Abstract
Interpersonal distance is central to communication and complex social behaviors but the neural correlates of interpersonal distance preferences are not defined. Previous studies suggest that damage to the orbitofrontal cortex (OFC) is associated with impaired interpersonal behavior. To examine whether the OFC is critical for maintaining appropriate interpersonal distance, we tested two groups of patients with OFC damage: Patients with OFC lesions and patients with behavioral variant frontotemporal dementia. These two groups were compared to healthy controls and to patients with lesions restricted to the dorsolateral prefrontal cortex. Only patients with OFC damage showed abnormal interpersonal distance preferences, which were significantly different from both controls and patients with dorsolateral prefrontal damage. The comfortable distances these patients chose with strangers were significantly closer than the other groups and resembled distances normally used with close others. These results shed light on the role of the OFC in regulating social behavior and may serve as a simple diagnostic tool for dementia or lesion patients.
Keywords: interpersonal distance, personal space, social norms, orbitofrontal cortex, behavioral variant frontotemporal dementia
Introduction
Interpersonal distance (IPD), the space between two people, plays a central role in communication and social interactions. At a proper interpersonal distance, people may signal responsiveness and feelings of comfort and safety to one another (Feeney, 1999; Kaitz et al., 2004). However, when IPD norms are violated, it can be construed as a threat and induce a state of anxiety (Lloyd, 2009).
Research on IPD originally derived from the ideas of the anthropologist Edward Hall, who described personal space as a series of spatial spheres with the individual as their center. The personal space zones surrounding the individual are referred to as ‘intimate’ (0–45 cm), ‘personal’ (45–120 cm), ‘social’ (120–360 cm) and ‘public’ (>360 cm; Hall, 1963). The sense and need for IPD gradually develops with age, and is stabilized at adult levels around the age of 12 (Aiello and Aiello, 1974). At this age, children acquire their adult preferred distances, as well as an understanding of the correlation between physical proximity and psychological closeness (Meisels and Guardo, 1969). Individuals with autism spectrum disorders show abnormalities in IPD, which are detectable across the lifespan and levels of functioning, with some adults even reporting a complete absence of personal space (Kennedy and Adolphs, 2014).
The neural correlates of IPD preferences are not clear. It has been shown to be related to sensory sensitivity and attentional mechanisms (e.g. how sensitive one is to smell, sound or touch; Perry et al., 2015), and also to amygdala activation, perhaps signaling threat when one is being approached (Kennedy et al., 2009). Indeed patients with amygdala damage showed closer IPD preferences, less feelings of threat and more feelings of trust towards approaching strangers (Harrison et al., 2015; Kennedy et al., 2009). While these automatic, low-level mechanisms undoubtedly play a crucial role in IPD preferences, maintaining personal space also strongly relates to respecting social norms, as can be seen by differences between cultures in interpersonal distance norms (Aiello, 1987). Since the orbitofrontal cortex (OFC) has been suggested as a critical brain region regulating social behavior and inhibiting inappropriate social conduct (Kringelbach and Rolls, 2004; Beer et al., 2006a, b), we hypothesized that IPD preferences may be further regulated by the OFC, and that people with damage to the OFC will not keep the expected distance from others.
Both clinical observations and empirical studies report that that OFC damage is associated with impaired interpersonal behavior, such as an impaired ability to prioritize solutions to interpersonal problems (Saver and Damasio, 1991), greeting strangers in an overly familiar manner (Rolls et al., 1994) or disclosing inappropriate personal information in a conversation with a stranger (Beer et al., 2006a). These studies suggest that patients with OFC damage behave with strangers in ways that are more appropriate for interactions with close others. In line with these results, neuroimaging studies with healthy participants show activation in the OFC in situations that violate social norms (Berthoz et al., 2002). Taken together this literature suggests that OFC damage may lead to failure in regulating IPD.
Although most of the research on the OFC and interpersonal behavior has utilized patients with OFC lesions, patients with behavioral-variant frontotemopral dementia (bvFTD) also have OFC damage and deficits in social behavior. Frontotemporal dementia is a neurodegenerative disease that selectively affects the frontal and anterior temporal lobes of the brain, regions that are crucial for proper social and emotional functioning (Rosen et al., 2005; Werner et al., 2007). Dramatic social and emotional changes (e.g. emotional blunting, lack of empathy, disinhibition and poor insight) are early and striking manifestations of this disease and serve as diagnostic criteria distinguishing it from other forms of dementia (Boxer and Miller, 2005; Neary et al., 1998, 2005). Frontotemporal dementia includes two major clinical syndromes: bvFTD and language variants known as primary progressive aphasias. In bvFTD, the subtype that primarily affects the OFC, early and profound social deficits, particularly impulsive and inappropriate behavior, are common (Boxer and Miller, 2005). In fact, a combination of OFC atrophy and behavioral disinhibition appears to be a powerful diagnostic tool in differentiating bvFTD from Alzheimer’s disease (Hornberger et al., 2011; Rascovsky et al., 2011).
To test our hypothesis that the OFC plays a critical role in maintaining interpersonal distance and differentiating between appropriate distances from strangers and from friends, we tested two groups of patients with OFC damage: patients with restricted OFC lesions following traumatic brain injury or tumor resection, and patients diagnosed with bvFTD. These two patient groups were compared to healthy controls and to patients with lesions to the dorsolateral prefrontal cortex (DLPFC). Including DLPFC patients enabled us to differentiate the role of the OFC from general prefrontal cortex control mechanisms. The bvFTD group was examined as part of a day-long assessment of emotional functioning (Levenson et al., 2007) that included involvement of their caregivers. This enabled us to differentiate between the patients’ preferred distance from a stranger and a close other.
Methods
Patients
Lesion Patients. We examined eight patients with focal OFC lesions because of resection of a primary intracranial tumor (meningioma; n = 2) or contusion because of traumatic brain injury (n = 6) and five focal DLPFC lesions following stroke. Patient inclusion was based on focal frontal brain lesions indicated on pre-existing CT and/or MRI scans. Participants with a history of serious psychiatric disease, drug, or alcohol abuse requiring treatment, pre-morbid head injury, pre-/comorbid neurological disease, IQ < 85, substantial aphasia, visual neglect or marked sensory impairment were excluded from participation. Testing took place at least 6 months after injury or surgery. The OFC group was tested at a mean of 14.75 years (s.d. = 13.34) after injury/surgery and the DLPFC group was tested at a mean of 11.9 years (s.d. = 5.98). For other demographic information, refer to Table 1. Patients gave written informed consent before participating in the studies and received payment for participation ($20 an hour + transportation fare and time).
Table 1.
Demographic information about all participants, including lesion information for lesion patients
| Group | Participant number | Age | Gender | Experimenter gender | Years of education | Cause of lesion (if relevant) | Years since lesion onset (if relevant) |
|---|---|---|---|---|---|---|---|
| OFC | 1 | 65 | F | F | 16 | Meningioma resection | 8 |
| 2 | 62 | M | F | 10 | Head trauma | 39 | |
| 3 | 59 | F | F | 16 | Head trauma | 9 | |
| 4 | 73 | F | F | 16 | Meningioma resection | 7 | |
| 5 | 48 | F | F | 13 | Head trauma | 2 | |
| 6 | 49 | F | F | 16 | Head trauma | 7 | |
| 7 | 53 | F | F | 16 | Head trauma | 36 | |
| 8 | 34 | F | F | 16 | Head trauma | 10 | |
| DLPFC | 1 | 57 | F | M | 20 | CVA | 18 |
| 2 | 53 | M | F | 18 | CVA | 13 | |
| 3 | 64 | F | M | 18 | CVA | 15 | |
| 4 | 34 | M | F | 18 | CVA | 1 | |
| 5 | 71 | F | F | 14 | CVA | 0.5 | |
| bvFTD | 1 | 67 | M | M | Not specified | – | – |
| 2 | 60 | F | M | 12 | – | – | |
| 3 | 71 | F | M | 12 | – | – | |
| 4 | 63 | M | F | 14 | – | – | |
| 5 | 57 | M | M | 14 | – | – | |
| 6 | 75 | M | F | 16 | – | – | |
| Controls | 1 | 65 | F | F | 16 | – | – |
| 2 | 61 | M | F | 14 | – | – | |
| 3 | 54 | F | F | 16 | – | – | |
| 4 | 63 | F | F | 18 | – | – | |
| 5 | 53 | M | F | 15 | – | – | |
| 6 | 62 | F | F | 16 | – | – | |
| 7 | 54 | M | F | 18 | – | – | |
| 8 | 59 | F | F | 16 | – | – | |
| 9 | 56 | M | F | 18 | – | – | |
| 10 | 60 | F | F | 18 | – | – |
Lesion Reconstruction. Lesion reconstructions were based on structural MRIs obtained after study inclusion. Lesions were outlined by drawing manually on Fluid Attenuated Inversion Recovery (FLAIR), T1 and T2 weighted images of each participant’s brain using MRIcron (www.mccauslandcenter.sc.edu/mricro/mricron/) and Adobe Photoshop CC 2015 (http://www.adobe.com/). High-resolution T1 and T2 weighted images were used as aids to determine the borders of the lesions. The resulting lesion masks were transferred to normalized space using the Statistical Parametric Mapping software's (SPM8:www.fil.ion.ucl.ac.uk/spm/) New Unified Segmentation routine. Individual participant lesion mask, T1, and FLAIR images were first co-registered to a template T1 image (normalized from 152 T1 scans) and the resulting transformation parameters subsequently applied to the lesion mask. Lesions were reconstructed under the supervision of a neurologist (RTK). Illustrations of the traced lesions are presented in Figure 1.
Fig. 1.
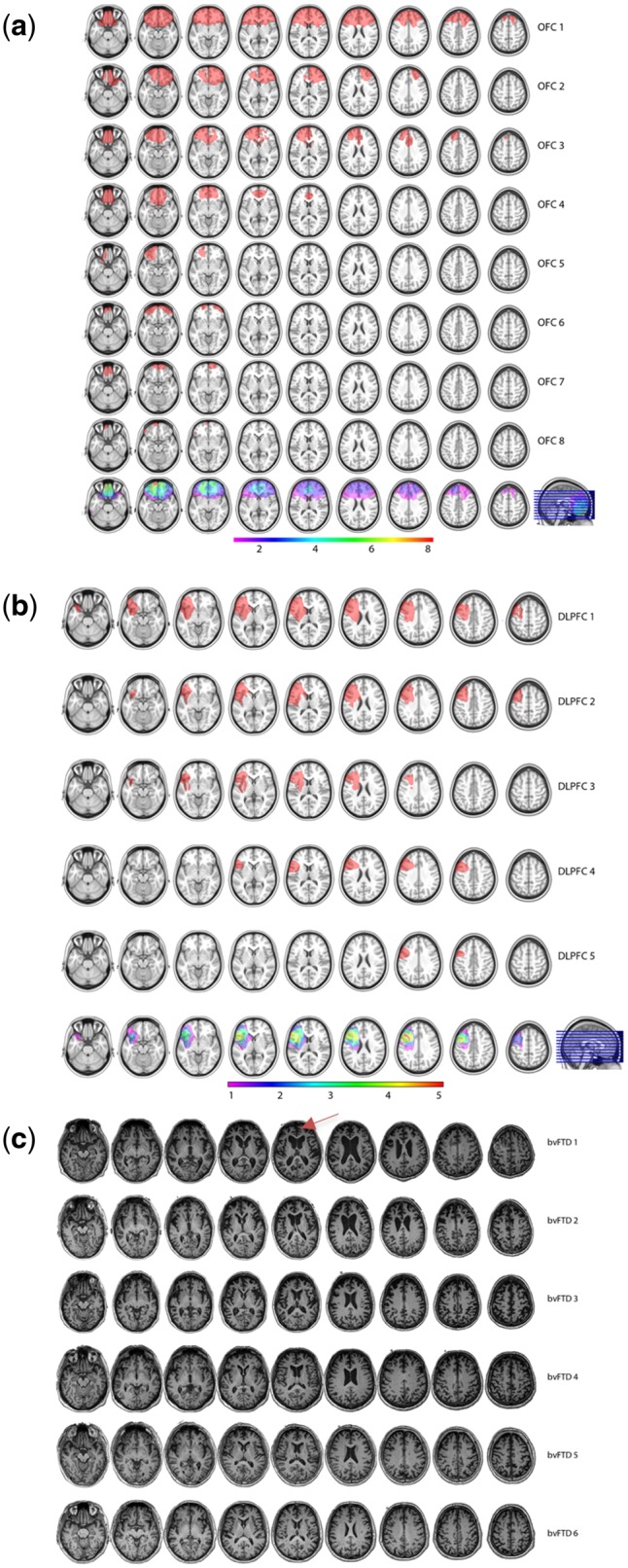
Reconstructions of (a) lesions for the OFC group. Individual patients (1–8) and group overlay (bottom row). The color code for the group overlay indicates the number of patients with damaged tissue in that area. (b): lesions for the DLPFC group. Individual patients (1–5) and group overlay (bottom row). The color code for the group overlay indicates the number of patients with damaged tissue in that area. (c) Frontal and temporal atrophy in bvFTD patients (1–6) in descending order of severity. Red arrow shows widening of the inter-hemispheric fissure in frontal regions.
bvFTD patients and their caregivers. bvFTD patients were recruited through the Memory and Aging Center at the University of California, San Francisco. All participants underwent extensive examinations that included neurological testing, neuropsychological testing and neuroimaging. Patient diagnoses were based on the Neary criteria for bvFTD (Neary et al., 1998). Patients participated in a day-long comprehensive assessment of emotional functioning at the University of California, Berkeley (Levenson et al., 2007). At the end of the day the patients were paid $120 for their participation. All patients were accompanied by a caregiver (4 spouses, 1 brother-in-law, 1 friend). There is a challenge in defining ‘lesion’ location in the bvFTD group, however, there is evidence that OFC is involved early and prominently in this group ( Perry et al., 2006). Moreover, the polar orbitofrontal regions show widening of the inter-hemispheric fissure in all bvFTD patients (see Figure 1c, red arrow pointing to the relevant slice in the first patient, and in all patients below it).
Controls. Ten healthy control participants were matched as closely as possible to the patients for age, sex and level of education. Refer to Table 1 for demographic information for all participants.
Sample size
Previous IPD studies have presented results on small sample sizes, e.g. n = 1 (amygdala damage; Kennedy et al., 2009), n = 3 (amygdala damage; Harrison et al., 2015), n = 13 (autism; Perry et al., 2015), n = 18 patients (autism; Kennedy and Adolphs, 2014). For this study, we were able to recruit 14 patients (8 OFC, 6 bvFTD). Patients in these groups are difficult to recruit, and being able to examine patients with focal OFC lesions alongside clearly diagnosed bvFTD patients is rare. For our control group, we tested a sample of five DLPFC patients and an additional 10 healthy controls. Note that the paradigm we used is highly similar to that used in other studies (Kennedy et al., 2009) and so results can be compared to healthy controls in these studies as well. Since each group size was relatively small, results were analyzed using both parametric and non-parametric statistical tests.
Procedure
Preferred distance from a stranger. A modified version of the stop-distance paradigm was used to assess IPD in OFC lesioned patients, DLPFC lesioned patients, bvFTD patients and controls. This procedure is a frequently used paradigm for assessing preferred or tolerated IPD under varied conditions, with high re-test reliability (Hayduk, 1983; Aiello, 1987). Testing began with the participant positioned at one end of the room with their toes against a measurement tape that was taped to the floor and an experimenter (not familiar to any of the participants) facing the participant from a distance of 2.8 m (9.19 feet). The participant was told that several measurements of distance between the participant and the experimenter would be recorded, and that there was no wrong answer. For distance 1, the participant was instructed to walk toward the experimenter and stop at a comfortable distance where they would normally interact with a person. After the participant stopped and the distance between the toes of the participant and the experimenter was recorded, the participant was instructed to keep walking toward the experimenter until the participant felt uncomfortable (Distance 2). After the two distances were recorded, the participant and experimenter switched places on the two ends of the measurement tape. The participant was told that the experimenter would now do the walking and that the participant should stop the experimenter at a comfortable distance (Distance 3). Then the experimenter kept walking toward the participant until the participant noted feeling uncomfortable (Distance 4). In all conditions, experimenters kept their eyes lowered (i.e. gazing down at the participant’s knees) and maintained a neutral facial expression. A second experimenter recorded the distances.
Preferred distance from a familiar other. Because bvFTD patients were accompanied by their caregivers, the caregivers were also asked to participate in the stop-distance task with the experimenter. In addition, bvFTD patients and their caregivers were asked to repeat the stop-distance task, but this time with each other. In this latter variant, the caregiver initially assumed the role of the experimenter described above with the patient approaching and stopping at a comfortable and uncomfortable distance (Distance 1 and 2). Then, again similar to above, the caregiver approached the patient until the patient told the caregiver to stop at a comfortable and an uncomfortable position (Distance 3 and 4). The patient and caregiver then switched roles, and preferred distances 1–4 were measured from the caregiver’s perspective.
Results
In order to reduce the number of statistical comparisons, and because the values for the two comfortable and the two uncomfortable distances were highly correlated, we computed a composite score by averaging the two values of Comfortable Distance (Distance 1 and 3, r = 0.834, P < 0.001) and Uncomfortable Distance (Distance 2 and 4, r = 0.805, P < 0.001).
To test our hypothesis that damage to the OFC, via either lesions or a neurodegenerative disorder, leads to choosing closer distances, a multivariate analysis of covariance (MANCOVA) was conducted with Comfortable and Uncomfortable distances as the dependent variables. In this analysis, Age of the participant was treated as a covariate, and Sex of the participant, Sex of the experimenter and Group (OFC, bvFTD, DLPFC and controls) were fixed factors. Bonferroni corrections were applied to all post-hoc comparisons. This enabled us to measure the effect of group on distance, taking into account possible interactions with sex and age (refer to Table 1 for a complete description of the demographic data for each participant).
Results showed no significant main effect for age for either Comfortable or Uncomfortable Distance measures (P > 0.25), and no significant main effect for participant sex [Wilks’ Lambda = 0.872, P > 0.25; Comfortable: F(1,19) = 0.503, P > 0.25; Uncomfortable: F(1,19) = 0.043, P > 0.25] or experimenter sex on either the multivariate or the univariate tests [Wilks’ Lambda = 0.838, P = 0.205; Comfortable: F(1,19) = 3.663, P = 0.071; Uncomfortable: F(1,19) = 2.634, P = 0.122].
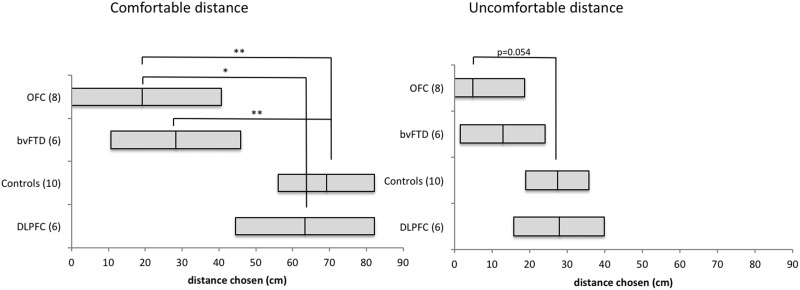
There was a significant main effect for Group (Wilks’ Lambda = 0.362, P = 0.004; Partial η2 = 0.398). Univariate analyses revealed that this effect was significant both for the Comfortable Distance [F(3,19) = 8.879, P = 0.001, Partial η2 = 0.584), and for the Uncomfortable Distance [F(3,19) = 3.980, P = 0.023, Partial η2 = 0.386]. Bonferroni corrected pairwise comparisons revealed significant differences in comfortable distance preferences between the Control group (Mean = 69.150 cm, 95% CI [56.103, 82.197]) and the OFC patients (Mean = 19.124 cm, 95% CI [0, 40.721], P = 0.003), between the Control group and the bvFTD group (Mean = 28.307 cm, 95% CI [10.744, 45.871], P = 0.006), between the OFC group and the DLPFC group (Mean = 63.315 cm, 95% CI [44.457, 82.173], P = 0.026), and a trend in comfortable distance difference between bvFTD and DLPFC (P = 0.077). For the uncomfortable distance, the difference between controls and OFC patients approached significance (Control Mean = 27.362 cm, 95% CI [19.002, 35.723], OFC Mean = 4.869 cm, 95% CI [0, 18.709], P = 0.054). No other differences were significant (see Figure 2 for all distances and 95% confident intervals).
Fig. 2.
Means and confidence intervals of the preferred comfortable distances (left) and uncomfortable distances (right) of each group. ** denotes significant differences P < 0.01 and * denotes significant differences P < 0.05.
To determine whether these results were skewed by the number of participants, the association between distance and group was re-examined with non-parametric tests (Kruskal–Wallis and Median Test). These analyses confirmed our parametric results, showing a significant difference between groups for both Comfortable Distance [Kruskal–Wallis χ2 = 13.932, P = 0.003; Median χ2= 13.749, P = 0.003] and Uncomfortable Distance [Kruskal–Wallis χ2= 10.807, P = 0.013; Median χ2= 12.547, P = 0.006].
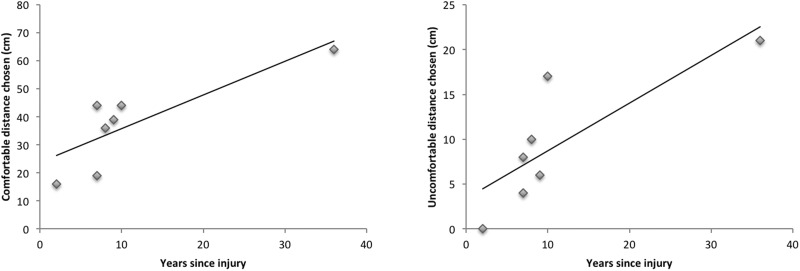
Correlation between time since injury and interpersonal distance preferences. All OFC patients were more than 1 year post-injury (range = 1–35 years). To determine whether time from injury had an effect on patients’ preferred IPD we conducted a partial correlation between time since injury and distance chosen, controlling for the participants’ gender. There was a strong correlation between years since injury and both comfortable distance (r = 0.821, P = 0.024) and uncomfortable distance, (r = 0.809, P = 0.027) indicating that patients may be able to relearn these social norms (Figure 3). These results stem from the analysis of the seven female participants. The one male participant (who was 30 years post-injury), chose a distance of 0 cm from the experimenter for both comfortable and uncomfortable distances, thus not following the female trend of greater time from injury correlating with greater distance.
Fig. 3.
Correlation between years since injury and comfortable distance chosen (left) or uncomfortable distance chosen (right) for the seven OFC female patients.
Correlation between extent of OFC damage and interpersonal distance preferences.
To determine whether the extent of OFC damage correlated with interpersonal distance preferences we tested the correlation between the percent of the OFC that was lesioned and comfortable or uncomfortable distance. Although both showed a negative direction, neither correlation was significant (comfortable distance: r = 0.234, P = 0.577; uncomfortable distance: r = 0.315, P = 0.447).
bvFTD patients do not differentiate between personal space with experimenters and caregivers. Next, for five bvFTD patients and their caregivers (one caregiver was not willing to participate), paired t-tests were used to compare the comfortable and uncomfortable interpersonal distances from a stranger (experimenter) to the distances they chose from a close other (their spouse/friend). Results revealed that caregivers’ comfortable interpersonal distances differed between stranger and close other (MDiff = 19.607 cm; 95% CI [3.051,36.165], t(4) = 3.288, P = 0.030; Figure 4); and a difference that was close to significant was found for uncomfortable interpersonal distance (MDiff = 9.652 cm; 95% CI [−0.799, 20.103], t(4) = 2.564, P = 0.062). In contrast, the bvFTD patients did not show differences between preferred interpersonal distances from a stranger and preferred interpersonal distances from their close other (Comfortable: MDiff = 6.985 cm; 95% CI [−5.127, 19.097], t(4) = 1.6, P = 0.185, see Figure 4; Uncomfortable: Mdiff = 3.683 cm 95% CI [−6.481,13.847], t(4) = 1.006, P > 0.250).
Fig. 4.
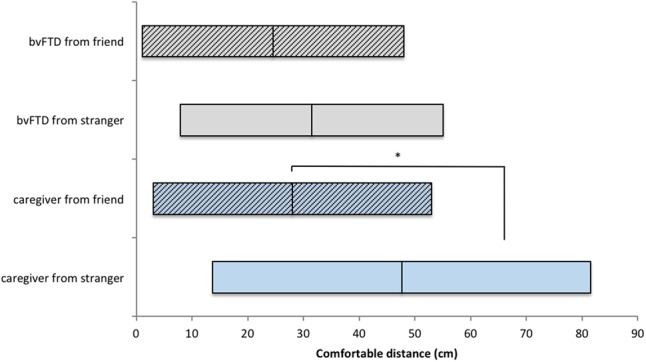
Comfortable distances chosen by bvFTD patients (gray) and their caregivers (light blue). While caregivers chose farther distances from strangers (clear bars) than from close others (striped), bvFTD patients did not and chose close distances from both close others and strangers.
Discussion
We examined interpersonal distance preferences in two groups of patients with damage to the OFC in comparison with a different frontal lesioned group and normal controls. We found that OFC damage reduces the need for interpersonal space, and in some cases eliminated it completely. Both groups with damage to the OFC preferred very close interpersonal distances [i.e. falling within the ‘intimate’ range (means of ∼19 cm for OFC and ∼28 cm for bvFTD)] regardless of whether they were approaching a stranger or a close other, whereas healthy controls and DLPFC patients preferred comfortable interpersonal distances within the expected ‘personal’ range (i.e. means of ∼69 cm for the healthy controls and ∼63 cm for DLPFC patients).
The addition of the DLPFC group is crucial in emphasizing the specific role of the OFC in this task, and ensuring that the deficits observed were not a general result of any brain lesion or a frontal lesion in any sector. Importantly, while the DLPFC group showed no deficits in the current task, partially overlapping cohorts were tested previously in our lab, and showed deficits in various tasks such as attentional control (Krämer et al., 2013) and honesty behavior (Zhu et al., 2014). In the latter, DLPFC patients were compared to OFC patients which showed no deficit in this paradigm. This strengthens the notion that the difference between the two groups in the current study is due to the role of the OFC in interpersonal distance, and not to a general tendency of these patients to do worse in laboratory testing.
These results fit in with a growing body of evidence showing that OFC patients do not conform to social norms, and do not use available cues in the social context for altering their social behavior (Rolls et al., 1994; Beer et al., 2003, 2006a). For example, in a study of patients with OFC lesions, patients were found to be aware of social norms of intimacy in a conversation with a stranger, but were unaware that their performance on a previous social task violated these norms (Beer et al., 2006a). Similarly, Stolk et al. (2015) demonstrated that patients with OFC damage were able to select effective communication behaviors during social interaction (describing to another player where to move their figure on a computer screen, using movements and gestures on the screen); however, their communicative decisions were not fine-tuned with their knowledge of the social partner (e.g. they did not change their behavior when told that the other player was a child versus an adult).
Why OFC patients violate social norms is still under debate. Theories regarding the role of the OFC in social cognition can be broadly categorized into two groups: lack of online self-monitoring, or deficient emotional systems (Beer et al., 2006). The first emphasizes the importance of the OFC for evaluating, not necessarily consciously, one’s behavior in the present moment to one’s higher order goals or to the reactions of other people (Prigatano, 1991; Stuss, 1991). Thus, OFC patients may choose close distances because they lack online self-insight into the inappropriateness of their behavior. Note that this implies that without an inhibition process that monitors social appropriateness, the default is for people to prefer close intimate distances from others, somewhat similar to utilization behaviors (a difficulty resisting the impulse to operate or manipulate objects that are within reach), common in frontal lesion patients (Lhermitte, 1986; Lhermitte et al., 1986).
Other theories propose that different forms of emotional deficits account for the impaired interpersonal behavior associated with OFC damage (Bechara et al., 2000; Elliott et al., 2000; Kringelbach and Rolls, 2004). For example, the somatic marker hypothesis proposes that the OFC is critical for interpreting somatic sensations that are needed to make decisions. Empirical support for this hypothesis comes from gambling studies that have found that patients with OFC damage do not show anticipatory anxiety before taking big risks (Bechara et al., 1997, 2000). Similarly, the OFC has been implicated in guiding behavior in ambiguous situations by incorporating intuition or ‘gut feelings’ into decision-making (Elliott et al., 2000). These somatic sensations are probably mediated by other cortical and subcortical structures, including the amygdala, the insula, the hypothalamus and the peripheral nervous system (Bechara et al., 2000). It is worth noting that Kennedy et al. (2009) showed that the amygdala also plays a role in maintaining interpersonal distance, and that individuals with amygdala damage do not experience the need for personal space. Therefore, according to the emotional deficit theories, OFC patients might choose closer distances because they lack an ‘alert’ system, possibly via extensive connections between the amygdala and OFC (Kringelbach and Rolls, 2004), warning them of the (physical or social) dangers of getting too close to another. In fact it may be that OFC patients suffer from both disinhibition caused by lack of online self-monitoring which is mediated by the OFC, and from an emotional deficit, caused by reduced input from limbic regions (Beer et al., 2006a). This makes OFC patients especially prone to deficits associated with evaluating the contextual relevance of emotional information for decision making (Beer et al., 2006b). Patients with lesions to the DLPFC, although suffering from other deficits, such as attention (Bidet-Caulet et al., 2014) or memory (Aly et al., 2011), do not show any sign of not conforming to social norms.
The correlation between time from lesion and behavior raises the possibility that keeping interpersonal distance can be taught over time, perhaps exploiting other brain mechanisms. Whether this results in a similar automatic feeling or is more controlled behavior is hard to determine. However, an anecdote from the testing session might shed some light on the phenomenon. The patient that chose the farthest distance, and had OFC damage for more than 30 years, explained when she stopped that ‘I know this is the distance that I should choose from a stranger, but in an evening seminar I take, where everyone is naked and does whatever they want, I always get very close to everyone’. This comment may suggest that keeping distance from strangers required a more explicit process for her, and was not determined by an intuitive feeling (see also estimates on Phineas Gage's psychosocial adaptation later in life, Macmillan and Lena, 2010). This also relates to studies showing that high functioning individuals with ASD often need to be explicitly taught how to maintain proper interpersonal space (Mitchell et al., 2007). Indeed, OFC abnormalities have been reported in ASD individuals (Bachevalier and Loveland, 2006; Girgis et al., 2007).
Simplicity is one of the strengths of this study. Using a simple behavioral task, clear differences were seen between different frontal-lesioned populations. Although requiring further studies and comparison to other dementia groups, this task has the potential of being beneficial as a first screening tool or diagnostic measure, differentiating between different forms of dementia as well as between sub-populations of ASD (Kennedy and Adolphs, 2014; Perry et al., 2015a). This study also has some limitations. Although together the OFC and bvFTD groups form a cohort of 14 patients, these groups are not identical in their damage or in their prognosis (the dementia patients’ behavior will most likely deteriorate while the OFC patients’ behavioral deficits will either stay the same or improve). Furthermore, as can been seen from Figure 1c, the bvFTD patients show a great variance in atrophy. Although all six patients were diagnosed with bvFTD, some still show minimal brain atrophy, while others show atrophy that extends beyond the OFC to additional frontal and temporal regions. In these cases, atrophy to other regions (e.g. the amygdala) may be affecting their interpersonal distance preferences as well.
To conclude, patients with damage to the OFC, either from focal lesions or from neurodegenerative disease, show abnormal IPD preferences compared to both healthy controls and patients with other frontal lesions. The comfortable distances these patients chose from strangers were significantly closer than controls, falling into a range that is thought to be normally used only with close others. These results shed light on the role of the OFC in implicit social decisions.
Funding
This work was supported by the EU Marie Curie Global Fellowship (to AP); NINDS R3721135 and the Nielsen Corporation (to RTK).
Conflict of interest. None declared.
References
- Aiello J.R. (1987). Human spatial behavior. Handbook of Environmental Psychology, 1, 389–504. [Google Scholar]
- Aiello J.R., Aiello T.D.C. (1974). The development of personal space: proxemic behavior of children 6 through 16. Human Ecology 2(3), 177–89. [Google Scholar]
- Aly M., Yonelinas A.P., Kishiyama M.M., Knight R.T. (2011). Damage to the lateral prefrontal cortex impairs familiarity but not recollection. Behavioural Brain Research, 225(1), 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J., Loveland K.A. (2006). The orbitofrontal–amygdala circuit and self-regulation of social–emotional behavior in autism. Neuroscience & Biobehavioral Reviews, 30(1), 97–117. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A. R. (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex, 10(3), 295–307. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Tranel D., Damasio A. R. (1997). Deciding advantageously before knowing the advantageous strategy. Science, 275(5304), 1293–4. [DOI] [PubMed] [Google Scholar]
- Beer J. S., Heerey E. A., Keltner D., Scabini D., Knight R. T. (2003). The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology, 85(4), 594.. [DOI] [PubMed] [Google Scholar]
- Beer J. S., John O. P., Scabini D., Knight R. T. (2006a). Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. Journal of Cognitive Neuroscience, 18(6), 871–9. [DOI] [PubMed] [Google Scholar]
- Beer J. S., Knight R. T., D'Esposito M. (2006b). Controlling the integration of emotion and cognition the role of frontal cortex in distinguishing helpful from hurtful emotional information. Psychological Science, 17(5), 448–53. [DOI] [PubMed] [Google Scholar]
- Berthoz S., Armony J.L., Blair R.J.R., Dolan R.J. (2002). An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain, 125(8), 1696–708. [DOI] [PubMed] [Google Scholar]
- Bidet-Caulet A., Buchanan K. G., Viswanath H., et al. (2014). Impaired facilitatory mechanisms of auditory attention after damage of the lateral prefrontal cortex. Cerebral Cortex, 25, 4126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer A. L., Miller B. L. (2005). Clinical features of frontotemporal dementia. Alzheimer Disease & Associated Disorders, 19, S3–6. [DOI] [PubMed] [Google Scholar]
- Elliott R., Dolan R. J., Frith C. D. (2000). Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex, 10(3), 308–17. [DOI] [PubMed] [Google Scholar]
- Feeney J.A. (1999). Adult romantic attachment and couple relationships In Classidy J., Shaver P. R. editors), Handbook of Attachment: Theory, Research, and Clinical Applications (pp. 355–77). New York, NY: Guilford Press. [Google Scholar]
- Girgis R. R., Minshew N. J., Melhem N. M., Nutche J. J., Keshavan M. S., Hardan A. Y. (2007). Volumetric alterations of the orbitofrontal cortex in autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 31(1), 41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E. T. (1963). A system for the notation of proxemic behavior1. American Anthropologist, 65(5), 1003–26. [Google Scholar]
- Harrison L. A., Hurlemann R., Adolphs R. (2015). An enhanced default approach bias following amygdala lesions in humans. Psychological Science, 26(10), 1543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayduk L. A. (1983). Personal-space—where we now stand. Psychological Bulletin, 94(2), 293–335. doi: 10.1037/0033-2909.94.2.293. [Google Scholar]
- Hornberger M., Geng J., Hodges J. R. (2011). Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain, 134(9), 2502–12. [DOI] [PubMed] [Google Scholar]
- Kaitz M., Bar-Haim Y., Lehrer M., Grossman E. (2004). Adult attachment style and interpersonal distance. Attachment & Human Development, 6(3), 285–304. [DOI] [PubMed] [Google Scholar]
- Kennedy D. P., Adolphs R. (2014). Violations of personal space by individuals with autism spectrum disorder. PLoS One, 9(8), e103369.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. P., Gläscher J., Tyszka J. M., Adolphs R. (2009). Personal space regulation by the human amygdala. Nature Neuroscience, 12(10), 1226–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer U. M., Solbakk A. K., Funderud I., Løvstad M., Endestad T., Knight R. T. (2013). The role of the lateral prefrontal cortex in inhibitory motor control. Cortex, 49(3), 837–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M. L., Rolls E. T. (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72(5), 341–72. [DOI] [PubMed] [Google Scholar]
- Levenson R. W., Ascher E., Goodkind M., McCarthy M., Sturm V., Werner K. (2007). Laboratory testing of emotion and frontal cortex. Handbook of Clinical Neurology, 88, 489–98. [DOI] [PubMed] [Google Scholar]
- Lhermitte F. (1986). Human autonomy and the frontal lobes. Part II: patient behavior in complex and social situations: the “environmental dependency syndrome”. Annals of Neurology, 19(4), 335–43. [DOI] [PubMed] [Google Scholar]
- Lhermitte F., Pillon B., Serdaru M. (1986). Human autonomy and the frontal lobes. Part I: Imitation and utilization behavior: a neuropsychological study of 75 patients. Annals of Neurology, 19(4), 326–34. [DOI] [PubMed] [Google Scholar]
- Lloyd D. M. (2009). The space between us: a neurophilosophical framework for the investigation of human interpersonal space. Neuroscience & Biobehavioral Reviews, 33(3), 297–304. [DOI] [PubMed] [Google Scholar]
- Macmillan M., Lena M. L. (2010). Rehabilitating Phineas gage. Neuropsychological Rehabilitation, 20(5), 641–58. [DOI] [PubMed] [Google Scholar]
- Meisels M., Guardo C. J. (1969). Development of personal space schemata. Child Development, 40(4), 1167–78. [PubMed] [Google Scholar]
- Mitchell P., Parsons S., Leonard A. (2007). Using virtual environments for teaching social understanding to 6 adolescents with autistic spectrum disorders. Journal of Autism and Developmental Disorders, 37(3), 589–600. [DOI] [PubMed] [Google Scholar]
- Neary D., Snowden J., Mann D. (2005). Frontotemporal dementia. The Lancet Neurology, 4(11), 771–80. [DOI] [PubMed] [Google Scholar]
- Neary D., Snowden J. S., Gustafson L., et al. (1998). Frontotemporal lobar degeneration A consensus on clinical diagnostic criteria. Neurology, 51(6), 1546–54. [DOI] [PubMed] [Google Scholar]
- Perry A., Levy-Gigi E., Richter-Levin G., Shamay-Tsoory S. G. (2015a). I nterpersonal distance and social anxiety in autistic spectrum disorders: a behavioral and ERP study. Social Neuroscience, 10(4), 354–65. [DOI] [PubMed] [Google Scholar]
- Perry A., Nichiporuk N., Knight R. T. (2015b). Where does one stand: a biological account of preferred interpersonal distance. Social Cognitive and Affective Neuroscience, 11(2), 317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. J., et al. (2006). Patterns of frontal lobe atrophy in frontotemporal dementia: a volumetric MRI study. Dementia and Geriatric Cognitive Disorders, 22(4), 278–87. [DOI] [PubMed] [Google Scholar]
- Prigatano G. P. (1991). Disturbances of self-awareness of deficit after traumatic brain injury. Awareness of Deficit after Brain Injury: Clinical and Theoretical Issues. New York: Oxford University Press, 111–26. [Google Scholar]
- Rascovsky K., Hodges J. R., Knopman D., et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E. T., Hornak J., Wade D., McGrath J. (1994). Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery & Psychiatry, 57(12), 1518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H. J., Allison S. C., Schauer G. F., Gorno-Tempini M. L., Weiner M. W., Miller B. L. (2005). Neuroanatomical correlates of behavioural disorders in dementia. Brain, 128(11), 2612–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver J. L., Damasio A. R. (1991). Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia, 29(12), 1241–9. [DOI] [PubMed] [Google Scholar]
- Stolk A., D’Imperio D., di Pellegrino G., Toni I. (2015). Altered communicative decisions following ventromedial prefrontal lesions. Current Biology, 25(11), 1469–74. [DOI] [PubMed] [Google Scholar]
- Stuss D.T. (1991). Self, awareness, and the frontal lobes: a neuropsychological perspective. In: Strauss, J., Goethals, G.R., editors The Self: Interdisciplinary Approaches. New York: Springer, 255–78. [Google Scholar]
- Werner K.H., Roberts N.A., Rosen H.J., et al. (2007). Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology, 69(2), 148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Jenkins A. C., Set E., et al. (2014). Damage to dorsolateral prefrontal cortex affects tradeoffs between honesty and self-interest. Nature Neuroscience, 17(10), 1319–21. [DOI] [PMC free article] [PubMed] [Google Scholar]