Abstract
Paying attention to others’ faces and eyes is a cornerstone of human social behavior. The µ-opioid receptor (MOR) system, central to social reward-processing in rodents and primates, has been proposed to mediate the capacity for affiliative reward in humans. We assessed the role of the human MOR system in visual exploration of faces and eyes of conspecifics. Thirty healthy males received a novel, bidirectional battery of psychopharmacological treatment (an MOR agonist, a non-selective opioid antagonist, or placebo, on three separate days). Eye-movements were recorded while participants viewed facial photographs. We predicted that the MOR system would promote visual exploration of faces, and hypothesized that MOR agonism would increase, whereas antagonism decrease overt attention to the information-rich eye region. The expected linear effect of MOR manipulation on visual attention to the stimuli was observed, such that MOR agonism increased while antagonism decreased visual exploration of faces and overt attention to the eyes. The observed effects suggest that the human MOR system promotes overt visual attention to socially significant cues, in line with theories linking reward value to gaze control and target selection. Enhanced attention to others’ faces and eyes represents a putative behavioral mechanism through which the human MOR system promotes social interest.
Keywords: eye-movements, social motivation, human µ-opioid receptor system, eye region
Introduction
The ability to detect and interpret information about other people is essential for successful navigation of the human social environment. The human face, particularly the eye region, is a rich source of information—a mere glance at someone’s eyes can suffice to determine their sex (Armann and Bulthoff, 2009), recognize identity (Althoff and Cohen, 1999) and capture nuances in emotional expression (Vassallo et al., 2009). The primate brain devotes a large proportion of neurons to processing eyes and faces (Issa and DiCarlo, 2012), enabling highly attuned sensitivity to these stimuli (Ghazanfar and Santos, 2004; Itier and Batty, 2009). During human face-processing, most visual attention is directed toward the eye region, as it typically contains more valuable social information than other facial parts (Althoff and Cohen, 1999). A number of neurological and psychiatric disorders, marked by deficits in social behavior, are characterized by disturbances in overt attention to the eyes (Dalton et al., 2005; Watson et al., 2010; Toh et al., 2011; Preller et al., 2014).
The µ-opioid receptor (MOR) system, central to reward and pain regulation across species (Fields, 2004), is also important for social reward such as bonding behaviors in rodents and primates (Herman and Panksepp, 1978; Panksepp, 1980; Moles et al., 2004; Machin and Dunbar, 2011; Løseth et al., 2014). Emerging evidence is linking MOR system function to social reward in humans (Chelnokova et al., 2014; Hsu et al., 2015). The present study investigates how the human MOR system affects visual attentional mechanisms to affectively neutral face stimuli.
Influential theories of attention propose that the utility and rewarding properties of attended visual information are intertwined in saccadic target selection (Maunsell, 2004; Schultz, 2006). Accordingly, the act of acquiring information is assigned a value of its own, as it increases the chance of making a better choice, and decreases uncertainty (Sprague and Ballard, 2003; Tatler et al., 2011). Gottlieb (2012) suggests that neurons responsible for target selection also encode information about the relative value of alternative targets. Gaze control may be directly moderated by dopamine- and opioid-rich nuclei of the basal ganglia and guided toward the location where reward is available (Hikosaka et al., 2006).
This study measured participants’ eye movements to address how the human MOR system modulates visual exploration of highly valuable social cues—the faces and eye region of conspecifics. Thirty healthy young males received a µ-opioid agonist morphine, a non-selective opioid antagonist naltrexone, or placebo per-oral on three separate days in a double-blind cross-over study, and viewed photos of female and male faces varying in attractiveness. The bidirectional pharmacological design, including both stimulation and inhibition of MOR signaling, enabled identification of behaviors promoted by the healthy human MOR system (as measured by the linear contrast Morphine > Placebo > Naltrexone). There were two main hypotheses. First, we expected that stimulating the MOR system with morphine would facilitate visual exploration of faces, i.e. increase the number of eye-fixations (Holmqvist et al., 2011), while naltrexone would diminish face exploration, in line with observations of MOR mediating exploratory behaviors in rodents (File, 1980; Vanderschuren et al., 1997). We also hypothesized that morphine would increase, and naltrexone decrease, overt attention to the eye region, as measured by proportion of total gaze time. In line with theories linking active visual scanning to latent decision processes (Tatler et al., 2011), such opioid-related changes in eye-movement behavior should reflect motivation to seek out information for social decision-making.
Secondarily, we assessed the potential behavioral function of MOR effects on gaze to the eye region through competing exploratory hypotheses. We reasoned that if the MOR system’s effects on overt attention reflected approach behavior, effects of MOR manipulations should be largest for the stimuli most likely to trigger approach (i.e. female gender, direct gaze, high attractiveness level). In contrast, if the effects of MOR manipulations were comparable across stimulus types, this would be more consistent with a role of the MOR system in promoting information-seeking behavior.
Materials and methods
Subjects
Of the 32 healthy males recruited for this study, one tested positive on the opiate urine screening, while another participant only completed one session. The final number of participants was 30 (mean age = 26.7, s.d. = 4.7 years). Exclusion criteria were a history of depression or other major psychiatric illness, ongoing treatment with medications, prior or ongoing substance dependence, and multiple complex allergies. Participants reported consuming an average of 5.5 alcoholic drinks per week. Previous recreational drug use was reported as follows: cannabinoids (23 participants), amphetamines (seven), stimulants (nine), hallucinogens (nine) and opiates (four; none had taken morphine in any form for at least 2 years prior to testing) (Saunders et al., 1993; Berman et al., 2005). All participants had normal or corrected-to-normal vision.
Procedure
Participants were tested on three separate days, with a minimum inter-session interval of 7 days. In each session, participants received one of three per-oral drugs [MOR agonist (morphine 10 mg), non-selective opioid receptor antagonist (naltrexone 50 mg) or placebo] in a double-blind, counterbalanced manner. Eye-tracking occurred between 70 and 140 min after drug treatment as part of a larger battery of reward tasks; the order of task administration was counterbalanced [details of drug administration and experimental timeline are presented in Chelnokova et al. (2014)]. Adding task order as a covariate to data analyses did not alter the pattern or statistical significance of the present results. Subjective state (including mood: happiness, anxiety, irritability, feeling good) was measured before and at 60, ∼100 and ∼150 min after drug administration. Subsequent analysis of mood ratings did not reveal any significant effects of either morphine or naltrexone on mood (see Supplementary Data for details), in line with previous observations using comparable or larger drug doses (Hanks et al., 1995; O’Neill et al., 2000; Zacny and Lichtor, 2008). To ensure that the results were not affected by drug effects on eye-hand coordination and motor function, we included a motor coordination task (Giovannoni et al., 1999) halfway through testing (∼110 min after drug intake; see Supplementary Data for the description of test and results, as well as for a discussion of potential drug effects on eye movement execution). At the end of the last session, participants were debriefed and asked to guess the identity of the drug received in each session. On average, participants identified the drug received correctly 34% of the time, indicating successful blinding.
Stimuli
Facial images were selected from the Oslo Face database, previously described in Chelnokova et al. (2014). A total of 240 images were used, depicting 60 females and 60 males with both direct and averted (half to the left and half to the right) gaze and a neutral facial expression. Forty unique images depicting 10 female and 10 male individuals [three most attractive, four attractive and three less attractive of each sex, as determined based on prior ratings from 20 independent male observers (mean age = 29.3, s.d. = 7.7 years); Table 1] with both direct and averted gaze (20 images of each gender) were presented in the task. No images were repeated across tasks or sessions. The direction of the averted gaze was counterbalanced. The order of presentation was pseudo-randomized and counterbalanced.
Table 1.
Means and standard deviations of prior attractiveness ratings of face categories used in the task, given by 20 independent male observers
| Male faces | Female faces | ||
|---|---|---|---|
| Less attractive | 2.99±0.34 | 3.00±0.37 | |
| Attractive | 4.18±0.21 | 4.88±0.18 | |
| Most attractive | 4.92±0.26 | 5.85±0.45 | |
Each image (19.5 × 19.5 cm) was presented on a computer screen located about 70 cm in front of the participant, with a resolution of 1680 × 1050 pixels. Models’ heads in the images subtended about 9.8 × 13 degrees of visual angle, comparable to the size viewed from a normal conversational distance (van Belle et al., 2010). A gray luminance-matched baseline image with a fixation cross was created for each of the facial stimuli. Fixation crosses were placed in either of the four corners of the image to avoid any central bias from the initial fixation.
The eye-tracking task
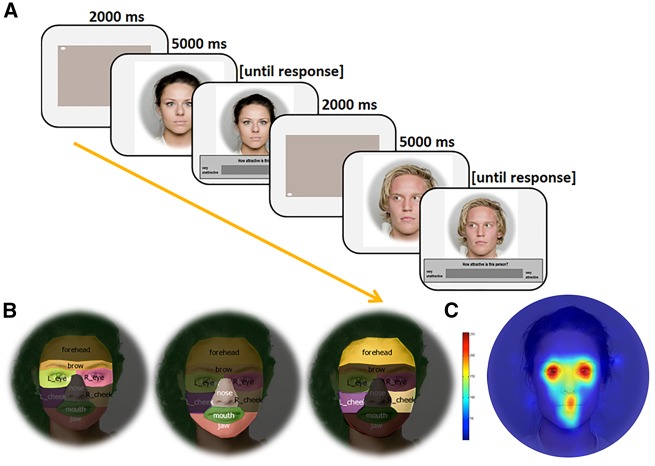
During the task, participants’ eye movements were recorded at 250 Hz with a binocular infrared Remote Eye Tracking Device, R.E.D. (SensoMotoric Instruments®; Teltow, Germany) in a windowless room with constant artificial lighting. Figure 1A illustrates the sequence of events for two subsequent trials. After presentation of a fixation point for 2 s, a facial image was presented on the computer screen for 5 s (viewing phase, for which eye-tracking data were analyzed) before a visual analog scale (VAS) appeared below the face (evaluation phase). Participants were requested to rate how attractive each face was on a VAS scale with the anchors ‘very unattractive’ and ‘very attractive’. After the response (or when 10 s elapsed), another baseline image was presented, followed by another facial image, and then by the VAS, etc. E-Prime 2.0® software (Psychology Software Tools Inc., Pittsburg, PA, USA) was used to present the stimuli and collect subjects’ VAS responses. Attractiveness ratings from a subset of the participants are reported in Chelnokova et al. (2014).
Fig. 1.
Overview of study design and AOIs. (A) Timeline of the events (two trials: the first trial showing a female stimulus face with direct gaze, and the second presenting a male stimulus face with averted gaze). (B) Illustration of the shape and extent of the AOIs of the face employed in the analysis of % of total eye fixation time (fix-t%). From left to right: Eye region, nose, mouth and jaw region, and forehead and cheek region. (C) A heat map illustrating a typical cumulative fixation pattern to a face observed in the current study. Individual fixation maps from all participants (N = 30) viewing the trials of the placebo condition were superimposed on a single face image and processed with a Gaussian filter, as described by Busey et al. (2010) to reveal the areas of highest fixation density (colored in red).
Data analysis
The following areas of interest (AOIs) were manually delineated for each of the faces using BeGaze (SensoMotoric Instruments®; Teltow, Germany) software: Eye region (comprising eyes and eyebrows); nose, mouth and jaw region; and forehead and cheek region, as in Guastella et al. (2008) (Figure 1B; AOI masks for the Oslo Face Database can be requested at sirileknes.com/oslo-face-database/). The number of eye-fixations (fix#) for the whole face and % of total fixation time (fix-t%), devoted to each of the three AOIs, were calculated for each participant and each stimulus. Note that since the % fixation time was calculated using the total fixation time to the entire image, the sum of the fix-t% for the three facial AOIs is not 100%. To control for variables such as session order, and to avoid data compression/aggregation, all eye-movement data were analyzed using linear multilevel/mixed effects models based on a maximum-likelihood approach (Baayen et al., 2008) in SPSS. To adjust for the dependency in the data, the models included a random effect for Subjects (random intercept). The following main/fixed factors were included in fix# data analysis: Drug (morphine, naltrexone or placebo), Gaze Direction (direct or averted gaze) and Face Attractiveness Level (most attractive, attractive and less attractive). The main factors for fix-t% data analysis included: AOI (eye region, nose–mouth–jaw region, forehead and cheeks), Drug (morphine, naltrexone or placebo), Gaze Direction (direct or averted gaze) and Face Attractiveness Level (most attractive, attractive and less attractive). Stimulus Order, Image Set and Session Number were included as regressors of no interest in all models. Main analyses of fix# and fix-t% data were run separately for female and male facial stimuli because evidence suggests differences in visual scanning of sexually relevant stimuli depending on the stimulus gender (Rupp and Wallen, 2007), and because male observers judged the most attractive males as significantly less attractive than the most attractive females (Table 1). To ensure robustness of the three-AOI fix-t% analysis, the model was also applied to log-transformed data. The transformation did not change the pattern of the results or statistical significance levels; therefore, outputs from the analyses on the primary data are reported. A follow-up analysis, which was restricted to the eye region, combined data from female and male faces (main factors Drug, Face Gender, Gaze Direction and Face Attractiveness Level) to enable comparison across stimulus gender. Model-estimated means, as well as within-subject standard deviations and standard errors of the mean calculated from the primary data by means of removing between-subject variability are reported throughout. As we expected the MOR manipulations to elicit bidirectional effects, the main contrast of interest (morphine vs naltrexone: M > N) is reported, along with significant drug-induced changes from placebo (P).
Results
The MOR system promotes visual exploration of faces
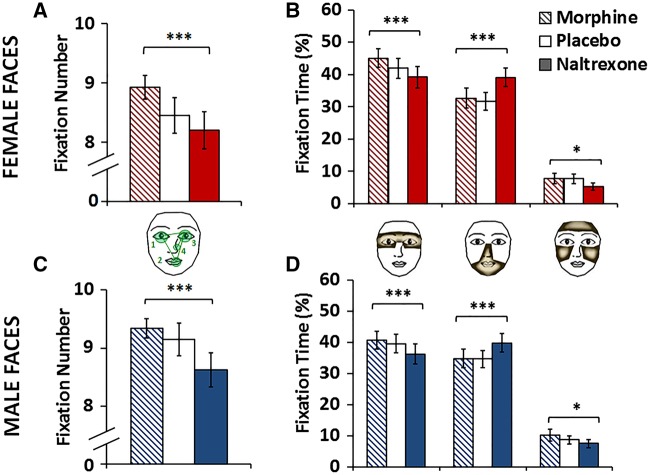
Linear multilevel regression analyses of total fix# to each face confirmed the hypothesis that the human MOR system promotes visual exploration of faces [main effect of Drug for female faces, F(2,1729)=12.67, P < 0.001 M > N, t = 4.95, P < 0.001, M > P, t = 3.25, P = 0.001; male faces, F(2,1727)=11.80, P < 0.001, M > N, t = 4.69, P < 0.001; P > N, t = 3.47, P = 0.001; Figure 2A and C, means and standard deviations reported in the Figure 2 caption]. No other significant main or interaction effects were observed in this analysis.
Fig. 2.
Morphine increased and naltrexone decreased visual attention to faces and eyes. (A) Visual exploration of facial stimuli, as measured by mean fix# for female faces (Morphine (M): Mean = 8.93 ± 1.08; Placebo (P): 8.45 ± 1.65; Naltrexone (N): 8.20 ± 1.71] and (C) male faces (M: 9.34 ± 0.94; P: 9.15 ± 1.54; N: 8.63 ± 1.61), was significantly modulated by the pharmacological manipulation of the MOR system. (B) Visual attention to the eye region was also modulated by the MOR manipulation, as illustrated by changes in fix-t% to selected AOI of female (Eye Region, M: 45.08 ± 15.18; P: 41.89 ± 16.42; N: 39.17 ± 18.22) and (D) male faces (Eye Region, M: 40.64 ± 15.52; P: 39.51 ± 16.35; N: 36.21 ± 17.73). Data for the female faces are presented in red, while data for the male faces are in blue. Error bars represent within-subjects SEM. ***P < 0.001, *P < 0.05. N = 30.
The MOR system promotes gaze to the eye region of faces
As expected, MOR manipulation significantly modulated visual attention (fix-t%) to both female [AOI*Drug F(4,5279) = 22.44, P < 0.001; Figure 2B] and male faces [AOI*Drug, F(4,5266)=12.29, P < 0.001; Figure 2D]. For the eye region, planned contrasts revealed that morphine increased, while naltrexone decreased fix-t% to the eye region of female (M > N, t = 5.53, P < 0.001; M > P, t = 3.00, P = 0.003; P > N, t = 2.54, P = 0.011) and male faces (M > N, t = 4.03, P < 0.001; P > N, t = 3.00, P = 0.003). Naltrexone also significantly affected visual attention to other face regions. Small decreases were observed for the forehead and cheeks (female: M > N, t = 2.39, P = 0.017; male: M > N, t = 2.43, P = 0.015), whereas fixation time to the nose, mouth and jaw region was increased (female: N > M, t = 5.98, P < 0.001; male: N > M, t = 4.51, P < 0.001). Means and standard deviations are reported in the Figure 2 caption.
Do MOR effects on eye gaze reflect increase in approach behavior or social interest?
A follow-up analysis, restricted to the eye region and assessing the effects of gender, gaze direction and attractiveness on fix-t% as a function of MOR manipulation was conducted to evaluate two competing exploratory hypotheses. As female gender, direct gaze, and high attractiveness level enhance the approach value of faces in male observers, we first confirmed that these factors increased visual attention to the eye region. Next, we evaluated the magnitude of drug effects for these stimuli. We reasoned that larger drug effects for such high approach value stimuli would support a specific MOR system promotion of social approach, whereas comparable drug effects across stimuli would favor the social interest hypothesis.
As expected, participants spent a larger proportion of fixation time on the eye region of female than male faces [main effect of Gender, F(1,3499)=36.62, P < 0.001; females: 41.27 ± 1.37; males: 37.62 ± 1.37]. However, drug effects on fixation time were comparable for male and female faces [Drug*Gender, F(2,3499)=1.08, P = 0.34].
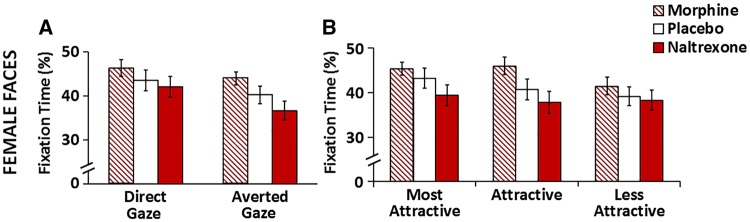
A larger proportion of fixation time to the eye region was also allocated to faces with direct gaze compared to faces with averted gaze [main effect of Gaze Direction, F(1,3499)=21.43, P < 0.001; direct: 40.81 ± 1.40; averted: 38.07 ± 1.40]. Planned contrasts revealed a significant increase of fix-t% to the eyes of both females and males looking directly at the observer (Females: Direct > Averted, t = 4.15, P < 0.001, direct: 43.06 ± 2.66; averted: 39.48 ± 2.15; Males: Direct > Averted, t = 2.35, P = 0.019, direct: 38.56 ± 2.24; averted: 36.67 ± 2.34). Nevertheless, drug effects on fixation time were comparable for faces with direct vs averted gaze [Drug*Gaze factors, F(2,3499)=0.07, P = 0.94; Figure 3A].
Table 2.
Means and standard deviations of fix-t% to the eye region of female faces for Drug*Gaze interaction
| Morphine | Placebo | Naltrexone | |
|---|---|---|---|
| Direct gaze | 45.40±10.64 | 42.72±12.90 | 41.06±12.95 |
| Averted gaze | 43.13±8.24 | 39.42±11.13 | 35.90±12.00 |
Table 3.
Means and standard deviations of fix-t% to the eye region of female faces for Drug*Attractiveness*Gender interaction
| Morphine | Placebo | Naltrexone | |
|---|---|---|---|
| Less attractive | 41.46±10.73 | 39.17±11.29 | 38.31±12.26 |
| Attractive | 45.99±10.91 | 40.77±12.76 | 37.77±13.65 |
| Most attractive | 45.34±8.03 | 43.26±12.55 | 39.35±12.77 |
Fig. 3.
Comparable effects of MOR manipulations on fix-t% to the eye region were observed across stimulus gender, gaze direction, and attractiveness level. (A) Comparable drug effects on fix-t% to the eye region of female faces with direct and averted gaze. (B) Similarly, drug effects on fix-t% to the eye region were comparable for female faces of varying attractiveness levels. Descriptive statistics are listed in Tables 2 and 3. Error bars represent within-subjects SEM. N = 30.
The main effect of attractiveness did not reach significance [F(2,3499) = 1.83, P = 0.16]. However, planned comparisons confirmed the expected increase of fix-t% to the eye region of the most attractive females compared with the less attractive ones (Most Attractive > Less Attractive, t = 2.80, P = 0.005, most attractive: 42.65 ± 2.93; less attractive: 39.65 ± 2.87). Drug effects were comparable across stimuli of varying attractiveness levels irrespective of face gender [Drug*Attractiveness*Gender, F(4,3499)=0.51, P = 0.73]; the illustration of comparable drug effects for female faces is presented in Figure 3B.
Furthermore, none of the three- or four-way interactions between attractiveness, gaze direction, face gender and drug was significant (F < 1.77, P > 0.17). Thus, we found little support for the MOR system specifically promoting social approach toward potential mating partners. The comparable drug effects for stimuli irrespective of face gender, gaze direction or attractiveness are more in accord with the view that MOR stimulation enhances attention to the eyes as a means of information-seeking.
Discussion
These results show that pharmacological manipulation of the human MOR system modulates overt attention to human faces. Specifically, we present causal, bi-directional evidence that the MOR system promotes visual exploration of faces, with morphine increasing and naltrexone decreasing the number of eye-fixations participants made to the photographs. Further, overt visual attention specifically to the eye region was also modulated by MOR system manipulation, such that morphine increased, while naltrexone decreased the proportion of time spent fixating on that information-rich facial region.
Consistent with the idea that distribution of eye-fixations reflects a drive to acquire information for perceptual decision-making (Tatler et al., 2011), more active visual exploration of faces should reflect greater motivation to obtain valuable socially relevant information as a basis for decision-making and behavior regulation. In light of current attentional theories (Maunsell, 2004; Gottlieb, 2012), the involvement of the MOR system in promoting visual exploration of faces and overt attention to the eye region can be understood from a perspective of facilitated extraction of socially relevant, and thus potentially rewarding, information. The observed effects on visual exploration constitute a possible behavioral mechanism for MOR-mediated social bonding in humans, thus supporting influential theories linking the human MOR system to social reward and affiliation (Depue and Morrone-Strupinsky, 2005; Machin and Dunbar, 2011). On the other hand, extracting information from faces and eyes is also important for many non-affiliative behaviors, such as determining whether someone may pose a threat. Furthermore, in rodents the MOR system appears to mediate both social and non-social aspects of exploratory behaviors (File, 1980; Vanderschuren et al., 1997). Only face stimuli were included in this study. We nevertheless speculate that future studies including non-social stimuli may find a similar MOR-enhancement of overt attention to areas rich in task-relevant information.
Human gaze is drawn toward the eyes of conspecifics (Birmingham and Kingstone, 2009; Levy et al., 2013). Indeed, the eye region provides rich, socially valuable information, diagnostic for determining and remembering identity (Henderson et al., 2005), gender (Saether et al., 2009), attractiveness (Baudouin and Tiberghien, 2004; Rhodes, 2006) and emotional state (often indicating the likelihood of threat or alliance) (Vassallo et al., 2009). Parallel to previous observations after intranasal oxytocin administration (Guastella et al., 2008), we showed that agonism of the µ-opioid system specifically promotes attention to the human eye region. Importantly, including both agonist and antagonist drugs enabled a bidirectional demonstration of the MOR system’s role. A similar demonstration is unfortunately lacking for oxytocin as there are at present no antagonists available for human testing. The present findings are thus more robust than evidence from treatment with either an agonist or antagonist alone. Note that oxytocin and µ-opioids are not the only neurotransmitters involved in visual attention to others’ faces and eyes (e.g. Jonassen et al., 2014). Here, blocking most of the MORs with naltrexone reduced, but did not eliminate eye fixations to the face and eye region.
With an exploratory analysis, we probed the functional relevance of MOR-induced changes in gaze to the eye region. The comparable effects of MOR manipulation across stimulus gender, gaze direction and levels of attractiveness did not support the hypothesis that MOR-enhanced attention to the eye region reflected increased approach motivation. Instead, we tentatively interpret the observed effects as reflecting motivation for gathering socially valuable information. Further research employing e.g. dynamic visual stimuli or joint attention paradigms (Schilbach et al., 2010), as well as different emotional facial expressions (Ipser et al., 2013) and individual difference measures of social function and attachment style (Nummenmaa et al., 2015), should elucidate the functional role of the MOR system in how people attend to others. In an effort to avoid potential drug interaction with circulating levels of estradiols and GnRH pulsability in females (Smith et al., 1998), only male participants were included in the test sample. As the current hypotheses are based on cross-species evidence consistent with an evolutionarily preserved function of MOR, we predict that future studies of the MOR system in women will reveal similar effects as the ones presented here in men.
Eye contact can both facilitate affiliation and induce stress, depending on the social context (Argyle and Dean, 1965; Kelly et al., 2010; Miellet et al., 2013). Involvement of the endogenous µ-opioid system in stress response regulation (Van Bockstaele and Valentino, 2013) could also contribute to the present results. For instance, a recent experimental study reported reduced social stress after treatment with the partial opioid agonist buprenorphine in humans (Bershad et al., 2015). Further, two human molecular imaging studies showed endogenous MOR regulation of affective responses to social acceptance and rejection (Hsu et al., 2013, 2015). However, the present results are unlikely to be explained by MOR stress regulation. Note that our design included neutral faces and no stress manipulation. Neither naltrexone nor morphine caused significant changes to the minimal levels of stress reported by participants (anxiety, irritability, etc.—see Supplementary Data for details). Debriefing confirmed that participants were fully blinded to the order of drug and placebo administration. Further, if stress regulation were the main mechanism underpinning the present findings, one would expect larger drug effects for direct gaze faces. Instead, MOR manipulation effects were comparable across stimuli with direct and averted gaze.
Two recent studies have linked reduced eye gaze to disruptions in reward processing (Watson et al., 2010; Preller et al., 2014). To our knowledge, the current findings are the first to causally demonstrate an association between disrupted MOR neurotransmission, and diminished visual attention to faces and eyes. Avoidance of the basic social behavior of looking someone in the eyes (even in photos) is observed in psychiatric disorders such as schizophrenia (Toh et al., 2011), social anxiety (Brunet et al., 2009), and autism spectrum disorders (Pelphrey et al., 2002; Dalton et al., 2005). Patients with major depressive disorder also showed reduced endogenous µ-opioid release in brain regions regulating stress, mood and motivation, combined with slower emotional recovery after social rejection, compared with healthy controls (Hsu et al., 2015). Future studies should investigate whether MOR system disruptions might underpin gaze avoidance and/or other aberrant social functioning observed in psychiatric disorders.
The present results are consistent with the idea that µ-opioid neurotransmission plays an important role in regulating healthy affiliative behavior across species, as suggested by studies in rodents (Moles et al., 2004; Resendez et al., 2013; Briand et al., 2015), as well as in both human and non-human primates (Nelson and Panksepp, 1998; Barr et al., 2008; Troisi et al., 2011; Hsu et al., 2013). Although the existing literature presents contradictory reports of the opposite effects of pharmacological manipulation of the MOR in rodents and primates, Løseth et al. (2014) showed that this discrepancy can be explained by taking into account differences in the animals’ initial motivational states. Opioid agonism primarily acts to soothe infants and primates after social isolation. In non-stressed animals however, the very same drugs promote social exploration and play behaviors (van Ree and Niesink, 1983; Guard et al., 2002; Trezza and Vanderschuren, 2008). As our study participants did not undergo aversive social isolation prior to testing, the current findings of MOR promotion of social interest are in consonance with this recently proposed model of state-dependent µ-opioid modulation of social motivation (SOMSOM; see Løseth et al., 2014).
By showing that the MOR system promotes visual exploration of others’ faces and overt attention to the eye region, we suggest a putative behavioral mechanism through which the MOR system promotes social motivation in the healthy human brain.
Supplementary Material
Acknowledgements
We thank J. Riegels, M. H. Sneve, M. Larsson, T. Karlsson and S. Aminihajibashi for technical assistance; I. Olsen, L. Bachs, V. Vindenes and E. Øiestad for pharmacological advice; and Dr L. Thomsen, Dr R. Jonassen, and Dr D. M. Ellingsen for helpful comments on earlier drafts of this manuscript.
Funding
The project was funded by grant number ES455867 to S.L. from the Research Council of Norway. The study was indirectly supported by Aleris Healthcare, Norway, financing the research position of F.W. at the University of Oslo.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Althoff R.R., Cohen N.J. (1999). Eye-movement-based memory effect: a reprocessing effect in face perception. Journal of Experimental Psychology: Learning, Memory, and Cognition, 25, 997–1010. [DOI] [PubMed] [Google Scholar]
- Argyle M., Dean J. (1965). Eye-contact, distance and affiliation. Sociometry, 28, 289–304. [PubMed] [Google Scholar]
- Armann R., Bulthoff I. (2009). Gaze behavior in face comparison: the roles of sex, task, and symmetry. Attention, Perception & Psychophysics, 71, 1107–26. [DOI] [PubMed] [Google Scholar]
- Baayen R.H., Davidson D.J., Bates D.M. (2008). Mixed-effects modeling with crossed random effects for subjects and items. Journal of Memory and Language, 59, 390–412. [Google Scholar]
- Barr C.S., Schwandt M.L., Lindell S.G., et al. (2008). Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proceedings of the National Academy of Sciences of the United States of America, 105, 5277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin J.Y., Tiberghien G. (2004). Symmetry, averageness, and feature size in the facial attractiveness of women. Acta Psychologica, 117, 313–32. [DOI] [PubMed] [Google Scholar]
- Berman A.H., Bergman H., Palmstierna T., Schlyter F. (2005). Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. European Addiction Research, 11, 22–31. [DOI] [PubMed] [Google Scholar]
- Bershad A.K., Jaffe J.H., Childs E., de Wit H. (2015). Opioid partial agonist buprenorphine dampens responses to psychosocial stress in humans. Psychoneuroendocrinology, 52, 281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham E., Kingstone A. (2009). Human social attention. Progress in Brain Research, 176, 309–20. [DOI] [PubMed] [Google Scholar]
- Briand L.A., Hilario M., Dow H.C., Brodkin E.S., Blendy J.A., Berton O. (2015). Mouse model of OPRM1 (A118G) polymorphism increases sociability and dominance and confers resilience to social defeat. Journal of Neuroscience, 35, 3582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet P.M., Heisz J.J., Mondloch C.J., Shore D.I., Schmidt L.A. (2009). Shyness and face scanning in children. Journal of Anxiety Disorders, 23, 909–14. [DOI] [PubMed] [Google Scholar]
- Busey T., Yu C., Wyatte D., Vanderkolk J., Parada F., Akavipat R. (2010). Consistency and variability among latent print examiners as revealed by eye tracking methodologies. Journal of Forensic Identification, 31, 60–91. [Google Scholar]
- Chelnokova O., Laeng B., Eikemo M., et al. (2014). Rewards of beauty: the opioid system mediates social motivation in humans. Molecular Psychiatry, 19, 746–7. [DOI] [PubMed] [Google Scholar]
- Dalton K.M., Nacewicz B.M., Johnstone T., et al. (2005). Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience, 8, 519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue R.A., Morrone-Strupinsky J.V. (2005). A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences, 28, 313–50. discussion 350–395. [DOI] [PubMed] [Google Scholar]
- Fields H. (2004). State-dependent opioid control of pain. Nature Reviews Neuroscience, 5, 565–75. [DOI] [PubMed] [Google Scholar]
- File S.E. (1980). Naloxone reduces social and exploratory activity in the rat. Psychopharmacology (Berlin), 71, 41–4. [DOI] [PubMed] [Google Scholar]
- Ghazanfar A.A., Santos L.R. (2004). Primate brains in the wild: the sensory bases for social interactions. Nature Reviews Neuroscience, 5, 603–16. [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Van Schalkwyk J., Fritz V., Lees A. (1999). Bradykinesia akinesia inco-ordination test (BRAIN TEST): an objective computerised assessment of upper limb motor function. Journal of Neurology, Neurosurgery & Psychiatry, 67, 624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J. (2012). Attention, learning, and the value of information. Neuron, 76, 281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard H.J., Newman J.D., Roberts R.L. (2002). Morphine administration selectively facilitates social play in common marmosets. Developmental Psychobiology, 41, 37–49. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Mitchell P.B., Dadds M.R. (2008). Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry, 63, 3–5. [DOI] [PubMed] [Google Scholar]
- Hanks G.W., O’Neill W.M., Simpson P., Wesnes K. (1995). The cognitive and psychomotor effects of opioid analgesics. II. A randomized controlled trial of single doses of morphine, lorazepam and placebo in healthy subjects. European Journal of Clinical Pharmacology, 48, 455–60. [DOI] [PubMed] [Google Scholar]
- Henderson J.M., Williams C.C., Falk R.J. (2005). Eye movements are functional during face learning. Memory & Cognition, 33, 98–106. [DOI] [PubMed] [Google Scholar]
- Herman B.H., Panksepp J. (1978). Effects of morphine and naloxone on separation distress and approach attachment: evidence for opiate mediation of social affect. Pharmacology, Biochemistry, and Behavior, 9, 213–20. [DOI] [PubMed] [Google Scholar]
- Hikosaka O., Nakamura K., Nakahara H. (2006). Basal ganglia orient eyes to reward. Journal of Neurophysiology, 95, 567–84. [DOI] [PubMed] [Google Scholar]
- Holmqvist K., Nyström M., Andersson R., Dewhurst R., Jarodzka H., Van de Weijer J. (2011). Eye Tracking: A Comprehensive Guide to Methods and Measures. Oxford: Oxford University Press. [Google Scholar]
- Hsu D.T., Sanford B.J., Meyers K.K., et al. (2013). Response of the mu-opioid system to social rejection and acceptance. Molecular Psychiatry, 18, 1211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D.T., Sanford B.J., Meyers K.K., et al. (2015). It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol Psychiatry, 20, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser J.C., Terburg D., Syal S., et al. (2013). Reduced fear-recognition sensitivity following acute buprenorphine administration in healthy volunteers. Psychoneuroendocrinology, 38, 166–70. [DOI] [PubMed] [Google Scholar]
- Issa E.B., DiCarlo J.J. (2012). Precedence of the eye region in neural processing of faces. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32, 16666–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier R.J., Batty M. (2009). Neural bases of eye and gaze processing: the core of social cognition. Neuroscience & Biobehavioral Reviews, 33, 843–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen R., Chelnokova O., Harmer C., Leknes S., Landrø N.I. (2014). A single dose of antidepressant alters eye-gaze patterns across face stimuli in healthy women. Psychopharmacology, 232, 953–8. [DOI] [PubMed] [Google Scholar]
- Kelly D.J., Miellet S., Caldara R. (2010). Culture shapes eye movements for visually homogeneous objects. Frontiers in Psychology 1, 6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., Foulsham T., Kingstone A. (2013). Monsters are people too. Biology Letters, 9, 20120850.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løseth G.E., Ellingsen D.-M., Leknes S. (2014). State-dependent µ-opioid Modulation of Social Motivation—a model. Frontiers in Behavioral Neuroscience, 8, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin A.J., Dunbar R.I.M. (2011). The brain opioid theory of social attachment: a review of the evidence. Behaviour, 148, 985–1025. [Google Scholar]
- Maunsell J.H.R. (2004). Neuronal representations of cognitive state: reward or attention? Trends in Cognitive Sciences, 8, 261–5. [DOI] [PubMed] [Google Scholar]
- Miellet S., Vizioli L., He L., Zhou X., Caldara R. (2013). Mapping face recognition information use across cultures. Frontiers in Psychology, 4, 34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A., Kieffer B.L., D’Amato F.R. (2004). Deficit in attachment behavior in mice lacking the μ-opioid receptor gene. Science, 304, 1983–6. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Panksepp J. (1998). Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neuroscience & Biobehavioral Reviews, 22, 437–52. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Manninen S., Tuominen L., et al. (2015). Adult attachment style is associated with cerebral mu-opioid receptor availability in humans. Human Brain Mapping, 36, 3621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill W.M., Hanks G.W., Simpson P., Fallon M.T., Jenkins E., Wesnes K. (2000). The cognitive and psychomotor effects of morphine in healthy subjects: a randomized controlled trial of repeated (four) oral doses of dextropropoxyphene, morphine, lorazepam and placebo. Pain, 85, 209–15. [DOI] [PubMed] [Google Scholar]
- Panksepp J. (1980). Brief social isolation, pain responsivity, and morphine analgesia in young rats. Psychopharmacology, 72, 111–2. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Sasson N.J., Reznick J.S., Paul G., Goldman B.D., Piven J. (2002). Visual scanning of faces in autism. Journal of Autism and Developmental Disorders, 32, 249–61. [DOI] [PubMed] [Google Scholar]
- Preller K.H., Herdener M., Schilbach L., et al. (2014). Functional changes of the reward system underlie blunted response to social gaze in cocaine users. Proceedings of the National Academy of Sciences of the United States of America, 111, 2842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez S.L., Dome M., Gormley G., et al. (2013). mu-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. Journal of Neuroscience, 33, 9140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G. (2006). The evolutionary psychology of facial beauty. Annual Review of Psychology, 57, 199–226. [DOI] [PubMed] [Google Scholar]
- Rupp H.A., Wallen K. (2007). Sex differences in viewing sexual stimuli: an eye-tracking study in men and women. Hormones and Behavior, 51, 524–33. [DOI] [PubMed] [Google Scholar]
- Saether L., Van Belle W., Laeng B., Brennen T., Overvoll M. (2009). Anchoring gaze when categorizing faces' sex: evidence from eye-tracking data. Vision Research, 49, 2870–80. [DOI] [PubMed] [Google Scholar]
- Saunders J.B., Aasland O.G., Babor T.F., De la Fuente J.R., Grant M. (1993). Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption II. Addiction, 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Wilms M., Eickhoff S.B., et al. (2010). Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. Journal of cognitive neuroscience, 22, 2702–15. [DOI] [PubMed] [Google Scholar]
- Schultz W. (2006). Behavioral theories and the neurophysiology of reward. Annual Review of Psychology, 57, 87–115. [DOI] [PubMed] [Google Scholar]
- Smith Y.R., Zubieta J.K., del Carmen M.G., et al. (1998). Brain opioid receptor measurements by positron emission tomography in normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. The Journal of Clinical Endocrinology and Metabolism, 83, 4498–505. [DOI] [PubMed] [Google Scholar]
- Sprague N., Ballard D.H. (2003) Eye movements for reward maximization. In: Proceedings of the 16th International Conference on Neural Information Processing Systems; Whistler, British Columbia, Canada. MIT Press, 1467–74.
- Tatler B.W., Hayhoe M.M., Land M.F., Ballard D.H. (2011). Eye guidance in natural vision: Reinterpreting salience. Journal of Vision, 11, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh W.L., Rossell S.L., Castle D.J. (2011). Current visual scanpath research: a review of investigations into the psychotic, anxiety, and mood disorders. Comprehensive Psychiatry, 52, 567–79. [DOI] [PubMed] [Google Scholar]
- Trezza V., Vanderschuren L.J.M.J. (2008). Cannabinoid and opioid modulation of social play behavior in adolescent rats: differential behavioral mechanisms. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 18, 519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi A., Frazzetto G., Carola V., et al. (2011). Social hedonic capacity is associated with the A118G polymorphism of the mu-opioid receptor gene (OPRM1) in adult healthy volunteers and psychiatric patients. Social Neuroscience—UK, 6, 88–97. [DOI] [PubMed] [Google Scholar]
- van Belle G., Ramon M., Lefevre P., Rossion B. (2010). Fixation patterns during recognition of personally familiar and unfamiliar faces. Frontiers in Psychology, 1, 20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele E.J., Valentino R.J. (2013). Neuropeptide regulation of the locus coeruleus and opiate-induced plasticity of stress responses. Advances in Pharmacology, 68, 405–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ree J.M., Niesink R.J. (1983). Low doses of beta-endorphin increase social contacts of rats tested in dyadic encounters. Life Sciences, 33(Suppl. 1), 611–4. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L.J., Niesink R.J., Van Ree J.M. (1997). The neurobiology of social play behavior in rats. Neuroscience & Biobehavioral Reviews, 21, 309–26. [DOI] [PubMed] [Google Scholar]
- Vassallo S., Cooper S.L., Douglas J.M. (2009). Visual scanning in the recognition of facial affect: is there an observer sex difference? Journal of Vision, 9, 11. [DOI] [PubMed] [Google Scholar]
- Watson K.K., Werling D.M., Zucker N.L., Platt M.L. (2010). Altered social reward and attention in anorexia nervosa. Frontiers in Psychology, 1, 36.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny J.P., Lichtor S.A. (2008). Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology (Berlin), 196, 105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.