Abstract
Brain circuitry underlying defensive behaviors includes forebrain modulatory sites, e.g. the amygdala and hypothalamus, and midbrain effector regions, such as the deep/intermediate layers of the superior colliculus (DLSC). When disinhibited, this network biases behavior towards reflexive defense reactions. While well characterized in rodent models, little is known about this system in the primate brain. Employing focal pharmacological manipulations, we have previously shown that activation of the DLSC triggers reflexive defensive responses, including cowering, escape behaviors and defensive vocalizations. Here, we show that activation of the DLSC also disrupts normal dyadic social interactions between familiar pairs of monkeys. When the basolateral complex of the amygdala (BLA) was inhibited concurrent with DLSC activation, cowering behavior was attenuated, whereas escape behaviors and defensive vocalizations were not. Moreover, inhibition of the BLA, previously shown to produce a profound increase in dyadic social interactions, was unable to normalize the decrease in social behavior resulting from DLSC activation. Together these data provide an understanding of forebrain–midbrain interactions in a species and circuit with translational relevance for the psychiatry of anxiety and post-traumatic stress disorders.
Keywords: tectum, macaque, microinjection
Introduction
The neural circuitry mediating reflexive defense reactions includes forebrain modulatory sites, such as the amygdala and hypothalamus, and midbrain effector regions, such as the periaqueductal grey (PAG), the deep/intermediate layers of the superior colliculus (DLSC) and the inferior colliculus (IC). Although all these structures (especially the amygdala and hypothalamus) are also involved in functions other than defensive, including appetitive and reward-related behaviors, this particular circuitry represents the core of the defensive system, referred to by some as ‘brain aversion system’ (Brandão et al., 1994). Activation of this system evokes species-typical escape behaviors across species (Brandao et al., 1986; Sahibzada et al., 1986; Bittencourt et al., 2005; DesJardin et al., 2013). These findings raise the possibility that midbrain structures (such as DLSC) may provide a critical (bottom up) source of pathological over activation in affective disorders, including post-traumatic stress disorder (PTSD).
Studies in human patients point to impaired top-down inhibition of amygdala in anxiety disorders and PTSD (Rauch et al., 2000; Hull, 2002). In particular, stress-induced impairment in prefrontal cortical function could be a basis for loss of cognitive control over amygdala-dependent processing. This dysfunction may manifest as a failure to extinguish trauma-related responses when no longer appropriate. In the face of compromised top-down control of this network, primitive defense mechanisms (e.g. the brainstem) may take on a greater role in driving affective processing.
While very well characterized in rodent models (Brandao et al., 1986; Sahibzada et al., 1986; Brandão et al., 1988; Dean et al., 1989; Brandão et al., 1993; Coimbra et al., 1996), this network remains significantly underexplored in primates. We recently reported that focal activation of the DLSC evoked dose-dependent emergence of defensive-like hyper-arousal behaviors in freely moving macaques (DesJardin et al., 2013). These behaviors included cowering, which resembles crouching from a looming stimulus, explosive escape behaviors and defensive-like vocalizations. These findings mirror those seen in rodents after activation of the colliculi or PAG.
The hypothesis that amygdala provides top-down inhibition of the midbrain defense network is supported by a series of studies in rodent models. For example, lesions or pharmacological inactivation of the basolateral complex of the amygdala (BLA) in rats exacerbated defensive behaviors evoked from the IC (Maisonnette et al., 1996; Macedo et al., 2006), whereas inactivation of the amygdala attenuated similar behaviors evoked from the DLSC (Wei et al., 2015). The degree to which midbrain-evoked defensive behaviors can be modified by manipulation of forebrain components of the network (e.g. the amygdala) remains unexamined in primates.
With respect to ethologically relevant unconditioned fear stimuli, the effect of BLA lesions differs between rodents and primates. In rats, for example, BLA lesions have no effect on freezing in response to fox odor (Rosen et al., 2008). In contrast, in monkeys, amygdala lesions disrupt behavioral responses to similarly relevant unconditioned stimulus presentation (i.e. snakes) (Meunier et al., 1999; Kalin et al., 2004). Thus, there is the potential for divergence in the role of amygdala between rodents and primates in the regulation of reflexive fear.
In addition to its role in fear processing, the amygdala is a critical regulator of social/affiliative interactions (Kling and Brothers, 1992). We recently found that transient pharmacological inhibition of either BLA or the central nucleus results in a profound increase in affiliative social behaviors in macaques (Wellman et al., 2016). Increased social interaction has also been reported after amygdala inactivation in rodents (Sanders and Shekhar, 1995; Sajdyk and Shekhar, 1997). In contrast to the aforementioned data, these data suggest that this network may be conserved across species. Understanding this circuitry may shed light on the neural substrates mediating anxiety and PTSD in humans.
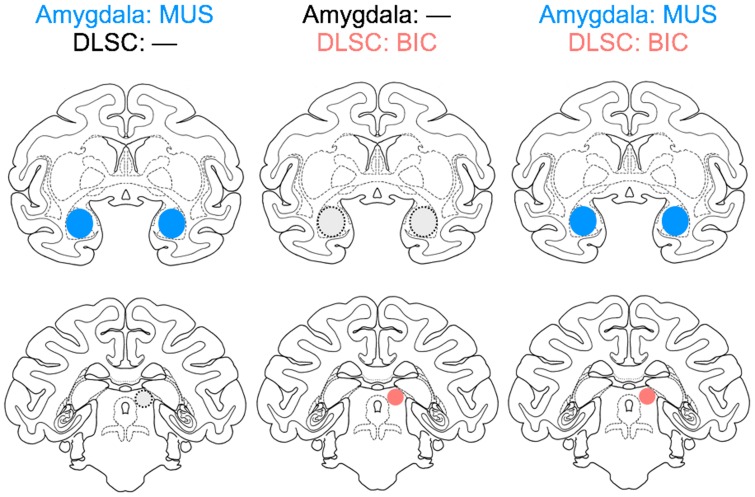
To resolve this apparent conflict, and provide an understanding of this circuitry in the primate, here, we tested the hypothesis that amygdala inhibition would attenuate aversive responses evoked from the DLSC. We examined two categories of behavior, defensive responses and social behaviors, while the animals were observed in familiar dyads. The effect of midbrain activation on social behavior is previously unexplored across species. Animals were tested after three experimental manipulations: (1) inhibition of BLA by muscimol infusion, (2) activation of the DLSC by bicuculline infusion and (3) simultaneous inhibition of BLA and activation of the DLSC (Figure 1).
Fig. 1.
Schematic of the experimental design. Intended drug infusion sites in the amygdala and superior colliculus are outlined on planes of a standard macaque atlas.
Materials and methods
Subjects
Seven animals were subjects in this study, five rhesus macaques (Macaca mulata) and two pigtail macaques (Macaca nemestrina). The rhesus macaques formed three experimental dyads, in which three (2 male: DE, DR; 1 female: WI) received drug infusions (see below) and two served as non-infused control partners (2 male: OL and DA). The pigtail macaques formed an additional dyad, with one animal drug-infused (male: GW) and one a non-infused control partner (female: JN). Thus, four experimental dyads were studied: two male-male (DE and OL, DR and DA), one female–male (WI and OL) and one male–female (GW and JN). To maximize the information obtained from each animal, two dyads were evaluated with two independent sets of infusion sites (DE and OL; GW and JN). Thus, a total of six experimental units made up our analysis. Animals were 3–4 years of age at the beginning of this study. In each pair, the monkeys were highly familiar with each other and well compatible.
DE, OL, DA, GW and JN also participated in prior studies (West et al., 2011; Holmes et al., 2012; DesJardin et al., 2013; Forcelli et al., 2014; Wellman et al., 2016), involving microinjections into one or more of the following sites: the DLSC, the substantia nigra pars reticulata, amygdala, hippocampus and the orbitofrontal cortex. DR and WI were experimentally naive.
All monkeys were pair-housed within two joined individual cages (61 × 74 × 76 cm each) in a room with regulated lighting (12 h light/dark cycle). They were maintained on primate LabDiet (5049; PMI Nutrition International, MD) supplemented with fresh fruit. Water was available ad libitum. The study was conducted under a protocol approved by the Georgetown University Animal Care and Use Committee and in accordance with the Guide for Care and Use of Laboratory Animals (National Research Council (U.S.) et al., 2011) adopted by the National Institutes of Health.
MRI-guided stereotaxic surgery
Using a sterile technique, four monkeys (DE, DR, WI and GW) were implanted with a stereotaxically positioned chronic infusion platform, following the procedures previously described in detail (Wellman et al., 2005; West et al., 2011). Animals were induced with ketamine (10 mg/kg), intubated, catheterized and maintained on isoflurane (2–4%, to effect) for the duration of the procedure. The surgery was followed by a post-operative regimen of analgesics and antibiotics determined in consultation with the facility veterinarian. The infusion platform allowed for a removable injector and cannula to be inserted into predetermined sites in the brain with 2 mm resolution in the anteroposterior and mediolateral planes and sub-mm resolution in the dorsoventral plane (Holmes et al., 2012; DesJardin et al., 2013; Dybdal et al., 2013).
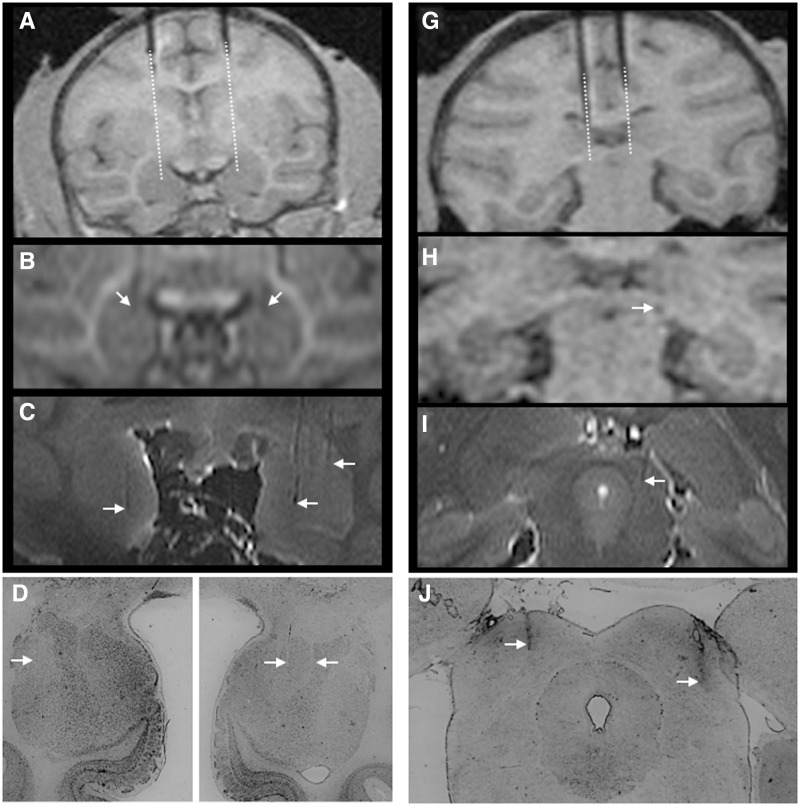
Pre- and post-operatively, each monkey received at least one T1-weighted scan to determine the coordinates for the infusion sites (Figure 2) as previously described (West et al., 2011). Additional scans were performed throughout the experiment as needed. To verify the location of the predetermined infusion sites, tungsten microelectrodes (FHC, Bowdoinham, ME), visible on the scan, were inserted through the infusion grid into the specified locations in each hemisphere, with the tip of the electrode placed at about 10 mm above the intended infusion site. Based on the position of the tip of the electrode on the MRI scan, the final coordinates for the drug infusions were adjusted with respect to the coordinates of the infusion grid (Figure 2).
Fig. 2.
MRI and histological confirmation of infusion sites. (A) pre-infusion positioning of tungsten microelectrodes dorsal to the amygdala. Dotted line indicates the extension of the cannula calculated from the tip of the electrode. (B) Electrode tip positioned bilaterally in the amygdala. (C) Post-mortem ex vivo imaging showing close correspondence to ante-mortem scans. Infusion tracks are visible in both hemispheres and are identified by arrows. (D) Histological confirmation of infusion sites in the amygdala of a single subject. Minimal damage is evident. Tracks are indicated by arrows. (G) Pre-infusion positioning of tungsten microelectrodes dorsal to the superior colliculus. Dotted line indicates the extension of the cannula calculated from the tip of the electrode. (H) Electrode tip positioned in the superior colliculus. (I) Post-mortem ex vivo imaging showing a cannula track in the DLSC. (J) Histological confirmation of infusion sites in the DLSC of a single subject; tracks are evident bilaterally and indicated by arrows.
Experimental design
All experiments were conducted in dyads of monkeys that were highly familiar with each other and each dyad had the experience of being housed together. Within each dyad, one monkey received intracerebral drug infusions and the other served as a non-injected partner. Experimental dyads included both same-sex and mixed-sex dyads. However, the small number of dyads precluded any systematic analysis of male-female differences in behavior. Each dyad was tested under three experimental conditions as outlined in Figure 1. One target was the basolateral complex of the amygdaloid nuclei (BLA), consisting of lateral, basal and accessory basal nuclei (Amaral et al., 1992). The second target was the DLSC. We assessed the effects of (1) bilateral infusion of muscimol in BLA, (2) unilateral infusion of bicuculline in DLSC and (3) bilateral infusion of muscimol in BLA and unilateral infusion of bicuculline in DLSC. The number of infusions per condition is provided in Table 1.
Table 1.
Number, site and doses of drug infusions
| Dyad | BLA: MUS | BLA: — | BLA: MUS | |
|---|---|---|---|---|
| DLSC: — | DLSC: BIC | DLSC: BIC | ||
| GW | JN | 1 | 1 (Right; 5 nmol) | 1 |
| GW2 | JN | 1 | 1 (Right; 5 nmol) | 1 |
| DR | DA | 2 | 2 (Left; 10 nmol) | 2 |
| DE | OL | 1 | 1 (Right, 7.5 nmol) | 1 |
| DE2 | OL | 1 | 1 (Right, 7.5 nmol) | 1 |
| WI | OL | 3 | 1 (Right, 10 nmol) | 2 |
For each dyad, the injected monkey is listed in the first column and the non-injected partner in the second column. Numbers indicate numbers of infusions per condition. For GW and DE, two complete pairs of experiments were conducted and are presented separately. For DR and WI, the infusion sessions were not performed in an order that allowed them to be separated into paired experiments, and thus data were averaged across infusion sessions for these animals.
In the present experiments, we followed the same design as in our previous study (Wellman et al., 2016). Because experience with an infused partner in a defensive state might have had carry-over effects on subsequent dyadic interactions, we employed two methods to address this: (1) we used a randomized order of infusions across subjects and (2) each infusion session was compared to a paired ‘baseline’ session recorded 24 h prior to the drug infusion. For that purpose, the experimental monkey was placed in a double-wide cage (twice the width of the home cage) with its partner and the pair was videotaped for 30 min. The pair was then separated to their home cages. Twenty-four hours later, the experimental monkey was drug-infused, and immediately after the infusion, the two monkeys were again placed in the observation cage and were videotaped for 1 h. Thus, the baseline session assessed 24 h before the infusion together with the behavioral assessment following the drug infusion formed a unit, in which each drug infusion session had its own preceding baseline control session. The number of drug infusions for each dyad is presented in Table 1. As indicated in the table, two dyads had multiple injections per condition. These infusion sessions were averaged for each condition to generate one number for each dyad for further processing. Because there were no significant differences between the baseline sessions within a dyad, we used an average of baselines for further statistical comparisons.
Compared to the use of indwelling cannula, permanently fixed in position, our approach gives us the flexibility to target multiple sites within an individual animal, however, insertion of the cannula for each drug infusion increases the likelihood of adverse effects, including mechanical damage to the site. Thus, there are a limited number of infusions that can be performed at each site. Using saline infusion in each session preceding the drug infusion would have increased the total number of infusions (and brain penetrations) to a prohibitively high level and we would have had to decrease the total number of drug infusions and/or the number of experimental conditions. Alternatively, if we used one saline infusion as a control for each drug infusion, we would lose the opportunity to determine if there were carry-over effects between the experimental conditions on baseline behavior. Our experimental design is consistent with maximizing data collection from each subject while minimizing potential adverse effects due to mechanical damage.
Intracerebral drug infusions
Microinfusions were conducted as previously described (Wellman et al., 2005). For each infusion, microinjection cannulae (29 gauge) were acutely inserted into target locations through the infusion platform. To transiently inactivate BLA, the GABAA agonist muscimol (Sigma-Aldrich Inc.) was administered at a dose of 9 nmol in 1 μl volume (9 mM solution), bilaterally. To transiently activate (disinhibit) DLSC, the GABAA antagonist bicuculline methiodide (Sigma-Aldrich Inc.) was used at a dose of 2.5–10 nmol, unilaterally (10 mM solution). Infusion volumes ranged from 0.25 to 1 µl, with drug delivered at a rate of 0.2 µl per minute. The doses of muscimol and bicuculline were selected based on our prior studies, which showed increases in social behavior after infusion of this dose and concentration of muscimol into the amygdala (Wellman et al., 2016) and the emergence of defensive responses after infusion of bicuculline doses within this range into the DLSC (DesJardin et al., 2013). In the latter experiment, we used a unilateral infusion and achieved a robust behavioral effect. We also performed dose response experiments and found that in most cases a dose of 5 nmol was sufficient to elicit a strong behavioral response. Since the goal of the experiment was to achieve a behavioral effect, we did not proceed with bilateral infusions.
After completion of drug infusion, 5 min were allowed to elapse prior to removal of the cannula to minimize drug reflux up the cannula track. The entire infusion procedure lasted 10–15 min.
Drug infusions in BLA were aimed at the center of the area as outlined in Figure 1, approximately at the border between the lateral and the basal nuclei, but without any intent to target a particular nucleus. Similarly, for DLSC, we targeted the center of the area outlined in Figure 1. In our previous study (Desjardin et al., 2013), we failed to find evidence for any rostrocaudal or mediolateral topography associated with defensive behaviors within the primate superior colliculus. This differs strikingly from the rodent, and may be a function of the ethology of these animals (predators and prey can come from both the upper and lower visual field in the monkey, whereas in the rat predators preferentially come from above). Because of this lack of topography, we aimed our injections for the middle of the DLSC in the present study.
In our previously published studies (DesJardin et al., 2013; Forcelli et al., 2014), we found that a 1 µl microinjection of the MRI contrast agent gadolinium (5 mM solution in saline), resulted in a hypersignal ∼3 mm in diameter when measured 60 min after infusion. We observed this pattern in several brain regions including superior colliculus. This pattern of diffusion is in agreement with previous gadolinium imaging in our laboratory and others (Wilke et al., 2010; Asthagiri et al., 2011) and matches the diffusion observed by Yoshida et al. (1991) using isotopic labeling to monitor the spread of bicuculline methyl chloride. Similarly, the diffusion of 1 µl of muscimol in rats (9mM) affected a ∼3mm in diameter sphere of tissue (Martin and Ghez, 1999) indicating similar spread for both drugs.
Behavioral assessment
All experimental session were video-recorded and the videotapes were subsequently analyzed using the software program The Observer (Noldus Information Technology, Wageningen, Netherlands) by two independent observers (one blind with respect to the treatment). The observers were trained to achieve a high level of inter-observer correlation (r = 0.9 or better) for each behavior. A list of behaviors and their definitions are presented in Table 2. We concentrated the analyses on two general categories: defensive behaviors (cowering, escapes and defensive vocalization) and social interactions. These categories, respectively, were identical (DesJardin et al., 2013) or similar to those used in our previous studies (Wellman et al., 2016). Additional behavioral category was distance between animals, consisting of contact, proximity and alone. This category was scored in parallel with the other behaviors, thus, for example, an animal engages in grooming while in contact or cowers while alone. In the social interactions category, frequency and duration were recorded for each behavior. Similarly, within the defensive behaviors category, cowering was analyzed as a ‘state’ variable (frequency, duration) but escapes and vocalizations were scored as ‘events’. Both social and defensive behaviors as well as distance between animals were scored from each videorecorded session. Both the baseline and post-infusion videotapes were scored in 15-min bins.
Table 2.
Operational definitions of behaviors analyzed
| Behavior | Description |
|---|---|
| Defensive behaviors | |
| Defensive vocalizations | Calls consisting of shrill barks, shrieks and squeaks |
| Cower | Withdrawal to the periphery of the cage in a crouched or recoiled position with an upward directed gaze |
| Escape | Sudden movement/startle response, typically consisting of moving explosively from one side of the cage to the other |
| Social behaviors | |
| Grooming-related behaviors | Subject presents for grooming, receives grooming from the conspecific or actively grooms the conspecific |
| Approach | Initiates social contact; moves body or head towards the conspecific |
| Mounting | Mounts the conspecific |
| Aggression | Makes threatening gestures (i.e. mouth threat, head or body lunge, cage shake) towards or hits, grabs or bites the conspecific |
| Play | In contact with the conspecific, includes chasing, wrestling and ‘rough and tumble’ behaviors |
| Withdrawal | Moves away from the conspecific when approached |
| General (non-social) behaviors | |
| Locomotion | Walk, runs, climbs or jumps |
| Passive | Inactive, stays in one location |
| Distance between animals (scored in parallel with the above categories) | |
| Social Contact | Non-incidental and consistent touch between subject and conspecific (can include grooming related behaviors, mounting, play or passive sitting when in contact with the conspecific) |
| Proximity | Subject sits or stands at arms length from the conspecific |
| Alone | Subject sits or stands isolated from the conspecific |
Previously, we assessed the time-course of defensive behaviors evoked by bicuculline infusions in DLSC (DesJardin et al., 2013). Because, depending on infusion site and drug dose, the timing of peak defensive responses differed from animal to animal, for each dyad in the present study we selected the 15 min time bin that resulted in the peak emergence of defensive responses. This time bin was then used for this dyad in all experimental conditions.
Statistical analysis
Defensive behaviors, cowering, escape and defensive vocalization, occurred only in those sessions when DLSC was disinhibited by bicuculline infusion. None of these behaviors occurred either during baselines or after muscimol infusion in BLA (without simultaneous DLSC activation) resulting in all scores being zero. Therefore, for statistical analyses, we excluded baseline and muscimol in BLA (without simultaneous DLSC activation) and analyzed the defensive behaviors by Wilcoxon's matched-pairs test, followed by Dunn's post-test; P values less than 0.05 were considered to be statistically significant. For sessions with DLSC activation, to determine whether the emergence of defensive behaviors is significantly different from zero, we used a Wilcoxon sign rank test.
Social behavior and distance between animals were analyzed by analysis of variance, with infusion as a repeated measure. Planned comparisons were evaluated using the two-stage step-up procedure of Benajmini, Krieger and Yekutieli, with a 10% false discovery rate (FDR). Analyses were run using GraphPad Prism (version 7, GraphPad, La Jolla, CA).
Verification of infusion sites
In addition to ante-mortem MRI analysis, a subset of animals was also examined using post-mortem MRI at high field strength (7 Tesla). Ex vivo scans of the fixed brain were performed on a Brucker Biospin Magnet using a Turbo-RARE pulse sequence, as previously described (Forcelli et al., 2014). The close correspondence of MRI-based positioning of the infusion sites to the intended targets was also verified histologically in thionin-stained sections for all animals, as previously described (Dybdal et al., 2013; Forcelli et al., 2014).
Results
Infusion sites verification
As shown in Figure 2, prior to microinjection, we determined infusion coordinates based on an MRI with tungsten microelectrodes placed dorsally to the intended infusion sites in the amygdala and DLSC (Figure 2A and G). These coordinates were used to calculate the final position for drug infusion (Figure 2B and H). Post-mortem ex vivo imaging showed a close correspondence to ante-mortem scans (Figure 2C and I) and a close co-registration with post-mortem histological analysis (Figure 2D and J). Thus, as with our prior studies, MRI-based and histological reconstruction of infusion sites verified the accuracy and reliability of our infusion procedures.
Behavioral effects
We first examined the timing of the emergence of defensive behaviors after infusion of bicuculline into DLSC. For DE and GW, the peak response occurred 0–15 min after drug infusion, for WI, 15–30 min and for both sites in DR, 30–45 min after drug infusion. These time bins were thus used for analyses below. While the time to peak differed between subjects, in all cases the emergence of defensive behaviors was evident within 15 min, strongly suggesting that responses were localized to SC and not to adjacent structures (e.g. periaqueductal grey, midbrain reticular formation).
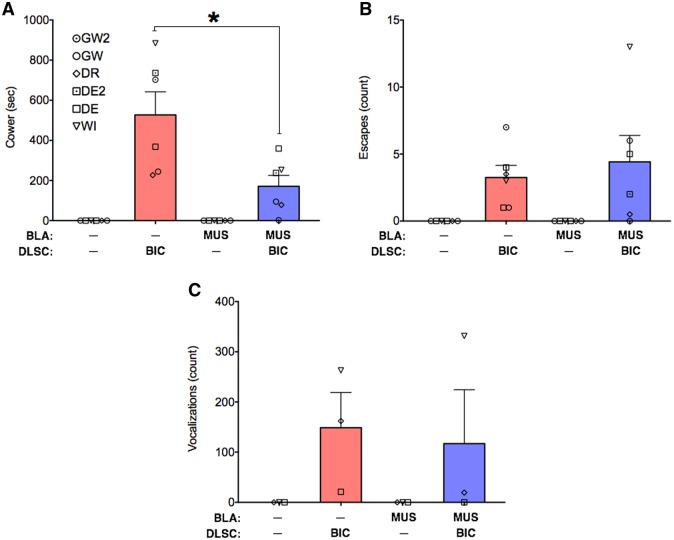
As shown in Figure 3, cowering (Figure 3A), escapes (Figure 3B) and defensive vocalizations (Figure 3C) were evoked by bicuculline microinfusion into DLSC. These behaviors were never detected under control infusion conditions, or after muscimol infusion in BLA. We examined these behaviors statistically using the Wilcoxon sign rank test (to determine if they occurred at a rate greater than 0), and found that the emergence of these behaviors reached the level of statistical significance for cowering (W = 21, P < 0.05) and escape (W = 21, P < 0.05), but not for defensive vocalizations (W = 6, P = 0.25). The fact that defensive vocalizations did not reach the level of statistical significance is likely due to the fact that only three of the six infusion sites evoked this behavior. This is consistent with our prior reports, which found cowering in all experimental subjects, but defensive vocalizations in only a subset of cases (DesJardin et al., 2013).
Fig. 3.
Amygdala inactivation selectively attenuates cowering evoked by activation of DLSC. (A) mean + SEM of time spent cowering, (B) mean + SEM of the number of escapes, (C) mean + SEM of the number of defensive vocalizations, under each drug infusion condition. Behaviors were cumulated over 15 min. Symbols indicate individual animals and correspond to the identity of animals in Figures 4 and 5 and Table 1. MUS = muscimol, BIC = bicuculline methiodide. * = significant difference between treatments (P < 0.05).
When we infused the amygdala with muscimol concurrent with bicuculline microinjection into the DLSC, we found a significant attenuation of cowering behavior (Wilcoxon matched-pairs test comparing DLSC to DLSC + AMY condition, W = −21, P = 0.03). Indeed, the median duration of cowering was 535 s after infusion into the DLSC alone, and 166 s after co-infusion into DLSC and amygdala. The duration of cowering was numerically decreased for all six infusion sites, across the four animals. The magnitude of this decrease ranged from 2% to 305%, with a mean reduction of 61%.
Unlike cowering, neither the number of escape responses, nor the number of defensive vocalizations were attenuated by inactivation of the amygdala + activation of the DLSC, as compared to activation of the DLSC alone (W = 1, P = 0.94 and W = −2, P = 0.75, respectively). It is worth noting, however, that the number of escape responses was numerically reduced in 4 of 6 infusion sites and the number of defensive vocalizations was reduced in 2 of 3 infusion sites.
We next examined the effects of our manipulations on social behavior. Animals within each dyad were highly familiar with each other and had a high rate of social interactions during baseline sessions (Figure 4).
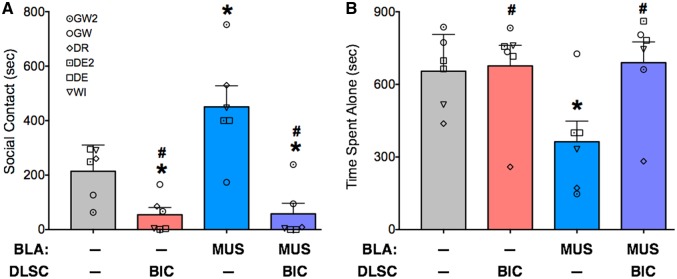
Fig. 4.
Amygdala inactivation fails to rescue social contact reduced by activation of DLSC. (A) Mean + SEM of social contact (sub-category of distance between animals as defined in Table 2). (B) Mean + SEM of time spent alone (as defined in Table 2). Symbols indicate the individual values for each injected monkey and correspond to the identities listed in Table 1. * = significantly different than baseline (P < 0.05); # significantly different than MUS in BLA (blue bar; P < 0.05).
We assessed time spent in social contact (Figure 4A) and time spent alone (Figure 4B). For social contact, analysis of variance, with treatment as a repeated measure, revealed a significant difference amongst the infusion conditions (F1.9,9.5 = 12.9, P = 0.0021). Bicuculline infusion into the DLSC reduced social behavior (P < 0.05, FDR adjusted). This reduction was seen in four of the six infusions; in three of the infusion sessions, it was almost completely abolished (4, 3 and 1 s, engaged in social behavior).
Consistent with our previous findings (Wellman et al., 2016), muscimol infusion into BLA increased social contact as compared with baseline. This increase was observed in all animals and its magnitude ranged from 35% to 1090%, with a mean increase of 230% (P < 0.05, one-tailed, FDR adjusted).
Co-infusion of muscimol into the amygdala and bicuculline into the DLSC did not rescue social interactions; indeed, this infusion condition was different from both the amygdala alone condition and the baseline condition (Ps < 0.05, FDR adjusted). Thus, this result was in contrast to the attenuation of defensive cowering (evoked by bicuculline in DLSC) seen with inhibiting the amygdala.
For time spent alone, ANOVA also revealed a main effect of treatment (F1.9, 9.3 = 8.9, P = 0.0076). Muscimol infusion into BLA reduced the time spent alone (P < 0.05, FDR adjusted); moreover, this condition differed from both conditions with bicuculline injection into DLSC (Ps < 0.05, FDR adjusted). Time spent in proximity to the conspecific did not differ as a function of treatment (F1.1, 3.3 = 1.005; P = 0.39).
Within the social behavior category, the most prominent behavior was grooming associated behavior, which represented close to 90% of social behavior activities under baseline. Thus, we examined the effect of our manipulations on this behavior. Analysis of variance, with treatment as a repeated measure, revealed a significant difference amongst the infusion conditions (F1.8,9.0 = 8.9, P = 0.0329). While grooming under muscimol infusion in BLA conditions did not differ significantly from baseline, it was reduced after bicuculline in DLSC and after bicuculline in DLSC combined with muscimol in BLA (Ps < 0.05, one-tailed, FDR adjusted) (Figure 5).
Fig. 5.
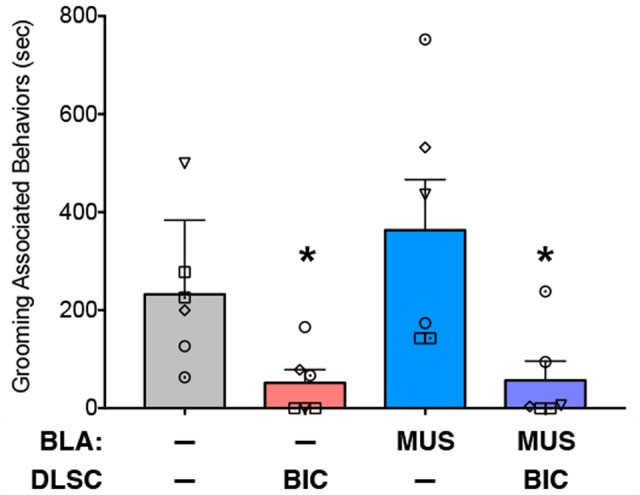
Amygdala inactivation fails to rescue grooming-associated behaviors reduced by activation of DLSC. Mean + SEM of time spent engaged in grooming associated behaviors (as defined in Table 2). Symbols indicate individual animals and correspond to those in Figures 3 and 4. * = significantly different than baseline (P < 0.05).
Discussion
Here we have found that activation of DLSC resulted in the emergence of defensive behaviors. This effect was observed in parallel with significantly decreased social interactions. Inactivation of the amygdala significantly attenuated cowering behavior evoked by activation of DLSC. This effect did not reach the level of statistical significance for the other defense behaviors examined (i.e. escape responses and vocalization), although a numeric reduction in the presence of these behaviors was detected in the majority of cases. Despite the efficacy of amygdala inactivation at suppressing cowering behavior, it did not normalize social interactions reduced by DLSC activation, as measured by either time spent in contact with the conspecific or grooming-related behaviors. This differing pattern of responses to amygdala inactivation raises several possibilities: (1) divergent pathways mediate DLSC effects on cowering and social behavior, and that amygdala can influence only a subset of these circuits, (2) that the presence of defensive behaviors evoked downstream from the amygdala (i.e. in DLSC) overrides pro-social motor commands, (3) that defensive behavior circuits are organized in such a manner to give priority to defense over other high priority behavioral domains, such as social behavior (and perhaps others such as feeding and reproduction). These non-mutually exclusive possibilities are all consistent with the notion that when faced with immediate threats, maximal survival benefit may be obtained by suppressing behaviors that are non-critical in the emergency situation.
While in the present study we focused on the DLSC, it is important to note that in rats, similar profiles of defensive responses can be evoked by electrical stimulation of PAG, DLSC and IC (Bittencourt et al., 2005). Together, these structures have been described as a ‘neural substrate of aversion in the midbrain tectum’ and have been suggested to ‘elaborate aversive motivational states underlying the behavioral and autonomic manifestations of defense reactions’ (Brandão et al., 1994).
Despite the similar profiles of innate defense behaviors evoked from these regions, the functional relationship between these structures and the amygdala differs substantially. The interactions of the rodent midbrain tectum with the amygdala have been investigated in great detail by Brandão, Coimbra and colleagues. Unconditioned fear responses evoked by stimulation of the IC were exacerbated by either inactivation or lesions of BLA (Maisonnette et al., 1996; Macedo et al., 2006). Moreover, lesions to BLA decreased the stimulation threshold needed to evoke these responses (Maisonnette et al., 1996). In contrast, lesions to the central nucleus of the amygdala increased the threshold for aversive responses evoked from the IC. A different profile was found for defense behaviors evoked from the PAG: lesions or inactivation of the amygdala attenuated unconditioned fear responses without altering stimulation thresholds (Oliveira et al., 2004; Martinez et al., 2006). A recent report employing optogenetic stimulation of the DLSC (Wei et al., 2015) found that amygdala inactivation with muscimol significantly attenuated the duration of freezing evoked from SC. The decrease in defensive responses caused by amygdala lesions or inactivation found in several of the above studies may be similar to the decrease in passive defensive responses (i.e. cowering) we observed with amygdala inactivation in the present study.
The degree to which other components of the defense response evoked from the midbrain tectum depend on forebrain structures has also been investigated in the rat (Müller-Ribeiro et al., 2015). In urethane anesthetized rats, forebrain removal (including amygdala and hypothalamus) was without effect on stimulus-evoked increases in blood pressure, respiratory rate and sympathetic nerve activity that are were seen after disinhibition of the colliculi (Müller-Ribeiro et al., 2014). These data suggest that forebrain modulatory sites are not necessary for autonomic components of the defense response, although this hypothesis has not been tested in primates.
While it is possible that the effects of amygdala and colliculus manipulations in the present study were due to parallel, rather than serial circuitry, there are compelling data suggesting that these structures communicate, albeit indirectly. Recent findings from Shang and colleagues showed that optogenetic activation of the superior colliculus triggers escape responses through descending projections to the parabigeminal nucleus. Interestingly, this nucleus projects to the central amygdala in mice (Shang et al., 2015). An alternate pathway was proposed by Wei et al. (2015), who found that activity within the lateral amygdala was temporally correlated with unconditioned fear responses (freezing) evoked from the superior colliculus. These authors propose that this effect was mediated by a relay in the lateral pulvinar, as pulvinar inactivation disrupted unconditioned freezing (Wei et al., 2015). A similar colliculus–pulvinar–amygdala pathway has been proposed in both humans and non-human primates. This has been suggested to be a subcortical route for visual threat processing; diffusion tensor imaging studies have lent credence to the presence of such a pathway in both species (Vuilleumier et al., 2003; Tamietto et al., 2012; Rafal et al., 2015), although this remains to be confirmed using conventional neuroanatomical methods. In the rodent, there is a striking mediolateral topography to behaviors evoked from the DLSC. Activation of medial domains (corresponding to the upper visual field) are strongly associated with defensive-like responses, whereas activation of the lateral SC (associated with the lower visual field) triggers approach responses. In our prior study, which initially characterized defensive responses evoked from the DLSC in monkeys, we failed to find any evidence for a mediolateral topography (even given the larger number of subjects and infusions in the prior experiment). Infusions in both the medial and lateral regions of the DLSC produced defensive behaviors, and each category of defense response was evoked from both medial and lateral sites with low latencies. This suggests a fundamental difference in either the output architecture or microcircutiry within the primate DLSC, as compared to rodents.
While interactions between amygdala and DLSC have not previously been evaluated in non-human primates, a great deal of attention has been paid to the role of amygdala and cortex in both learned and unlearned fear (see Murray and Wise, 2010 for a review). In non-human primates, lesions to the amygdala (either developmental or adult) result in impaired processing of unconditioned fear stimuli, such as snakes, human faces, human intruders and conspecific monkey faces (Meunier et al., 1999; Prather et al., 2001; Izquierdo and Murray, 2004; Kalin et al., 2004; Izquierdo et al., 2005; Chudasama et al., 2009; Machado et al., 2009;). Similarly, amygdala lesions reduced the acquisition, but not the retention or expression of condition fear in macaques (Antoniadis et al., 2009). Together, these data demonstrate a role for the primate amygdala in the processing of unconditioned (visual) threat, as well as conditioned threat.
With respect to the neurobiology of social behavior, the primate amygdala has been investigated extensively. In contrast, only one study, to the best of our knowledge, has examined the role of the midbrain in social behavior. In capuchin monkeys, bilateral lesions to superior colliculus transiently disrupted social behavior (Maior et al., 2012). This may be related to impaired threat processing, as these animals also failed to respond appropriately to unconditioned threats such as rubber snakes (Maior et al., 2011). Here, we found that activation of the DLSC also disrupted social behavior. This disruption may be due to the emergence of defensive behaviors, which precluded affiliative social interactions. Interestingly, the BLA inactivation attenuated defensive behaviors evoked from DLSC, but did not rescue the observed deficit in social interactions found after DLSC activation. These data may be of particular relevance to PTSD, as social stimuli have been reported to trigger increased activation in the SC of individuals with PTSD (Steuwe et al., 2014). Our findings underscore the importance of both the amygdala and the midbrain tectum (DLSC) in the regulation of emotional and social behavior in primates.
Acknowledgements
LM designed the study and conducted experiments, PAF, JD, EW, AH and LW conducted experiments, JD and CE analyzed videos, PAF and LM performed statistical analysis and wrote the paper, which all authors revised. The authors wish to thank Carrie Silver, Mena Teferra, Ashley Decker, Claire Cole and Mark Niedringhaus for technical support.
Funding
This work was supported in part by the National Institutes of Health [R01MH099505 to L.M., R01MH084069 to L.M., R21NS058253 to L.M., T32HD046388 to P.A.F., KL2TR001432 to P.A.F., F31NS061623 to A.H., F31DA026705 to E.A.W., T32NS041231 to C.E., F31MH067414 to L.L.W.].
Conflict of interest: The authors declare no competing financial interests.
References
- Amaral D., Price J.L., Pitkanen A., Carmichael S. (1992). Anatomical organization of the primate amygdaloid complex In: Aggleton J.P., editor, The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. New York, NY: Wiley-Liss, 1–66. [Google Scholar]
- Antoniadis E.A., Winslow J.T., Davis M., Amaral D.G. (2009). The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biological Psychiatry, 65, 241–8. doi:10.1016/j.biopsych.2008.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthagiri A.R., Walbridge S., Heiss J.D., Lonser R.R. (2011). Effect of concentration on the accuracy of convective imaging distribution of a gadolinium-based surrogate tracer. Journal of Neurosurgery, 115, 467–73. doi:10.3171/2011.3.JNS101381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt A.S., Nakamura-Palacios E.M., Mauad H., Tufik S., Schenberg L.C. (2005). Organization of electrically and chemically evoked defensive behaviors within the deeper collicular layers as compared to the periaqueductal gray matter of the rat. Neuroscience, 133, 873–92. doi:10.1016/j.neuroscience.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Brandão M.L., Cardoso S.H., Melo L.L., Motta V., Coimbra N.C. (1994). Neural substrate of defensive behavior in the midbrain tectum. Neuroscience and Biobehavioral Reviews, 18, 339–46. [DOI] [PubMed] [Google Scholar]
- Brandao M.L., Di Scala G., Bouchet M.J., Schmitt P. (1986). Escape behavior produced by the blockade of glutamic acid decarboxylase (GAD) in mesencephalic central gray or medial hypothalamus. Pharmacology, Biochemistry and Behavior, 24, 497–501. [DOI] [PubMed] [Google Scholar]
- Brandão M.L., Melo L.L., Cardoso S.H. (1993). Mechanisms of defense in the inferior colliculus. Behavioral Brain Researsch, 58, 49–55. [DOI] [PubMed] [Google Scholar]
- Brandão M.L., Tomaz C., Borges P.C., Coimbra N.C., Bagri A. (1988). Defense reaction induced by microinjections of bicuculline into the inferior colliculus. Physiology and Behavior, 44, 361–5. [DOI] [PubMed] [Google Scholar]
- Chudasama Y., Izquierdo A., Murray E.A. (2009). Distinct contributions of the amygdala and hippocampus to fear expression. European Journal of Neuroscience, 30, 2327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra N.C., Eichenberger G.C., Gorchinski R.T., Maisonnette S. (1996). Effects of the blockade of opioid receptor on defensive reactions elicited by electrical stimulation within the deep layers of the superior colliculus and DPAG. Brain Research, 736, 348–52. [DOI] [PubMed] [Google Scholar]
- Dean P., Redgrave P., Westby G.W. (1989). Event or emergency? Two response systems in the mammalian superior colliculus. Trends in Neurosciences, 12, 137–47. [DOI] [PubMed] [Google Scholar]
- DesJardin J.T., Holmes A.L., Forcelli P.A., et al. (2013). Defense-like behaviors evoked by pharmacological disinhibition of the superior colliculus in the primate. Journal of Neuroscience, 33, 150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdal D., Forcelli P.A., Dubach M., et al. (2013). Topography of dyskinesias and torticollis evoked by inhibition of substantia nigra pars reticulata. Movement Disorders, 28, 460–8. [DOI] [PubMed] [Google Scholar]
- Forcelli P.A., Palchik G., Leath T., et al. (2014). Memory loss in a nonnavigational spatial task after hippocampal inactivation in monkeys. Proceedings of the National Academy of Sciences of the United States of America, 111(11), 4315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A.L., Forcelli P.A., DesJardin J.T., et al. (2012). Superior colliculus mediates cervical dystonia evoked by inhibition of the substantia nigra pars reticulata. Journal of Neuroscience, 32, 13326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull A.M. (2002). Neuroimaging findings in post-traumatic stress disorder Systematic review. British Journal of Psychiatry, 181, 102–10. [PubMed] [Google Scholar]
- Izquierdo A., Murray E.A. (2004). Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. Journal of Neurophysiology, 91, 2023–39. [DOI] [PubMed] [Google Scholar]
- Izquierdo A., Suda R.K., Murray E.A. (2005). Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. Journal of Neuroscience, 25, 8534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin N.H., Shelton S.E., Davidson R.J. (2004). The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience, 24, 5506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling A., Brothers L. (1992). The amygdala and social behavior In: Aggleton J.P., editor, The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. New York, NY: Wiley-Liss, 353–77. [Google Scholar]
- Macedo C.E., Martinez R.C.R., Brandão M.L. (2006). Conditioned and unconditioned fear organized in the inferior colliculus are differentially sensitive to injections of muscimol into the basolateral nucleus of the amygdala. Behavioral Neuroscience, 120, 625–31. [DOI] [PubMed] [Google Scholar]
- Machado C.J., Kazama A.M., Bachevalier J. (2009). Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion, 9, 147–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maior R.S., Hori E., Barros M., et al. (2011). Superior colliculus lesions impair threat responsiveness in infant capuchin monkeys. Neuroscience Letters, 504, 257–60. [DOI] [PubMed] [Google Scholar]
- Maior R.S., Hori E., Uribe C.E., et al. (2012). A role for the superior colliculus in the modulation of threat responsiveness in primates: toward the ontogenesis of the social brain. Reviews in the Neurosciences, 23, 697–706. [DOI] [PubMed] [Google Scholar]
- Maisonnette S.S., Kawasaki M.C., Coimbra N.C., Brandão M.L. (1996). Effects of lesions of amygdaloid nuclei and substantia nigra on aversive responses induced by electrical stimulation of the inferior colliculus. Brain Research Bulletin, 40, 93–8. [DOI] [PubMed] [Google Scholar]
- Martinez R.C.R., de Oliveira A.R., Brandão M.L. (2006). Conditioned and unconditioned fear organized in the periaqueductal gray are differentially sensitive to injections of muscimol into amygdaloid nuclei. Neurobiology of Learning and Memory, 85, 58–65. doi:10.1016/j.nlm.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Martin J.H., Ghez C. (1999). Pharmacological inactivation in the analysis of the central control of movement. Journal of Neuroscience Methods, 86, 145–59. [DOI] [PubMed] [Google Scholar]
- Meunier M., Bachevalier J., Murray E.A., Málková L., Mishkin M. (1999). Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. European Journal of Neuroscience, 11, 4403–18. [DOI] [PubMed] [Google Scholar]
- Müller-Ribeiro F.C.F., Dampney R.A.L., McMullan S., Fontes M.A.P., Goodchild A.K. (2014). Disinhibition of the midbrain colliculi unmasks coordinated autonomic, respiratory, and somatomotor responses to auditory and visual stimuli. American Journal of Physiology Regulatory Integrative and Comparative Physiology, 307, R1025–35. [DOI] [PubMed] [Google Scholar]
- Müller-Ribeiro F.C.F., Goodchild A.K., McMullan S., Fontes M.A.P., Dampney R.A.L. (2015). Coordinated autonomic and respiratory responses evoked by alerting stimuli: role of the midbrain colliculi. Respiratory Physiology and Neurobiology, 226, 87–93.doi:10.1016/j.resp.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Murray E.A., Wise S.P. (2010). Interactions between orbital prefrontal cortex and amygdala: advanced cognition, learned responses and instinctive behaviors. Current Opinion in Neurobiology 20, 212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.), Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) (2011). Guide for the care and use of laboratory animals, 8th ed. Washington, DC: National Academies Press. [Google Scholar]
- Oliveira L.C., Nobre M.J., Brandão M.L., Landeira-Fernandez J. (2004). Role of amygdala in conditioned and unconditioned fear generated in the periaqueductal gray. Neuroreport, 15, 2281–5. [DOI] [PubMed] [Google Scholar]
- Prather M.D., Lavenex P., Mauldin-Jourdain M.L., et al. (2001). Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience, 106, 653–8. [DOI] [PubMed] [Google Scholar]
- Rafal R.D., Koller K., Bultitude J.H., et al. (2015). Connectivity between the superior colliculus and the amygdala in humans and macaque monkeys: virtual dissection with probabilistic DTI tractography. Journal of Neurophysiology, 114, 1947–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S.L., Whalen P.J., Shin L.M., et al. (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry, 47, 769–76. [DOI] [PubMed] [Google Scholar]
- Rosen J.B., Pagani J.H., Rolla K.L.G., Davis C. (2008). Analysis of behavioral constraints and the neuroanatomy of fear to the predator odor trimethylthiazoline: a model for animal phobias. Neuroscience and Biobehavioral Reviews, 32, 1267–76. [DOI] [PubMed] [Google Scholar]
- Sahibzada N., Dean P., Redgrave P. (1986). Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. Journal of Neuroscience, 6, 723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk T.J., Shekhar A. (1997). Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Research, 764, 262–4. [DOI] [PubMed] [Google Scholar]
- Sanders S.K., Shekhar A. (1995). Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacology, Biochemistry and Behavior, 52, 701–6. [DOI] [PubMed] [Google Scholar]
- Shang C., Liu Z., Chen Z., et al. (2015). BRAIN CIRCUITS A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science, 348, 1472–7. [DOI] [PubMed] [Google Scholar]
- Steuwe C., Daniels J.K., Frewen P.A., et al. (2014). Effect of direct eye contact in PTSD related to interpersonal trauma: an fMRI study of activation of an innate alarm system. Social Cognitive & Affective Neuroscience, 9, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M., Pullens P., de Gelder B., Weiskrantz L., Goebel R. (2012). Subcortical connections to human amygdala and changes following destruction of the visual cortex. Current Biology, 22, 1449–55. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J.L., Driver J., Dolan R.J. (2003). Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience, 6, 624–31. [DOI] [PubMed] [Google Scholar]
- Wei P., Liu N., Zhang Z., Liu X., Tang Y., et al. (2015). Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nature Communications, 6, 6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman L.L., Forcelli P.A., Aguilar B.L., Malkova L. (2016). Bidirectional control of social behavior by activity within basolateral and central amygdala of primates. Journal of Neuroscience, in press. Doi:10.1523/JNEUROSCI.0333-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman L.L., Gale K., Malkova L. (2005). GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. Journal of Neuroscience, 25, 4577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West E.A., DesJardin J.T., Gale K., Malkova L. (2011). Transient inactivation of orbitofrontal cortex blocks reinforcer devaluation in macaques. Journal of Neuroscience, 31, 15128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke, M., Turchi, J., Smith, K., Mishkin, M., Leopold, D.A. (2010). Pulvinar inactivation disrupts selection of movement plans. Journal of Neuroscience, 30, 8650 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Nagatsuka Y., Muramatsu S., Niijima K. (1991). Differential roles of the caudate nucleus and putamen in motor behavior of the cat as investigated by local injection of GABA antagonists. Neuroscience Research, 10, 34–51. [DOI] [PubMed] [Google Scholar]