Abstract
The capacity to suppress inappropriate thoughts, emotions and actions in favor of appropriate ones shows marked changes throughout childhood and adolescence. Most research has focused on pre-frontal circuit development to explain these changes. Yet, subcortical circuitry involving the amygdala and ventral striatum (VS) has been shown to modulate cue-triggered motivated behaviors in rodents. The nature of the interaction between these two subcortical regions in humans is less well understood, especially during development when there appears to be heightened sensitivity to emotional cues. In the current study, we tested how task-based cortico-subcortical and subcortico-subcortical functional connectivity in 155 participants ages from 5 to 32 impacted cognitive control performance on an emotional go/nogo task. Functional connectivity between the amygdala and VS was inversely correlated with age and predicted cognitive control to emotional cues, when controlling for performance to neutral cues. In contrast, increased medial pre-frontal-amygdala connectivity was associated with better cognitive control to emotional cues and this cortical-subcortical connectivity mediated the association between amygdala-VS connectivity and emotional cognitive control. These findings suggest a dissociation in how subcortical-subcortical and cortical-subcortical connectivity impact cognitive control across development.
Keywords: emotion, connectivity, amygdala, ventral striatum, pre-frontal cortex, adolescent, development
Introduction
As individuals grow and develop, responses to, and regulation of emotional information mature. Although children’s emotions are typically buffered and regulated by the presence of parents (Moriceau and Sullivan, 2006; Gee et al., 2014), the transitional developmental period of adolescence is characterized by emotional lability and sensitivity to both positive and negative emotional information (Galvan et al., 2006; Hare et al., 2008; Geier et al., 2009; Steinberg, 2010; Cohen-Gilbert and Thomas, 2013; Dreyfuss et al., 2014). This emotional lability during adolescence is due in part to changes in cognitive control capacity (Casey, 2016).
An imbalance model of adolescent neurobiology (Casey et al., 2008), proposes that regional neurochemical, structural and functional brain changes lead to imbalances within neural circuitry that underlie these behavioral and emotional changes during development. The development of specific cortical and subcortical neural systems is thought to underlie motivated behavior and the regulation of that motivated behavior (Ernst et al., 2006; Casey et al., 2008; Steinberg, 2010; Mills et al., 2014). Development of these neural circuits underlies increases in cognitive control in both positive and negative emotional contexts (Steinberg, 2010; van Leijenhorst et al., 2010; Cohen-Gilbert and Thomas, 2013; Grose-Fifer et al., 2013).
A core feature of the imbalance model is that pre-frontal cortical circuitry undergoes substantial maturation from childhood to young adulthood (Goddings et al., 2014; Mills et al., 2014; Giedd et al., 2015). In adulthood, the medial pre-frontal cortex has dense projections to the intercalated cells of the amygdala as well as to the GABAergic medium spiny neurons of the ventral striatum (VS) that modulate motivated behaviors (Quirk et al., 2003). Tract tracing studies in rodents show that amygdala-to-Pre-frontal Cortex (PFC) projections emerge earlier than PFC-to-amygdala projections (Bouwmeester et al., 2002a,b). These efferent connections from the PFC mature through adolescence (Verwer et al., 1996; Cunningham et al., 2002; Cressman et al., 2010). Effective and functional connectivity imaging research in humans and rodents corroborate the role of PFC-amygdala connectivity in emotional and behavioral regulation (Perlman and Pelphrey, 2011; Gee et al., 2013b; Dincheva et al., 2015; Fareri et al., 2015), although these methods are unable to precisely infer causality or directionality.
Much of the extant research has focused on the maturation of cortical-subcortical connectivity implicated in emotion regulation (e.g. Monk et al., 2008; Gee et al., 2013b). Yet, the processing of emotional cues and execution of cognitive control also relies on subcortical circuitry. These circuits include the monosynaptic connection between the amygdala and VS. Robust unidirectional glutamatergic projections from the amygdala to the VS exist (Haber and Knutson, 2010), and subserve motivated behaviors in rodents (Stuber et al., 2011). Thus, subcortical connectivity may impact emotional reactivity and behavior. While the amygdala and striatum have oftentimes been considered to be valence specific regions that operate in isolation (i.e. the amygdala for negative affect; the VS for reward processing), computational (Li et al., 2011), animal (Paton et al., 2006) and human imaging (Levita et al., 2009) studies suggest otherwise. How interactions between these subcortical regions develop, whether subcortical-subcortical connectivity impacts affective processing in humans, and how cortical-subcortical connectivity interacts with subcortical-subcortical connectivity has yet to be examined. In particular, examining the development and behavioral impact of subcortical-subcortical and cortical-subcortical connectivity may provide a broader context for understanding how the development of these networks relates to cognitive control.
To test how subcortico-subcortical and cortical-subcortical circuitry impacts affective processing and cognitive control, we utilized task-based fMRI from a large, cross-sectional sample of human children, adolescents and young adults (n = 155), who performed a go/no-go task to emotional faces. We first examined neurodevelopmental changes in subcortical-subcortical functional connectivity to emotional cues. To probe the behavioral implications of the neurodevelopmental changes in this circuitry, we next related cortical-subcortical and subcortical-subcortical task-based functional connectivity to behavior in the task. Based on the previous developmental imaging evidence (Hare et al., 2008), we predicted that more robust PFC-subcortical connectivity would be predictive of better cognitive control. Given emerging evidence for subcortical-subcortical interactions in motivated action (Stuber et al., 2011), we predicted that amygdala-VS connectivity would decrease during development and that decreases in connectivity would be associated with improvements in cognitive control. Lastly, we integrated the cortical-subcortical and subcortical-subcortical associations with behavior using mediation. Specifically, we examined whether associations between subcortical-subcortical connectivity and behavior were mediated, at least in part, by cortical-subcortical connectivity.
Materials and methods
Subjects
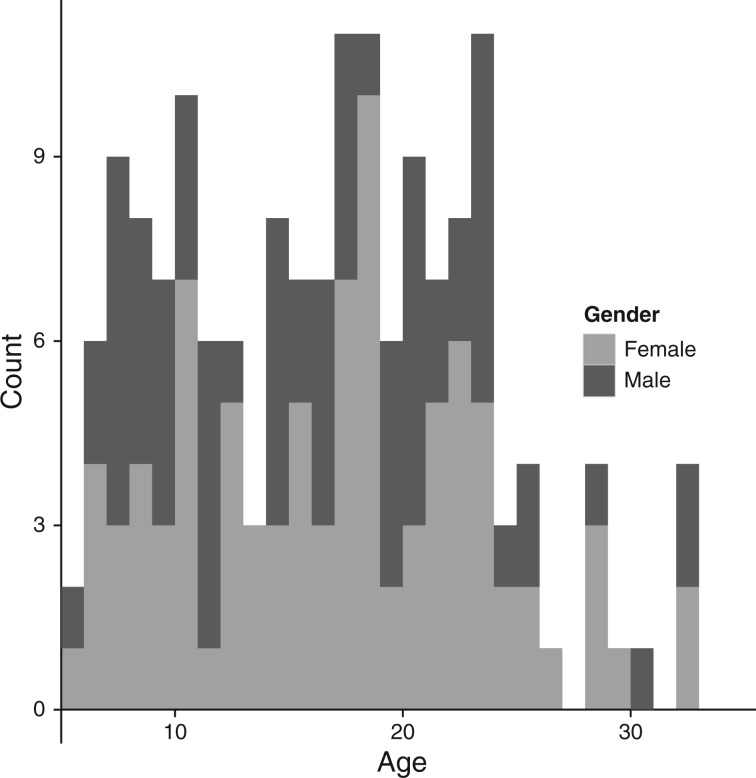
Imaging data was collected from 155 children, adolescents and young adults {88 female; age range: 5.40–32.8 years, mean age: 16.59, s.d. = 6.65 years; [55 children (5.4–13.0 years); 36 adolescents (13.1–18.0 years); 69 adults (>18.0 years)]; see Figure 1 for the sample distribution} over the course of 5.5 years (December 2008 to July 2014). Participants were scanned at the same site on either a GE Signa 3T (n = 61) or a SIEMENS Magnetom 3T (n = 94). Data was excluded for too much head motion (defined as no more than 10% of echoplanar imaging (EPI) volumes censored with a threshold of half a voxel movement).
Fig. 1.
Histogram representing the distributions of age and gender for participants in the study.
Non-target trial data (not examined in this manuscript) from a subset of this sample has been published previously (Hare et al., 2008; Somerville et al., 2011; Dreyfuss et al., 2014). No data has been reported examining functional connectivity from this full sample. All participants provided informed written consent (parental consent and subject assent for children and adolescents) approved by the institutional review board of Weill Cornell Medical College.
Behavioral paradigm
Participants completed six runs of a go/no-go task using fearful, happy and calm facial expressions as target (go) or non-target (no-go) stimuli. Within each run, two types of facial emotions were presented, one serving as the target (go) stimulus, to which they were instructed to press a button, and the other serving as a non-target (no-go) stimulus, for which they were instructed to withhold a button press. Facial expressions were pseudorandomized across the run to control for presentation order, and all combinations of expression were used as both targets and non-targets, resulting in a 2 (response: go, no-go) × 3 (emotion: fear, calm, happy) factorial design. All participants experienced all six conditions in the same scanning session. Prior to each run, participants were instructed as to which expression served as the target (go) stimulus and that they should respond with a button press only to that expression. Participants were also instructed to respond as fast as possible but to try to avoid making errors.
Stimuli and apparatus
The stimuli consisted of fearful, happy and calm faces from the NimStim set of facial expressions (Tottenham et al., 2009). Calm (i.e. mildly pleasant neutral) faces were used because developmental populations may perceive neutral faces as negative (Gross and Ballif, 1991; Thomas et al., 2001; Herba and Phillips, 2004). Button responses and reaction times were logged using E-Prime software.
Task parameters
The data was acquired in six functional imaging runs that combined each emotion (happy, calm and fear) and response (go and no-go; Figure 1) using a rapid event-related design. On each trial, a face appeared for 500 ms followed by a jittered intertrial interval between 2 s and 14.5 s (mean: 5.2 s) during which participants were presented with a fixation crosshair. A total of 48 trials were presented per run in pseudorandomized order (36 go and 12 no-go). A total of 72 go and 24 no-go trials were acquired for each expression type.
Image acquisition
Data from participants scanned on the GE used a quadrature head coil; the SIEMENS, a 12-channel head coil. On the GE, a high-resolution, T1-weighted anatomical scan (256 × 256 in-plane resolution, 240-mm field of view, 120 1.5-mm axial slices) was acquired for each subject for transformation and localization of data to MNI space. A spiral in and out sequence was used to acquire EPI functional data (repetition time= 2500 ms, echo time = 30 ms, flip angle = 90, skip 0, 64 × 64 matrix). In all, 34 4 mm-thick coronal slices (3.125 × 3.125 mm resolution) covering the entire brain except for the posterior portion of the occipital lobe were acquired. On the SIEMENS, a high resolution, T1-weighted anatomical scan (256 × 256 in-plane resolution, 240-mm field of view, 160 1.2-mm sagittal slices) was acquired for each subject for transformation and localization of data to MNI space. SIEMENS EPI data consisted of 34, 4 mm thick slices through the coronal plane with a 3.125 × 3.125mm in plane resolution covering the entire brain (repetition time = 2500 ms, TE = 30 ms, flip angle = 90, 64 × 64 matrix).
Behavioral analysis
Behavioral data from the emotional go/no-go task was analyzed for accuracy and cognitive control using d-prime. D-prime incorporates both accuracy in hits and false alarms. To examine the development of affective behavior generally, we examined brain-behavior associations by collapsing across happy and fear cues to compute d-prime across valence. To ensure that no one valence was driving brain-behavior effects, we also examined these associations for each valence separately. D-prime was calculated by subtracting normalized false alarm rate from normalized go accuracy using z-scores. Accuracy rate, false alarm rate and reaction times are reported for comparison. In all analyses, behavior in response to neutral cues was controlled for when examining associations between d-prime to emotional cues and connectivity.
Imaging analysis
A study-specific template from subjects’ T1s was created using ANTS (Avants and Gee, 2004; Klein et al., 2009) with a resolution of 1 mm3 based upon the MNI 152 atlas. For each subject, a linear transformation (FLIRT) and a non-linear, diffeomorphic transformation (ANTS) were performed to spatially register each participant’s T1 to the template. The transformation matrix was saved for use with functional data. For functional connectivity analyses using structural information as a covariate (to examine whether effects could be accounted for by differences in anatomical structure), the log of the Jacobian determinant was extracted using within the VS and amygdala masks described below. The Jacobian determinant is a metric representing how much a voxel had to expand or contract to fit the template. It takes the form of a ratio with 1 being no change in size for that voxel to fit the template.
Functional imaging data preprocessing was performed using Analysis of Functional NeuroImages (AFNI). Functional imaging data was slice-time corrected, realigned and coregistered with each participant’s T1. Normalization of EPI data was performed as follows: A study specific template was created (based on the MNI 152 template brain) using a combination of FSL’s FLIRT and ANTS in which FLIRT was used for linear registration (rigid-body), whereas ANTS was used for non-linear registration. Once the study specific template was created, each participant’s T1 was normalized to this study specific template in two steps: using FLIRT and ANTS. Both the linear and non-linear transformation matrices were saved and were subsequently applied to the EPI data (again using FLIRT and ANTS) for registration of EPI data to the template. EPI data was smoothed 6 mm FWHM using 3dFWHMx). AFNI’s 3dFWHMx allows for the accurate estimation of cluster size necessary for multiple comparison correction using 3dClustSim. Within-subjects connectivity analyses were performed using the beta-series method (Rissman et al., 2004) and bilateral amygdala and ventral striatal seed ROIs from the Harvard-Oxford atlas at a 25% threshold (Desikan et al., 2006). Connectivity analyses were performed using the beta series correlation method described in (Rissman et al., 2004). Briefly, this approach requires that separate parameter estimates (beta values) be computed for each ‘correct’ trial time-locked to stimulus onset. Trials on which an incorrect response was made were modeled separately. Blood Oxygen Level Dependent (BOLD) responses during stimulus onset were modeled as brief epochs of neural activity convolved with a canonical hemodynamic response function. Nuisance covariates included the second-order polynomial used to model the baseline and slow signal drift, as well as six motion estimate covariates. Beta values were sorted by trial type (e.g. each happy-go trial has it’s own beta estimate). The extent to which brain regions interact during a particular task stage is quantified by the extent to which their respective beta series from that condition are correlated. Subsequently, a within subjects correlation was performed between the mean of the seed region (the amygdala) and every other voxel across the whole brain. For Amygdala-VS connectivity, the mean across both ROIs was computed separately and the within subjects correlation between these two regions was estimated for each condition. Error trials were grouped and modeled separately. We combined the happy and fear go trials to examine the development of affective processing at second-level analyses.
To examine associations of subcortical connectivity with development and behavior, we regressed amygdala-VS connectivity in response to valenced cues on age (collapsing across happy and fear cues). Followed-up analyses examined whether connectivity between these subcortical regions predicted behavior (d-prime). Scanner type is controlled for in all analyses, and connectivity to calm cues was included as a covariate to test the specificity of the findings to the emotional cues.
To examine development of pre-frontal regulatory control of subcortical processing, we performed a voxelwise connectivity analysis using the amygdala as the seed and regressed this on d-prime. Voxel-wise data was thresholded at P < 0.005 (k > 60 2 mm3 voxels), which corresponds to a significance level of P < 0.05 corrected for multiple comparisons based on Monte Carlo simulation across the whole brain. Monte Carlo simulations were conducted using AFNI’s 3dClustSim program (http://afni.nimh.nih.gov/).
Lastly, we examined if cortical-subcortical connectivity mediated the effect of subcortico-subcortical connectivity on behavior regardless of age. We reasoned that one role for cortical-subcortical connectivity in the regulation of emotion and behavior may be via mediating the association between subcortical connectivity and behavior. As such, we performed a Sobel mediation test in which amygdala-VS connectivity to emotional cues was the independent variable, d-prime to emotional cues was the dependent variable and mPFC-amygdala connectivity to emotional cues was the mediator.
Results
Behavioral results
Emotional cue-triggered cognitive control (d-prime) was associated with age (r = 0.46, t(154) = 6.42, P<0.001) such that d-prime (performance) improved with age. The association between emotional cue-triggered cognitive control and age remained significant after controlling for d-prime to calm cues [B = 0.02, t(153) = 1.75, P = 0.04 (one-tailed)]. There was no significant association between date of the scan and behavior (P = 0.34), and including scanner site in the analysis did not attenuate the association between behavioral performance and age (B = 0.097, t(153) = 5.7, P<0.001). This suggests that neither when the scan was run nor which scanner was used had any effect on behavioral performance in the task. Accuracy, RT and false alarm rate to emotional cues were each also associated with age (Accuracy: r = 0.34, t(154) = 4.57, P<0.001; RT: r = −0.42, t(154) = −5.83, P<0.001; False Alarm Rate: (r = 0.46, t(154) = −6.37, P<0.001), indicating that across these additional measures, cognitive control increased and accuracy increased with age, respectively.
Imaging results: development of subcortico-subcortical connectivity
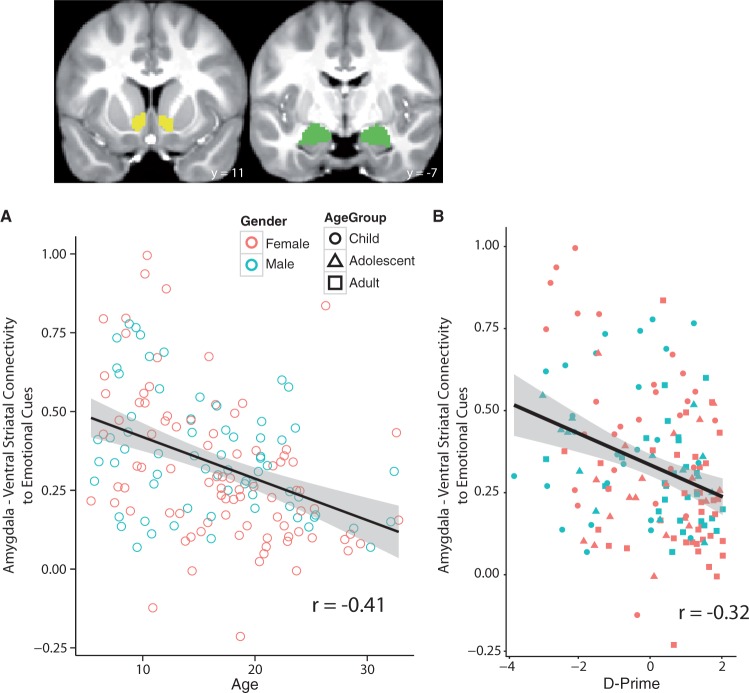
We examined associations between subcortico-subcortical connectivity and age. Amygdala-VS connectivity to emotional cues (the happy and fear go cues combined) showed a linear effect by age (Figure 2; B = −0.01; t(153) = −5.50; P<0.001). This association with age was significant when controlling for scanner type and amygdala-VS connectivity to calm cues (B = −0.004; t(151) = −2.11; P = 0.04). This effect appeared to be primarily a linear effect as including the quadratic term (Age2) in the regression was not significant (P = 0.34) and the linear term remained significant (P = 0.003). A similar analysis examining amygdala-VS connectivity to the calm go cues, controlling for scanner type and connectivity when emotional cues were presented was not significant (B = −0.003; t(151) = −1.32; P = 0.19), suggesting that this connectivity is not completely attributable to overall changes in connectivity. Associations between amygdala-VS connectivity and age were also significant when examining subcortico-subcortical connectivity to the happy cues (r = −0.40, t(153) = −5.45, P<0.001) and the fear (r = −0.40, t(153) = −4.02, P<0.001) cues independently, suggesting that one specific valence did not drive these effects.
Fig. 2.
Subcortical-subcortical (amygdala-ventral striatal [VS]) connectivity is associated with age and behavior during the imaging task. (A) Amygdala-VS connectivity to the emotional target (i.e the “go”) cues decreases linearly as a function of age (B = −0.01; t(153) = −5.50; P<0.001). (B) This subcortical-subcortical connectivity in response to emotional cues predicts cue-triggered cognitive control, with higher amygdala-VS connectivity predicting less cognitive control as measured by d-prime (r = −0.32, t(151) = −4.16, P<0.001). For age groups—children: less than 13.1 years [n = 55 participants], adolescents: 13.1–18.0 years [n = 36 participants], adults: greater than 18.0 years [n = 69 participants].
To further examine whether this developmental subcortical connectivity effect could be accounted for by differences in anatomical structure, we performed a regression in which we utilized the mean Jacobian determinant of amygdala and the mean Jacobian determinant of the VS in the same regression. Including these two variables in the model (while also controlling for connectivity to neutral ‘calm’ cues) did not attenuate the association between amygdala-VS connectivity to emotional cues and age (B = −0.006; t(150) = −3.10; P = 0.002). Taken together, these data suggest that over development, amygdala-VS functional connectivity in response to emotional cues becomes less positive and cannot be accounted for by structural changes in the size of amygdala or VS.
Imaging results: associations between subcortico-subcortical connectivity and behavior
We next tested whether subcortico-subcortical connectivity extended to emotional cue-triggered cognitive control. Amygdala—VS connectivity to emotional cues predicted d-prime to emotional cues (Figure 3; r = −0.32, t(151) = −4.16, P<0.001), indicating that greater amygdala—VS connectivity to emotional cues results in worse behavioral performance. Despite the high correlation between d-prime and age (r = 0.46), this association remained when including age as a covariate [B = −0.02, t(150) = −1.95, P = 0.026 (one-tailed)]. To examine whether this association was specific to emotional stimuli we performed a regression that included scanner type and amygdala—VS connectivity to calm cues as covariates. This association remained significant [B = −0.02, t(149) = −1.92, P = 0.023 (one-tailed)], indicating that the association between subcortico-subcortical connectivity to emotional cues and cognitive control is not due to general differences subcortico-subcortical connectivity. To further examine emotional specificity, we performed the same regression but examined the association of amygdala—VS connectivity to calm cues with d-prime to calm cues, now controlling for scanner type and connectivity to emotional cues. This analysis revealed that amygdala-VS connectivity to calm cues was not associated with d-prime to calm cues (B = 0.0008, t(149) = 0.08, P = 0.94). Once again, amygdala—VS connectivity to happy (r = −0.20, t(152) = −2.58, P = 0.01) and fear (r = −0.44, t(152) = 6.13, P<0.001) cues were independently associated with d-prime suggesting this effect was not driven by connectivity to a specific valence. Taken together, these data suggest that greater amygdala—VS connectivity to emotional cues irrespective of valence is predictive of poorer cognitive control as indexed by d-prime to emotional faces. We also performed this regression analysis including all covariates in the same model: we tested the association between d-prime to emotional cues and amygdala-VS connectivity to emotional cues including age, differences in normalization and scanning site as covariates in the model. The association was reduced, but remained [B = −0.02, t(147) = −1.72, P = 0.044 (one-tailed)], consistent with our original hypothesis.
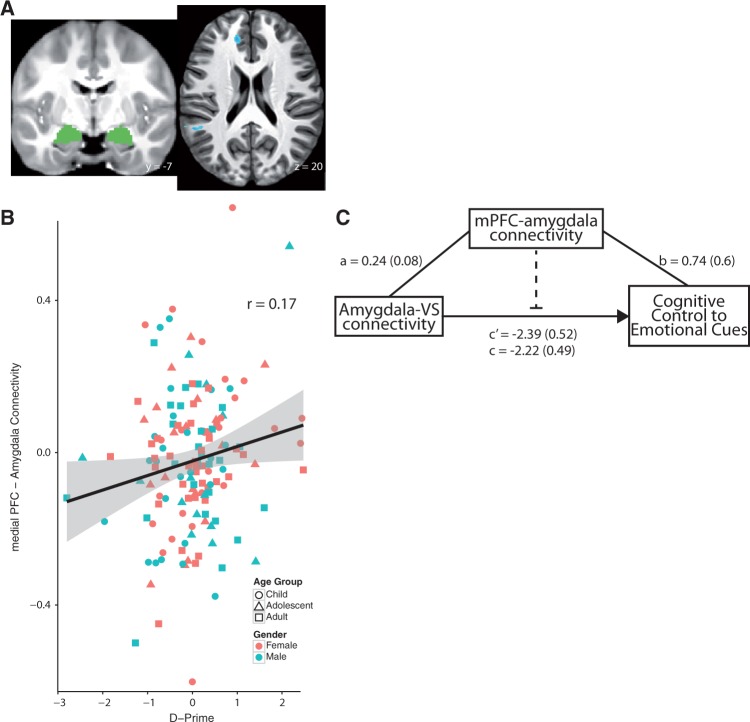
Fig. 3.
Cortical-subcortical connectivity predicts cognitive control to emotional cues. (A) Localization of mPFC-amygdala connectivity. The same amygdala ROI used in Figure 2 is used here to examine the effects of cortical-subcortical connectivity with behavior; (B) mPFC-amygdala connectivity is associated with enhanced cognitive control to emotional cues controlling for connectivity to calm cues; (C) A model of how cortical-subcortical connectivity between the medial PFC and amygdala mediates the relationship between subcortical-subcortical connectivity and cognitive control. Subcortical-subcortical connectivity is associated with less cognitive control (Figure 2), whereas greater cortical-subcortical (medial PFC-amygdala) connectivity is associated with better cognitive control. Values represent beta values.
To address whether this association was specific to amygdala-VS connectivity, we performed the identical connectivity analyses as that described above, but examined connectivity between the amygdala and the dorsal striatum (a bilateral dorsal striatum ROI from the same Harvard-Oxford anatomical mask—see Materials and methods). Including connectivity to calm cues as a covariate, there was not a significant association between amygdala-dorsal striatum connectivity to emotional cues and age (B = −0.0018, t(142) = −1.08, P = 0.28). This suggests that the association between amygdala-VS connectivity to emotional cues and behavior is specific to the ventral portion of the striatum.
Imaging results: cortico-subcortical connectivity
We then examined the association between cortical-subcortical (PFC-amygdala) connectivity with d-prime. A whole-brain voxelwise regression, examining amygdalar connectivity in response to emotional (happy and fear cues combined) cues vs calm cues was regressed on d-prime to emotional vs calm cues. When correcting for multiple comparisons, an area of medial PFC (mPFC) was significant such that the greater connectivity between the amygdala and this region of mPFC on emotional cues, the better the d-prime toward emotional cues (Figure 3B; max x, y, z = [14, 40, 18], BA 24/32; r = 0.17, t(148) = 2.1, P = 0.037). To ensure this was not due to an effect of outliers, we removed potential d-prime outliers (mean +/− 3 s.d.). This did not attenuate the relationship between amygdala-mPFC connectivity and d-prime (r = 0.16, t(147) = 2.025, P = 0.045). Using the mPFC supra-threshold cluster ROI from the voxelwise analyses, the association between amygdala-mPFC connectivity and d-prime remained significant when including age and scanner type as covariates (B = 0.04, t(146) = 2.11, P = 0.04).
To examine specificity of the mPFC-amygdala connectivity, we also examined whether mPFC-VS connectivity predicted d-prime. Including age and scanner as covariates, mPFC-VS connectivity in response to emotional cues was not significantly predictive of d-prime (B = −0.01, t(146) = −1.62, P = 0.11), although was in the same (positive) direction as the mPFC-amygdala association.
Imaging results: mediation of cortical-subcortical connectivity on subcortico-subcortical connectivity on behavior
We explicitly examined if cortical-subcortical connectivity mediated the effect of subcortico-subcortical connectivity on behavior regardless of age. We performed a Sobel mediation test in which amygdala-VS connectivity to emotional cues was the independent variable, d-prime to emotional cues was the dependent variable and mPFC-amygdala connectivity to emotional cues was the mediator. Mediation was calculated two ways, first was with no covariates included and second with covariates including connectivity to calm cues, behavior to calm cues, age and scanner type. In both instances, the mediation was significant: MPFC-amygdala connectivity significantly mediated the association between amygdala-VS connectivity and d-prime in response to emotional cues, controlling for age (Sobel test = 2.15, P = 0.03; Figure 3C). This provides further evidence for an effect of subcortical connectivity on motivated behavior that is regulated in part by cortical-subcortical connectivity.
Discussion
Marked changes in the capacity to override motivated behaviors occur during adolescence. A unidirectional subcortico-subcortical pathway from the amygdala to the VS modulates cue-triggered motivated behaviors in adult rodents (Stuber et al., 2011). However, our understanding of the interaction between these two brain regions during development in humans has not been well characterized. In the current study, we examined how engagement of this subcortico-subcortical circuit changes across development, how activity in this circuit impacts cognitive control to emotional triggers, and how pre-frontal modulation of this circuit may help to regulate emotional cognitive control.
We build on existing research indicating that cortical-subcortical neural circuits undergo dramatic functional changes across development (Steinberg, 2005; Ernst et al., 2006; Casey et al., 2008). In a large sample, we show that the magnitude of subcortico-subcortical connectivity in response to emotional cues decreased with age. Greater amygdala-VS connectivity was associated with poorer behavioral performance and cognitive control, regardless of age, to emotional cues but not neutral cues. In line with a role for engagement of cortical-subcortical networks in behavioral regulation, medial PFC-amygdala connectivity predicted better cognitive control in response to emotional cues. Importantly, the impact of subcortical-subcortical connectivity on behavior was mediated by magnitude of engagement of cortical-subcortical circuitry.
Developmental changes in motivated actions involve the refinement of multiple circuits that change in hierarchical and dynamic ways (Casey et al., 2016). The development of subcortico-subcortical and cortical-subcortical networks may have complimentary roles in motivated behavior across development. Previous work suggests that subcortical regions may functionally sensitize during adolescence (Monk et al., 2003; Galvan et al., 2006; Guyer et al., 2008; Geier et al., 2009; van Leijenhorst et al., 2010). These subcortical regions are regulated by the PFC (Hare et al., 2008; Somerville et al., 2011; Gee et al., 2013a). Here, we show how moving away from examination of isolated subcortical regions and towards connectivity within these subcortical circuits across development may provide a basis for changes in cognitive control to emotional cues that are modulated by cortical inputs.
Significant cortical reorganization occurs during development. At rest, this reorganization occurs as a change from a preponderance of short, local connections to long-range connectivity as individuals enter adulthood (Dosenbach et al., 2010; Di Martino et al., 2014). The emergence of a greater proportion of long-range connections may enhance cognitive control when emotional stimuli are present. Many studies examining connectivity over development have mapped these functional interactions using resting-state fMRI. Complementing these resting state fMRI studies are investigations requiring individuals to respond to salient stimuli. These paradigms challenge neural systems with emotional cues. These task-based paradigms also permit experimental control, an understanding of how neural circuits mature over development in response to salient stimuli, and ground patterns of connectivity to task-related behavior. Studies examining the development of cortical and subcortical connectivity at rest (e.g. Gee et al., 2013b; Gabard-Durnam et al., 2014; Fareri et al., 2015; Van Duijvenvoorde et al., 2015) show similar, but not identical patterns of results and suggest that resting state reveals an important, but incomplete picture of neural development. This study highlights the utility of a task-based approach that examines changes in functional connectivity over development.
It is important to note that inferring directionality of connectivity effects and distinguishing between inhibitory and excitatory influences using fMRI are not possible. Some evidence from fMRI studies has suggested that both negative (Hariri et al., 2003; Kim et al., 2003; Hare et al., 2008) and positive (Milad et al., 2007; Delgado et al., 2008; Linnman et al., 2011) cortical-subcortical connectivity likely reflect cortical influences on subcortical structures. Nonetheless, imaging methods in humans are inherently correlational and therefore we are unable to determine for certain whether the circuit level effects described here are cortical-to-subcortical or subcortical-to-cortical, or excitatory or inhibitory in nature. As such, we cannot conclude the directionality in which these circuits are impacting individual differences in behavior. Future work, particularly using approaches that allow for causal inferences, is needed to further elucidate the nature of cortical-subcortical changes as well as subcortico-subcortical connectivity in development and its associations with behavior.
Another primary limitation of the current study is that it is cross-sectional with no longitudinal data. As such, we are unable to identify precisely how maturation of these pre-frontal, amygdalar and striatal circuits is involved in affective processing, and behavior. For example, does the temporal course of engagement of these regulatory circuits precede, follow, or emerge concomitantly with the behavioral phenomena? Nascent and ongoing large-scale longitudinal studies such as the Adolescent Brain and Cognitive Development and Lifespan Human Connectome Projects are two such projects that will be able to test exactly these types of questions. A second limitation is that data was acquired from two separate scanners. That data from these two scanners was acquired at different points in time may have increased noise in our data simply by virtue of technological improvements having arisen when the newer (Siemens) scanner was installed with newer sequences. That said, there were no behavioral differences between sites and we attempted to account for this potential limitation by controlling for scanner in all analyses.
The present study examined the development and behavioral consequences of functional interactions within subcortical regions and between pre-frontal and subcortical regions. We demonstrate, in addition to developmental changes in subcortico-subcortical circuitry, that heightened connectivity between the amygdala and VS is predictive of poorer cognitive control to emotional stimuli across age. These findings are complemented by findings that enhanced cortical-subcortical functional connectivity is associated with greater cognitive control and that the association between subcortico-subcortical connectivity and cognitive control is mediated by cortical-subcortical connectivity. Overall, the findings suggest that traditional, valence-based accounts of subcortical functioning, assessing the role of the amygdala and VS independently, do not fully capture the dynamic functional changes that occur across development (Casey et al., 2016). Changes in functional maturation of subcortical-subcortical and cortical-subcortical interactions complement one another and simultaneously contribute to behavioral responses to emotional stimuli. Examining functional connectivity within and between these networks across development and grounding these associations with specific measures of task-related behavior will facilitate characterization of the neural development pertaining to cognitive control.
Funding
This work has been supported by NIMH P50 MH 079513 (BJC), a Medical Scientist Training Program grant to M.D. (T32 GM007739), and a National Science Foundation Graduate Research Fellowship award to A.O.C.
Conflict of interest: None declared.
References
- Avants B., Gee J.C. (2004). Geodesic estimation for large deformation anatomical shape averaging and interpolation. NeuroImage, 23(Suppl 1), S139–50. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Smits K., Van Ree J.M. (2002a). Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. Journal of Comparative Neurology, 450, 241–55. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Wolterink G., van Ree J.M. (2002b). Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. Journal of Comparative Neurology, 442, 239–49. [DOI] [PubMed] [Google Scholar]
- Casey B.J. (2016). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66, 295–319. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. (2008). The adolescent brain. Developmental Review, 28, 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Galván A., Somerville L.H. (2016). Beyond simple models of adolescence to an integrated circuit-based account: A commentary. Developmental Cognitive Neuroscience, 17, 128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gilbert J.E., Thomas K.M. (2013). Inhibitory control during emotional distraction across adolescence and early adulthood. Child Development, 84, 1954–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman V.L., Balaban J., Steinfeld S., et al. (2010). Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. Journal of Comparative Neurology, 518, 2693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. (2002). Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. Journal of Comparative Neurology, 453, 116–30. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Nearing K.I., Ledoux J.E., Phelps E.A. (2008). Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron, 59, 829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31, 968–80. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Fair D.A., Kelly C., et al. (2014). Unraveling the miswired connectome: a developmental perspective. Neuron, 83, 1335–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincheva I., Drysdale A.T., Hartley C.A., et al. (2015). FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nature Communications, 6, 6395.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Nardos B., Cohen A.L., et al. (2010). Prediction of individual brain maturity using fMRI. Science, 329, 1358–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss M., Caudle K., Drysdale A.T., et al. (2014). Teens impulsively react rather than retreat from threat. Developmental Neuroscience, 36, 220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. (2006). Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine, 36, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Gabard-Durnam L., Goff B., et al. (2015). Normative development of ventral striatal resting state connectivity in humans. NeuroImage, 118, 422–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., et al. (2014). The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage, 95, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., et al. (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience, 26, 6885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., et al. (2013a). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America, 110, 15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L., Telzer E.H., et al. (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science, 25, 2067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., et al. (2013b). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience, 33, 4584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F., Terwilliger R., Teslovich T., Velanova K., Luna B. (2009). Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex, 20, 1613–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J. N., Raznahan A., Alexander-Bloch A., Schmitt E., Gogtay N., Rapoport J. L. (2015). Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40, 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A.-L., Mills K. L., Clasen L. S., Giedd J. N., Viner R. M., Blakemore S.-J. (2014). The influence of puberty on subcortical brain development. NeuroImage, 88, 242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101, 8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose-Fifer J., Rodrigues A., Hoover S., Zottoli T. (2013). Attentional capture by emotional faces in adolescence. Advances in Cognitive Psychology, 9, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A.L., Ballif B. (1991). Children’s understanding of emotion from facial expressions and situations: a review. Developmental Review, 11, 368–98. [Google Scholar]
- Guyer A., Monk C., McClure-Tone E., et al. (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20, 1565–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63, 927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Fera F., Weinberger D.R., James W. (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry, 53, 494–501. [DOI] [PubMed] [Google Scholar]
- Herba C., Phillips M. (2004). Annotation: development of facial expression recognition from childhood to adolescence: behavioural and neurological perspectives. Journal of Child Psychology and Psychiatry, 45, 1185–98. [DOI] [PubMed] [Google Scholar]
- Kim H., Somerville L.H., Johnstone T., Alexander A.L., Whalen P.J. (2003). Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport, 14, 2317–22. [DOI] [PubMed] [Google Scholar]
- Klein A., Andersson J., Ardekani B.A., et al. (2009). Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage, 46, 786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L., Hare T.A., Voss H.U., Glover G., Ballon D.J., Casey B.J. (2009). The bivalent side of the nucleus accumbens. NeuroImage, 44, 1178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Schiller D., Schoenbaum G., Phelps E.A., Daw N.D. (2011). Differential roles of human striatum and amygdala in associative learning. Nature Neuroscience, 14, 1250–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C., Zeffiro T.A., Pitman R.K., Milad M.R. (2011). An fMRI study of unconditioned responses in post-traumatic stress disorder. Biology of Mood and Anxiety Disorders, 1, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR., Wright C.I., Orr S.P., Pitman R.K., Quirk G.J., Rauch S.L. (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry, 62, 446–54. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.-L., Clasen L.S., Giedd J.N., Blakemore S.-J. (2014). The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience, 36(3–4), 147–60. [DOI] [PubMed] [Google Scholar]
- Monk C.S., McClure E.B., Nelson E.E., et al. (2003). Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage, 20, 420–8. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Telzer E.H., Mogg K., et al. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65, 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. (2006). Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience, 9, 1004–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J.J., Belova M.A., Morrison S.E., Salzman C.D. (2006). The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature, 439, 865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Pelphrey K.A. (2011). Developing connections for affective regulation: age-related changes in emotional brain connectivity. Journal of Experimental Child Psychology, 108, 607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Likhtik E., Pelletier J.G., Paré D. (2003). Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. Journal of Neuroscience, 23, 8800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J., Gazzaley A., D’Esposito M. (2004). Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage, 23, 752–63. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. (2011). Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience, 23, 2123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. (2005). Cognitive and affective development in adolescence. Trends in Cognitive Sciences, 9, 69–74. [DOI] [PubMed] [Google Scholar]
- Steinberg L. (2010). A dual systems model of adolescent risk-taking. Developmental Psychobiology, 52, 216–24. [DOI] [PubMed] [Google Scholar]
- Stuber G.D., Sparta D.R., Stamatakis A.M., et al. (2011). Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature, 475, 377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K.M., Drevets W.C., Dahl R.E., et al. (2001). Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry, 58, 1057–63. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., et al. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168, 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duijvenvoorde A.C.K., Achterberg M., Braams B.R., Peters S., Crone E.A. (2015). Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. NeuroImage, 124(Pt A), 409–20. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L., Zanolie K., Meel C.S.V., Westenberg P.M., Rombouts S.A.R.B, Crone E.A. (2010). What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex, 20, 61–9. [DOI] [PubMed] [Google Scholar]
- Verwer R., Van Vulpen E., Van Uum J. (1996). Postnatal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. Journal of Comparative Neurology, 376, 75–96. [DOI] [PubMed] [Google Scholar]