Abstract
The structure of the mask stimulus is crucial in backward masking studies and we recently demonstrated such an effect when masking faces. Specifically, we showed that activity of the amygdala is increased to fearful facial expressions masked with neutral faces and decreased to fearful expressions masked with a pattern mask—but critically both masked conditions discriminated fearful expressions from happy expressions. Given this finding, we sought to test whether masked fearful eye whites would produce a similar profile of amygdala response in a face vs non-face context. During functional magnetic resonance imaging scanning sessions, 30 participants viewed fearful or happy eye whites masked with either neutral faces or pattern images. Results indicated amygdala activity was increased to fearful vs happy eye whites in the face mask condition, but decreased to fearful vs happy eye whites in the pattern mask condition—effectively replicating and expanding our previous report. Our data support the idea that the amygdala is responsive to fearful eye whites, but that the nature of this activity observed in a backward masking design depends on the mask stimulus.
Keywords: amygdala, backward masking, eyes, fMRI, fear
Introduction
We automatically attend to signals in the environment that convey salient information, especially information predicting potential harm. Behaviorally, this attention to threat manifests in fast orientation and quick responses to threat-related stimuli, such as images of snakes or emotional facial expressions (Mogg and Bradley, 1999; Ohman et al., 2001). These effects are observed even when the level of awareness or attention is restricted (Mogg and Bradley, 1999; Ohman et al., 2001). In order to investigate this automaticity, numerous laboratories have used the backward masking paradigm (Morris et al., 1998; Whalen et al., 1998; Etkin et al., 2004; Armony et al., 2005; Pessoa et al., 2006; Williams et al., 2006; Ohman et al., 2007; Jessen and Grossman, 2014; Kanat et al., 2015). In a typical backward masking experiment using emotional facial expressions, a target stimulus (e.g., a fearful face) is presented briefly, then immediately replaced by a mask stimulus (e.g. a neutral face). Original reports presented the fearful target stimulus for 33 ms (after Ohman et al., 1993), while more recent reports observe similar effects at a 17 ms presentation rate (Whalen et al., 2004; Kim et al., 2010). Though participants are subjectively unaware of the target stimuli, the blood oxygen level dependent (BOLD) signal in the amygdala is preferentially increased to masked fearful faces, compared to happy or neutral faces (Morris et al., 1998; Whalen et al., 1998; Rauch et al., 2000; Williams et al., 2006). The amygdala can even detect low-level, unrefined components of fearful faces, such as eye whites, when masked by neutral faces (Whalen et al., 2004). These findings support the idea that the amygdala is sensitive to crude representations of threat-related stimuli (LeDoux, 1996).
In a previous study using backward masking, we addressed whether it was necessary to use a human face as the masking stimulus in order to observe amygdala response to the target fear stimulus. Asked another way, does the participant have to be subjectively aware that they are in a ‘face’ experiment? What if a random non-face pattern mask was used as the mask stimulus, making subjects unaware that faces were in the experiment? In this study (Kim et al., 2010) we reported an interactive effect between facial expression (fearful, happy faces) and mask stimulus type (neutral faces, pattern images) on amygdala BOLD activity (Kim et al., 2010). Specifically, we demonstrated that amygdala BOLD activity to fearful vs happy faces was increased when masked with neutral faces (replicating previous reports), but was decreased when they were masked with non-face pattern images. Critically, the amygdala clearly discriminated between the hidden fearful vs happy faces in both cases, but also showed an opposite response between mask conditions in terms of the direction of signal change. While it is clear that the amygdala is sensitive to the hidden target stimuli per se, the direction of signal change is also influenced by an interaction of this information with the explicit mask stimulus.
Given that we have previously shown that masked fearful eye whites are sufficient to produce amygdala activation (Whalen et al., 2004), here we sought to use these stimuli in place of whole fearful faces in an attempt to replicate BOLD signal increases and decreases to face and pattern masked fearful vs happy eye whites, respectively. We predicted that the amygdala would exhibit increased activity to fearful vs happy eye whites when the faces were followed by neutral face masks. Conversely, we also predicted that the amygdala would exhibit decreased activity to fearful vs happy eye whites when the faces were followed by a pattern image.
Materials and methods
Participants
A total of 37 healthy volunteers (21 women, 19.5 ± 1.6 years of age, 34 right-handed) were screened for current or past psychiatric illness (Axis I or II) using an abbreviated version of the Structured Clinical Interview for DSM-IV (First et al., 1997). None of the participants had a history of taking psychotropic medications. Following the fMRI scanning sessions, we assessed handedness with the Edinburgh Handedness Inventory (Oldfield, 1971). The Committee for the Protection of Human Subjects at Dartmouth College approved the study protocol. We obtained written, informed consent from the participants prior to the experiment.
Stimuli
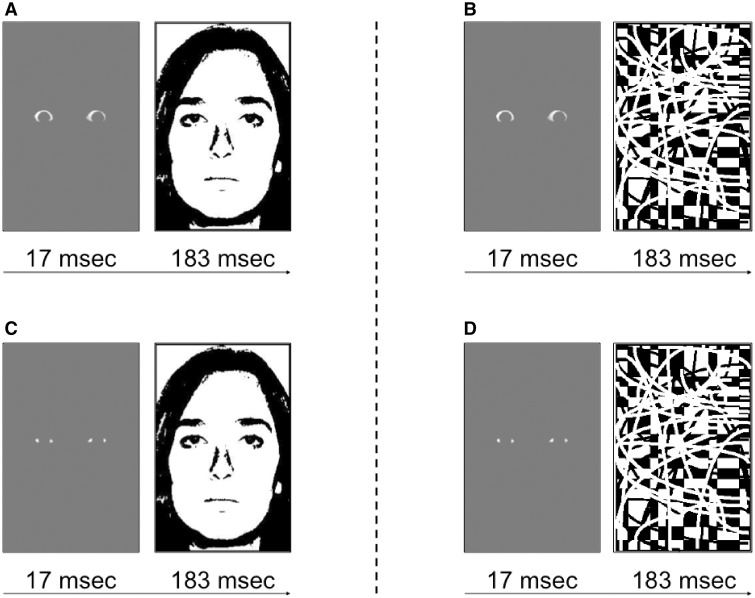
We adapted the eye white stimuli from our previous study (Whalen et al., 2004). To create the target stimuli, we used eye whites from faces of eight different individuals with fearful and happy expressions (four males and four females; Ekman and Friesen, 1976). In our previous study, the eye whites were set against a black background for maximum contrast. Here we aimed to demonstrate the generalizability and sensitivity of this effect by utilizing a gray background (Figure 1). For the face masks, we used the neutral expressions of the same eight individuals. We grayscaled each face, and then thresholded to produce black and white binary images (i.e., line drawings). The pattern mask image was the same as used previously (Kim et al., 2010) and was specifically designed to mask fearful faces as effectively as neutral faces—an assertion supported by our previous study. All of the stimuli were back-projected (Panasonic PT-D4000U DLP) onto a screen, which the participants viewed using a mirror that was mounted on the head coil. Given that the exact timing of the stimulus presentation was crucial in our experiment, we used a photodiode-oscilloscope system (Tektronix, 2012) to ensure that the precise timing. By observing 100 trials we verified that all target stimuli were presented between 16 and 17 ms, which corresponds to < 2 refresh rate in a 60 Hz display.
Fig. 1.
Examples of (A) face-masked fearful eye white trials, (B) pattern-masked fearful eye white trials, (C) face-masked happy eye white trials and (D) pattern-masked happy eye white trials. Trials were repeated 36 times within each 18 s block.
One potentially confounding difference between our face and pattern mask condition used in our previous report (Kim et al., 2010) is that the face condition consists of eight stimulus face identities while the pattern mask condition comprises a single mask image. Since the pattern mask condition produced an unanticipated decrease in amygdala activity, we wished to address the possibility that this was due to the single repeating image mask, rather than a non-face pattern image per se. For this reason, we altered the face mask condition used here so that half of the participants (n = 19; 10 women, 19.2 ± 1.2 years of age, 17 right handed) viewed all eight identities as the mask stimulus, while the other half (n = 18; 11 women, 19.8 ± 1.82 years of age, 18 right-handed) saw only one randomly chosen identity. In order to maximize statistical power, we first report the results derived from all participants, and then show that there are no significant differences in brain activity between the two datasets. We note that the absence of significant differences may not necessarily imply that there were no differences between groups; it may be the case where the difference was too small to be detected, especially given the relatively small sample size. We also note here that in order to control for the different number of masks across the face and pattern conditions, we chose to use a single neutral face instead of eight pattern images. This decision was based on pragmatic reasons—since the contours in the pattern images are not random, but rather deliberately designed to successfully mask facial features, the development of varying pattern images was beyond the scope of the current study. Still, it would be important to develop and acquire varying types of pattern masks for future backward masking studies that aim to take face vs non-face context into consideration.
Paradigm
We adapted the procedures from our previous backward masking studies (Whalen et al., 2004; Kim et al., 2010). In the scanner, we instructed participants to passively view blocked presentations of masked images that appeared on the screen during three functional scans. Importantly, the participants were not informed about the hidden masked eye whites, since we prefer to test participants when they are naïve, and then to test them again while they perform an objective forced choice test of awareness (See Whalen et al., 2004, Supplementary Material).
Since there were two types of masks (neutral face, pattern image) and two types of targets (fearful, happy eye whites), there were four possible target-mask trial types: (i) face-masked fearful, (ii) face-masked happy, (iii) pattern-masked fearful and (iv) pattern-masked happy. In the scanner, each individual passively viewed blocked presentations of these four trial types across three functional runs. Each functional run, which was 5 min and 14 s long, consisted of two 18 s blocks of each of the four trial types, with 18 s fixation blocks interleaved between them. The order of the trial blocks was counterbalanced across participants. Within each 18 s block, a target stimulus (fearful or happy eye whites) was presented on the screen for 17 ms, and then immediately replaced by a mask stimulus (neutral face or pattern image) that was on the screen for 183 ms, followed by a fixed interstimulus interval of 300 ms. Thus, each trial was 500 ms long, which allowed for a total of 36 masked fearful or happy eye white stimuli in each block. In the face mask condition, the identity of the face mask never matched the identity of the eye whites on any given trial, consistent with our previous study (Whalen et al., 2004). The order of the faces within a block was pseudorandomized to ensure that the same face was not presented more than twice in a row. The pattern mask was designed and subsequently shown (Kim et al., 2010) to produce similarly effective masking compared to the face masks, and this one pattern mask was used throughout the present experiment.
Subject debriefing
We assessed subjective awareness with post-scan interview sessions. Immediately after the participants exited the scanner, we asked them what they thought the purpose of the study was. Then, we instructed them to describe what was presented on the screen during the fMRI scanning sessions. Next, we asked the participants to comment on any aspects of the faces and pattern images. Finally, we asked them if they had seen any parts of fearful or happy expressions during the fMRI scanning sessions. If any participants reported seeing even a single pair of fearful or happy eye whites (out of 864 total pairs presented during the scanning sessions), we considered them to be subjectively aware of the target stimuli and thus removed their data from further analysis, consistent with our previous studies (Whalen et al., 1998; Whalen et al., 2004; Kim et al., 2010).
Immediately following this post-scan interview, we debriefed the participants and explained that there were fearful or happy eye whites before each mask stimulus. With this knowledge, participants were exposed to a total of 40 experimental blocks again (10 of each trial type), and were asked to actively search for the ‘hidden’ eye whites. This post-scan test was performed outside the MRI scanner using an LCD display with 60 Hz refresh rate that matched the capabilities of the projector that was used inside the scanner, and was also verified using a photodiode-oscilloscope system (Tektronix, 2012). We instructed the participants to evaluate the blocks instead of individual trials to reflect the blocked stimulus presentations in the scanner. Specifically, we instructed participants to report whether the masked eye whites of a block were fearful or happy in a two alternative forced choice task. This allowed us to assess their objective awareness—the ability to correctly discriminate whether the masked eye whites were fearful or happy even without subjective awareness (Etkin et al., 2004; Whalen et al., 2004; Pessoa et al., 2006; Kim et al., 2010). Adopting the operational definition from past studies, objective awareness was quantified based on signal detection theory by calculating a sensitivity index (d’) based upon the percentage of trials a masked stimulus was correctly identified when presented (hits), adjusted for the percentage of trials a masked stimulus was ‘identified’ when not presented (false alarms), using the following formula: [d’ = z-score (% hits) – z-score (% false alarms), with chance performance = 0 ± 1.74] (Whalen et al., 2004; Kim et al., 2010). Thus, greater absolute d’ values correspond to increased ability to discriminate fearful vs happy eye whites, even when the participants were not subjectively aware of them.
Image acquisition
Brain data from all participants were acquired at the Dartmouth Brain Imaging Center, using a 3.0 Tesla Philips Intera Achieva Scanner (Philips Medical Systems, Bothell, WA) equipped with an 8-channel head coil. Functional T2*-weighted images were acquired using echo-planar imaging sequence, with 36 interleaved 3-mm thick slices with 0.5 mm interslice gap for each brain volume (echo time [TE] = 35 ms, repetition time [TR] = 2000 ms, field of view [FOV] = 240 mm, flip angle = 90°, voxel size = 3 × 3 × 3.5 mm). Anatomical T1-weighted images were scanned using a high-resolution 3D magnetization-prepared rapid gradient echo sequence, with 160 contiguous 1-mm thick sagittal slices (TE = 4.6 ms, TR = 9.8 ms, FOV = 240 mm, flip angle = 8°, voxel size = 1 × 0.94 × 0.94 mm).
fMRI data analysis
All fMRI data were processed using the Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuroscience, London, UK). Following the procedures we used in our previous study (Kim et al., 2010), raw fMRI data were preprocessed by adjusting for any head movement that occurred during the scanning sessions. Head movement was less than 1.5 mm in any direction for all participants. Next, each individual’s high-resolution anatomical image was coregistered with the functional images. Coregistered anatomical images were then spatially normalized using the Montreal Neurological Institute (MNI)-152 template. Normalization parameters derived from this step were applied to the functional images, in order to transform them into standard space, and resampled to 3 × 3 × 3 mm voxels. Spatially normalized functional images were smoothed using a Gaussian kernel of 6 mm full width at half maximum. For each participant, linear contrast maps [target emotion (fearful, happy eye whites)] × [mask type (non-face pattern, face)] were constructed using a boxcar function convolved with the hemodynamic response function and covariates of no interests (a session mean, a linear trend for each run to account for low-frequency drift, and six movement parameters derived from head motion corrections). Individual contrast maps were then entered into a random effects model to enable population-based inferences from our data. To accommodate the 2 × 2 factorial design (target emotion × mask type), a voxelwise analysis of variance (ANOVA) model was constructed. Based on our previous finding (Kim et al., 2010), we were primarily interested in the interaction contrast (emotion × mask).
Given that our goal was to investigate how the amygdala in particular responds to threat-related stimuli in the absence of explicit awareness, we imposed a significance threshold of P < 0.05 corrected for multiple comparisons over the bilateral amygdala volume (∼4500 mm3, defined using the Automated Anatomical Labeling atlas; Maldjian et al., 2003), as determined by Monte Carlo simulations (n = 10 000) implemented in 3dClustSim within AFNI software (Cox, 1996). The corrected P < 0.05 threshold corresponded to uncorrected P < 0.005, k ≥ 5 voxels (135 mm3). Building upon the findings from our previous backward masking study (Kim et al., 2010), we sought to identify voxels in the amygdala that showed a significant interaction, characterized by increased BOLD signal to face-masked fearful vs happy eye whites, and decreased BOLD signal to pattern-masked fearful vs happy eye whites. In addition, we also report the results from the whole brain voxelwise ANOVA. Monte Carlo simulations determined that whole brain-corrected P < 0.05 threshold corresponded to uncorrected P < 0.001, k ≥ 36 voxels (972 mm3). Specifically, post hoc analyses were performed on the brain regions that were significant from the voxelwise ANOVA. To this end, spherical region-of-interest (ROI) with a 10-mm radius were defined around the each of peak voxels and average parameter estimates from all significant voxels (P < 0.001) within the ROI were extracted for further statistical analyses.
Results
Behavioral data
Post-scan assessment identified that out of the initial 37 participants, seven had seen at least one pair of masked eye whites during the scanning sessions. The observed subjective detection rate of ∼20% is consistent with our previous backward masking studies (Whalen et al., 1998; Kim et al., 2010) and consistent with these reports we excluded these subjects, analyzing the remaining 30 who were subjectively unaware of the eye white stimuli. Thus, we report the results from 30 participants (19 women, 19.4 ± 1.6 years of age, 28 right-handed). Four out of the remaining 30 participants demonstrated above chance performance (d’ > 1.74) on the objective awareness measure administered after scanning. These participants were included in the presented data because the main focus of the current study was on the effects of subjective awareness, and this approach was consistent with our previous studies (Whalen et al., 2004; Kim et al., 2010), allowing for a more precise replication attempt. In addition, the results did not differ when they were excluded from this analysis, consistent with our previous report (Whalen et al., 2004).
We also note here that data from all 30 participants are presented together despite the two different face mask conditions (i.e. eight identities vs one identity), because there was no significant group difference in objective awareness between the participants who saw eight neutral faces (n = 15) versus one neutral face (n = 15) (face-masked: t = -1.28, P = 0.212; pattern-masked: t = -0.77, P = 0.449).
fMRI data
Region-of-interest analysis of the amygdala
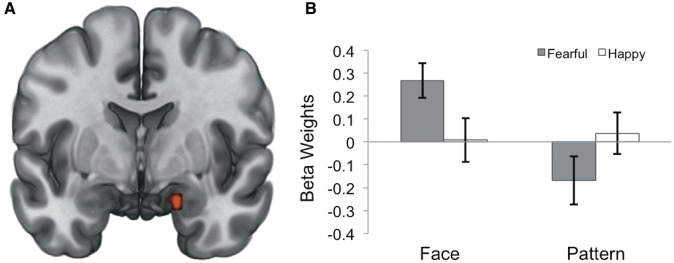
Activation in the right amygdala (MNI 18, -3, -21; F = 10.55, P < 0.05 corrected for multiple comparisons, cluster size = 162 mm3) was shown to have an interactive effect between target emotion (fearful or happy eye whites) and mask type (non-face pattern or neutral face) (Figure 2). Specifically, this interaction was characterized by increased activity to fearful vs happy eye whites in the face mask condition (t = 2.58, P = 0.015), and decreased activity to fearful vs happy eye whites in the pattern mask condition (t = -2.07, P = 0.048). There were no significant differences in right amygdala activity to fearful vs happy eye whites between groups who saw eight neutral faces versus one neutral face in the face-masked condition (face-masked: t = 0.77, P = 0.448; pattern-masked: t = -0.59, P = 0.558). Objective awareness (d’) to face and pattern mask conditions was not significantly correlated with right amygdala activity to face-masked fearful vs happy eye whites (r = 0.07, P = 0.722) or pattern-masked fearful vs happy eye whites (r = −0.001, P = 0.996).
Fig. 2.
(A) Statistical map derived from a 2 × 2 voxelwise analysis of variance model showing right amygdala voxel clusters (red) with a significant target × mask interaction effect (MNI 18, −3, −21; F = 10.55, P < 0.05 corrected). (B) Right amygdala activity to each condition displaying a significant signal increase to face-masked fearful vs happy eye whites, as well as signal decrease to pattern-masked fearful vs happy eye whites. Spherical ROI with a 3-mm radius was defined around the peak voxel and average parameter estimates were extracted. Error bars represent the standard error of the mean.
Whole brain voxelwise ANOVA
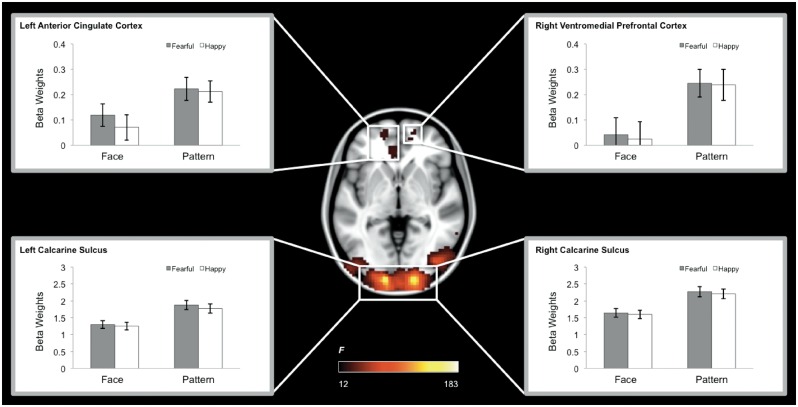
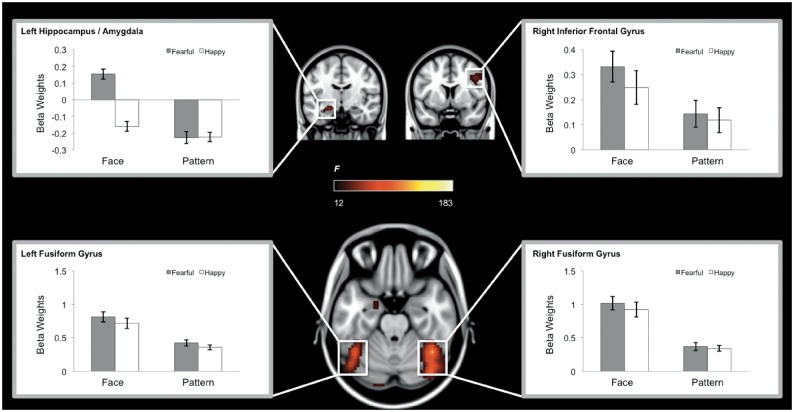
A main effect of Mask was observed within the occipital lobe centered at the calcarine sulcus, as well as the fusiform gyrus, amygdala, hippocampus, inferior frontal gyrus, the ventromedial prefrontal cortex and the anterior cingulate cortex (Table 1). Post hoc analyses revealed that this effect was due to greater activity in the pattern mask compared to face mask condition in the bilateral calcarine sulci, ventromedial prefrontal cortex and the anterior cingulate cortex (Figure 3); and greater activity in the face compared to mask condition in the fusiform gyrus, amygdala, hippocampus, and inferior frontal gyrus (Figure 4), which was consistent with a previously known neural system for face perception (e.g. fusiform gyrus, amygdala; Haxby et al., 2001). In contrast, no brain region showed a significant main effect of emotion or an interaction between mask and emotion.
Table 1.
Whole brain voxelwise ANOVA results (P < 0.05 whole brain-corrected)
| Brain region | Side | F | x | y | z | |
|---|---|---|---|---|---|---|
| Main effect of mask (face vs pattern masks) | ||||||
| Calcarine sulcus | R | 183.51 | 18 | −99 | 0 | |
| Calcarine sulcus | L | 157.37 | −15 | −102 | −3 | |
| Fusiform gyrus | R | 110.36 | 42 | −54 | −24 | |
| Fusiform gyrus | L | 77.95 | −42 | −57 | −21 | |
| Hippocampus | L | 35.80 | −21 | −12 | −15 | |
| Amygdalaa | L | 14.79 | −21 | −3 | −24 | |
| Inferior frontal gyrus | R | 31.24 | 45 | 6 | 36 | |
| Ventromedial prefrontal cortex | R | 18.18 | 12 | 54 | −9 | |
| Anterior cingulate cortex | L | 18.13 | −6 | 36 | −3 | |
| Main effect of emotion (fearful vs happy eye whites) | ||||||
| No brain regions were observed | ||||||
| Mask × emotion interaction | ||||||
| No brain regions were observed | ||||||
Amygdala voxels were part of a bigger cluster encompassing the hippocampus.
Fig. 3.
Brain regions showing a significant main effect of Mask (Pattern > Face), which includes the bilateral calcarine sulci, left anterior cingulate cortex, and right ventromedial prefrontal cortex. We note that the left calcarine sulcus also exhibited a main effect of emotion (Fearful > Happy), although it did not survive the corrected threshold imposed on the voxelwise ANOVA for the main effect of Emotion.
Fig. 4.
Brain regions showing a significant main effect of Mask (Face > Pattern), which includes the bilateral fusiform gyri, left hippocampus/amygdala, and right inferior frontal gyrus. We note that the left hippocampus/amygdala ROI also exhibited a main effect of emotion (Fearful > Happy) as well as a Mask × Emotion interaction, and the left fusiform gyrus ROI showed a main effect of emotion (Fearful > Happy), although these regions did not survive the corrected threshold imposed on the voxelwise ANOVA for the main effect of Emotion and the Mask × Emotion interaction, respectively.
Discussion
Here we replicated human amygdala BOLD signal increases in response to face-masked fear (Morris et al., 1998; Whalen et al., 1998, 2004; Rauch et al., 2000; Etkin et al., 2004; Pessoa et al., 2006; Williams et al., 2006; Kim et al., 2010; Straube et al., 2011) and decreases in response to pattern-masked fear (Kim et al., 2010). Specifically, we observed this effect to masked fearful eye whites in a similar region of right amygdala where we observed previously using whole fearful faces (Kim et al., 2010). These results again show that the amygdala is sensitive to the hidden target information per se because BOLD signal changes here discriminated between fearful and happy eye whites regardless of mask type. But, that said, since the direction of BOLD signal changes differed depending on mask type, it is clear that amygdala activity is also influenced by the mask stimulus itself.
Increased amygdala activity to face-masked fearful eye whites is consistent with findings from previous studies (Whalen et al., 2004; Straube et al., 2010; Kanat et al., 2015) and further supports the notion that the amygdala is sensitive to crude, low-level representations of threat-related stimuli even when the level of awareness is restricted (LeDoux, 1996). By showing an increase in amygdala activity to face-masked fearful eye whites that are comparable to other reports of increased amygdala activity to face-masked fearful faces (Morris et al., 1998; Whalen et al., 1998; Rauch et al., 2000; Etkin et al., 2004; Pessoa et al., 2006; Williams et al., 2006; Kim et al., 2010), the present findings further support the idea that the eyes—especially the eye whites (i.e., sclera)—are a face region from which meaningful predictive information is extracted (Whalen, 1998; Morris et al., 2002; Sekuler et al., 2004; Adolphs et al., 2005). The present data also extend our previous report using eye white stimuli contrasted with a black background—here we used a gray background to show that eye white stimuli presented with a lower contrast produce a similar effect. It is also worth noting that the spatial location of amygdala activity in our previous study (see Figure 2 in Kim et al., 2010) was strikingly similar to that found in our current study (Figure 2)—both studies show activity in the medial aspect of the right amygdala (the peak voxels were [18, −3, −18] and [18, −3, −21], respectively).
When presented on their own, eye white stimuli are not interpreted naturally. That is, when these same eye white stimuli are presented overtly, participants describe their strange experiences when viewing them (e.g. ‘floating eyes’, ‘looked like cat-eyes’; see Whalen et al., 2004, Supplementary Material). There are two important points here. First, the face mask condition provides a plausible context for the rapid eye white presentation. Second, if one is interested in studying any component of the face, backward masking is a useful strategy since it can mitigate the subjective problem—namely, the weirdness of perceiving facial components devoid of a naturally accurate face context.
The pattern mask condition provides evidence that the amygdala is responsive to fearful eye whites in the absence of relevant contextual information (i.e. the rest of the face). These data differ from the findings of Straube and colleagues (2010), who did not observe amygdala activity that discriminated between fearful and happy eye whites when masked by non-face masks (i.e. flowers). Perhaps the different mask type between these two studies is the basis for this difference in results. Alternatively, we note that though there was no contextual ‘face’ information in the pattern mask condition, there were overtly viewable faces present in the greater experimental context, which could be important.
Two widely accepted theoretical frameworks explain the mechanisms behind backward masking: stimulus substitution and stimulus integration (see Bachmann and Allik, 1976; Bachmann et al., 2005 for extensive discussion). To briefly summarize, the stimulus substitution account suggests that the mask stimulus (e.g. neutral faces or pattern masks) replaces the target stimulus (e.g. fearful or happy eye whites), and thus the target stimulus fails to reach the level of subjective awareness (Bachmann and Allik, 1976; Rolls and Tovee, 1994; Di Lollo et al., 2000). Stimulus integration, on the other hand, predicts that the target stimulus gets amalgamated with the mask stimulus and perceived as a single object. Thus, if the amygdala follows the stimulus substitution account, one might expect increased activity to fearful eye whites regardless of the mask-type. In contrast, stimulus integration might predict amygdala responses to be dependent on the mask type, such that the eye whites would only be amalgamated in the face-masked versus pattern masked condition. Of course this is an assumption, and given classic studies showing that masked face stimuli can affect preferences for subsequently presented ideographic stimuli (Murphy et al., 1995), it certainly is possible that the pattern mask is also perceived differently depending the preceding eye white condition. What is clear from the present results together with our previous study (Kim et al., 2010) is that both accounts may be at work and what remains to be explained is—why do these two mask condition contexts produce such radical differences in the way that amygdala signal changes discriminate between fearful and happy eye whites?
We implemented two face mask conditions where we compared repeating eight face identities with one repeating face identity—because there was only one pattern mask. This differed from our previous report (Kim et al., 2010), where we wondered if decreases in amygdala activity in the pattern mask condition were related to the repetition of one single image, based on the findings showing that amygdala BOLD signals are reduced through repetition suppression (i.e., when the same images are repeatedly presented; Ishai et al., 2004). In this study, we did not find evidence that amygdala activity was affected when the eye whites were masked by one face identity compared to eight. As we have with our previous study (Kim et al., 2010), we propose that one possible explanation for the observed suppression of amygdala BOLD activity may be due to the mismatch between the pattern masks and eye white targets. Given that decreased BOLD signal does not necessarily correspond to suppressed neuronal activity (Maier et al., 2008), electrophysiological studies of the amygdala using pattern masks would provide confirmation of these predictions.
This replication suggests that pattern-masked fear may be a reliable way to produce BOLD signal decreases in the amygdala. Since it is not at all clear what exactly a BOLD signal decrease means, this paradigm might be used in an attempt to elucidate this phenomenon. For example, this paradigm could be used as an opportunity to better understand the relationship between BOLD signal increases vs decreases and behavior—if a behavioral effect could be developed on trials preceded by masked fearful vs happy face stimuli. To elaborate, Maier and colleagues (2008) used a repetition suppression study in monkeys to show that the cortical region that initially showed BOLD signal increases and then subsequent BOLD signal decreases to re-presented pictures, exhibited increased neuronal spike activity throughout in later electrophysiological recordings. Thus, BOLD signal decreases cannot be assumed to solely reflect less activity or output. In fact, as we learn more about the synchronizing of neuronal firing (e.g. oscillations), it is possible that a brain region that exhibits less neuronal firing, but in a synchronous manner, would extract less oxygen from the blood (i.e. show a BOLD signal decrease) but could actually be producing more efficient output (see Balduzzi et al., 2008). Future behavioral and imaging studies will be needed to address this important issue.
It is important to acknowledge that the underlying mechanism of the amygdala BOLD signal decrease to pattern-masked fearful eye whites is still poorly understood. We expected this behavior of amygdala activity to emerge only because we have already observed it in our previous study (Kim et al., 2010). While this largely unexpected phenomenon could be interpreted in the context of the stimulus substitution and stimulus integration accounts, it still does not provide a full explanation of the observed amygdala activity patterns. Given the converging empirical data showing that a subregion of the right amygdala is responding to masked fearful vs happy eye whites by increasing its activity in a face context and decreasing its activity in a non-face context, we hope that the current series of findings could act as a starting point in the formal investigation of this particular nature of amygdala BOLD responses in order to fully understand its underlying mechanisms.
One thing to consider when interpreting the current data is the potential differences in attention caused by the different mask-types. In addition to the face vs non-face context the masks explicitly provide, it is plausible to think that the participants’ focus of attention may also depend on the mask. That is, while viewing faces, it is more than likely that the participants’ attention was centered on the eye region, which could carry over to the next set of trials. Consequentially, participants might have been more prone to be affected by the masked eye whites when face masks were used, compared to non-face pattern masks—contributing to the differential activity of the amygdala observed in the current study. This possibility could be directly addressed in future studies by employing an eye-tracking measure while the participants are viewing blocks of eye whites backward-masked with face and pattern images.
While the aim of the current study, consistent with many other backward masking studies, was to investigate the effects of masked fearful eye whites while mitigating subjective awareness, what could also be interesting is the characteristics of participants who have successfully detected these masked presentation of eye whites. Interestingly, we have observed that about 20% of the participants recruited for backward masking studies became subjectively aware of the masked faces/eye whites (Whalen et al., 1998; Kim et al., 2010). Given that the presentation time for the masked images was very brief (17 or 33 ms), there is a possibility that these individuals may be hypersensitive or hyper-vigilant to threat-related cues. This hypothesis could be tested in future studies tailored to explore the behavioral and neural characteristics of such individuals. One difficulty is that the participants’ behavior might fundamentally change once they become subjectively aware of the masked images during an experiment (e.g. they will start to expect and actively search for other hidden images). This issue could be mitigated by a two-stage study where participants are first identified as subjectively aware or unaware in an initial backward masking study, and then subsequently investigating their behavioral and neural characteristics.
Most generally, this study design attempted to appreciate the notion that the amygdala is at once cue reactive, but contextually bound. Amygdala responses to rapidly presented naturally predictive cues (i.e. eye whites) in the absence of explicit knowledge, is consistent with a role for the amygdala in automatic responses to predictive environmental cues that would serve to increase the alertness and efficiency of other brain systems in order to determine the nature of the prediction and potential outcomes (see Whalen et al., 2009 for a review). That said, the fact that these responses can be readily modulated based upon the explicit context present when these cues were encountered, speaks to the highly integrative nature of cue and contextual processing in the amygdala (Phillips and LeDoux, 1992; Alvarez et al., 2008). The current findings expand the general view of amygdala function—that it automatically and rapidly processes threat-related information—by highlighting that this automaticity can be affected by context.
To summarize, the present data illustrate how face versus non-face contextual information influences amygdala activity to masked fearful eye whites. Building upon our previous findings that showed an interactive effect in the amygdala towards masked fearful vs happy faces (Kim et al., 2010), we observed similar amygdala activity to masked fearful vs happy eye whites—increased activity towards face-masked fearful vs happy eye whites, and decreased activity towards pattern-masked fearful vs happy eye whites. Converging evidence from our previous and current studies suggests that the right amygdala may contain a subpopulation of neurons that are sensitive to threat-related information processed under restricted subjective awareness. These findings may be used to guide future electrophysiological investigations, which could offer further insight to the nature of amygdala BOLD signal suppression due to the mismatch between threat-related targets and masks.
Acknowledgements
We thank Rebecca A. Loucks and Ashly A. McLean for assisting with stimulus development and data collection. This work was supported by the National Institute of Mental Health (MH080716 to P.J.W., MH090672 to M.J.K.).
Conflict of interest. None declared.
References
- Adolphs R., Gosselin F., Buchanan T.W., Tranel D., Schyns P., Damasio A.R. (2005). A mechanism for impaired fear recognition after amygdala damage. Nature, 433, 68–72. [DOI] [PubMed] [Google Scholar]
- Alvarez R.P., Biggs A., Chen G., Pine D.S., Grillon C. (2008). Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. Journal of Neuroscience, 28, 6211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann T., Allik J. (1976). Intergration and interruption in the masking of form by form. Perception, 5, 79–97. [DOI] [PubMed] [Google Scholar]
- Bachmann T., Luiga I., Poder E. (2005). Variations in backward masking with different masking stimuli: II. The effects of spatially quantised masks in the light of local contour interaction, interchannel inhibition, perceptual retouch, and substitution theories. Perception, 34, 139–53. [DOI] [PubMed] [Google Scholar]
- Balduzzi D., Riedner B.A., Tononi G. (2008). A BOLD window into brain waves. Proceeding of the National Academy of Sciences USA, 105, 15641–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computer and Biomedical Research, 29, 162–73. [DOI] [PubMed] [Google Scholar]
- Davis F.C., Johnstone T., Mazzulla E.C., Oler J.A., Whalen P.J. (2009). Regional response differences across the human amygdaloid complex during social conditioning. Cerebral Cortex, 60, 512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lollo V., Enns J.T., Rensink R.A. (2000). Competition for consciousness among visual events: the psychophysics of reentrant visual processes. Journal of Experimental Psychology: General, 129, 481–507. [DOI] [PubMed] [Google Scholar]
- Ekman P.F., Friesen W.V. (1976). Pictures of Facial Affect. Palo Alto: Consulting Psychologists Press. [Google Scholar]
- Etkin A., Klemenhagen K.C., Dudman J.T., et al. (2004). Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron, 44, 1043–55. [DOI] [PubMed] [Google Scholar]
- Ishai A., Pessoa L., Bikle P.C., Ungerleider L.G. (2004). Repetition suppresion of faces is modulated by emotion. Proceeding of the National Academy of Sciences USA, 101, 9827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen S., Grossmann T. (2014). Unconscious discrimination of social cues from eye whites in infants. Proceeding of the National Academy of Sciences USA, 111, 16208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T., Somerville L.H., Alexander A.L., et al. (2005). Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage, 25, 1112–23. [DOI] [PubMed] [Google Scholar]
- Kanat M., Heinrichs M., Mader I., van Elst L.T., Domes G. (2015). Oxytocin modulates amygdala reactivity to masked fearful eyes. Neuropsychopharmacology, 40, 2632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Somerville L.H., Johnstone T., Alexander A.L., Whalen P.J. (2003). Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport, 14, 2317–22. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Loucks R.A., Neta M., et al. (2010). Behind the mask: the influence of mask-type on amygdala response to fearful faces. Social Cognitive and Affective Neuroscience, 5, 363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E. (1996). The Emotional Brain. New York: Simon and Shuster. [Google Scholar]
- Maier A., Wilke M., Aura C., Zhu C., Ye F.Q., Leopold D.A. (2008). Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nature Neuroscience, 11, 1193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P. (1999). Some methodological issues in assessing attentional biases for threatening faces in anxiety: a replication study using a modified version of the probe detection task. Behaviour Research and Therapy, 37, 595–604. [DOI] [PubMed] [Google Scholar]
- Morris J.S., deBonis M., Dolan R.J. (2002). Human amygdala responses to fearful eyes. Neuroimage, 17, 214–22. [DOI] [PubMed] [Google Scholar]
- Morris J.S., Ohman A., Dolan R.J. (1998). Conscious and unconscious emotional learning in the human amygdala. Nature, 393, 467–70. [DOI] [PubMed] [Google Scholar]
- Murphy S.T., Monahan J.L., Zajonc R.B. (1995). Additivity of non-conscious affect: Combined effects of priming and exposure. Journal of Personality and Social Psychology, 69, 589–602. [DOI] [PubMed] [Google Scholar]
- Ohman A., Flykt A., Esteves F. (2001). Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: General, 130, 466–78. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Japee S., Sturman D., Ungerleider L.G. (2006). Target visibility and visual awareness modulate amygdala responses to fearful faces. Cerebral Cortex, 16, 366–75. [DOI] [PubMed] [Google Scholar]
- Phillips R.G., LeDoux J.E. (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106, 274–85. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Whalen P.J., Shin L.M., et al. (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry, 47, 769–76. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Tovee M.J. (1994). Processing speed in the cerebral cortex and the neurophysiology of visual masking. Proceedings: Biological Sciences, 257, 9–15. [DOI] [PubMed] [Google Scholar]
- Sekuler A.B., Gaspar C.M., Gold J.M., Bennett P.J. (2004). Inversion leads to quantitative, not qualitative, changes in face processing. Current Biology, 14, 391–6. [DOI] [PubMed] [Google Scholar]
- Straube T., Dietrich C., Mothes-Lasch M., Mentzel H.J., Miltner W.H. (2010). The volatility of the amygdala response to masked fearful eyes. Human Brain Mapping, 31, 1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen P.J. (1998). Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7, 177–88. [Google Scholar]
- Whalen P.J., Davis F.C., Oler J.A., Kim H., Kim M.J., Neta M. (2009). Human amygdala responses to facial expressions of emotion In: Whalen P. J., Phelps E. A., editors. The Human Amygdala, pp. 265–88. New York: Guilford Press. [Google Scholar]
- Whalen P.J., Kagan J., Cook R.G., et al. (2004). Human amygdala responsivity to masked fearful eye whites. Science, 306, 2061. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Rauch S.L., Etcoff N.L., McInerney S.C., Lee M.B., Jenike M.A. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18, 411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M., Liddell B.J., Kemp A.H., et al. (2006). Amygdala-prefrontal dissociation of subliminal and supraliminal fear. Human Brain Mapping, 27, 652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]