Abstract
Aim:
To clinically and radiographically evaluate the reparative dentin formation in indirect pulp treatment (IPT) using mineral trioxide aggregate (MTA) and light cured calcium silicate (TheraCal) in primary molars over a period of 6 months.
Materials and Methods:
A clinical trial on IPT on 43 primary molars in 21 patients between the age of 4–7 years, divided into two groups: 22 teeth in MTA group and 21 in TheraCal group. Measurement of the variation in dentin thickness was done on the digitalized radiograph at baseline, 3 months and 6 months using CorelDRAW X3 software.
Results:
Statistical analysis using an independent t-test for intragroup and intergroup comparison showed a significant increase in dentin thickness in both the MTA and TheraCal group (intragroup comparison [P < 0.05]). However, intergroup comparison between MTA and TheraCal showed no statistical difference in reparative dentin formation (P > 0.05).
Conclusion:
Clinically and radiographically, both MTA and TheraCal are good IPT materials. The better handling characteristics and comparable reparative dentin-forming ability of TheraCal make this material an alternative to MTA in pediatric restorative procedures.
Keywords: Indirect pulp treatment, mineral trioxide aggregate, primary teeth, reparative dentin, TheraCal
Introduction
Maintaining the deciduous teeth in the dental arch till it exfoliates is pivotal in maintaining the integrity of the arch, establishing occlusion and function of the permanent dentition. The main reasons for premature loss of primary teeth are dental trauma or dental caries.[1] Every effort should be taken to preserve the vitality of the pulp as it leads to more favorable treatment outcomes. Indirect pulp treatment (IPT) is one of the most preferred vital pulp treatment modality in restorative dentistry today which may be attributed to the introduction of new biocompatible materials and a better understanding of pulp biology.
Dorfman et al. in 1943 stated that decalcification of dentin precedes bacterial invasion within the dentin.[2] Removing the outer layer of carious dentin, that contain the majority of the microorganisms, reducing the continued demineralization of the deeper dentin layers from bacterial toxins, and sealing the lesion to allow the pulp to generate reparative dentin is the mechanism on which IPT procedure is based.[2] Ca(OH)2 has been the material of choice for indirect pulp capping for over 200 years. It served as the gold standard to which newly introduced pulp capping materials were compared.[3] The introduction of newer materials with bioactive properties such as mineral trioxide aggregate (MTA), light cured calcium silicate (TheraCal LC), biodentin helped surpass the demerits of Ca(OH)2 such as nonadherence to dentin, dissolution over time, and tunnel defects and ensured increased success rate for IPT procedure.
MTA is a hydraulic silicate cement, relying primarily on hydration reactions for setting. The major constituents of this material are dicalcium silicate, tricalcium silicate, tricalcium aluminate, gypsum, and tetracalcium aluminoferrite. It has good reparative dentin-forming ability as confirmed by many in vivo studies using this material in vital pulp therapies. The drawbacks of MTA include longer setting time, difficult handling characteristics, high cost, and solubility demonstrating 24% loss after 78 days of storage in water.[4] TheraCal LC is a new class of materials called resin modified calcium silicates that have been reported to stimulate apatite formation and the formation of reparative dentin. TheraCal contains approximately 45% wt mineral material (Type III Portland cement), 10% wt radiopaque component, 5% wt hydrophilic thickening agent (fumed silica), and approximately 45% hydrophobic resin.
Both MTA and TheraCal have been proved to have the ability to induce reparative dentin formation. In a literature search, no studies were found evaluating IPT using MTA and TheraCal in primary molars. Hence, the present study was done to evaluate the reparative dentin-forming ability of MTA and TheraCal in IPT in primary molars.
Materials and Methods
The study design was a clinical trial in a sample of 43 primary molars in 21 patients divided into two groups by simple random sampling method. Informed consent was obtained from the parents of the patients selected for the study. The institutional ethical clearance was obtained before the initiation of this work.
Selection criteria
Forty-three healthy primary molars in 21 patients aged between 4 and 7 years with deep carious lesion on either occlusal or proximal surface with no history of spontaneous pain and with a remaining dentin thickness (RDT) of more than 0.25 mm in the bitewing radiograph, as measured using CorelDRAW X3 software (Corel Corporation, Ottawa, Ontario, Canada) (mean RDT in MTA group – 0.52 ± 0.18 mm and in TheraCal group – 0.52 ± 0.17 mm) were included in the study. Patients were selected from the OP of the Department of Pedodontics in our institute.
Periodontally sound teeth with large carious lesion with no history of spontaneous pain, with more than 2/3rd of the root present, with no periapical changes and absence of sinus tract, were selected for the study. Teeth with definite pulpal involvement, discoloration, mobility and radiographically signs of internal resorption, pulpal calcification, periapical or furcation involvement were excluded.
Sample preparation and experimentation
The teeth with deep carious lesion with no history of spontaneous pain were subjected to routine standardized periapical and posterior bitewing radiograph to ascertain the inclusion criteria. The selected teeth were randomly divided into two groups depending on the material used.
The author did the IPT procedure. After administration of topical anesthetic, the tooth to be treated was isolated using rubber dam. Carious lesion was removed using slow speed handpiece with a large round carbide bur. Care was taken to remove caries completely from the lateral walls of the cavity and cavosurface margin. Soft caries at the pulpal floor of the cavity was removed preserving the affected dentin as identified by visual and tactile method. Following caries removal, the cavity was flushed with saline for 10 s and dried with cotton pellets. In patients belonging to Group I, MTA powder was mixed with sterile water for 30 s so as to get a sandy consistency and carried with a ball ended condenser tip and applied by light pressure with moist cotton pellet. The cotton pellet was placed in the cavity, and temporary restoration with ZnOE was done. A base with resin-modified glass ionomer cement (RMGIC) followed by composite restoration was given post 45 min once the cotton pellet and temporary was removed. In patients belonging to Group II, TheraCal LC was applied directly to the dentin surface using the dispensing syringe and light cured for 20 s, followed by RMGIC base and composite restoration.
A baseline radiograph was made after the restoration using an extension cone paralleling (XCP) film holder. Subsequently, at 3 and 6 months, the teeth were clinically examined and radiographically evaluated. All the radiographs were standardized using XCP technique and scanned and transferred to the computer for digital analysis. Dentin thickness was measured at the baseline, 3rd and 6th months using CorelDRAW X3 software [Figures 1–4] by an independent examiner who was blinded to the study. The digitalized images were overlapped using the software keeping cementoenamel junction and the highest point in the floor of pulp chamber as reference points, and increment in dentin thickness was measured.
Figure 1.
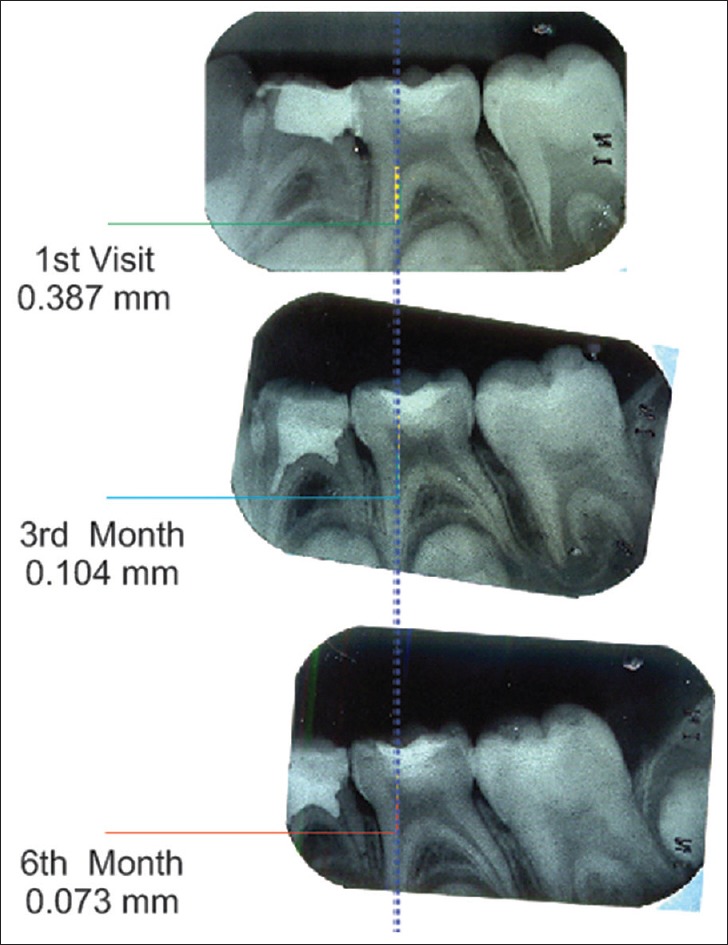
Measurement of reparative dentin formation – mineral trioxide aggregate group
Figure 4.

Measurement of reparative dentin formation – TheraCal group [magnified]
Figure 2.

Measurement of reparative dentin formation – mineral trioxide aggregate group [magnified]
Figure 3.
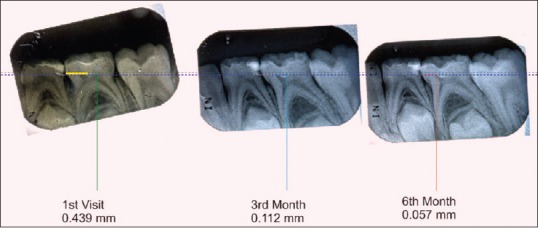
Measurement of reparative dentin formation – TheraCal group
Intragroup comparison of dentin increment in both the test groups was done between baseline value and baseline to 3 months value (A), baseline value and 3–6 months value (B), baseline to 3 months value and 3–6 months value (C), and baseline value and baseline to 6 months value (D).
Criteria for clinical evaluation included the absence of spontaneous pain, sensitivity to pressure, fistula, edema, and abnormal mobility. Treatment was considered to be successful when the pulp remained vital with no signs of spontaneous pain and no radiographic evidence of pathologic change at the end of study period. Statistical analysis was performed using SPSS software version 20 (IBM Corporation, Armonk, New York, United States). Intragroup and intergroup comparison for an increase in dentin thickness was carried out using independent t-test. P < 0.05 was considered statistically significant.
Results
Of a total of 22 specimens included in the MTA group, four specimens were excluded due to loss of follow-up. Deposition of tertiary dentin in MTA group at the end of 3 months varied between 0.073 mm and 0.119 mm. From 3–6 months, it varied between 0.041 mm and 0.073 mm. The mean increment in dentin thickness in first 3 months was 0.095 ± 0.014 mm and from 3–6 months was 0.056 ± 0.009 mm. The average increase in dentin thickness in a total 6 months was 0.151 ± 0.022 mm.
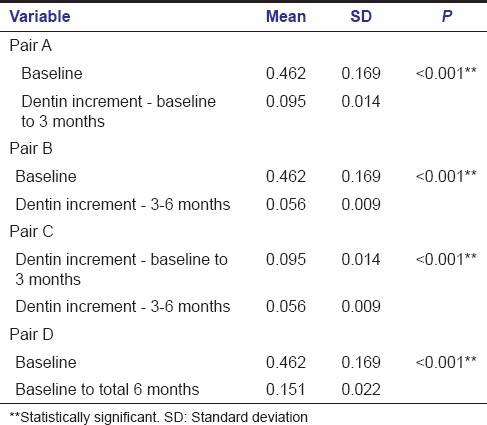
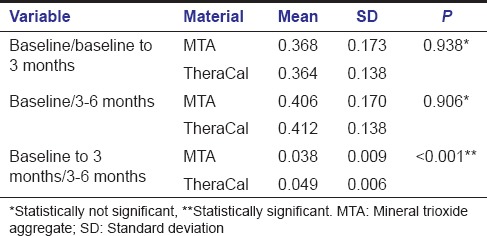
Intragroup comparison of dentin increment in MTA group showed a statistically significant increase in dentin thickness when the baseline value was compared with baseline to 3 months value with a P < 0.001 (A) [Table 1]. Statistically significant increase in dentin thickness was noted when baseline value was compared with that of 3–6 months (B) [Table 1]. Comparison of baseline to 3 months value with 3–6 months value also gave an increase in dentin thickness with a P < 0.001 which was statistically significant (C) [Table 1]. Similarly when baseline value was compared with total 6 months value, a statistically significant increase in dentin thickness was found with a P < 0.001 (D) [Table 1].
Table 1.
Intragroup comparison - mineral trioxide aggregate group
Three of the total 21 specimens included in the TheraCal group were excluded; one due to a loss of follow-up and two due to dislodged restoration. Deposition of tertiary dentin in the TheraCal group at the end of 3 months varied between 0.088 mm and 0.115 mm; from 3 to 6 months it varied from 0.046 mm to 0.059 mm. The mean increase in dentin thickness in the first 3 months was 0.101 mm ± 0.008 mm and from 3 months to 6 months was 0.053 mm ± 0.004 mm. The average increase in dentin thickness in total 6 months was 0.154 mm ± 0.012 mm.
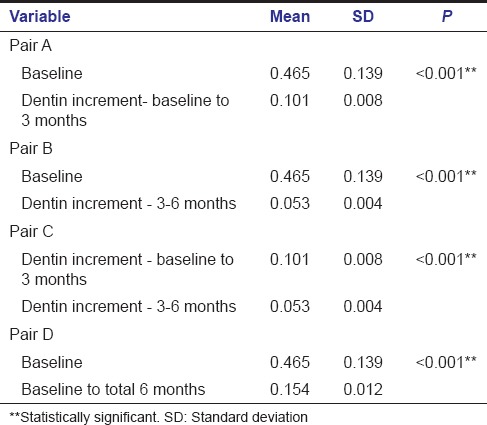
Intragroup comparison of dentin increment in TheraCal group also showed a statistically significant increase in dentin thickness when the baseline value was compared with a baseline to 3 months (A) [Table 2] and also while comparing baseline value with that of 3–6 months (B) [Table 2]. Comparison of baseline to 3 months value with 3–6 months value gave a statistically significant result with P < 0.001 (C) [Table 2]. Statistically significant result was obtained when the baseline value was compared with total 6 months value (D) [Table 2].
Table 2.
Intragroup comparison - TheraCal group
Intergroup comparison between MTA and TheraCal using independent t-test was done between baseline to 3 months, 3–6 months, and total 6 months. Intergroup analysis of dentin increment in the first 3 months and next 3 months between MTA and TheraCal were not significant yielding a P = 0.091 and 0.149 respectively. Increase in dentin increment in a total of 6 months was also compared between both the materials (P = 0.611) [Table 3]. These results showed that even though there is increase in dentin thickness within each group, there is no significant difference in dentin increment when comparing both groups. Intergroup comparison comparing the difference in dentin increment in both groups showed that dentin increment in first 3 months was statistically significant when compared to dentin increment in 3–6 months (P < 0.001) [Table 4].
Table 3.
Intergroup comparison - mineral trioxide aggregate and TheraCal
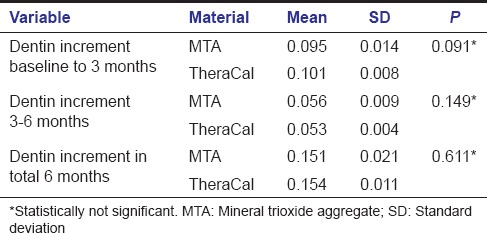
Table 4.
Intergroup comparison (difference) between mineral trioxide aggregate and TheraCal
Discussion
Indications for IPT and pulpotomy procedures are identical.[5] However, many studies have proved that the success of IPT was more compared to that of pulpotomy.[3,6,7] A retrospective study by Farooq et al. showed normal exfoliation pattern for teeth treated with IPT compared to teeth treated by pulpotomy procedure, which showed early exfoliation.[6] Careful diagnosis of the pulpal status is essential for the success of any conservative pulp treatment. The samples for this study were selected based on the clinical and radiographic criteria for IPT. Ricucci et al. correlated clinical and radiographic evaluation of pulp status with histologic findings and found that findings using defined criteria for clinical and histological classification of pulp conditions revealed a good agreement especially for cases with no pulpal disease or reversible disease.[8]
The mean RDT determined from the bitewing radiographs in the MTA group was 0.515 ± 0.182 mm and that of the TheraCal group was 0.522 ± 0.179 mm. As reported by Murray and Smith preparation of deep cavities with an RDT of 0.25–0.004 mm injures underlying odontoblasts, preventing the secretion of reactionary dentin beneath the cavity preparation.[9] Assessment of dentin thickness was done at the baseline, at the end of 3 months and 6 months in the digitalized radiographs keeping cement-enamel junction and highest point on the floor of the pulp chamber as reference points. In an in vitro study in extracted teeth, Krasner and Rankow proposed the presence of identifiable reference points for locating the floor of pulp chamber. The authors demonstrated that the floor of the pulp chamber is always located in the center of the tooth at the level of the cement-enamel junction and the cement-enamel junction is the most consistent, repeatable landmark for locating the position of the pulp chamber.[10]
Large round bur in a slow speed handpiece was used for the caries removal in this study. Falster in an in vivo study comparing the pulp protecting ability of calcium hydroxide and adhesive resin demonstrated that the use of large, round carbide burs allow for better control of the “partial caries removal step” at the site of potential pulp exposure when compared to the use of spoon excavator. With spoon excavators, removal of affected dentin from deeper layers may cause accidental pulp exposure more commonly than with round burs at low speed.[11]
MTA is available as grey MTA (GMTA) and white MTA (WMTA). The occurrence of discoloration and longer setting time in association with GMTA led to the introduction of WMTA with the elimination of iron, preventing alumina-ferrite formation which was responsible for the discoloration. Smaller particle size of WMTA provides greater specific surface area which increases wetting volume, water-binding capacity and hydration rate. MTA Angelus has less amount of calcium sulfate which decreases its setting time.[12] No significant difference between GMTA and WMTA in terms of calcified bridge thickness and pulp inflammatory response to the capping materials was observed in the study by Eskandarizadeh et al.[13] Owing to its superior properties, WMTA was used as one of the test material in this study. The drawbacks of MTA include longer setting time, difficult handling characteristics, high cost, and solubility demonstrating 24% loss after 78 days of storage in water.[4]
TheraCal has high calcium releasing ability, lower solubility, and comparable shear bond strength when compared with MTA.[14] Makkar et al. studied the sealing ability of TheraCal, biodentin and MTA using confocal laser scanning microscopy and found out that TheraCal exhibits better sealing ability as a pulp capping agent than MTA and biodentin.[15] In the histologic study of direct pulp capping with TheraCal, Cannon et al. proved that TheraCal demonstrated a complete hard tissue formation, adhesiveness to moist substrate and more positive response to the pulp exposure and bacterial contamination.[16] TheraCal has higher bond strength than MTA in alkaline environment of the dentin as corroborated by the in vitro study comparing the push-out bond strength of biodentin, glass ionomer cement (GIC), MTA and TheraCal.[17] There was no statistical difference in dentin deposition at the end of the first 3 months, second 3 months, and total 6 months between MTA and TheraCal. This may be attributed to the fact that TheraCal is chemically similar to MTA formulation with the addition of hydrophobic resin.[15]
Between the groups, the dentin increment in first 3 months in TheraCal group was higher when compared to that of the MTA group, though not statistically significant. In TheraCal, there is increased calcium ion release at the initial period of 28 days, which decreases later. The alkaline pH of TheraCal decreases from 10-11 to 8-8.5 in 7–14 days creating a favorable environment for pulp cell viability and metabolic activity with the formation of reparative tertiary dentin.[14,18] The average dentin increment in the first 3 months in both groups was significantly higher when compared to second 3 months which may be attributed to decrease in calcium release by both materials over time as explained by in vitro study by Gandolfi et al.[14]
Present study showed high tertiary dentin formation by both the test materials owing to the fact that both materials release calcium ions in sufficient quantities to promote reparative dentin formation.[19] The average reparative dentin formation in this study after MTA placement in 6 months was found to be 0.151 ± 0.022 mm. This result is in agreement with the study done by George et al. comparing the reparative dentin formation between MTA and Ca(OH)2 in which the dentin increment in 6 months in the MTA group was found to be 0.143 ± 0.045 mm.[20] The mean reparative dentin formation in the TheraCal group in 6 months was found to be 0.154 mm ± 0.012 mm.
Vij et al. evaluated the effect of restoration placed at the time of pulp therapy. The 83% success rate of IPT restored with stainless steel crowns was not statistically different from the 87% success in teeth restored with intracoronal restoration.[21] In vitro study done by Kasraei et al. proved that RMGIC shows reduced microleakage compared to composites.[22] Shikha et al. in a comparative study of shear bond strength between RMGIC and conditioned and unconditioned MTA surface concluded that RMGIC can be applied as a base over MTA when used in direct pulp treatment or furcal repair. The RMGIC will not affect the MTA setting once the initial setting has occurred.[23] GIC placed over 45 min as opposed to 72 h old MTA, showed no effect on crazing, setting time or shear bond strength with a noticeable formation of calcium salts at the interface.[12] RMGIC also has a better bonding to composite resin than the conventional GIC due to the similar chemistry between RMGIC and composite resin. Both are cured by a free radical initiator system which provides a potential for chemical bonding between these two materials.[24] As suggested in the above studies, RMGIC was placed over both MTA and TheraCal before placing a composite restoration to obtain an optimum marginal seal.
In the present study, failure due to dislodged restorations was recorded in the second primary molars in the TheraCal group. Based on the literature, IPT is more successful in second primary molars.[5] Tooth position in the arch, the restoration size with a large configuration factor, and self-etching primer bonded to enamel, high caries activity challenge may possibly explain the failure rate of occlusal restorations of second primary molars.[25]
Based on the results of the present study, both MTA and TheraCal showed statistically significant tertiary dentin deposition. The above-mentioned results are based on a short-term clinical trial. Further long-term studies with larger sample size and histologic evaluation are required to confirm these results. More researches directed toward the development of better economical and biocompatible materials which do not affect the pulp vitality and normal resorption are needed for providing less invasive, quality dental treatment for the pediatric patients.
Conclusion
Based on the results of this short-term clinical and radiographic study, the following conclusions were drawn:
Clinically and radiographically, both MTA and TheraCal are good IPT medicaments with significant reparative dentin formation
Radiographically, both MTA and TheraCal showed increased dentin deposition in first 3 months when compared to the second 3 months.
Due to the ease of application, comparable tertiary dentin formation, better sealing ability and the convenience to provide the final restoration in a single appointment, TheraCal may be recommended as an alternative to MTA in pediatric restorative dentistry.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are grateful to the faculty, postgraduates and the nonteaching staffs of the Department of Pedodontics and Preventive Dentistry for their valuable support toward this clinical research.
References
- 1.Ritwik P. A review of pulp therapy for primary and immature permanent teeth. J Calif Dent Assoc. 2013;41:585–95. [PubMed] [Google Scholar]
- 2.Dorfman A, Stephan RM, Muntz JA. In vitro studies of carious dentin. II. Extent of infection in carious lesions. J Am Dent Assoc. 1943;30:1901. [Google Scholar]
- 3.Al-Zayer MA, Straffon LH, Feigal RJ, Welch KB. Indirect pulp treatment of primary posterior teeth: A retrospective study. Pediatr Dent. 2003;25:29–36. [PubMed] [Google Scholar]
- 4.Hilton TJ. Keys to clinical success with pulp capping: A review of the literature. Oper Dent. 2009;34:615–25. doi: 10.2341/09-132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coll JA. Indirect pulp capping and primary teeth: Is the primary tooth pulpotomy out of date? J Endod. 2008;34(7 Suppl):S34–9. doi: 10.1016/j.joen.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Farooq NS, Coll JA, Kuwabara A, Shelton P. Success rates of formocresol pulpotomy and indirect pulp therapy in the treatment of deep dentinal caries in primary teeth. Pediatr Dent. 2000;22:278–86. [PubMed] [Google Scholar]
- 7.Seale NS. Indirect pulp therapy: An alternative to pulpotomy in primary teeth. Tex Dent J. 2010;127:1175–83. [PubMed] [Google Scholar]
- 8.Ricucci D, Loghin S, Siqueira JF., Jr Correlation between clinical and histologic pulp diagnoses. J Endod. 2014;40:1932–9. doi: 10.1016/j.joen.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Murray PE, Smith AJ. Saving pulps – A biological basis. An overview. Prim Dent Care. 2002;9:21–6. doi: 10.1308/135576102322547511. [DOI] [PubMed] [Google Scholar]
- 10.Krasner P, Rankow HJ. Anatomy of the pulp-chamber floor. J Endod. 2004;30:5–16. doi: 10.1097/00004770-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Falster CA, Araujo FB, Straffon LH, Nör JE. Indirect pulp treatment: In vivo outcomes of an adhesive resin system vs calcium hydroxide for protection of the dentin-pulp complex. Pediatr Dent. 2002;24:241–8. [PubMed] [Google Scholar]
- 12.Darvell BW, Wu RC. “MTA” – An hydraulic silicate cement: Review update and setting reaction. Dent Mater. 2011;27:407–22. doi: 10.1016/j.dental.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Eskandarizadeh A, Shahpasandzadeh MH, Shahpasandzadeh M, Torabi M, Parirokh M. A comparative study on dental pulp response to calcium hydroxide, white and grey mineral trioxide aggregate as pulp capping agents. J Conserv Dent. 2011;14:351–5. doi: 10.4103/0972-0707.87196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandolfi MG, Siboni F, Prati C. Chemical-physical properties of TheraCal, a novel light-curable MTA-like material for pulp capping. Int Endod J. 2012;45:571–9. doi: 10.1111/j.1365-2591.2012.02013.x. [DOI] [PubMed] [Google Scholar]
- 15.Makkar S, Kaur H, Aggarwal A, Vashisht R. A confocal laser scanning microscopic study evaluating the sealing ability of mineral trioxide aggregate, biodentine and a new pulp capping agent-TheraCal. Dent J Adv Stud. 2015;3:20–5. [Google Scholar]
- 16.Cannon M, Gerodias N, Viera A, Percinoto C, Jurado R. Primate pulpal healing after exposure and TheraCal application. J Clin Pediatr Dent. 2014;38:333–7. doi: 10.17796/jcpd.38.4.m585322121536q71. [DOI] [PubMed] [Google Scholar]
- 17.Makkar S, Vashisht R, Kalsi A, Gupta P. The effect of altered pH on push-out bond strength of biodentin, glass ionomer cement, mineral trioxide aggregate and TheraCal. Serbian Dent J. 2015;62:7–11. [Google Scholar]
- 18.Jack DG. Utilizing bioactive liners: Stimulating post-traumatic dentin formation. J Dent Mater. 2012;29:2–6. [PubMed] [Google Scholar]
- 19.Poggio C, Arciola CR, Beltrami R, Monaco A, Dagna A, Lombardini M, et al. Cytocompatibility and antibacterial properties of capping materials. ScientificWorldJournal 2014. 2014:181945. doi: 10.1155/2014/181945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George V, Janardhanan SK, Varma B, Kumaran P, Xavier AM. Clinical and radiographic evaluation of indirect pulp treatment with MTA and calcium hydroxide in primary teeth (in-vivo study) J Indian Soc Pedod Prev Dent. 2015;33:104–10. doi: 10.4103/0970-4388.155118. [DOI] [PubMed] [Google Scholar]
- 21.Vij R, Coll JA, Shelton P, Farooq NS. Caries control and other variables associated with success of primary molar vital pulp therapy. Pediatr Dent. 2004;26:214–20. [PubMed] [Google Scholar]
- 22.Kasraei S, Azarsina M, Majidi S. In vitro comparison of microleakage of posterior resin composites with and without liner using two-step etch-and-rinse and self-etch dentin adhesive systems. Oper Dent. 2011;36:213–21. doi: 10.2341/10-215-L. [DOI] [PubMed] [Google Scholar]
- 23.Shikha S, Shenoy VU, Margasahayam SV. Comparison of shear bond strength of resin-modified glass ionomer to conditioned and unconditioned mineral trioxide aggregate surface: An in vitro study. J Conserv Dent. 2014;17:440–3. doi: 10.4103/0972-0707.139832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandak MG, Pattanaik N, Das A. Comparative study to evaluate shear bond strength of RMGIC to composite resin using different adhesive systems. Contemp Clin Dent. 2012;3:252–5. doi: 10.4103/0976-237X.103613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casagrande L, Bento LW, Dalpian DM, García-Godoy F, de Araujo FB. Indirect pulp treatment in primary teeth: 4-year results. Am J Dent. 2010;23:34–8. [PubMed] [Google Scholar]


