Abstract
Most of our social interaction is naturally based on emotional information derived from the perception of faces of other people. Negative facial expressions of a counterpart might trigger negative emotions and initiate emotion regulatory efforts to reduce the impact of the received emotional message in a perceiver. Despite the high adaptive value of emotion regulation in social interaction, the neural underpinnings of it are largely unknown. To remedy this, this study investigated individual differences in emotion regulation effectiveness during the reappraisal of angry faces on the underlying functional activity using functional magnetic resonance imaging (fMRI) as well as the underlying functional connectivity using resting-state fMRI. Greater emotion regulation ability was associated with greater functional activity in the ventromedial prefrontal cortex. Furthermore, greater functional coupling between activity in the ventrolateral prefrontal cortex and the amygdala was associated with emotion regulation success. Our findings provide a first link between prefrontal cognitive control and subcortical emotion processing systems during successful emotion regulation in an explicitly social context.
Keywords: reappraisal, resting-state, fMRI, neuroimaging, amygdala, inferior frontal gyrus
Introduction
Due to our highly social environment, the human brain is specialized in the processing of others’ emotional facial expressions (Palermo and Rhodes, 2007; Tamietto and de Gelder, 2010). However, the perception of emotional facial expressions in others does not automatically trigger emotional responses in a perceiver; cognitive control mechanisms support appropriate and flexible emotional responding according to a given social context (Blechert et al., 2012). Thus, successful emotion regulation plays a fundamental role in interpersonal functioning and social interaction. However, surprisingly the ability to successfully regulate one’s emotions in response to a socio-emotional stimulus like emotional facial expressions has been understudied so far.
Previous neuroimaging studies primarily focused on the down-regulation of emotion using reappraisal in response to complex emotionally evocative scenes taken from the international affective picture system (IAPS) (Bradley and Lang, 2007) (for an overview please see Ochsner et al., 2012; Buhle et al., 2013; Kohn et al., 2014). Reappraisal refers to the cognitive re-evaluation of a potentially emotionally arousing event by altering its emotional impact (Gross and Thompson, 2007). The emotional impact of an event on our emotional state can either be increased or decreased, e.g. one could emotionally engage to great extent with an arousing situation and feel more anxious or one could try to distance oneself from the emotional situation by framing it in another (e.g. more positive) way. This up- or down-regulation of affective responses represents two distinct reappraisal goals (McRae et al., 2012a; Ochsner et al., 2012; Gross, 2013); however, most studies have focused on down-regulation. Based on numerous neuroimaging studies a well-established network of brain regions has been associated with reappraisal (Ochsner and Gross, 2005; Phillips et al., 2008; Kalisch, 2009; Diekhof et al., 2011; Ochsner et al., 2012; Buhle et al., 2013; Frank et al., 2014; Kohn et al., 2014). Emotion regulation involves frontal [dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC)], temporal [temporal pole, superior and middle temporal gyrus (STG and MTG), temporo-parietal junction], medial [dorsomedial and ventromedial PFC (VMPFC), medial orbitofrontal cortex, anterior cingulate cortex (ACC), posterior cingulate cortex (PCC)], parietal [inferior and superior parietal lobe (IPL and SPL)] as well as subcortical regions (ventral striatum, amygdala) and the insula.
Only few studies so far investigated explicit emotion regulation, i.e. reappraisal, in response to facial expressions or dynamic social stimuli (Goldin et al., 2009; Blechert et al., 2012; McRae et al., 2012b; Ziv et al., 2013; Otto et al., 2014; Nelson et al., 2015). These studies either found an increase in response (McRae et al., 2012b; Nelson et al., 2015) or no significant changes in response (Goldin et al., 2009; Ziv et al., 2013; Otto et al., 2014) in the amygdala during reappraisal. Furthermore, enhanced activity was reported in the DLPFC, VLPFC and medial PFC in some studies (Goldin et al., 2009; Nelson et al., 2015), while others report more widespread activity in parietal as well as temporal regions, ACC and PCC in addition to frontal areas (Ziv et al., 2013; Otto et al., 2014). These inconsistent findings might be related to individuals’ reappraisal ability (i.e. task-related regulation success), which has not been considered in these studies.
Individual differences in the ability to reappraise one’s emotions are likely to change the neural responses during emotion processing: previous studies demonstrated that such abilities go along with increased activity in prefrontal cognitive control regions (Ochsner et al., 2002, 2004b; Phan et al., 2005; Urry et al., 2006; Wager et al., 2008) and in the amygdala (Eippert et al., 2007). Furthermore, individuals who are more successful in emotion regulation demonstrate greater effective connectivity between the amygdala and lateral and medial PFC (Banks et al., 2007). Recently, a link between emotion regulation ability and intrinsic functional brain architecture was found: successful reappraisal was correlated with greater activity in medial and lateral prefrontal regions and lesser resting-state functional connectivity (rsFC) between right amygdala and both medial prefrontal and PCCs as well as between bilateral DLPFC and posterior visual cortices (Uchida et al., 2015). These findings suggest that individual differences in reappraisal success might not only affect functional activity within emotion regulation related brain regions during active task engagement but also its intrinsic functional connectivity at rest.
In this study, we investigated individual differences in emotion regulation effectiveness during the reappraisal of negative faces (anger expression) using functional magnetic resonance imaging (fMRI) and rsFC analysis. To probe the regulatory flexibility, i.e. the ability to adopt different regulatory goals, participants were asked to either engage with the depicted emotion (Increase) or to down-regulate their emotion (Decrease). In the control condition, participants were asked to passively view the faces (Observe). After each trial, participants rated their emotional state, which was used as measure for reappraisal success. On a behavioural level, emotional state ratings are expected to follow regulation goals: up-regulation leads to an increase of negative feelings, while down-regulation is associated with a decrease in negative affect. On a neural level, we hypothesized an increase in activity in prefrontal cognitive control regions such as DLPFC and VLPFC including inferior frontal gyrus (IFG), medial PFC, ACC as well as temporal regions (STG, MTG) during the down-regulation of emotions in response to negative facial expressions. Similar regions might be recruited during up-regulation; however, no study has so far examined this, precluding firm predictions. Neither did the pervious literature warrant directional hypothesis for amygdala responding during the down-regulation of emotions in response to faces.
Subsequent functional connectivity analyses focused on regions identified during the emotion regulation task (contrasting Decrease vs Observe-angry) that correlated with task-related reappraisal success including the VMPFC. In addition, we used the left and right amygdala as seed regions as both have consistently been implicated in emotion generative processes, the processing of social information in faces (Phan et al., 2002; Sergerie et al., 2008; Adolphs, 2010; Ray and Zald, 2012; Murray et al., 2014) as well as emotion regulation (Diekhof et al., 2011; Buhle et al., 2013; Frank et al., 2014). We expected less resting-state connectivity between right amygdala and medial PFC and PCC based on a recent study (Uchida et al., 2015).
Materials and methods
Participants
Sixty healthy, right-handed participants with normal or corrected to normal vision gave written, informed consent and participated in the fMRI experiment (30 females, mean age = 30.48 years, s.d. = 11.10, range = 18–57). Due to technical problems with data acquisition, 12 participants did not participate in the resting-state experiment. The final sample for the resting-state experiment consisted of 48 participants (25 females, mean age = 29.70 years, s.d. = 11.08). All participants reported no history of neurological or psychiatric disorders based on the German version of the structural clinical interview (Wittchen et al., 1997). To control for potential differences based on neurocognitive abilities, participants were administered neuropsychological tests tapping verbal intelligence (Wortschatztest, WST) (Schmidt and Metzler, 1992) and executive functions (trail-making test, TMT-A/B) (Reitan, 1956).
To assure that our sample did not differ from the population average, we obtained several questionnaires relating to emotion processing and regulation, which were not used as covariates for further analyses. Participants rated their state and trait anxiety using the State Trait Anxiety Inventory(STAI) (Spielberger et al., 1970; Laux et al., 1981), and we also tested for severity of depressive symptoms [Becks Depression Scale (BDI 2)] (Hautzinger et al., 2006). Alexithymia, the inability to describe and regulate one’s emotions, was assessed using the Toronto Alexithymia Scale (TAS)-20 (Bach et al., 1996). Individual differences in habitual emotion regulation strategies (suppression and reappraisal) was assessed using the emotion regulation questionnaire (Gross and John, 2003; Abler and Kessler, 2009) and the emotion regulation inventory (König, 2011). Details on sample characteristics are listed in Table 1. The study was approved by the ethics committee of the Medical Faculty of the RWTH Aachen University.
Table 1.
Sample description
| Females (s.d.) | Males (s.d.) | |
|---|---|---|
| N | 30 | 30 |
| Age | 31.66 (12.62) | 29.30 (9.42) |
| Verbal intelligence (WST) | 32.83(2.19) | 33.06 (2.76) |
| TMT-A | 20.55 (8.78) | 22.17 (7.84) |
| TMT-B | 36.18 (13.52) | 37.88 (20.10) |
| BDI 2 | 2.23 (3.24) | 1.37 (2.35) |
| Trait anxiety | 33.83 (8.78) | 30.67 (4.47) |
| State anxiety | 35.60 (7.99) | 32.20 (5.45) |
| ERQ suppression | 3.29 (0.94) | 3.36 (1.22) |
| ERQ reappraisal | 4.85 (0.96) | 4.74 (1.02) |
| ERI negative | 46.33 (7.51) | 44.13 (5.77) |
| ERI positive | 28.23 (5.65) | 26.07 (5.91) |
| ERI total | 74.53 (11.73) | 70.13 (9.73) |
| TAS difficulty identifying feelings | 10.33 (3.52) | 10.27 (3.24) |
| TAS difficulty describing feelings | 12.47 (3.07) | 12.23 (3.24) |
| TAS externally oriented thinking | 17.43(3.76) | 19.60 (4.52) |
s.d., standard deviation; WST, Wortschatztest; TMT, trail-making test; BDI, Becks Depression Scale; ERQ, emotion regulation questionnaire; ERI, emotion regulation inventory; TAS, Toronto Alexithymia Scale.
Experimental design
Stimuli
Stimuli consisted of 140 images from the FACES set (60 angry male, 60 angry female, 10 neutral male, 10 neutral female facial expressions) (Ebner et al., 2010). In a behavioural pre-test, the stimuli were evaluated by a different group of 31 participants (16 females, mean age = 31.00 years, s.d. = 14.41). Participants rated the faces on valence and arousal on a nine-point Likert scale from 1 (very positive/calm) to 9 (very negative/highly arousing). The results showed a main effect of valence [F(1,30) = 67.24, P < 0.001] and arousal [F(1,30) = 631.63, P < 0.001] but no significant interaction effect [F(1,30) = 1.77, P = 0.193]. Angry faces were rated as more negative [t(30) = 19.80, P < 0.001] and arousing [t(30) = 15.75, P < 0.001] than neutral faces.
During the fMRI experiment, images were presented in the centre of the screen with an 800 × 600 pixel display subtending 32° × 24° visual angle on dual display goggles (VisuaStim, MR Research, USA) using the stimulation software Presentation (Version 14.1, Neurobehavioural Systems, USA).
Reappraisal task.
The task design has been adapted from previous studies on emotion regulation using reappraisal (Ochsner et al., 2004b; Eippert et al., 2007; Kim & Hamann, 2007; Domes et al., 2010; Morawetz et al., 2016a). Three task conditions were implemented in the experiment (Figure 1). In the Observe condition, participants were presented with either angry (Observe-angry) or neutral (Observe-neutral) faces and were asked to view the stimuli attentively and allow to experience/feel any emotional responses, which these might elicit without trying to manipulate them. In the Increase condition, participants were asked to imagine that the depicted person was associated and angry/upset with them, because they had done something wrong (Ziv et al., 2013). In contrast, in the Decrease condition, participants were instructed to imagine that the depicted person was unknown to them and just had a bad day (Blechert et al., 2012; Ziv et al., 2013). All participants received a training session to practice the reappraisal strategies before scanning. The training session consisted of 1 Observe-neutral, 1 Observe-angry, 3 Increase and 3 Decrease trials and lasted ∼5–10 min.
Fig. 1.
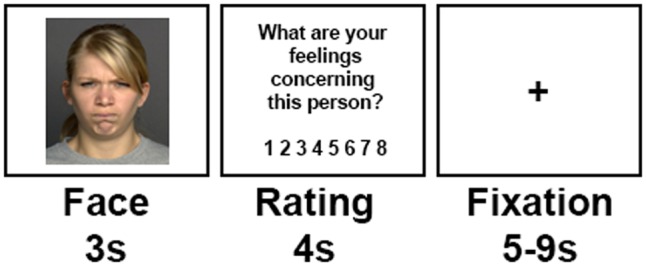
Task design. Each block started with an instruction, introducing the experimental condition. (Increase, Decrease, Observe). Each trial started with the presentation of a neutral or angry face for 3 s. During this time period, participants were supposed to either up-regulate (Increase) or down-regulate (Decrease) their emotions or observe the image and not regulate their emotions (Observe-angry/Observe-neutral). Subsequently, participants were asked to rate their current emotional state (‘What are your feelings concerning this person?’) on a scale from 1 (unpleasant/bad) to 8 (pleasant/well) followed by a fixation phase of 5–9 s.
A mixed block/event-related design was used implementing the three task conditions in a fixed order to allow for the simultaneous modelling of the transient, trial-related and the sustained, task-related blood-oxygen level dependent (BOLD) signals (Petersen and Dubis, 2012). Each block started with an auditory instruction via headphones indicating the experimental condition (first block: Observe; second and third block: Increase/Decrease). The experimental conditions of the second and third block were counterbalanced across participants, i.e. either starting with Increase followed by Decrease or vice versa. Each trial started with a face displayed for 3 s, followed by an emotional state rating (4 s). Participants were asked to rate their current emotional state with regard to the depicted person on a scale from 1 (unpleasant/bad) to 8 (pleasant/well) by pressing a button on a button fibre optic response pad (Cambridge Research Systems Ltd, England), providing a measure of trial-by-trial emotional state. Finally, a fixation cross presented in the centre of the screen for 5–9 s concluded the trial.
The first run (Observe condition) consisted of 60 trials and lasted 15 min (Observe-angry: 40 trials, Observe-neutral: 20 trials). The second and third run (Increase/Decrease condition) consisted of 40 trials each, lasting 10 min. Resting-state data were collected prior to the fMRI experiment.
MRI procedures
fMRI data acquisition
Whole-brain functional and anatomical images were acquired using a 3.0 T Magnetom TimTrio MRI scanner (Siemens, Erlangen, Germany) and a 12-channel head coil. A high-resolution 3D T1-weighted dataset was acquired for each participant (176 sagittal sections, 1 × 1 × 1 mm³; 256 × 256 data acquisition matrix). Functional images were acquired using a T2*-weighted, gradient-echo echo planar imaging (EPI) pulse sequence recording 36 sections oriented parallel to the anterior and posterior commissure at an in-plane resolution of 3.2 × 3.2 × 3.2 mm³ (interslice gap = 3.84; echo time (TE) = 30 ms; repetition time (TR) = 2.2 s; flip angle (FA) = 77°; FoV = 192 × 192 mm2; 64 × 64 data acquisition matrix). For the first experimental run 430 whole-brain volumes and for the second/third run 291 whole-brain volumes were recorded.
rsFC data (rs-fMRI) were acquired using single-shot GR-EPI (TE = 30 ms; TR = 2.2 s; FA = 77°) resulting in 36 axial slices at an in-plane resolution of 3.1 × 3.1 × 3.2 mm3 aligned to the anterior and posterior commissure (slice gap). Participants were instructed to relax in the scanner, stay awake with eyes closed (at low-level illumination) and ‘allow thoughts to come and go freely’. Two hundred and ten whole-brain volumes were recorded for the rs-fMRI.
Data analyses.
Behavioural data
As we were interested in successful emotion regulation, we calculated reappraisal success scores based on the affect ratings acquired after each trial. Reappraisal success was defined as either a decrease or increase in reported emotion when applying a cognitive reappraisal strategy relative to the individual’s mean affect ratings of the Observe-angry condition representing the ‘natural’ emotional response to the stimuli. On this basis, each reappraisal trial (Increase or Decrease) was categorized as either successful or unsuccessful by subtracting the affect rating from the mean baseline (Observe-angry) (Wager et al., 2008; Morawetz et al., 2016a,b). Hence, negative values during Increase represent successful trials (participant reported stronger negative affect) while positive values represent unsuccessful trials (participant reported weaker negative affect) and vice versa for Decrease. Reappraisal success scores were calculated as the total number of successful reappraisal trials for each participant for both reappraisal conditions separately.
fMRI data.
Functional imaging data analysis was performed using SPM8 (Statistical Parametric Mapping, Wellcome Institute for Cognitive Neurology, London, UK) and Matlab 8.0.0 (MathWorks, Natick, MA, USA).
For all task-based analyses, preprocessing of fMRI data included slice time correction, realignment to the mean image, co-registration to the individual T1-weighted anatomical images as well as spatial normalization to the standard EPI template, reslicing to 3 × 3 × 3 mm voxels (Montreal Neurological Institute, MNI template, as implemented in SPM8). Spatial smoothing was performed using a 8 mm full-width at half-maximum (FWHM) isotopic Gaussian kernel.
The first-level fixed effects model consisted of a set of five regressors (Increase, Decrease, Observe-angry, Observe-neutral and Rating) convolved with the haemodynamic response function and six regressors describing head motion. In a second-level, random effects group analyses effects of (i) emotional reactivity (Observe-angry > Observe-neutral), (ii) reappraisal condition (Observe-angry vs Increase, Observe-angry vs Decrease, Observe-angry vs Increase + Decrease) and (iii) reappraisal success were tested using reappraisal success scores for each reappraisal goal separately as covariates. To assess random-effects across participants, one-sample t-tests were computed.
Functional connectivity analysis.
Functional connectivity analysis was carried out by using the Data Processing Assistant for Resting-State fMRI (DPARSF) toolbox (Chao-Gan and Yu-Feng, 2010). In addition to standard preprocessing (slice time correction, realignment, spatial normalization to the standard EPI template), resting-state data were corrected using linear regression to correct for changes in cerebrospinal fluid and white matter using the CompCor method (Behzadi et al., 2007) (as implemented in DPARSF, Chao-Gan and Yu-Feng, 2010) rather than global signal regression, a widely used preprocessing method known to mathematically generate negative correlations and systematically alter network structure (Murphy et al., 2009; Saad et al., 2012). Data were band-pass filtered (0.01–0.08 Hz) to eliminate low frequency fluctuations and smoothed with 6 mm FWHM isotopic Gaussian kernel. The first 10 volumes of the rs-fMRI run were discarded to allow for equilibrium effects.
Seed definitions.
Functional connectivity analysis was performed by applying a seed-region approach (Biswal et al., 1995). We selected left and right amygdala as defined in the Anatomy Toolbox (Eickhoff et al., 2005) (left amygdala: −23, −4, −20; 1546 voxels; right amygdala: 27, −4, −20, 1424 voxels). The amygdala has previously been implicated in emotion generative processes as well as in the processing of social information in faces (Phan et al., 2002; Sergerie et al., 2008; Derntl et al., 2009, 2012; Adolphs, 2010; Ray and Zald, 2012; Murray et al., 2014) and thus has been used as seed region in our analyses. In addition, we defined one more seed region, namely the VMPFC (7, 25, −6), based on the results of the fMRI analysis using a 10 mm sphere around the peak voxel of the fMRI group contrast Decrease > Observe-angry correlated with Decrease success.
rsFC and reappraisal.
For each participant, time-courses of all voxels within the seeds were extracted and expressed as the first eigenvariate. Linear (Pearson) correlation coefficients were computed between the ensuing characteristic time series of the seed regions and the time series of all other grey matter voxels of the brain to quantify rsFC. The voxel-wise correlation coefficients of each participant and seed were transformed into Fisher’s Z-scores. Then, the Z-scores for the different seeds were fed into a second-level general linear model (GLM) incorporating regressors for the within-participant coupling of activity between the seed region and other brain areas. To examine the neural correlates of individual differences in reappraisal success, we performed a whole-brain regression between reappraisal success scores and rsFC with the anatomically defined amygdala and functionally defined VMPFC seed regions. Following GLM estimation, random-effects analyses were performed, as described earlier for the task-related whole-brain analyses, with contrasts for regions showing significant coupling with the seed region that correlates with reappraisal success. This analysis resulted in three regression models.
Overlap of default mode network and intrinsic connectivity networks related to emotions with our amygdala seeded clusters.
Previous resting-state studies (Banks et al., 2007; Johnstone et al., 2007; Uchida et al., 2015) demonstrated an association between the amygdala and major nodes (PCC and medial PFC) of the default mode network (DMN) and suggested an important role for these regions in emotion regulation. Thus, we calculated the mean DMN rsFC for all participants and overlaid it with the amygdala seed based clusters that significantly correlated with reappraisal success. For this analysis, we defined the three DMN seeds as 10 mm spheres around the peak coordinates of the medial PFC, PCC and parietal cortex obtained from the literature (Fox et al., 2005). For each participant, time-courses of all voxels within the seeds were extracted and expressed as the first eigenvariate. Linear (Pearson) correlation coefficients were computed between the ensuing characteristic time series of the seed regions and the time series of all other grey matter voxels of the brain to quantify rsFC. The voxel-wise correlation coefficients of each participant and seed were transformed into Fisher’s Z-scores. Then, the Z-scores for the different seeds were fed into a second-level GLM incorporating regressors for the within-participant coupling of activity between the seed region and other brain areas resulting in three resting-state DMN maps. The mean of these three seed-based maps was calculated to define one overall group DMN. In a final step, the amygdala seed based clusters that significantly correlated with reappraisal success were overlaid onto the mean group-based DMN.
Furthermore, we overlaid the amygdala seed based clusters that correlated with reappraisal success onto intrinsic connectivity networks (ICNs) related to emotion processing and interoception obtained from the literature (Laird et al., 2011a). We used the first five (ICNs 1–5) out of 20 spatially co-occurring maps of ICNs as these have been described to be strongly related to a collective range of emotional and autonomic processes (brainmap.org/icns). This analysis was performed to serve for interpreting the functional significance of our resting-state connectivity results.
Statistical thresholds.
Data were visualized and statistically thresholded using NeuroElf. Whole-brain family-wise error (FWE) multiple-comparison correction thresholds were determined using AlphaSim (Forman et al., 1995) and significance was set at FWE P < 0.05. This technique controls for the FWE by simulating null datasets with the same spatial autocorrelation found in the residual images and creates a frequency distribution of different cluster sizes. Clusters larger than the minimum size corresponding to the a priori chosen FWE are then retained for additional analysis. This cluster-based method of thresholding is often more sensitive to activation when one can reasonably expect multiple contiguous activated voxels (Forman et al., 1995; Petersson et al., 1999) and is widely used in fMRI research. Clustering thresholds were estimated for each contrast separately and are reported in the tables. Anatomical labels were determined by converting MNI coordinates to Talairach space (Talairach and Tournoux, 1988) and using the Talairach Daemon brain atlas (Lancaster et al., 2000). Reported coordinates are in MNI space. The same procedure was used for the task-related and resting-state analysis. All trials were included in the task-related analysis regardless whether it was a successful or unsuccessful trial.
Results
Behavioural results
Reappraisal task.
Participants reported significantly greater negative affect during the Increase (Mean = 2.92, s.d. = 0.86) compared with the Decrease (M = 4.71 ± 1.05) [t(59) = −11.67, P < 0.001, Cohen’s d = 1.86] and the Observe-angry (M = 3.19 ± 0.85) [t(59) = −2.43, P = 0.02, Cohen’s d = 0.31] as well as the Observe-neutral (M = 5.28 ± 1.00) condition [t(59) = −16.30, P < 0.001, Cohen’s d = 2.53] condition. During Decrease, participants reported that reappraisal significantly reduced negative affect compared with the Observe-angry condition [t(59) = 12.38, P < 0.001, Cohen’s d = 1.59]. The Observe-neutral condition significantly differed from the Decrease [t(59) = −4.40, P < 0.001, Cohen’s d = 0.55] and the Observe-angry [t(59) = 20.27, P < 0.001, Cohen’s d = 2.25] condition indicating least negative affect.
To test for gender effects, we performed a 2 (gender) * 4 (task conditions) repeated measures analysis of variance (ANOVA) and observed a significant main effect of task [F(1,29) = 37.20, P < 0.001] but no significant gender effect [F(1,29) = 0.17, P = 0.67] and no significant task-by-gender interaction [F(1,29) = 0.08, P = 0.77].
Neuroimaging results
Emotional reactivity.
Observe-angry > Observe-neutral
The direct comparison resulted in a widespread network of regions including prefrontal cortex [right medial frontal gyrus (medFG), right middle frontal gyrus (MFG)], temporal regions [bilateral MTG, left STG], parietal regions (left angular gyrus), bilateral insula, cingulate cortex, bilateral caudate and left postcentral gyrus (Supplementary Material, Figure S1 and Table S1).
Observe-neutral > Observe-angry.
The reverse contrast revealed increased activity in the occipital cortex, prefrontal regions (left medFG, left MFG), parietal regions [left SPL, left IPL], temporal regions (right STG), subcortical areas [left amygdala, left parahippocampal gyrus, putamen, thalamus], left insula, bilateral precentral gyrus, cingulate cortex, right cuneus and right fusiform gyrus.
Reappraisal effects.
Decrease vs Observe-angry
The down-regulation of emotion compared with the control condition resulted in enhanced activity in the left angular gyrus, left medFG and right PCC (Supplementary Material, Figure S2 and Table S2).
Observe-angry vs Decrease.
The reverse contrast demonstrated increased activity in a number of regions including prefrontal areas [right IFG, left MFG, right medFG], bilateral pre- and postcentral gyrus, parietal regions (right IPL, left SPL), temporal regions (right STG, left MTG), left cingulate gyrus, bilateral amygdalae, bilateral thalamus and left insula (Supplementary Material, Figure S2 and Table S2).
Increase vs Observe-angry.
Similar results to the down-regulation of emotion were obtained during up-regulation of negative affect: increased activity was found for the left angular gyrus, right postcentral gyrus and left precuneus (Supplementary Material, Figure S3 and Table S3).
Observe-angry vs Increase.
During Observe-angry (compared with Increase) stronger activation of the prefrontal areas (bilateral IFG, left MFG, left medFG), bilateral pre- and postcentral gyrus, left MTG, right supramarginal gyrus, right hippocampus, left cingulate cortex and left insula emerged (Supplementary Material, Figure S3 and Table S3).
Increase + Decrease vs Observe-angry.
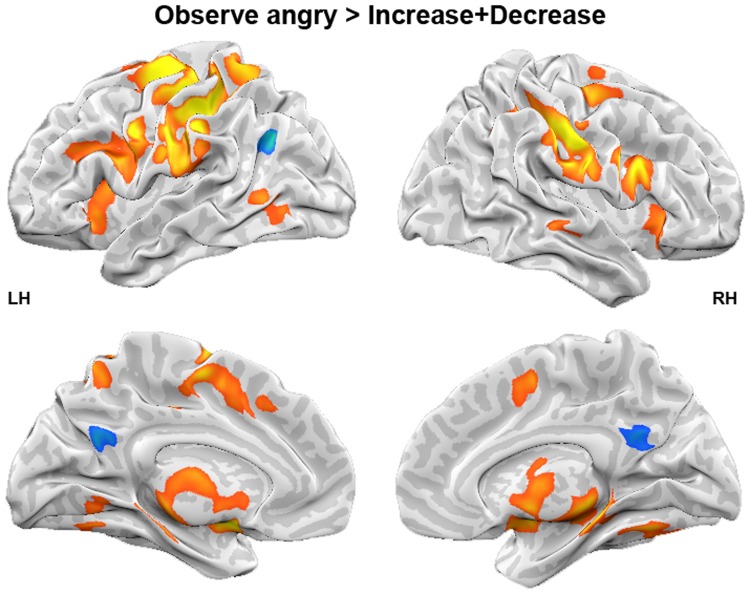
Finally, we tested for reappraisal effects in general independent of reappraisal goal. Reappraisal was associated with higher activity in the left angular gyrus and left precuneus. In contrast, the Observe-angry condition elicited stronger activation in frontal regions such as right IFG, left MFG and left medFG, in left SPL, bilateral pre- and postcentral gyrus, subcortical regions (bilateral amygdalae, left thalamus, right hippocampus), left insula, left cingulate gyrus and left precuneus than the reappraisal conditions (Figure 2, Table 2).
Fig. 2.
Effect of reappraisal. Whole-brain analysis for the contrast Observe-angry > Increase + Decrease. LH, left hemisphere; RH, right hemisphere.
Table 2.
Reappraisal effects: Increase + Decrease
| Brain region | Hemisphere | x | y | z | T | k |
|---|---|---|---|---|---|---|
| Observe-angry > Increase + Decrease (yellow − red) | ||||||
| Postcentral gyrus (BA 2) | RH | 62 | −22 | 30 | 6.65 | 921 |
| IFG (BA 9) | RH | 58 | 5 | 26 | 5.21 | LM |
| Postcentral gyrus (BA 2) | LH | −49 | −29 | 36 | 6.42 | 5135 |
| Precentral gyrus (BA 6) | LH | −29 | −12 | 56 | 6.28 | LM |
| Postcentral gyrus (BA 3) | LH | −41 | −19 | 55 | 6.10 | LM |
| Precentral gyrus (BA 6) | LH | −57 | −2 | 33 | 5.73 | LM |
| Amygdala | LH | −19 | 1 | −7 | 5.15 | LM |
| Amygdala | RH | 19 | −3 | −8 | 5.12 | LM |
| Thalamus | RH | 11 | −26 | −4 | 5.06 | LM |
| medFG (BA 6) | LH | −4 | −6 | 53 | 4.64 | LM |
| SPL (BA 7) | LH | −27 | −50 | 56 | 4.60 | LM |
| SPL (BA 7) | LH | −12 | −62 | 54 | 4.56 | LM |
| Thalamus | RH | 6 | −3 | 2 | 4.27 | LM |
| Hippocampus | RH | 30 | −25 | −5 | 4.24 | LM |
| Midbrain Substania Nigra | LH | −14 | −20 | −8 | 4.21 | LM |
| Thalamus | LH | −8 | −28 | 0 | 4.08 | LM |
| Thalamus | LH | −3 | −12 | 14 | 3.85 | LM |
| Insula (BA 13) | LH | −33 | 13 | 13 | 3.77 | LM |
| Insula (BA 13) | LH | −36 | 18 | −1 | 3.75 | LM |
| IFG (BA 45) | RH | 33 | 24 | 4 | 3.73 | LM |
| Precentral gyrus (BA 44) | LH | −55 | 11 | 4 | 3.50 | LM |
| Thalamus (ventral anterior nucleus) | RH | 13 | −7 | 14 | 3.47 | LM |
| Cingulate gyrus (BA 24) | LH | −11 | 6 | 44 | 3.46 | LM |
| IFG (BA 47) | RH | 48 | 16 | −2 | 3.45 | LM |
| Insula (BA 13) | LH | −40 | −9 | 15 | 3.43 | LM |
| MFG (BA 9) | LH | −46 | 23 | 28 | 3.38 | LM |
| Cingulate gyrus (BA 32) | LH | −4 | 20 | 39 | 3.28 | LM |
| IFG (BA 47) | RH | 36 | 20 | −9 | 3.25 | LM |
| Precuneus (BA 7) | LH | −19 | −69 | 40 | 3.13 | LM |
| Cuneus (BA 18) | LH | −20 | −70 | 16 | 2.89 | LM |
| Precentral gyrus (BA 6) | RH | 32 | −14 | 56 | 4.84 | 202 |
| MTG | RH | 53 | −31 | 2 | 3.66 | 54 |
| MTG (BA 37) | LH | −53 | −65 | 7 | 3.58 | 111 |
| Increase + Decrease > Observe-angry (green − blue) | ||||||
| Angular gyrus (BA 39) | LH | −45 | −68 | 30 | −4.56 | 82 |
| Precuneus (BA 31) | LH | −3 | −60 | 25 | −3.63 | 123 |
Peaks are identified with MNI coordinates. K, cluster size; BA, Brodmann area; LM, local maxima; LH, left hemisphere; RH, right hemisphere. Significance level of P < 0.01, FWE corrected P < 0.05 at 178 voxels.
Gender effects.
To test for gender effects, we repeated the previous analysis with respect to gender. These analyses revealed no significant clusters. Thus, data were collapsed across gender.
Correlation between reappraisal success and reappraisal-related activity.
Across all participants, greater success in down-regulating emotions correlated significantly and positively with greater activity in VMPFC during decreasing emotions compared with the control condition (Figure 3, Table 3). Thus, this region has been used as seed region for further rsFC analyses. The correlation between Increase success and activity during the up-regulation of emotion compared with the control condition did not reveal any significant results.
Fig. 3.
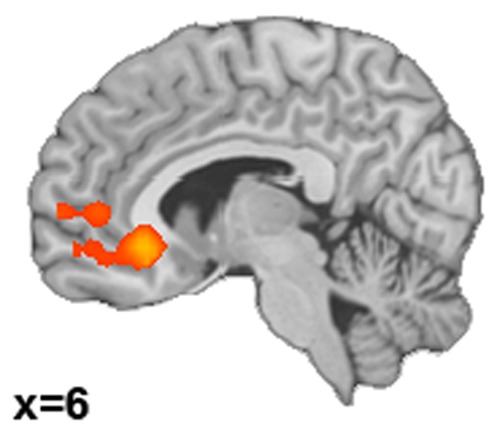
Brain activations correlating with reappraisal success. Correlation between success in decreasing emotion (Decrease success scores) and activity during down-regulating emotion compared with the control condition (Decrease > Observe-angry).
Table 3.
Correlation between reappraisal success and reappraisal-related activity
| Brain region | Hemisphere | x | y | z | T | k |
|---|---|---|---|---|---|---|
| Decrease > Observe-angry correlated with Decrease success | ||||||
| VMPFC/anterior cingulate (Brodmann area 32)a | RH | 7 | 25 | −6 | 3.96 | 614 |
| Anterior cingulate (Brodmann area 32) | RH | 7 | 25 | −6 | 3.96 | LM |
| Anterior cingulate (Brodmann area 10) | LH | −13 | 31 | −7 | 3.48 | LM |
| MFG (Brodmann area 10) | LH | −29 | 43 | 12 | 3.16 | LM |
| Frontal lobe | RH | 0 | 57 | 3 | 3.02 | LM |
| Anterior cingulate (Brodmann area 32) | RH | 11 | 44 | 5 | 2.72 | LM |
| medFG (Brodmann area 10) | LH | −15 | 52 | 10 | 2.72 | LM |
| MFG (Brodmann area 10) | LH | −32 | 60 | 13 | 2.72 | LM |
| medFG (Brodmann area 11) | RH | 4 | 51 | −11 | 2.66 | LM |
| Increase > Observe-angry correlated with Increase success | ||||||
| no significant results | ||||||
Peaks are identified with MNI coordinates. K, cluster size; BA, Brodmann area; LM, local maxima; LH, left hemisphere; RH, right hemisphere. Significance level of P < 0.05, FWE corrected P < 0.05 at 337 voxels.
Indicates ROI used for rs-fMRI analyis.
Correlation between down-regulating success and rsFC.
Amygdala seed.
Across participants, greater success in down-regulating emotions correlated significantly and positively with functional connectivity between the right amygdala seed and clusters in left IPL, left insula and left IFG (Figure 4, Table 4) (there was no correlation with the left amygdala). The clusters of the left IFG and left insula overlapped with an ICN associated with a complex set of functions including language, executive function, affective and interoceptive processes (ICN 4 obtained from brainmap.org/icns) (Figure 5). None of these regions overlapped with the groups’ mean DMN (Supplementary Material, Figure S4).
Fig. 4.
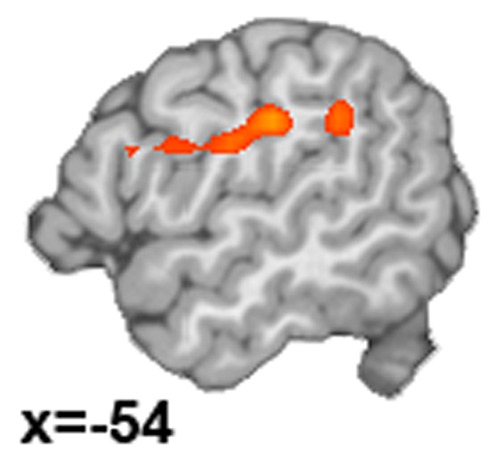
Right amygdala rsFC correlated with Decrease success.
Table 4.
Correlations of rsFC with reappraisal success
| Brain region | Hemisphere | x | y | z | T | k |
|---|---|---|---|---|---|---|
| Right amygdala seed: positive correlation with Decrease success | ||||||
| IPL (Brodmann area 40) | LH | −52 | −27 | 29 | 4.17 | 205 |
| IPL (Brodmann area 40) | LH | −52 | −27 | 29 | 4.17 | LM |
| Insula (Brodmann area 13) | LH | −42 | −18 | 24 | 3.85 | LM |
| Insula (Brodmann area 13) | LH | −47 | −38 | 26 | 3.71 | LM |
| IFG (Brodmann area 44) | LH | −50 | 10 | 23 | 3.18 | LM |
| Right amygdala seed: negative correlation with Decrease success | ||||||
| no significant results | ||||||
| Left amygdala seed: positive correlation with Decrease success | ||||||
| no suprathreshold clusters | ||||||
| Left amygdala seed: negative correlation with Decrease success | ||||||
| no suprathreshold clusters | ||||||
| VMPFC seed: positive correlation with Decrease success | ||||||
| no suprathreshold clusters | ||||||
| VMPFC seed: negative correlation with Decrease success | ||||||
| no suprathreshold clusters | ||||||
Peaks are identified with MNI coordinates. K, cluster size; BA, Brodmann area; LM, local maxima; LH, left hemisphere; RH, right hemisphere. Significance level of P < 0.01, FWE corrected P < 0.05 at 150 voxels.
Fig. 5.
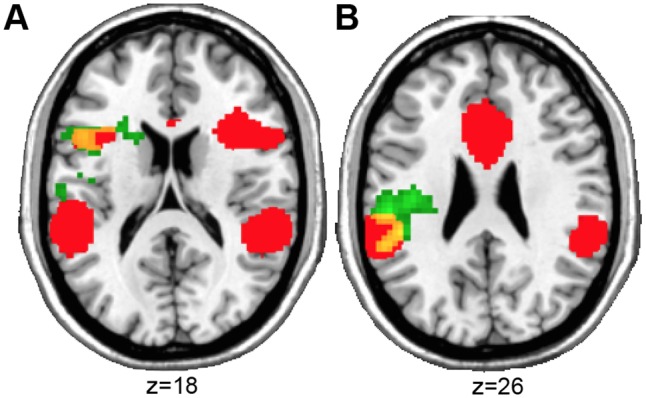
Overlap (yellow) of the ICN involved in emotion processing obtained from the literature (Laird et al., 2011) (ICN 4) (red) and the clusters that significantly correlated with reappraisal success using the amygdala as seed region for the rsFC analysis (green). (A) Overlap with left IFG and (B) left insula.
VMPFC seed
The correlation between Decrease success and functional connectivity between the VMPFC seed and the rest of the brain did not reveal any significant results applying our threshold.
Discussion
Previous research on emotion regulation primarily focused on the down-regulation of negative emotions in response to highly aversive and complex scene images. Based on these findings, several models on emotion regulation evolved, supporting the idea that prefrontal regions, especially the DLPFC, down-regulate activity in subcortical regions implicated in the processing of emotions, such as the amygdala (Ochsner and Gross, 2005; Quirk and Beer, 2006; Urry et al., 2006; Johnstone et al., 2007; Phillips et al., 2008; Wager et al., 2008; Kober et al., 2010). However, it remains unclear if such a top–down model of emotion regulation also applies to socially relevant stimuli such as emotional facial expressions.
This study investigated the individual differences in emotion regulation ability during the reappraisal of angry faces and its underlying functional activity as well as connectivity. During scanning, participants performed a reappraisal task in which they had to either up- or down-regulate their emotions. On the behavioural level, emotion regulation was successful: participants rated their emotional state more negatively after the up-regulation and less negatively after the down-regulation compared with the control condition.
Reappraisal of negative emotions in faces
On a neural level, reappraisal, i.e. up- and down-regulation of emotions in response to negative faces went along with activity in left angular gyrus and precuneus. The angular gyrus as part of the emotion regulation system has been implicated in representing the perceptual and semantic features of a stimulus (Ochsner et al., 2012), in social cognition (Kohn et al., 2014) and semantic processes (Messina et al., 2015). Both regions have been shown to be involved in theory of mind, i.e. reasoning about and attributing mental states (Schurz et al., 2014), which may be particularly triggered through the instructed reappraisal strategy.
Interestingly, in contrast to reappraisal studies using emotional scenes, we did not observe increased activity in prefrontal control regions, i.e. DLPFC or VLPFC, neither during the up- nor the down-regulation of negative emotions. This might either be explained by the stimulus material or by individual differences in emotion regulation success. Indeed, using reappraisal success scores as covariate in our analysis revealed increased activity in VMPFC; a region involved in stimulus evaluation rather than cognitive control per se (Ochsner et al., 2012). Although VMPFC has been found to mediate diminished negative affect in a domain-independent fashion (Diekhof et al., 2011), a recent meta-analysis mainly based on reappraisal of emotional scenes did not find consistent activation of VMPFC during reappraisal and suggested that VMPFC is involved in processes related to emotion generation, such as self-reflection (Denny et al., 2012). Therefore, it could be argued that the reappraisal of social stimuli, i.e. faces, might engage different cognitive functions, e.g. theory of mind, mentalizing and self-reflection to a greater extent as the reappraisal of emotional scenes, and thus involves different prefrontal cortex regions, i.e. VMPFC instead of DLPFC and VLPFC. Furthermore, participants had explicit instructions how to reappraise the emotional stimuli leaving not much room for associations and new interpretations. In contrast, in studies using more complex aversive scenes, participants are given relative freedom to enact reappraisal. This means that in our study participants might have followed the instructions and used only one specific tactic to reappraise whereas in studies of unrestricted reappraisal a variety of different reappraisal tactics might be used depending on the content of the images, e.g. distancing, reality change, change future consequences (McRae et al., 2012a). Thus, this might be associated with increased activity in prefrontal attention and memory systems due to an enhanced recruitment of cognitive resources. This issue relates to adaptation effects associated with stimulus content. In this study, the ‘same’ stimulus content, i.e. angry faces, was presented during all reappraisal trials, though presented by different actors, while studies using IAPS pictures provide much more variety of stimulus content ranging from, e.g. car accidents, mutilation, war scenes to operations and injuries. Therefore, the restricted instructed reappraisal tactic and the rather consistent stimulus content together might explain the reduced activity in prefrontal regions during reappraisal. It is noteworthy that we observed an effect of reappraisal in the amygdala, which has been associated with the processing of emotion (Zald, 2003; Sergerie et al., 2008) and has previously been shown to be modulated by reappraisal (Buhle et al., 2013; Frank et al., 2014). This indicates that emotion regulation was also successful on a neural level.
Only few studies investigated reappraisal of negative emotional facial expressions in an explicit emotion regulation task providing inconsistent results. One study found increased amygdala activity during reappraisal of negative faces using a region of interest (ROI) approach (McRae et al., 2012b), while the whole-brain analysis of the same study revealed increased activity in prefrontal regions including the DLPFC, VLPFC, medial PFC, ACC, PCC and temporal cortex (Otto et al., 2014). In line with that, a recent study reported increased activity in the DLPFC, VLPFC, medial PFC and amygdala during reappraisal of negative faces using a priori ROIs (Nelson et al., 2015). Furthermore, Goldin et al. (2009) observed enhanced activation of medial PFC, ACC, PCC, insula, occipital, parietal and temporal regions in healthy controls compared with individuals with social anxiety disorder during reappraisal of harsh facial expressions compared with passive viewing of neutral non-social scenes. In line with this, Ziv et al. (2013) demonstrated increased activity in dorsomedial PFC, VLPFC, parietal and temporal regions during reappraisal of negative faces. Our results are partly in line with these studies, as we also observed increased activity in parietal regions (Goldin et al., 2009; Ziv et al., 2013) and in the VMPFC (Goldin et al., 2009; Otto et al., 2014; Nelson et al., 2015). However, a direct comparison of our findings with previous results is clearly limited due to methodological differences between studies. Two of the studies used a priori ROIs to investigate effects of reappraisal (McRae et al., 2012b; Nelson et al., 2015), while another two studies used a whole-brain analysis approach (Ziv et al., 2013; Otto et al., 2014). However, Ziv et al. did not contrast reappraisal vs a control condition (Observe) but rather compared different reappraisal goals with each other. Goldin et al. did not report findings for healthy controls alone and did not use neutral faces as baseline condition constricting a direct comparison of results. Furthermore, previous studies used negative facial expressions including anger, fear and contempt, while in our study we only used angry faces. Another important aspect concerns the experimental design: we used a mixed block/event-related design, while the other studies used event-related designs. Finally, we implemented two different reappraisal goals in our study, the up- and down-regulation of emotion.
Reappraisal success and its association with functional activation and intrinsic connectivity
We observed a positive correlation between reappraisal success during Decrease and activity in VMPFC. VMPFC has been shown to play a key role in conceptually driven affect (Roy et al., 2012). Reappraisal as a conceptually driven form of emotion regulation, i.e. with the goal to generate positive (Decrease) or negative (Increase) conceptual frames, has been linked to VMPFC activity (Diekhof et al., 2011). Previous imaging studies also implicated the VMPFC in the positive and negative valuation of stimuli in a context- and goal-dependent manner (Hare et al., 2009; Hutcherson et al., 2012).
Based on our resting-state correlations, we were able to identify intrinsically connected brain regions implicated in successful emotion regulation. This means the higher the ability to regulate one’s emotions, the higher the intrinsic functional coupling between these regions. We extend previous findings by demonstrating a positive association between reappraisal success and functional coupling of the right amygdala with left insular cortex, left parietal regions and left IFG. The functional importance of these regions in emotion regulation is further corroborated by the link to previously described ICNs. Our findings overlap with an ICN that has been proposed to represent a transitional network linking cognition and emotion/interoception (Laird et al., 2011b). This aligns well with the idea that the IFG plays an intermediary role between PFC and the emotion generative system thereby linking the cortical cognitive control system to the subcortical affect processing system (Ochsner et al., 2012; Morawetz et al., 2016b).
The insula, together with the amygdala and the ventral striatum, have been proposed to constitute the emotion generative system (Ochsner et al., 2012). The insula has been implicated in the processing of negative affect and emotion regulation (Wager and Barrett, 2004). More specifically, the anterior insula has been associated with interoceptive awareness of the body and in executive functions such as the control of attention (Wager and Barrett, 2004). It has been involved in tasks that require the manipulation of information in working memory (Wager and Smith, 2003), response inhibition (Nee et al., 2007) and shifting attention (Wager et al., 2004), which represent relevant processes in emotion regulation. The IPL along with the DLPFC plays a key role in selective attention and working memory during the reappraisal of emotional responses (Miller, 2000; Wager and Smith, 2003; Wager et al., 2004). As previously shown, the VLPFC, including the IFG, provides moderate projections to the amygdala (Ray and Zald, 2012). These findings support the importance of the IFG as relay node within the emotion regulation network and corroborate its suggested intermediary role to link the prefrontal cortex with the amygdala (Ochsner et al., 2004a, 2012; Morawetz et al., 2016a,b). In support of recent models of emotion regulation (Ochsner et al., 2012; Etkin et al., 2015; Smith and Lane, 2015) and in accordance with recent meta-analyses highlighting the functional role of the IFG in emotion regulation (Kohn et al., 2014; Messina et al., 2015), we propose that successful emotion regulation is mediated by the functional interplay between VLPFC and amygdala.
Limitations
It has to be noted that we only used angry faces in our study. Thus, a generalization of our results to other basic emotions such as sadness or fear is not possible. Although entirely distinct neural networks do not represent basic emotions in facial expressions, they are at least partially separable (Fusar-Poli et al., 2009). Thus, we cannot rule out that differences in processing basic emotions in faces might be associated with differences in the recruitment of the emotion regulation network. Furthermore, future studies using positive stimuli, such as happy faces, could extend our present findings.
Moreover, it is important to note that the presented faces were only static and not dynamic. Facial expressions are naturally dynamic and vary rapidly in relation to situational requirements. Dynamic compared with static faces lead to more activity in the face-processing system as well as the IFG (Kilts et al., 2003; Trautmann et al., 2009) and might provide a more appropriate approach to examine the processing of emotional face perception and its regulation than static stimuli thereby increasing ecologic validity (Wiggert et al., 2015).
Another methodological limitation concerns the order of the blocks that was fixed with respect to the Observe condition, which always preceeded the reappraisal blocks. This might have led to habituation effects and diminished neural activation, especially in the reappraisal blocks. This issue needs to be addresses in future studies using a counterbalanced approach.
Since this was the first approach to examine intrinsic functional connectivity with emotion regulation success, our data are awaiting replication in independent samples, particularly given multiple-comparison/detection threshold issues. Future studies will have to further probe the link between prefrontal cognitive control and subcortical emotion processing systems to gain more insight on (successful) emotion regulation and the neural mechanisms.
Conclusion
In summary, our study provides evidence that activation of the medial prefrontal cortex, a region associated with stimulus evaluation and mentalizing, during emotion regulation of negative facial expressions is correlated with individual differences in reappraisal success. Furthermore, a set of intrinsically connected brain regions implicated in successful emotion regulation could be determined demonstrating enhanced connectivity between the right amygdala and the VLPFC, parietal regions and the insular cortex. Thus, our findings provide a first link between prefrontal cognitive control, e.g. VLPFC and subcortical emotion processing systems, e.g. amygdala during successful emotion regulation in an explicitly social context.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the Jülich-Aachen Research Alliance (JARA BRAIN) and the Brain Imaging Facility of the Interdisciplinary Center for Clinical Research within the Faculty of Medicine at the RWTH Aachen University.
Supplementary Material
References
- Abler B., Kessler H. (2009). Emotion Regulation Questionnaire – Eine deutschsprachige Fassung des ERQ von Gross und John. Diagnostica, 55, 144–52. [Google Scholar]
- Adolphs R. (2010). What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences, 1191, 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M., Bach D., De Zwaan M., Serim M., Bohmer F. (1996). Validierung der deutschen Version der 20-Item Toronto Alxithymie Skala bei Normalpersonen und psychiatrischen Patienten. Psychotherapie, Psychosomatik, Medizinische Psychologie, 46, 23–8. [PubMed] [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2, 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34, 537–41. [DOI] [PubMed] [Google Scholar]
- Blechert J., Sheppes G., Di Tella C., Williams H., Gross J.J. (2012). See what you think: reappraisal modulates behavioral and neural responses to social stimuli. Psychological Science, 23, 346–53. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. (2007). The international affective picture system (IAPS) in the study of emotion and attention In: Coan J.A., Allen J.J., editors. Handbook of Emotion Elicitation and Assessment, Series in Affective Science, 29–46, New York: Oxford University Press. [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2013). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience, 4, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny B.T., Kober H., Wager T., Ochsner K.N. (2012). A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience, 24, 1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Habel U., Robinson S., et al. (2012). Culture but not gender modulates amygdala activation during explicit emotion recognition. BMC Neuroscience, 13, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Habel U., Windischberger C., et al. (2009). General and specific responsiveness of the amygdala during explicit emotion recognition in females and males. BMC Neuroscience, 10, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Geier K., Falkai P., Gruber O. (2011). Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage, 58, 275–85. [DOI] [PubMed] [Google Scholar]
- Domes G., Schulze L., Böttger M., et al. (2010). The neural correlates of sex differences in emotional reactivity and emotion regulation. Human Brain Mapping, 31, 758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner N.C., Riediger M., Lindenberger U. (2010). FACES—a database of facial expressions in young, middle-aged, and older women and men: development and validation. Behavior Research Methods, 42, 351–62. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage, 25, 1325–35. [DOI] [PubMed] [Google Scholar]
- Eippert F., Veit R., Weiskopf N., Erb M., Birbaumer N., Anders S. (2007). Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping, 28, 409–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Büchel C., Gross J.J. (2015). The neural bases of emotion regulation. Nature Reviews. Neuroscience, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine, 33, 636–47. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102, 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D.W., Dewitt M., Hudgens-Haney M., et al. (2014). Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews, 45, 202–11. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., et al. (2009). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience, 34, 418–32. [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., Manber T., Hakimi S., Canli T., Gross J.J. (2009). Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry, 66, 170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. (2013). Emotion regulation: taking stock and moving forward. Emotion, 13, 359–65. [DOI] [PubMed] [Google Scholar]
- Gross J.J., John O.P. (2003). Individual Differences in Two Emotion Regulation Processes: Implications for Affect, Relationships, and Well-Being. Journal of Personality and Social Psychology, 85, 348–62. [DOI] [PubMed] [Google Scholar]
- Gross J.J., Thompson R.A. (2007). Emotion regulation: Conceptual foundations In: Gross J.J., editors. Handbook of Emotion Regulation, 3–26, New York: Guilford Press [Google Scholar]
- Hare T.A., Camerer C.F., Rangel A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324, 646–8. [DOI] [PubMed] [Google Scholar]
- Hautzinger M., Keller F., Kühner C. (2006). Beck Depression Inventar II (BDI 2). Frankfurt am Main: Harcourt Test Service. [Google Scholar]
- Hutcherson C.A., Plassmann H., Gross J.J., Rangel A. (2012). Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. The Journal of Neuroscience, 32, 13543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. (2007). Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience, 27, 8877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. (2009). The functional neuroanatomy of reappraisal: time matters. Neuroscience and Biobehavioral Reviews, 33, 1215–26. [DOI] [PubMed] [Google Scholar]
- Kilts C.D., Egan G., Gideon D.A., Ely T.D., Hoffman J.M. (2003). Dissociable neural pathways are involved in the recognition of emotion in static and dynamic facial expressions. Neuroimage, 18, 156–68. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Hamann S. (2007). Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience, 19, 776–98. [DOI] [PubMed] [Google Scholar]
- Kober H., Mende-Siedlecki P., Kross E.F., et al. (2010). Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America, 107, 14811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. (2014). Neural network of cognitive emotion regulation - an ALE meta-analysis and MACM analysis. Neuroimage, 87, 345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König D. (2011). Die Regulation von negativen und positiven Emotionen. Entwicklung des Emotionsregulations-Inventars und Vergleich von Migränikerinnen mit Kontrollpersonen. Universität Wien.
- Laird A.R., Eickhoff S.B., Fox P.M., et al. (2011a). The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Research Notes, 4, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A.R., Fox P.M., Eickhoff S.B., et al. (2011b). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23, 4022–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., et al. (2000). Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping, 10, 120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux L., Glanzmann P., Schaffner P., Spielberger C.D. (1981). Das State-Trait-Angstinventar (STAI), Weinheim Beltz. Weinheim, Germany: Beltz.
- McRae K., Ciesielski B., Gross J.J. (2012a). Unpacking cognitive reappraisal: goals, tactics, and outcomes. Emotion, 12, 250–5. [DOI] [PubMed] [Google Scholar]
- McRae K., Misra S., Prasad A.K., Pereira S.C., Gross J.J. (2012b). Bottom-up and top-down emotion generation: implications for emotion regulation. Social Cognitive and Affective Neuroscience, 7, 253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina I., Bianco S., Sambin M., Viviani R. (2015). Executive and semantic processes in reappraisal of negative stimuli: insights from a meta-analysis of neuroimaging studies. Frontiers in Psychology, 6, 974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K. (2000). The prefrontal cortex and cognitive control. Nature Reviews. Neuroscience, 1, 59–65. [DOI] [PubMed] [Google Scholar]
- Morawetz C., Bode S., Baudewig J., Jacobs A.M., Heekeren H.R. (2016a). Neural representation of emotion regulation goals. Human Brain Mapping, 37, 600–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C., Bode S., Baudewig J., Kirilina E., Heekeren H.R. (2016b). Changes in effective connectivity between dorsal and ventral prefrontal regions moderate emotion regulation. Cerebral Cortex, 26, 1923–37. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage, 44, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R.J., Brosch T., Sander D. (2014). The functional profile of the human amygdala in affective processing: insights from intracranial recordings. Cortex, 60, 10–33. [DOI] [PubMed] [Google Scholar]
- Nee D.E., Wager T.D., Jonides J. (2007). Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective & Behavioral Neuroscience, 7, 1–17. [DOI] [PubMed] [Google Scholar]
- Nelson B.D., Fitzgerald D.A., Klumpp H., Shankman S.A., Phan K.L. (2015). Prefrontal engagement by cognitive reappraisal of negative faces. Behavioural Brain Research, 279, 218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D.E. (2002). Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14, 1215–29. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–9. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Knierim K., Ludlow D.H., et al. (2004a). Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience, 16, 1746–72. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004b). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage, 23, 483–99. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B., Misra S., Prasad A., McRae K. (2014). Functional overlap of top-down emotion regulation and generation: an fMRI study identifying common neural substrates between cognitive reappraisal and cognitively generated emotions. Cognitive, Affective & Behavioral Neuroscience, 14, 923–38. [DOI] [PubMed] [Google Scholar]
- Palermo R., Rhodes G. (2007). Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia, 45, 75–92. [DOI] [PubMed] [Google Scholar]
- Petersen S., Dubis J. (2012). The mixed block/event-related design. Neuroimage, 62, 1177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson K.M., Nichols T.E., Poline J.B., Holmes A.P. (1999). Statistical limitations in functional neuroimaging. II. Signal detection and statistical inference. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 354, 1261–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. (2005). Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry, 210–9. doi:10.1016/j.biopsych.2004.10.030. [DOI] [PubMed]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage, 16, 331–48. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13, 829–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Beer J.S. (2006). Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology, 16, 723–7. [DOI] [PubMed] [Google Scholar]
- Ray R.D., Zald D.H. (2012). Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience and Biobehavioral Reviews, 36, 479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. (1956). Trail Making Test: Manual for Administration, Scoring and Interpretation. Indianapolis, IN: Indiana University Medical Center. [Google Scholar]
- Roy M., Shohamy D., Wager T.D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16, 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z.S., Gotts S.J., Murphy K., et al. (2012). Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain connectivity, 2, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K., Metzler P. (1992). Wortschatztest (WST). Beltz Test GmbH. Weinheim, Germany.
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 32, 811–30. [DOI] [PubMed] [Google Scholar]
- Smith R., Lane R. (2015). The neural basis of one’s own conscious and unconscious emotional states. Neuroscience and Biobehavioral Reviews, 57, 1–29 [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. (1970). The State-Trait Anxiety Inventory. MANUAL 1–23.
- Talairach J., Tournoux P. (1988). Co-Planar Stereotaxic Atlas of the Human Brain (Thieme Classics). Thieme.
- Tamietto M., de Gelder B. (2010). Neural bases of the non-conscious perception of emotional signals. Nature Reviews. Neuroscience, 11, 697–709. [DOI] [PubMed] [Google Scholar]
- Trautmann S.A., Fehr T., Herrmann M. (2009). Emotions in motion: dynamic compared to static facial expressions of disgust and happiness reveal more widespread emotion-specific activations. Brain Research, 1284, 100–15. [DOI] [PubMed] [Google Scholar]
- Uchida M., Biederman J., Gabrieli J.D.E., et al. (2015). Emotion regulation ability varies in relation to intrinsic functional brain architecture. Social Cognitive and Affective Neuroscience, 10, 1738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., Reekum V., Marije C., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience, 26, 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Barrett L.F. (2004). From affect to control: functional specialization of the insula in motivation and regulation. Emotion, 129, 2865. [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Jonides J., Reading S. (2004). Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage, 22, 1679–93. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Smith E.E. (2003). Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience, 3, 255–74. [DOI] [PubMed] [Google Scholar]
- Wiggert N., Wilhelm F.H., Reichenberger J., Blechert J. (2015). Exposure to social-evaluative video clips: neural, facial-muscular, and experiential responses and the role of social anxiety. Biological Psychology, 110, 59–67. [DOI] [PubMed] [Google Scholar]
- Wittchen H., Zaudig M., Fydrich T. (1997). Strukturiertes Klinisches Interview für DSM-IV. Göttingen, Germany: Hogrefe.
- Zald D.H. (2003). The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews, 41, 88–123. [DOI] [PubMed] [Google Scholar]
- Ziv M., Goldin P.R., Jazaieri H., Hahn K.S., Gross J.J. (2013). Emotion regulation in social anxiety disorder: behavioral and neural responses to three socio-emotional tasks. Biology of Mood & Anxiety Disorders, 3, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.