Abstract
Aims:
Oral cancer is the most life-threatening disease of oral tissues. In societies where the incidence of oral cancer is high, clinically recognizable premalignant lesions are particularly common. Diagnosing oral cancers at an early stage is critical in improving the survival rate and reducing the morbidity associated with the disease. Alterations in the sialic acid levels in cancer patients have stimulated interest in this sugar residue as a possible tumor marker.
Settings and Design:
The purpose of this study was to estimate the salivary sialic acid levels in patients with oral premalignancy and squamous cell carcinoma and to correlate it with their grades to develop a cost-effective and noninvasive diagnostic parameter.
Materials and Methods:
Unstimulated whole saliva was collected from the groups under study and subjected to biochemical analysis for determination of sialic acid levels.
Statistical Analysis Used:
The salivary sialic acid levels were correlated with the clinical stage and histological grade by one-way ANOVA (SPSS software version 15).
Results:
Salivary sialic acid was elevated in oral squamous cell carcinoma (OSCC) compared to oral premalignancy and control group. A statistically significant correlation was observed between the grades of squamous cell carcinoma, grades of dysplasia in premalignancy, and sialic acid level.
Conclusion and Clinical Significance:
Evaluation of salivary sialic acid levels in premalignant and malignant lesions can serve as a screening tool. The mortality and morbidity of OSCC can be reduced if the lesions are diagnosed in early precancerous states using such noninvasive diagnostic methods for screening and monitoring of the population.
Keywords: Leukoplakia, oral squamous cell carcinoma, saliva, sialic acid
Introduction
Oral squamous cell carcinoma (OSCC) is the sixth most common human cancer with a high morbidity rate and a 5-year mortality rate of about 50%.[1] The incidence as well as mortality rate of cancer have shown a sharp acceleration since the last two decades.[2]
It is now well accepted that the transition from normal mucosa to invasive squamous cell carcinoma (SCC) is a complex and multistep process, with a multifactorial etiology. Clinically recognizable premalignant lesions are particularly common in societies where the incidence of oral cancer is high.[3] The mortality rates remain unchanged in spite of the vast amount of research and the advances accomplished in the field of oncology and surgery.[4] Therefore, more strenuous efforts are required for the fight against this life-threatening disease.[2]
Early detection of oral cancer is of paramount importance to decrease the morbidity and mortality of the disease. This motivates the search of parameters which will help in the early diagnosis to better tailor the individual management of OSCC patients.[4]
Tumor markers are biochemical substances elaborated by tumor cells either due to the cause or effect of malignant process.[5] Thus, these substances that are expressed by cancer cells could be of appreciable diagnostic and prognostic value in cancer patients.[6] Selected glycoproteins and glycolipids may prove to be tumor markers since these are the major constituents of the cell membrane. Increased levels of glycopeptides containing mannose, galactose, sialic acid, and fucose are reported in several types of transformed cells and solid tumors, suggesting an association between malignant transformation and changes in cell-surface glycoconjugates.[7]
Sialic acid, a family of acylated derivatives of neuraminic acid, usually occurs as a terminal component at the nonreducing end of carbohydrate chains of glycoproteins and glycolipids.[8] It is thought to be important in determining the surface properties of cells and has been implicated in cellular invasiveness, adhesiveness, and immunogenicity.[7] Alterations in the sialic acid levels in cancer patients have stimulated interest in this sugar residue as a possible tumor marker.[5]
Interest in saliva as a diagnostic fluid has grown exponentially in recent years. There has been a growing appreciation that saliva can reflect virtually the entire spectrum of normal and disease states.[9] This has suggested the potential utility of salivary sialic acid as a parameter in the diagnosis of the malignant disease. Furthermore, there is a paucity of studies using saliva as diagnostic fluid for oral cancer.[10]
Therefore, the purpose of this study was to estimate the salivary sialic acid levels in patients with oral premalignant lesions and SCC and to correlate it with grades of epithelial dysplasia and SCC, to develop a cost-effective and noninvasive diagnostic parameter.
Materials and Methods
The study comprised the following groups:
Group I: Thirty patients with OSCC
Group II: Thirty patients with premalignant lesions such as leukoplakia, erythroplakia, smokeless tobacco keratosis, and smokers’ palate (tobacco-related lesions were included)
Group III (control group): Thirty healthy volunteers with good oral hygiene and no systemic disorders.
Patients with a history of diabetes mellitus, cardiovascular diseases, rheumatoid arthritis, and any systemic ailments were excluded from the study. Pregnant females were also excluded from the study.
Group I was subjected to clinical staging by tumor-node-metastasis (TNM) system developed by the American Joint Committee for Cancer Staging and End Result Reporting[11] and histological grading according to Bryne et al.[12] Leukoplakia in Group II was grouped according to lesion size, site of lesion, clinical aspects, pathological aspects (LSCP) classification and staging system by van der Waal et al.[13] Histopathologic assessment of all clinically diagnosed premalignant lesions was done for the degree of epithelial dysplasia according to the WHO classification.[14]
Collection of saliva
The unstimulated whole saliva was collected and analyzed in this study. Saliva samples were collected during 10 am–12 pm, 2 h after the patients usual breakfast time. The saliva was collected by draining method, in which saliva was allowed to drip off the lower lip according to Navazesh.[15] Before the collection of saliva, the patient was asked to rinse the mouth thoroughly, to remove any food debris.
Biochemical analysis of saliva for sialic acid estimation
Frozen saliva was brought back to room temperature and was centrifuged at 1000 rpm for 15 min, and the resulting supernatant was used for biochemical estimation of sialic acid. The estimation of sialic acid was done by the method described by Yao et al.[16,17,18]
One way ANOVA test (SPSS software - version 15, Illinois, chicago, USA) was used for finding the correlation between salivary sialic acid levels and different grades of premalignant and malignant lesions.
Results and Observations
The demographic data of the groups under study were evaluated. The age group with a maximum predilection for oral malignancy in the present study was found to be between 61 and 70 years, constituting 33.3% of the patients of Group I included in the study. Premalignant lesions were most commonly seen in the age group of 31–40 years.
OSCC group (n = 30) comprised 18 (60%) male and 12 (40%) female patients; premalignant group (n = 30) comprised 28 (93.3%) males and 2 (6.6%) females.
On distribution of the thirty patients of OSSC group according to TNM staging, 13 (43.3%) patients were categorized into stage III. Eleven (36.7) cases were categorized into stage IV, whereas stage I and stage II encompassed 3 (10%) cases each [Graph 1].
Graph 1.
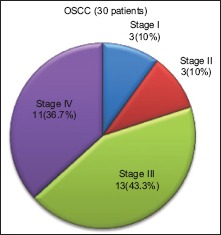
Distribution of oral squamous cell carcinoma cases according to the tumor-node-metastasis staging system by the American Joint Committee for Cancer Staging and End Result Reporting (1967)
Of thirty cases of premalignant lesions, 28 cases were diagnosed as leukoplakia and two cases as tobacco pouch keratosis. The distribution of the 28 cases of leukoplakia based on clinical staging according to the LSCP system showed that 10 (35.7%) cases of leukoplakia belonged to stage II followed by eight (28.6%) cases each in stage I and stage IV and only two (7.1%) cases in stage III [Graph 2].
Graph 2.
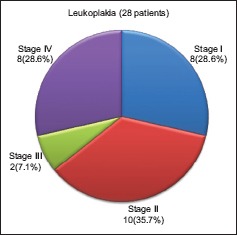
Distribution of cases of leukoplakia based on clinical staging according to the lesion size, site of lesion, clinical aspects, pathological aspects-classification system
According to the Bryne's grading system, 16 (53.3%) cases of OSSC (n = 30) were categorized under Grade I (well differentiated [WD]), whereas 10 (33.3%) cases fell under the category of Grade II (moderately differentiated [MD]) and 4 (13.3%) cases were categorized as Grade III (poorly differentiated [PD]) [Graph 3].
Graph 3.
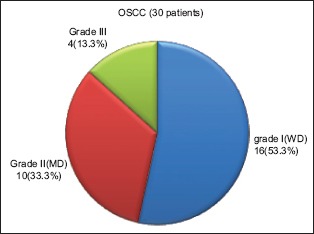
Distribution of cases of oral squamous cell carcinoma group according to Bryne's grading system
On histopathological examination, 11 (36.7%) cases with premalignant lesions (n = 30) showed only hyperkeratosis without any evidence of dysplasia, whereas 10 (33.3%), 7 (23.3%), and 2 (6.7%) cases were categorized under mild, moderate, and severe dysplasia, respectively [Graph 4].
Graph 4.
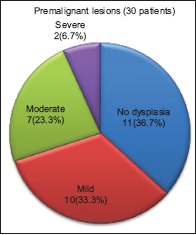
Distribution of cases in premalignant group according to histopathologic grade of dysplasia by the WHO
Results of salivary analysis
Saliva obtained from the study groups was analyzed to find any significant difference in the values of free sialic acid (FSA) and protein-bound sialic acid (PBSA) between the three groups. The analysis was also done to find any correlation in the levels of FSA and PBSA between different histopathological grades of premalignant and OSCC lesions. They were tested using One-way ANOVA test (SPSS software version-15). The results thus obtained are presented in the tables given below.
The mean levels of salivary FSA and PBSA were found to be elevated in malignant as compared to premalignant followed by the control group. A highly significant difference (at 95% confidence interval, i.e., two-tailed) between OSCC, premalignant, and control group was found.
The mean FSA and PBSA levels progressively increased from Grade I OSCC to Grade III OSCC with statistically significant difference (P < 0.05) between Grade I (WD), Grade II (MD), and Grade III (PD).
The mean salivary FSA and PBSA levels were progressively elevated in accordance with the grade of dysplasia, i.e., the levels were most elevated in severe followed by moderate, mild, and no dysplasia. Statistical analysis revealed a significant difference between the grades of dysplasia.
On correlation of the stage of OSCC, there was no significant difference of FSA and PBSA with respect to all four stages of OSCC.
In cases of leukoplakia staged by LSCP classification system, a significant difference was observed in the levels of FSA and PBSA with respect to different stages of leukoplakia. However, the mean salivary sialic acid levels were less in stage II as compared to stage I followed by stage III and stage IV.
Discussion
Oral cancer is the most life-threatening disease of oral tissues. Early detection followed by appropriate treatment, can increase cure rates to 80% or 90%, and can greatly improve the quality of life by minimizing extensive, debilitating treatments.[19] Studies of malignant cells have revealed alterations in cell surfaces and membranes in terms of sialic acid content of glycoproteins and glycolipids.[20] Sialic acid has been found to be released into circulation during tumor progression.[21]
The significance of serum sialic acid as a sensitive tumor marker has been established in previous studies. However, its specificity is not yet determined.[16] Saliva wins over blood as a diagnostic fluid to monitor health and disease because of compelling reasons such as being inexpensive, noninvasive (basically an ultrafiltrate of blood), and easy to handle. For patients, the noninvasive collection technique dramatically reduces anxiety, discomfort, and simplifies procurement of repeated samples for longitudinal monitoring over time. The transfer of serum constituents, which are not part of the normal salivary constituents, into the saliva is related to the physiochemical characteristics of these molecules. For accurate diagnosis, a defined relationship is required between the concentration of the biomarker in serum and the concentration in saliva. With new and highly sensitive technologies, the lower level of analytes in saliva is no longer a limitation.[22]
Thus, we estimated the salivary sialic acid levels in patients with oral premalignant and malignant lesions and correlated it with grades of oral premalignant and malignant lesions to develop a noninvasive diagnostic parameter.
In the present study, the mean salivary FSA and PBSA levels were found to be elevated in malignant as compared to premalignant followed by the control group. The levels also showed statistically significant difference (P < 0.05) between the three groups. The findings were in accordance with the findings of Sanjay et al.[10] who compared the levels of salivary FSA and PBSA in control and malignant group. The study, however, did not include a premalignant group. Shivshankara and Prabhu[16] similarly showed significantly elevated levels of FSA and PBSA in malignant group as compared to normal and premalignant group.
Baxi et al.,[21] Baxi et al.,[7] Rao et al.,[23] Raval et al.,[24] Rajpura et al.,[2] and Joshi and Patil[25] demonstrated elevated levels of serum sialic acid in oral cancer group as compared to premalignant and control group. Xing et al.[26] and Bathi et al.[27] also showed elevated levels of serum sialic acid in oral cancer group as compared to the control group but did not include premalignant group. According to the above-mentioned studies and the findings of our study, a definite relationship is established between the concentration of sialic acid in serum and saliva in cancer patients. This is due to the fact that saliva is ultimately an ultrafiltrate of serum; thus, saliva can be used as a potentially diagnostic tool as compared to serum. Increased levels of sialic acid in cancer patients can be explained by spontaneous release or shedding of aberrant sialic acid-rich glycoproteins.[28] This fact is confirmed by the findings of our study.
Correlation of sialic acid levels in saliva with histopathologic grading of oral cancer was also done. The mean FSA and PBSA levels progressively increased from Grade I to Grade III with statistically significant difference (P < 0.05) between Grade I (WD), Grade II (MD), and Grade III (PD). Similarly, Sanjay et al.[10] found that salivary FSA values were significantly higher in WD cases as compared to MD cases suggesting the correlation of elevated salivary sialic acid levels to the progression of OSCC. However, PBSA did not differ significantly among WD and MD cases. Shivshankara and Prabhu[16] also found elevated levels of FSA and PBSA in WD cases as compared to MD cases.
The possible reason for such variation in the results could be subjective variation between histopathologic grading and the grading system used. Furthermore, in the present study, only four cases of Grade III were studied. If we consider the clinical staging of these histopathologically diagnosed Grade III cases, all four cases belonged to stage IV. Hence, tumor burden and lesser degree of differentiation might be the causes of higher levels of free and PBSA in Grade III cases.
Most cancer cells carry more negative surface charges on their cell surface than their normal counterparts. Due to the higher content of negative charges, the cancer cells tend to repel each other resulting in lesser adhesiveness.[29] Lesser adhesiveness of cancer cells is seen in higher grades of OSCC, where the cells are arranged in small groups or are present as isolated cells. This abnormality is due to the higher sialic acid content of the cancer cell membrane, i.e., ultimately released into the circulation.
Correlation of histopathologic grading of dysplasia of premalignant lesions with salivary sialic acid levels was done. To the best of our knowledge, correlation of salivary sialic acid levels with histopathological grading of dysplasia has not been done. Grading of premalignant lesions was done according to the WHO[14] histopathological grading. The mean salivary FSA and PBSA levels showed a statistically significant increase with the grade of dysplasia.
In a similar study by Joshi and Patil,[25] mean serum total sialic acid level increased along with the grade of dysplasia similar to the findings of our study, but the levels were not found to be statistically significant when compared between the grades of dysplasia.
It has been reported that mild, moderate, and severe dysplasias develop into malignancy in 3%, 4%, and 43%, respectively.[30] The level of salivary sialic acid increases with the degree of dysplasia in oral premalignant lesions indicating its association with malignant transformation. Furthermore, increased content of sialic acid is responsible for the lack of cohesion between the cells, which contributes to the histopathological appearance of severe dysplasia.
In addition, the levels of salivary sialic acid were also correlated with clinical staging of OSCC according to TNM staging and also with clinical stages of leukoplakia according to the LSCP classification. The levels were higher in stage II, followed by stage IV, III, and I. However, no statistically significant difference was found between the stages of OSCC group. This discrepancy could be because the TNM classification is a clinical staging system and hence may not always be significantly correlated with an increase in sialic acid, which is an indication of lack of adhesiveness at cellular level.
The levels of salivary sialic acid were also correlated with clinical staging of leukoplakia lesions. The mean salivary levels of FSA and PBSA showed a significant difference, but the levels were found to be less in stage II as compared to stage I. However, the levels significantly correlated with the clinical staging of leukoplakia. No previous study has shown such correlation of serum or salivary sialic acid with LSCP staging system for leukoplakia. The possible reason for decrease in the level of salivary sialic acid in stage II of leukoplakia could be because four out of eight cases showed no dysplasia in stage I and six out of ten cases showed no dysplasia in stage II. LSCP classification system has three clinical factors and one histological factor, and hence that could possibly explain this observation.
Thus, in the present study, the level of sialic acid was significantly elevated in oral cancer patients compared to premalignant and control group, suggesting the role of sialic acid in determining malignant changes in the cell.
Conclusion
Elevated sialic acid levels in oral cancer and precancer indicate its importance as a tumor marker. The method used in the study to detect sialic acid in saliva was comparatively easy and cost-effective.
The mortality and morbidity of OSCC can be reduced if the lesions are diagnosed in early precancerous states using such noninvasive diagnostic methods for screening and monitoring of the population.
However, the levels have also shown elevation in other cancer groups, so a comparative study between the levels of salivary sialic acid in oral as well as other cancer groups is required. This will help in increasing the specificity of salivary sialic acid as a significant tumor marker.
Evaluation of salivary sialic acid levels in premalignant and malignant lesions can serve as a screening tool. The diagnostic capacity of saliva in oral and oropharyngeal lesions may be enhanced because of the intimate contact of the saliva with the lesional tissue.
The mortality and morbidity of OSCC can be reduced if the lesions are diagnosed in early precancerous states using such noninvasive diagnostic methods for screening and monitoring of the population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shpitzer T, Bahar G, Feinmesser R, Nagler RM. A comprehensive salivary analysis for oral cancer diagnosis. J Cancer Res Clin Oncol. 2007;133:613–7. doi: 10.1007/s00432-007-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajpura KB, Patel PS, Chawda JG, Shah RM. Clinical significance of total and lipid bound sialic acid levels in oral pre-cancerous conditions and oral cancer. J Oral Pathol Med. 2005;34:263–7. doi: 10.1111/j.1600-0714.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 3.Scully C, Sudbø J, Speight PM. Progress in determining the malignant potential of oral lesions. J Oral Pathol Med. 2003;32:251–6. doi: 10.1034/j.1600-0714.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 4.Massano J, Regateiro FS, Januário G, Ferreira A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 5.Malati T. Tumour markers: An overview. Indian J Clin Biochem. 2007;22:17–31. doi: 10.1007/BF02913308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tewarson SL, Mittal VP, Singh M, Gupta GP. Serum sialic acid – An important cancer marker. Indian J Cancer. 1993;30:125–31. [PubMed] [Google Scholar]
- 7.Baxi BR, Patel PS, Adhvaryu SG, Dayal PK. Usefulness of serum glycoconjugates in precancerous and cancerous diseases of the oral cavity. Cancer. 1991;67:135–40. doi: 10.1002/1097-0142(19910101)67:1<135::aid-cncr2820670124>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Erbil KM, Jones JD, Klee GG. Use and limitations of serum total and lipid-bound sialic acid concentrations as markers for colorectal cancer. Cancer. 1985;55:404–9. doi: 10.1002/1097-0142(19850115)55:2<404::aid-cncr2820550219>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Segal A, Wong DT. Salivary diagnostics: Enhancing disease detection and making medicine better. Eur J Dent Educ. 2008;12(Suppl 1):22–9. doi: 10.1111/j.1600-0579.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanjay PR, Hallikeri K, Shivashankara AR. Evaluation of salivary sialic acid, total protein, and total sugar in oral cancer: A preliminary report. Indian J Dent Res. 2008;19:288–91. doi: 10.4103/0970-9290.44529. [DOI] [PubMed] [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al., editors. AJCC Cancer Staging Manual. 6th ed. Chicago: Springer; 2009. Purposes and principles of staging; pp. 6–7. [Google Scholar]
- 12.Bryne M, Nielsen K, Koppang HS, Dabelsteen E. Reproducibility of two malignancy grading systems with reportedly prognostic value for oral cancer patients. J Oral Pathol Med. 1991;20:369–72. doi: 10.1111/j.1600-0714.1991.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 13.van der Waal I, Schepman KP, van der Meij EH, Smeele LE. Oral leukoplakia: A clinicopathological review. Oral Oncol. 1997;33:291–301. doi: 10.1016/s1368-8375(97)00002-x. [DOI] [PubMed] [Google Scholar]
- 14.Gale N, Pilch BZ, Sidransky D, Westra WH, Califano J. Epithelial precursor lesions. In: Barnes L, Eveson J, Reichart P, Sidransky D, editors. W.H.O. Classification of Tumors. Pathology & Genetics Head and Neck Tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 15.Navazesh M. Methods for collecting saliva. Ann NY Acad Sci. 1993;20:72–7. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 16.Shivashankara AR, Prabhu MK. Salivary total protein, sialic acid, lipid peroxidation and glutathione in oral squamous cell carcinoma. Biomed Res. 2011;22:355–9. [Google Scholar]
- 17.Shetty PK, Pattabiraman TN. Salivary glycoproteins as indicators of oral diseases. Indian J Clin Biochem. 2004;19:97–101. doi: 10.1007/BF02872400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao K, Ubuka T, Masuoka N, Kinuta M, Ikeda T. Direct determination of bound sialic acids in sialoglycoproteins by acidic ninhydrin reaction. Anal Biochem. 1989;179:332–5. doi: 10.1016/0003-2697(89)90138-3. [DOI] [PubMed] [Google Scholar]
- 19.Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3:27. doi: 10.1186/1479-5876-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadam CY, Katkam RV, Suryakar AN, Kumbar KM, Kadam DP. Biochemical markers in oral cancer. Biomed Res. 2011;22:76–80. [Google Scholar]
- 21.Baxi BR, Patel PS, Adhvaryu SG. A report on clinical importance of serum glycoconjugates in oral cancer. Indian J Clin Biochem. 1990;5:139–44. [Google Scholar]
- 22.Li Y, Denny P, Ho CM, Montemagno C, Shi W, Qi F, et al. The Oral Fluid MEMS/NEMS Chip (OFMNC): Diagnostic and translational applications. Adv Dent Res. 2005;18:3–5. doi: 10.1177/154407370501800102. [DOI] [PubMed] [Google Scholar]
- 23.Rao VR, Krishnamoorthy L, Kumaraswamy SV, Ramaswamy G. Circulating levels in serum of total sialic acid, lipid-associated sialic acid, and fucose in precancerous lesion and cancer of the oral cavity. Cancer Detect Prev. 1998;22:237–40. doi: 10.1046/j.1525-1500.1998.0oa04.x. [DOI] [PubMed] [Google Scholar]
- 24.Raval GN, Patel DD, Parekh LJ, Patel JB, Shah MH, Patel PS. Evaluation of serum sialic acid, sialyltransferase and sialoproteins in oral cavity cancer. Oral Dis. 2003;9:119–28. doi: 10.1034/j.1601-0825.2003.01795.x. [DOI] [PubMed] [Google Scholar]
- 25.Joshi M, Patil R. Estimation and comparative study of serum total sialic acid levels as tumor markers in oral cancer and precancer. J Cancer Res Ther. 2010;6:263–6. doi: 10.4103/0973-1482.73339. [DOI] [PubMed] [Google Scholar]
- 26.Xing RD, Chen RM, Wang ZS, Zhang YZ. Serum sialic acid levels in patients with oral and maxillofacial malignancy. J Oral Maxillofac Surg. 1991;49:843–7. doi: 10.1016/0278-2391(91)90013-c. [DOI] [PubMed] [Google Scholar]
- 27.Bathi RJ, Nandimath K, Kannan N, Shetty P. Evaluation of glycoproteins as prognosticators in head and neck malignancy. Indian J Dent Res. 2001;12:93–9. [PubMed] [Google Scholar]
- 28.Raval GN, Parekh LJ, Patel DD, Jha FP, Sainger RN, Patel PS. Clinical usefulness of alterations in sialic acid, sialyltransferase and sialoproteins in breast cancer. Indian J Clin Biochem. 2004;19:60–71. doi: 10.1007/BF02894259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traving C, Schauer R. Structure, function and metabolism of sialic acids. Cell Mol Life Sci. 1998;54:1330–49. doi: 10.1007/s000180050258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arduino PG, Surace A, Carbone M, Elia A, Massolini G, Gandolfo S, et al. Outcome of oral dysplasia: A retrospective hospital-based study of 207 patients with a long follow-up. J Oral Pathol Med. 2009;38:540–4. doi: 10.1111/j.1600-0714.2009.00782.x. [DOI] [PubMed] [Google Scholar]


