Abstract
The aim of this study was to examine differences in the neural processing of social information about kin and friends at different levels of closeness and social network level. Twenty-five female participants engaged in a cognitive social task involving different individuals in their social network while undergoing functional magnetic resonance imaging scanning to detect BOLD (Blood Oxygen Level Dependent) signals changes. Greater levels of activation occurred in several regions of the brain previously associated with social cognition when thinking about friends than when thinking about kin, including the posterior cingulate cortex (PCC) and the ventral medial prefrontal cortex (vMPFC). Linear parametric analyses across network layers further showed that, when it came to thinking about friends, activation increased in the vMPFC, lingual gyrus, and sensorimotor cortex as individuals thought about friends at closer layers of the network. These findings suggest that maintaining friendships may be more cognitively exacting than maintaining kin relationships.
Keywords: social networks, kin, friendship, fMRI, social cognition
INTRODUCTION
Modern humans live in social communities that are much larger and more complex than those that characterize monkeys and apes (Dunbar, 1993). Nonetheless, in both cases, community size is limited by a combination of cognitive processing power (the social brain hypothesis) (Dunbar, 1992) and time constraints (Miritello et al., 2013). Time limits the size of social groups because, in both monkeys and humans, relationship quality depends on the time invested in the relationship (Sutcliffe et al., 2012). Since the time that can be devoted to social interaction each day is limited (Dunbar, 1993; Lehmann et al., 2007), the number of relationships that can be maintained at the required level of emotional closeness will inevitably be limited. One mechanism that might have allowed humans to overcome this constraint is kinship, perhaps because kinship allows at least some relationships to be stored as schemas rather than as detailed blow-by-blow behavioral histories (Brashears, 2013). Kinship schemas allow an automated response when deciding how one should act toward an individual, whereas in the case of friendships, such decisions might require the actor to review the history of the relationship in some detail before deciding on an appropriate response. If so, then we might expect to find neurophysiological differences in the way individuals process decisions about kin compared to those about friends.
Members of an individual’s social network are organized as a set of hierarchically inclusive layers, roughly consisting of a “support clique” (4–5 individuals), a “sympathy group” (12–15 individuals), an “affinity group” (∼50 individuals), and the active network (∼150 individuals) (Zhou et al., 2005; Sutcliffe et al., 2012; Dunbar et al., 2015). Although each consecutive layer increases in size, relationships with members of successive layers are characterized by decreasing levels of emotional closeness and decreasing frequency of interaction (Roberts et al., 2009). The reason for this is likely due to the fact that maintaining social networks imposes considerable cognitive load on individuals (Meyer et al., 2012). Indeed, it has been shown that the size of an individual’s social network is related to both mentalizing competences (Stiller and Dunbar, 2007; Powell et al., 2012) and the volume of key brain regions associated with aspects of social cognition like theory of mind [e.g. TPJ (temporo-parietal junction), vLPFC (ventrolateral prefrontal cortex), and two regions of the ventral medial prefrontal cortex (vMPFC)] (Kanai et al., 2012; Powell et al., 2012, 2010). While traditional communities are closely bound by interconnected kin relationships (Barnard, 2009), modern urbanized communities consist of a mix of kin and non-kin (friendship) relationships (Roberts et al., 2009). This has further implications for social network size and structure, since relationships with kin have been found to be both more stable over time and more intimate, and to require less time investment, than comparable non-kin (i.e. friendship) relationships (Roberts and Dunbar, 2011; Roberts et al., 2014).
In effect, these findings suggest that not all relationships are created equal and that the cognitive underpinnings for managing relationships are likely to differ between relationship types and across the layers of the network. It is already known that some types of relationships, such as romantic vs maternal love relationships, while showing considerable overlap, also show some activations in different brain areas (Bartels and Zeki, 2000, 2004; Fisher et al., 2005). For example, while both romantic relationships and maternal ‘love’ relationships showed activations in dopaminergic and reward and emotion processing centres of the brain—including the caudate, ventral tegmental area, putamen, insula, and the anterior cingulate cortex—maternal relationships (mothers thinking about their child) showed additional activation in the periaqueductal (central) gray matter. It is possible that friends and kin may similarly involve slightly different cognitive processes, which in turn may vary depending on the layer of the network.
Aside from facial recognition tasks (DeBruine et al., 2005; Platek and Kemp, 2009), kinship has been virtually ignored in social cognitive neuroscience. Indeed, social networks, and the cognitive demands of managing these, have attracted only limited interest in either psychology or the neurosciences (Kanai et al., 2012; Powell et al., 2012, 2010). Yet, kinship and kinship naming play a singularly important role in the management of our social world in both traditional small-scale societies and the modern, post-industrial world (Roberts and Dunbar, 2011; Roberts et al., 2014). In this respect, we cover new ground in asking questions about the neural processing of kinship and the role this might play in facilitating the maintenance of our large, complex social networks.
We use functional magnetic resonance imaging (fMRI) neuroimaging to test the hypothesis that kinship relationships involve different cognitive processes than friendship-based relationships. By doing this, we aim to explore whether and how brain activations might be differentiated when dealing with two important types of relationship: kin vs friends. Furthermore, we ask whether activations vary when thinking about kin and friends depending on where in the social network these individuals lie, with network layer location assessed by frequency of contact and subjective feelings of intimacy. As robust sex differences have previously been observed in both mentalizing and empathizing abilities (Baron-Cohen et al., 2001; Stiller and Dunbar, 2007) and in neural activations associated with “theory of mind” tasks (Krach et al., 2009), it is possible that relationship processing differs between the sexes; we therefore sought to minimize gender effects by considering only one sex and chose females as they are the most intensely social.
Methods
Participants
Twenty-five healthy, right-handed, female participants with no history of neurological or psychiatric disorders or reported MRI contraindications (i.e. metal in their bodies, claustrophobia, and pregnancy) were recruited from the University of Oxford student body. To ensure equal possibility for frequent contact and closeness with both established friends and family members, participants were limited to individuals who have lived in the UK for at least 15 years and stated that they have the majority of their friends and family living in the same country. Mean age of participants was 23.2 years (s.d. = 5.0), all participants stated either that they spoke English as their native language (23 participants) or that their understanding of English was “excellent” (two participants). Written informed consent was obtained in accordance with Oxford University’s Institutional Review Board (CUREC). Participants were remunerated £25 for their time and travel expenses.
Measures
Before attending each scanning session, participants were asked to fill in a computer-based questionnaire about various individuals in their social network. Participants were asked to name two to three friends and two to three family members (excluding romantic partners) at five different network levels, with network location determined by frequency of contact (following Roberts et al., 2009). Individuals whom the participants contacted about once a week were Level 1 (“support clique” of 4–5 individuals), once a month Level 2 (the “sympathy group” of 12–15 individuals), once every 6 months Level 3 (“affinity group” of ∼50 individuals), once a year Level 4 (and the active network of ∼150 individuals), or less than once a year Level 5 (extended network of ∼500 individuals). Participants also stated their level of “emotional closeness” with each individual on a Likert-type scale (1 = “not at all close”, 10 = “extremely close”). Past research has found that these groupings correspond closely with decreasing levels of intimacy with individuals across the network levels (Roberts et al., 2009). Two individuals (one friend and one kin) were chosen from each network level, ensuring that the stated emotional closeness for those individuals was matched and equal to or lower than that for the individuals chosen at the previous level. In total, 10 individuals from each participant’s social network (five friends and five kin, one at each level of their social network) were selected as “target” stimuli in the social decision task to be carried out by the participant while in the MRI scanner.
Procedure
Before the scanning session, participants were first familiarized with the scanning procedure and the task they were to perform. The task was a social processing task that involved answering multiple questions about each of the previously selected target individuals, as well as about themselves. The stimuli comprised 20 statements about motivations, mind-states, and behaviors associated with each of the target individuals, adapted from the MinIPIP Big Five personality inventory (Donnellan et al., 2006). Example statements included “I think ‘X’ generally is not interested in abstract ideas” and “I think ‘X’ generally feels other’s emotions” (with ‘X’ replaced by the name provided by the participant for each target individual). Participants were asked to what extent they agreed with each statement as it relates to each target individual, with responses assessed using a four-point Likert-type scale (“strongly disagree,” “disagree,” “agree,” and “strongly agree”). Inferring the beliefs or preferences of other individuals is a core component of mentalizing and is a type of social cognition often assessed in imaging research (Jenkins and Mitchell, 2010; Wang et al., 2012).
The stimuli were presented using Neurobehavioral Systems’ Presentation software on a projected screen behind the magnet bore via a mirror, with responses collected using a four-button box with which participants were familiarized before the experiment. The study consisted of one continuous block, with a 21-second pause in the middle, during which the participants viewed and answered their agreement to the 20 statements as they related to each of the 10 target individuals and to themselves. Each trial consisted of a 3-second inter-stimulus interval, followed by a time-jittered cue (a fixation cross, cue times varying from 0.9 to 1.5 seconds), followed by a stimulus statement displayed alongside a response key. Each statement remained on screen until the participant made their response using the response box (or for a maximum of 8 seconds), at which time the next trial was presented. Reaction times (RT) were collected for the time between stimulus question onset to response button press. Trials (questions and target individuals) were pseudo-randomized for each participant, with each participant completing a total of 220 trials (20 for each target individual). Mean total scanning time was 27.8 minutes (s.d. = 2.9).
Image acquisition and data analysis
Functional images were acquired using a Siemens 3.0 Tesla MAGNETOM Trio MRI device with a 32-channel TIM head array at the University of Oxford Centre for Clinical Magnetic Resonance Imaging. At the beginning of each scanning session, 192 high-resolution structural T1-weighted gradient echo images were acquired to be used for localization of function for each participant (TR = 2.04 ms, TE = 4.7 ms, flip angle 8°, FOV = 192 mm, voxel size = 1 × 1 × 1 mm). For the functional data, a total of between 595 and 900 functional T2*-weighted echo-planar images were acquired for participants (volume variability due to variation in total response-times), in volumes of 41 3.5 mm transverse echo-planar slices covering the whole brain (TR = 2.41 s, TE = 28 ms, flip angle = 90°, FOV = 192 mm, matrix = 64 × 64, voxel size 3.0 × 3.0 × 3.5 mm). The first four volumes of each run were treated as dummy runs for equilibration purposes and deleted. At the end of the session, a fieldmap (B0) image was acquired for use during the registration phase of the analysis (TR = 488 ms, TE(1) = 5.19 ms, TE(2) = 7.65 ms).
Data were pre-processed and analyzed using FMRIB’s Linear Image Registration Tool (MCFLIRT, Jenkinson et al., 2002), a motion correction algorithm, with automatic brain extraction, slice-time correction, B0 field-map unwarping, 7 mm full-width-at-half-maximum Gaussian kernel smoothing, and a high-pass filter cutoff point of 90 ms applied to improve registration and signal-to-noise ratio. FSL software (Smith et al., 2004) was used to analyze the functional data and register it to the corresponding structural image using linear (BBR) registration, with each participant’s structural image further registered to the Montreal Neurological Institute 2 mm space using nonlinear registration (warp resolution 10 mm). Motion parameters were inspected to ensure motion never exceeded one voxel size (3 mm) on any axis.
Functional images were analyzed with general linear models (GLM) using FSLs FEAT software (Smith et al., 2004; Jenkinson et al., 2012). For the within-subject, first-level analysis, the entire length of each trial (RT) was modeled and convolved with a canonical double-gamma hemo-dynamic response function. For the group (second-level) analysis, a General Linear Model(GLM) model was created with one regressor for mean activation during all Friend trials and one for mean activation during Kin trials, with contrasts examining mean kin > friend activation and mean friend > kin activation. For the parametric analyses, a design matrix was created to detect any positive or negative linear activation across the five network layers with either Friends or Kin. Group level analysis to calculate mean activations across participants was performed using FSL’s Local Analysis of Mixed Effects higher-level modeling. To account for multiple comparisons, cluster thresholding (using GRF (Gaussian Random Field) theory) was applied at a z threshold of 2.3 (P < 0.05), as this type of correction is more sensitive than voxel-based methods.
Results
RT differences
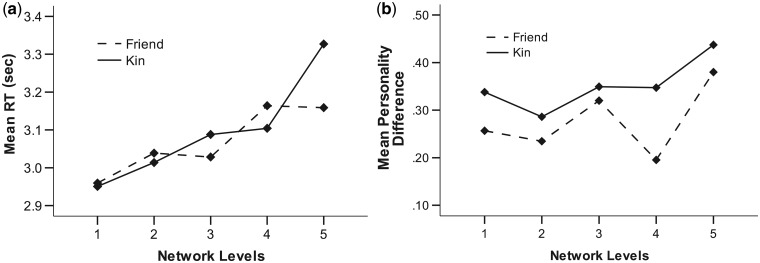
To ensure that any differences in observed activation were not due to attention or engagement effects, the RTs for responses to trials in the Kin and Friend conditions across the network levels were compared (Figure 1A). Kin and Friend RTs were highly correlated (Pearson’s r = 0.947; P < 0.001). Differences in RTs across relationship type and network level were compared using a 2 × 5 repeated-measures analysis of variance (ANOVA), with RTs for kin and friends as one factor, and RTs for the five network levels as the other repeated measures factor. There were no overall RT differences between friends and kin (F(1,24) = 0.40, P = 0.533, partial η2 = 0.02), but RTs did vary with network level (F(4,21) = 4.37, P = 0.004, partial η2 = 0.51), within-subject contrasts suggesting that this was a linear trend (F = 17.75, P < 0.001, partial η2 = 0.43), i.e. RTs increased linearly the further in the network layer a target individual was placed. No interaction effects were found between kin vs friends and network levels (F(4,21) = 1.44, P = 0.257, partial η2 = 0.22).
Fig. 1.
(A) Differences in response RT for friends and kin at the five levels of the social network. (B) Mean differences in personality scores between the participants and kin and friends at the five levels of the social network.
Personality differences
As participants rated both their own and target individuals on various personality variables, it was possible to examine how dissimilar each target group was to each rating individuals. Dissimilarity was assessed by first calculating mean personality scores for each of the big five personality factors (openness, conscientiousness, extraversion, agreeableness, and neuroticism) and then taking the mean absolute differences on these scores between the participant and each target individual (Figure 1B). Personality differences were then analyzed using a 2 × 5 repeated-measures ANOVA, with differences between kin and friends as one repeated-measures factor and differences at the five network levels as the other repeated-measures factor. Results suggest that while participants were more similar to friends (M = 0.28, SE = .04) than to kin (M = 0.36, SE = .04) overall (F(1,24) = 7.26, P = 0.013, partial η2 = 0.22), there were no significant differences across the network levels (F(4,21) = 3.51, P = 0.073, partial η2 = 0.32) and there were no interaction effects between kin vs friends and network levels (F(4,21) = 0.98, P = 0.440, partial η2 = 0.16).
Main effects of friend vs kin mentalizing
When examining the kin > friend activations across participants, there were no significant mean activations after cluster thresholding. However, when looking at the friend > kin contrast, three distinct clusters of activation were found (Table 1). These roughly correspond to (i) the bilateral posterior cingulate cortex (PCC), including the retrosplenial cortex; (ii) the bilateral vMPFC; and (iii) the left sensorimotor cortex (Figure 2).
Table 1.
Main activation clusters in the friend > kin condition
| Cluster index | Cluster size | Max z value | P (corrected) | MNI local maxima | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Bilateral posterior cingulate cortex | 2512 | <0.0001 | ||||
| Left posterior cingulate | 4.74 | −6 | −56 | 6 | ||
| Left posterior cingulate | 4.66 | −14 | −48 | 8 | ||
| Left posterior cingulate | 4.61 | −8 | −56 | 10 | ||
| Right posterior cingulate | 4.38 | 10 | −54 | 26 | ||
| Right posterior cingulate | 4.38 | 18 | −48 | 8 | ||
| Bilateral ventral medial prefrontal cortex | 1514 | <0.001 | ||||
| Left ventral medial prefrontal cortex | 3.98 | −6 | 62 | 0 | ||
| Left ventral medial prefrontal cortex | 3.81 | −6 | 42 | −10 | ||
| Right ventral medial prefrontal cortex | 3.47 | 8 | 58 | −10 | ||
| Sensorimotor cortex | 1002 | 0.012 | ||||
| Left sensorimotor cortex | 3.53 | −8 | −22 | 64 | ||
| Left sensorimotor cortex | 3.26 | −32 | −32 | 60 | ||
| Right sensorimotor cortex | 3.15 | 12 | −18 | 66 | ||
Note: Total cluster size (in voxels) alongside the corresponding corrected significance level, as well as local maxima at various regional sub-peaks with z scores (coordinates reported in Montreal Neurological Institute [MNI] space). Cluster thresholding was applied at a z threshold of 2.3 (P < 0.05).
Fig. 2.
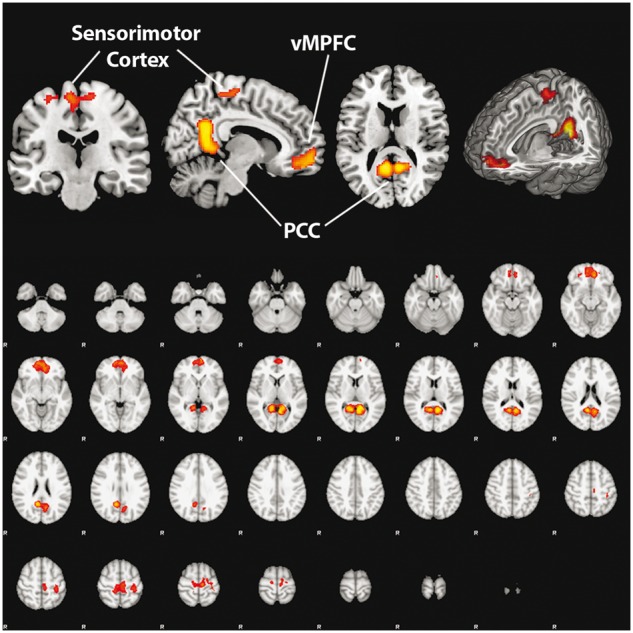
Activations in the friend > kin condition, showing regions with significantly greater activations when performing social cognition relating to friends as compared to social cognition relating to kin.
Parametric activation across network layers for friends and kin
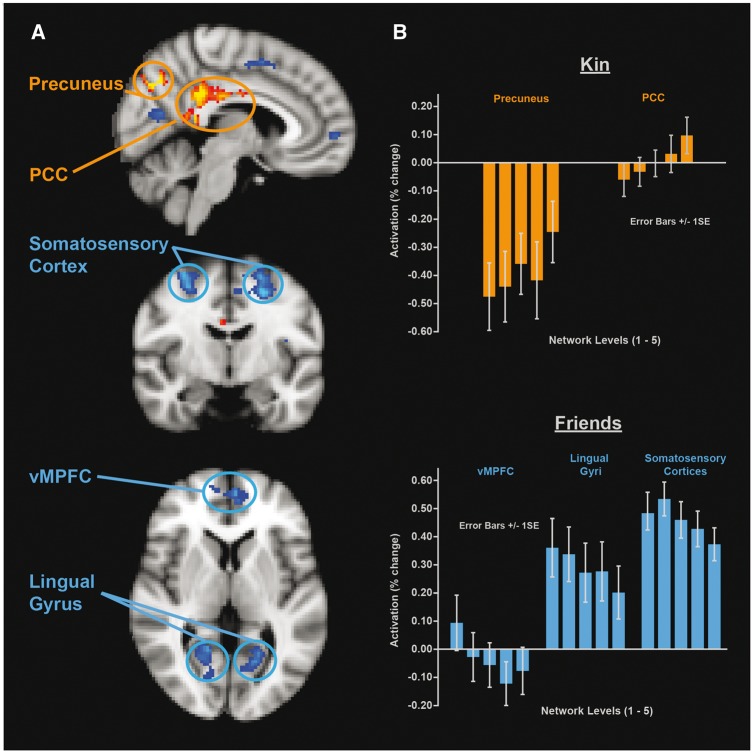
To explore activations associated with social cognition for individuals at different levels of the social network (i.e. network levels 1–5), linear parametric activation analyses were carried out separately for friends and for kin. For friends, significant negative linear parametric activations were found across the network layers (Figure 3), with no positive linear activations detected after thresholding. The analysis suggested that as individuals were thinking about attributes associated with friends who were increasingly closer to them in their social network (in terms of both frequency of contact and emotional closeness), greater activation occurred in the bilateral somatosensory cortices, lingual gyri, and the vMPFC (Table 2).
Fig. 3.
Parametric linear activations at the five different levels of the social network (z > 3.3 shown, P < 0.001). (A) Blue shows negative linear parametric activation with friends, i.e. greater activation associated with thinking about closer friends, red shows positive linear parametric activation when thinking about kin, i.e. greater activation occurring when thinking about ever more distant kin. (B) Percentage activation change for kin (precuneus and PCC) and for friends (vMPFC, lingual gyri, and somatosensory gyri) across the five levels of the social network (mean of ROI (Region of Interest) masks based on z > 3.3 activation clusters).
Table 2.
Linear parametric activation clusters for friends and kin across different social network levels
| Cluster size | Local Maxima z value | Cluster P (corrected) | MNI local maxima | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Friends: negative activation | ||||||
| Bilateral somatosensory cortices | 11,735 | <0.0001 | ||||
| Right sensorimotor cortex (finger) | 4.39 | 50 | −26 | 44 | ||
| Right sensorimotor cortex | 4.09 | 32 | −9 | 62 | ||
| Left sensorimotor cortex | 4.14 | −24 | −8 | 50 | ||
| Bilateral lingual gyri | 10,909 | |||||
| Left lingual gyrus | 4.11 | −14 | −66 | −4 | ||
| Right lingual gyrus | 3.97 | 16 | −64 | 10 | ||
| Right cerebellum (motor) | 3.94 | 26 | −52 | −22 | ||
| Right retrosplenial cortex | 3.92 | 16 | −56 | 4 | ||
| Bilateral prefrontal cortices | 4123 | <0.001 | ||||
| Left ventral media prefrontal cortex | 4.15 | −4 | 56 | 2 | ||
| Right ventral media prefrontal cortex | 3.94 | 10 | 56 | 0 | ||
| Left thalamus | 3.71 | −8 | −14 | −2 | ||
| Kin: positive activation | ||||||
| Precuneus and posterior cingulate | 4598 | <0.001 | ||||
| Right precuneus | 4.79 | 8 | −70 | 38 | ||
| Left precuneus | 4.35 | −4 | −76 | 42 | ||
| Right PCC | 4.67 | 4 | −38 | 30 | ||
| Left PCC | 4.14 | −2 | −26 | 26 | ||
Note: Total cluster size (in voxels) alongside the corresponding corrected significance level, as well as local maxima at various regional sub-peaks with z scores (coordinates reported in Montreal Neurological Institute [MNI] space). Cluster thresholding was applied at a z threshold of 2.3 (P < 0.05).
When looking at activations across the different network levels with kin, significant positive linear parametric activations were found in two regions, with no significant negative linear activations occurring (Figure 3). This analysis suggested that as individuals thought about kin members at more distant network layers, greater activation occurred in the precuneus, as well as in the PCC (Table 2).
Discussion
This study examined differences in the processing of social information as it relates to different members of an individual’s social network, namely members who are “kin” vs members who are “friends,” at different levels of the social network. Whole-brain analyses showed that, when processing information about friends as compared to kin, greater levels of activation occurred in several regions of the brain previously associated with social cognition, including the PCC and the vMPFC, as well as the sensorimotor cortex. Linear parametric analyses across network layers showed that, when it came to thinking about friends, activation increased in the vMPFC, lingual gyrus, and sensorimotor cortex as individuals thought about friends at progressively closer layers of the network, while with kin activation increased in the precuneus and PCC as individuals thought about kin at more distal levels of their social network.
Areas of the MPFC play a role in multiple, complex cognitions and affective processes and have been implicated in the processing of self-referential thought and social information, i.e. taking the first person perspective and the third person perspective (Vogeley et al., 2001). Research has found, for example, that seeing friends being socially excluded as compared to strangers was related to greater activation in the MPFC and dorsal anterior cingulate cortex (Meyer et al., 2013), that making judgments about self and friends vs strangers is related to general increased midline responses (rostral anterior cingulate cortex, anterior MPFC) (Krienen et al., 2010) and that regions of the medial frontal lobes are activated specifically by familiar faces but not unknown or self-faces (Platek et al., 2006). A recent parametric functional fMRI study found that the MPFC (and the PCC) shows social load-dependent increases in activity that also correlate with an individual’s perspective taking ability (Meyer et al., 2012). Looking at different medial frontal regions in more detail, the ventral aspect of the MPFC has been associated more closely with both self-referential thought and thought about similar others, while the dorsal MPFC has been associated with thinking (or “mentalizing”) about dissimilar others (Mitchell et al., 2006; Denny et al., 2012; Wagner et al., 2012). While historically this region had also been associated with general “metacognitive” executive functions (Damasio et al., 1993; Vendrell et al., 1995; Goldman-Rakic, 1996; Curtis and D’Esposito, 2003; Ardila, 2008), a recent meta-analysis of cognitive reasoning tasks suggests that core mentalizing areas (including the MPFC) are most active primarily when the task involves some form of social evaluation, such as reasoning about human agency or human traits (Van Overwalle, 2011). It has been suggested that individuals may be referencing the self to infer the mental states of others when they are sufficiently similar to the self (Mitchell et al., 2005), since the self is more likely to be an appropriate reference point for reasoning about more similar individuals (Krienen et al., 2010). However, dorsal PFC activation may also suggest that subjects may simply be engaged in more basic cognitive processing of facts relating to those particular relationships.
It is likely that this type of self-referential cognition was occurring among participants in this study, since all target individuals were familiar, with some likely to be similar as well. We found significantly greater activation in the vMPFC region when thinking about friends vs kin, probably because friends are more similar to participants, and may thus have activated more self-referential thought. Friends are typically “chosen” by an individual, and such choices are often based on homophily—friends tend to have more similar personalities and are more likely to share interests than non-friends (i.e. “birds of a feather flock together”) (Kandel, 1978; McPherson et al., 2001; Machin and Dunbar, 2013; Curry and Dunbar, 2013a,b), a finding that was confirmed by the personality data collected in this study. While there are genetic and environmental components of personality that can lead to similarities amongst kin (at innermost network layers in particular), kin cannot be selected for similarity, and therefore this homophily effect is less pronounced. It is possible that this increased similarity in personality among friends explains the differential activation found here. It is also possible that activation might be reversed (i.e. greater for kin than friends) if individuals were looking at faces of network members, rather than thinking about their personalities, since kin are likely to share greater facial similarity because of genetic relatedness (DeBruine et al., 2005; Platek and Kemp, 2009).
This study also found differences in activation when processing information about friends compared to kin in the medial posterior regions of the brain, particularly the PCC. The PCC has been previously associated with the retrieval of autobiographical memories and self-referential processing across various sensory moduli (Lou et al., 2004). Previous research has found activation in this region during recall of family members and friends (Maddock et al., 2001), when viewing personally familiar rather than just familiar (i.e. celebrity) faces (Gobbini et al., 2004), and when viewing own children vs familiar but unrelated children (Leibenluft et al., 2004), with activations in this region found to rise parametrically alongside personal stimulus familiarity (Gobbini and Haxby, 2007). That friends were associated with greater activity in this area than kin perhaps suggests that either there is greater “personal familiarity” with friends across the social network, or that friends activated autobiographical memories to a greater extent than kin (even though both were matched for frequency of contact and emotional closeness in this study). This might be explained by the possibility that thinking about friend relationships involves recruiting more autobiographical memories, whereas kin relationships invoke more generic schema-type cognitions that are not as dependent on actual autobiographical memories.
The differential activations reported here suggest that friend relationships are somehow qualitatively different to kin relationships, activating brain regions to differing extents. This finding may go some way to explaining how humans have managed to maintain increasingly large and otherwise cognitively taxing social networks—that is, by creating complex kin networks that allowed them to maintain closeness with individuals labeled as “kin” without having to undertake more cognitively involved individual-by-individual assessment of relationship history. This kind of efficiency in processing may be carried out by utilizing kinship schema that allows individuals to use simple and general kinship-based rules as compression heuristics to store social information more efficiently in place of the more detailed information associated with a particular relationship (Brashears, 2013). The use of such schemas would explain why kinship relationships have been found to require less maintenance to remain at similar levels of closeness (Roberts and Dunbar, 2011). It is possible that similar “schemas” might also exist for different types of friendships, or any other type of social relationship for that matter. However, when one considers that schemas associated with kin are likely to have been established at the earliest developmental stages, and be more consistent than the various friend-relationship schemas that would need to be recalled and employed for various types of friends, it is likely that “kin” schemas would remain the more efficient way of processing relationship information. It is particularly interesting to note that even though the overall RTs for the two relationship types show no differences between kin and friends, underlying differences in processing still exist. This may be because RTs are a very crude proxy for cognitive effort and are not as revealing as actual brain activity in elucidating differences in processing of different types of social relationships.
Small activation differences in the friend > kin condition were also found in the somatosensory cortex, particularly in the left hemisphere. As all participants were right handed, we speculate that this difference may represent increased activation related to hand movements necessary to respond to the behavioral task and is thus not especially informative. Perhaps the increased levels of cognition complexity associated with friends vs kin mentalizing led to greater uncertainty in decision making, leading participants to make more changes in their responses, thereby causing greater fluctuations in hand movement. If this was the case, we might expect to see differences in RTs between the friend and kin conditions, with friend assessments taking longer due to this increased uncertainty; however, as no such differences were found, this finding may warrant future investigation.
When looking at the parametric activations across different levels of the social network, different regions were found to activate within friends than within kin at different network levels. It must be noted that these analyses do not directly examine quantitative differences between kin and friend activation levels (i.e. the role of the “Main Effects” analyses above), but rather show how brain activity varies linearly across the different network layers within friends, and within kin, demonstrating how these patterns are unique to each relationship group. As participants thought about friends at closer network levels (i.e. those with whom they had more frequent contact and were emotionally closer to) activation increased in the vMPFC, lingual gyri, and somatosensory cortices. As mentioned earlier, the vMPFC has been associated with self-referential thought about similar others and general mentalizing (theory of mind) cognitions (Gallagher and Frith, 2003; Mitchell et al., 2006). Such activation is in line with past findings that not only are friends more similar than non-friends but also that the closer a friend is in the social network the more similar they are likely to be (. McPherson et al., 2001; Curry and Dunbar, 2013a) and thus more likely to trigger self-referential thought. While the data on personality differences collected here did not find significant personality differences across the layers, the non-significant trend (Figure 1B), combined with the medium effect size and previous findings to this effect (ibid.), suggests that this remains a possibility. Since family members are not selected for personality traits, increases in similarity may not be present at closer levels of the kin network. The lingual gyrus, in turn, has been associated with processing of visual information, including perception and short-term memory for human faces, word identification, and theory-of-mind cognitions such as ascribing intentions to others (Brunet et al., 2000; Mechelli et al., 2000; Kozlovskiy et al., 2014).
Both the vMPC and bilateral sections of the lingual gyri are regions previously associated with theory of mind cognition. The fact that thinking about closer friends activates these regions to a greater extent than thinking about more distant friends also suggests that individuals may be expending more effort in evaluating emotions and intentions with respect to friends at these closer layers. The same parametric activation does not occur with kin, implying again that social cognition about friends, and particularly close friends, is qualitatively different than social cognition relating to kin. It is these friends at the closest layers of the social network who may require the greatest amount of relationship maintenance, and cognitive resources, to preserve their most intimate network position (Roberts and Dunbar, 2011). As in the friend > kin contrast, the sensorimotor cortex was also activated to a greater extent with friends at closer network layers than at distant layers, suggesting that close friends may be driving the overall differences found in earlier whole-brain analyses. The reason for this differential activation, however, remains unclear at this point.
When it came to social cognition about kin at different network layers, parametric activation showed that as individuals thought about more distant kin, activation increased in the Precuneus and PCC. The posterior cingulate has been associated with viewing personally familiar stimuli and recall of family/friends (Maddock et al., 2001; Gobbini and Haxby, 2007), while the precuneus region has been implicated in perspective taking, thinking about both the self and others (Cavanna and Trimble, 2006; Araujo et al., 2013), and has been found to be more strongly engaged during mentalizing about emotions as opposed to intentions (Atique et al., 2011). The fact participants had greater activation in this region with ever more distant kin may suggest that they are expending more effort in thinking about them and are possibly focusing on emotional aspects of the relationship to a greater extent. We speculate that this could be the result of a greater obligation felt toward kin than toward friends, inducing higher motivation to evaluate aspects of the relationship even at the most distal levels of the social network and irrespective of how emotionally close to the participant they actually are. This may be driven by the fact that there likely exists greater dissonance between knowledge of a kin member and felt obligation to know that kin member—dissonance that may increase as we think about ever more distant kin. We may have equally poor knowledge of distant friends, but, with equally low levels of obligation felt toward those friends, there is less dissonance. It is this dissonance that may be underlying the parametric activation in the PCC.
The current study showed, for the first time, that friendship relationships seem to be cognitively unique to similar kin relationships. This finding also suggests that maintaining kin relationships may be less cognitively involved overall—a finding that may explain previous observations that kin relationships are less likely to degrade over time and with reduced levels of contact, compared to friend relationships (Roberts and Dunbar, 2011). This study was, of course, performed using only female participants, and it could be that males behave differently. Past research suggests that females differ from males in their mentalizing and theory of mind abilities (Baron-Cohen et al., 2001; Krach et al., 2009), as well as the structure and composition of their social networks (Stiller and Dunbar, 2007). Because of this, this particular study was limited to females so to as to eliminate possible sex confounds, thus limiting the generalizability of these results. It would be interesting to see whether males also differ in the processing of social information of friends vs kin, and therefore in whether kinship schema are used in the same way by both sexes. If this is found to be the case, it would suggest that kin schema may be used to help extend human social networks beyond natural community sizes previously imposed by cognitive constraints.
Acknowledgements
The data are available upon request from Rafael Wlodarski (email: rafael.wlodarski@psy.ox.ac.uk). We wish to thank Emily Connolly for valuable input into the imaging protocol design and analysis. RW designed the research, performed the research, analyzed the data and wrote the article. R.D. designed the research and wrote the article.
Funding
R.W. and R.D. are funded by an ERC (European Research Council) Advanced Investigator grant (grant no. 295663) awarded to R.D.
Conflict of interest. None declared.
References
- Araujo H.F., Kaplan J., Damasio A.R. (2013). Cortical midline structures and autobiographical-self processes: An activation-likelihood estimation meta-analysis. Frontiers in Human Neuroscience 7, 548.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A. (2008). On the evolutionary origins of executive functions. Brain Cognition 68, 92–9. [DOI] [PubMed] [Google Scholar]
- Atique B., Erb M., Gharabaghi A., Grodd W., Anders S. (2011). Task-specific activity and connectivity within the mentalizing network during emotion and intention mentalizing. Neuroimage 55, 1899–911. [DOI] [PubMed] [Google Scholar]
- Barnard A. 2009. Social origins: sharing exchange and kinship In: Botha R., Knight C., editors. The Cradle of Language. Oxford: Oxford University Press, pp. 219–35. [Google Scholar]
- Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. (2001). The “Reading the Mind in the Eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry 42, 241–51. [PubMed] [Google Scholar]
- Bartels A., Zeki S. (2000). The neural basis of romantic love. Neuroreport 11, 3829–34. [DOI] [PubMed] [Google Scholar]
- Bartels A., Zeki S. (2004). The neural correlates of maternal and romantic love. Neuroimage 21, 1155–66. [DOI] [PubMed] [Google Scholar]
- Brashears M.E. (2013). Humans use compression heuristics to improve the recall of social networks. Science Report 3, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet E., Sarfati Y., Hardy-Baylé M.C., Decety J. (2000). A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage 11, 157–66. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–83. [DOI] [PubMed] [Google Scholar]
- Curry O.S., Dunbar R.I.M. (2013a). Do birds of a feather flock together?: The relationship between similarity and altruism in social networks. Human Nature 24, 336–47. [DOI] [PubMed] [Google Scholar]
- Curry O.S., Dunbar R.I.M. (2013b). Sharing a joke: the effects of a similar sense of humor on affiliation and altruism. Evolution and Human Behavior 34, 125–9. [Google Scholar]
- Curtis C.E., D’Esposito M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences 7, 415–23. [DOI] [PubMed] [Google Scholar]
- Damasio A.R., Anderson S.W., Tranel D. (1993). The frontal lobes In: Heilman K., Valenstein E., editors. Clinical Neuropsychology. New York: Oxford University Press, pp. 417–65. [Google Scholar]
- DeBruine L.M., Jones B.C., Perrett D.I. (2005). Women’s attractiveness judgments of self-resembling faces change across the menstrual cycle. Hormones and Behavior 47, 379–83. [DOI] [PubMed] [Google Scholar]
- Denny B.T., Kober H., Wager T.D., Ochsner K.N. (2012). A Meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan M.B., Oswald F.L., Baird B.M., Lucas R.E. (2006). The mini-IPIP scales: Tiny-yet-effective measures of the Big Five factors of personality. Psychological Assessment 18, 192–203. [DOI] [PubMed] [Google Scholar]
- Dunbar R.I.M. (1992). Neocortex size as a constraint on group size in primates. Journal of Human Evolution 22, 469–93. [Google Scholar]
- Dunbar R.I.M. (1993). Coevolution of neocortical size, group size and language in humans. Behavioral and Brain Sciences 16, 681–735. [Google Scholar]
- Dunbar R.I.M., Arnaboldi V., Conti M., Passarella A. (2015). The structure of online social networks mirrors those in the offline world. Social Networks 43, 39–47. [Google Scholar]
- Fisher H.E., Aron A., Brown L.L. (2005). Romantic love: an FMRI study of a neural mechanism for mate choice. Journal of Comparative Neurology 493, 58–62. [DOI] [PubMed] [Google Scholar]
- Gallagher H.L., Frith C.D. (2003). Functional imaging of “theory of mind.”. Trends in Cognitive Sciences 7, 77–83. [DOI] [PubMed] [Google Scholar]
- Gobbini M.I., Haxby J.V. (2007). Neural systems for recognition of familiar faces. Neuropsychologia 45, 32–41. [DOI] [PubMed] [Google Scholar]
- Gobbini M.I., Leibenluft E., Santiago N., Haxby J.V. (2004). Social and emotional attachment in the neural representation of faces. Neuroimage 22, 1628–35. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. (1996). The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philosophical Transactions of the Royal Society B 351, 1445–53. [DOI] [PubMed] [Google Scholar]
- Jenkins A.C., Mitchell J.P. (2010). Mentalizing under uncertainty: dissociated neural responses to ambiguous and unambiguous mental state inferences. Cerebral Cortex 20, 404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P.R., Brady M., Smith S.M. (2002). Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–41. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. (2012). FSL. Neuroimage 62, 782–90. [DOI] [PubMed] [Google Scholar]
- Kanai R., Bahrami B., Roylance R., Rees G. (2012). Online social network size is reflected in human brain structure. Proceedings of the Royal Society B: Biological Sciences 279, 1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D.B. (1978). Homophily, selection, and socialization in adolescent friendships. American Jouranl of Sociology 84, 427–36. [Google Scholar]
- Kozlovskiy S.A., Pyasik M.M., Korotkova A.V., Vartanov A.V., Glozman J.M., Kiselnikov A.A. (2014). Activation of left lingual gyrus related to working memory for schematic faces. International Journal of Psychophysiology 94, 241. [Google Scholar]
- Krach S., Blümel I., Marjoram D., et al. (2009). Are women better mindreaders? Sex differences in neural correlates of mentalizing detected with functional MRI. BMC Neuroscience 10, 9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen F.M., Tu P.C., Buckner R.L. (2010). Clan mentality: evidence that the medial prefrontal cortex responds to close others. Journal of Neuroscience 30, 13906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J., Korstjens A.H., Dunbar R.I.M. (2007). Group size, grooming and social cohesion in primates. Animal Behaviour 74, 1617–29. [Google Scholar]
- Leibenluft E., Gobbini M.I., Harrison T., Haxby J.V. (2004). Mothers’ neural activation in response to pictures of their children and other children. Biological Psychiatry 56, 225–32. [DOI] [PubMed] [Google Scholar]
- Lou H.C., Luber B., Crupain M., et al. (2004). Parietal cortex and representation of the mental Self. Proceedings of the National Academy Sciences 101, 6827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin A., Dunbar R.I.M. (2013). Sex and gender as factors in romantic partnerships and best friendships. Jouranl of Relationships Research 4, 1–10. [Google Scholar]
- Maddock R.J., Garrett A.S., Buonocore M.H. (2001). Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104, 667–76. [DOI] [PubMed] [Google Scholar]
- McPherson M., Smith-Lovin L., Cook J.M. (2001). Birds of a feather: homophily in social networks. Annual Review of Sociology 27, 415–44. [Google Scholar]
- Mechelli A., Humphreys G.W., Mayall K., Olson A., Price C.J. (2000). Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proceedings of the Royal Society B: Biological Sciences 267, 1909–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.L., Masten C.L., Ma Y., et al. (2013). Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Social Cognitive and Affective Neuroscience 8, 446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.L., Spunt R.P., Berkman E.T., Taylor S.E., Lieberman M.D. (2012). Evidence for social working memory from a parametric functional MRI study. Proceedings of the National Academy Sciences 109, 1883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miritello G., Moro E., Lara R., et al. (2013). Time as a limited resource: communication strategy in mobile phone networks. Social Networks 35, 89–95. [Google Scholar]
- Mitchell J.P., Banaji M.R., Macrae C.N. (2005). The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience 17, 1306–15. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Macrae C.N., Banaji M.R. (2006). Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 50, 655–63. [DOI] [PubMed] [Google Scholar]
- Platek S.M., Kemp S.M. (2009). Is family special to the brain? An event-related fMRI study of familiar, familial, and self-face recognition. Neuropsychologia 47, 849–58. [DOI] [PubMed] [Google Scholar]
- Platek S.M., Loughead J.W., Gur R.C., et al. (2006). Neural substrates for functionally discriminating self-face from personally familiar faces. Human Brain Mapping 27, 91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.L., Lewis P.A., Dunbar R.I.M., García-Fiñana M., Roberts N. (2010). Orbital prefrontal cortex volume correlates with social cognitive competence. Neuropsychologia 48, 3554–62. [DOI] [PubMed] [Google Scholar]
- Powell J.L., Lewis P.A., Roberts N., García-Fiñana M., Dunbar R.I.M. (2012). Orbital prefrontal cortex volume predicts social network size: an imaging study of individual differences in humans. Proceedings of the Royal Society B: Biological Sciences 279, 2157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S.G.B., Dunbar R.I.M. (2011). The costs of family and friends: an 18-month longitudinal study of relationship maintenance and decay. Evolution and Human Behavior 32, 186–97. [Google Scholar]
- Roberts S.G.B., Arrow H., Gowlett J., Lehmann J., Dunbar R.I.M. (2014). Close social relationships: towards an integrated perspective In: Dunbar R.I.M., Gowlett J., editors. Lucy to Language: The Benchmark Papers. Oxfors: Oxford University Press, pp. 1–58. [Google Scholar]
- Roberts S.G.B., Dunbar R.I.M., Pollet T.V., Kuppens T. (2009). Exploring variation in active network size: Constraints and ego characteristics. Social Networks 31, 138–46. [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, 208–19. [DOI] [PubMed] [Google Scholar]
- Stiller J., Dunbar R.I.M. (2007). Perspective-taking and memory capacity predict social network size. Social Networks 29, 93–104. [Google Scholar]
- Sutcliffe A., Dunbar R.I.M., Binder J., Arrow H. (2012). Relationships and the social brain: Integrating psychological and evolutionary perspectives. British Journal of Psychology 103, 149–68. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. (2011). A dissociation between social mentalizing and general reasoning. Neuroimage 54, 1589–99. [DOI] [PubMed] [Google Scholar]
- Vendrell P., Junqué C., Pujol J., Jurado M.A., Molet J., Grafman J. (1995). The role of prefrontal regions in the Stroop task. Neuropsychologia 33, 341–52. [DOI] [PubMed] [Google Scholar]
- Vogeley K., Bussfeld P., Newen A., et al. (2001). Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage 14, 170–81. [DOI] [PubMed] [Google Scholar]
- Wagner D.D., Haxby J.V., Heatherton T.F. (2012). The representation of self and person knowledge in the medial prefrontal cortex. Wiley Interdisciplinary Reviews: Cognitive Science 3, 451–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Mao L., Ma Y., et al. (2012). Neural representations of close others in collectivistic brains. Social Cognitive and Affective Neuroscience 7, 222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W.X., Sornette D., Hill R.A., Dunbar R.I.M. (2005). Discrete hierarchical organization of social group sizes. Proceedings of the Royal Society B: Biological Sciences 272, 439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]