 6996/1-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alzu A, Bermejo R, Begnis M, Lucca C, Piccini D, Carotenuto W, Saponaro M, Brambati A, Cocito A, Foiani M, et al. Senataxin associates with replication forks to protect fork integrity across RNA-polymerase-II-transcribed genes. Cell. 2012;151:835–846. doi: 10.1016/j.cell.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azvolinsky A, Giresi PG, Lieb JD, Zakian VA. Highly transcribed RNA polymerase II genes are impediments to replication fork progression in Saccharomyces cerevisiae. Mol. Cell. 2009;34:722–734. doi: 10.1016/j.molcel.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow JH, Faryabi RB, Callén E, Wong N, Malhowski A, Chen HT, Gutierrez-Cruz G, Sun H-WW, McKinnon P, Wright G, et al. Identification of early replicating fragile sites that contribute to genome instability. Cell. 2013;152:620–632. doi: 10.1016/j.cell.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos de Oliveira FM, Kim D, Cussiol JRR, Das J, Jeong MC, Doerfler L, Schmidt KH, Yu H, Smolka MB. Phosphoproteomics reveals distinct modes of Mec1/ATR signaling during DNA replication. Mol. Cell. 2015;57:1124–1132. doi: 10.1016/j.molcel.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo R, Capra T, Jossen R, Colosio A, Frattini C, Carotenuto W, Cocito A, Doksani Y, Klein H, Gómez-González B, et al. The replication checkpoint protects fork stability by releasing transcribed genes from nuclear pores. Cell. 2011;146:233–246. doi: 10.1016/j.cell.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo R, Lai MS, Foiani M. Preventing replication stress to maintain genome stability: resolving conflicts between replication and transcription. Mol. Cell. 2012;45:710–718. doi: 10.1016/j.molcel.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Bhatia V, Barroso SI, García-Rubio MLL, Tumini E, Herrera-Moyano E, Aguilera A. BRCA2 prevents R-loop accumulation and associates with TREX-2 mRNA export factor PCID2. Nature. 2014;511:362–365. doi: 10.1038/nature13374. [DOI] [PubMed] [Google Scholar]
- Blythe SA, Wieschaus EF. Zygotic genome activation triggers the DNA replication checkpoint at the midblastula transition. Cell. 2015;160:1169–1181. doi: 10.1016/j.cell.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper AM, Nghiem P, Arlt MF, Glover TW. ATR regulates fragile site stability. Cell. 2002;111:779–789. doi: 10.1016/s0092-8674(02)01113-3. [DOI] [PubMed] [Google Scholar]
- Castel SE, Ren J, Bhattacharjee S, Chang A-YY, Sánchez M, Valbuena A, Antequera F, Martienssen RA. Dicer promotes transcription termination at sites of replication stress to maintain genome stability. Cell. 2014;159:572–583. doi: 10.1016/j.cell.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano-Pozo M, Santos-Pereira JMM, Rondón AG, Barroso S, Andújar E, Pérez-Alegre M, García-Muse T, Aguilera A. R loops are linked to histone H3 S10 phosphorylation and chromatin condensation. Mol. Cell. 2013;52:583–590. doi: 10.1016/j.molcel.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Chan YA, Aristizabal MJ, Lu PY, Luo Z, Hamza A, Kobor MS, Stirling PC, Hieter P. Genome-wide profiling of yeast DNA:RNA hybrid prone sites with DRIP-chip. PLoS Genet. 2014;10:e1004288. doi: 10.1371/journal.pgen.1004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-ZZ, Bennett CL, Huynh HM, Blair IP, Puls I, Irobi J, Dierick I, Abel A, Kennerson ML, Rabin BA, et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4) Am. J. Hum. Genet. 2004;74:1128–1135. doi: 10.1086/421054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AC, Cramer P. Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature. 2011;471:249–253. doi: 10.1038/nature09785. [DOI] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol. Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova DS. DNA replication initiation patterns and spatial dynamics of the human ribosomal RNA gene loci. J. Cell. Sci. 2011;124:2743–2752. doi: 10.1242/jcs.082230. [DOI] [PubMed] [Google Scholar]
- Duch A, Felipe-Abrio I, Barroso S, Yaakov G, García-Rubio M, Aguilera A, de Nadal E, Posas F. Coordinated control of replication and transcription by a SAPK protects genomic integrity. Nature. 2013;493:116–119. doi: 10.1038/nature11675. [DOI] [PubMed] [Google Scholar]
- Dutta D, Shatalin K, Epshtein V, Gottesman ME, Nudler E. Linking RNA polymerase backtracking to genome instability in E. coli. Cell. 2011;146:533–543. doi: 10.1016/j.cell.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epshtein V, Kamarthapu V, McGary K, Svetlov V, Ueberheide B, Proshkin S, Mironov A, Nudler E. UvrD facilitates DNA repair by pulling RNA polymerase backwards. Nature. 2014;505:372–377. doi: 10.1038/nature12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felipe-Abrio I, Lafuente-Barquero J, García-Rubio MLL, Aguilera A. RNA polymerase II contributes to preventing transcription-mediated replication fork stalls. EMBO J. 2015;34:236–250. doi: 10.15252/embj.201488544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French S. Consequences of replication fork movement through transcription units in vivo. Science (New York, N.Y.) 1992;258:1362–1365. doi: 10.1126/science.1455232. [DOI] [PubMed] [Google Scholar]
- Gan W, Guan Z, Liu J, Gui T, Shen K, Manley JL, Li X. R-loop-mediated genomic instability is caused by impairment of replication fork progression. Genes Dev. 2011;25:2041–2056. doi: 10.1101/gad.17010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A, Spivak G, Hanawalt PC. Transcription-coupled DNA repair in prokaryotes. Progress in Molecular Biology and Translational Science. 2012;110:25–40. doi: 10.1016/B978-0-12-387665-2.00002-X. [DOI] [PubMed] [Google Scholar]
- García-Gómez S, Reyes A, Martínez-Jiménez MII, Chocrón ES, Mourón S, Terrados G, Powell C, Salido E, Méndez J, Holt IJ, et al. PrimPol, an archaic primase/polymerase operating in human cells. Mol. Cell. 2013;52:541–553. doi: 10.1016/j.molcel.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rubio MLL, Pérez-Calero C, Barroso SI, Tumini E, Herrera-Moyano E, Rosado IVV, Aguilera A. The Fanconi Anemia Pathway Protects Genome Integrity from R-loops. PLoS Genet. 2015;11:e1005674. doi: 10.1371/journal.pgen.1005674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottipati P, Cassel TN, Savolainen L, Helleday T. Transcription-associated recombination is dependent on replication in Mammalian cells. Mol. Cell. Biol. 2008;28:154–164. doi: 10.1128/MCB.00816-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar J, Leela JK, Anupama K. R-loops in bacterial transcription: their causes and consequences. Transcription. 2013;4:153–157. doi: 10.4161/trns.25101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh M, Gromak N. Out of balance: R-loops in human disease. PLoS Genet. 2014;10:e1004630. doi: 10.1371/journal.pgen.1004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh M, Lufino MM, Wade-Martins R, Gromak N. R-loops associated with triplet repeat expansions promote gene silencing in Friedreich ataxia and fragile X syndrome. PLoS Genet. 2014;10:e1004318. doi: 10.1371/journal.pgen.1004318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J, Kumar C, Lynch G, Yadav T, Whitehouse I, Remus D. Post-licensing Specification of Eukaryotic Replication Origins by Facilitated Mcm2-7 Sliding along DNA. Mol. Cell. 2015;60:797–807. doi: 10.1016/j.molcel.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy L, Roten C-AHA. Genometric analyses of the organization of circular chromosomes: a universal pressure determines the direction of ribosomal RNA genes transcription relative to chromosome replication. Gene. 2004;340:45–52. doi: 10.1016/j.gene.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Guy CP, Atkinson J, Gupta MK, Mahdi AA, Gwynn EJ, Rudolph CJ, Moon PB, van Knippenberg IC, Cadman CJ, Dillingham MS, et al. Rep provides a second motor at the replisome to promote duplication of protein-bound DNA. Mol. Cell. 2009;36:654–666. doi: 10.1016/j.molcel.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynn EJ, Smith AJ, Guy CP, Savery NJ, McGlynn P, Dillingham MS. The conserved C-terminus of the PcrA/UvrD helicase interacts directly with RNA polymerase. PLoS ONE. 2013;8:e78141. doi: 10.1371/journal.pone.0078141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-González B, Felipe-Abrio I, Aguilera A. The S-phase checkpoint is required to respond to R-loops accumulated in THO mutants. Mol. Cell. Biol. 2009;29:5203–5213. doi: 10.1128/MCB.00402-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-González B, García-Rubio M, Bermejo R, Gaillard H, Shirahige K, Marín A, Foiani M, Aguilera A. Genome-wide function of THO/TREX in active genes prevents R-loop-dependent replication obstacles. EMBO J. 2011;30:3106–3119. doi: 10.1038/emboj.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler AR, Donnelly CJ, Periz G, Simko EA, Shaw PG, Kim M-SS, Maragakis NJ, Troncoso JC, Pandey A, Sattler R, et al. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature. 2014;507:195–200. doi: 10.1038/nature13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangauer MJ, Vaughn IW, McManus MT. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 2013;9:e1003569. doi: 10.1371/journal.pgen.1003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchi E, Skourti-Stathaki K, Ventz S, Pinello L, Yen A, Kamieniarz-Gdula K, Dimitrov S, Pathania S, McKinney KM, Eaton ML, et al. BRCA1 recruitment to transcriptional pause sites is required for R-loop-driven DNA damage repair. Mol. Cell. 2015;57:636–647. doi: 10.1016/j.molcel.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmrich A, Ballarino M, Tora L. Collisions between replication and transcription complexes cause common fragile site instability at the longest human genes. Mol. Cell. 2011;44:966–977. doi: 10.1016/j.molcel.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Helmrich A, Ballarino M, Nudler E, Tora L. Transcription-replication encounters, consequences and genomic instability. Nat. Struct. Mol. Biol. 2013;20:412–418. doi: 10.1038/nsmb.2543. [DOI] [PubMed] [Google Scholar]
- Jones RM, Mortusewicz O, Afzal I, Lorvellec M, García P, Helleday T, Petermann E. Increased replication initiation and conflicts with transcription underlie Cyclin E-induced replication stress. Oncogene. 2013;32:3744–3753. doi: 10.1038/onc.2012.387. [DOI] [PubMed] [Google Scholar]
- Labib K, Hodgson B. Replication fork barriers: pausing for a break or stalling for time? EMBO Rep. 2007;8:346–353. doi: 10.1038/sj.embor.7400940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linskens MH, Huberman JA. Organization of replication of ribosomal DNA in Saccharomyces cerevisiae. Molecular and Cellular Biology. 1988;8:4927–4935. doi: 10.1128/mcb.8.11.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis EW, Sanz LA, Chédin F, Hagerman PJ. Transcription-associated R-loop formation across the human FMR1 CGG-repeat region. PLoS Genet. 2014;10:e1004294. doi: 10.1371/journal.pgen.1004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffee SR, Smith DJ, Whitehouse I. Quantitative, genome-wide analysis of eukaryotic replication initiation and termination. Mol. Cell. 2013;50:123–135. doi: 10.1016/j.molcel.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BC, Becerril C, Spronck JC, Ljungman M. Ultraviolet light-induced apoptosis is associated with S-phase in primary human fibroblasts. DNA Repair. 2002;1:811–820. doi: 10.1016/s1568-7864(02)00109-x. [DOI] [PubMed] [Google Scholar]
- Merrikh CN, Brewer BJ, Merrikh H. The B. subtilis Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units. PLoS Genet. 2015;11:e1005289. doi: 10.1371/journal.pgen.1005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrikh H, Machón C, Grainger WH, Grossman AD, Soultanas P. Co-directional replication-transcription conflicts lead to replication restart. Nature. 2011;470:554–557. doi: 10.1038/nature09758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrikh H, Zhang Y, Grossman AD, Wang JD. Replication-transcription conflicts in bacteria. Nat. Rev. Microbiol. 2012;10:449–458. doi: 10.1038/nrmicro2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meryet-Figuiere M, Alaei-Mahabadi B, Ali MM, Mitra S, Subhash S, Pandey GK, Larsson E, Kanduri C. Temporal separation of replication and transcription during S-phase progression. Cell Cycle. 2014;13:3241–3248. doi: 10.4161/15384101.2014.953876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkin EV, Mirkin SM. Mechanisms of transcription-replication collisions in bacteria. Molecular and Cellular Biology. 2005;25:888–895. doi: 10.1128/MCB.25.3.888-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkin EV, Castro Roa D, Nudler E, Mirkin SM. Transcription regulatory elements are punctuation marks for DNA replication. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7276–7281. doi: 10.1073/pnas.0601127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischo HE, Gómez-González B, Grzechnik P, Rondón AG, Wei W, Steinmetz L, Aguilera A, Proudfoot NJ. Yeast Sen1 helicase protects the genome from transcription-associated instability. Mol. Cell. 2011;41:21–32. doi: 10.1016/j.molcel.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira M-CC, Klur S, Watanabe M, Németh AH, Le Ber I, Moniz J-CC, Tranchant C, Aubourg P, Tazir M, Schöls L, et al. Senataxin, the ortholog of a yeast RNA helicase, is mutant in ataxia-ocular apraxia 2. Nat. Genet. 2004;36:225–227. doi: 10.1038/ng1303. [DOI] [PubMed] [Google Scholar]
- Nguyen VC, Clelland BW, Hockman DJ, Kujat-Choy SL, Mewhort HE, Schultz MC. Replication stress checkpoint signaling controls tRNA gene transcription. Nat. Struct. Mol. Biol. 2010;17:976–981. doi: 10.1038/nsmb.1857. [DOI] [PubMed] [Google Scholar]
- Nudler E. RNA polymerase backtracking in gene regulation and genome instability. Cell. 2012;149:1438–1445. doi: 10.1016/j.cell.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opalka N, Chlenov M, Chacon P, Rice WJ, Wriggers W, Darst SA. Structure and function of the transcription elongation factor GreB bound to bacterial RNA polymerase. Cell. 2003;114:335–345. doi: 10.1016/s0092-8674(03)00600-7. [DOI] [PubMed] [Google Scholar]
- Paeschke K, Bochman ML, Garcia PD, Cejka P, Friedman KL, Kowalczykowski SC, Zakian VA. Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature. 2013;497:458–462. doi: 10.1038/nature12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Million-Weaver S, Chattopadhyay S, Sokurenko E, Merrikh H. Accelerated gene evolution through replication-transcription conflicts. Nature. 2013;495:512–515. doi: 10.1038/nature11989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Vangeloff AD, Landick R. Bacterial transcription terminators: the RNA 3’-end chronicles. J. Mol. Biol. 2011;412:793–813. doi: 10.1016/j.jmb.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryk N, Kahli M, d’ Aubenton-Carafa Y, Jaszczyszyn Y, Shen Y, Silvain M, Thermes C, Chen C-LL, Hyrien O. Replication landscape of the human genome. Nat Commun. 2016;7:10208. doi: 10.1038/ncomms10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli J, Gerhold C-BB, Tosi A, Hustedt N, Seeber A, Sack R, Herzog F, Pasero P, Shimada K, Hopfner K-PP, et al. Mec1, INO80, and the PAF1 complex cooperate to limit transcription replication conflicts through RNAPII removal during replication stress. Genes Dev. 2016;30:337–354. doi: 10.1101/gad.273813.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz RT, O’Donnell M. The replisome uses mRNA as a primer after colliding with RNA polymerase. Nature. 2008;456:762–766. doi: 10.1038/nature07527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz RT, O’Donnell M. Direct restart of a replication fork stalled by a head-on RNA polymerase. Science. 2010;327:590–592. doi: 10.1126/science.1179595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SK, MacAlpine HK, Prinz JA, Li Y, Belsky JA, MacAlpine DM. Dynamic loading and redistribution of the Mcm2-7 helicase complex through the cell cycle. EMBO J. 2015;34:531–543. doi: 10.15252/embj.201488307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado F, Aguilera A. Impairment of replication fork progression mediates RNA polII transcription-associated recombination. EMBO J. 2005;24:1267–1276. doi: 10.1038/sj.emboj.7600602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proshkin S, Rahmouni AR, Mironov A, Nudler E. Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science. 2010;328:504–508. doi: 10.1126/science.1184939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghanian M, Zenkin N, Yuzenkova Y. Bacterial global regulators DksA/ppGpp increase fidelity of transcription. Nucleic Acids Res. 2015;43:1529–1536. doi: 10.1093/nar/gkv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat. Rev. Cancer. 2012;12:68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabouri N, McDonald KR, Webb CJ, Cristea IM, Zakian VA. DNA replication through hard-to-replicate sites, including both highly transcribed RNA Pol II and Pol III genes, requires the S. pombe Pfh1 helicase. Genes Dev. 2012;26:581–593. doi: 10.1101/gad.184697.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar TS, Wastuwidyaningtyas BD, Dong Y, Lewis SA, Wang JD. The nature of mutations induced by replication-transcription collisions. Nature. 2016;535:178–181. doi: 10.1038/nature18316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Pereira JMM, Aguilera A. R loops: new modulators of genome dynamics and function. Nat. Rev. Genet. 2015;16:583–597. doi: 10.1038/nrg3961. [DOI] [PubMed] [Google Scholar]
- Sanz LA, Hartono SR, Lim YW, Steyaert S, Rajpurkar A, Ginno PA, Xu X, Chédin F. Prevalent, Dynamic, and Conserved R-Loop Structures Associate with Specific Epigenomic Signatures in Mammals. Mol. Cell. 2016;63:167–178. doi: 10.1016/j.molcel.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saponaro M, Kantidakis T, Mitter R, Kelly GP, Heron M, Williams H, Söding J, Stewart A, Svejstrup JQ. RECQL5 controls transcript elongation and suppresses genome instability associated with transcription stress. Cell. 2014;157:1037–1049. doi: 10.1016/j.cell.2014.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab RA, Nieminuszczy J, Shah F, Langton J, Lopez Martinez D, Liang C-CC, Cohn MA, Gibbons RJ, Deans AJ, Niedzwiedz W. The Fanconi Anemia Pathway Maintains Genome Stability by Coordinating Replication and Transcription. Mol. Cell. 2015;60:351–361. doi: 10.1016/j.molcel.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekedat MD, Fenyö D, Rogers RS, Tackett AJ, Aitchison JD, Chait BT. GINS motion reveals replication fork progression is remarkably uniform throughout the yeast genome. Molecular Systems Biology. 2010;6:353. doi: 10.1038/msb.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skourti-Stathaki K, Proudfoot NJ, Gromak N. Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol. Cell. 2011;42:794–805. doi: 10.1016/j.molcel.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skourti-Stathaki K, Kamieniarz-Gdula K, Proudfoot NJ. R-loops induce repressive chromatin marks over mammalian gene terminators. Nature. 2014;516:436–439. doi: 10.1038/nature13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollier J, Cimprich KA. Breaking bad: R-loops and genome integrity. Trends Cell Biol. 2015;25:514–522. doi: 10.1016/j.tcb.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollier J, Stork CT, García-Rubio MLL, Paulsen RD, Aguilera A, Cimprich KA. Transcription-coupled nucleotide excision repair factors promote R-loop-induced genome instability. Mol. Cell. 2014;56:777–785. doi: 10.1016/j.molcel.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsan A, Tehranchi A, MacAlpine DM, Wang JD. Co-orientation of replication and transcription preserves genome integrity. PLoS Genet. 2010;6:e1000810. doi: 10.1371/journal.pgen.1000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz EJ, Warren CL, Kuehner JN, Panbehi B, Ansari AZ, Brow DA. Genome-wide distribution of yeast RNA polymerase II and its control by Sen1 helicase. Mol. Cell. 2006;24:735–746. doi: 10.1016/j.molcel.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Stirling PC, Chan YA, Minaker SW, Aristizabal MJ, Barrett I, Sipahimalani P, Kobor MS, Hieter P. R-loop-mediated genome instability in mRNA cleavage and polyadenylation mutants. Genes Dev. 2012;26:163–175. doi: 10.1101/gad.179721.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork CT, Bocek M, Crossley MP, Sollier J, Sanz LA, Chédin F, Swigut T, Cimprich KA. Co-transcriptional R-loops are the main cause of estrogen-induced DNA damage. Elife. 2016:5. doi: 10.7554/eLife.17548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckey R, García-Rodríguez N, Aguilera A, Wellinger RE. Role for RNA:DNA hybrids in origin-independent replication priming in a eukaryotic system. Proc. Natl. Acad. Sci. U.S.A. 2015;112:5779–5784. doi: 10.1073/pnas.1501769112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tallec B, Millot GAA, Blin ME, Brison O, Dutrillaux B, Debatisse M. Common fragile site profiling in epithelial and erythroid cells reveals that most recurrent cancer deletions lie in fragile sites hosting large genes. Cell Rep. 2013;4:420–428. doi: 10.1016/j.celrep.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Le Tallec B, Koundrioukoff S, Wilhelm T, Letessier A, Brison O, Debatisse M. Updating the mechanisms of common fragile site instability: how to reconcile the different views? Cell. Mol. Life Sci. 2014;71:4489–4494. doi: 10.1007/s00018-014-1720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehranchi AK, Blankschien MD, Zhang Y, Halliday JA, Srivatsan A, Peng J, Herman C, Wang JD. The transcription factor DksA prevents conflicts between DNA replication and transcription machinery. Cell. 2010;141:595–605. doi: 10.1016/j.cell.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Platas AA, Hawley DK. Transcriptional fidelity and proofreading by RNA polymerase II. Cell. 1998;93:627–637. doi: 10.1016/s0092-8674(00)81191-5. [DOI] [PubMed] [Google Scholar]
- Tuduri S, Crabbé L, Conti C, Tourrière H, Holtgreve-Grez H, Jauch A, Pantesco V, De Vos J, Thomas A, Theillet C, et al. Topoisomerase I suppresses genomic instability by preventing interference between replication and transcription. Nat. Cell Biol. 2009;11:1315–1324. doi: 10.1038/ncb1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba L, Amon JD, Koshland D, Vuica-Ross M. RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol. Cell. 2011;44:978–988. doi: 10.1016/j.molcel.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter D, Lier A, Geiselhart A, Thalheimer FB, Huntscha S, Sobotta MC, Moehrle B, Brocks D, Bayindir I, Kaschutnig P, et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520:549–552. doi: 10.1038/nature14131. [DOI] [PubMed] [Google Scholar]
- Washburn RS, Gottesman ME. Transcription termination maintains chromosome integrity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:792–797. doi: 10.1073/pnas.1009564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P-CC, Chang AN, Kao J, Du Z, Meyers RM, Alt FW, Schwer B. Long Neural Genes Harbor Recurrent DNA Break Clusters in Neural Stem/Progenitor Cells. Cell. 2016;164:644–655. doi: 10.1016/j.cell.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Samarabandu J, Devdhar RS, Siegel AJ, Acharya R, Berezney R. Segregation of transcription and replication sites into higher order domains. Science. 1998;281:1502–1506. doi: 10.1126/science.281.5382.1502. [DOI] [PubMed] [Google Scholar]
- Weissman IL, Gage FH. A Mechanism for Somatic Brain Mosaicism. Cell. 2016;164:593–595. doi: 10.1016/j.cell.2016.01.048. [DOI] [PubMed] [Google Scholar]
- Wellinger RE, Prado F, Aguilera A. Replication fork progression is impaired by transcription in hyperrecombinant yeast cells lacking a functional THO complex. Mol. Cell. Biol. 2006;26:3327–3334. doi: 10.1128/MCB.26.8.3327-3334.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MD, Harreman M, Taschner M, Reid J, Walker J, Erdjument-Bromage H, Tempst P, Svejstrup JQ. Proteasome-mediated processing of Def1, a critical step in the cellular response to transcription stress. Cell. 2013;154:983–995. doi: 10.1016/j.cell.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüce Ö, West SC. Senataxin, defective in the neurodegenerative disorder ataxia with oculomotor apraxia 2, lies at the interface of transcription and the DNA damage response. Mol. Cell. Biol. 2013;33:406–417. doi: 10.1128/MCB.01195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]