Abstract
A pragmatic comparative effectiveness trial examined whether extending the duration of a cost-effective, intensive tobacco-dependence intervention designed to support autonomy will facilitate long-term tobacco abstinence. Participants were randomly assigned to one of three tobacco-dependence interventions based on self-determination theory, namely, Intensive Treatment (IT; six contacts over 6 months), Extended Need Support (ENS; eight contacts over 12 months) and Harm Reduction (HR; eight contacts over 12 months with medication use if willing to reduce cigarette use by half). Among participants who completed the interventions, analyses revealed beneficial effects of ENS (15.7 versus 3.8%; χ 2(1) = 6.92, P < 0.01) and HR (13.6 versus 3.8%; χ 2(1) = 5.26, P < 0.05), relative to IT, on 12-month prolonged abstinence from tobacco. Also, analyses revealed beneficial effects of ENS (77.7 versus 43.0%; χ 2(1) = 24.90, P < 0.001) and HR (84.0 versus 43.0%; χ 2(1) = 37.41, P < 0.001), relative to IT, on use of first-line medications for smoking cessation. Hence, two new interventions were found to be efficacious particularly among participants who completed the interventions. Smokers who stay in treatment for an additional 6 months may benefit from an additional two contacts with practitioners, and thus it seems reasonable for policy makers to offer additional contacts given the health benefits associated with prolonged tobacco abstinence.
Introduction
Tobacco use remains the leading cause of preventable death in the USA [1]. Smokers who quit permanently live longer and have better quality of life than those who continue to smoke [2, 3]. Despite the plethora of behavioral, pharmacological and public-policy efforts to promote smoking cessation, most smokers who quit relapse within 1 year after treatment [4], thereby undermining the physical and psychological health benefits [5] associated with long-term tobacco abstinence. Indeed, 12-month prolonged abstinence from tobacco increases the likelihood of achieving permanent tobacco abstinence [6, 7] and decreases the risk of myocardial infarction by 50% [8, 9]. Hence, the importance of understanding the factors associated with maintenance of tobacco abstinence is apparent [4, 10].
In 2003, the National Institutes of Health funded 21 studies via the Health Maintenance Consortium [11] to examine maintenance of health-behavior change. Around the same time, respect for patient autonomy was elevated to the highest level of biomedical ethics and medical professionalism [12–14]—a definitive responsibility among medical professionals that is equivalent to enhancing patient well-being and reducing social injustice. The present research describes outcomes associated with one of those studies, namely, the Smoker’s Health Project (SHP). The SHP is a pragmatic comparative effectiveness trial informed by self-determination theory (SDT) [15–17] and designed to facilitate long-term tobacco abstinence via three SDT-based intensive tobacco-dependence interventions [4, 10] that were developed around the concept of support for patient autonomy. (Pragmatic trials select relevant clinical interventions for comparison, include patients from a diverse clinical population who are recruited from heterogeneous settings, and assess various health outcomes to answer practical questions for policy makers [18].)
SDT is a macro-theory of human motivation that specifies support for satisfaction of the basic psychological needs for autonomy, competence and relatedness as necessary to promote health-behavior change and its maintenance. When in social contexts that support these basic psychological needs, people tend to perceive themselves as autonomous (choiceful) and competent (capable) concerning their behavior. Thus, the mechanisms by which need-supportive health care contexts promote health-behavior change and its maintenance are autonomous self-regulation (ASR) and perceived competence (PC). Indeed, a recent meta-analysis revealed support for this set of associations across 184 studies in the health domain [19].
Previously, we randomized 1006 adult smokers into a cessation-induction trial in which participants in the intervention condition met four times with counselors over 6 months, and the counselors engaged in a discussion about participants’ health in a manner that was intended to support autonomy and PC. In that trial, we demonstrated the efficacy [20–22] and cost-effectiveness [23] of an intensive tobacco-dependence intervention based on SDT and consistent with the Public Health Service (PHS) Guideline for Treating Tobacco Use and Dependence [4, 10] in facilitating long-term tobacco abstinence, relative to community care. That intervention was translated into care and became part of usual care in the region to which we compared two new interventions in the current, pragmatic comparative effectiveness trial. These new interventions were designed to determine whether long-term tobacco abstinence is increased through (1) extending need support by offering two additional contacts with study practitioners between 6 and 12 months post-randomization and by teaching important others to be need supportive, and (2) recommending first-line smoking-cessation medications to smokers who do not want to stop smoking completely within 30 days. The SDT-based interventions are described in detail below [24].
An efficacious, cost-effective intervention that supports patient autonomy and increases long-term tobacco abstinence via ASR and PC would attain the highest standards of medical care. In the current pragmatic comparative effectiveness trial, we recruited smokers using a diverse array of strategies; accepted smokers into the study whether or not they intended to quit; included smokers with a history of depression, anxiety and/or chemical dependency; randomized smokers at their initial visit, rather than after a run-in period; and assessed a variety of health outcomes, including ASR and PC for smoking cessation, medication use and smoking status.
We specified four hypotheses based on the literature reviewed earlier:
Hypothesis 1. The new SDT-based interventions, relative to the previously validated SDT-based intervention, will increase the likelihood of attaining 12-month prolonged abstinence from tobacco, the number of consecutive days since last cigarette, and 7-day point prevalence tobacco abstinence.
Hypothesis 2. The new SDT-based interventions, relative to the previously validated SDT-based intervention, will increase the likelihood of using first-line medications for smoking cessation and the number of days using such medications.
Hypothesis 3. The number of days using first-line medications for smoking cessation will relate positively to the likelihood of attaining 12-month prolonged abstinence from tobacco and the number of consecutive days since last cigarette.
Hypothesis 4. The components of the SDT Model of Health Behavior (changes in ASR and PC for smoking cessation from 6 to 18 months) will relate positively to the likelihood of attaining 12-month prolonged abstinence from tobacco and the number of consecutive days since last cigarette.
Methods
As reported elsewhere [24], smokers were accepted into the study regardless of whether they intended to quit, and participants were eligible if they had smoked at least 100 cigarettes in their lifetime, had smoked at least five cigarettes per day during the 4 weeks prior to enrollment, were at least 18 years of age, could read and speak English, reported no prior history of psychotic illness (anxiety and depression were allowed), had a life expectancy of at least 24 months and did not plan to leave the area during the study period. Participants were recruited using bus, newspaper and radio advertisements; signs in medical facilities; and mailings to patients in local physicians’ practices. After informed consent was obtained, participants were randomized to study condition (20% to Intensive Treatment (IT), 40% to Extended Need Support (ENS), 40% to Harm Reduction (HR)) and were told about the number of visits that was expected and drug side effects and potential toxicities. With this randomization scheme, the study was powered to detect differences between IT and the two new SDT-based interventions, as previous research demonstrated the efficacy [20–22] and cost-effectiveness [23] of IT in facilitating long-term tobacco abstinence, relative to community care. All clinical visits occurred in a community health center. Participants were paid $100 for completion of the study, and payment was pro-rated based on the percentage of questionnaires and visits that was completed. The University of Rochester Human Subjects Review Board approved the study protocol used in this trial. The Federal Drug Administration approved the medication protocol used in this trial.
The SHP interventions
A full description of the SHP interventions is presented elsewhere [24].
Intensive treatment
The IT condition (n = 172) was similar to the translated 6-month intensive tobacco-dependence intervention based on SDT and consistent with the PHS Guideline, which was validated in our previous trial [20–23, 25]. (The IT condition was referred to as “Community Care” elsewhere [24].) The clinical goal of IT was to guide participants toward autonomous decision-making about tobacco use, including not stopping smoking. If willing to stop, the tobacco-dependence counselor (four contacts) provided skills building and problem solving, and the prescriber (two contacts) guided participants toward autonomous decision-making about use of effective medications. Participants who were not willing to stop smoking within 30 days were asked to call back when they were ready to do so.
Extended need support
The ENS condition (n = 324) provided the same content as the IT condition, and the intervention was extended to 12 months and included eight contacts. Participants were encouraged to have at least eight contacts even if they were not ready to stop smoking, and they were asked to bring one important other (non-health-care professional) to a 50-min session on how to support the participant’s autonomy around tobacco use and cessation.
Harm reduction
The HR condition (n = 324) provided the same critical features as the ENS condition. Participants who were not willing to stop smoking within 30 days were recommended first-line smoking-cessation medications for the duration of the intervention if they were willing to reduce their cigarette use by half. Participants were informed that there is no evidence to suggest that reducing cigarette use improves health, although doing so may increase confidence for stopping smoking.
Procedure and time line for assessments
At baseline, participants completed a questionnaire packet assessing demographic information, medical history, smoking history, addiction severity and intention to stop smoking within 30 days. At 2, 4, 6, 12, 18 and 24 months post-randomization, participants were mailed a questionnaire packet assessing ASR and PC for smoking cessation, medication use and smoking status. Non-responsive participants were contacted twice by mail and three times by phone, if necessary.
Measures
ASR for smoking cessation
The Treatment Self-Regulation Questionnaire [26] presented participants with the following stem: “The reason I would stop smoking or continue not smoking permanently is …” Participants rated preselected responses that assessed autonomous reasons for smoking cessation (six items; e.g. because I personally believe it is the best thing for my health). Responses were made on a 7-point scale from 1 (not at all true) to 7 (very true). The mean, standard deviation, and reliability for this measure were as follows: M (SD) = 6.13 (1.14), α = 0.91 at 6 months; M (SD) = 6.09 (1.13), α = 0.90 at 18 months.
PC for smoking cessation
The Perceived Competence Scale [26] assessed participants’ feeling able to stop smoking permanently (four items; e.g. I am able to stop smoking permanently now). Responses were made on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree). The mean, standard deviation and reliability for this measure were as follows: M (SD) = 4.79 (1.96), α = 0.95 at 6 months; M (SD) = 4.66 (2.04), α = 0.96 at 18 months.
Medication use
Participants were asked whether they had used any of the first-line, FDA-approved medications that were available at the time of this trial (nicotine replacement therapies, Bupropion SR, varenicline). At each time point, participants reported the number of days that they had used each type of medication during the intervention by providing the “date of first use” and the “date of last use” for the medication(s). The variable days using medication was derived from participants’ last report within the intervention period (6 months post-randomization for IT, 12 months post-randomization for ENS and HR).
Smoking status
The primary outcome was 12-month prolonged abstinence (12mPA) from tobacco [7], which was assigned if a participant indicated that he or she had not used tobacco at all (including denying the use of a pipe, cigars, snuff and chewing tobacco) between the end of the intervention and 12 months post-intervention. The secondary outcome was self-reported number of consecutive days since last cigarette at 12 months post-intervention. The tertiary outcome was 7-day point prevalence (7dPP) tobacco abstinence [6] at 12 months post-intervention. Participants responded either “yes” or “no” to having smoked a cigarette, even a puff, in the past 7 days and to having used a pipe, cigars, snuff and chewing tobacco. All smoking status outcomes were assessed at 18 months post-randomization for IT and at 24 months post-randomization for ENS and HR.
Analytic overview
We tested Hypotheses 1–3 in two stages. Our primary analysis was conducted using treated-as-intended, which included only participants who completed the study. The use of treated-as-intended analyses is preferred in pragmatic trials (such as the present research), and this type of analysis offers to the clinician important information on whether the treatment is effective among participants who complete the study protocol. Our secondary analysis was conducted using intention-to-treat, which included all participants who were randomized, and participants who did not complete the study were considered to be smokers. The use of intention-to-treat analyses is preferred in efficacy trials, and this type of analysis offers to the researcher important information on whether the treatment is effective relative to a control condition. We tested Hypothesis 4 with as-reported data, which included only data provided by participants. A P value of 0.05 was used to determine statistical significance in all analyses.
Results
Recruitment, randomization and retention
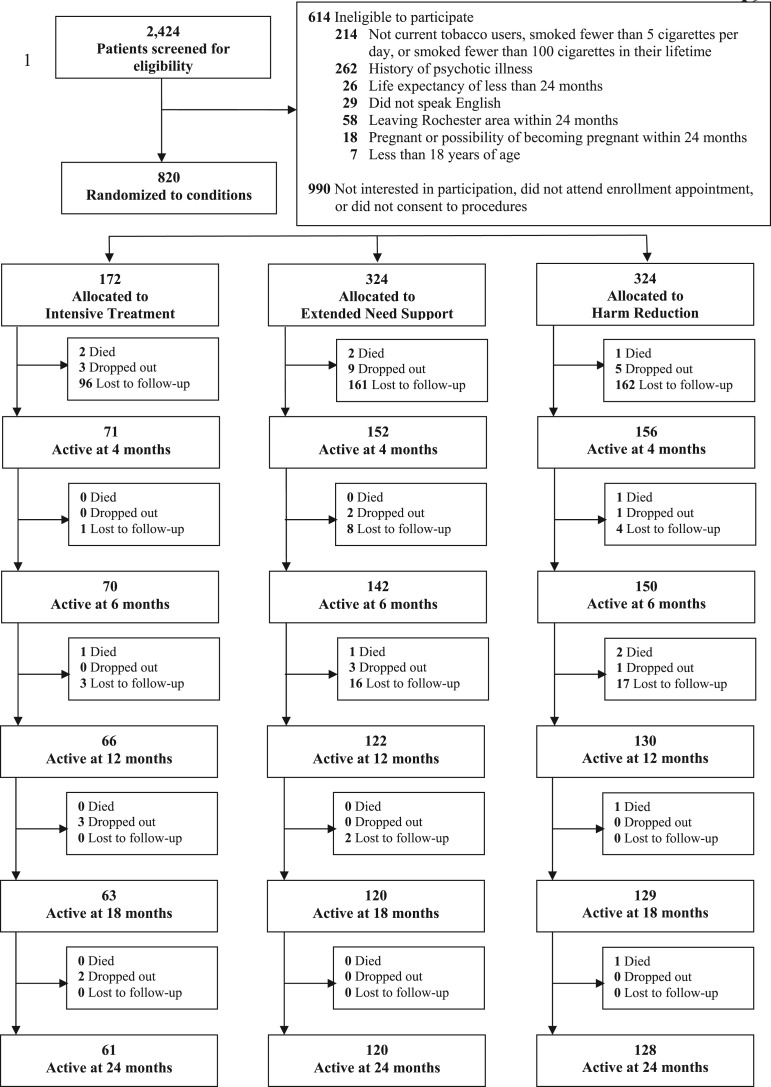
Between August 2004 and September 2008, 2424 smokers were screened for eligibility, 1810 (75%) were eligible and 820 (45%) of those eligible came to an initial appointment, provided full informed consent, completed the baseline questionnaires and were randomized to treatment condition. Baseline characteristics of the study participants are displayed in Table I. Randomization was only partially effective, as participants in the HR condition reported smoking more cigarettes per day at baseline than those in the IT condition [M (SD) = 19.95 (10.85) versus 17.71 (8.67); t (790) = 2.39, P < 0.05]. Figure 1 depicts participant flow through the 24-month study period.
Table I.
Baseline characteristics of the study participants
| Characteristic | Overall (N = 820) | IT (n = 172) | ENS (n = 324) | Harm reduction (n = 324) |
|---|---|---|---|---|
| Sex (% female) | 59.8 | 57.4 | 60.1 | 60.7 |
| Age (M) | 47.39 | 47.74 | 46.27 | 48.32 |
| SES (1–9) | 4.31 | 4.35 | 4.35 | 4.24 |
| Marital status (% married) | 36.0 | 35.5 | 34.9 | 37.4 |
| Ethnicity (% White) | 71.8 | 73.4 | 67.6 | 75.2 |
| Cigarettes per Day (M) | 18.87 | 17.71 | 18.43 | 19.95 |
| Fagerstrom AS (M) | 7.57 | 7.43 | 7.51 | 7.72 |
SES, socioeconomic status; AS, addiction severity
Fig. 1.
CONSORT recruitment and retention of participants.
Primary analyses: effect of treatment condition on maintenance of tobacco abstinence
Hypothesis 1 stated that the new SDT-based interventions, relative to the previously validated SDT-based intervention, will increase the likelihood of attaining 12mPA from tobacco, the number of consecutive days since last cigarette, and 7dPP tobacco abstinence. Results are displayed in Table II.
Table II.
Primary hypotheses: effects of treatment condition (versus IT) on maintenance of tobacco abstinence and medication use
| Intervention versus IT | Treated-as-intended |
Intention-to-treat |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12mPA from tobacco | ||||||||||
| Attain (%) | χ 2(1) | NNT | OR | 95% CI | Attain (%) | χ 2(1) | NNT | OR | 95% CI | |
| ENS | 15.7 versus 3.8 | 6.92** | 8.4 | 4.72 | 1.35, 16.53 | 8.4 versus 7.1 | 0.26 | — | 1.20 | 0.59, 2.44 |
| Harm reduction | 13.6 versus 3.8 | 5.26* | 10.2 | 3.99 | 1.13, 14.09 | 7.9 versus 7.1 | 0.09 | — | 1.12 | 0.55, 2.28 |
| Intervention versus IT | Days since last cigarette | |||||||||
| Mean (SD) | t(322) | Mean (SD) | t(805) | |||||||
| ENS | 115.42 (248.07) versus 22.48 (90.46) | 3.00** | 61.67 (186.04) versus 39.38 (126.82) | 1.37 | ||||||
| Harm reduction | 100.33 (233.83) versus 22.48 (90.46) | 2.53* | 55.99 (177.60) versus 39.38 (126.82) | 1.02 | ||||||
| Intervention versus IT | 7dPP tobacco abstinence | |||||||||
| Attain (%) | χ2(1) | NNT | OR | 95% CI | Attain (%) | χ2(1) | NNT | OR | 95% CI | |
| ENS | 22.3 versus 12.7 | 2.96 + | 10.4 | 1.98 | 0.90, 4.36 | 13.1 versus 14.8 | 0.27 | — | 0.87 | 0.51, 1.48 |
| Harm reduction | 20.8 versus 12.7 | 2.21 | — | 1.81 | 0.82, 4.00 | 12.3 versus 14.8 | 0.62 | — | 0.81 | 0.47, 1.38 |
| Intervention versus IT | Medication use | |||||||||
| Attain (%) | χ2(1) | NNT | OR | 95% CI | Attain (%) | χ2(1) | NNT | OR | 95% CI | |
| ENS | 77.7 versus 43.0 | 24.90*** | 2.9 | 4.61 | 2.49, 8.55 | 44.5 versus 36.1 | 3.26 + | 11.9 | 1.42 | 0.97, 2.09 |
| Harm reduction | 84.0 versus 43.0 | 37.41*** | 2.4 | 6.95 | 3.62, 13.36 | 52.2 versus 36.1 | 11.50*** | 6.2 | 1.93 | 1.32, 2.84 |
| Intervention versus IT | Days using medication | |||||||||
| Mean (SD) | t(322) | Mean (SD) | t(805) | |||||||
| ENS | 86.50 (140.85) versus 21.33 (51.04) | 3.90*** | 37.38 (97.97) versus 21.41 (58.13) | 1.94 + | ||||||
| Harm reduction | 75.67 (117.60) versus 21.33 (51.04) | 3.27*** | 36.17 (86.75) versus 21.41 (58.13) | 1.79 + | ||||||
Note. 12mPA from tobacco = 12-month prolonged abstinence from tobacco; 7dPP tobacco abstinence = 7-day point prevalence tobacco abstinence; Attain (%) = percentage who attained outcome; NNT = number needed to treat; OR = odds ratio; 95% CI = 95% confidence interval
P < 0.10,
*P < 0.05,
** P < 0.01,
*** P < 0.001
12mPA from tobacco
With treated-as-intended, Chi-square analysis revealed a significant effect of treatment condition on the likelihood of attaining 12mPA from tobacco [χ 2(2) = 6.91, P < 0.05]. 12mPA from tobacco was higher in the ENS condition and the HR condition, relative to the IT condition. With intention-to-treat, Chi-square analysis did not reveal a significant effect of treatment condition on the likelihood of attaining 12mPA from tobacco [χ 2(2) = 0.26, ns].
Days since last cigarette
With treated-as-intended, analysis of variance revealed a significant effect of treatment condition on the number of consecutive days since last cigarette [F(2, 322) = 4.88, P < 0.01]. Days since last cigarette was higher in the ENS condition and the HR condition, relative to the IT condition. With intention-to-treat, analysis of variance did not reveal a significant effect of treatment condition on the number of consecutive days since last cigarette [F(2, 805) = 0.94, ns].
7dPP tobacco abstinence
With treated-as-intended, Chi-square analysis did not reveal a significant effect of treatment condition on the likelihood of attaining 7dPP tobacco abstinence [χ 2(2) = 3.11, ns]. With intention-to-treat, Chi-square analysis did not reveal a significant effect of treatment condition on the likelihood of attaining 7dPP tobacco abstinence [χ 2(2) = 0.62, ns].
Primary analyses: effect of treatment condition on medication use
Hypothesis 2 stated that the new SDT-based interventions, relative to the previously validated SDT-based intervention, will increase the likelihood of using first-line medications for smoking cessation and the number of days using such medications. Results are displayed in Table II.
Medication use
With treated-as-intended, Chi-square analysis revealed a significant effect of treatment condition on the likelihood of using first-line medications for smoking cessation [χ 2(2) = 43.43, P < 0.001]. Medication use was higher in the ENS condition and the HR condition, relative to the IT condition. With intention-to-treat, Chi-square analysis revealed a significant effect of treatment condition on the likelihood of using first-line medications for smoking cessation [χ 2(2) = 11.87, P < 0.01]. Medication use was higher in the HR condition and somewhat higher in the ENS condition, relative to the IT condition.
Days using medication
With treated-as-intended, analysis of variance revealed a significant effect of treatment condition on the number of days using first-line medications for smoking cessation [F(2, 322) = 8.24, P < 0.001]. Days using medication were higher in the ENS condition and the HR condition, relative to the IT condition. With intention-to-treat, analysis of variance did not reveal a significant effect of treatment condition on the number of days using first-line medications for smoking cessation [F(2, 805) = 2.12, ns].
Primary analyses: relation of days using medication to maintenance of tobacco abstinence
Hypothesis 3 stated that the number of days using first-line medications for smoking cessation will relate positively to the likelihood of attaining 12mPA from tobacco and the number of consecutive days since last cigarette.
12mPA from tobacco
With treated-as-intended, logistic regression analysis revealed a significant positive relation of days using medication [b = 0.01; Wald z(1) = 18.81, P < 0.001; 95% CI 1.003–1.007] to the likelihood of attaining 12mPA from tobacco. With intention-to-treat, logistic regression analysis revealed a significant positive relation of days using medication [b = 0.01; Wald z(1) = 43.69, P < 0.001; 95% CI 1.005–1.009] to the likelihood of attaining 12mPA from tobacco.
Days since last cigarette
With treated-as-intended, linear regression analysis revealed a significant positive relation of days using medication [β = 0.30, P < 0.001] to the number of consecutive days since last cigarette. With intention-to-treat, linear regression analysis revealed a significant positive relation of days using medication [β = 0.33, P < 0.001] to the number of consecutive days since last cigarette.
Primary analyses: SDT model of health behavior
Hypothesis 4 stated that the components of the SDT Model of Health Behavior (changes in ASR and PC for smoking cessation from 6 to 18 months) will relate positively to the likelihood of attaining 12mPA from tobacco and the number of consecutive days since last cigarette.
12mPA from tobacco
With as-reported data, logistic regression analyses revealed significant positive relations of changes in ASR [b = 0.60; Wald z(1) = 5.09, P < 0.05; 95% CI 1.08–3.05] and PC [b = 1.60; Wald z(1) = 19.51, P < 0.001; 95% CI 2.43–10.03] for smoking cessation to the likelihood of attaining 12mPA from tobacco.
Days since last cigarette
With as-reported data, linear regression analyses revealed significant positive relations of changes in ASR [β = 0.19, P < 0.05] and PC [β = 0.44, P < 0.001] for smoking cessation to the number of consecutive days since last cigarette.
An integrated model—12mPA from tobacco
12mPA from tobacco was regressed simultaneously onto change in ASR, change in PC, and days using medication. With as-reported data, logistic regression analysis revealed a significant positive relation of change in PC for smoking cessation [b = 1.78; Wald z(1) = 17.83, P < 0.001; 95% CI 2.60–13.53] to the likelihood of attaining 12mPA from tobacco, whereas days using medication [b = 0.001; Wald z(1) = 0.11, ns; 95% CI 0.99–1.00] and change in ASR for smoking cessation [b = 0.39; Wald z(1) = 0.52, ns; 95% CI 0.51–4.35] were non-significant.
An integrated model—days since last cigarette
Days since last cigarette was regressed simultaneously onto change in ASR, change in PC and days using medication. With as-reported data, linear regression analysis revealed marginal or significant positive relations of days using medication [β = 0.11, P < 0.10] and change in PC for smoking cessation [β = 0.44, P < 0.001] to the number of consecutive days since last cigarette, whereas change in ASR for smoking cessation [β = 0.03, ns] was non-significant.
Discussion
The SHP is a pragmatic comparative effectiveness trial that examined the impact of (1) extending need support by offering two additional contacts with study practitioners between 6 and 12 months post-randomization and by teaching important others to be need supportive, and (2) recommending first-line smoking-cessation medications to smokers who do not want to stop smoking completely within 30 days. With treated-as-intended, the ENS and HR conditions, relative to the IT condition, were found to increase maintenance of long-term tobacco abstinence and use of first-line medications for smoking cessation. With intention-to-treat, little difference was observed among the three SDT-based interventions. There appears to be a beneficial effect of offering two additional contacts, teaching important others to be need supportive and recommending medications if smokers are willing to reduce their cigarette use by half, and the observed differences among study conditions are likely to be of clinical importance because smokers who attain 12mPA from tobacco are less likely to relapse [6, 7] and have a heart attack [8, 9].
Changes in ASR and PC for smoking cessation related positively to the likelihood of attaining 12mPA from tobacco and the number of consecutive days since last cigarette. Change in PC for smoking cessation predicted maintenance of tobacco abstinence over and above days using medication and change in ASR for smoking cessation. Such results underscore the importance of creating need-supportive clinical contexts that facilitate ASR and PC for smoking cessation.
The major limitation of this study was the low completion rate, which was noted despite using similar methods of recruitment and retention from our previous trial [20–22] that resulted in 82% completion. Hence, smokers may be less willing to stay in treatment for as long as was indicated at the beginning of the trial. This high drop-out rate reflects the complexity of retaining participants in longer-term interventions, and offers somewhat less confidence in our finding of between-group differences. That being said, our pragmatic trial was designed to respect participants’ autonomy around their decision to remain in treatment. Notwithstanding the scientific limitation reflected in this (possible) real-world phenomenon, policy makers may adjust staffing requirements according to low rates of completion, thereby reducing cost and possibly enhancing support for autonomy.
Conclusion
In our previous trial, we demonstrated the efficacy [20–22] and cost-effectiveness [23] of an intensive tobacco-dependence intervention designed to support autonomy in facilitating long-term tobacco abstinence, relative to community care, among all participants who were randomized. In the current, pragmatic comparative effectiveness trial, we examined whether extending the duration of our previous treatment will facilitate long-term tobacco abstinence and medication use. Two new interventions were found to be effective among participants who completed the interventions, and changes in ASR and PC for smoking cessation were confirmed as predictors of maintenance of tobacco abstinence.
Practice implications
The new SDT-based interventions, relative to the previously validated SDT-based intervention, were found to be effective in facilitating long-term tobacco abstinence using treated-as-intended (but not intention-to-treat) analyses. Policy makers face a difficult dilemma around the type of analysis to use to guide their recommendations, especially insofar as pragmatic analyses can clarify the efficacy of later components of clinical interventions. Our pragmatic analysis (treated-as-intended) indicated that smokers who stay in treatment for an additional 6 months may benefit from an additional two contacts with practitioners. More pragmatic trials are needed to confirm these findings, yet it seems reasonable for policy makers to offer additional contacts given the health benefits associated with prolonged tobacco abstinence and the cost-effectiveness of intensive tobacco-dependence interventions relative to other health-care interventions [10, 27].
Funding
This study was supported by grants from the National Cancer Institute [R01-CA106668] awarded to G.C.W.; the National Institute of Mental Health and the National Cancer Institute [R01-MH059594] awarded to G. C. W.; the National Center for Research Resources [M01-RR00044] awarded to the University of Rochester’s General Clinical Research Center; and the National Center for Research Resources ARRA Supplement [UL1RR024160] awarded to the University of Rochester’s Clinical and Translational Science Institute.
Trial registration: ClinicalTrials.gov number NCT00178685.
Conflict of interest statement
None declared.
References
- 1. Mokdad AH, Marks JS, Stroup DF. et al. Actual causes of death in the United States, 2000. J Am Med Assoc 2004; 291:1238–45. [DOI] [PubMed] [Google Scholar]
- 2. Doll R, Peto R, Boreham J. et al. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer 2005; 92:426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strandberg AY, Strandberg TE, Pitkälä K. et al. The effect of smoking in midlife on health-related quality of life in old age: a 26-year prospective study. Arch Intern Med 2008; 168:1968–74. [DOI] [PubMed] [Google Scholar]
- 4. Fiore MC, Bailey WC, Cohen SJ. et al. Treating Tobacco Use and Dependence. Rockville, MD: U.S. Department of Health and Human Services (DHHS), 2000. [Google Scholar]
- 5. Niemiec CP, Ryan RM, Patrick H. et al. The energization of health-behavior change: examining the associations among autonomous self-regulation, subjective vitality, depressive symptoms, and tobacco abstinence. J Positive Psychol 2010; 5:122–38. [Google Scholar]
- 6. Hughes JR, Keely JP, Niaura RS. et al. Measures of abstinence in clinical trials: issues and recommendations. Nico Tob Res 2003; 5:13–25. [PubMed] [Google Scholar]
- 7. Pierce JP, Gilpin EA. A minimum 6-month prolonged abstinence should be required for evaluating smoking cessation trials. Nico Tob Res 2003; 5:151–3. [DOI] [PubMed] [Google Scholar]
- 8. U.S. Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
- 9. U.S. Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- 10. Fiore MC, Jaen CR, Baker TB. et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, 2008. [Google Scholar]
- 11. Ory MG, Smith ML, Mier N. et al. The science of sustaining health behavior change: the health maintenance consortium. Am J Health Behav 2010; 34:647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beauchamp TL, Childress JF. Principles of Biomedical Ethics, 5th edn. New York: Oxford University Press, Inc, 2001. [Google Scholar]
- 13. Beauchamp TL, Childress JF. Principles of Biomedical Ethics, 6th edn. New York: Oxford University Press, Inc, 2009. [Google Scholar]
- 14. ABIM, ACP-ASIM, Medicine EFoI. Medical professionalism in the new millennium: a physician charter. Ann Intern Med 2002; 136:243–6. [DOI] [PubMed] [Google Scholar]
- 15. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self-determination of behavior. Psychol Inquiry 2000; 11:227–68. [Google Scholar]
- 16. Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol 2000; 55:68–78. [DOI] [PubMed] [Google Scholar]
- 17. Niemiec CP, Ryan RM, Deci EL. Self-determination theory and the relation of autonomy to self-regulatory processes and personality development In: Hoyle RH. (ed). Handbook of Personality and Self-Regulation. Malden, MA: Blackwell Publishing Ltd, 2010, 169–91. [Google Scholar]
- 18. Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. J Am Med Assoc 2003; 290:1624–32. [DOI] [PubMed] [Google Scholar]
- 19. Ng JYY, Ntoumanis N, Thøgersen-Ntoumani C. et al. Self-determination theory applied to health contexts: a meta-analysis. Perspect Psychol Sci 2012; 7:325–40. [DOI] [PubMed] [Google Scholar]
- 20. Williams GC, McGregor HA, Sharp D. et al. Testing a self-determination theory intervention for motivating tobacco cessation: supporting autonomy and competence in a clinical trial. Health Psychol 2006; 25:91–101. [DOI] [PubMed] [Google Scholar]
- 21. Williams GC, McGregor H, Sharp D. et al. A self-determination multiple risk intervention trial to improve smokers’ health. J Gen Intern Med 2006; 21:1288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams GC, Niemiec CP, Patrick H. et al. The importance of supporting autonomy and perceived competence in facilitating long-term tobacco abstinence. Ann Behav Med 2009; 37:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pesis-Katz I, Williams GC, Niemiec CP. et al. Cost-effectiveness of intensive tobacco dependence intervention based on self-determination theory. Am J Manag Care 2011; 17:e393–8. [PMC free article] [PubMed] [Google Scholar]
- 24. Williams GC, Patrick H, Niemiec CP. et al. The Smoker’s Health Project: a self-determination theory intervention to facilitate maintenance of tobacco abstinence. Contemp Clin Trials 2011; 32:535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams GC, Minicucci DS, Kouides RW. et al. Self-determination, smoking, diet and health. Health Educ Res 2002; 17:512–21. [DOI] [PubMed] [Google Scholar]
- 26. Williams GC, McGregor HA, Zeldman A. et al. Testing a self-determination theory process model for promoting glycemic control through diabetes self-management. Health Psychol 2004; 23:58–66. [DOI] [PubMed] [Google Scholar]
- 27. Tengs TO, Adams ME, Pliskin JS. et al. Five-hundred life-saving interventions and their cost-effectiveness. Risk Anal 1995; 15:369–90 [DOI] [PubMed] [Google Scholar]