Abstract
Context:
Phosphatase and tensin homolog (PTEN) is a tumor suppressor gene located on chromosome 10q23. PTEN has its major function in the regulation of cell adhesion, cell cycle arrest, migration, apoptosis programming, and differentiation. This genomic region suffers loss of heterozygosity in many human cancers.
Aims:
The aim of this study was to compare the immunohistochemical expression of PTEN in normal oral mucosa and oral squamous cell carcinoma (OSCC) and to correlate the PTEN expression in gradings of OSCC.
Materials and Methods:
Thirty cases of paraffin tissue sections of previously diagnosed OSCC were taken. Of thirty cases, ten were well differentiated, ten were moderately differentiated, and ten were poorly differentiated. As a control, ten paraffin sections of oral normal mucosa tissue specimens were taken from patients undergoing extractions. The sections were stained for immunohistochemical expression of PTEN. The cells stained by PTEN antibody were counted, and an immunohistochemical score was obtained.
Statistical Analysis Used:
Statistical analysis was done using Mann–Whitney's test and Kruskal–Wallis test.
Results:
Statistical analysis revealed that there was a significant difference between normal mucosa and OSCC in immunohistochemistry staining. However, there was no significant difference in PTEN expression among gradings of OSCC.
Conclusions:
The study concluded that there was a decrease in PTEN expression in OSCC than normal mucosa. It also concluded that PTEN is a tumor suppressor gene which has a wide role in oral carcinogenesis.
Keywords: Oral squamous cell carcinoma, phosphatase and tensin homolog, tumor suppressor
Introduction
Oral cancer constitutes 1%–2% of all cancers in the body. The majority of oral cancers are squamous cell carcinoma.[1] Oral cancer ranks the eighth position worldwide and is the third most common cancer in South-East Asia.[2] The known classic risk factor of oral cancer is tobacco use,[3] and other etiological factors include alcohol, infections, dietary factors, chemical irritants, etc.[4]
Oral carcinogenesis is a process involving various steps in which several genetic events change the regular functions of oncogenes and tumor suppressor genes. These changes cause an increase in the production of transcription factors, growth factors, cell surface receptors, and intracellular messenger signaling. This leads to the failure of tumor suppressor activity, which in turn leads to a cell phenotype which can cause an increase in cell proliferation and reduction in cell cohesion with the capacity of infiltrating local tissues and spreading to distant sites.[5]
Many irreversible changes in deoxyribonucleic acid (DNA) sequence such as mutations, amplifications, and gene deletions may either activate oncogenes or inactivate tumor suppressor genes. These genetic alterations may lead to the development of oral premalignancy and its progression to oral cancer.[6]
Tumor suppressor genes prevent the development of cancer by directly controlling the cell growth through genes such as p16 and p53. Hence, tumor suppressor genes are considered to be the gatekeepers. These genes have been reported to be inactivated in many tumors. The various changes in tumor suppressor genes such as loss of heterozygosity, mutation, and microsatellite instability cause increase in genetic vulnerability for malignant transformation.[7]
Phosphatase and tensin homolog (PTEN) is the tumor suppressor gene which has an important role in negative regulation of cell survival signaling.[8] This gene is inactivated in tumors involving the brain, breast, and prostate cancers. This gene is considered to be the main cause of autosomal disorders of hamartomatous polyposis syndrome such as Cowden disease and Bannayan–Zonana syndrome.[9]
Studies have shown that PTEN mutations along with the loss of heterozygosity of the PTEN locus are seen in some cases of head and neck squamous cell carcinoma. In addition, head and neck squamous cell carcinoma cases have shown some molecular alteration in the phosphoinositide 3-kinase (PI3K) or Akt or protein kinase B pathway with downregulation of PTEN protein. Reduction in PTEN expression was also reported in some tongue cancers. These studies suggest the important role of the PTEN or PI3K or Akt signaling pathways in the carcinogenesis of head and neck squamous cell carcinoma.[10]
The 5-year survival rate of OSCC patients varies from 40% to 50%. In spite of easy access to the oral cavity for clinical examination, oral squamous cell carcinoma (OSCC) is diagnosed in advanced stages.[11]
There is a dearth of knowledge in relation to the development and progression of oral cancer. The development of cancer may involve a change in expression of various proteins. The study of proteins which change in oral cancer will provide valuable information and will help in the understanding of the disease. It also identifies the proteins which can be potentially utilized as markers for early cancer detection.[12] Cancer research advancement has provided ample knowledge about molecular biology and cellular processes OSCC. Our knowledge about carcinogenesis, recognition of biological markers, and molecularly targeted therapies are advancing through various translational research activities and clinical trials.[13]
Materials and Methods
Source of data
The present study was conducted on thirty cases (ten well-differentiated OSCC, ten moderately differentiated OSCC, and ten poorly differentiated OSCC) of formalin-fixed, paraffin-embedded tissue sections of previously diagnosed cases of OSCC. Ten normal oral mucosal tissue specimens were taken as control group from patients undergoing minor oral surgical procedures after taking informed consent from the patients. Previously treated cases and recurrent lesions of OSCCs were excluded from the study.
Methodology
The obtained forty tissue blocks were sectioned at 4-µ thickness using automatic microtome and subjected to the routine hematoxylin and eosin examination to confirm the diagnosis. Fresh sections of same thickness were subjected for the immunohistochemistry analysis using PTEN antibody. The PTEN primary antibody was purchased from Biocare and secondary horse peroxidase sensitive kit from Leica. Antigen retrieval buffer and wash buffer were prepared. The sections were cut and mounted at 4-µ thickness on slides coated with a poly-L-lysine and incubated overnight at 58°C.
Antigen retrieval was done by immersing the sections in antigen retrieval buffer and kept inside the microwave oven for four cycles. The sections were treated with peroxidase block for 5 min to block the endogenous peroxidase enzyme activity. Sections were then drained and covered with primary antibody against PTEN with a dilution of 1:100 for 1 h to identify tumor marker by an antigen-antibody reaction. Enzymes were labeled by treating the sections with NovaLink polymer secondary antibody for 30 min. Washing with tris buffer saline was done for 5 min in between the procedure. Chromogen was then added to the sections for 5 min to give color to the antigen. Finally, the sections were counterstained with hematoxylin, dehydrated, cleared in xylene, and mounted with DPX.
Counting criteria
The cells which are stained brown color are considered to be Positive cells [Figures 1–4]. Quantitative analysis of PTEN expression was done according to the scoring method by Kurasawa et al.[14] The mean of the percentages of positive cells was calculated in 10 fields at ×40 magnification. The cells were counted with the help of Image J software.
Figure 1.
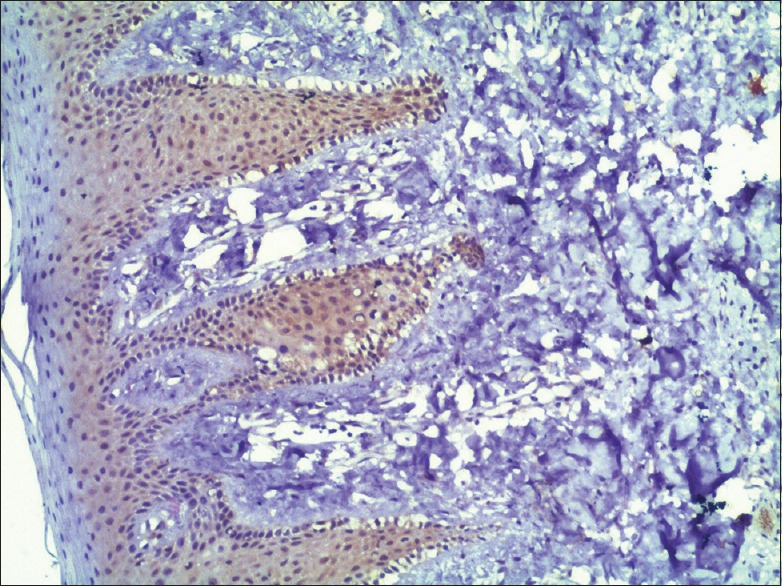
Phosphatase and tensin homolog expression in normal mucosa with intense staining (×10)
Figure 4.
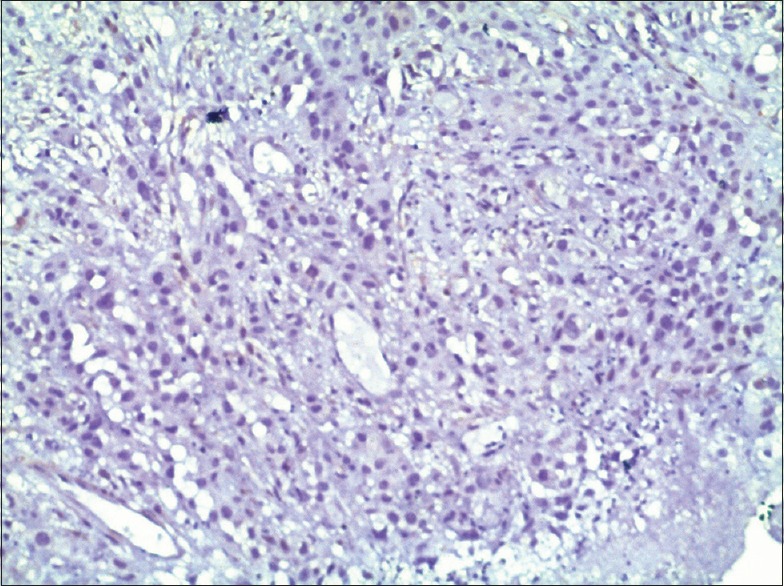
Phosphatase and tensin homolog expression in poorly differentiated (oral squamous cell carcinoma) with negative staining (×10)
Figure 2.
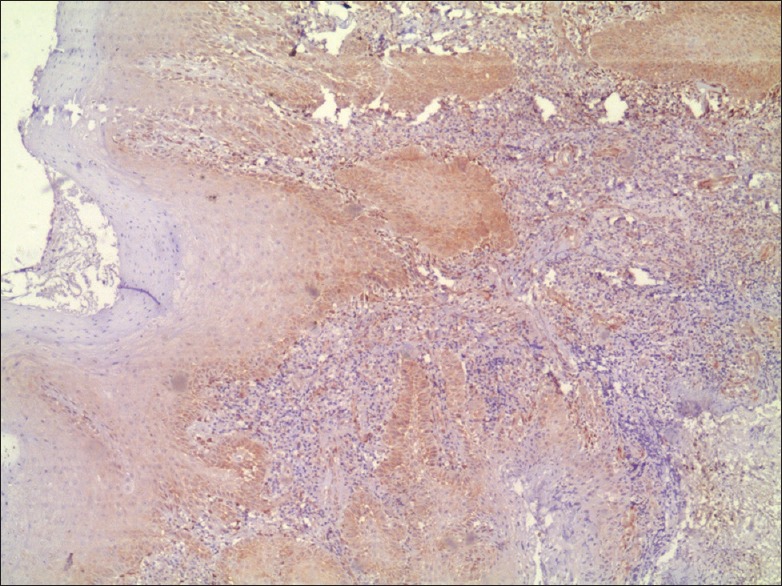
Phosphatase and tensin homolog expression in well-differentiated oral squamous cell carcinoma with moderate staining (×4)
Figure 3.
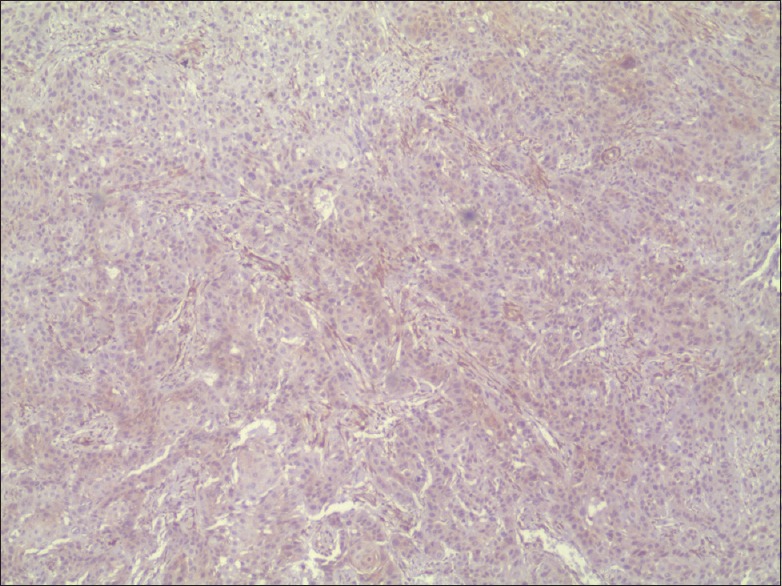
Phosphatase and tensin homolog expression in moderately differentiated oral squamous cell carcinoma with mild staining (×4)
The scoring of the intensity of PTEN immunoexpression was also done as follows:
0 - Negative staining
1 - Mild staining
2 - Moderate staining
3 - Intense staining.
The percentage of positive cells and intensity of staining were multiplied to produce an immunohistochemical score for each case. The staining in normal oral mucosa was taken as a positive control, and endothelial staining of endothelial cells of blood vessels within the connective tissue was taken as an internal control. The obtained immunohistochemistry scores were used for statistical analysis and the significance was assessed.
Statistical analysis
The analysis of the obtained data was done using the Statistical Package for Social Science software (IBM Corporation, USA). Data analysis was performed using Mann–Whitney test and Kruskal–Wallis test to correlate the immunohistochemistry scores and intensity of staining in normal mucosa and gradings of OSCC.
Results and Observations
The present study consisted of forty samples, ten each of normal mucosa, well-, moderately, and poorly differentiated OSCC. PTEN immunohistochemistry staining was done, and the stained slides were observed under light microscope. The total number of cells and positive cells were counted, and percentages of stained cells were calculated. The scoring of the intensity of staining was also done. The immunohistochemistry scores for each sample were obtained by multiplying the stained cells percentage and the intensity. There was statistically significant difference of PTEN expression between normal mucosa and OSSC in case of both immunohistochemistry scores [Table 1 and Graph 1] and intensity of staining. No significant difference in PTEN expression was seen between the gradings of OSCC [Table 2 and Graph 2].
Table 1.
Comparison of immunohistochemical score between normal and oral squamous cell carcinoma

Graph 1.
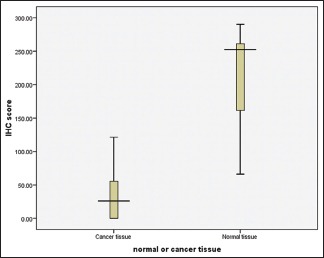
Comparison of immunohistochemical score between normal and oral squamous cell carcinoma
Table 2.
Comparison of immunohistochemical score between well-differentiated, moderately differentiated, and poorly differentiated oral squamous cell carcinoma

Graph 2.
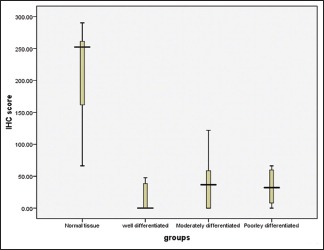
Comparison of immunohistochemical score among well-, moderately, and poorly differentiated oral squamous cell carcinoma
Discussion
Cancer is a group of heterogeneous diseases characterized by various clinical and biological characteristics. Hence, recognition of predictive and prognostic markers is clinically important. In recent times, even though molecular studies showed a strong prognostication, immunohistochemistry remains a convenient and powerful prognostic power as it is cheaper and easier to perform.[15] The importance of PTEN in oral carcinogenesis is strongly highlighted in this study.
Kurasawa et al. and Lee et al. reported cytoplasmic staining of PTEN. Angadi et al. and Alyasiri et al. reported nuclear staining, whereas Rahmani et al. and Hu et al. reported both nuclear and cytoplasmic staining.[8,14,15,16,17,18] Our study showed predominantly cytoplasmic staining. Few normal mucosa slides showed nuclear staining along with cytoplasmic staining. PTEN staining was seen in basal and spinous layers but predominantly in spinous layer with a reduction in intensity toward the granular layer. No staining was seen in stratum corneum layer.
Studies on PTEN in OSCC had shown that there was frequent genetic alterations and loss of expression. Keeping the marker profile of PTEN in view, the immunoexpression pattern was analyzed with the histological grade. The difference in the expression pattern of PTEN in histological grade did not reach statistical significance. This was in agreement with the studies done by Alyasiri et al., Lee et al., and Rahmani et al.
The study showed a highly significant difference of PTEN expression between normal mucosa and OSCC. Loss of PTEN expression is seen in OSCC samples.
The downregulation of PTEN expression can be explained by decreased protein synthesis, elevated protein degradation, or other posttranslational modifications.[16] PTEN mutation has been demonstrated in many cancers; however, a rare PTEN mutation has been demonstrated in OSCC.[19,20,21] Loss of heterozygosity at band 10q.23 was also detected in OSCC.[19] The genetic alterations in the PI3K signaling pathway may also play a major role in PTEN downregulation.[22] PTEN loss with increased Akt phosphorylation and reduced apoptosis was also demonstrated in oral cancer.[8] Another possible mechanism for loss of PTEN expression might be due to gene inactivation by hypermethylation of promoter region.[14]
Some studies have shown that PTEN gene alterations have been related to advanced disease. PTEN may also be involved in regulating tumor cell invasion and metastasis.[23] Studies have also shown PTEN status as an independent predictor of poor outcome in case of tumor stage and nodal status. Since AKT pathway involves many downregulations such as inhibition of tumor cell proliferation, apoptosis, and DNA repair and it is also associated with radioresistance mechanisms, inactivation of PTEN can affect the efficiency of anticancer therapy. These factors can create an interest in PTEN and other elements of the AKT pathway to be used as a potential prognostic and predictive markers for combined modality treatment and can also help targeting new drugs.[24]
The study concluded that downregulation of PTEN is seen in OSCC, indicating major role of PTEN as a tumor suppressor in oral carcinogenesis.
Conclusions
This study was done to compare the PTEN expression in normal mucosa and OSCC patients, and a very significant difference was obtained between normal mucosa and OSCC. OSCC is characterized by loss of PTEN expression; however, the intergroup comparison of PTEN expression between well, moderately, and poorly differentiated was found to be not statistically significant. This tumor suppressor marker should be thoroughly explored for their use as a prognostic indicator and for monitoring the progression of OSCC. In future, investigation of PTEN inactivation in OSCC can be used in novel therapeutic approaches.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.van der Waal I. Are we able to reduce the mortality and morbidity of oral cancer; some considerations. Med Oral Patol Oral Cir Bucal. 2013;18:e33–7. doi: 10.4317/medoral.18486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massano J, Regateiro FS, Januário G, Ferreira A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 3.Attar E, Dey S, Hablas A, Seifeldin IA, Ramadan M, Rozek LS, et al. Head and neck cancer in a developing country: A population-based perspective across 8 years. Oral Oncol. 2010;46:591–6. doi: 10.1016/j.oraloncology.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meurman JH. Infectious and dietary risk factors of oral cancer. Oral Oncol. 2010;46:411–3. doi: 10.1016/j.oraloncology.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Williams HK. Molecular pathogenesis of oral squamous carcinoma. Mol Pathol. 2000;53:165–72. doi: 10.1136/mp.53.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mascolo M, Siano M, Ilardi G, Russo D, Merolla F, De Rosa G, et al. Epigenetic disregulation in oral cancer. Int J Mol Sci. 2012;13:2331–53. doi: 10.3390/ijms13022331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Kikuchi K, Takano Y. ING genes work as tumor suppressor genes in the carcinogenesis of head and neck squamous cell carcinoma. J Oncol 2011. 2011:963614. doi: 10.1155/2011/963614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alyasiri NS, Mehdi SJ, Alam MS, Ali A, Mandal AK, Gupta S, et al. PTEN-mediated AKT activation contributes to the reduced apoptosis among Indian oral squamous cell carcinoma patients. J Cancer Res Clin Oncol. 2012;138:103–9. doi: 10.1007/s00432-011-1077-y. [DOI] [PubMed] [Google Scholar]
- 9.Kohno T, Takahashi M, Manda R, Yokota J. Inactivation of the PTEN/MMAC1/TEP1 gene in human lung cancers. Genes Chromosomes Cancer. 1998;22:152–6. doi: 10.1002/(sici)1098-2264(199806)22:2<152::aid-gcc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Bian Y, Hall B, Sun ZJ, Molinolo A, Chen W, Gutkind JS, et al. Loss of TGF-ß signaling and PTEN promotes head and neck squamous cell carcinoma through cellular senescence evasion and cancer-related inflammation. Oncogene. 2012;31:3322–32. doi: 10.1038/onc.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markopoulos AK. Current aspects on oral squamous cell carcinoma. Open Dent J. 2012;6:126–30. doi: 10.2174/1874210601206010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karsani SA, Saihen NA, Zain RB, Cheong SC, Abdul Rahman M. Comparative proteomics analysis of oral cancer cell lines: Identification of cancer associated proteins. Proteome Sci. 2014;12:3. doi: 10.1186/1477-5956-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tegginamani AS. Evaluation of p53 expression in oral squamous cell carcinoma with and without tobacco habits. A comparative study. Indian J Dent Adv. 2011;3:723–6. [Google Scholar]
- 14.Kurasawa Y, Shiiba M, Nakamura M, Fushimi K, Ishigami T, Bukawa H, et al. PTEN expression and methylation status in oral squamous cell carcinoma. Oncol Rep. 2008;19:1429–34. [PubMed] [Google Scholar]
- 15.Rahmani A, Alzohairy M, Babiker AY, Rizvi MA, Elkarimahmad HG. Clinicopathological significance of PTEN and Bcl2 expressions in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2012;5:965–71. [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JI, Soria JC, Hassan KA, El-Naggar AK, Tang X, Liu DD, et al. Loss of PTEN expression as a prognostic marker for tongue cancer. Arch Otolaryngol Head Neck Surg. 2001;127:1441–5. doi: 10.1001/archotol.127.12.1441. [DOI] [PubMed] [Google Scholar]
- 17.Angadi PV, Krishnapillai R. Evaluation of PTEN immunoexpression in oral submucous fibrosis: Role in pathogenesis and malignant transformation. Head Neck Pathol. 2012;6:314–21. doi: 10.1007/s12105-012-0341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu TH, Huang CC, Lin PR, Chang HW, Ger LP, Lin YW, et al. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer. 2003;97:1929–40. doi: 10.1002/cncr.11266. [DOI] [PubMed] [Google Scholar]
- 19.Shao X, Tandon R, Samara G, Kanki H, Yano H, Close LG, et al. Mutational analysis of the PTEN gene in head and neck squamous cell carcinoma. Int J Cancer. 1998;77:684–8. doi: 10.1002/(sici)1097-0215(19980831)77:5<684::aid-ijc4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Mavros A, Hahn M, Wieland I, Koy S, Koufaki ON, Strelocke K, et al. Infrequent genetic alterations of the tumor suppressor gene PTEN/MMAC1 in squamous cell carcinoma of the oral cavity. J Oral Pathol Med. 2002;31:270–6. doi: 10.1034/j.1600-0714.2002.310504.x. [DOI] [PubMed] [Google Scholar]
- 21.Poetsch M, Lorenz G, Kleist B. Detection of new PTEN/MMAC1 mutations in head and neck squamous cell carcinomas with loss of chromosome 10. Cancer Genet Cytogenet. 2002;132:20–4. doi: 10.1016/s0165-4608(01)00509-x. [DOI] [PubMed] [Google Scholar]
- 22.Chiosea SI, Grandis JR, Lui VW, Diergaarde B, Maxwell JH, Ferris RL, et al. PIK3CA, HRAS and PTEN in human papillomavirus positive oropharyngeal squamous cell carcinoma. BMC Cancer. 2013;13:602. doi: 10.1186/1471-2407-13-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvesen HB, Stefansson I, Kalvenes MB, Das S, Akslen LA. Loss of PTEN expression is associated with metastatic disease in patients with endometrial carcinoma. Cancer. 2002;94:2185–91. doi: 10.1002/cncr.10434. [DOI] [PubMed] [Google Scholar]
- 24.Snietura M, Jaworska M, Mlynarczyk-Liszka J, Goraj-Zajac A, Piglowski W, Lange D, et al. PTEN as a prognostic and predictive marker in postoperative radiotherapy for squamous cell cancer of the head and neck. PLoS One. 2012;7:e33396. doi: 10.1371/journal.pone.0033396. [DOI] [PMC free article] [PubMed] [Google Scholar]


