Abstract
Muscle microvasculature critically regulates endothelial exchange surface area to facilitate transendothelial delivery of insulin, nutrients, and oxygen to myocytes. Insulin resistance blunts insulin-mediated microvascular recruitment and decreases muscle capillary density; both contribute to lower microvascular blood volume. Glucagon-like peptide 1 (GLP-1) and its analogs are able to dilate blood vessels and stimulate endothelial cell proliferation. In this study, we aim to determine the effects of sustained stimulation of the GLP-1 receptors on insulin-mediated capillary recruitment and metabolic insulin responses, small arterial endothelial function, and muscle capillary density. Rats were fed a high-fat diet (HFD) for 4 wk with or without simultaneous administration of liraglutide and subjected to a euglycemic hyperinsulinemic clamp for 120 min after an overnight fast. Insulin-mediated muscle microvascular recruitment and muscle oxygenation were determined before and during insulin infusion. Muscle capillary density was determined and distal saphenous artery used for determination of endothelial function and insulin-mediated vasodilation. HFD induced muscle microvascular insulin resistance and small arterial vessel endothelial dysfunction and decreased muscle capillary density. Simultaneous treatment of HFD-fed rats with liraglutide prevented all of these changes and improved insulin-stimulated glucose disposal. These were associated with a significantly increased AMPK phosphorylation and the expressions of VEGF and its receptors. We conclude that GLP-1 receptor agonists may exert their salutary glycemic effect via improving microvascular insulin sensitivity and muscle capillary density during the development of insulin resistance, and early use of GLP-1 receptor agonists may attenuate metabolic insulin resistance as well as prevent cardiovascular complications of diabetes.
Keywords: glucagon-like peptide-1, endothelial function, microvascular recruitment, vasodilation, insulin resistance
insulin increases muscle blood flow, and this action is paralleled by changes in insulin-mediated glucose disposal in humans (4). Muscle microvasculature is the critical link that couples insulin's vascular and metabolic actions (5). To act on myocytes, insulin has to be first delivered into the capillaries nourishing the myocytes and then transported through the endothelium to enter the interstitial space, two rate-limiting steps in muscle insulin action (22, 45). Insulin regulates its own delivery to and actions in muscle by recruiting muscle microvasculature, thus expanding the endothelial surface area available for insulin transport from the plasma compartment to muscle insterstitum (12, 43) and facilitating its own transendothelial transport (44). This feed-forward mechanism contributes up to 40% of insulin-mediated glucose uptake (43) but is impaired in the insulin-resistant states (28, 29).
Another major determinant of the muscle endothelial surface area is the number of capillaries in muscle, which directly correlates with insulin-stimulated glucose uptake (21, 27) and decreases in the insulin-resistant state (17, 27). The vascular endothelial growth factor (VEGF) family of proteins regulates angiogenesis (33). VEGF recruits and differentiates endothelial progenitor cells and induces endothelial cell proliferation and migration, leading to increased formation of new vessels (33). In the insulin-resistant states, VEGF actions in muscle vascular beds are impaired, which triggers muscle capillary regression (17, 20). This is consistent with the observation that muscle-specific VEGF deletion induces muscle capillary rarefaction and muscle insulin resistance (8, 41).
Vascular endothelium expresses abundant GLP-1 receptors (GLP-1R) (32), and GLP-1 exerts multitude vascular actions in addition to its well-characterized glycemic actions. GLP-1 infusion increases acetylcholine-induced vasodilatation in healthy humans (7) and improves flow-mediated dilatation in patients with type 2 diabetes mellitus (32). GLP-1 also regulates insulin action and glucose metabolism via its vascular effects. Indeed, GLP-1 increases coronary blood flow and myocardial uptake of glucose in the Langendorff-perfused rat heart during low-flow ischemia (49) and myocardial glucose uptake in dogs with cardiomyopathy (31) and regulates muscle glucose uptake independent of its ability to enhance insulin secretion (3). Recently, we have shown that acute GLP-1 infusion increases muscle microvascular recruitment and glucose use independent of insulin secretion (10, 40), likely via protein kinase A-mediated endothelial nitric oxide synthase (eNOS) activation (10, 15). Importantly, GLP-1's microvascular action is preserved in both acute and chronic insulin-resistant states (13). Furthermore, GLP-1 and its analogs are able to stimulate endothelial proliferation and angiogenesis. Exendin-4 stimulates endothelial cell proliferation (16) and increases cell migration in scratch wound assays as well as vessel sprouting in three-dimensional bead assays (24). Incubation of human umbilical vein endothelial cells with GLP-1 dose-dependently promotes angiogenesis (2).
In the current study, we determined the effects of the long-acting GLP-1 analog liraglutide on insulin-mediated capillary recruitment and metabolic insulin responses, small arterial endothelial function, and muscle capillary density in rats fed a high-fat diet (HFD) for 4 wk. Our results indicate that sustained GLP-1R stimulation with liraglutide prevented HFD-induced muscle microvascular insulin resistance and small arterial vessel endothelial dysfunction, preserved muscle capillary density, and significantly improved insulin's metabolic action during HFD feeding.
RESEARCH DESIGN AND METHODS
Animal Preparation and Experimental Protocols
Adult male Sprague-Dawley rats (170–190 g) were purchased from Charles River Laboratories (Wilmington, MA) and housed at 22 ± 2°C on a 12-h light-dark cycle. Only male rats were used, as both endothelial cells and vascular smooth muscle cells possess estrogen receptors and estrogen functionally modulates vasomotion (25). Rats were fed either a chow diet (CD; 28 kcal% protein, 60 kcal% carbohydrate, and 12 kcal% fat) or HFD (20 kcal% protein, 20 kcal% carbohydrate, and 60 kcal% fat; Research Diets) with or without daily subcutaneous administration of liraglutide (200 μg/kg twice daily; Novo Nordisk) for 4 wk. Rats were pair-fed, and each rat was limited to a food intake of 15–20 g/day and had water access ad libitum. To improve tolerance, liraglutide was given at a lower dose (50 μg/kg twice/day) initially, and the dose was titrated up to 200 μg/kg twice/day over 8 days (i.e., an increase of 100 μg·kg−1·day−1 every 2 days). Control rats received an equal amount of saline injection subcutaneously twice daily. At the end of 4 wk of dietary intervention, rats weighed 350–400 g and were studied under the following two protocols. HFD treatment for 4 wk is sufficient to induce significant insulin resistance in muscle microvasculature and resistance arterioles as well as myocytes in rats (48). Because liraglutide has a half-life of ∼4 h in rats (39), the last dose of liraglutide was given 24 h prior to the infusion experiment to exclude acute effects from liraglutide.
Protocol 1.
Rats were fasted overnight, anesthetized with pentobarbital sodium (50 mg/kg ip; Abbott Laboratories, North Chicago, IL), and intubated to maintain a patent airway. They were placed in a supine position on a heating pad to ensure euthermia. The carotid artery and jugular vein were cannulated with polyethylene tubing (PE-50; Fisher Scientific, Newark, DE) for arterial blood pressure monitoring, arterial blood sampling, and various infusions. After a 30- to 45-min baseline period to ensure hemodynamic stability and a stable level of anesthesia, each rat received a euglycemic hyperinsulinemic clamp (3 mU·kg−1·min−1) for 120 min, with arterial blood glucose determined every 10 min using an Accu-Chek Advantage glucometer (Roche Diagnostics, Indianapolis, IN). Dextrose (30% wt/vol) was infused at a variable rate to maintain blood glucose within 10% of basal, and whole body glucose disposal rates were calculated. Hindleg skeletal muscle microvascular blood volume (MBV) and microvascular blood flow velocity (MFV) were determined using contrast-enhanced ultrasound at times 0, 30, 60, and 120 min, as described previously (10, 12). Muscle microvascular blood flow (MBF) was derived as product of MBV and MFV (i.e., MBF = MBV × MFV). Muscle oxygen saturation and plasma nitric oxide (NO), endothelin-1 (ET-1), and insulin concentrations were determined at each time point. At the end of the study, rats were euthanized by anesthetic overdose, and gastrocnemius muscle was dissected, freeze clamped, and stored at −70°C for later determination of protein kinase B (PKB or Akt) and extracellular signal regulated protein kinase 1 and 2 (ERK1/2) phosphorylation.
Throughout the study, mean arterial pressure was monitored via a sensor connected to the carotid arterial catheter (Harvard Apparatus, Holliston, MA, and ADInstruments, Colorado Springs, CO). Pentobarbital sodium was infused at a variable rate to maintain steady levels of anesthesia and blood pressure throughout the study.
Protocol 2.
After an overnight fast, rats were euthanized using CO2 overdose. Gastrocnemius muscle was collected for the determination of capillary density, 5′-adenosine monophosphate-activated protein kinase (AMPK) phosphorylation, and mRNA expressions of VEGF, VEGF receptor 1 (VEGFR1), and VEGF receptor 2 (VEGFR2). The distal saphenous artery (≤250 μm) was dissected and used for determination of endothelial function and insulin-mediated vasodilation.
This investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Publication No. 85-23, revised 1996). The study protocols were approved by the Animal Care and Use Committee of the University of Virginia.
Measurement of Skeletal Muscle Capillary Density
Muscle was fixed in 4% paraformaldehyde, embedded in paraffin, and then sectioned transversely at 1- to 2-μm thickness. Tissue sections were deparaffinized and rehydrated, and endogenous peroxidase was inhibited by methanol-30% H2O2 (100 ml/1.5 ml) for 60 min. The sections were then incubated with fluorescein Griffonia Simplcifolia Lectin I (Vector Laboratories) overnight at 4°C in a humidity chamber to stain endothelial cells. Capillaries were observed under a fluorescence microscope (Olympus SZX12), and images were obtained at ×200 magnification. For each sample section, capillaries and muscle fibers were counted in six randomly selected ×200 fields, and capillary density was expressed as capillaries/muscle fibers.
Determination of Protein Phosphorylation in Muscle
Total AMPK, Akt, and ERK1/2 and phosphorylation of AMPK, Akt, and ERK1/2 in gastrocnemius were determined using Western blot analysis, as described previously (13, 46). Primary antibodies against phospho-AMPKα (Thr172), total AMPK, phospho-Akt (Ser473), total Akt, phospho-ERK (Thr202/Tyr204), and total ERK1/2 were purchased from Cell Signaling Technology. All blots were developed using ECL (GE Healthcare Bio-Sciences). Chemiluminescence blot images were captured using the UVP imaging system and quantified using ImageQuant 3.3 software. For protein phosphorylation analyses, both the total and the phosphospecific densities were quantified and the ratios of phosphospecific to total density calculated.
Quantitative RT-PCR
Total RNA extraction and reverse transcription.
Muscle tissue was homogenized in TRIZOL reagent (Ambion), and total RNA was extracted and treated with Dnase (Direct-zol RNA MiniPrep Kit; Zymo Research, Irvine, CA). Reverse transcription (RT) was performed using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) with random hexamers primers. The reaction time was 5 min at 65°C (Denature), 10 min at 25°C (anneal), 50 min at 50°C (cDNA synthesis), and 5 min at 85°C (terminate reaction).
Real-time quantitative PCR of muscle VEGF, VEGFR1, and VEGFR2.
PCR amplification was performed on a iCycler instrument (Bio-Rad, Hercules, CA) using ABsolute Blue qPCR SYBR Green Fluorescein Mix (Thermo Scientific). Primer sequences were as follows: VEGF: forward 5′-CAA TGA TGA AGC CCT GG AGT-3′, reverse 5′-TCT CCT ATG TGC TGG CTT TG-3′; VEGFR1: forward 5′-GTC ACT ACA ACC ACT CCA AAG A-3′, reverse 5′-CCC TCG ATT CTG TTC CTA TGT-3′; VEGFR2: forward 5′-GTA CCA AAC CAT GCT GGA TGC 3′, reverse 5′-CTT GCA GGA GAT TTC CCA AGT G-3′. All samples were done in triplicate under the following conditions for each primer set: 15 min at 95°C, followed by 40 cycles of 15 s at 95°C, 1 min at 60°C, and 30 s at 72°C. A total of 25 ng RNA was used per reaction. Each assay included a standard curve of six serial dilutions of rat muscle cDNA to assess reaction efficiency and negative control. The expression of respective mRNA was expressed as percentage of the control.
Determination of endothelial function and vascular insulin responses.
Rat distal saphenous artery was cut into segments of ∼2 mm in length and mounted in a Multi Myograph System (Danish Myo Technology, Aarhus, Denmark) for isometric tension recordings. The organ chamber was filled with 6 ml of physiological salt solution (130 mM NaCl, 4.7 mM KCl, 1.6 mM CaCl2, 1.17 mM MgSO4, 1.18 mM KH2PO4, 14.9 mM NaHCO3, 0.026 mM EDTA, and 5.5 mM glucose; pH, 7.4), oxygenated with 95% O2-5% CO2, and maintained at 37°C, as described previously (46, 47). The arterial ring was initially stretched to 5 mN, allowed to stabilize at baseline tone, and then preconstricted with phenylephrine (PE; 2 μM). Changes in vascular tone to increasing concentrations of acetylcholine (ACh), sodium nitroprusside (SNP), and insulin were recorded and expressed in percent of the tension to PE.
Measurement of Plasma NO, ET-1, and Insulin Levels
Plasma NO levels were measured using 280i Nitric Oxide Analyzer (GE Analytical Instruments) according to the manufacturer's instructions. In brief, ice-cold ethanol was added into plasma samples at a ratio of 2:1. The mixture was kept at 0°C for 30 min and then centrifuged at 16,000 g for 5 min. The supernatant was then used for NO analysis based on a gas phase chemiluminescent reaction between NO and ozone. Plasma ET-1 concentrations were assayed using an endothelin 1 ELISA kit (Abcam). Plasma insulin concentrations were determined using a rat insulin ELISA assay kit (Mercodia, Uppsala, Sweden).
Quantification of Muscle Interstitial Oxygen Saturation
Muscle interstitial oxygen saturation was measured using a fiber-optic oxygen measurement system (OXYMICRO; World Precision Instruments) based on the effect of dynamic luminescence quenching by molecular oxygen. In brief, a needle housing the fibro-optic oxygen microsensor was inserted into the hindleg skeletal muscle, and the glass fiber with its oxygen-sensitive tip inside the needle was extended into the muscle by carefully pressing the syringe plunger. Measurements were taken every 10 s, and the values were averaged.
Statistical Analysis
All data are presented as means ± SE. Statistical analyses were performed with SigmaStat 3.1.1 software (Systat Software), using Student t-test or repeated-measures ANOVA with post hoc analysis as appropriate. A P value of <0.05 was considered statistically significant.
RESULTS
Liraglutide Treatment Improves Metabolic Insulin Responses in HFD Rats
We first examined the effects of HFD and liraglutide on metabolic actions of insulin in the study animals using a 2-h insulin clamp. Rats fed a HFD had higher plasma insulin concentrations but normal plasma glucose concentrations, consistent with insulin resistance, and administration of liraglutide lowered plasma insulin concentrations back to the levels seen in control rats (Table 1). Insulin infusion raised plasma insulin concentrations by ∼300 pM across all groups. Figure 1 shows that HFD feeding depressed insulin-stimulated whole body glucose disposal by 62% (P < 0.01), which was associated with a marked suppression of insulin-stimulated muscle Akt phosphorylation (P < 0.05) and a twofold increase in ERK phsophorylation. Liraglutde treatment improved insulin-stimulated glucose disposal by 35% (from 3.67 ± 0.34 to 4.95 ± 0.31 mg·kg−1·min−1, P < 0.05) and muscle Akt phosphorylation and suppressed insulin-stimulated ERK phosphorylation.
Table 1.
MAP and plasma glucose and insulin concentrations during insulin clamp
| Minutes |
||||
|---|---|---|---|---|
| 0 | 30 | 60 | 120 | |
| MAP, mmHg | ||||
| CD | 106 ± 2 | 104 ± 7 | 100 ± 7 | 103 ± 3 |
| HFD | 117 ± 6 | 117 ± 5 | 115 ± 6 | 117 ± 7 |
| HFD + liraglutide | 109 ± 3 | 111 ± 2 | 104 ± 3 | 107 ± 4 |
| Plasma glucose, mM | ||||
| CD | 5.2 ± 0.3 | 4.6 ± 0.3 | 5.2 ± 0.2 | 5.4 ± 0.3 |
| HFD | 5.1 ± 0.2 | 5.1 ± 0.2 | 4.7 ± 0.2 | 4.9 ± 0.2 |
| HFD + liraglutide | 4.8 ± 0.1 | 4.8 ± 0.1 | 5.0 ± 0.2 | 4.9 ± 0.2 |
| Plasma insulin, pM | ||||
| CD | 103 ± 15 | 368 ± 31* | 405 ± 15* | 475 ± 37* |
| HFD | 276 ± 31# | 507 ± 21* | 497 ± 21* | 577 ± 28* |
| HFD + liraglutide | 164 ± 30 | 486 ± 31* | 499 ± 37* | 533 ± 34* |
Values are means ± SE; n = 5-9 each.
MAP, mean arterial pressure; CD, chow diet; HFD, high-fat diet.
P < 0.05 compared with 0 min;
P < 0.05 compared with CD (ANOVA).
Fig. 1.
Liraglutide (Lira) treatment improves insulin responses in high-fat diet (HFD)-fed rats. Each rat was fed either a chow diet (CD) or HFD ± Lira for 4 wk and received a 2-h euglycemic insulin clamp (3 mU·kg−1·min−1) after an overnight fast. A: time course of glucose infusion rate (GIR) and GIR during steady state (90–120 min). B: muscle Akt phosphorylation (p-Akt). C: muscle ERK phosphorylation (p-ERK); n = 5–8/group. *P < 0.05 compared with CD; #P < 0.05 compared with HFD. t-Akt, total Akt; t-ERK, total ERK.
HFD Causes Muscle Microvascular Insulin Resistance, Which is Prevented by Liraglutide Treatment
Because insulin recruits skeletal muscle microvasculature and microvascular insulin resistance is an early event in HFD-induced insulin resistance (48), the above findings prompted us to examine whether liraglutide treatment can prevent or at least attenuate microvascular insulin resistance in the HFD setting. Figure 2, A–C, shows the changes in muscle MBV, MFV, and MBF during insulin infusion. Basal muscle MBV did not differ among all three groups of animals (CD: 1.79 ± 0.21; HFD: 1.85 ± 0.31; HFD ± Lira: 1.29 ± 0.32 in video intensity; P > 0.05). Insulin promptly increased muscle MBV without changing MFV in CD rats, leading to a significant increase in muscle MBF (P < 0.05). This insulin-mediated muscle microvascular recruitment was completely abolished by HFD feeding. Insulin even moderately depressed muscle MBV in HFD rats (by 20%, P < 0.05). Simultaneous treatment with liraglutide fully rescued insulin's microvascular actions in HFD-fed animals.
Fig. 2.
Effect of liraglutide (Lira) on insulin-mediated muscle microvascular perfusion and muscle oxygenation during HFD feeding. Each rat was fed either a CD or HFD ± Lira for 4 wk and received a 2-h euglycemic insulin clamp (3 mU·kg−1·min−1) after an overnight fast. A: microvascular blood volume (MBV). B: microvascular blood flow velocity (MFV). C: microvascular blood flow (MBF). D: muscle oxygen saturation; n = 5–7/group. *P < 0.05 compared with respective baseline (0 min). VI, video intensity.
Because changes of muscle MBV usually parallel the changes in muscle interstitial oxygen saturation, we concurrently measured muscle interstitial oxygen saturation in the current study (Fig. 2D). Insulin infusion steadily increased muscle interstitial oxygen saturation in control animals (P = 0.002, ANOVA), and this effect was similarly abolished by HFD feeding. Consistent with the changes in muscle microvascular perfusion, liraglutide treatment fully restored insulin-stimulated increase in muscle interstitial oxygen saturation (P < 0.01, ANOVA). Not surprisingly, insulin increased plasma NO contents in control but not in HFD-fed rats, and liraglutide treatment restored insulin-mediated increases in plasma NO contents. On the contrary, insulin markedly increased levels of plasma ET-1, a potent vasoconstrictor, in HFD-fed rats, which was prevented by liraglutide treatment (Fig. 3).
Fig. 3.
Effect of liraglutide (Lira) on insulin-induced nitric oxide (NO) and endothelin-1 (ET-1) production. Each rat was fed either a CD or HFD ± Lira for 4 wk and received a 2-h euglycemic insulin clamp (3 mU·kg−1·min−1) after an overnight fast. A: plasma NO concentrations. B: plasma ET-1 concentrations; n = 4–8/group. *P < 0.05 compared with respective baseline (0 min).
Liraglutide Prevents HFD-Induced Endothelial Dysfunction and Insulin Resistance in Small Resistance Arterioles
Because endothelial dysfunction and endothelial insulin resistance coexist in the state of metabolic insulin resistance, we next examined the effects of HFD with or without liraglutide administration on endothelial function and insulin responses in small resistance arteriole that feeds into the microvasculature. HFD feeding significantly decreased both Ach- and insulin-induced vasorelaxation (P < 0.05 for both), whereas these effects were restored in arterioles isolated from HFD rats given 4-wk liraglutide (Fig. 4). Neither HFD nor HFD + liraglutide affected arterial responsiveness to PE (data not shown) or SNP (Fig. 4B). Thus, sustained activation of GLP-1 receptor with liraglutide prevented both endothelial dysfunction and insulin resistance in small resistance arterioles during HFD feeding.
Fig. 4.
Liraglutide (Lira) prevents HFD-induced endothelial dysfunction and insulin resistance in isolated saphenous artery. Each rat was fed either a CD or HFD ± Lira for 4 wk. Distal saphenous artery was isolated and preconstricted with phenylephrine, and the vasodilatory responses to acetylcholine (ACh; A), sodium nitroprusside (SNP; B), or insulin (C) were measured; n = 6/group. *P < 0.05 compared with CD.
Liraglutide Preserves Muscle Capillary Density in HFD-Fed Rats
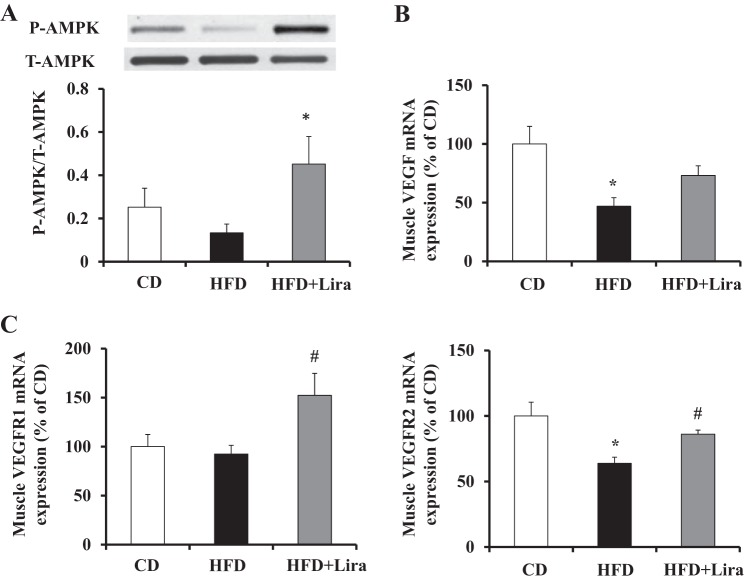
We finally examined muscle capillary density in rats with or without liraglutide administration, as muscle capillarization is another determinant of endothelial surface area and insulin action in muscle. As shown in Fig. 5, HFD feeding for 4 wk reduced muscle capillary density by ∼25% (P < 0.0002), whereas administration of liraglutide in HFD-fed rats preserved muscle capillary density. This was associated with a marked increase in muscle AMPK phosphorylation (P < 0.05, Fig. 6). The reduction in muscle capillary density in HFD-fed rats was associated with a significant decrease in muscle VEGF and VEGFR2 expression (53 and 36% respectively, P < 0.05 in both) but not the expression of VEGFR1. Liraglutide treatment restored muscle expressions of both VEGF and VEGFR2 and significantly increased the VEGFR1 expression in HFD-fed rats.
Fig. 5.
Liraglutide (Lira) treatment prevents HFD-induced decrease in muscle capillary density. Rats were fed a HFD either with or without Lira for 4 wk. Control rats were fed a CD for 4 wk. A: representative images of capillary content in gastrocnemious. B: Muscle capillary density (capillaries/muscle fiber); n = 12/group. *P < 0.0002 compared with CD; #P = 0.001 compared with HFD.
Fig. 6.
Effect of liraglutide (Lira) treatment on muscle AMP-activated protein kinase (AMPK) phosphorylation (p-AMPK) and VEGF expression in HFD-fed rats. Each rat was fed either a CD or HFD ± Lira for 4 wk. A: muscle p-AMPK; n = 6/group. B: VEGF mRNA expression; n = 10–11/group. C: VEGFR1 and -2 mRNA expressions; n = 9–10/group. *P < 0.05 compared with CD; #P < 0.05; compared with HFD. t-AMPK, total AMPK.
DISCUSSION
Novel findings of the current study include that simultaneous treatment with liraglutide effectively prevented HFD-induced 1) endothelial dysfunction and insulin resistance in small arterioles, 2) microvascular insulin resistance, 3) decrease in insulin-mediated muscle oxygenation, and 4) decrease in muscle capillarization in rats. These findings are of great importance as GLP-1R agonists have been widely used to control glycemia in patients with type 2 diabetes but the underlying mechanisms remain to be fully defined. Our results argue strongly that GLP-1R agonists may exert their salutary glycemic effect via improving microvascular insulin sensitivity and that early use of GLP-1R agonists may attenuate metabolic insulin resistance and prevent diabetes and its complications in addition to improving glycemic control.
Muscle microvasculature provides endothelial exchange surface area to facilitate insulin and nutrient exchanges between plasma and muscle interstitium and plays a critical role in regulating muscle metabolism and insulin responses (5, 6). We and others have demonstrated repeatedly that expansion of muscle microvascular volume through the microvascular recruitment process increases muscle insulin action (11, 13, 35, 46, 47). Insulin per se actively regulates its own delivery to muscle microvasculature and facilitates its own transendothelial transport via a receptor-mediated pathway (5, 6). These actions contribute up to 40% of insulin-mediated glucose disposal and are impaired in the insulin-resistant states (43). Our current observation, together with our prior reports that GLP-1 acutely recruits muscle microvasculature in both insulin-sensitive and -resistant states (10, 13, 15, 40), strongly suggests that muscle microvasculature is a therapeutic target of GLP-1 and its analogs. However, although liraglutide can directly stimulate endothelial cell NO production (19) and exenatide can acutely increase capillary perfusion in humans (37), it does not appear that the increase in insulin-mediated microvascular recruitment in HFD-fed rats was secondary to an acute, direct action of liraglutide, as the last dose of liraglutide was given 24 h before insulin clamp and tissue harvest, and in rodents the half-life of liraglutide is only ∼4 h (39). In addition, in the ex vivo experiments, distal saphenous artery isolated from liraglutide-treated animals exhibited improved endothelial function (response to acetylcholine) and insulin responses in the absence of liraglutide in the incubation chamber. Although the underlying mechanisms remain to be defined, a possible explanation is the anti-inflammatory effect of GLP-1R agonists in the vasculature. Inflammation induces microvascular insulin resistance, and we have demonstrated recently that inflammation-induced microvascular insulin resistance is an early event in diet-induced obesity and plays a critical role in the pathogenesis of metabolic insulin resistance (48). That liraglutide treatment completely restored insulin-mediated muscle microvascular recruitment but only partially increased insulin-stimulated muscle Akt phosphorylation and glucose disposal (by ∼35%) suggests that HFD induces insulin resistance in both vasculature and myocytes, and liraglutide acts mainly in restoring insulin responses in the microvascular component. This is consistent with the fact that insulin's microvascular actions contribute up to 40% of insulin-mediated glucose disposal (43).
In the insulin-sensitive state, insulin fine-tunes vascular tone via balanced production of vasodilator NO and vasoconstrictor ET-1. Insulin resistance results in a decreased NO bioavailability and increased ET-1 production, thus tilting the balance toward an increased vasoconstrictive activity (30). Indeed, in the current study, we observed that insulin failed to increase plasma NO content and significantly increased ET-1 production in HFD-fed rats. Importantly, both defects were corrected with liraglutide treatment. These results are certainly in line with our observation of liraglutide preventing microvascular insulin resistance during HFD feeding and provide a logical explanation to clinical observations that GLP-1R agonists improve tissue perfusion and decrease blood pressure in addition to improving glycemic control. The sharp increase in muscle oxygenation after insulin stimulation in liraglutide-treated animals is consistent with an improved insulin response in muscle microvasculature. It also implies that GLP-1R agonists might be beneficial in managing patients with insulin resistance and tissue ischemia such as peripheral arterial diseases or coronary artery disease.
Muscle capillary content also critically regulates endothelial exchange surface area. Prior studies have demonstrated that muscle capillary density directly correlates with insulin-stimulated glucose uptake (21, 27), and insulin resistance is associated with a reduction of muscle capillary content (capillary rarefaction) (17, 27), which is proportional to the severity of insulin resistance (27, 38). Insulin-stimulated muscle glucose disposal increases by ∼30%, concomitant with an ∼20% increase in skeletal muscle capillarizatin (1). Similar to a prior report (42), we observed that 4-wk HFD feeding significantly reduced capillary density in muscle. However, simultaneous administration of liraglutide completely prevented HFD-induced reduction in muscle capillarization. This is not surprising, as insulin resistance-associated capillary rarefaction occurs early in the process, and GLP-1R agonists have been shown to potently stimulate endothelial proliferation and angiogenesis (2, 16, 24).
VEGF and its receptors are crucial regulators of vasculogenesis and angiogenesis (33). Whereas VEGFR1 is required for the recruitment of hematopoietic precursors, VEGFR2 is essential for the functions of vascular endothelial cells and neovascularization in physiological or pathological conditions (33). In addition to promoting endothelial cell survival and angiogenesis, VEGF also prevents apoptosis of microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling (18). In internal mammary artery samples collected from patients with diabetes, capillary density was only 30% of that in the nondiabetic vessels, whereas VEGF expression was reduced by 48% (14). Targeted inhibition of muscle VEGF expression reduced a 64% decrease in capillary density and capillary-to-fiber ratio (41). Our data show that HFD reduced the expressions of both VEGF and VEGFR2, which was prevented by liraglutide treatment. Interestingly, whereas VEGFR1 expression did not decrease with HFD, simultaneous treatment with liraglutide significantly increased VEGFR1 expression in muscle. It is likely that both VEGFR1 and VEGFR2 signaling are involved in liraglutide prevention of capillary rarefaction in HFD-fed rats.
In the current study, liraglutide treatment was associated with a more than threefold increase in muscle AMPK phosphorylation, which is potentially the common denominator under all current findings. First, AMPK is a key regulator of energy metabolism in muscle, and its activation leads to increased fatty acid oxidation and glucose uptake (36). Second, AMPK activation improves mitochondria function and decreases oxidative stress, endoplasmic reticulum stress, and inflammation (36), and inhibition of inflammation reverses HFD-induced microvascular insulin resistance (48). Third, activation of AMPK by AICAR increases eNOS activity and vasodilates the resistance arteries ex vivo and recruits muscle microvasculature in vivo (9). Finally, AMPK signaling stimulates VEGF expression, regulates skeletal muscle capillarization (34, 50), and inhibits hyperglycemia-induced endothelial apoptosis (23) and mitochondria reactive oxygen species production (26).
In conclusion, our results demonstrate that HFD feeding is associated with microvascular insulin resistance and decreased capillarization in muscle, and sustained activation of GLP-1R with liraglutide prevents microvascular insulin resistance and restores capillarization in muscle with a likely involvement of AMPK and VEGF signaling. Because microvascular recruitment and muscle capillarization determine the endothelial exchange surface area available for transendothelial transport of insulin, nutrients, and oxygen in muscle, patients with insulin resistance and diabetes may benefit from early use of GLP-1R agonists. Further validation in humans is warranted.
GRANTS
This work was supported by the American Diabetes Association Grant 1-15-CE-32 (to Z. Liu) and National Institutes of Health Grants R01-HL-094722 and R01-DK-102359 (to Z. Liu). Z. Fu was supported by a fellowship award from the American Diabetes Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.C., E.J.B., and Z.L. conception and design of research; W.C., Z.F., K.A., and Z.L. performed experiments; W.C., Z.F., and Z.L. analyzed data; W.C., Z.F., E.J.B., and Z.L. interpreted results of experiments; W.C. and Z.L. prepared figures; W.C. and Z.L. drafted manuscript; W.C., Z.F., K.A., E.J.B., and Z.L. approved final version of manuscript; E.J.B. and Z.L. edited and revised manuscript.
REFERENCES
- 1.Akerstrom T, Laub L, Vedel K, Brand CL, Pedersen BK, Lindqvist AK, Wojtaszewski JF, Hellsten Y. Increased skeletal muscle capillarization enhances insulin sensitivity. Am J Physiol Endocrinol Metab 307: E1105–E1116, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Aronis KN, Chamberland JP, Mantzoros CS. GLP-1 promotes angiogenesis in human endothelial cells in a dose-dependent manner, through the Akt, Src and PKC pathways. Metabolism 62: 1279–1286, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala JE, Bracy DP, James FD, Julien BM, Wasserman DH, Drucker DJ. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology 150: 1155–1164, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron AD. Hemodynamic actions of insulin. Am J Physiol Endocrinol Metab 267: E187–E202, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Barrett E, Eggleston E, Inyard A, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52: 752–764, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab 301: E252–E263, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab 293: E1289–E1295, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP, Wasserman DH. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes 62: 572–580, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley EA, Eringa EC, Stehouwer CDA, Korstjens I, van Nieuw Amerongen GP, Musters R, Sipkema P, Clark MG, Rattigan S. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside in the muscle microcirculation increases nitric oxide synthesis and microvascular perfusion. Arterioscler Thromb Vasc Biol 30: 1137–1142, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 61: 888–896, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chai W, Wang W, Dong Z, Cao W, Liu Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes 60: 2939–2946, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai W, Wang W, Liu J, Barrett EJ, Carey RM, Cao W, Liu Z. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension 55: 523–530, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai W, Zhang X, Barrett EJ, Liu Z. Glucagon-like peptide 1 recruits muscle microvasculature and improves insulin's metabolic action in the presence of insulin resistance. Diabetes 63: 2788–2799, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung AW, Hsiang YN, Matzke LA, McManus BM, van Breemen C, Okon EB. Reduced expression of vascular endothelial growth factor paralleled with the increased angiostatin expression resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in human type 2 diabetic arterial vasculature. Circ Res 99: 140–148, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Dong Z, Chai W, Wang W, Zhao L, Fu Z, Cao W, Liu Z. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am J Physiol Endocrinol Metab 304: E222–E228, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erdogdu Ö, Nathanson D, Sjöholm Å, Nyström T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol 325: 26–35, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Gavin TP, Stallings HW, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA, Pofahl WE, Hickner RC. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol 98: 315–321, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, Law PY, Hebbel RP. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res 247: 495–504, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Hattori Y, Jojima T, Tomizawa A, Satoh H, Hattori S, Kasai K, Hayashi T. A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia 53: 2256–2263, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res 101: 948–956, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Hedman A, Berglund L, Essén-Gustavsson B, Reneland R, Lithell H. Relationships between muscle morphology and insulin sensitivity are improved after adjustment for intra-individual variability in 70-year-old men. Acta Physiol Scand 169: 125–132, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Herkner H, Klein N, Joukhadar C, Lackner E, Langenberger H, Frossard M, Bieglmayer C, Wagner O, Roden M, Müller M. Transcapillary insulin transfer in human skeletal muscle. Eur J Clin Invest 33: 141–146, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes 51: 159–167, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Kang HM, Kang Y, Chun HJ, Jeong JW, Park C. Evaluation of the in vitro and in vivo angiogenic effects of exendin-4. Biochem Biophys Res Commun 434: 150–154, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Koh KK. Effects of estrogen on the vascular wall: vasomotor function and inflammation. Cardiovasc Res 55: 714–726, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 55: 120–127, 2006. [PubMed] [Google Scholar]
- 27.Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Järvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80: 415–424, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab 96: 438–446, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab 94: 3543–3549, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen Yt, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation 110: 955–961, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, Sjöholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 287: E1209–E1215, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 7: 359–371, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res 96: 838–846, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Rattigan S, Wallis MG, Youd JM, Clark MG. Exercise training improves insulin-mediated capillary recruitment in association with glucose uptake in rat hindlimb. Diabetes 50: 2659–2665, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest 123: 2764–2772, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smits MM, Muskiet MH, Tonneijck L, Kramer MH, Diamant M, van Raalte DH, Serné EH. GLP-1 receptor agonist exenatide increases capillary perfusion independent of nitric oxide in healthy overweight men. Arterioscler Thromb Vasc Biol 35: 1538–1543, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Solomon TPJ, Haus JM, Li Y, Kirwan JP. Progressive hyperglycemia across the glucose tolerance continuum in older obese adults is related to skeletal muscle capillarization and nitric oxide bioavailability. J Clin Endocrinol Metab 96: 1377–1384, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sturis J, Gotfredsen CF, Rømer J, Rolin B, Ribel U, Brand CL, Wilken M, Wassermann K, Deacon CF, Carr RD, Knudsen LB. GLP-1 derivative liraglutide in rats with β-cell deficiencies: influence of metabolic state on β-cell mass dynamics. Br J Pharmacol 140: 123–132, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subaran SC, Sauder MA, Chai W, Jahn LA, Fowler DE, Aylor KW, Basu A, Liu Z. GLP-1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin Sci (Lond) 127: 163–170, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics 18: 63–69, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Thomas MM, Trajcevski KE, Coleman SK, Jiang M, Di Michele J, O'Neill HM, Lally JS, Steinberg GR, Hawke TJ. Early oxidative shifts in mouse skeletal muscle morphology with high−fat diet consumption do not lead to functional improvements. Physiol Rep 2: e12149, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285: E123–E129, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Wang AX, Liu Z, Barrett EJ. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes 57: 540–547, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest 84: 1620–1628, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao L, Chai W, Fu Z, Dong Z, Aylor KW, Barrett EJ, Cao W, Liu Z. Globular adiponectin enhances muscle insulin action via microvascular recruitment and increased insulin delivery. Circ Res 112: 1263–1271, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L, Fu Z, Wu J, Aylor KW, Barrett EJ, Cao W, Liu Z. Globular adiponectin ameliorates metabolic insulin resistance via AMPK-mediated restoration of microvascular insulin responses. J Physiol 593: 4067–4079, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao L, Fu Z, Wu J, Aylor KW, Barrett EJ, Cao W, Liu Z. Inflammation-induced microvascular insulin resistance is an early event in diet-induced obesity. Clin Sci (Lond) 129: 1025–1036, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther 317: 1106–1113, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP. AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. J Physiol 586: 6021–6035, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]