Abstract
Negative life events (NLE) contribute to anxiety and depression disorders, but their relationship with brain functioning in adolescence has rarely been studied. We hypothesized that neural response to social threat would relate to NLE in the frontal–limbic emotional regions. Participants (N = 685) were drawn from the Imagen database of 14-year-old community adolescents recruited in schools. They underwent functional MRI while viewing angry and neutral faces, as a probe to neural response to social threat. Lifetime NLEs were assessed using the ‘distress’, ‘family’ and ‘accident’ subscales from a life event dimensional questionnaire. Relationships between NLE subscale scores and neural response were investigated. Links of NLE subscales scores with anxiety or depression outcomes at the age of 16 years were also investigated. Lifetime ‘distress’ positively correlated with ventral-lateral orbitofrontal and temporal cortex activations during angry face processing. ‘Distress’ scores correlated with the probabilities of meeting criteria for Generalized Anxiety Disorder or Major Depressive Disorder at the age of 16 years. Lifetime ‘family’ and ‘accident’ scores did not relate with neural response or follow-up conditions, however. Thus, different types of NLEs differentially predicted neural responses to threat during adolescence, and differentially predicted a de novo internalizing condition 2 years later. The deleterious effect of self-referential NLEs is suggested.
Keywords: adolescence, anxiety, depression, fMRI, negative life events, social threat
Introduction
Negative life events (NLEs) occurring during childhood are among the strongest predictors of internalizing disorders, especially Generalized Anxiety Disorder (GAD) and Major Depressive Disorder (MDD) (Vollebergh et al., 2001).
Most reports of a relation between NLE and brain correlates have mainly documented severe chronic events such as psychosocial deprivation, or child abuse as a paradigm of repeated traumatic events. For instance, in severely neglected children, alterations in brain structure have been detected in amygdala and hippocampus (Hanson et al., 2015), corpus callosum, frontal regions and striatum (Bick et al., 2015), all of which being regions that contribute to emotional regulation. Similarly, a history of childhood abuse has inconsistently been related with volume changes in amygdala, hippocampus, orbitofrontal or prefrontal cortex in children and adults (Hanson et al., 2010).
Functional alterations have also been reported in youths with emotional neglect aged 9–18 years, in the left amygdala and right anterior hippocampus, during an angry face processing task (Maheu et al., 2010). In childhood maltreatment, atypical activation of amygdala, anterior cingulate cortex (ACC) and frontal regions have been found (McCrory et al., 2011) using tasks notably tapping the responsiveness to threat-related facial expressions (Dannlowski et al., 2012). In addition, exposure to enduring childhood abuse and neglect during sensitive periods has been related with marked effects in regions involved in the pathophysiology of depressive disorders (Teicher and Samson, 2016).
However, more common NLEs have rarely been considered. It has been suggested that the trauma type might moderate the regional response (Grant et al., 2011), supporting the need to investigate the type of NLE, but it is still unclear whether findings of atypical emotional processing may generalize to juveniles who have experienced other, more common, NLE.
Besides child abuse or neglect, other NLE can also contribute to the vulnerability to depression (Green et al., 2010). Indeed, NLE such as life-threatening diseases, sudden death of a loved one, parent separation, dysfunctional home, or poverty, have been emphasized in stress models during adolescence (Compas et al., 1993). The overwhelming of the coping capacities may ultimately lead to symptoms of anxiety, depression and loss of control (Dohrenwend et al., 1980; Veit and Ware 1983), as well as to poor self-esteem (Isomaa et al., 2013). These NLE could impact the functioning of the middle frontal cortex in children (Demir-Lira et al., 2016). Still, the long-term relation of those less traumatic NLE with brain function during adolescence is yet to be determined.
Here, we hypothesized that previous exposure to these less traumatic NLE would relate with lasting functional cerebral changes in emotional processing. To investigate this relationship, we measured the NLE using the Newcomb Questionnaire (LEQ) (Newcomb et al., 1981), and assessed emotional processing using functional magnetic resonance imaging (fMRI) and a face task. We also hypothesized that adolescents with high NLEs scores at the age of 14 years would be more prone to develop anxiety or depressive disorders at follow-up at the age of 16 years.
The LEQ clusters 39 life events specific to adolescence. It encompasses seven subscales with positive, mixed or negative valence. In order to focus on events with negative valence, we retained the ‘distress’, ‘family’ and ‘accident’ subscales. The ‘distress’ scale includes self-referential NLE (e.g. physical appearance and academic achievement) inducing low self-esteem. The scale ‘family’ includes items relating to problems within the family, and the scale ‘accident’ includes items regarding accidents or illness, both scales reporting external threatening events.
We used an fMRI paradigm involving angry face processing, a task that has recurrently been studied in depressive patients (Monk et al., 2006). Angry faces are indeed a robust social threat signal (Grosbras and Paus, 2006). Angry faces processing involves limbic regions, such as insula, amygdala, ACC and prefrontal cortices including ventrolateral prefrontal (VLPFC) and orbitofrontal cortex (OFC) (Fusar-Poli et al., 2009). These regions have been implicated in the pathophysiology of depressive disorders (Price and Drevets, 2010).
We analyzed a large sample of 14-year-old community adolescents carefully screened for the absence of psychiatric conditions to avoid potential confounding effects of the neural correlates of pathophysiology (Bick and Nelson 2016).
Our first aim was to investigate whether the neural response to viewing facial signals of anger was modulated by variation in lifetime experienced NLE. We studied whether the different kinds of NLE would differentially modulate the neural response. Based on the literature above, we a priori hypothesized that the ‘distress’, ‘family’ or ‘accident’ subscales scores would correlate with the frontal and limbic regions (OFC, ACC and amygdala).
Second, we examined whether the lifetime ‘distress’, ‘family’ or ‘accident’ scores assessed in those healthy adolescents at the age of 14 years would correlate with the development of a diagnosis of GAD or MDD at the age of 16 years. Indeed, at variance with other anxiety disorders, both disorders are closely related because generalized anxiety often precedes, associates with or follows MDD (Moffitt et al., 2007).
Material and methods
Participants selection and assessment
Participants were drawn from the IMAGEN database that combines behavioural and neuropsychological characterization, functional and structural neuroimaging data in 2223 adolescents recruited in middle schools between the age 13 and 15 years. A detailed description of recruitment and research procedures has been published elsewhere (Schumann et al., 2010). Particularly, clinical and behavioural assessments—except for diagnostic assessment—were performed using Psytools software (Delosis Ltd, London, UK) via its Internet-based platform. The assessment battery of questionnaires and cognitive tasks was self-administered both in participants’ homes and at the neuroimaging facilities. Participants and their parents provided written informed consent and assent. Ethics committees of all participating institutions approved the study. Consistently with the legislation, French adolescents were not financially compensated for their participation.
Using the following inclusion criteria, 685 healthy adolescents (368 girls) were found eligible for the present study. First, none of them met criteria for a psychiatric disorder according to their diagnostic ratings using the DAWBA (Development and Well-Being Assessment Interview, www.dawba.com, Goodman et al., 2000). The DAWBA is a computerized self-report assessment completed by the participants and their parents that generates computerized probability levels of meeting DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) and ICD-10 (International Statistical Classification of Diseases and Related Health Problems) diagnoses, called ‘DAWBA bands’ (Goodman et al., 2011; Supplementary Methods). Second, in order to minimize the multisite variability in fMRI datasets due to scanner manufacturers, only participants who were scanned using a Siemens scanner (Paris, Mannheim, Hamburg and Dresden) were included, after visual quality control of their images (Supplementary Table S1). Alcohol and drug consumptions were, respectively, assessed with the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993) and the ESPAD (European School Survey Project on Alcohol and Drugs, www.espad.org; Paschall et al., 2009). Participants with an AUDIT score higher than 6 were excluded (Conigrave et al., 1995), as well as participants who had tried a substance more than once in their lifetime, except for cannabis whose exclusion criterion was more than five times during lifetime. Consumption of any drug during the month preceding the scanning (except for tobacco) was also an exclusion criterion. In addition, adolescents treated with psychoactive drugs or having acute medical conditions at the time of the study were excluded.
Pubertal Developmental Status (PDS) scores were computed according to a specific self-rated questionnaire (Petersen et al., 1988).
NLE assessments
Lifetime NLEs were assessed using the LEQ administered on line. This multidimensional self-questionnaire validated in adolescents (Newcomb et al., 1981) allows screening for positive and NLEs through 39 items. The authors individualized seven subscales from the factorial analysis of the 39 items: ‘family’, ‘accident’, ‘distress’, ‘autonomy’, ‘deviance’, ‘sexuality’ and ‘other’. For each item, the desirability and the occurrence are noted. The desirability of each item permits to label each life event into a positive or a negative one. As we were interested in life event stressors, we only considered the subscales with NLEs. The subscales with mixed life events, such as the ‘sexuality’ scale, were not analysed. Three subscales were retained for analysis, including the ‘distress’ NLE subscale with six items (‘Face broke out with pimples', ‘Started seeing a therapist', 'Thought about suicide', ‘Ran away from home', ‘Got poor grades in school' and ‘Gained a lot of weight'), the ‘family’ subscale with five items (‘Parents divorced', ‘Family had money problems', ‘Parents argued or fought', ‘Parent remarried' and 'Parent abused alcohol') and the ‘accident’ subscale with four items (‘Family accident or illness', ‘Given medication by physician', ‘Death in family' and 'Serious accident or illness'). Each participant rated the lifetime occurrence of each event with ‘never’ rated ‘0’ and ‘ever’ rated ‘1’. For each subscale, the sum of the events lifetime scores was computed.
Follow-up assessment
Neuroimaging acquisitions were not repeated. Participants were re-assessed 2 years after completion of the baseline study, using the same web-based self-report questionnaires as at baseline (DAWBA, LEQ, PDS, AUDIT and ESPAD). DAWBA bands were available for 523 adolescents (274 girls) aged 16.11 ± 0.58 years.
MRI data acquisition
Task design
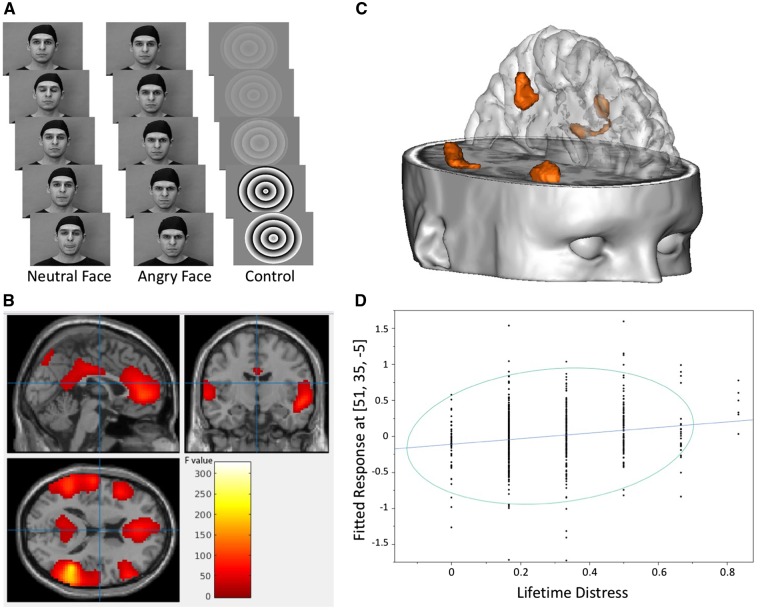
We used the Face task (Figure 1A), an fMRI task displaying dynamic angry faces derived from Grosbras and Paus (2006), that we previously used in another study with adolescents (Tahmasebi et al., 2012). Two male and two female young actors were employed to enhance empathy and identification (Somerville et al., 2010). Participants were requested to passively view a sequence of short (2-5s) black and white video clips with three conditions (neutral, angry and control). Video clips were preferred to static faces as they allow a better recruitment of cerebral regions implicated in facial processing (Arsalidou et al., 2011). The first two conditions were organized in two blocks of 18 seconds each. Each block includes four to seven video clips depicting faces. The faces were always neutral at the beginning and then either turned angry or stayed neutral (e.g. blinking their eyes, opening their mouth and twitching their nose). In both conditions, the actor’s gaze was direct and forward.
Fig. 1.
(A) Face task. Blocks of videos with faces turning from neutral to angry expression or staying neutral intercalated with blocks of control stimuli. Printed with permission from Grosbras and Paus (2006). (B). F test. Statistical parametric map showing significant neural response during the ‘angry vs neutral’ comparison of the Face task in 685 healthy adolescents (P < 0.05, family-wise error (FWE) corrected for multiple tests and a cluster size >200 voxels). Color bar indicates F values. (C) Clusters of voxels (detailed in Table 2) where correlations were detected between the lifetime ‘distress’ NLE mean scores and the t-statistic activation maps during the contrast ‘angry minus neutral’. (D) Correlation plot between the lifetime ‘distress’ NLE scores and neural response at the peak voxel MNI (Montreal Neurological Institute) coordinates within the right ventrolateral prefrontal cortex (PFWE = 0.005).
The control condition was a control video clip adapted from a study by Beauchamp et al. (2002) and lasting 18 seconds with contracting and expanding concentric circles with different black-and-white contrasts matching the contrast and motion characteristics of the faces clips. These control images were not relevant to the present study as they were initially intended to account for neural activity associated with viewing non-biological motion, which is not the aim of the present study (Supplementary Figure S1 and Table S4).
Five blocks of angry faces and five blocks of neutral faces were presented during 6 minutes. They were interleaved with nine blocks of the control condition (Figure 1A).
Before the scanning, participants were asked to watch the video clips carefully and to lie very still during the task.
Imaging parameters and processing
Magnetic resonance images were obtained on 3 Tesla imaging systems (Trio; Siemens, Erlangen, Germany). To avoid large motions, the head was immobilized with fitting foam padding. Standardized hardware for visual stimulus presentation (Nordic Neurolab, Bergen, Norway) was used at all sites. A hundred and sixty volumes were collected per subject, each comprising 40 slices parallel to the anterior–posterior bicommissural line (2.4 mm thickness, 1 mm gap, RelaxationTime RT = 2.20 s, matrix: 642, echo time ET = 30 ms) at the spatial resolution of 3.4 mm isotropic and temporal resolution of 2.2 seconds.
FMRI pre-processing and single-subject analyses were performed within SPM8 (Statistical Parametric Mapping [SPM]; http://www.fil.ion.ucl.ac.uk/spm/) as described in Thyreau et al. (2012). Time series data were first corrected for slice-timing and then corrected for movement (spatial realignment) to the first volume and non-linearly warped on the Montreal Neurological Institute (MNI) space, using a custom EPI template. Images were then smoothed with a Gaussian Kernel of 5 mm full-width half maximum. Single-subject analyses were performed within the framework of the general linear model (GLM), using SPM default hemodynamic response function.
fMRI statistical analysis
In order to minimize multicentre and different scanner biases, we studied datasets acquired with 3T Siemens imagers with the same MRI sequences because they were implemented in most participating centers. Indeed, some reports have suggested that multicenter MRI studies should achieve higher compatibility among scanners by using equipments of the same manufacturer. Potential biases due to different scanner manufacturers have previously been described (Zou et al., 2005; Friedman et al., 2008; Reig et al., 2009) and exceed the bias due to multicentre scanning (Brown et al., 2011).
As sex differences in face processing during adolescence have been described (Schneider et al., 2011); an exploratory analysis was conducted to search for an interaction between LEQ scores and sex during the Face Task. As none was found, sex was added as a confounding covariate in the SPM random-effect model.
We used the ‘angry vs neutral’ comparison to specifically focus on the neural correlates of anger and to remove the brain activity related to face processing. Using SPM8 and the GLM, analyses were performed on the single-subject ‘angry vs neutral’ comparisons with lifetime ‘distress’ mean scores as covariate of interest, and sex, PDS scores, age and the four imaging centers as confounding covariates. The angry vs neutral global comparison (F test) was used to extract a regions-of-interest mask. Correlations between neural response during the angry minus neutral contrast and each NLE score were performed within that mask, leading to three different analyses (angry minus neutral and ‘distress’ score, angry minus neutral and ‘family’ score and angry minus neutral and ‘accident’ score).
All statistical maps were thresholded for voxel and cluster levels at P < 0.05 family-wise error corrected for multiple comparisons.
Correlations between NLE at the age of 14 years and psychiatric outcome at the age of 16 years
Diagnostic probabilities (i.e. DAWBA bands) derived from DAWBA self-ratings performed by the participants 2 years after the first scanning were retrieved to assess diagnostic outcomes. As the distribution of DAWBA bands was not Gaussian (after application of the Shapiro–Wilk test), we used the non-parametric Kendall rank correlation (two sided) to explore a potential association between lifetime LEQ scores (‘distress’, ‘family’ and ‘accident’) and the probability of meeting criteria for the following diagnoses: GAD and MDD. In addition, we performed a causal mediation analysis to inform on the putative impact of brain response to angry faces on the link between NLE and the follow-up diagnoses (Supplementary Material).
The participants’ descriptive and follow-up analyses were performed using R software (http://cran.r-project.org). Statistical significance was set at P < 0.05, two tailed.
Results
Participant characteristics
No significant differences in handedness, age, lifetime ‘family’, ‘accident’ and ‘distress’ mean scores were found between girls and boys (Table 1). As expected, PDS scores differed in boys and girls. Lifetime ‘family’ and ‘accident’ mean scores differed across imaging centers with lower scores in Paris adolescents (Supplementary Table S2). The ‘distress’ score correlated with the ‘family’ score (Pearson’s correlation test, r = 0.23, P < 0.001) and with the ‘accident’ score (r = 0.27, P < 0.001). The ‘family’ score also correlated with the ‘accident’ score (r = 0.26, P < 0.001).
Table 1.
Participant characteristics.
| Overall | Girls | Boys | Statistical | |
|---|---|---|---|---|
| N = 685 | N = 368 | N = 317 | Comparison girls vs boys | |
| Age, mean (s.d.) | 14.48 (0.46) | 14.49 (0.45) | 14.47 (0.45) | t = 0.58* |
| PDS score, mean (s.d.) | 2.89 (0.55) | 3.19 (0.38) | 2.25 (0.51) | t = 18.35 *** |
| ‘Distress’a score, mean (s.d.) | 0.29 (0.17) | 0.29 (0.18) | 0.28 (0.16) | t = 1.03* |
| ‘Accident’a score, mean (s.d.) | 0.54 (0.26) | 0.54 (0.26) | 0.53 (0.27) | t = 0.50* |
| ‘Family’a score, mean (s.d.) | 0.24 (0.22) | 0.25 (0.22) | 0.22 (0.22) | t = 1.58* |
| Handedness, right handers (N) | 612 | 336 | 277 | χ2 = 2.89* |
PDS, pubertal developmental status.
Subscales of the Life Event Questionnaire.
P > 0.05.
P < 0.001.
fMRI results
Angry vs neutral faces comparison (F test) was associated with neural response in a set of regions including the cingulate gyrus (anterior, middle and posterior), the right hippocampal gyrus, the inferior and middle bilateral frontal gyri, and the middle and superior temporal gyri (Figure 1B and Supplementary Table S3). These regions were used as a mask for the correlation analyses.
Correlations between neural response during the ‘angry-neutral’ comparison and lifetime ‘distress’ score were detected in the right VLPFC/OFC, the middle frontal gyrus in the dorsolateral prefrontal cortex (DLPFC) and in the bilateral middle and superior temporal gyri. No significant correlation was found between lifetime ‘distress’ mean scores and neural response in the ACC (Table 2 and Figure 1C and 1D). Finally, lifetime ‘family’ or ‘accident’ mean scores did not correlate with the neural response.
Table 2.
Correlations between neural response during the ‘angry vs neutral’ contrast and lifetime ‘distress’ NLE subscale score in 685 adolescents
| Brain regions | BA | MNI coordinatesa | Cluster sizeb | t Valuec df = 676 | P FWEd | ||
|---|---|---|---|---|---|---|---|
| x | y | z | Peak level | ||||
| R Inf frontal gyrus orbital part (VLPFC/OFC) | 47 | 51 | 35 | −5 | 47 | 4.54 | 0.005 |
| R Middle frontal gyrus (DLPFC) | 6 | 42 | 2 | 55 | 51e | 4.49 | 0.006 |
| R Middle frontal gyrus | 9 | 42 | 2 | 43 | …e | 4.44 | 0.007 |
| L Superior temporal gyrus (Wernicke) | 22 | −66 | −46 | 19 | 12 | 4.44 | 0.007 |
| R Middle temporal gyrus | 21 | 63 | −43 | 1 | 23 | 4.25 | 0.015 |
| L Middle temporal gyrus | 21/37 | −48 | −46 | 4 | 5 | 4.17 | 0.021 |
Montreal Neurological Institute (MNI) coordinates in millimetres for significant peak voxels, in the left–right, anterior-posterior and inferior-superior dimensions, respectively.
Cluster size is expressed in number of voxels, with voxel size = 9 mm3.
t refers to the t-score at those coordinates (local maxima).
Probabilities, Family Wise Error corrected for multiple tests.
Peaks in the same cluster.
FWE, family-wise error; BA, brodmann area; L, left; R, right; df, degrees of freedom.
Correlations between NLE at the age of 14 years and psychiatric outcome at follow-up (age 16 years)
The lifetime ‘distress’ mean score at baseline correlated with the probability of meeting criteria for GAD (P = 0.03, z = 2.13, τ = 0.08) and MDD (P = 0.05, z = 1.94, τ = 0.08) at follow-up.
No significant correlations were found between the lifetime ‘family’ or ‘accident’ mean scores at baseline and the follow-up probabilities of meeting criteria for GAD or MDD.
Post hoc mediation analysis
The neural responses to angry faces did not mediate the relationship between NLE scores at the age of 14 years, and GAD or MDD probabilities at follow-up (Supplementary Material, post hoc exploratory analysis).
Discussion
This is the first report of a relation between NLE and regional brain function in a cohort of 685 healthy 14-year-old adolescents during angry face processing, a stimulus tapping on threat detection (Feldmann-Wüstefeld et al., 2011). The different types of NLE were differentially associated with neural responses to social threat in those young adolescents, so that the higher the scores on the ‘distress’ NLE subscale, the more the neural response in frontal and temporal subregions. In contrast, no link was detected between the ‘family’ or ‘accident’ subscales and response to angry faces. Moreover, only the ‘distress’ score at the age of 14 years was related to diagnosis of a de novo internalizing condition, GAD or MDD, at the age of 16 years.
To our knowledge, only one fMRI study has investigated the impact of NLE on the adolescent healthy brain (Ganzel et al., 2013). At variance with our work, the authors used an emotional task (fearful face photographs) and an a priori-defined region-of-interest (ROI) approach in a sample of 14 adolescents aged 10–15 years and found that reactivity to emotional faces covaried with life events in multiple regions, including the amygdala, insula and prefrontal cortex. In contrast with the present study, they assessed severe external traumatic events such as sexual violence and did not explore self-referential NLE, at variance with the items of the LEQ ‘distress’ scale used here.
We used a much larger cohort with a smaller age range. A major methodological drawback of fMRI studies during adolescence is indeed the large age-range of the participants, leading to variability in developmental stage (Choudhury et al., 2006). Age at inclusion in the present study ranged between 13 and 15 years. Indeed, by considerably narrowing the age range and implementing pubertal status as a confounding covariate into the analyses, we expected to minimize such biases by stratifying the fMRI sample. This strengthens the robustness of the results. We also performed a whole-brain analysis, to grasp all the potential regions that would covariate with the studied NLE and not only the regions we a priori retained.
Here, the ‘distress’ NLE score was related with the neural response to angry faces in the middle frontal gyrus, in the superior and middle temporal gyrus, and in the temporoparietal junction. These regions are part of the so-called social brain (Blakemore, 2012) including the mentalizing network, and we speculate that this finding is consistent with the self-referential feature of the NLE in the ‘distress’ subscale.
Mentalizing consists in perceiving, representing and reasoning about the intentions and psychological disposition of one's self and others. It engages the superior temporal sulcus, the inferior frontal gyrus and premotor areas (Nolte et al., 2013). This network is active, while processing social threat like angry faces. The temporoparietal junction is notably implicated in appropriately attributing a mental state to a person's facially expressed anger in order to infer their intentions (Kret et al., 2011).
Also, the ‘distress’ NLE score correlated with the neural response in the right VLPFC/OFC. Of note, the literature highlights that the VLPFC is preferentially engaged during recall of negative affects (Markowitsch et al., 2003), evaluation of negative outcomes (Ursu and Carter, 2005), and reappraisal of negative scenes or emotions (Ochsner et al., 2004). Moreover, the VLPFC/OFC has an inhibitory function on amygdala in the context of social threat (Monk et al., 2008; Nelson and Guyer, 2011). This might account for the present lack of correlation with the amygdala.
At variance with our prediction, we did not observe any correlation between the lifetime NLE subscales and ACC response, although involvement of the ACC has previously been reported during the angry faces task, and has been associated with distress. However, in the present study, the assessment was focused on past events, whereas correlations with ACC activity have been reported in relation with current distress (Masten et al., 2009).
Overall, the lifetime ‘distress’ NLE score correlated during social threat processing with VLPFC/OFC and with temporal regions involved in mentalizing and self-referential concepts (Blakemore, 2012).
The second finding of this study was that lifetime ‘distress’ NLE score assessed at the age of 14 years was associated with GAD or MDD outcome, at the age of 16 years, whereas ‘family’ and ‘accident’ scores were not. The correlation detected here raises the hypothesis that the experience of ‘distress’ NLE plays a role in the risk for these conditions.
Emotion regulation deficits are increasingly understood as important predictors of internalizing symptoms (as in GAD and MDD) among adolescents. Herein, the correlations between the ‘distress’ NLE subscale and the neural response to angry faces were detected in regions implicated in the pathophysiology of internalizing disorders, notably VLPFC/OFC and DLPFC (BA 9), which are dysfunctional in studies using analogous fMRI methods in juvenile patients with GAD (Monk et al., 2006; Beesdo et al., 2009) or MDD (Hulvershorn et al., 2011).
In this study, we tested the hypothesis that different type of NLE would have differential neural effects on social threat processing. Interestingly, only the ‘distress’ NLE subscale correlated with neuroimaging data, whereas the ‘family’ or the ‘accident’ scale did not. On a caution note, we speculate that the ‘distress’ subscale encompasses self-referential NLE, i.e. NLE ‘internal’ to the person, regarding the self-image components (Supplementary Methods). The ‘family’ and ‘accident’ subscales, rather account for external NLEs, occurring outside the subject's mind. Thus, only the self-referential NLE seemed to have an interaction with social threat processing. Future studies might investigate whether self-referential NLE might lead to a propensity to mentalize with negative thoughts and emotions and impair self-esteem. The same interpretation holds for the finding that only the ‘distress’ NLE subscale correlated with GAD or MDD diagnostic outcomes in the cohort of adolescents. Indeed, this propensity during adolescence may be a vulnerability dimension to future onset of anxiety and depressive disorders (Newton-Howes et al., 2015).
The present findings must be viewed in the light of some technical considerations and limitations. First, the present adolescent sample was recruited at the age of 14 years, a sensible period to enquire about their perception of NLE. Indeed, regarding changes in stress perception over time, overall, most adolescents experience high levels of stress during early adolescence up to the age of 15 years, after which there is a decrease in perception (Brown and Spencer, 2013; Niwa et al., 2016). Therefore, the present results cannot be extended to late adolescence or adulthood.
Second, the passive viewing condition during the fMRI task might question the ability of the subject to engage in the task. However, it has been shown that emotion processing can occur even when the subject is not focused on the task through unconscious priming (Telzer et al., 2008) and that passive viewing enables to find subtler differences in emotional processing than an emotional task with a constrained attention condition.
Third, only angry faces were displayed, whereas adding another negatively valenced facial expression (e.g. fearful or sad face stimuli) would have explored more thoroughly the specificity of the findings. This limitation is inherent to feasibility issues since this would have required much longer MR acquisition time, which might compromise robustness in a large multicenter study. Also, disentangling the brain networks processing various negative emotional stimuli was not the purpose of the present study, inasmuch as some negative stimuli might be intricate. For instance, at variance with angry faces, fearful faces might provide less information about the source of danger, and not convey direct threat to the person viewing the expression (Whalen et al., 2001).
Fourth, the NLEs assessed by the ‘distress’ subscale do not overlap the ‘accident’ and ‘family’ subscale items (Newcomb et al., 1981). The factor analysis by Newcomb et al. (1981) reported that these subscales segregate in different dimensions of stress. Here, we acknowledge that the NLE subscales were not completely independent in the present adolescent sample since Pearson’ statistics retrieved significant correlations, but the values of the correlation coefficients show that the NLE subscales were far from being equivalent (undistinct) since their correlation coefficients well below 0.90, Actually, the NLE subscales had partial correlations below 0.27 in the present sample.
Finally, the present correlative analysis was designed neither to draw causal inferences nor to examine the mechanisms of the detected associations, notably their molecular determinants. Nevertheless, aspects of sensitization, as well as a recent report of telomere length changes in children exposed to chronic social stress (Mitchell et al., 2014), may be relevant to the present findings as perspectives for translational research.
Conclusion
This is the first study to show that different types of NLE differentially interfere with neural responses to social threat in healthy adolescents, emphasizing the impact of self-referential life stressors, which may harm emotional regulation in juveniles. Indeed, this NLE type also participated to the onset of de novo internalizing conditions (MDD or GAD) 2 years later.
OTHER MEMBERS OF THE IMAGEN CONSORTIUM
Reed L, Cattrell A, Nymberg C, Stueber K, Mallik C, Clarke TK, Stacey D, Desrivieres S, Schilling C, Ströhle A, Wolff E, Schubert F, McCabe E, Whelan R, Spanagel R, Muller K, Bordas N, Bricaud Z, Galinowski A, Massicotte J, Rogers J, Schwartz Y, Poline JB, Speiser C, Constant P, Mignon X and Thomsen T.
Acknowledgements
Pr Stephane Lehéricy and the team at the CENIR (Centre de Neuro-imagerie de Recherche, Institut du Cerveau et de la Moëlle Epinière, Hôpital Pitié-Salpétrière, Paris, France) are acknowledged for their support in the acquisition of follow-up data.
Funding
This work was supported by the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement- related behaviour in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the FP7 project IMAGEMEND (IMAgingGEnetics for MENtal Disorders) and the Innovative Medicine Initiative Project EU-AIMS (115300-2), Medical Research Council Programme Grant “Developmental pathways into adolescent substance abuse” (93558), as well as the Swedish funding agency FORMAS. Support was also provided by the Bundesministerium für Bildung und Forschung (grant # 01EV0711, and the project AERIAL grant # 01EE1406C) and the Deutsche Forschungsgemeinschaft (grant # SFB 940) Further support was provided by an APHP/INSERM interface grant to MLPM, a grant “poste accueil” AP-HP–CEA 2012 to FGB, a Paris-Descartes University collaborative-project-2010 grant, a Paris Sud University IDEX 2012 PhD grant to HV, two ANR grants (Samenta Adodep project, – Eranet NEURON project AF12-NEUR0008-01-WM2NA), a grant from the Fondation de France- (2012-00033703) “Développement de l'Enfant” program, and a grant from the Mission Interministérielle de Lutte contre les drogues et les conduites addicitives (MILDECA).
Disclaimer
This article reflects only the author's views and the Community is not liable for any use that may be made of the information contained therein.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. Robert Goodman is the owner of Youthinmind, Ltd., which provides no-cost and low-cost software and websites related to the Development and Well-Being Assessment and the Strengths and Difficulties Questionnaire. Trevor Robbins receives compensation as a consultant for Cambridge Cognition. Gareth Barker received honoraria for teaching from General Electric during the course of this work. The other authors declare no conflict of interest.
References
- Arsalidou M., Morris D., Taylor M.J. (2011). Converging evidence for the advantage of dynamic facial expressions. Brain Topography 24, 149–63. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.S., Lee K.E., Haxby J.V., Martin A. (2002). Parallel visual motion processing streams for manipulable objects and human movements. Neuron 34, 149–59. [DOI] [PubMed] [Google Scholar]
- Beesdo K., Lau J.Y.F., Guyer A.E., et al. (2009). Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry 66, 275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J., Fox N., Zeanah C., Nelson C.A. (2015). Early deprivation, atypical brain development, and internalizing symptoms in late childhood. Neuroscience. doi: 10.1016/j.neuroscience.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J., Nelson C.A. (2016). Early adverse experiences and the developing brain. Neuropsychopharmacology 41, 177–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J. (2012). Development of the social brain in adolescence. Journal of the Royal Society of Medicine 105, 111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.G., Mathalon D.H., Stern H., et al. (2011). Multisite reliability of cognitive BOLD data. NeuroImage 54, 2163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.R., Spencer K.A. (2013). Steroid hormones, stress and the adolescent brain: A comparative perspective. Neuroscience 249, 115–28. [DOI] [PubMed] [Google Scholar]
- Choudhury S., Blakemore S.J., Charman T. (2006). Social cognitive development during adolescence. Social Cognitive and Affective Neuroscience 1, 165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas B.E., Orosan P.G., Grant K.E. (1993). Adolescent stress and coping: implications for psychopathology during adolescence. Journal of Adolescence 16(3),331–49. [DOI] [PubMed] [Google Scholar]
- Conigrave K.M., Hall W.D., Saunders J.B. (1995). The AUDIT questionnaire: choosing a cut-off score. Alcohol Use Disorder Identification Test. Addiction (Abingdon, England) 90, 1349–56. [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Stuhrmann A., Beutelmann V., et al. (2012). Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry 71(4),286–93. [DOI] [PubMed] [Google Scholar]
- Demir-Lira ÖE., Voss J.L., O’Neil J.T., Briggs-Gowan M.J., Wakschlag L.S., Booth J.R. (2016). Early-life stress exposure associated with altered prefrontal resting-state fMRI connectivity in young children. Developmental Cognitive Neuroscience 19, 107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend B.P., Shrout P.E., Egri G., Mendelsohn F.S. (1980). Nonspecific psychological distress and other dimensions of psychopathology: Measures for use in the general population. Archives of General Psychiatry 37, 1229–36. [DOI] [PubMed] [Google Scholar]
- Feldmann-Wüstefeld T., Schmidt-Daffy M., Schubö A. (2011). Neural evidence for the threat detection advantage: differential attention allocation to angry and happy faces. Psychophysiology 48, 697–707. [DOI] [PubMed] [Google Scholar]
- Friedman L., Stern H., Brown G.G., et al. (2008). Test–retest and between-site reliability in a multicenter fMRI study. Human Brain Mapping 29, 958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., et al. (2009). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience 34, 418–32. [PMC free article] [PubMed] [Google Scholar]
- Ganzel B.L., Kim P., Gilmore H., Tottenham N., Temple E. (2013). Stress and the healthy adolescent brain: evidence for the neural embedding of life events. Development and Psychopathology 25(4 Pt 1), 879–89. [DOI] [PubMed] [Google Scholar]
- Goodman A., Heiervang E., Collishaw S., Goodman R. (2011). The « DAWBA bands » as an ordered-categorical measure of child mental health: description and validation in British and Norwegian samples. Social Psychiatry and Psychiatric Epidemiology 46, 521–32. [DOI] [PubMed] [Google Scholar]
- Goodman R., Ford T., Richards H., Gatward R., Meltzer H. (2000). The development and well-being assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines 41(5),645–55. [PubMed] [Google Scholar]
- Grant M.M., Cannistraci C., Hollon S.D., Gore J., Shelton R. (2011). Childhood trauma history differentiates amygdala response to sad faces within MDD. Journal of Psychiatric Research 45, 886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., McLaughlin K.A., Berglund P.A. &., et al. (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication i: associations with first onset of dsm-iv disorders. Archives of General Psychiatry 67, 113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras M.H., Paus T. (2006). Brain networks involved in viewing angry hands or faces. Cerebral Cortex 16, 1087–96. [DOI] [PubMed] [Google Scholar]
- Hanson J.L., Chung M.K., Avants B.B., et al. (2010). Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. The Journal of Neuroscience 30, 7466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Nacewicz B.M., Sutterer M.J., et al. (2015). Behavior problems after early life stress: contributions of the hippocampus and amygdala. Biological Psychiatry 77, 314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn L.A., Cullen K., Anand A. (2011). Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging and Behavior 5, 307–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomaa R., Väänänen J.M., Fröjd S., Kaltiala-Heino R., Marttunen M. (2013). How low is low? Low self-esteem as an indicator of internalizing psychopathology in adolescence. Health Education & Behavior 40, 392–9. [DOI] [PubMed] [Google Scholar]
- Kret M.E., Denollet J., Grèzes J., de Gelder B. (2011). The role of negative affectivity and social inhibition in perceiving social threat: an fMRI study. Neuropsychologia 49, 1187–93. [DOI] [PubMed] [Google Scholar]
- Maheu F.S., Dozier M., Guyer A.E., et al. (2010). A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective & Behavioral Neuroscience 10, 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch H.J., Vandekerckhove M.M.P., Lanfermann H., Russ M.O. (2003). Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior 39, 643–65. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., et al. (2009). Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience 4, 143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E., De Brito S.A., Viding E. (2011). The impact of childhood maltreatment: a review of neurobiological and genetic factors. Frontiers in Psychiatry 28(2),48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C., Hobcraft J., McLanahan S.S., et al. (2014). Social disadvantage, genetic sensitivity, and children’s telomere length. Proceedings of the National Academy of Sciences of the United States of America 111, 5944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E., Harrington H., Caspi A. &., et al. (2007). Depression and generalized anxiety disorder: Cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Archives of General Psychiatry 64, 651–60. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Klein R.G., Telzer E.H., et al. (2008). Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. The American Journal of Psychiatry 165, 90–8. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Nelson E.E., McClure E.B., et al. (2006). Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry 163, 1091–7. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Guyer A.E. (2011). The development of the ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience 1, 233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb M.D., Huba G.J., Bentler P.M. (1981). A multidimensional assessment of stressful life events among adolescents: derivation and correlates. Journal of Health and Social Behavior 22, 400–15. [Google Scholar]
- Newton-Howes G., Horwood J., Mulder R. (2015). Personality characteristics in childhood and outcomes in adulthood: findings from a 30 year longitudinal study. The Australian and New Zealand Journal of Psychiatry 49, 377–86. [DOI] [PubMed] [Google Scholar]
- Niwa M., Lee R.S., Tanaka T., Okada K., Kano S.I., Sawa A. (2016). A critical period of vulnerability to adolescent stress: epigenetic mediators in mesocortical dopaminergic neurons. Human Molecular Genetics 25, 1370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte T., Bolling D.Z., Hudac C.M., Fonagy P., Mayes L., Pelphrey K.A. (2013). Brain mechanisms underlying the impact of attachment-related stress on social cognition. Frontiers in Human Neuroscience 7, 816.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage 23, 483–99. [DOI] [PubMed] [Google Scholar]
- Paschall M.J., Grube J.W., Kypri K. (2009). Alcohol control policies and alcohol consumption by youth: a multi-national study. Addiction (Abingdon, England) 104, 1849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence 17, 117–33. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology 35, 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig S., Sánchez-González J., Arango C., et al. (2009). Assessment of the increase in variability when combining volumetric data from different scanners. Human Brain Mapping 30, 355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J.B., Aasland O.G., Babor T.F., de la Fuente J.R., Grant M. (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction (Abingdon, England) 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Schneider S., Peters J., Bromberg U., Brassen S., Menz M.M., Miedl S.F.; IMAGEN Consortium (2011). Boys do it the right way: sex-dependent amygdala lateralization during face processing in adolescents. NeuroImage 56, 1847–53. [DOI] [PubMed] [Google Scholar]
- Schumann G., Loth E., Banaschewski T., Barbot A., Barker G., Büchel C.; IMAGEN Consortium (2010). The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Molecular Psychiatry 15, 1128–39. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Casey B. (2010). A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition 72, 124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi A.M., Artiges E., Banaschewski T., Barker G.J., Bruehl R., Büchel C.; IMAGEN Consortium (2012). Creating probabilistic maps of the face network in the adolescent brain: a multicentre functional MRI study. Human Brain Mapping 33, 938–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. (2016). Annual research review: enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry, and Allied Disciplines 57, 241–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Mogg K., Bradley B.P., et al. (2008). Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biological Psychology 79, 216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyreau B., Schwartz Y., Thirion B., Frouin V., Loth E., Vollstädt-Klein S.; IMAGEN Consortium (2012). Very large fMRI study using the IMAGEN database: sensitivity-specificity and population effect modeling in relation to the underlying anatomy. NeuroImage 61, 295–303. [DOI] [PubMed] [Google Scholar]
- Ursu S., Carter C.S. (2005). Outcome representations, counterfactual comparisons and the human orbitofrontal cortex: implications for neuroimaging studies of decision-making. Brain Research. Cognitive Brain Research 23, 51–60. [DOI] [PubMed] [Google Scholar]
- Veit C.T., Ware J.E. (1983). The structure of psychological distress and well-being in general populations. Journal of Consulting and Clinical Psychology 51, 730–42. [DOI] [PubMed] [Google Scholar]
- Vollebergh W.M., Iedema J., Bijl R.V., de Graaf R., Smit F., Ormel J. (2001). The structure and stability of common mental disorders: The nemesis study. Archives of General Psychiatry 58, 597–603. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Shin L.M., McInerney S.C., Fischer H., Wright C.I., Rauch S.L. (2001). A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion 1, 70–83. [DOI] [PubMed] [Google Scholar]
- Zou K.H., Greve D.N., Wang M., et al. (2005). Reproducibility of Functional MR Imaging: Preliminary Results of Prospective Multi-institutional Study Performed by Biomedical Informatics Research Network. Radiology 237, 781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.