Abstract
To our knowledge, no study has investigated the association of long-term exposure to traffic pollution with markers of atherosclerosis in 4 vascular beds simultaneously in an all-African-American cohort. Among participants in the Jackson Heart Study (Jackson, Mississippi; baseline mean age = 55.5 (standard deviation, 12.7) years), we used linear regression to estimate percent differences in carotid intima-media thickness (CIMT) at baseline (2004) and used modified Poisson regression (robust error variance) to estimate prevalence ratios for peripheral artery disease (PAD), coronary artery calcification (CAC), and abdominal aortic calcification (AAC) at the first follow-up visit (2005–2008) for persons living less than 150 m (versus more than 300 m) from major roadways, adjusting for confounders. Living less than 150 m from such roadways was associated with a significant 6.67% (95% confidence interval: 1.28, 12.35) increase in CIMT (4,800 participants). PAD prevalence among persons living less than 150 m from a major roadway was 1.17 (95% confidence interval: 0.73, 1.86) times that of persons living more than 300 m away (4,443 participants), but this result was not statistically significant. There was no association for CAC or AAC. The association with CIMT was stronger in participants with a cardiovascular disease history than in those without one (P = 0.04). We observed an association in the carotid vascular beds but not the coronary, abdominal, or peripheral vascular beds. Our results highlight the need to consider residential proximity to roadways as a potential cardiovascular disease risk factor for blacks.
Keywords: abdominal aortic calcification, African Americans, air pollution, atherosclerosis, carotid intima-media thickness, coronary artery calcification, peripheral artery disease, traffic pollution
Numerous epidemiologic studies have shown that ambient air pollution increases the risk of cardiovascular morbidity and mortality (1–3). Local traffic is a major source of within-city variation in air pollution levels (4), and exposure to traffic-related pollution may be an important contributor to adverse health impacts (5–7). Previous studies have shown that short-term exposure (i.e., days or months) to traffic pollution is associated with indicators of cardiovascular health (8–10). Animal studies on long-term associations with exposure (i.e., years) have demonstrated pro-atherosclerotic effects of diesel exhaust particles and concentrated ambient urban particles (11–13). Epidemiologic studies have also found associations between markers of atherosclerosis and particulate matter or residential proximity to major roadways (14–25). However, all of these studies investigated the associations in urban populations that were entirely or predominately white. To our knowledge, no study has examined the associations for African Americans in a mixed urban-rural area of the Deep South. African Americans in general, and particularly those living in the US South, have higher cardiovascular disease (CVD) risk and prevalence and may experience twice as much exposure to traffic-related pollution as other subpopulations (26). It is not clear whether previous results from white populations are generalizable to African Americans with a different CVD risk profile and disease burden.
We examined the hypothesis that residential proximity to major roadways, a marker of long-term exposure to traffic pollution, is associated with atherosclerosis in the Jackson Heart Study (JHS), the largest prospective all-African-American cohort study to examine CVD etiology to date. We evaluated the hypothesis in 4 vascular beds, including the carotid, coronary, abdominal, and peripheral vascular beds.
METHODS
Between 2000 and 2004, the JHS recruited a total of 5,301 noninstitutionalized African-American men and women aged 21–95 years residing in the tricounty Jackson, Mississippi, Metropolitan Statistical Area through 4 recruitment strategies, including random sampling of the Jackson subpopulation of the Atherosclerosis Risk in Communities Study (22%), random sampling of eligible households in the Jackson Metropolitan Statistical Area (17%), structured community-based volunteer programs (30%), and recruitment of eligible family members aged 21 years or more (31%), as previously described (27, 28). The structured community-based volunteer programs used a community sampling frame with enrollment units stratified by socioeconomic status (SES) proxy to recruit volunteers who met JHS eligibility criteria. The JHS cohort has been shown to be representative of the underlying African-American population living in the Jackson area (29). Previous studies have indicated that pollution from vehicle combustion is the primary contributor to air pollution in the Jackson area (30), in the absence of major industries.
At baseline, participants completed an in-home interview followed by a clinic visit. At the time of the first follow-up (2005–2008) after the initial recruitment period (2000–2004), participants returned to complete a follow-up clinic visit. All participants provided written informed consent. All JHS protocols were reviewed and approved by the institutional review boards at Jackson State University, Tougaloo College, and the University of Mississippi Medical Center. This analysis was approved by the institutional review boards at Indiana University and Brown University.
Markers of atherosclerosis
We assessed atherosclerosis in 4 vascular beds using carotid intima-media thickness (CIMT), ankle-brachial index (ABI), coronary artery calcification (CAC), and abdominal aortic calcification (AAC). During the baseline clinical examination, JHS investigators measured the intima-media thickness of the far-wall common carotid artery using B-mode ultrasound. We took the average of the CIMTs measured at the left and right sides of the neck as a marker of atherosclerosis in the carotid vascular bed (31). In addition, JHS staff measured systolic blood pressure (mm Hg) at the posterior tibial artery in the leg and the brachial artery in the arm on both sides of the body using Doppler ultrasound. We calculated ABI, the ratio of the systolic blood pressure of the posterior tibial artery to that of the brachial artery, separately for each side of the body. We then took the lower value of the 2 ABIs for each participant as a marker of atherosclerosis in the peripheral vascular bed (32). We defined peripheral artery disease (PAD) as present if ABI ≥1.3 or ABI ≤0.9 and absent otherwise (33).
During the first follow-up clinic visit, we assessed atherosclerosis using CAC and AAC in the coronary and abdominal vascular beds, respectively. JHS staff measured CAC and AAC using chest and abdomen computed tomography (CT) with a cardiac-gated electron-beam CT scanner based on the CT scanning and interpretation protocol used in the Multi-Ethnic Study of Atherosclerosis (MESA), as previously described (34). Experienced radiologists at the JHS core reading center (Wake Forest University School of Medicine) performed all CT scan reading and scoring, with an interobserver scoring agreement of 0.99 (32). We defined CAC or AAC as present if the Agatston score was greater than 0 (32).
Residential proximity to nearest major roadways
We calculated residential distance to the nearest major roadway as a marker of long-term exposure to traffic pollution. We used ArcGIS (version 9.2; Esri, Inc., Redlands, California) to geocode addresses available at baseline and at the first follow-up visit (35). We calculated the Euclidean distance from each participant's residence to the nearest major roadway, defined as roads with US Census Feature Class Code A1 (primary highway with limited access; interstate highways) or A2 (primary road without limited access; US highways and state roads between major towns), as previously described (36). We used residential distance to A1 or A2 roads as the marker of long-term exposure to traffic pollution in primary analyses, and we used residential distance to A1 roads in secondary analyses.
Covariates
During the baseline in-home interview, JHS investigators obtained detailed information on participant age, sex, education, household income, physical activity, and smoking history, as previously described (28). Household income was self-reported in 11 categories (≤$5,000–≥$100,000), and participants were classified into 4 categories (low, lower middle, upper middle, and high) based on family size, number of children under 18 years of age, and US Census-designated poverty level, as previously shown (37). Self-reported physical activities during leisure time, at work, at home, and in sports were measured, with a total score that could range from 3 to 20, using the validated JHS Physical Activity Cohort instrument (38).
During the baseline clinic examination, JHS investigators collected additional information on medical history, current medications, and alcohol consumption. JHS staff also measured height, weight, and supine blood pressure and collected a fasting venous blood sample, as previously described (28). Participants were classified as hypertensive if their blood pressure was ≥140/90 mm Hg or they were taking blood pressure-lowering medication (39, 40). Participants were classified as having diabetes if they reported a past diagnosis of diabetes, if they reported using any diabetes medications (self-reported and actual use of medications brought in by patients that were used during the 2 weeks prior to clinic examination), if their measured hemoglobin A1c level was ≥6.5%, or if their fasting glucose measurement was ≥126 mg/dL (41). Participants were classified as having hyperlipidemia if their total cholesterol level was ≥200 mg/dL, their low-density lipoprotein cholesterol level was ≥130 mg/dL, or they were taking lipid-lowering medication (36).
Neighborhood-level SES is an important and significant predictor for a variety of outcomes related to cardiovascular health (42–44). We derived neighborhood SES at the census tract level using a z-score-based index of 6 variables obtained from the 2010 US Census (45). We assigned the neighborhood SES index to each participant based on the census tract of residence, with higher scores indicating more affluent tracts. In addition, we also calculated the numbers of fast-food outlets and grocery stores per 1,000 people within 1.5 miles (2.4 km) (a distance that covers most errands and short transportation trips (46)) of each participant's residence (45). Previous studies have shown that neighborhood food environment, such as lack of access to fruit and vegetables, is associated with cardiovascular health (47–49).
Statistical analyses
In all analyses, we excluded participants whose addresses were not geocoded at the street level. We categorized distance to major roadways (A1/A2) as <150 m, 150–300 m, or >300 m, because 1) the concentration of particulate matter from highway traffic pollution decreases by 50% at 150 m and fades to background level after 300 m (50) and 2) natural splines of associations between continuous distance (with 3 degrees of freedom (df)) and prevalence ratios for PAD, CAC, and AAC and log CIMT suggest a linear dose response (see Web Figures 1–4, available at http://aje.oxfordjournals.org/). We modeled distance to major roadways 1) as a categorical variable, testing for linear trend by assigning the median distance to each category and including the term as a continuous variable in regression models, and 2) as a continuous variable on both the original scale and after taking the natural logarithm. We transformed CIMT data to improve the normality of the outcome distribution. We modeled log CIMT in linear regression analysis to estimate the percent difference in CIMT for persons living less than 150 m from major roadways compared with those living more than 300 m from such roadways, per 150-m increase in distance or for every doubling of the increase in distance. We used modified Poisson regression with robust error variance to estimate the prevalence ratio for PAD at baseline and for CAC and AAC at the first follow-up visit among persons living less than 150 m from major roadways compared with those living more than 300 m away, per 150-m increase in distance or for every doubling of the increase in distance. Modified Poisson regression provides a direct estimate of the prevalence ratio, whereas standard logistic regression would instead provide an estimate of the prevalence odds ratio (51).
We incrementally adjusted for potentially confounding factors, including 1) only age (years; natural spline with 3 df) and sex in a model with minimal adjustment; 2) additionally household income (low, lower middle, upper middle, high), education (high school or less, college or vocational school, graduate school), and neighborhood SES; 3) in a fully adjusting model, additionally (linear continuous) body mass index (weight (kg)/height (m)2; natural spline with 3 df), smoking (never, past, or current smoker), physical activity (natural spline with 3 df), alcohol consumption in the past year (yes vs. no), history of self-reported and physician-diagnosed coronary heart disease (CHD), myocardial infarction, stroke, and carotid angioplasty, and neighborhood food environment, including number of fast-food restaurants (linear continuous variable) and number of grocery stores (linear continuous variable); and 4) additional adjustment for diabetes mellitus (yes vs. no), hypertension (yes vs. no), and hyperlipidemia (yes vs. no), because these variables may be confounding risk factors but might also plausibly be intermediates along the causal pathway from traffic pollution to atherosclerosis. Data on all covariates were available at both baseline and follow-up except for sex, education, and household income (only available at baseline).
Among the fully adjusting models, where there were significant associations between atherosclerosis and distance to roadways, we further attempted to identify subgroups more likely to have a higher prevalence of atherosclerosis associated with long-term exposure to traffic pollution. We stratified the results from the full-adjustment models by age, sex, smoking, alcohol consumption, hypertension, diabetes, hyperlipidemia, and history of CHD, myocardial infarction, stroke, or carotid angioplasty, based on an a priori hypothesis that these major CVD risk factors may confer additional susceptibility to traffic pollution. Some prior studies also explored the possibility that SES indicators such as household income may modify the association between air pollution and blood pressure (52). We also tested the interactions between these factors and residential distance.
Sensitivity analyses
We conducted sensitivity analyses to evaluate the robustness of our findings. First, we redefined outcomes one at a time: 1) We defined PAD as present if ABI ≥1.3 or ABI ≤0.9 and absent if ABI equaled 1.0–1.3; 2) we excluded participants with ABI >1.4; 3) we defined CAC as present if the Agatston score was greater than 100 for males and greater than 0 for females; 4) we defined CAC and AAC as present if the Agatston score was greater than 20. Second, we modified exposure classifications by dichotomizing distance to roadways using a cutoff of 150 m or 300 m or by assessing distance to A1 roads. Third, we adjusted for fast-food outlets and grocery stores within a radius of 0.75 miles (1.2 km) or 1.5 miles (2.4 km) from each residence. These distances were chosen on the basis of urban planning literature (46) to capture both a walkable distance (0.75 miles) and a larger distance that encompasses the majority of a person's daily activities (1.5 miles), as previously used for participants in the Women's Health Initiative (45, 53). Fourth, we restricted our analyses to 1) participants living in urban areas, as defined by the 2010 US Census Urban and Rural Classification (54); 2) participants free of a history of self-reported and physician-diagnosed CHD, stroke, myocardial infarction, and carotid angioplasty; or 3) participants living within 4,000 m (≤4,000 m) of major roads.
All analyses were performed using R statistical software (version 2.13; R Foundation for Statistical Computing, Vienna, Austria). A 2-sided P value less than 0.05 was considered statistically significant.
RESULTS
Analyses related to CIMT and ABI at baseline
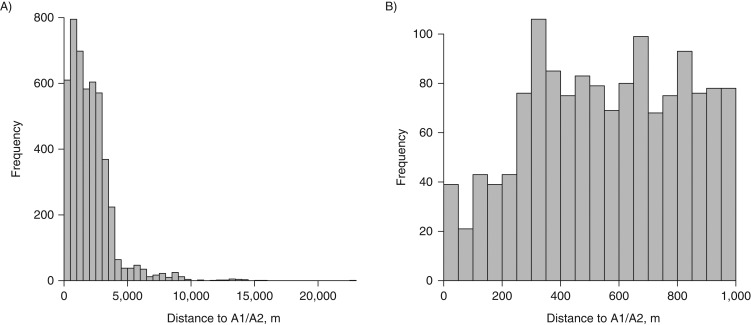
We had 4,800 participants for analysis related to CIMT after excluding 195 participants with addresses that were not geocoded at the street level (Table 1). Participants in the CIMT analysis were predominately female (63.7%), with a mean age of 55.5 (standard deviation (SD), 12.7) years and a mean body mass index of 31.6 (SD, 7.2). The mean CIMT was 0.8 mm (SD, 0.2). Participants living less than 150 m from the nearest major (A1/A2) roadways were more likely to be female, less educated, and current smokers and more likely to have higher body mass index, hyperlipidemia, and a history of CHD, myocardial infarction, stroke, or cardiac angioplasty but a lower income and lower prevalences of diabetes and hypertension. Most participants lived within 4,000 m of A1/A2 roads (Figure 1).
Table 1.
Baseline Characteristics of Participants With Carotid Intima-Media Thickness Measurements, by Residential Distance to Major Roadways, Jackson Heart Study, 2004
| Characteristic | Residential Distance to Major Roadwaysa | |||||||
|---|---|---|---|---|---|---|---|---|
| All (n = 4,800)b | <150 m (n = 103) | 150–300 m (n = 158) | >300 m (n = 4,539) | |||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | |
| Age, years | 55.5 (12.7) | 55.4 (12.9) | 59.1 (12.5) | 55.4 (12.7) | ||||
| Female sex | 63.7 | 68.0 | 63.8 | 63.6 | ||||
| Body mass indexc | 31.6 (7.2) | 32.9 (8.4) | 30.8 (6.7) | 31.6 (7.1) | ||||
| Education | ||||||||
| High school or less | 38.0 | 43.1 | 38.0 | 38.0 | ||||
| College or vocational school | 29.1 | 30.4 | 24.1 | 29.2 | ||||
| Graduate school | 32.9 | 26.5 | 37.9 | 32.8 | ||||
| Household income | ||||||||
| Low | 15.1 | 20.0 | 8.4 | 15.2 | ||||
| Lower middle | 24.1 | 24.0 | 25.2 | 24.0 | ||||
| Upper middle | 29.9 | 21.3 | 29.8 | 30.1 | ||||
| High | 30.9 | 34.7 | 36.6 | 30.7 | ||||
| Alcohol consumption | 45.6 | 40.8 | 38.2 | 46.0 | ||||
| Ever smoking | ||||||||
| Never smoker | 68.2 | 66.0 | 75.3 | 68.0 | ||||
| Past smoker | 18.9 | 13.6 | 15.8 | 19.0 | ||||
| Current smoker | 12.9 | 20.4 | 8.9 | 12.8 | ||||
| Hypertension | 62.6 | 56.4 | 67.7 | 62.5 | ||||
| Diabetes mellitus | 21.6 | 16.5 | 21.8 | 21.7 | ||||
| Hyperlipidemia | 54.7 | 60.2 | 58.9 | 54.4 | ||||
| History of CHD, stroke, or MI | 5.3 | 7.8 | 3.2 | 5.3 | ||||
| No. of fast-food outlets per square mile | 1.6 (2.5) | 1.7 (2.4) | 1.9 (2.6) | 1.5 (2.5) | ||||
| No. of grocery stores per square mile | 2.7 (4.3) | 4.0 (5.3) | 3.9 (5.1) | 2.6 (4.2) | ||||
| Carotid intima-media thickness, mm | 0.8 (0.2) | 0.7 (0.2) | 0.8 (0.3) | 0.8 (0.2) | ||||
Abbreviations: CHD, coronary heart disease; MI, myocardial infarction; SD, standard deviation.
a Major roadways were defined as US Census Feature Class Code A1 or A2 roads.
b Missing data: body mass index, n = 5; education, n = 17; household income, n = 742; alcohol consumption, n = 16; ever smoking, n = 32; hypertension, n = 42; diabetes, n = 52.
c Weight (kg)/height (m)2.
Figure 1.
Residential distance to US Census Feature Class Code A1/A2 roads among 4,800 participants with data on carotid intima-media thickness (A) and among participants living within 1,000 m (≤1,000 m) of A1/A2 roads (B), Jackson Heart Study, 2000–2008.
Similarly, we had 4,443 participants for analysis related to ABI after excluding 40 participants with addresses not geocoded at the street level. Participants in the ABI analysis had similar characteristics as those in the CIMT analysis (see Web Table 1).
We found that, compared with living more than 300 m from A1/A2 roads, living within 150 m of such roads was associated with a significant 6.67% (95% confidence interval (CI): 1.28, 12.35) increase in CIMT after adjustment for confounders (Table 2). The prevalence of PAD among persons living less than 150 m from A1/A2 roads was 1.17 (95% CI: 0.73, 1.86) times that of persons living more than 300 m from such roads, but this result was not statistically significant. When considering only distance to A1 roads, the results were similar but not statistically significant (Web Table 2).
Table 2.
Associations Between Residential Distance to the Nearest Major Roadways and Carotid Intima-Media Thickness and the Presence of Peripheral Artery Disease at Baseline and the Presence of Coronary Artery Calcification or Abdominal Aortic Calcification at the First Follow-up Visit, Jackson Heart Study, 2000–2008a
| Outcome and Model | Residential Distance to Major Roadwaysb | Ptrend | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <150 m | 150–300 m | >300 m | ||||||||
| % Difference | PR | 95% CI | % Difference | PR | 95% CI | % Difference | PR | 95% CI | ||
| Baseline | ||||||||||
| CIMT | (n = 103) | (n = 158) | (n = 4,539) | |||||||
| 1c | 4.13 | −0.29, 8.74 | 0.03 | −3.44, 3.61 | 0 | 1 | Referent | 0.23 | ||
| 2d | 5.06 | −0.17, 10.58 | 0.12 | −3.69, 4.09 | 0.19 | |||||
| 3e | 5.93f | 0.63, 11.52 | −0.45 | −4.34, 3.60 | 0.22 | |||||
| 4g | 6.67f | 1.28, 12.35 | 0.04 | −3.89, 4.14 | 0.12 | |||||
| PAD | (n = 90) | (n = 149) | (n = 4,204) | |||||||
| 1 | 0.93 | 0.60, 1.44 | 0.86 | 0.61, 1.22 | 0 | 1 | Referent | 0.41 | ||
| 2 | 1.12 | 0.69, 1.79 | 0.83 | 0.56, 1.24 | 0.66 | |||||
| 3 | 1.11 | 0.70, 1.78 | 0.90 | 0.60, 1.34 | 0.90 | |||||
| 4 | 1.17 | 0.73, 1.86 | 0.88 | 0.58, 1.32 | 0.92 | |||||
| First Follow-up Visit | ||||||||||
| CAC | (n = 56) | (n = 140) | (n = 2,542) | |||||||
| 1 | 0.86 | 0.66, 1.12 | 0.81 | 0.63, 1.02 | 0 | 1 | Referent | 0.04 | ||
| 2 | 0.88 | 0.63, 1.24 | 0.81 | 0.62, 1.06 | 0.09 | |||||
| 3 | 0.85 | 0.60, 1.22 | 0.82 | 0.62, 1.07 | 0.09 | |||||
| 4 | 0.90 | 0.62, 1.31 | 0.80 | 0.61, 1.05 | 0.11 | |||||
| AAC | (n = 56) | (n = 140) | (n = 2,541) | |||||||
| 1 | 0.90 | 0.76, 1.06 | 1.00 | 0.99, 1.00 | 0 | 1 | Referent | 0.19 | ||
| 2 | 0.91 | 0.74, 1.11 | 1.03 | 0.90, 1.19 | 0.85 | |||||
| 3 | 0.92 | 0.73, 1.11 | 1.07 | 0.93, 1.23 | 0.90 | |||||
| 4 | 0.92 | 0.74, 1.13 | 1.06 | 0.92, 1.22 | 0.87 | |||||
Abbreviations: AAC, abdominal aortic calcification; CAC, coronary artery calcification; CI, confidence interval; CIMT, carotid intima-media thickness; PAD, peripheral artery disease; PR, prevalence ratio.
a Estimates represent relative percent difference in CIMT modeled as a continuous outcome and PRs for PAD, CAC, and AAC modeled as categorical outcomes.
b Major roadways were defined as US Census Feature Class Code A1 or A2 roads.
c Model 1 adjusted for age (natural spline, 3 df) and sex.
d Model 2 additionally adjusted for household income, education, and neighborhood socioeconomic status.
e Model 3 additionally adjusted for body mass index (natural spline, 3 df), smoking, physical activity (natural spline, 3 df), alcohol consumption, history of self-reported and physician-diagnosed coronary heart disease, myocardial infarction, stroke, and carotid angioplasty, and neighborhood food environment (number of fast-food restaurants or grocery stores within 1.5 miles (2.4 km) of residence).
f P < 0.05.
g Model 4 additionally adjusted for diabetes mellitus, hypertension, and hyperlipidemia.
When modeling exposure as a continuous distance on the logarithmic scale, CIMT decreased by 0.02% for every doubling of the distance to A1/A2 roads, but the results were not statistically significant (Table 3). We also observed decreased prevalence of PAD for every doubling of distance to A1/A2 roads. The results were not materially different when exposure was modeled on the natural scale (Table 3) or when distance to A1 roads was considered (Web Table 3).
Table 3.
Associations Between Continuous Residential Distance to the Nearest Major Roadways and Carotid Intima-Media Thickness and the Presence of Peripheral Artery Disease at Baseline and the Presence of Coronary Artery Calcification or Abdominal Aortic Calcification at the First Follow-up Visit, Jackson Heart Study, 2000–2008a
| Outcome and Model | Residential Distance to Major Roadwaysb | |||||
|---|---|---|---|---|---|---|
| Per Doubling in Distance | Per 150-m Increase in Distance | |||||
| % Difference | PR | 95% CI | % Difference | PR | 95% CI | |
| Baseline | ||||||
| CIMT | ||||||
| 1c | −0.33 | −0.75, 0.09 | −0.020 | −0.074, 0.035 | ||
| 2d | −0.04 | −0.51, 0.44 | 0.017 | −0.042, 0.077 | ||
| 3e | −0.02 | −0.51, 0.47 | 0.022 | −0.039, 0.083 | ||
| 4f | −0.05 | −0.54, 0.44 | 0.022 | −0.039, 0.083 | ||
| PAD | ||||||
| 1 | 1.00 | 0.96, 1.04 | 1.00 | 0.99, 1.006 | ||
| 2 | 0.98 | 0.94, 1.03 | 1.00 | 0.99, 1.006 | ||
| 3 | 0.98 | 0.94, 1.02 | 0.99 | 0.99, 1.005 | ||
| 4 | 0.98 | 0.94, 1.03 | 1.00 | 0.99, 1.006 | ||
| First Follow-up Visit | ||||||
| CAC | ||||||
| 1 | 1.01 | 0.96, 1.04 | 0.99 | 0.99, 1.003 | ||
| 2 | 1.00 | 0.97, 1.03 | 0.99 | 0.99, 1.001 | ||
| 3 | 1.00 | 0.97, 1.04 | 0.99 | 0.99, 1.003 | ||
| 4 | 1.00 | 0.97, 1.04 | 0.99 | 0.99, 1.003 | ||
| AAC | ||||||
| 1 | 1.02 | 0.99, 1.03 | 1.00 | 0.99, 1.003 | ||
| 2 | 1.00 | 0.98, 1.02 | 1.00 | 0.99, 1.003 | ||
| 3 | 0.99 | 0.98, 1.02 | 1.00 | 0.99, 1.003 | ||
| 4 | 0.99 | 0.98, 1.02 | 1.00 | 0.99, 1.003 | ||
Abbreviations: AAC, abdominal aortic calcification; CAC, coronary artery calcification; CI, confidence interval; CIMT, carotid intima-media thickness; PAD, peripheral artery disease; PR, prevalence ratio.
a Estimates represent relative percent difference in CIMT and PRs for PAD, CAC and AAC per 150-m increase in distance when distance is modeled on the natural scale or per doubling in distance when distance is modeled on a logarithmic scale.
b Major roadways were defined as US Census Feature Class Code A1 or A2 roads.
c Model 1 adjusted for age (natural spline, 3 df) and sex.
d Model 2 additionally adjusted for household income, education, and neighborhood socioeconomic status.
e Model 3 additionally adjusted for body mass index (natural spline, 3 df), smoking, physical activity (natural spline, 3 df), alcohol consumption, history of self-reported and physician-diagnosed coronary heart disease, myocardial infarction, stroke, and carotid angioplasty, and neighborhood food environment (number of fast-food restaurants or grocery stores within 1.5 miles (2.4 km) of residence).
f Model 4 additionally adjusted for diabetes mellitus, hypertension, and hyperlipidemia.
Analyses related to CAC and AAC measured at the first follow-up visit
We had 2,682 participants for analysis related to CAC, excluding 201 participants with addresses not geocoded at the street level (Table 4). Compared with those with CIMT measurements (Table 1), participants with CAC measurements were more educated, more likely to have a higher income and hypertension, and less likely to have a history of CHD, myocardial infarction, stroke, or cardiac angioplasty. Participants living less than 150 m from A1/A2 roads versus those living more than 300 m from such roads had lower prevalences of CAC (42.9% vs. 48.7%) and AAC (58.9% vs. 66.1%).
Table 4.
Characteristics of Participants With Coronary Artery Calcification Measurements at the First Follow-up Visit, by Residential Distance to Major Roadways, Jackson Heart Study, 2005–2008
| Characteristic | Residential Distance to Major Roadwaysa | |||||||
|---|---|---|---|---|---|---|---|---|
| All (n = 2,682)b | <150 m (n = 56) | 150–300 m (n = 84) | >300 m (n = 2,542) | |||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | |
| Age, years | 60.5 (11.1) | 60.8 (12.4) | 62.5 (10.3) | 60.5 (11.0) | ||||
| Female sex | 65.5 | 66.1 | 63.1 | 65.7 | ||||
| Body mass indexc | 31.6 (6.4) | 29.9 (5.2) | 31.1 (5.7) | 31.7 (6.5) | ||||
| Education | ||||||||
| High school or less | 33.9 | 29.1 | 27.4 | 34.1 | ||||
| College or vocational school | 46.1 | 52.7 | 44.0 | 46.1 | ||||
| Graduate school | 20.0 | 18.2 | 28.6 | 19.8 | ||||
| Household income | ||||||||
| Low | 11.0 | 9.8 | 9.8 | 11.1 | ||||
| Lower middle | 22.0 | 31.7 | 16.9 | 22.0 | ||||
| Upper middle | 31.6 | 17.1 | 26.8 | 32.1 | ||||
| High | 35.3 | 41.4 | 46.5 | 34.8 | ||||
| Alcohol consumption | 46.2 | 57.1 | 50.0 | 45.9 | ||||
| Ever smoking | ||||||||
| Never smoker | 70.2 | 69.6 | 76.2 | 70.0 | ||||
| Past smoker | 19.1 | 16.1 | 13.1 | 19.4 | ||||
| Current smoker | 10.7 | 14.3 | 10.7 | 10.6 | ||||
| Hypertension | 71.2 | 50.0 | 72.6 | 71.6 | ||||
| Diabetes mellitus | 21.8 | 14.3 | 23.8 | 22.0 | ||||
| Hyperlipidemia | 58.6 | 67.9 | 56.0 | 58.4 | ||||
| History of CHD, stroke, or MI | 0.4 | 0 | 0 | 0.4 | ||||
| No. of fast-food outlets per square mile | 1.6 (2.7) | 1.6 (2.3) | 2.1 (2.8) | 1.5 (2.8) | ||||
| No. of grocery stores per square mile | 2.5 (4.8) | 3.9 (5.6) | 3.5 (4.8) | 2.5 (4.7) | ||||
| Coronary artery calcification | 48.7 | 42.9 | 42.9 | 49.0 | ||||
Abbreviations: CHD, coronary heart disease; MI, myocardial infarction; SD, standard deviation.
a Major roadways were defined as US Census Feature Class Code A1 or A2 roads.
b Missing data: body mass index, n = 37; education, n = 3; household income, n = 400; alcohol consumption, n = 8; ever smoking, n = 19.
c Weight (kg)/height (m)2.
All participants with CAC measurements had AAC measurements, except 1 participant. Thus, we had a sample of 2,681 participants for analysis related to AAC (data not shown).
We did not observe an elevated prevalence of atherosclerosis as shown above for CIMT and PAD among persons living less than 150 m from A1/A2 roads (Table 2). We observed similar results when considering A1 roads only (Web Table 2), and none of those associations were statistically significant.
We also observed lower prevalences of CAC and AAC for every doubling of residential distance to A1/A2 roads (Table 3). The results were not materially different when modeling exposure on the natural scale (Web Table 3) or when considering distance to A1 roads (Web Table 3).
Effect modification
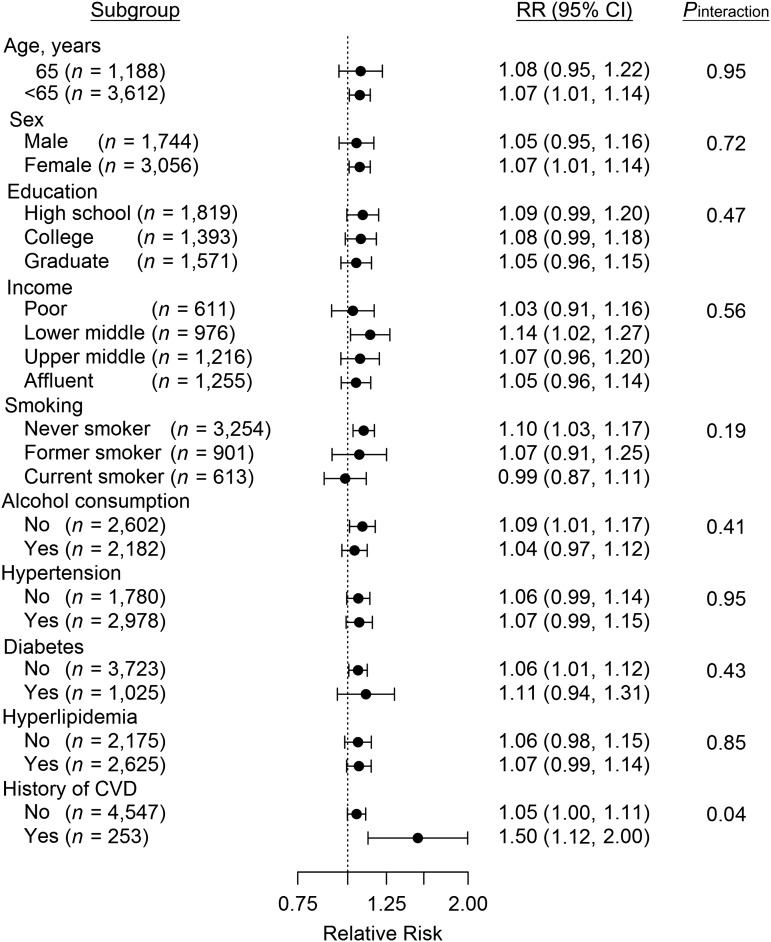
We tested whether the association between markers of atherosclerosis and residential distance differed among subgroups. We found positive increases in CIMT associated with proximity to A1/A2 roads for mostly all strata, but there was little evidence of interaction, except for participants with a history of CHD, myocardial infarction, stroke, or cardiac angioplasty (Figure 2).
Figure 2.
Stratified relative risk (RR) of atherosclerosis (measured by carotid intima-media thickness) for participants living less than 150 m from US Census Feature Class Code A1/A2 roads as compared with those living more than 300 m from A1/A2 roads, Jackson Heart Study, 2000–2008. Bars, 95% confidence intervals (CIs).
Sensitivity analysis
When restricting our analyses to participants living in urban areas, we found that the increase in CIMT for persons living less than 150 m from A1/A2 roads was elevated by 1%–2% and remained statistically significant (data not shown). The results for PAD, CAC, and AAC were not materially different after analyses were restricted to urban residents (data not shown). When we restricted analyses to participants free of a history of CHD, stroke, or cardiac angioplasty, the results were attenuated but did not change materially (Web Table 4). The results were not materially different in any other sensitivity analyses, including restriction of the analyses to persons living within 4,000 m of A1/A2 roads (data not shown).
DISCUSSION
We found that, compared with living more than 300 m from A1/A2 roadways, participants living less than 150 m from A1/A2 roads had a significant 6.67% (95% CI: 1.28, 12.35) increase in CIMT after adjustment for confounders. The prevalence of PAD among participants living less than 150 m from A1/A2 roads was 1.17 (95% CI: 0.73, 1.86) times that for participants living more than 300 m from such roads, but this association was not statistically significant. We did not observe an elevated prevalence of CAC or AAC among persons living less than 150 m from A1/A2 roadways. These results were robust to a number of sensitivity analyses.
Our conclusion that long-term exposure to traffic pollution, marked by residential proximity to A1/A2 roads, increases CIMT was consistent with the findings in large, predominately white cohorts such as MESA, the Heinz Nixdorf Recall Study, and the ESCAPE Study (14–19). Previous investigation in the Normative Aging Study also found positive but nonsignificant increases in CIMT associated with residential proximity to A1/A2 roads (20).
Very few investigators have studied the association between long-term exposure to traffic pollution and PAD. We observed that living close to major roads increased PAD prevalence. Though it was not statistically significant, this result for persons living less than 150 m from major roads was consistent overall with the findings of Hoffmann et al. (22), in whose study persons living less than 50 m and 50–100 m from a major road had odds ratios of 1.77 (95% CI: 1.01, 2.10) and 1.02 (95% CI: 0.58, 1.80), respectively, for PAD as compared with those living more than 200 m away from such roads.
In the coronary vascular bed, our null results for CAC were not consistent with previous significant findings from the Heinz Nixdorf Recall Study (23) and the Danish National Registry (25). The American Heart Association has concluded that CIMT is a good surrogate measure of subclinical atherosclerosis (55), and many studies using CIMT have shown that long-term exposure to traffic pollution increases risk of atherosclerosis in white populations. However, in comparison with CIMT, CAC as a surrogate measure of subclinical atherosclerosis may be different in African Americans. Despite the American Heart Association's consensus on CAC being a good surrogate for coronary atherosclerosis, the use of CAC in African Americans may have limited discriminatory power, because the absence of CAC does not necessarily imply an absence of CVD risk in African Americans (56, 57). Previous studies have shown that African Americans who have higher systolic blood pressure and rates of CHD events have a lower prevalence of CAC (58). It is plausible that JHS participants with CHD at the first follow-up visit had little or no CAC, leading to underestimation of CAC cases and biasing the results towards the null.
In the abdominal vascular bed, Allen et al. (24) reported no association between residential proximity to major roadways and AAC in MESA participants, which was consistent with our results. AAC is associated with increased risk of vascular atherosclerosis at nonaortic sites and is considered to have additive value over other surrogate measures.
The differences in CIMT across strata defined by potential susceptibility factors were small and not statically significant, with the exception of history of CHD, myocardial infarction, stroke, or cardiac angioplasty. The larger increase in CIMT associated with living closer to A1/A2 roads was consistent with previous studies for females (16, 17), never smokers (18), and persons with a history of CHD (17). As one might expect, there was no association with CIMT among current smokers, suggesting that the associations seen in other strata may largely reflect the associations with current smoking. The lower increase found in this study was also observed in previous studies for nondiabetic participants (16), although the exact reason is unclear.
Our study had some limitations. First, the use of residential proximity to major roadways as a surrogate measure of long-term exposure to traffic-related pollution may lead to exposure misclassification. This crude surrogate measure may not reflect the spatial distribution of pollutant concentrations due to differences in traffic volume and vertical concentration gradients. It also does not account for other potential confounders, such as traffic-related noise. However, many studies using residential proximity have shown positive results for persons living closest to major roadways, which is consistent with associations found in studies using sophisticated air pollution modeling (59). Second, we did not have information on residential history and the duration of residence at each location prior to each study visit. However, any resulting exposure misclassification was probably nondifferential. Third, this was a cross-sectional study, from which we were not able to discern the temporal relationship between long-term exposure to traffic-related pollution and atherosclerosis.
The study also had some strengths. To our knowledge, this was the first study to simultaneously evaluate markers of atherosclerosis in 4 different vascular beds associated with long-term exposure to traffic pollution. This was also the first attempt to replicate previous findings in a large, all-African-American population, although the findings may not be generalizable to the general African-American population of the United States, due in part to a different CVD risk factor profile unique to African Americans living in the South.
In conclusion, among African Americans in the JHS, long-term exposure to traffic pollution is associated with CIMT, a marker of atherosclerosis in the carotid vascular bed, but not with CAC, AAC, or PAD in the coronary, abdominal, and peripheral vascular beds, respectively. Our results highlight the need to consider residential proximity to major roadways a potential CVD risk factor for African Americans, especially when tackling environmental injustice issues, although the traffic-atherosclerosis association may be relatively small compared with other established risk factors.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Environmental Health, Fairbanks School of Public Health, Indiana University, Indianapolis, Indiana (Yi Wang); Department of Epidemiology, School of Public Health, Brown University, Providence, Rhode Island (Gregory A. Wellenius, Annie Gjelsvik); Center for Research, Evaluation and Environmental & Policy Change, My Brother's Keeper, Inc., Jackson, Mississippi (DeMarc A. Hickson); Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi (DeMarc A. Hickson); Department of Family Medicine, Alpert Medical School, Brown University, Providence, Rhode Island (Charles B. Eaton); and School of Nursing, University of Mississippi Medical Center, Jackson, Mississippi (Sharon B. Wyatt).
This work was supported by grant R21 NR013231 from the National Institute of Nursing Research and the National Institute of Minority Health and Health Disparities. The Jackson Heart Study was supported by contracts HHSN268201300046C, HSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities.
We thank the Jackson Heart Study staff for data collection.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring institutions.
Conflict of interest: none declared.
REFERENCES
- 1.Dockery DW, Pope CA III, Xu X, et al. . An association between air pollution and mortality in six U.S. Cities. N Engl J Med. 1993;329(24):1753–1759. [DOI] [PubMed] [Google Scholar]
- 2.Pope CA III, Dockery DW.. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56(6):709–742. [DOI] [PubMed] [Google Scholar]
- 3.Brook RD, Rajagopalan S, Pope CA III, et al. . Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. [DOI] [PubMed] [Google Scholar]
- 4.Brugge D, Durant JL, Rioux C.. Near-highway pollutants in motor vehicle exhaust: a review of epidemiologic evidence of cardiac and pulmonary health risks. Environ Health. 2007;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters A, von Klot S, Heier M, et al. . Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351(17):1721–1730. [DOI] [PubMed] [Google Scholar]
- 6.Laden F, Neas LM, Dockery DW, et al. . Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect. 2000;108(10):941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Künzli N, Kaiser R, Medina S, et al. . Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet. 2000;356(9232):795–801. [DOI] [PubMed] [Google Scholar]
- 8.Delfino RJ, Tjoa T, Gillen DL, et al. . Traffic-related air pollution and blood pressure in elderly subjects with coronary artery disease. Epidemiology. 2010;21(3):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delfino RJ, Staimer N, Tjoa T, et al. . Association of biomarkers of systemic inflammation with organic components and source tracers in quasi-ultrafine particles. Environ Health Perspect. 2010;118(6):756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madrigano J, Baccarelli A, Wright RO, et al. . Air pollution, obesity, genes and cellular adhesion molecules. Occup Environ Med. 2010;67(5):312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quan CL, Sun QH, Lippmann M, et al. . Comparative effects of inhaled diesel exhaust and ambient fine particles on inflammation, atherosclerosis, and vascular dysfunction. Inhal Toxicol. 2010;22(9):738–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LC, Nadziejko C.. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. V. CAPs exacerbate aortic plaque development in hyperlipidemic mice. Inhal Toxicol. 2005;17(4-5):217–224. [DOI] [PubMed] [Google Scholar]
- 13.Sun Q, Yue P, Kirk RI, et al. . Ambient air particulate matter exposure and tissue factor expression in atherosclerosis. Inhal Toxicol. 2008;20(2):127–137. [DOI] [PubMed] [Google Scholar]
- 14.Perez L, Wolf K, Hennig F, et al. . Air pollution and atherosclerosis: a cross-sectional analysis of four European cohort studies in the ESCAPE study. Environ Health Perspect. 2015;123(6):597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gan WQ, Allen RW, Brauer M, et al. . Long-term exposure to traffic-related air pollution and progression of carotid artery atherosclerosis: a prospective cohort study. BMJ Open. 2014;4:e004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adar SD, Sheppard L, Vedal S, et al. . Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the Multi-Ethnic Study of Atherosclerosis and Air Pollution. PLoS Med. 2013;10(4):e1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer M, Moebus S, Möhlenkamp S, et al. . Urban particulate matter air pollution is associated with subclinical atherosclerosis: results from the HNR (Heinz Nixdorf Recall) study. J Am Coll Cardiol. 2010;56(22):1803–1808. [DOI] [PubMed] [Google Scholar]
- 18.Künzli N, Jerrett M, Mack WJ, et al. . Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113(2):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonne C, Yanosky JD, Beevers S, et al. . PM mass concentration and PM oxidative potential in relation to carotid intima-media thickness. Epidemiology. 2012;23(3):486–494. [DOI] [PubMed] [Google Scholar]
- 20.Wilker EH, Mittleman MA, Coull BA, et al. . Long-term exposure to black carbon and carotid intima-media thickness: the Normative Aging Study. Environ Health Perspect. 2013;121(9):1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann B, Moebus S, Dragano N, et al. . Residential traffic exposure and coronary heart disease: results from the Heinz Nixdorf recall study. Biomarkers. 2009;14(suppl 1):74–78. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann B, Moebus S, Kröger K, et al. . Residential exposure to urban air pollution, ankle-brachial index, and peripheral arterial disease. Epidemiology. 2009;20(2):280–288. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann B, Moebus S, Möhlenkamp S, et al. . Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116(5):489–496. [DOI] [PubMed] [Google Scholar]
- 24.Allen RW, Criqui MH, Diez Roux AV, et al. . Fine particulate matter air pollution, proximity to traffic, and aortic atherosclerosis. Epidemiology. 2009;20(2):254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambrechtsen J, Gerke O, Egstrup K, et al. . The relation between coronary artery calcification in asymptomatic subjects and both traditional risk factors and living in the city centre: a DanRisk substudy. J Intern Med. 2012;271(5):444–450. [DOI] [PubMed] [Google Scholar]
- 26.Houston D, Wu J, Ong P, et al. . Structural disparities of urban traffic in Southern California: implications for vehicle-related air pollution exposure in minority and high-poverty neighborhoods. J Urban Aff. 2004;26(5):565–592. [Google Scholar]
- 27.Fuqua SR, Wyatt SB, Andrew ME, et al. . Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15(4 suppl 6):S6-18–S6-29. [PubMed] [Google Scholar]
- 28.Taylor HA Jr, Wilson JG, Jones DW, et al. . Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 suppl 6):S6-4–S6-17. [PubMed] [Google Scholar]
- 29.Hickson DA, Waller LA, Gebreab SY, et al. . Geographic representation of the Jackson Heart Study cohort to the African-American population in Jackson, Mississippi. Am J Epidemiol. 2011;173(1):110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yerramilli A, Dodla VB, Desamsetti S, et al. . Air quality modeling for the urban Jackson, Mississippi region using a high resolution WRF/Chem model. Int J Environ Res Public Health. 2011;8(6):2470–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein JH, Korcarz CE, Post WS.. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: summary and discussion of the American Society of Echocardiography consensus statement. Prev Cardiol. 2009;12(1):34–38. [DOI] [PubMed] [Google Scholar]
- 32.Tullos BW, Sung JH, Lee JE, et al. . Ankle-brachial index (ABI), abdominal aortic calcification (AAC), and coronary artery calcification (CAC): the Jackson Heart Study. Int J Cardiovasc Imaging. 2013;29(4):891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera M, Basagaña X, Aguilera I, et al. . Association between long-term exposure to traffic-related air pollution and subclinical atherosclerosis: the REGICOR study. Environ Health Perspect. 2013;121(2):223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carr JJ, Nelson JC, Wong ND, et al. . Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. [DOI] [PubMed] [Google Scholar]
- 35.Robinson JC, Wyatt SB, Hickson D, et al. . Methods for retrospective geocoding in population studies: the Jackson Heart Study. J Urban Health. 2010;87(1):136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Eliot MN, Koutrakis P, et al. . Ambient air pollution and depressive symptoms in older adults: results from the MOBILIZE Boston study. Environ Health Perspect. 2014;122(6):553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebreab SY, Diez-Roux AV, Hickson DA, et al. . The contribution of stress to the social patterning of clinical and subclinical CVD risk factors in African Americans: the Jackson Heart Study. Soc Sci Med. 2012;75(9):1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smitherman TA, Dubbert PM, Grothe KB, et al. . Validation of the Jackson Heart Study physical activity survey in African Americans. J Phys Act Health. 2009;6(suppl 1):S124–S132. [DOI] [PubMed] [Google Scholar]
- 39.Chobanian AV, Bakris GL, Black HR, et al. . The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 40.Wyatt SB, Akylbekova EL, Wofford MR, et al. . Prevalence, awareness, treatment, and control of hypertension in the Jackson Heart Study. Hypertension. 2008;51(3):650–656. [DOI] [PubMed] [Google Scholar]
- 41.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bird CE, Seeman T, Escarce JJ, et al. . Neighbourhood socioeconomic status and biological ‘wear and tear’ in a nationally representative sample of US adults. J Epidemiol Community Health. 2010;64(10):860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merkin SS, Basurto-Davila R, Karlamangla A, et al. . Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults: NHANES III. Ann Epidemiol. 2009;19(3):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diez Roux AV, Merkin SS, Arnett D, et al. . Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. [DOI] [PubMed] [Google Scholar]
- 45.Dubowitz T, Ghosh-Dastidar M, Eibner C, et al. . The Women's Health Initiative: the food environment, neighborhood socioeconomic status, body mass index, and blood pressure. Obesity (Silver Spring). 2012;20(4):862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu PS, Young J. Summary of Travel Trends: 1995 Nationwide Personal Transportation Survey. Washington, DC: Federal Highway Administration, US Department of Transportation; 1999. [Google Scholar]
- 47.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 48.Kawakami N, Li X, Sundquist K. Health-promoting and health-damaging neighbourhood resources and coronary heart disease: a follow-up study of 2 165 000 people. J Epidemiol Community Health. 2011;65(10):866–872. [DOI] [PubMed] [Google Scholar]
- 49.Morgenstern LB, Escobar JD, Sánchez BN, et al. . Fast food and neighborhood stroke risk. Ann Neurol. 2009;66(2):165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Y, Hinds WC, Kim S, et al. . Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52(9):1032–1042. [DOI] [PubMed] [Google Scholar]
- 51.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 52.Hicken MT, Adar SD, Diez Roux AV, et al. . Do psychosocial stress and social disadvantage modify the association between air pollution and blood pressure? The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2013;178(10):1550–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirwa K, Eliot MN, Wang Y, et al. . Residential proximity to major roadways and prevalent hypertension among postmenopausal women: results from the Women's Health Initiative San Diego Cohort. J Am Heart Assoc. 2014;3(5):e000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bureau of the Census, US Department of Commerce. 2010 Census urban and rural classification and urban area criteria. https://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed October 10, 2016.
- 55.Smith SC Jr, Greenland P, Grundy SM. Prevention conference V. Beyond secondary prevention: identifying the high-risk patient for primary prevention. Executive summary. Circulation. 2000;101(1):111–116. [DOI] [PubMed] [Google Scholar]
- 56.McClelland RL, Chung H, Detrano R, et al. . Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113(1):30–37. [DOI] [PubMed] [Google Scholar]
- 57.Greenland P, LaBree L, Azen SP, et al. . Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291(2):210–215. [DOI] [PubMed] [Google Scholar]
- 58.Doherty TM, Tang W, Detrano RC. Racial differences in the significance of coronary calcium in asymptomatic black and white subjects with coronary risk factors. J Am Coll Cardiol. 1999;34(3):787–794. [DOI] [PubMed] [Google Scholar]
- 59.Brook RD, Rajagopalan S. Particulate matter air pollution and atherosclerosis. Curr Atheroscler Rep. 2010;12(5):291–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.