Abstract
Purpose
Future advances in ophthalmology will see a paradigm shift in diagnostics from a focus on dysfunction and disease to better measures of psychophysical function and health. Practical methods to define genotypes will be increasingly important and non-invasive nanotechnologies are needed to detect molecular changes that predate histopathology.
Methods
This is not a review nor meant to be comprehensive. Specific topics have been selected to illustrate the principles of important paradigm shifts that will influence the future of ophthalmic diagnostics. It is our impression that future evaluation of vision will go beyond visual acuity to assess ocular health in terms of psychophysical function. The definition of disease will incorporate genotype into what has historically been a phenotype-centric discipline. Non-invasive nanotechnologies will enable a paradigm shift from disease detection on a cellular level to a sub-cellular molecular level.
Results
Vision can be evaluated beyond visual acuity by measuring contrast sensitivity, color vision, and macular function, as these provide better insights into the impact of aging and disease. Distortions can be quantified and the psychophysical basis of vision can be better evaluated than in the past by designing tests that assess particular macular cell function(s). Advances in our understanding of the genetic basis of eye diseases will enable better characterization of ocular health and disease. Non-invasive nanotechnologies can assess molecular changes in the lens, vitreous, and macula that predate visible pathology. Oxygen metabolism and circulatory physiology are measurable indices of ocular health that can detect variations of physiology and early disease.
Conclusions
This overview of paradigm shifts in ophthalmology suggests that the future will see significant improvements in ophthalmic diagnostics. The selected topics illustrate the principles of these paradigm shifts and should serve as a guide to further research and development. Indeed, successful implementation of these paradigm shifts in ophthalmology may provide useful guidance for similar developments in all of healthcare.
I. INTRODUCTION
Medicine is a discipline that traditionally uses patient evaluations to find disease.1 In the absence of abnormal findings, ‘health’ is assumed. This is especially true in ophthalmology where physical examination, diagnostic tests, and imaging are designed to seek abnormalities. Indeed, it is often glibly said that in ophthalmology “what you see is what you get”. Consequently, the philosophy of our approach is often shaped by direct visualization of disease. ‘Health” is defined as the absence of visible manifestations of disease. While this has certainly been useful to date, future advances in health care will depend upon establishing health as a diagnosis2, so as to enable health promotion and preventive care, the ultimate goal. Doing so in ophthalmology will require a departure from our current concepts of eye disease, which are predominantly focused on tissue and cellular histopathology. Advances will shift our focus to the molecular level for assessment of structure and function. Such a paradigm shift enables the establishment of ocular health as a distinct entity (diagnosis). Better measurements will lead to better understanding of the psychophysical basis of vision and better metrics of structure and physiologic function will allow for better management including preventive treatments. With this shift, a new paradigm of health promotion could provide a powerful means to not only prevent disease and the visual consequences associated with aging, but also relate (link) disease prevention and health promotion initiatives to performance-based measures such as reading speed, driving simulators, and tests of actual life function such as CAARV.3
The theme of the 2016 Knapp Symposium of the American Ophthalmological Society was “Innovative Paradigm Shifts in Ophthalmology”. Paradigm Shift is a term coined in 1962 by T.S. Kuhn to mean “a fundamental change in the basic concepts and experimental practices of a scientific discipline”.4 The term has now become part of the business and political lexicons, but it was initially used as a means of re-considering established scientific models to provide better explanations of available information. The 2016 Knapp symposium addressed both diagnostic and therapeutic paradigm shifts in ophthalmology. Although technologic advances in therapy, such as laser therapy to replace surgery in glaucoma,5,6 the transition from surgery to pharmacotherapy with anti-VEGF injections for exudative maculopathies,7 pharmacologic vitreolysis to replace vitrectomy for vitreo-maculopathies8,9, and new technologies for drug delivery10 were important parts of this symposium, these topics are beyond the scope of this white paper. Further, it should be emphasized that this is not a comprehensive review article. Rather, the topics presented have been selected to illustrate the principles espoused and demonstrate the initial implementation of the paradigm shifts that will influence the future of ophthalmology.
This treatise thus begins with a consideration of how vision is measured, as a reflection of the need in the future to define eye health in terms of various psychophysical functions. Also important is the question of the relative roles of phenotype and genotype in our future concepts of disease. Lastly, non-invasive nanotechnologies are being developed to enable a departure from the disease-oriented, histopathologic perspectives of disease that currently dominate ophthalmology.
In 1936, the renowned physicist Max Planck stated that
“An important scientific innovation rarely makes its way by gradually winning over and converting its opponents - it rarely happens that Saul becomes Paul. What does happen is that its opponents gradually die out and that the growing generation is familiarized with the idea from the beginning.”11
It is hoped that this white paper will help prove Max Planck wrong, and that we need not wait for the next generation to adopt the tenets espoused herein.
II. BEYOND VISUAL ACUITY – TOMORROW’S MEASURES OF VISION
“Science is measurement”
Francis Bacon12
A. INTRODUCTION
Visual acuity (VA) has long been the cornerstone of measuring vision. Snellen acuity is common, convenient and reliable, yet this method of measuring vision is also problematic. For example, VA is just a sampling of the central few degrees of visual field and generally it is only measured at 100% contrast. There are many occasions when patients with VA of 20/20 are, in fact, functionally blind. In the future, we will see a paradigm shift to assessments that evaluate other aspects of vision. These will better characterize the patient’s visual abilities, will better detect the early onset of disease, and will better measure response to therapy than VA alone. Going beyond VA will afford better phenotype characterization for both research and clinical care. A better understanding of the physiology of vision as reflected by these new testing paradigms may even enable new methods by which to improve vision beyond what is considered ‘normal’ today.
B. Utility and Limitations of Visual Acuity (VA)
The measurement of VA is typically performed with black letters against a white background. However, a prospective study13 comparing mean Snellen VA and ETDRS VA found scores were significantly better on ETDRS charts especially in patients with poor vision. Although VA testing is required by the U.S. Food and Drug Administration as a measure of vision for any clinical trial, the Snellen chart is not suitable for patients who have severe vision loss.
VA can be considered as a measure of the minimal angle of resolution for a high contrast target. However, the issue of contrast is important in this paradigm because letters of less contrast may need to be bigger to be seen (see section on contrast sensitivity). And yet, in the real world, rarely do we get 100% contrast (with the exception of reading high quality magazines). Furthermore, the measure of VA is largely the measure of the central visual field and correspondingly, macular function. So, in trials concerning optic nerve function (such as glaucoma or optic neuritis), VA can be very misleading. Neuro-ophthalmologists know that you can’t predict VA from optic atrophy. So, if not VA, where should we turn?
C. Testing Contrast Sensitivity Function
A good test of vision should satisfy three functions: To detect early disease; to characterize the disease; to monitor the disease for progression or response to therapy. Contrast sensitivity testing has the potential to meet all three requirements.
1. DEFINITION AND METHODS OF MEASUREMENT
The measurement of contrast sensitivity is the measurement of the visual ability to detect small differences in the luminance between the object of regard and the background. Testing for such usually involves establishing the minimum difference in luminance necessary for the subject to detect a difference by discerning an object or pattern. The purest test of this involves sinusoidal grating patterns. However, there are quicker and more convenient tests such as forced choice gratings at different orientations that vary in size and contrast, which is the basis of the Freiburg Acuity Contrast Test (FrACT). Another is the Pelli-Robson test where letters are similar to Snellen chart letters but with diminishing contrast, or low contrast testing of VA. Each test is easier than the former but less psychophysically “pure” (sharp edges are of a different spatial frequency than the whole objects) in measuring contrast sensitivity.
The human visual system has the highest contrast sensitivity at medium-level angular frequency, approximately 5–7 cycles per degrees. This then decreases with angle and approaches the need for 100% contrast at the highest angular frequencies.14 In this sense, VA could be defined as the point where the contrast sensitivity curve touches the “X” axis or that spatial frequency near 0% contrast sensitivity. In a variety of diseases, there may be diminished contrast sensitivity with consequent decreased visual function in the presence of normal visual acuity.15
2. CONTRAST SENSITIVITY FUNCTION IN OPTIC NEUROPATHIES
Optic neuropathies can produce a variety of visual impairments with a primary effect on the central (eg. Leber’s hereditary optic neuropathy), or peripheral (e.g. Optic disc drusen) visual fields. In the case of the former, there is an immediate and profound effect on VA that may not change further despite progression of the optic nerve damage (floor effect). In the case of the latter, there may be profound progression without any change in VA as the optic nerve fibers arising from the macula are never involved. In both cases, as well as in a variety of other optic neuropathies, visual fields and contrast sensitivity functions are more useful than visual acuities.16,17 Recent work in patients with multiple sclerosis has determined that mild damage to the optic nerve, as measured by low contrast testing, can be used to monitor the patient for response to immunomodulation therapy.18
3. CONTRAST SENSITIVITY FUNCTION IN VITREO-RETINAL DISORDERS
a. Vitreous and Posterior Vitreous Detachment
Contrast sensitivity function (CSF) is known to decrease with age, a phenomenon previously thought to reflect aging changes and degenerations within the retina, optic nerve and visual cortex19,20 Nonetheless, CSF is not commonly measured in clinical practice. Recent studies by Sebag et al. have shown that changes in the vitreous body may also alter CSF. Patients without any ocular abnormalities, except bothersome vitreous floaters primarily due to posterior vitreous detachment, had reductions of CSF of about 67% in comparison to that found in age-matched controls.21 It is believed that in addition to provoking symptomatic floaters, these vitreous degenerations produce optical effects that reduce CSF but with normal or nearly normal visual acuities.22 That this impairment strikingly normalized within one week after limited vitrectomy strongly suggests that vitreous was the cause of the impairment.21,22 Subsequent studies confirmed that age-related gel liquefaction with intravitreal fiber formation and posterior vitreous detachment are associated with diminished CSF.23 In young myopic individuals there is similar aggregation of collagen fibrils and gel liquefaction constituting myopic vitreopathy that also reduces CSF. It is likely that this phenomenon underlies the measureable negative impact on the quality of life experienced by patients with vitreous floaters.24 Thus, CSF testing is a useful measure of the impact of various ocular as well as central nervous system disorders on vision. Future advances will hopefully see the integration of CSF testing into clinical practice alongside visual acuity testing.
b. Retinopathies
In addition to being directly measured, CSF can be integrated into the measurement of visual fields. In this sense, contrast can be the “Z” axis for the construction of a 3-dimensional visual field display done through a computerized presentation and analysis, called 3-dimensionnal threshold Amsler Grid (3-D TAG) testing.25 This system has shown characteristic contrast/visual field defects in diabetic retinopathy26, plaquenil toxicity27, glaucoma28,29, age-related macular degeneration30, and vitreo-maculopathies.31,32 In glaucoma, 3D-TAG can detect changes prior to conventional visual field loss (Figure 1, left), as can Pelli-Robson, Mars, Arden Gratings, SPARCS, and other tests of contrast sensitivity function. In maculopathies, disease severity and the response to therapy can be quantified with 3D-TAG, as has been shown for pharmacologic vitreolysis therapy of vitreo-macular traction30 and surgery for macular pucker.31 (Figure 1, right) Further advances could be realized by relating such visual function to quality of life and ability to function in daily living. 3
FIGURE 1.
3-DIMENSIONAL THRESHOLD AMSLER GRID (3D-TAG)
Left: 3-dimensional Threshold Amsler Grid testing is found superior and inferior nasal steps in the right eye of glaucoma-suspect patient with normal (B) Humphrey visual field. The 3D-TAG is much more sensitive. Grayscale figure on right is a second patient tested by 3-D TAG on two subsequent days that shows identical stair-case pattern of inferior nasal steps in the right eye only seen at lower contrast levels (the lower the contrast, the larger the field defect). Again this was in a patient with normal Humphrey visual field. The similarity between these two tests on subsequent days confirms the test/retest reproducibility.
[Adapted from Nazemi et al. Early detection of glaucoma by means of a novel 3D computer-automated visual field test. Br J Ophthalmol. 2007;91(10):1331–36.]
Right: Volumetric 3-dimensional plots demonstrate the location and extent of visual distortions before and after surgery for macular pucker. The volume inside the 3-dimensional tracing is calculated and expressed as the percent of the hill-of-vision afflicted with metamorphopsia. Displayed are the plots of the patient’s metamorphopsia before surgery (pre-op) and at 1, 3, 6, and 12 months after surgery (post-op). The distortions index (percent volume loss relative to the entire hill of vision) reduced from 15.80% before surgery to 4.74% at 1 month, 1.33% at 3 months, and 0.34% 12 months after surgery. [Reprinted from Nguyen J, Yee KMP, Sadun AA, Sebag J: Quantifying visual dysfunction and the response to surgery in macular pucker. Ophthalmology 2016; 123:1500–10.]
D. COLOR VISION
Neuro-ophthalmologists refer to a triad of tests that are particularly sensitive to optic nerve dysfunction - color vision, afferent pupillary defect and brightness-sense. Acquired impairments of color (dyschromatopsia) often reflect optic nerve dysfunction, especially in the presence of good visual acuity.33 The Ishihara pseudoisochromatic color plates are commonly used because of the simplicity of this test. However, such testing may not detect subtle disease. More sophisticated testing, such as with the Cambridge Colour testing system, the Farnsworth D-15, the Lanthony 15-Hue Desaturated Test, or the Farnsworth-Munsell 100 Hues test is tedious, as it requires specialized lighting and a commitment of time. But such testing may reveal early glaucoma, autosomal dominant optic atrophy, optic neuritis, and even Alzheimer’s optic neuropathy.34, 35 Furthermore, color vision may also be reduced in macular disease.36,37 Thus, future paradigms of measuring vision will benefit from developing simple yet more sensitive tests of color vision.
E. MACULAR FUNCTION
Measuring macular function can provide insight into the early manifestation(s) of many diseases.36 Deficient macular function can produce impairments of contrast sensitivity, dyschromatopsia, and especially central depressions on 3-D TAG testing. Furthermore, metamorphopsia are common in macular disease and this too can be characterized, quantitated, and monitored with 3-D TAG testing.31,38 Indeed, the three-dimensional shape of a metamorphopsia defect may look very different from that of scotomatous defect. The latter is often cylindric (the same at all contrast levels) while the former is often conical with the tip pointing “down” (denotes increased central visual field abnormality with lower contrast levels between the grid and the background). The 3-dimensional zone of metamorphopsia may be cylindrical, conical, or even in an hour-glass shape at times, as the larger zone of metamorphopsia may be brought out by higher or lower contrast levels. Diabetic macular edema was found to be associated with multi-focal, conical 3D-TAG abnormalities, while exudative age-related macular degeneration was associated with unifocal, quasi-cylindric abnormalities.37 3D-TAG was also able to distinguish between wet and dry macular degeneration.29 Insofar as amplification of the zone of metamorphopsia can occur at various contrast levels, it may be reasonable to obtain an index reflecting the total volume of the 3-D cylinder or cone of metamorphopsia. This has been done for macular pucker where the distortions index (%volume lost of the hill-of-vision) decreased progressively following vitreous surgery31. (Figure 1, right)
The problem of quantifying distortions is made even more difficult when adding the fourth dimension of spatial frequency. In the future, this should be integrated, since certain lesions may produce more metamorphopsia when looking at finer lines, while others induce more distortions when tested with coarser and broader lines. Thus, future paradigms of measuring vision may be provide even greater insight into the physiology of vision by utilizing 4-dimensional assessments.
1. Retinal Ganglion Cells
Different types of retinal ganglion cells subserve separate visual functions. One major distinction is defined by the layers of the lateral geniculate nucleus (LGN) to which the cells project. M-cells are fast-conducting and project to the Magnocellular layers of the LGN as well as the superior colliculus, while P-cells are slow firing and project to the Parvocellular layers of the LGN. Distinguishing the different functions of M-cells from the P-cells is both complicated and controversial. However, in the simplest sense, P-cells furnish what we would consider as conscious visual elements needed for discrimination, since they provide high contrast, high spatial frequency information, and color vision.39 Since the proportion of P-cells is highest in the macula, high contrast/high spatial frequency information and color vision are often considered macular functions. M-cells, in contradistinction, help us perceive stimuli that deserve our attention and may provoke eye movements. Hence, they provide better contrast sensitivity to low spatial frequencies and motion detection. So the M-cells, may be tuned to determine “where” things are happening (a moving shadow in the dark), while P-cells in the macula allow one to determine “what” it is and therefore are critical to read, perform eye surgery, or pick out your favorite scarf design.
A third type of retinal ganglion cell, constituting about 1% of the total, is distributed about evenly across the retina. Melanopsin-expressing retinal ganglion cells (mRGCs) represent a new class of photoreceptors that subserve the photoentrainment of circadian rhythms, the pupillary reflex, and other non-image forming functions of the eye.40 They do not contribute to image resolution. There has been a growing research interest in these cells (Figure 2), including a few studies on the relevance of the mRGC system in humans in aging and in different optic neuropathies.41 For example, mRGCs resist neurodegeneration in inherited mitochondrial disorders that cause blindness.42,43 Measuring their dysfunction requires specialized psychophysics such as pupillometry with light stimuli at different wavelengths, representing another paradigm to measure vision beyond visual acuity.39
FIGURE 2.
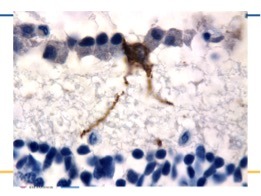
MELANOPSIN RETINAL GANGLION CELL
A melanopsin retinal ganglion cell (brown) positioned along a line of normal retinal ganglion cells (blue) in a normal human retina. Note the extensions (dendrites) that are knobby, beaded and full of melanopsin which course down towards the inner nuclear layer.
F. OTHER MEANS OF VISUAL ASSESSMENTS
There are at least eight different retinal projections to different primary nuclei in the human brain, corresponding to different visual functions.44 To some extent, different visual testing modalities can parse out these pathways and functions. However, the physiology of other projections, such as to the superior colliculus and pulvinar, is still obscure. Visual parallel processing continues in the human cortex. Hence, there can be relatively specific visual impairments in the face of normal VA. Some techniques, like frequency doubling technology perimetry or quantitative layer-by-layer perimetry lay claim to separating out the pathways.45,46 But the former is probably not as specific for M-cell function as purported, and the latter rather too tedious to perform. There are old electrophysiological tests, such as critical flicker fusion frequency (the speed at which flickering light is perceived as steady) and new ones, such as the “C” wave or photopic negative response, that may lead to more reliable measures for the detection of specific inner as well as outer retinal dysfunctions.47
G. CONCLUSIONS
There will never be a shortage of new investigational tools for the assessment of human visual function, but the tests that matter most to ophthalmologists will be those that possess several critical attributes: they should be quantitative and reliable; they should be low maintenance in terms of cost, hardware and time; they should be sensitive and specific and provide insights into pathophysiology; but most of all, effective tests will allow for the early detection, accurate characterization and accurate monitoring of the progression or resolution of various ocular and visual diseases. The measurement of visual acuity has served us well, but it is now time to expand our basic armamentarium to include new assessments for the evaluation of vision. As an exciting byproduct, the subsequent increased understanding of vision provided by these developments may lead to the enhancement of ‘normal’ vision beyond visual acuity.
III. PHENOTYPE/GENOTYPE - TOMORROW’S DEFINITION OF DISEASE
“Only with the discovery of the double helix and the ensuing genetic revolution have we had grounds for thinking that the powers held traditionally to be the exclusive property of the gods might one day be ours.”
James D. Watson, The Daily Telegraph, London, March 19, 2003
A. CLASSIC CONCEPTS OF DISEASE
Medicine, in general, and ophthalmology, in particular, have a long history of describing diseases based on the observable signs in affected patients. For example, at different times during recorded history the disease we now know as tuberculosis (TB) has been called phthisis and consumption, among other names, based on the features care providers felt were most prominent.48,49 Similarly, inherited retinal degenerative disorders have been defined by changes detected in pupil responses and observed on fundus exam, producing names such as Leber congenital amaurosis (LCA) and retinitis pigmentosa (RP), respectively. In short, diseases have been defined by their phenotypes.50 There are problems with this approach, however, as phenotypic descriptions often do not contain information about disease etiology, and thus are limited with regard to informing medical care providers and patients about prognosis and potential therapies for their disorders. For example, only with the discovery of the TB bacillus (Mycobacterium tuberculosis) was it possible to make some headway with treatment and control this disease.47,48 Improved understanding of the etiologies of different diseases provides opportunities for a paradigm shift to define diseases differently, with a focus on etiology rather than disease manifestation(s) alone.
Historically, methods to define diseases have changed as new information about disease etiologies have been discovered. For example, since the identification of infectious etiologic agents provides critical information about therapy and prognosis, the World Health Organization issued updated guidelines for naming infectious disorders in 2015.51 These guidelines (http://www.who.int/topics/infectious_diseases/naming-new-diseases/en/) indicate that terms used for naming infectious diseases should:
be descriptive
include the etiologic agent, if known
avoid eponyms, names of people or places or animals
For example, the disease previously called “swine flu” would now be called H1N1 influenza or Legionnaires’ disease would be called Legionella associated pneumonia. The premise of this treatise is that the same approach used for infectious etiologies should be applied to non-infectious disorders, particularly eye diseases. Specifically, it is proposed that the genetic etiologies of eye disorders should and will be included in their names going forward. The example of inherited retinal degenerations will be used to illustrate the reasons for this recommendation, which the authors hope can also be applied more broadly.
B. GENETICS OF INHERITED RETINAL DEGENERATIONS
Inherited retinal degenerations (IRDs) are important causes of vision loss. This group of Mendelian disorders is characterized by progressive dysfunction and death of rod and cone photoreceptor cells.52 Several types of IRD, such as those that have been called LCA, cause vision loss in infancy or childhood.53,54,55 Other IRD subtypes, such as RP, account for up to 25% of blindness or visual impairment in working age people (21–60 years).56,57,58,59 Since the identification of rhodopsin (RHO) as an IRD disease gene in 1990, over 250 genes that harbor mutations which cause retinal degenerative disorders have been identified, making IRDs one of the most genetically diverse groups of inherited disorders.60,61,62
In the past several years, there has been increasing interest in identifying the genetic cause of disease for patients with IRDs, since several clinical trials have demonstrated the potential benefit of gene augmentation therapy for patients with specific genetic forms of IRD.63,64,65,66,67,68,69 Given the genetic heterogeneity of IRDs, next generation sequencing (NGS) methods are now being used for genetic diagnostic testing of patients with IRDs.70,71,72,73,74,75,76 The use of these unbiased testing approaches, which usually include all known IRD disease genes, has confirmed that the traditional phenotype-based definitions of disease do not provide accurate information regarding disease etiology. That is, mutations in disease genes originally identified to be associated with one phenotypic sub-type of IRD can cause disease that manifests clinically as a different sub-type of IRD. As an example of this, we recently evaluated two siblings with teenage onset retinal degeneration whose disease was found to be caused by mutations in the CEP290 gene (Figure 3). The clinical manifestations in these patients with teenage onset of disease were bone spicule pigmentation on fundus exam, mid-peripheral visual field loss, and relative preservation of retinal function as measured by electroretinography (ERG) are consistent with a clinical diagnosis of RP. Yet, mutations in CEP290 have been predominantly associated with early onset, severe retinal degeneration since mutations in CEP290 were originally reported to cause LCA, as well as syndromic ciliopathies.77,78 But, until NGS-based approaches for genetic testing of patients with IRDs became available, patients with other phenotypes such as RP were generally not tested for mutations in CEP290. Thus, the range of phenotypes produced by mutations in CEP290 has not been fully appreciated.
FIGURE 3.
CEP290-ASSOCIATED RETINAL DEGENERATION
Wide field fundus (top, left) and autofluorescence images (top, right), and optical coherence tomography image (bottom) from the right eye of a 15 year old with retinal degeneration due to two null mutations in the CEP290 gene. Note the mid-peripheral bone spicule pigmentation (Top, left and right) and relative preservation of the photoreceptor layer centrally (bottom), consistent with a clinical diagnosis of RP. At the time these images were taken, the patient had visual acuity of 20/20 and 20/25, but mid-peripheral visual field loss. Full field ERG testing showed reduced but relatively well preserved scoptopic and photopic responses.
Similarly, mutations in genes originally described to be associated with mild or stationary disease can cause severe, early onset retinal degeneration. An example is the recent identification of two patients with early onset, severe retinal degeneration (LCA) due to mutations in the CACNA1F gene, which was initially associated with congenital stationary night blindness (CSNB), a notably milder form of retinal degeneration.79 In both of these cases, the patients presented with nystagmus in infancy, and were found to have reduced or absent ERG responses (Figure 4). These findings led to the clinical diagnosis of LCA in both patients. NGS-based genetic testing subsequently identified mutations in CACNA1F in both patients.78 The observation that mutations in the same gene can cause clinically different forms of retinal degeneration should not be surprising, since many diseases display a spectrum of severity, and the distinction between the disorders we have called LCA and RP or LCA and CSNB is based on the severity of the disease phenotype. An example from outside of ophthalmology is the clinical presentation of cystic fibrosis, which can range from chronic sinusitis in adulthood to chronic respiratory tract infections and failure to thrive starting during infancy, yet all forms of the disease are due to mutations in the CFTR gene.80 What these findings demonstrate is that LCA and RP are not different disorders, but rather the opposite ends of the phenotypic spectrum of the same genetic diseases that cause vision loss via dysfunction and death of the photoreceptor and RPE cells of the retina.
FIGURE 4.
CACNA1F-ASSOCIATED RETINAL DEGENERATION
Top: Fundus images from a 4 year old with CACNA1F-associated retinal degeneration
Bottom: Full-field flash electroretinography according to ISCEV standards at age 3.5 months. ERG responses were recording in response to single flashes of 0.01 cd.s/m2 (rod response), 3.0 cd.s/m2 (rod-cone responses), or repeated flashes of 3.0 cd.s/m2 (30Hz cone responses). All responses were severely attenuated, consistent with the clinical diagnosis of LCA.
[adapted from Brennan ML, Schrijver I. Cystic Fibrosis: A review of associated phenotypes, use of molecular diagnostic approaches, genetic characteristics, progress, and Dilemmas. J Mol Diag 2016;18:3–14]
C. PROPOSAL TO UPDATE DEFINITIONS OF INHERITED EYE DISEASES
The genetic data described above are important for several reasons. First, they demonstrate that broad genetic testing is more accurate than focused testing, which can be limited by traditional phenotypic diagnostic categories. At present, options for broad testing include panel-based tests and medical exomes. It is likely that whole genome sequencing will be used more routinely in the future given the ability of this approach to accurately detect structural variants.81 Second, the data indicate that historical definitions of disease are inadequate because the underlying cause of disease cannot be predicted by clinical findings. Information provided to patients about prognosis and possible treatments based on clinical diagnoses alone may be inaccurate, because phenotypic clinical definitions of disease alone are not precise.
Finally, the advent of gene therapies for some genetic forms of IRD makes it important to determine the precise genetic basis of disease, and to include this in the description of disease. Moreover, the inclusion of a description of etiology in disease names has therapeutic importance as well. It is proposed that genetic diagnostic testing is part of optimal care for patients with IRDs, and that the descriptions of different forms of IRD be updated to include genetic etiology, when this is available. Examples include:
RHO-associated retinal degeneration (or RHO-associated RP)
CEP290-associated early onset retinal degeneration (or CEP290-associated LCA); CEP290-associated retinal degeneration if the disease has a later onset or less severe phenotype; or CEP290-associated ciliopathy if the disease is syndromic
ABCA4-associated macular degeneration (or ABCA4-associated Stargardt disease)
There have also been similar suggestions for updated descriptions of other genetic disorders such as cardiomyopathies, liver disease and cancers. 82,83,84,85,86 These definitions may be further refined in the future, as information about genetic modifiers of disease severity becomes available.
D. BROADER APPLICATION OF GENETIC DISEASE DEFINITIONS TO OPHTHALMOLOGY
The same paradigm can be applied to other inherited forms of eye disease, such as glaucoma and other optic neuropathies. For example, mutations in 6 genes have been identified to cause early-onset glaucoma, and account for approximately 20% of patients with early-onset disease.87 Identification of individuals with mutations in one of these genes is valuable, as appropriate monitoring and timely treatment of early disease can reduce vision loss in many cases.88 Further, early studies suggest that early-onset glaucoma due to mutations in the myocilin (MYOC) gene may be amendable to treatment with agents that reduce endoplasmic reticulum stress.89 Identification of patients with MYOC-associated glaucoma may thus help identify individuals who could be eligible to participate in clinical trials of novel therapies for this form of disease.87
Identification of the genetic cause of disease for patients with other forms of optic neuropathy can be similarly, worthwhile. In addition to defining the inheritance pattern so that accurate risk assessment for family members can be provided, clinical trials of gene augmentation therapy for ND4-associated optic neuropathy (Leber Hereditary Optic Neuropathy) due to mutations in the ND4 gene are currently underway.90,91 Patients with ND4-associated disease may also benefit from avoidance of certain environmental exposures such as smoking and alcohol consumption.92
It may also be desirable to classify common, complex disorders by the genetic contributors to their etiology, although this will be more difficult to achieve because multiple genetic variants with small effect sizes are likely to contribute to these disorders. As an example, over 34 genetic variants have been associated with age-related macular degeneration (AMD).93 As these and other genetic associations are studied in more detail, it is likely that some will be found to influence the response to therapy. Lores-Motta and colleagues reported that single nucleotide polymorphisms in the NRP1 and VEGFR2 genes are significantly associated with response to anti-VEGF therapy with ranibizumab, and thus can be used to guide treatment decisions for individual patients.94 It is worth pointing out that numerous other genetic risk scores for AMD have been developed, and many are predictive of the measured outcome when applied to subsets of patients. It has been suggested that additional progress in this area will depend on use of more uniform phenotype and genotype information so that the resulting risk scores are useful for a wide variety of patients.95 For example, obtaining consistent genotype information for a larger numbers of patients with AMD could allow for development of more robust risk scores related to disease progression or differential response to therapies. Similarly, adoption of consistent methods for assessing phenotype, such as one of the contrast sensitivity or macular function measures described above, could facilitate aggregation of data from multiple centers. Thus, it is possible that tomorrow’s definition of common diseases such as AMD and glaucoma will also include genetic etiology.
IV. OPHTHALMIC NANOTECHNOLOGIES - TOMORROW’S EYE DIAGNOSTICS
Any sufficiently advanced technology is indistinguishable from magic.
Arthur C. Clarke, “Profiles of The Future”, 1961 (Clarke’s third law)
Throughout history, ophthalmology has benefited from technologic advances in imaging. Indeed, it could be argued that ophthalmology was born as a result of imaging. The invention of ophthalmoscopy by Purkinje in 182396 and the subsequent development of the ophthalmoscope by Babbage in 184797 and Helmholz in 185198 enabled practitioners to actually visualize structures within the eye.99 Gullstrand’s slit lamp biomicroscope additionally enabled detailed examination of the anterior segment.100 Hans Goldmann’s classic works employed biomicroscopy and contact lens viewing of the posterior segment to generate great advances in or understanding of eye diseases.101
Yet, in the wake of great progress during the past century and a half, the fundamental philosophy of our approach to ophthalmic diagnostics has regrettably plateaued. Even modern imaging technologies such as optical coherence tomography are idled at the level of cell and tissue pathology. This is quite limiting, as disease begins on the molecular level and thus our current approach is only able to diagnose ocular disease at fairly advanced stages in the natural history. Consider, for example, the case of a patient with diabetes. Our current approach to evaluating such a patient’s eyes is basically an effort to identify cataracts, iris neovascularization, vitreous membranes and/or hemorrhage, retinal microhemorrhages, retinal microaneurysms, macular hard exudates/cysts/ thickening, and neovascularization of the retina and/or optic disc. In the absence of these findings we advise the patient that there is no evidence of diabetic eye disease. Yet, it is well-known that this disease begins on a physiologic and biochemical level with molecular changes that predate the aforementioned cellular changes by years, if not decades.102,103,104,105,106 Thus, in search of identifying/defining ocular health as a diagnosis (see above) and thereby enabling identification of the earliest departure from this ideal state, new diagnostic technologies are necessary. Given the need for molecular diagnostics, nanotechnology offers great promise. Of particular importance is the ability of laser light to access and leave the eye, providing great opportunities for laser-based nanotechnologies to play an important role in tomorrow’s eye diagnostics. Lastly, with tissues representative of nearly every tissue type in the body, the eye can serve as a window to the body, if not indeed the soul.
A. Anterior Segment
The tear film is a complex structure that plays an important role in ocular health.107,108 Non-invasive spectral analysis of the ocular surface could be performed with relatively high levels of incident light energy since off axis laser irradiation poses little risk to the inner eye.
Conjunctival blood vessels can be safely illuminated with relatively high levels of energy and circulatory physiology could be readily evaluated in various conditions of health and disease. 109,110,111,112,113
The cornea consists largely of extracellular matrix molecules, primarily collagen. As such, it is representative of extracellular matrix biology throughout the body and can be a useful source of information regarding the molecular effects of systemic diseases such as diabetes on the extracellular matrix. One of the most fundamental molecular effects of diabetes is the formation of advanced glycation endproducts of collagen, which is ubiquitous in the human body. 114,115,116 Thus, evaluation of the changes in corneal collagen induced by diabetes might provide an index of protein glycation throughout the body, just as the glycated hemoglobin test provides important information regarding recent (2 – 3 months) glycemic control. Corneal collagen, however, does not have the rapid turnover of red blood cells and thus glycation effects here would represent chronic influences. Since glycated collagen demonstrates autofluorescence, a system has been developed to measure corneal autofluorescence117 and studies have evaluated diabetic patients117,118,119 with the results correlated to diabetic eye disease.121 Future studies of corneal autofluorescence and other biophysical properties of key molecules in diabetes should be directed to detecting molecular changes prior to the onset of complications such as diabetic retinopathy, in order to develop new strategies to prevent disease, perhaps with agents that inhibit protein glycation.122,123,124
Aqueous is an ultrafiltrate of blood. Thus, the anterior chamber offers a unique opportunity to non-invasively assay a close representative of blood. For example, aqueous glucose levels have been indexed non-invasively by measuring the polarization properties of aqueous in vivo.125 Raman spectroscopy offers an even more broad-based non-invasive nanotechnology that could potentially be developed to replace the need for drawing blood.126,127,128
The lens is largely acellular, composed primarily of proteins that undergo pathologic cross-linking and aggregation, resulting in cataracts. Studies have suggested that cataract formation may be irreversible once lens opacities develop.129 Early detection is thus needed to enable preventative therapy that could obviate the need for surgery.130 Dynamic light scattering is a laser-based nanotechnology131 that has been developed to provide non-invasive quantitative assessment of particle size distribution in the human lens (Figure 5, left) and detect precataractous alpha-crystallin chaperone reserve in the lens nucleus, reflecting the risk of cataract formation.132 Longitudinal studies in a cohort of 45 subjects identified the progression of lens opacification in those individuals with diminishing alpha-crystallin.133 (Figure 5, right)
FIGURE 5.
DYNAMIC LIGHT SCATTERING OF THE HUMAN LENS IN VIVO
Left: Particle size distribution of the human lens at different ages
The histograms display the particle size distribution obtained with non-invasive dynamic light scattering in 3 individuals of different ages. There is a shift to the right denoting larger particle sizes with advancing age. [courtesy of Dr. Rafat Ansari and Dr. Manuel Datiles]
Right: Progression of Nuclear Sclerosis in a 43 year-old patient over 20 months. There is a decrease in the a-crystallin index (ACI) corresponding to an increase in lens opacification (NO=nuclear opacification) as assessed by AREDS nuclear opacification grading.
[AREDS: Age-Related Eye Disease Study; OD=right eye; OS=left eye]
[Reprinted with permission from: Datiles MB 3rd, Ansari RR, Yoshida J, et al: Longitudinal study of age-related cataract using dynamic light scattering: loss of α-crystallin leads to nuclear cataract development. Ophthalmology. 2016;123(2):248–54.]
B. Vitreous
Invisible by design, the vitreous body represents perhaps the most challenging of all ocular structures to image, owing to the very low concentration of structural macromolecules in this highly hydrated (98% water) tissue.134 Although swept source OCT has recently improved clinical evaluation of the posterior vitreous, it is to date unable to image the entire vitreous body and no studies have demonstrated the ability to enable quantitative analysis on a molecular level. Ultrasound is able to image the entire vitreous body and studies have recently shown efficacy in quantifying vitreous echodensity as a metric of vitreous pathology.135 These are relatively macroscopic changes, however, and more detailed molecular changes need to be measured for health assessment and early diagnosis of disease. Experimentally, dynamic light scattering has been employed to non-invasively evaluate the molecular structure of vitreous, but no clinical studies have been performed to date.136,137,138,139
C. Fundus
1. Blood Flow and Oxygenation
Normal physiology in the retina, choroid, and optic nerve is critical to good visual function. There are currently no non-invasive technologies that are routinely employed in patient care, but these are available. Based upon the pioneering work of George Benedek at MIT, the non-invasive nanotechnology of laser Doppler velocimetry has been used to measure blood flow in the human retina140,141,142, optic nerve 142,143,144,145, and choroid.147 Also available are in vivo measures of oxygenation as developed by Delori et al.148,149 and Stefánsson. 150,151 Clinical application of non-invasive evaluation of circulatory physiology and ocular metabolism will enable the paradigm shift in diagnostics from its current focus on histopathology to defining health and disease on the basis of physiopathology, rather than histopathology, hopefully enabling intervention at more reversible stages of disease. Non-invasive evaluation of ocular circulation is currently increasing substantially with the development of OCT angiography.152,153,154 Applications to date include glaucoma (Figure 6) and age-related macular degeneration. (Figure 7)
FIGURE 6.

OCT ANGIOGRAPHY (OCTA) IN GLAUCOMA
Disc photographs (A1, A2), optical coherence tomography (OCT) reflectance (B1, B2), whole-depth OCT angiograms (C1, C2, en face maximum projection), and cross-sectional angiograms (D1, D2, overlaying on OCT reflectance in gray scale) in the right eye of a normal subject (A1) and the left eye of a perimetric glaucoma (PG) subject (A2). Disc margins are marked by the black elliptical outlines (B1, B2, C1, C2). The position of the cross-section is shown by dotted blue lines (C1, C2). A dense microvascular network is visible on the OCTA of the normal disc (C1). This network was greatly attenuated in the glaucomatous disc (C2).
[courtesy of Dr. Liang Liu, Dr. Yali Jia, and Dr. David Huang;
reprinted from: Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, Lombardi LH: Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014;121(7):1322–32.]
FIGURE 7.
OCT ANGIOGRAPHY (OCTA) IN AGE-RELATED MACULAR DEGENERATION
(Top, left) En face OCTA of the inner retina. (Top, center) En face OCTA of the outer retina showing the CNV. (Top, right) Early-phase fluorescein angiography (FA). (Bottom, left) Composite en face OCTA showing intraretinal fluid (light blue) over the CNV (yellow). (Bottom, center) Retinal thickness deviation map showing thickening over the CNV.
[courtesy of Dr. Liang Liu, Dr. Yali Jia, and Dr. David Huang;
Reprinted with permission from Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, Potsaid B: Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 2014;121(7):1435–44]
2. Macula
Age-related macular degeneration (AMD) is believed to result from light-induced and metabolism-related oxidative injury to retinal and retinal pigment epithelial cells. Genetic predisposition(s) influences the contributory inflammatory sequelae. It has been proposed that dietary carotenoids, such as lutein and zeaxanthine, could impart protection against AMD owing to the blue light-filtering properties of carotenoids.155,156 Non-invasive technologies are being developed to measure the levels of macular pigment as a means of identifying individuals at risk as well as monitoring the response to therapy.157,158 Such an approach would truly embody the principles of quantitatively characterizing relative levels of wellness and enabling monitored strategies to promote better health.
D. Implementation Platform
The implementation of advanced non-invasive nanodiagnostics via tele-ophthalmology platforms is an important aspect of this paradigm shift. Such a platform will enable disease (ophthalmic and systemic) detection remotely rather than in the office or clinic.159 Not only will this provide better outreach into the community, but should enable earlier disease detection and therapy during more reversible stages of disease, which is the ultimate paradigm shift in Medicine.
CONCLUDING REMARKS
The foregoing provides an overview of paradigm shifts that promise to improve not only ophthalmic diagnostics, but all aspects of eye and vision care. This white paper is not intended as a review nor to be comprehensive. Rather, topics have been selected to illustrate the principles of the paradigm shifts the authors felt were important for the future of ophthalmic diagnostics. Indeed, successful development and implementation of these paradigm shifts may also provide useful guidance for similar developments in all of healthcare. These transformations must not only be embraced by the ophthalmic community but actively promoted by those most skilled in the art, for the betterment of the science as well as patient care. Eventually, powerful diagnostic capabilities will shift our focus from disease detection to prevention and health promotion, enabling us to help people feel and function better, thereby fulfilling what should be the ultimate mission of medicine:
“...to help people die young, as late in life as possible.
Ernst L. Wynder, President American Health Foundation, 1976
ACKNOWLEDGEMENTS
Dr. Sebag wishes to thank Dr. Rafat Ansari for presenting information on Future Diagnostic Technologies at the 2016 Knapp Symposium of the American Ophthalmological Society and Derek Nguyen who assisted with literature search and manuscript preparation.
Dr. Pierce acknowledges support from the National Eye Institute [EY012910 and P30EY014104 (MEEI core support)], the Foundation Fighting Blindness (USA), and would like to thank the patients and their family members for their participation in this study, members of the Ocular Genomics Institute for their experimental assistance, and Dr. Janey Wiggs, Dr. Kinga Bujakowska and Emily Place for their comments on the manuscript.
Footnotes
Presented in part as The Knapp Symposium of the 152nd Annual Meeting of the American Ophthalmological Society, on May 21, 2016 in Colorado Springs, Colorado, USA
REFERENCES
- 1.Dubos R. Man Adapting: The Silliman Lectures. New Haven, CT: Yale Univ. Press; 1965. [Google Scholar]
- 2.Sebag J. The Diagnosis of Health. First Prize, National Essay Competition. Preventive Medicine. 1979;8:76–78. doi: 10.1016/0091-7435(79)90031-8. [DOI] [PubMed] [Google Scholar]
- 3.Ekici F, Loh R, Waisbourd M, Sun Y, Martinez P, Nayak N, Wizov SS, Hegarty S, MPhil, Hark Lisa A, PhD, RD, Spaeth GL. Relationships between measures of the ability to perform vision-related activities, vision-related quality of life, and clinical findings in patients with glaucoma. JAMA Ophthalmol. 2015;133(12):1377–85. doi: 10.1001/jamaophthalmol.2015.3426. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962. [Google Scholar]
- 5.Meyer JJ, Lawrence SD. What’s new in laser treatment for glaucoma? Curr Opin Ophthalmol. 2012;23(2):111–7. doi: 10.1097/ICU.0b013e32834f1887. [DOI] [PubMed] [Google Scholar]
- 6.Alon S. Selective Laser Trabeculoplasty: A Clinical Review. J Curr Glaucoma Pract. 2013 May-Aug;7(2):58–65. doi: 10.5005/jp-journals-10008-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon SD, Lindsley K, Vedula SS, et al. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;29(8):CD005139. doi: 10.1002/14651858.CD005139.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebag J. Pharmacologic vitreolysis – premise and promise of the first decade. (Guest Editorial) Retina. 2009;29:871–4. doi: 10.1097/IAE.0b013e3181ac7b3c. [DOI] [PubMed] [Google Scholar]
- 9.Khoshnevis M, Sebag J. Pharmacologic vitreolysis with ocriplasmin: rationale for use and therapeutic potential in vitreo-retinal disorders. BioDrugs. 2015;29(2):103–12. doi: 10.1007/s40259-015-0120-y. [DOI] [PubMed] [Google Scholar]
- 10.Lin P, Menda S, de Juan E., Jr . Principles and Practice of Intravitreal Application of Drugs. In: Sebag J, editor. Vitreous: in Health and Disease. New York, NY: Springer Science+Business; 2014. [Google Scholar]
- 11.Planck M. The Philosophy of Physics. New York, NY: W. W. Norton; 1936. [Google Scholar]
- 12.Spedding J, Ellis RL, Heath DD. The Works of Francis Bacon. Vol. 6. Cambridge, UK: Cambridge University Press; 2011. p. 503. [Google Scholar]
- 13.Kaiser P. Prospective Evaluation of Visual Acuity Assessment: A Comparison of Snellen Versus ETDRS Charts in Clinical Practice (An AOS Thesis) Trans Am Ophthalmol Soc. 2009;107:311–24. [PMC free article] [PubMed] [Google Scholar]
- 14.Leguire LE, Algaze A, Kashou NH, et al. Relationship among fMRI, contrast sensitivity and visual acuity. Brain Res. 2011;1367:162–9. doi: 10.1016/j.brainres.2010.10.082. [DOI] [PubMed] [Google Scholar]
- 15.Hashemi H, Khabazkhoob M, Jafarzadehpur E, et al. Contrast sensitivity evaluation in a population-based study in Shahroud, Iran. Ophthalmology. 2012;119(3):541–6. doi: 10.1016/j.ophtha.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Sadun AA, Borchert M, DeVita E, et al. Assessment of visual impairment in patients with Alzheimer’s disease. Am J Ophthalmol. 1987;104(2):113–20. doi: 10.1016/0002-9394(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 17.Risacher SL, Wudunn D, Pepin SM, et al. Visual contrast sensitivity in Alzheimer’s disease, mild cognitive impairment, and older adults with cognitive complaints. Neurobiol Aging. 2013;34(4):1133–44. doi: 10.1016/j.neurobiolaging.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balcer LJ, Galetta SL, Polman CH, et al. Low-contrast acuity measures visual improvement in phase 3 trial of natalizumab in relapsing MS. Neurol Sci. 2012;318(1–2):119–24. doi: 10.1016/j.jns.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 19.McKendrick AM, Weymouth AE, Battista J. Visual form perception from age 20 through 80 years. Invest Ophthalmol Vis Sci. 2013;54(3):1730–9. doi: 10.1167/iovs.12-10974. [DOI] [PubMed] [Google Scholar]
- 20.Lek JJ, Vingrys AJ, McKendrick AM. Rapid contrast adaptation in glaucoma and in aging. Invest Ophthalmol Vis Sci. 2014;55(5):3171–8. doi: 10.1167/iovs.13-13229. [DOI] [PubMed] [Google Scholar]
- 21.Sebag J, Yee KM, Wa CA, et al. Vitrectomy for floaters: prospective efficacy analyses and retrospective safety profile. Retina. 2014;34(6):1062–8. doi: 10.1097/IAE.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 22.Huang LC, Yee KMP, Wa CA, et al. Vitreous floaters and vision: Current concepts and management paradigms. In: Sebag J, editor. Vitreous - in Health & Disease. Springer; New York: 2014. pp. 771–788. [Google Scholar]
- 23.Garcia G, Khoshnevis M, Yee KM, Nguyen-Cuu J, Nguyen JH, Sebag J. Degradation of contrast sensitivity following posterior vitreous detachment. Am J Ophthalmol. 2016 doi: 10.1016/j.ajo.2016.09.005. http://dx.doi.org/10.1016/j.ajo.2016.09.005. [e-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Milston R, Madigan MC, Sebag J. Vitreous Floaters: Etiology, Diagnostics, and Management. Surv Ophthalmol. 2016;61(2):211–27. doi: 10.1016/j.survophthal.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Fink W, Sadun AA. Three-dimensional computer-automated threshold Amsler grid test. J Biomed Opt. 2004;9(1):149–53. doi: 10.1117/1.1625952. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe KA, Sadun AA. Threshold Amsler grid testing in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1991;229(3):219–23. doi: 10.1007/BF00167871. [DOI] [PubMed] [Google Scholar]
- 27.Almony A, Garg S, Peters RK, et al. Threshold Amsler grid as a screening tool for asymptomatic patients on hydroxychloroquine therapy. Br J Ophthalmol. 2005;89(5):569–74. doi: 10.1136/bjo.2004.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazemi PP, Fink W, Sadun AA, et al. Early detection of glaucoma by means of a novel 3D computer-automated visual field test. Br J Ophthalmol. 2007;91(10):1331–6. doi: 10.1136/bjo.2007.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen DT, Fahimi A, Fink W, et al. Novel 3D computer-automated threshold Amsler grid visual field testing of scotomas in patients with glaucoma. Eur J Ophthalmol. 2009;19(5):776–82. doi: 10.1177/112067210901900515. [DOI] [PubMed] [Google Scholar]
- 30.Robison CD, Jivrajka RV, Bababeygy SR, et al. Distinguishing wet from dry age-related macular degeneration using three-dimensional computer-automated threshold Amsler grid testing. Br J Ophthalmol. 2011;95(10):1419–23. doi: 10.1136/bjo.2010.194886. [DOI] [PubMed] [Google Scholar]
- 31.Tozer KR, Fink W, Sadun AA, et al. Prospective three-dimensional analysis of structure and function in vitreomacular adhesion cured by pharmacologic vitreolysis. Retina Cases Brief Rep. 2013;7(1):57–61. doi: 10.1097/ICB.0b013e318263d3ee. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen J, Yee KMP, Sadun AA, Sebag J. Quantifying visual dysfunction and the response to surgery in macular pucker. Ophthalmology. 2016;123:1500–10. doi: 10.1016/j.ophtha.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Riordan-Eva P. Clinical assessment of optic nerve disorders. Eye. 2004;18:1161–1168. doi: 10.1038/sj.eye.6701575. [DOI] [PubMed] [Google Scholar]
- 34.Katz B. The dyschromatopsia of optic neuritis: a descriptive analysis of data from the optic neuritis treatment trial. Trans Am Ophthalmol Soc. 1995;93:685–708. [PMC free article] [PubMed] [Google Scholar]
- 35.Takeda S, Tajime K, Taniguchi T. The Takeda three colors combination test: a screening test for detection of very mild Alzheimer’s disease. Scientific World Journal. 2014;2014:907316. doi: 10.1155/2014/907316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Downie LE, Cheng AS, Vingrys AJ. Color vision deficits in intermediate age-related macular degeneration. Optom Vis Sci. 2014;91(8):932–8. doi: 10.1097/OPX.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 37.Dimitrov PN1, Robman LD, Varsamidis M, et al. Visual function tests as potential biomarkers in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(13):9457–69. doi: 10.1167/iovs.10-7043. [DOI] [PubMed] [Google Scholar]
- 38.Jivrajka RV, Kim JK, Fink W, et al. Quantitative analysis of central visual field defects in macular edema using three-dimensional computer-automated threshold Amsler grid testing. Graefes Arch Clin Exp Ophthalmol. 2009;247(2):165–70. doi: 10.1007/s00417-008-0971-8. [DOI] [PubMed] [Google Scholar]
- 39.Sadun AA. Dyslexia at The New York Times: (mis)understanding of parallel visual processing. Arch Ophthalmol. 1992;110(7):933–4. doi: 10.1001/archopht.1992.01080190039026. [DOI] [PubMed] [Google Scholar]
- 40.Moura AL, Nagy BV, La Morgia C, et al. The pupil light reflex in Leber’s hereditary optic neuropathy: evidence for preservation of melanopsin-expressing retinal ganglion cells. Invest Ophthalmol Vis Sci. 2013;54(7):4471–7. doi: 10.1167/iovs.12-11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.La Morgia C, Ross-Cisneros FN, Koronyo Y, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. 2016;79(1):90–109. doi: 10.1002/ana.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.La Morgia C, Ross-Cisneros FN, Hannibal J, et al. Melanopsin-expressing retinal ganglion cells: implications for human diseases. Vision Res. 2011;51(2):296–302. doi: 10.1016/j.visres.2010.07.023. (review) [DOI] [PubMed] [Google Scholar]
- 43.La Morgia C, Ross-Cisneros FN, Sadun AA, et al. Melanopsin retinal ganglion cells are resistant to neurodegeneration in mitochondrial optic neuropathies. Brain. 2010;133(8):2426–38. doi: 10.1093/brain/awq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadun AA. Vision: a multimodal sense. Bull Clin Neurosci. 1985;50:61–8. [PubMed] [Google Scholar]
- 45.Boland MV, Gupta P, Ko F, et al. Evaluation of Frequency-Doubling Technology Perimetry as a Means of Screening for Glaucoma and Other Eye Diseases Using the National Health and Nutrition Examination Survey. JAMA Ophthalmol. 2016;134(1):57–62. doi: 10.1001/jamaophthalmol.2015.4459. [DOI] [PubMed] [Google Scholar]
- 46.Enoch JM. Quantitative layer-by-layer perimetry: an update. Am J Optom Physiol Opt. 1982;59(12):952–3. doi: 10.1097/00006324-198212000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Moss HE, Park JC, McAnany JJ. The photopic negative response in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci. 2015;56(6):3709–14. doi: 10.1167/iovs.15-16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koehler CW. Consumption, the great killer. Modern Drug Discovery. 2002;5:47–9. [Google Scholar]
- 49.Fogel N. Tuberculosis: a disease without boundaries. Tuberculosis (Edinb) 2015;95:527–31. doi: 10.1016/j.tube.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 50.Bell J. The new genetics in clinical practice. BMJ. 1998;316:618–20. doi: 10.1136/bmj.316.7131.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kupferschmidt K. Infectious diseases. Rules of the name. Science. 2015;348:745. doi: 10.1126/science.348.6236.745. [DOI] [PubMed] [Google Scholar]
- 52.Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Progress Retinal Eye Research. 2010;29:335–75. doi: 10.1016/j.preteyeres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Weleber RG. Infantile and childhood retinal blindness: a molecular perspective. Ophthalmic Genetics. 2002;23:71–97. doi: 10.1076/opge.23.2.71.2214. [DOI] [PubMed] [Google Scholar]
- 54.Michaelides M, Hardcastle AJ, Hunt DM, et al. Progressive cone and cone-rod dystrophies: phenotypes and underlying molecular genetic basis. Survey of Ophthalmology. 2006;51:232–58. doi: 10.1016/j.survophthal.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Chung DC, Traboulsi EI. Leber congenital amaurosis: clinical correlations with genotypes, gene therapy trials update, and future directions. Journal of AAPOS. 2009;13:587–92. doi: 10.1016/j.jaapos.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Hata H, Yonezawa M, Nakanishi T, et al. Causes of entering institutions for visually handicapped persons during the past fifteen years. Japanese Journal Clinical Ophthalmology. 2003;57:259–62. [Google Scholar]
- 57.Buch H, Vinding T, La CM, et al. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology. 2004;111:53–61. doi: 10.1016/j.ophtha.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Al-Merjan JI, Pandova MG, Al-Ghanim M, et al. Registered blindness and low vision in Kuwait. Ophthalmic Epidemiol. 2005;12:251–7. doi: 10.1080/09286580591005813. [DOI] [PubMed] [Google Scholar]
- 59.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 60.Dryja TP, McGee TL, Reichel E, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–6. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- 61.RetNet. RetNet web site. http://www.sph.uth.tmc.edu/Retnet/2016.
- 62.Hsiau TH, Diaconu C, Myers CA, et al. The cis-regulatory logic of the mammalian photoreceptor transcriptional network. PLoS ONE. 2007;2:e643. doi: 10.1371/journal.pone.0000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. New England Journal of Medicine. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. New England Journal of Medicine. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 65.Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proceedings National Academy Sciences USA. 2008;105:15112–7. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobson SG, Cideciyan AV, Ratnakaram R, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Archives Ophthalmology. 2012;130:9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bennett J, Ashtari M, Wellman J, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4:120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacLaren RE, Groppe M, Barnard AR, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song J, Smaoui N, Ayyagari R, et al. High-throughput retina-array for screening 93 genes involved in inherited retinal dystrophy. Investigative Ophthalmology & Visual Science. 2011;52:9053–60. doi: 10.1167/iovs.11-7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Audo I, Bujakowska KM, Leveillard T, et al. Development and application of a next-generation-sequencing (NGS) approach to detect known and novel gene defects underlying retinal diseases. Orphanet Journal Rare Diseases. 2012;7:8. doi: 10.1186/1750-1172-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neveling K, Collin RW, Gilissen C, et al. Next-generation genetic testing for retinitis pigmentosa. Human Mutation. 2012;33:963–72. doi: 10.1002/humu.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eisenberger T, Neuhaus C, Khan AO, et al. Increasing the Yield in Targeted Next-Generation Sequencing by Implicating CNV Analysis, Non-Coding Exons and the Overall Variant Load: The Example of Retinal Dystrophies. PLoS One. 2013;8:e78496. doi: 10.1371/journal.pone.0078496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang F, Wang H, Tuan HF, et al. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Human Genetics. 2014;133:331–45. doi: 10.1007/s00439-013-1381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ge Z, Bowles K, Goetz K, et al. NGS-based Molecular diagnosis of 105 eyeGENE((R)) probands with Retinitis Pigmentosa. Sci Rep. 2015;5:18287. doi: 10.1038/srep18287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Consugar M, Navarro-Gomez D, Place EM, et al. Panel-based Genetic Diagnostic Testing for Inherited Eye Diseases is Highly Accurate and Reproducible and More Sensitive for Variant Detection Than Exome Sequencing. Genetics In Medicine. 2015;17:253–61. doi: 10.1038/gim.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.den Hollander AI, Koenekoop RK, Yzer S, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. American Journal of Human Genetics. 2006;79:556–61. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sayer JA, Otto EA, O’Toole JF, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. NatGenet. 2006;38:674–81. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 79.Men CJ, Bujakowska KM, Comander J, et al. Two cases of CACNA1F-associated retinal degeneration demonstrate the phenotypic overlap between LCA and CSNB. Submitted. 2016 [Google Scholar]
- 80.Brennan ML, Schrijver I. Cystic Fibrosis: A Review of Associated Phenotypes, Use of Molecular Diagnostic Approaches, Genetic Characteristics, Progress, and Dilemmas. The Journal of molecular diagnostics : JMD. 2016;18:3–14. doi: 10.1016/j.jmoldx.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 81.Christensen KD, Dukhovny D, Siebert U, et al. Assessing the Costs and Cost-Effectiveness of Genomic Sequencing. J Pers Med. 2015;5:470–86. doi: 10.3390/jpm5040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ashrafian H, Watkins H. Reviews of translational medicine and genomics in cardiovascular disease: new disease taxonomy and therapeutic implications cardiomyopathies: therapeutics based on molecular phenotype. Journal of the American College of Cardiology. 2007;49:1251–64. doi: 10.1016/j.jacc.2006.10.073. [DOI] [PubMed] [Google Scholar]
- 83.de Koning TJ, Tijssen MA. Movement disorders in 2014. Genetic advances spark a revolution in dystonia phenotyping. Nat Rev Neurol. 2015;11:78–9. doi: 10.1038/nrneurol.2014.254. [DOI] [PubMed] [Google Scholar]
- 84.Horlings HM, Flanagan AM, Huntsman DG. Categorization of cancer through genomic complexity could guide research and management strategies. The Journal of pathology. 2015;236:397–402. doi: 10.1002/path.4542. [DOI] [PubMed] [Google Scholar]
- 85.Karlsen TH, Lammert F, Thompson RJ. Genetics of liver disease: From pathophysiology to clinical practice. J Hepatol. 2015;62:S6–S14. doi: 10.1016/j.jhep.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 86.Chia NY, Tan P. Molecular classification of gastric cancer. Ann Oncol. 2016;27(5):763–9. doi: 10.1093/annonc/mdw040. [DOI] [PubMed] [Google Scholar]
- 87.Fan BJ, Wiggs JL. Glaucoma: genes, phenotypes, and new directions for therapy. Journal Clinical Investigation. 2010;120:3064–72. doi: 10.1172/JCI43085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wiggs JL, Pierce EA. Genetic testing for inherited eye disease: who benefits? JAMA Ophthalmology. 2013;131:1265–6. doi: 10.1001/jamaophthalmol.2013.4509. [DOI] [PubMed] [Google Scholar]
- 89.Zode GS, Kuehn MH, Nishimura DY, et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2015;125:3303. doi: 10.1172/JCI82799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wan X, Pei H, Zhao MJ, et al. Efficacy and Safety of rAAV2-ND4 Treatment for Leber’s Hereditary Optic Neuropathy. Sci Rep. 2016;6:21587. doi: 10.1038/srep21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feuer WJ, Schiffman JC, Davis JL, et al. Gene Therapy for Leber Hereditary Optic Neuropathy: Initial Results. Ophthalmology. 2016;123:558–70. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies - disease mechanisms and therapeutic strategies. Progress in retinal and eye research. 2011;30:81–114. doi: 10.1016/j.preteyeres.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48:134–43. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lores-Motta L, van Asten F, Muether PS, et al. A genetic variant in NRP1 is associated with worse response to ranibizumab treatment in neovascular age-related macular degeneration. Pharmacogenet Genomics. 2016;26:20–7. doi: 10.1097/FPC.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cooke Bailey JN, Hoffman JD, Sardell RJ, et al. The Application of Genetic Risk Scores in Age-Related Macular Degeneration: A Review. J Clin Med. 2016;5 doi: 10.3390/jcm5030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Albert DM, Miller WH. Jan Purkinje and the ophthalmoscope. Am J Ophthalmol. 1973;76(4):494–9. doi: 10.1016/0002-9394(73)90737-x. [DOI] [PubMed] [Google Scholar]
- 97.Keeler CR. 150 years since Babbage’s ophthalmoscope. Arch Ophthalmol. 1997;115:1456–7. doi: 10.1001/archopht.1997.01100160626017. [DOI] [PubMed] [Google Scholar]
- 98.Shastid TH. The description of an ophthalmoscope - a translation of Von Helmhotz’s “Beschreibung eines Augenspiegels”. Chicago, IL: Cleveland Press; 1916. [original work published 1851] [Google Scholar]
- 99.Friedenwald J. A critical study of the modern ophthalmoscope. Trans Am Ophthalmol Soc. 1928;26:381–426. [PMC free article] [PubMed] [Google Scholar]
- 100.Ehinger B, Grzybowski A. Allvar Gullstrand (1862–1930) - the gentleman with the lamp. Acta Ophthalmol. 2011;89(8):701–8. doi: 10.1111/j.1755-3768.2011.02235.x. [DOI] [PubMed] [Google Scholar]
- 101.Fankhauser F. Remembrance of Hans Goldmann, 1899–1991. Surv Ophthalmol. 1992;37(2):137–42. doi: 10.1016/0039-6257(92)90077-7. [DOI] [PubMed] [Google Scholar]
- 102.Wan TT, Li XF, Sun YM, et al. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. Biomed Pharmacother. 2015;74:145–7. doi: 10.1016/j.biopha.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 103.Gale J, Aiello LP, Sebag J. Diabetic vitreopathy. In: Sebag J, editor. Vitreous - in Health & Disease. Springer; New York: 2014. pp. 57–80. [Google Scholar]
- 104.Ahsan H. Diabetic retinopathy--biomolecules and multiple pathophysiology. Diabetes Metab Syndr. 2015;9(1):51–4. doi: 10.1016/j.dsx.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 105.Yamagishi S, Matsui T. Advanced glycation end products (AGEs), oxidative stress and diabetic retinopathy. Curr Pharm Biotechnol. 2011;12(3):362–8. doi: 10.2174/138920111794480534. [DOI] [PubMed] [Google Scholar]
- 106.Lutty GA. Effects of diabetes on the eye. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF81–7. doi: 10.1167/iovs.13-12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.You J, Willcox MD, Madigan MC, et al. Tear fluid protein biomarkers. Adv Clin Chem. 2013;62:151–96. doi: 10.1016/b978-0-12-800096-0.00004-4. [DOI] [PubMed] [Google Scholar]
- 108.Zhou L, Beuerman RW. Tear analysis in ocular surface diseases. Prog Retin Eye Res. 2012;31(6):527–50. doi: 10.1016/j.preteyeres.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 109.Khansari MM, Wanek J, Felder AE, et al. Automated Assessment of Hemodynamics in the Conjunctival Microvasculature Netowrk. IEEE Trans Med Imaging. 2016;35(2):605–11. doi: 10.1109/TMI.2015.2486619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang H, Ye Y, DeBuc DC, et al. Human conjunctival microvasculature assessed with a retinal function imager (RFI) Microvasc Res. 2013;85:134–7. doi: 10.1016/j.mvr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ohtani N. Laser Doppler flowmetry of the bulbar conjunctiva as a monitor of the cerebral blood flow. Nihon Kyobu Geka Gekkai Zasshi. 1996;44(9):1721–8. [PubMed] [Google Scholar]
- 112.Van Zijderveld R, Ince C, Schlingemann RO. Orthogonal polarization spectral imaging of conjunctival microcirculation. Graefes Arch Clin Exp Ophthalmol. 2014;252(2):773–9. doi: 10.1007/s00417-014-2603-9. [DOI] [PubMed] [Google Scholar]
- 113.Arzhimatova GS, Kheilo TS. Assessment of microvascular bulbar conjunctiva in patients with type 1 diabetes. Vestn Oftalmol. 2015;131(6):85–90. doi: 10.17116/oftalma2015131685-90. [DOI] [PubMed] [Google Scholar]
- 114.Sveen KA, Dahl-Jorgensen K, Stensaeth KH, et al. Glucosepane and oxidative markers in skin collagen correlate with intima media thickness and arterial stiffness in long-term type 1 diabetes. J Diabetes Complications. 2015;29(3):407–12. doi: 10.1016/j.jdiacomp.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 115.Monnier VM, Sun W, Gao X, et al. Skin collagen advanced glycation endproducts (AGEs) and the long-term progression of sub-clinical cardiovascular disease in type 1 diabetes. Cardiovasc Diabetol. 2015;14:118. doi: 10.1186/s12933-015-0266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gautieri A, Redaelli A, Buehler MJ, Vesentini S. Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: candidate amino acids involved in Advanced Glycation End-products. Matrix Biol. 2014;34:89–95. doi: 10.1016/j.matbio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 117.Rovati L, Docchio F, Van Best J. Autofluorescence methods in ophthalmology. J Biomed Opt. 2004;9(1):9–21. doi: 10.1117/1.1628241. [DOI] [PubMed] [Google Scholar]
- 118.Calvo-Maroto AM, Perez-Cambrodi RJ, Garcia-Lazaro S. Ocular autofluorescence in diabetes mellituis. A review. J Diabetes. 2016 doi: 10.1111/1753-0407.12423. [DOI] [PubMed] [Google Scholar]
- 119.Van Schaik HJ, Alkemade C, Smart W, Van Best JA. Autofluorescence of the diabetic and healthy human cornea in vivo at different excitation wavelengths. Exp Eye Res. 1999;68(1):1–8. doi: 10.1006/exer.1998.0575. [DOI] [PubMed] [Google Scholar]
- 120.Van Schaik HJ, Coppens J, Van den Berg TJ, Van Best JA. Autofluorescence distribution along the corneal axis in diabetic and healthy humans. Exp Eye Res. 1999;79(5):505–510. doi: 10.1006/exer.1999.0733. [DOI] [PubMed] [Google Scholar]
- 121.Rovati L, Fankhauser F, Docchio F, Van Best J. Diabetic retinopathy assessed by dynamic light scattering and corneal autofluorescence. J Biomed Opt. 1998;3(3):357–63. doi: 10.1117/1.429882. [DOI] [PubMed] [Google Scholar]
- 122.Borg DJ, Forbes JM. Targeting advanced glycation with pharmaceutical agents: where are we now? Glycoconj J. 2016 doi: 10.1007/s10719-016-9691-1. [DOI] [PubMed] [Google Scholar]
- 123.Ahmad S, Khan MS, Akhter F, et al. Glycoxidation of biological macromolecules: a critical approach to halt the menace of glycation. Glycobiology. 2014;24(11):979–990. doi: 10.1093/glycob/cwu057. [DOI] [PubMed] [Google Scholar]
- 124.Ashraf JM, Shahab U, Tabrez S, et al. Quercetin as a finer substitute to aminoguanidine in the inhibition of glycation products. Int J Biol Macromol. 2015;77:188–92. doi: 10.1016/j.ijbiomac.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 125.Ansari R, Bockle S, Rovati L. New optical scheme for a polarimetric-based glucose sensor. J Biomed Opt. 2004;9(1):103–115. doi: 10.1117/1.1626664. [DOI] [PubMed] [Google Scholar]
- 126.Sebag J, Nie S, Reiser KA. Raman spectroscopy of human vitreous in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1994;35:2976–2980. [PubMed] [Google Scholar]
- 127.March WF, Rabinovich B, Adams RL. Noninvasive glucose monitoring of the aqueous humor of the eye: Part I. Measurement of very small optical rotations. Diabetes Care. 1982;5(3):254–8. doi: 10.2337/diacare.5.3.254. [DOI] [PubMed] [Google Scholar]
- 128.March WF, Rabinovich B, Adams RL. Noninvasive glucose monitoring of the aqueous humor of the eye: Part II. Animal studies and the scleral lens. Diabetes Care. 1982;5(3):259–65. doi: 10.2337/diacare.5.3.259. [DOI] [PubMed] [Google Scholar]
- 129.Kupfer C. Bowman Lecture: the conquest of cataract: a global challenge. Trans Ophthalmol Soc U K. 1985;104( Pt 1):1–10. [PubMed] [Google Scholar]
- 130.Congdon NG. Prevention strategies for age related cataract: present limitations and future possibilities. Br J Ophthalmol. 2001;85(5):516–520. doi: 10.1136/bjo.85.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ansari RR. Ocular static and dynamic light scattering: a non-invasive diagnostic tool for eye research and clinical practice. J Biomed Optics. 2004;9(1):22–37. doi: 10.1117/1.1626663. [DOI] [PubMed] [Google Scholar]
- 132.Datiles MB, III, Ansari RR, Suh KI, Vitale S, Reed GF, Zigler SJ, Jr, Ferris F., III Clinical detection of precataractous lens protein changes using dynamic light scattering. Arch Ophthalmol. 2008;126(12):1687–93. doi: 10.1001/archophthalmol.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Datiles MB, 3rd, Ansari RR, Yoshida J, et al. Longitudinal study of age-related cataract using dynamic light scattering: loss of α-crystallin leads to nuclear cataract development. Ophthalmology. 2016;123(2):248–54. doi: 10.1016/j.ophtha.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sebag J, Silverman RH, Coleman DJ. To see the invisible - the quest of imaging vitreous. In: Sebag J, editor. Vitreous - in Health & Disease. Springer; New York: 2014. pp. 193–222. [Google Scholar]
- 135.Mamou J, Wa CA, Yee KM, et al. Ultrasound-based quantification of vitreous floaters correlates with contrast sensitivity and quality of life. Invest Ophthalmol Vis Sci. 2015;56:1611–1617. doi: 10.1167/iovs.14-15414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sebag J, Ansari RR, Dunker S, Suh SI. Dynamic light scattering of diabetic vitreopathy. Diabetes Technology & Therapeutics. 1999;1:169–176. doi: 10.1089/152091599317387. [DOI] [PubMed] [Google Scholar]
- 137.Ansari RR, Dunker S, Suh K, et al. Quantitative molecular characterization of bovine vitreous and lens with non-invasive dynamic light scattering. Exp Eye Res. 2001;73:859–866. doi: 10.1006/exer.2001.1097. [DOI] [PubMed] [Google Scholar]
- 138.Sebag J. Molecular biology of pharmacologic vitreolysis. Trans Am Ophthalmol Soc. 2005;103:473–494. [PMC free article] [PubMed] [Google Scholar]
- 139.Sebag J, Ansari RR, Suh KI. Pharmacologic vitreolysis with microplasmin increases vitreous diffusion coefficients. Graefes Arch Clin Exp Ophthalmol. 2007;245(4):576–80. doi: 10.1007/s00417-006-0394-3. [DOI] [PubMed] [Google Scholar]
- 140.Riva CE, Grunwald JE, Petrig BL. Autoregulation of human retinal blood flow. An investigation with laser Doppler velocimetry. Invest Ophthalmol Vis Sci. 1986;27(12):1706–12. [PubMed] [Google Scholar]
- 141.Feke GT, Tagawa H, Deupree DM, et al. Blood flow in the normal human retina. Invest Ophthalmol Vis Sci. 1989;30:58–65. [PubMed] [Google Scholar]
- 142.Grunwald JE, Riva CE, Sinclair SH, et al. Laser Doppler velocimetry study of retinal circulation in diabetes mellitus. Arch Ophthalmol. 1986;104(7):991–6. doi: 10.1001/archopht.1986.01050190049038. [DOI] [PubMed] [Google Scholar]
- 143.Riva CE, Grunwald JE, Sinclair SH. Laser Doppler measurement of relative blood velocity in the human optic nerve head. Invest Ophthalmol Vis Sci. 1982;22(2):241–8. [PubMed] [Google Scholar]
- 144.Sebag J, Feke GT, Delori FC, et al. Anterior optic nerve blood flow in experimental optic atrophy. Invest Ophthalmol Vis Sci. 1985;26:1415–1422. [PubMed] [Google Scholar]
- 145.Sebag J, Delori F, Feke G, et al. Anterior optic nerve blood flow decreases in clinical neurogenic optic atrophy. Ophthalmology. 1986;93:858–865. doi: 10.1016/s0161-6420(86)33671-6. [DOI] [PubMed] [Google Scholar]
- 146.Zink JM, Grunwald JE, Plitz-Seymour J, et al. Association between lower optic nerve laser Doppler blood volume measurements and glaucomatous visual field progression. Br J Ophthalmol. 2003;87(12):1487–91. doi: 10.1136/bjo.87.12.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Geiser MH, Diermann U, Riva CE. Compact laser doppler choroidal flowmeter. J Biomed Opt. 1999;4(4):459–464. doi: 10.1117/1.429960. [DOI] [PubMed] [Google Scholar]
- 148.Delori FC. Noninvasive technique for oximetry of blood in retinal vessels. Appl Opt. 1988;27(6):1113–25. doi: 10.1364/AO.27.001113. [DOI] [PubMed] [Google Scholar]
- 149.Sebag J, Delori FC, Feke GT. Effects of inner retinal degeneration on blood flow and oxygen saturation in humans. Arch Ophthalmol. 1989;107:222–226. doi: 10.1001/archopht.1989.01070010228027. [DOI] [PubMed] [Google Scholar]
- 150.Geirsdottir A, Palsson O, Hardarson SH, et al. Retinal vessel oxygen saturation in healthy individuals. Invest Ophthalmol Vis Sci. 2012 Aug 13;53(9):5433–42. doi: 10.1167/iovs.12-9912. [DOI] [PubMed] [Google Scholar]
- 151.Kristjansdottir JV1, Hardarson SH, Harvey AR, et al. Choroidal oximetry with a noninvasive spectrophotometric oximeter. Invest Ophthalmol Vis Sci. 2013;54(5):3234–9. doi: 10.1167/iovs.12-10507. [DOI] [PubMed] [Google Scholar]
- 152.Huang D, Jia Y, Gao SS, Lumbroso B, Rispoli M. Optical Coherence Tomography Angiography Using the Optovue Device. Dev Ophthalmol. 2016;56:6–12. doi: 10.1159/000442770. [DOI] [PubMed] [Google Scholar]
- 153.Hwang TS, Gao SS, Liu L, et al. Automated Quantification of Capillary Nonperfusion Using Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmol. 2016;134(4):367–73. doi: 10.1001/jamaophthalmol.2015.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fang PP, Lindner M, Steinberg JS, et al. Clinical applications of OCT angiography. Ophthalmologe. 2016;113(1):14–22. doi: 10.1007/s00347-015-0192-6. [DOI] [PubMed] [Google Scholar]
- 155.Sabour-Pickett S, Nolan JM, Loughman J, Beatty S. A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Mol Nutr Food Res. 2012;56(2):270–86. doi: 10.1002/mnfr.201100219. [DOI] [PubMed] [Google Scholar]
- 156.Loskutova E, Nolan J, Howard A, Beatty S. Macular pigment and its contribution to vision. Nutrients. 2013;5(6):1962–9. doi: 10.3390/nu5061962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gellermann W, Bernstein P. Noninvasive detection of macular pigments in the human eye. J Biomed Opt. 2004;9(1):75–85. doi: 10.1117/1.1628240. [DOI] [PubMed] [Google Scholar]
- 158.Bernstein PS, Delori FC, Richer S, et al. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vision Res. 2010;50(7):716–28. doi: 10.1016/j.visres.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shaw J. TeleOphthalmology - ready for prime time? Eyenet Magazine. 2016 Jun;:41–5. [Google Scholar]







