Abstract
Previous studies demonstrated that excitatory (high frequency) offline transcranial magnetic stimulation (TMS) over the left and right dorsolateral prefrontal cortex (DLPFC) modulates attention allocation on threatening stimuli in non-clinical samples. These studies only employed offline TMS protocol that did not allow investigating the effect of the stimulation on the early stage of threat processing. In this study, the role of the right and left dorsolateral prefrontal cortex in early threat processing was investigated in high and low anxious individuals by means of an inhibitory single-pulse online TMS protocol. Our results demonstrated the role of the left DLPFC in an early stage of threat processing and that this effect is modulated by individuals’ anxiety level. The inhibitory stimulation of the left DLPFC determined a disengagement bias in high anxious individuals, while the same stimulation determined an attentional avoidance in low anxious individuals. The findings of the present study suggest that right and left DLPFC are differently involved in early threat processing of healthy individuals.
Keywords: attention, anxiety, DLPFC, TMS, threat
Introduction
Neurocognitive models of selective attention to threat (Bishop et al., 2004; Bishop, 2008) foresee two systems responsible for attention allocation. The first system would be related to a bottom-up, stimulus-driven, rapid allocation of attention toward potential threat in the environment and would be associated with the activity of limbic areas, including the amygdala. The second system would be related to a top-down attentional control, responsible for maintenance of attention via the inhibition of task-irrelevant threat-related information, and would be linked with activity of the dorsolateral prefrontal cortex (DLPFC). In this perspective, the attentional biases for threat (ABTs) frequently observed in clinical (patients with anxiety disorders) and non-clinical population (for a review, see Cisler and Koster, 2010) would be related to an increase of amygdala activation (stimulus-driven system) and a reduced activity of the DLPFC (top-down control; Bishop et al., 2004).
Several neuroimaging data (Bishop et al., 2004; Bishop et al., 2007; Fales et al., 2008; Peers et al., 2013) are consistent with this kind of models. Bishop et al. (2004) reported that healthy people with high state anxiety levels show reduced recruitment of the left lateral prefrontal cortex when processing task-irrelevant threatening distractors, compatible with the idea that prefrontal cortex can allocate attention on less salient task-related stimuli in presence of salient emotional distractors. Similarly, Bishop et al. (2007) revealed that high trait anxiety is associated with a reduced left DLPFC activity in response to threatening distractors. Both these studies suggested that DLPFC, in particular in the left hemisphere, is involved in the attentional control in presence of threatening stimuli. On the other hand, Fales et al. (2008) observed an increase of activity in the right DLPFC when healthy subjects have to ignore fear-related stimuli in an attention task. According to the authors, the enhanced right DLPFC activation could be due either to increased selective attention for threat or to an attempt to suppress amygdala activity. In a similar vein, Peers et al. (2013) confirmed that DLPFC is involved in controlling deployment of attention over threatening stimuli, but did not specifically assess the lateralization of DLPFC involvement.
Taken together, such neuroimaging data supported the hypothesis that DLPFC and related areas (ACC and orbitofrontal cortex), particularly in the right hemisphere, are involved in attention allocation, and are related to the ability to control the attentional focus in presence of threatening stimuli. Nevertheless, these neuroimaging studies did not clarify the specific role of right and left DLPFC in attention allocation. Brain stimulation studies, instead, provided direct information about the complementary, if not opposite, roles played by the DLPFC of the two hemispheres in attentional control for threatening information. It has been shown that offline high-frequency (excitatory) repetitive transcranial magnetic stimulation (rTMS) over the right DLPFC can reduce the ability to inhibit processing of negative information (sad faces) in healthy individuals performing a Negative Affective Priming task (Leyman et al., 2009). Similarly, in a combined rTMS and fMRI study, De Raedt et al. (2010) observed that high-frequency rTMS over the right DLPFC resulted in impaired disengagement from threat (angry faces) in healthy women, and was associated with decreased activation within the right DLPFC, dorsal anterior cingulate cortex and left superior parietal gyrus, and with increased activity within the right amygdala. In the same study (De Raedt et al., 2010), high-frequency rTMS over the left DLPFC determined a reduction of attentional engagement toward threat and was associated with increased activation of the right DLPFC, right superior parietal gyrus, dorsal anterior cingulate cortex and the left orbitofrontal cortex (OFC). The different effects of rTMS over the right and the left DLPFC suggested that these areas play a different role in ABTs. In particular, the right DLPFC might be responsible for attentional allocation on threatening stimuli, whereas the left DLPFC might reduce attentional engagement toward threat. The role of right DLPFC in processing negative stimuli (such as threatening stimuli) is compatible with predictions of the ‘valence-asymmetry hypothesis’, according to which negative emotions are preferentially processed by the right hemisphere (Davidson and Irwin, 1999). This hypothesis also foresees that positive emotions would be processed by the left hemisphere (Davidson and Irwin, 1999), suggesting that the two hemispheres are differently involved in emotional processing, but no available TMS studies employed positive emotional stimuli.
In all the previous studies rTMS was applied using an offline protocol and assessing selective attention (attentional biases for threat) before and after brain stimulation. However, offline protocols did not allow investigating which stage of attention allocation toward threatening stimuli is affected by rTMS. Indeed, in a recent study combining rTMS and magnetoencephalography, Zwanzger et al. (2014) showed that stimulation over the right DLPFC led to differential emotional responses in early (110 –170 ms) affective processing (particularly in temporal cortex regions) during the presentation of fearful compared to neutral faces. These results are compatible with the idea that DLPFC is selectively related to an early top–down control of attentional process, and would require further elaboration of the attentional control model.
The aim of this study is to test whether the left and the right DLPFC stimulation could affect attention allocation at early processing stages in healthy volunteers. For this purpose, we adopted a within-subject online stimulation protocol in which we delivered single-pulse (inhibitory) TMS (spTMS) over the right or left DLPFC 100 or 200 ms after stimulus onset. If TMS over the DLPFC can affect early fear-related attention (time-interval between 110 and 170 ms; Zwanzger et al., 2014), we hypothesized that spTMS affects attention allocation when delivered 100 ms but not 200 ms after stimulus onset. On the basis of previous rTMS studies, we also expected that left stimulation should determine an increase of attention allocation on threatening stimuli (e.g. disengagement bias), whereas the right stimulation should determine a reduction of attentional bias (or attentional avoidance) for threat. Moreover, since previous studies (Vanderhasselt et al., 2011) on healthy individuals showed that ABT induction after HF-rTMS over the right DLPFC is stronger in individuals with high anxiety levels, we tested both high and low anxious participants to evaluate whether baseline anxiety levels modulated spTMS effects. It is possible to expect that high anxious individuals showed an increase of attention allocation on threatening stimuli during left DLPFC stimulation, whereas no specific prediction can be made for low anxious individuals.
In our experimental paradigm, we presented threatening stimuli for 700 ms. In non-clinical individuals highly threatening stimuli typically elicit ABTs at short presentation times (Koster et al., 2006), whereas at longer presentation times (e.g. 1500 ms, Mogg et al., 1997) they usually do not. Therefore by presenting threatening stimuli for 700 ms, and recording individuals’ responses thereafter, we could expect a strongly reduced likelihood of observing ABTs in the sham condition, thus ascribing any ABTs after TMS to a direct effect of the experimental manipulation.
Materials and methods
Participants
Participants were carefully screened prior to inclusion in the study. Participants with current or past psychiatric or neurological disorders (investigated by a self-administered questionnaire complemented by an interview conducted by a trained psychologist) were excluded. All participants were medication free and had no history of neurosurgical interventions, pace-maker implantation or other metal or magnetic objects in the body in line with the safety guidelines for TMS use (Rossi et al., 2009).
Twenty-five healthy right-handed female participants aged between 19 and 30 years (mean age= 21.50 years, SE= .52) were recruited to participate in this study. Consistent with previous studies (e.g. Vanderhasselt et al., 2011), only female participants were included for reasons of homogeneity and sex differences in brain activation during emotional-related tasks.
All participants received a complete description of the study procedure, which was approved by the institutional ethics committee of the Department of Psychology, Second University of Naples and was conducted in accordance with the ethical standards of Helsinki Declaration. All participants gave their written informed consent.
State-Trait Anxiety Scale
State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983) consists of two 20-item scales assessing state and trait anxiety. The STAI-State subscale requires respondents to rate how they feel ‘right now … at this moment’ using a four-point scale (1= not at all, 4= very much so) in response to a series of self-descriptive statements. The STAI-Trait subscale asks respondents to rate how they ‘generally’ feel using a four-point scale (1= almost never, 4= almost always) in response to a series of self-descriptive statements. These subscales have been demonstrated to be valid and to have solid psychometric properties.
Exogenous cueing task
All participants underwent an exogenous spatial cueing task with threatening and non-threatening cues.
Threatening (n = 20) and non-threatening (n = 20) images were selected from the International Affective Picture System (Lang et al., 2008), on the basis of their valence and arousal ratings: threatening stimuli were selected among those with negative valence (<4.5) and high arousal (6–9) scores, whereas selected non-threatening stimuli had intermediate valence and arousal scores (>4.5 and < 5.5). Each stimulus was presented 4 times, for a total number of 160 trials.
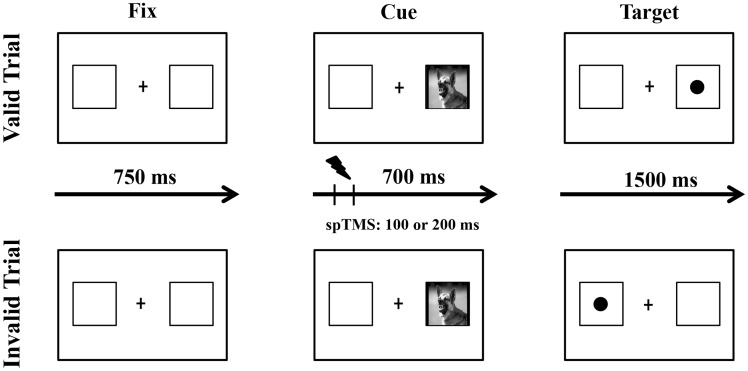
Each trial consisted in a fixation cross (+) flanked by two blank squares (340 × 340 pixel) on its right and left side (300 pixel from the fixation cross; visual angle: 11.30° at a viewing distance of 50 cm) presented for 750 ms and followed by a cue (a threatening or non-threatening image; 300 × 300 pixel) which randomly appeared for 700 ms in one of the two squares. Each image appeared twice in the right and twice in the left square. After cue presentation, a dot (1 cm) appeared in one of the two squares, in the same (valid trial) or in the opposite (invalid trial) position as the cue for 1500 ms or until participants’ response (Figure 1). Inter-trial interval was 750 ms. In the present study, we presented 128 valid trials (80%; 64 threatening and 64 non-threatening) and 32 invalid trials (20%; 16 threatening and 16 non-threatening) in a randomized order. Although some studies also included uncued trials (Stormark et al., 1995; Amir et al., 2003), we only employed cued trials in our experimental design to increase comparability with previous TMS studies on ABT (e.g. De Raedt et al., 2010; Vanderhasselt et al., 2011).
Fig. 1.
Schematic overview of the stimulation protocol during the exogenous cueing task. Examples of threatening valid (top row) and invalid trials (bottom row) are depicted. The trial duration was of 2950 ms.
Neuronavigation and TMS
On-line single pulse TMS was delivered 100 ms or 200 ms after stimulus onset by means of a 70-mm figure-eight coil connected to a Magstim Rapid 2 stimulator (Magstim Company) producing a maximum output of 3.5 T at the coil surface. In keeping with safety recommendations (Rossi et al., 2009), stimulation intensity was set at 110% of individual motor threshold and ranged from 51 to 70% of the maximum stimulator output. Motor threshold was defined as the lowest TMS intensity capable of evoking a muscle twitch in the contralateral hand in 8 of 10 consecutive trials. The brain targets and the correspondent scalp sites were localized by means of Softaxic Optic (EMS) neuronavigation system. Neuronavigation was carried out on estimated-MRI stereotaxic brain templates based on a sample of 65 scalp points digitized by means of a Polaris Vicra (Northern Digital) digitizer.
Mean Talairach coordinates of brain targets were as follows: left DLPFC: x= −45, y = 30, z = 31; right DLPFC: x = 45, y = 30, z = 31.
In the sham condition, the coil was placed at an angle of 90° on the vertex (x = 08, y = −22, z = 74), resting on the scalp with only one edge, so that the coil focus was directed away from participant head. Coil position on the stimulation sites was continuously monitored by means of the neuronavigator system during the entire experimental session. In all conditions, a mechanical arm fixed to a tripod held the coil.
Procedure
Each participant underwent all three stimulation conditions, with 1-week interval between two subsequent sessions to avoid carry-over effects from the previous stimulation.
Participants completed the State Trait Anxiety Inventory (only in the first session) and then received spTMS while they performed the exogenous cueing task. The spTMS was delivered over the right or the left DLPFC (real stimulation) or orthogonally to the vertex (sham stimulation). The order of stimulation conditions was counterbalanced across subjects.
In all sessions, participants were seated comfortably in a quite room with the head on a chinrest, facing the monitor at a distance of about 50 cm.
During the task, participants were required to keep their eyes on the fixation cross and to respond, as fast and accurately as possible, pressing a right key (l) on the keyboard when the target (dot) appeared on the right and a left key (a) when the target appeared on the left. For each trial, both accuracy and response times (RTs) were recorded.
Results
Group characteristics
Three participants were excluded from the final sample because they did not complete all the stimulation sessions. In the remaining 22 participants, mean trait and state anxiety scores were 42.14 (SE = 1.68) and 37.41 (SE = 1.54), respectively. Participants were divided into two subgroups (low anxious and high anxious) based on a median split of their trait anxiety scores (median STAI = 41.50). Eleven participants were included in low anxious group (trait anxiety: M = 35.73, SE = 0 .95) and the remaining 11 participants were included in high anxious group (trait anxiety: M = 48.55, SE= 1.64).
RTs analysis
Data were cleaned removing errors trials (1% of the total) and RTs outliers (TRs < 150 and >1000; 6% of the total) as in previous studies (Sagliano et al., 2014). The proportion of discarded trials did not significantly differ across the three conditions (chi-square < 1).
A preliminary analysis has been conducted to exclude the effect of stimulation order on RTs. We run two separate 3 × 2 × 2 × 2 ANOVAs with three within-subject factors (Stimulation: right DLPFC, left DLPFC, sham; valence: threatening, non-threatening; validity: valid, invalid) and a between-subject factor (stimulation order: right stimulation before left stimulation, left stimulation before right stimulation) on RTs for the two spTMS timings (100 and 200 ms). The results (see Supplementary Table 1) showed that the main effect of stimulation order was not significant, and that this variable did not interact significantly with the remaining variables. On this basis we did not consider stimulation order in all the following analyses.
To investigate the effect of anxiety, two separate 2 × 2 × 2 ANOVAs with two within-subject factors (valence: threatening, non-threatening; validity: valid, invalid) and one between-subject factor (anxiety: low, high) on RTs in sham condition were conducted for the two spTMS timings (100 and 200 ms). These analyses revealed a significant main effect of validity on RTs when sham pulse was delivered at 100 ms, F(1,20)= 11.30, P < 0 .01, η2p = 0.36, and when it was delivered at 200 ms, F(1,20)= 5.82, P = 0 .03, η2p = .23, as participants were faster to respond to valid (100 ms: M = 348.21, SE = 9.98; 200 ms: M = 355.97, SE = 10.31) compared than invalid trials (100 ms: M= 376.06, SE= 14.13; 200 ms: 380.13, SE= 14.96). These ANOVAs did not reveal any other main effects or interactions (P > 0.05).
Two separate 3 × 2 × 2 × 2 ANOVAs with three within-subject factors (Stimulation: right DLPFC, left DLPFC, sham; valence: threatening, non-threatening; validity: valid, invalid) and one between-subject factor (anxiety: low, high) on RTs were conducted for the two spTMS timings (100 and 200 ms). All significant effects were investigated by means of Bonferroni corrected comparisons (observed power for all the following analyses is reported in the Supplementary Table 2).
Table 1 shows mean reaction times and standard error on the exogenous cueing task. The ANOVA on RTs when spTMS was delivered at 100 ms showed a significant main effect of validity, F(1,20)= 21.14, P < .001, η2p = .51, as individuals were faster to respond to valid trials (M = 338. 37, SE = 9.08) compared to invalid trials (M = 367.01, SE = 11.89), and a significant stimulation × valence × validity × anxiety group interaction, F(2,40)= 7.43, P = 0 .002, η2p = 0 .27. Bonferroni corrected comparisons revealed that, during the left DLPFC stimulation, individuals with low levels of anxiety were slower to respond to threatening valid trials (M = 365.13, SE = 16.92) compared to non-threatening valid trials (M = 350.46, SE = 15.35; P = 0 .02), whereas high anxious individuals were slower to respond to threatening invalid trials (M = 377.89, SE = 19.70) compared to non-threatening invalid trials (M = 353.90, SE = 18.48; P = 0.01). No other effect resulted significant (P > 0 .05).
Table 1.
Mean and Standard Error of the RTs in the exogenous cueing task as a function of anxiety group, stimulation condition, validity and valence
| LTA | HTA | |||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Invalid | ||||
| Threat | ||||
| L-DLPFC | 360.11 | 19.7 | 377.77 | 19.7 |
| R-DLPFC | 362.24 | 19.09 | 359.85 | 19.09 |
| Sham | 388.16 | 23.62 | 373.69 | 23.62 |
| Non-threat | ||||
| L-DLPFC | 369.21 | 18.48 | 353.9 | 18.48 |
| R-DLPFC | 351.13 | 18.45 | 365.67 | 18.45 |
| Sham | 362.03 | 18.34 | 380.37 | 18.34 |
| Valid | ||||
| Threat | ||||
| L-DLPFC | 365.14 | 16.92 | 323.69 | 16.92 |
| R-DLPFC | 328.29 | 15.86 | 324.7 | 15.86 |
| Sham | 347.89 | 14.54 | 349.54 | 14.54 |
| Non-threat | ||||
| L-DLPFC | 350.46 | 15.35 | 320.19 | 15.35 |
| R-DLPFC | 330.94 | 16.58 | 324.23 | 16.58 |
| Sham | 349.14 | 14.4 | 346.26 | 14.4 |
The same ANOVA conducted on RTs when spTMS was delivered at 200 ms only showed a significant main effect of validity F(1,20) = 14.29, P < 0 .001, η2p = 0.42, as individuals were faster to respond to valid trials (M = 347. 61, SE = 9.2) compared to invalid trials (M = 372.48, SE = 12.19). No other significant main effects or interactions resulted significant (P > 0 .05).
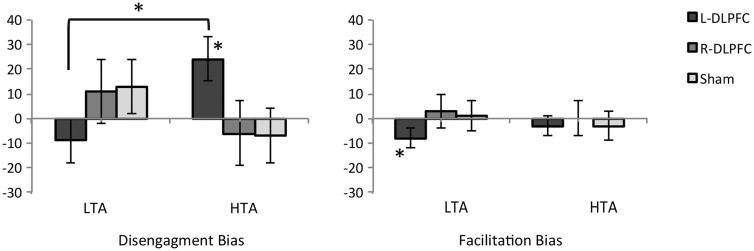
To further test our hypotheses, bias scores were calculated according to the procedure used by Koster et al. (2006): facilitation score (RTvalid/non-threatening cue - RTvalid/threatening cue) and disengagement score (RTinvalid/threatening cue - RTinvalid/non-threatening cue). Because previous analysis on RTs for the trials in which the pulse was delivered at 200 ms after stimulus onset only showed a significant main effect of validity, bias scores were calculated only for trials in which the pulse was delivered at 100 ms. Mean bias scores and standard error are reported in Figure 2.
Fig. 2.
Means (and standard errors) of facilitation bias (FB) and disengagement bias scores (DB; negative values for both scores would indicate an avoidance bias) as a function of anxiety group (low trait anxiety: LTA; high: HTA trait anxiety) and stimulation condition (right: R-DLPFC; left: L-DLPFC; sham).
*means significantly different from zero or between groups P < 0.05.
A 3 × 2 ANOVA with one within-subject factor (Stimulation: right DLPFC, left DLPFC, sham) and one between-subject factor (anxiety: low and high) was separately conducted on facilitation and disengagement scores (observed power for this analysis is reported in the Supplementary Table 3). This analysis did not show any significant main effect or interaction on facilitation score (P < 0 .05), whereas it showed a significant stimulation × anxiety group interaction on disengagement score, F(2,40)= 4.56, P = 0 .02, η2p = 0 .19. Bonferroni corrected pairwise comparisons revealed that the high anxious individuals showed a greater disengagement bias compared to low anxious individuals only during left DLPFC stimulation (P = 0 .02). Moreover, disengagement bias was greater after left DLPFC stimulation compared to sham condition only in high anxious group (P = 0 .02).
Single-sample t-test comparison was also used to verify if bias scores were significantly greater than zero. This analysis revealed that only two significant bias scores in the left DPLFC condition were greater than zero: facilitation bias (negative score indicating attentional avoidance) in low anxious individuals, t(10) = −2.65, P = 0.02, and disengagement bias in high anxious individuals, t(10) = −2.65, P = 0 .01. No score resulted significantly different from zero during the right DPLFC stimulation.
Discussion
The aim of this study was to elucidate the role of right and left DLPFC in modulating selective attention for threat in healthy individuals. Our main results can be summarized as follows: (1) spTMS affected threat elaboration when delivered during an early stage of stimulus processing; (2) only the stimulation over the left DLPFC was effective in modulating attention allocation for threatening stimuli; (3) the effect of stimulation over the left DLPFC differed in participants with high or low trait anxiety level. In particular, the left DLPFC stimulation led to a disengagement bias in high anxious individuals and to an avoidance bias in low anxious individuals.
Before tackling possible implications of our main stimulation findings, however, it is worth underlining the lack of attentional biases in both high and low anxious individuals in the sham stimulation condition. This finding was likely related to the presentation time used in the present study (700 ms), and reinforced the idea that our findings of ABTs during left DLPFC stimulation were related to the stimulation protocol employed in this study.
Our first main finding demonstrated that the spTMS over the DLPFC affected early threat elaboration. This result supports the hypothesis that the DLPFC is involved in a top-down process of early attention allocation to threat, as suggested by a recent combined TMS-magnetoencephalography study (Zwanzger et al., 2014). Such findings would confirm that DLPFC plays a role in top-down amygdala regulation (De Raedt et al., 2010). However, the present study would demonstrate that early involvement of DLPFC in threat processing is dependent on hemispheric functional asymmetry in emotional processing.
The finding of a selective effect of the left DLPFC inhibition in modulating ABTs would be in line with previous models positing a different role of the left and right hemisphere in emotion processing (Davidson and Irwin, 1999). In our experiment, left DLPFC inhibition could have indirectly increased right DLPFC activity leading to ABTs, as demonstrated by previous TMS-fMRI study (De Raedt et al., 2010). Instead, the absence of any effect on ABTs following right DLPFC could be due to a general interference on threat processing (with reduced attention allocation on negative stimuli), in line with our predictions. Further studies should employ high-frequency online stimulation protocol over the right DLPFC in order to verify this interpretation.
For a full comprehension of the present findings, however, it is necessary to consider that the effect of inhibition of the left DLPFC differed substantially as a function of individual trait anxiety. During TMS stimulation of the left DLPFC, high anxious individuals manifested a tendency to process threatening stimuli longer. This disengagement bias is in line with our predictions and with the findings from previous TMS studies. Recently, Balconi and Ferrari (2012) observed greater accuracy and lower RTs for positive stimuli compared to negative ones in retrieving emotional words from memory in high anxious individuals after (excitatory) HF-rTMS over the left DLPFC. As Balconi and Ferrari suggested, such results would suggest that enhanced activation of the left DLPFC might reduce the assumed superiority of the right DLPFC in high anxious individuals, restoring balance between the two hemispheres. In this view, we obtained a complementary pattern of results using inhibitory online stimulation of the left DLPFC, with an imbalance in favour of the right DLPFC and an increased elective attention to threatening stimuli. This interpretation is also compatible with findings reported by De Raedt et al. (2010), where the (excitatory) HF-rTMS stimulation over the left DLPFC reduced attentional engagement associated with high activity of the right DLPFC. In the same study, De Raedt et al. (2010) showed that high-frequency (excitatory) rTMS over the right DLPFC determined an impaired disengagement from threatening stimuli (angry faces) associated with reduced activity of the right DLPFC and the cingulate cortex, but with increased activity in the amygdala.
This pattern of dynamic interplay between the two hemispheres is consistent with the valence-asymmetry hypothesis (Davidson and Irwin, 1999) and with the idea that selective attention for threat shown by clinical anxious individuals may be due to an unbalance between right and left DLPFC (see also Balconi and Ferrari, 2012).
In low anxious individuals the left (inhibitory) DLPFC stimulation determined an avoidance bias, i.e. it led participants to allocate their attention away from the threat. We had no specific prediction for low anxious individuals, but we could explain the appearance of the ABT pattern in this subsample by positing that the left (inhibitory) DLPFC stimulation enhanced right DLPFC activity, thus allowing to observe the behaviour pattern typical of such individuals, i.e. a tendency to move attention away from threatening stimuli (e.g., MacLeod and Mathews, 1988; Mogg et al., 1994; Sagliano et al., 2014), even at longer presentation times (700 ms).
Taken together, our findings are in line with the idea that baseline level of anxiety could modulate selective attention for threat (Vanderhasselt et al., 2011), and would demonstrate that the same stimulation could induce an opposite effect on attentional control depending on individuals’ low or high trait anxiety level. In healthy individuals, amygdala response during threat processing is normally regulated by top-down control of the DLPFC. Anxious individuals, on the contrary, show high baseline amygdala activation due to a dysfunction of the DLPFC-amygdala circuitry leading to ABTs (Bishop et al., 2004; Vanderhasselt et al., 2011). Previous TMS studies demonstrated that interfering with the DLPFC-amygdala circuitry via DLPFC stimulation it is possible to affect the activity of the amygdala and to increase ABTs in healthy individuals (De Raedt et al., 2010). The different levels of amygdala activation in high and low anxious individuals could explain why the same pattern of stimulation is associated with an increased disengagement bias in high anxious individuals and with an increase of attentional avoidance in low anxious individuals. Nevertheless, further studies integrating inhibitory spTMS and fMRI are needed to ascertain this phenomenon.
An alternative interpretation of the difference between the effects of left stimulation in low and high anxious individuals comes from the cognitive-motivational model proposed by (Mogg and Bradley 1998). These authors proposed that ABTs are related to the valence evaluation system responsible for the attentional allocation toward threatening stimuli. This system would be involved in strategic appraisal of the stimuli, the disengagement from threat and the switch of the attention toward task-related activities. The authors suggested that anxiety might be related to a dysfunction of the valence evaluation system. In this perspective, the left DLPFC stimulation could differently affect ABTs in high and low anxious individuals due to the pre-existing differences in the valence evaluation system. However, this interpretative framework needs to be supported by empirical evidence directly examining the involvement of the DLPFC in the valence evaluation system.
Our finding of a DLPFC involvement in early threat processing may have potential clinical implications. Recent studies (Clarke et al., 2014; Heeren et al., 2015) showed that Transcranial Direct Current Stimulation over the left DLPFC could enhance the positive effects of the attentional bias modification (ABM) procedure in training individuals to allocate attention away from threat. In line with such studies, our data suggests that brain stimulation techniques might be a promising tool to enhance treatment of anxiety disorders. However, further studies should investigate the possible long-lasting effects of online spTMS combined with ABM in attentional bias and anxiety reduction. In this perspective, our finding of an early DLPFC involvement in ABT emphasizes the need to take into account the stimulation timing in reducing attention allocation on threat.
The present study also has some limitations. We acknowledge that we enrolled a relatively small number of participants, and this implied a relatively low statistical power. This, however, did not preclude the observation of a significant effect of stimulation over the left DLPFC. Moreover, to ensure homogeneity of the sample, we only included female participants and this choice might limit the generalization of our findings. Additionally, in our study we only investigated the effect of spTMS on threat processing. It would be interesting to investigate whether right and left DLPFC are differently involved in early processing of emotionally positive stimuli.
Summarizing, thanks to its good temporal resolution, TMS allowed us to evaluate how attention is allocated on threatening stimuli in the early stage of processing (100 ms). Data from the present study confirmed that online single-pulse stimulation over the left DLPFC could modify top-down early attentional control of threat as a function of baseline individual anxiety level, with apparently opposite patterns (a difficulty to remove attention from threat in high anxious individuals, and an increased attentional avoidance in low anxious individuals). These findings provide novel insights on brain mechanisms related to selective attention for threat and might be of interest for possible clinical applications.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Amir N., Elias J., Klumpp H., Przeworski A. (2003). Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat?. Behaviour Research and Therapy, 41, 1325–35. [DOI] [PubMed] [Google Scholar]
- Balconi M., Ferrari C. (2012). rTMS stimulation on left DLPFC affects emotional cue retrieval as a function of anxiety level and gender. Depress Anxiety, 29, 976–82. [DOI] [PubMed] [Google Scholar]
- Bishop S., Duncan J., Brett M., Lawrence A.D. (2004). Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience, 7,184–8. ' p [DOI] [PubMed] [Google Scholar]
- Bishop S.J. (2008). Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences, 1129, 141–52. [DOI] [PubMed] [Google Scholar]
- Bishop S.J., Jenkins R., Lawrence A.D. (2007). Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cerebral Cortex, 17, 1595–603. [DOI] [PubMed] [Google Scholar]
- Cisler J.M., Koster E.H. (2010). Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review'. Clinical Psychology Review, 30, 203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P.J., Browning M., Hammond G., Notebaert L., MacLeod C. (2014). The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: evidence from transcranial direct current stimulation'. Biological Psychiatry, 76, 946–52. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Irwin W. (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences, 3, 11–21. [DOI] [PubMed] [Google Scholar]
- De Raedt R., Leyman L., Baeken C., et al. (2010). Neurocognitive effects of HF-rTMS over the dorsolateral prefrontal cortex on the attentional processing of emotional information in healthy women: an event-related fMRI study. Biological Psychology, 85, 487–95. [DOI] [PubMed] [Google Scholar]
- Fales C.L., Barch D.M., Rundle M.M., et al. (2008). Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression'. Biological Psychiatry, 63, 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren A., Baeken C., Vanderhasselt M.A., Philippot P., de Raedt R. (2015). Impact of Anodal and Cathodal Transcranial Direct Current Stimulation over the Left Dorsolateral Prefrontal Cortex during Attention Bias Modification: An Eye-Tracking Study'. PLoS One, 10, e0124182.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster E.H., Crombez G., Verschuere B., Van Damme S., Wiersema J.R. (2006). Components of attentional bias to threat in high trait anxiety: Facilitated engagement, impaired disengagement, and attentional avoidance. Behaviour research and therapy, 44, 1757–71. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (2008) International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical report A-8. [Google Scholar]
- Leyman L., De Raedt R., Vanderhasselt M.a, Baeken C. (2009). Influence of high-frequency repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex on the inhibition of emotional information in healthy volunteers. Psychological Medicine, 39, 1019–28. [DOI] [PubMed] [Google Scholar]
- MacLeod C., Mathews A. (1988). Anxiety and the allocation of attention to threat. Quarterly Journal of Experimental Psychology A, 40, 653–70. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P., Hallowell N. (1994). Attentional bias to threat: Roles of trait anxiety, stressful events, and awareness'. The Quarterly Journal of Experimental Psychology Section A, 47,841–64. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P., De Bono J., Painter M. (1997). Time course of attentional bias for threat information in non-clinical anxiety. Behaviour research and therapy, 35, 297–303. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P. (1998). A cognitive-motivational analysis of anxiety. Behaviour research and therapy, 36, 809–48. [DOI] [PubMed] [Google Scholar]
- Peers P.V., Simons J.S., Lawrence A.D. (2013). Prefrontal control of attention to threat. Frontiers in Human Neuroscience, 7, 24.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research.'. Clinical Neurophysiology, 120, 2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagliano L., Trojano L., Amoriello K., Migliozzi M., D'Olimpio F. (2014). Attentional biases toward threat: the concomitant presence of difficulty of disengagement and attentional avoidance in low trait anxious individuals. Frontiers in Psychology 5, 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene P.R., Vagg P.R., Jacobs A.G. (1983) 'Manual for the State-Trait Anxiety Inventory (Form Y). Manual for the Statetrait Anxiety Inventory STAI, pp. 4–6. [Google Scholar]
- Stormark K.M., Nordby H., Hugdahl K. (1995). Attentional shifts to emotionally charged cues: Behavioural and ERP data. Cognition & Emotion, 9, 507–23. [Google Scholar]
- Vanderhasselt M.A., Baeken C., Hendricks M., Raedt D.R. (2011). The effects of high frequency rTMS on negative attentional bias are influenced by baseline state anxiety. Neuropsychologia, 49, 1824–30. [DOI] [PubMed] [Google Scholar]
- Zwanzger P., Steinberg C., Rehbein M.A., et al. (2014). Inhibitory repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex modulates early affective processing'. Neuroimage, 101, 193–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.