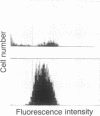
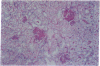
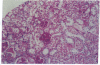
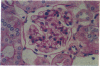
Abstract
Hematopoietic stem cell defects are thought to be involved in the etiopathogenesis of systemic autoimmune disease. Positively selected, stem cell-enriched populations of wheat germ agglutinin-positive (WGA+) low-density bone marrow and fetal liver cells from normal and autoimmune-prone mice were used to determine whether reciprocal transplantation of stem cells between normal and autoimmune-prone mice inhibits or causes development of autoimmune disease. NZB recipients of DBA/2 stem cell populations analyzed greater than 100 days after bone marrow or fetal liver cell transplantation showed decreased levels of anti-DNA antibodies and decreased glomerular lesions when compared with nontreated NZB mice or NZB recipients of NZB stem cell preparations. Female DBA/2 recipients of WGA+ NZB bone marrow cell or fetal liver cell transplants exhibited elevated serum autoantibody levels and developed glomerular lesions characteristic of NZB mice when analyzed greater than 100 days after transplantation. These pathological disturbances were not observed in DBA/2 recipients of DBA/2 stem cell preparations. The data indicate that WGA+ stem cells from autoimmune-prone NZB mice contain the genetic defects responsible for the development of systemic autoimmune disease.
Full text
PDF


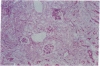
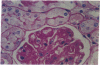
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akizuki M., Reeves J. P., Steinberg A. D. Expression of autoimmunity by NZB/NZW marrow. Clin Immunol Immunopathol. 1978 Jul;10(3):247–250. doi: 10.1016/0090-1229(78)90177-0. [DOI] [PubMed] [Google Scholar]
- Berman J. W., Basch R. S. Thy-1 antigen expression by murine hematopoietic precursor cells. Exp Hematol. 1985 Dec;13(11):1152–1156. [PubMed] [Google Scholar]
- Boswell H. S., Wade P. M., Jr, Quesenberry P. J. Thy-1 antigen expression by murine high-proliferative capacity hematopoietic progenitor cells. I. Relation between sensitivity to depletion by Thy-1 antibody and stem cell generation potential. J Immunol. 1984 Dec;133(6):2940–2949. [PubMed] [Google Scholar]
- DeHeer D. H., Edgington T. S. Evidence for a B lymphocyte defect underlying the anti-X anti-erythrocyte autoantibody response of NZB mice. J Immunol. 1977 May;118(5):1858–1863. [PubMed] [Google Scholar]
- Frederickson G. G., Basch R. S. L3T4 antigen expression by hemopoietic precursor cells. J Exp Med. 1989 Apr 1;169(4):1473–1478. doi: 10.1084/jem.169.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitsu T., Sakuma S., Seki N., Senoh H., Mori J., Kikuchi H. Effect of auranofin on autoimmune disease in a mouse model. Int J Immunopharmacol. 1986;8(8):897–910. doi: 10.1016/0192-0561(86)90091-3. [DOI] [PubMed] [Google Scholar]
- Himeno K., Good R. A. Marrow transplantation from tolerant donors to treat and prevent autoimmune diseases in BXSB mice. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2235–2239. doi: 10.1073/pnas.85.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S., Good R. A., Nakamura T., Sekita K., Inoue S., Oo M. M., Muso E., Ogawa K., Hamashima Y. Rationale for bone marrow transplantation in the treatment of autoimmune diseases. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2483–2487. doi: 10.1073/pnas.82.8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S., Kawamura M., Takao F., Inaba M., Yasumizu R., Than S., Hisha H., Sugiura K., Koide Y., Yoshida T. O. Organ-specific and systemic autoimmune diseases originate from defects in hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8341–8344. doi: 10.1073/pnas.87.21.8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S., Yasumizu R., Inaba M., Izui S., Hayakawa K., Sekita K., Toki J., Sugiura K., Iwai H., Nakamura T. Long-term observations of autoimmune-prone mice treated for autoimmune disease by allogeneic bone marrow transplantation. Proc Natl Acad Sci U S A. 1989 May;86(9):3306–3310. doi: 10.1073/pnas.86.9.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H., Kincade P. W., Good R. A., Fernandes G. Reciprocal transfer of abnormalities in clonable B lymphocytes and myeloid progenitors between NZB and DBA/2 mice. J Immunol. 1981 Sep;127(3):1232–1235. [PubMed] [Google Scholar]
- Lapidot T., Terenzi A., Singer T. S., Salomon O., Reisner Y. Enhancement by dimethyl myleran of donor type chimerism in murine recipients of bone marrow allografts. Blood. 1989 May 15;73(7):2025–2032. [PubMed] [Google Scholar]
- Lord B. I., Spooncer E. Isolation of haemopoietic spleen colony forming cells. Lymphokine Res. 1986 Winter;5(1):59–72. [PubMed] [Google Scholar]
- Miyama-Inaba M., Ogata H., Toki J., Kuma S., Sugiura K., Yasumizu R., Ikehara S. Isolation of murine pluripotent hemopoietic stem cells in the Go phase. Biochem Biophys Res Commun. 1987 Sep 15;147(2):687–694. doi: 10.1016/0006-291x(87)90985-5. [DOI] [PubMed] [Google Scholar]
- Morton J. I., Siegel B. V. Letter: Early autoantibody formation in lethally irradiated or drug-treated H-2-compatible recipients of pre-autoimmune NZB bone marrow or fetal liver cells. Transplantation. 1974 Jun;17(6):624–626. doi: 10.1097/00007890-197406000-00012. [DOI] [PubMed] [Google Scholar]
- Morton J. I., Siegel B. V., Moore R. D. Transplantation of autoimmune potential. II. Glomerulonephritis in lethally irradiated DBA/2 recipients of NZB bone marrow cells. Transplantation. 1975 Jun;19(6):464–469. [PubMed] [Google Scholar]
- Morton J. I., Siegel B. V. Transplantation of autoimmune potential. I. Development of antinuclear antibodies in H-2 histocompatible recipients of bone marrow from New Zealand Black mice. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2162–2165. doi: 10.1073/pnas.71.6.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J. I., Siegel B. V. Transplantation of autoimmune potential. III. Immunological hyper-responsiveness and elevated endogenous spleen colony formation in lethally irradiated recipients of NZB bone marrow cells. Immunology. 1978 May;34(5):863–868. [PMC free article] [PubMed] [Google Scholar]
- Morton J. I., Siegel B. V. Transplantation of autoimmune potential. IV. Reversal of the NZB autoimmune syndrome by bone marrow transplantation. Transplantation. 1979 Feb;27(2):133–134. [PubMed] [Google Scholar]
- Mulder A. H., Bauman J. G., Visser J. W., Boersma W. J., van den Engh G. J. Separation of spleen colony-forming units and prothymocytes by use of a monoclonal antibody detecting an H-2K determinant. Cell Immunol. 1984 Oct 15;88(2):401–410. doi: 10.1016/0008-8749(84)90173-4. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Ikehara S., Good R. A., Inoe S., Sekita K., Furukawa F., Tanaka H., Oo M. M., Hamashima Y. Abnormal stem cells in autoimmune-prone mice are responsible for premature thymic involution. Thymus. 1985;7(3):151–160. [PubMed] [Google Scholar]
- Nicola N. A., Burgess A. W., Metcalf D., Battye F. L. Separation of mouse bone marrow cells using wheat germ agglutinin affinity chromatographyy. Aust J Exp Biol Med Sci. 1978 Dec;56(6):663–679. doi: 10.1038/icb.1978.74. [DOI] [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. Isolation of hemopoietic stem cell subsets from murine bone marrow: I. Radioprotective ability of purified cell suspensions differing in the proportion of day-7 and day-12 CFU-S. Exp Hematol. 1988 Jan;16(1):21–26. [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. Isolation of hemopoietic stem cell subsets from murine bone marrow: II. Evidence for an early precursor of day-12 CFU-S and cells associated with radioprotective ability. Exp Hematol. 1988 Jan;16(1):27–32. [PubMed] [Google Scholar]
- Reisner Y., Itzicovitch L., Meshorer A., Sharon N. Hemopoietic stem cell transplantation using mouse bone marrow and spleen cells fractionated by lectins. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2933–2936. doi: 10.1073/pnas.75.6.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Sugiura K., Ikehara S., Gengozian N., Inaba M., Sardiña E. E., Ogata H., Seong S. M., Good R. A. Enrichment of natural suppressor activity in a wheat germ agglutinin positive hematopoietic progenitor-enriched fraction of monkey bone marrow. Blood. 1990 Mar 1;75(5):1125–1131. [PubMed] [Google Scholar]
- Sugiura K., Inaba M., Ogata H., Yasumizu R., Inaba K., Good R. A., Ikehara S. Wheat germ agglutinin-positive cells in a stem cell-enriched fraction of mouse bone marrow have potent natural suppressor activity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4824–4826. doi: 10.1073/pnas.85.13.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K., Inaba M., Ogata H., Yasumuzu R., Sardiña E. E., Inaba K., Kuma S., Good R. A., Ikehara S. Inhibition of tumor cell proliferation by natural suppressor cells present in murine bone marrow. Cancer Res. 1990 May 1;50(9):2582–2586. [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Visser J. W., Bol S. J. A two-step procedure for obtaining 80-fold enriched suspensions of murine pluripotent hemopoietic stem cells. Stem Cells. 1982;1(4-5):240–249. [PubMed] [Google Scholar]
- Visser J. W., de Vries P. Isolation of spleen-colony forming cells (CFU-s) using wheat germ agglutinin and rhodamine 123 labeling. Blood Cells. 1988;14(2-3):369–384. [PubMed] [Google Scholar]