Abstract
This review summarizes the American Physiological Society (APS) Presidential Symposium 1 entitled “Physiological Processes Underlying Organ Injury in Alcohol Abuse” at the 2016 Experimental Biology meeting. The symposium was organized by Dr. Patricia Molina, past president of the APS, was held on April 3 at the Convention Center in San Diego, CA, and was funded by the National Institute on Alcohol Abuse and Alcoholism. The “Physiological Processes Underlying Organ Injury in Alcohol Abuse Symposium” assembled experts and leaders in the field and served as a platform to discuss and share knowledge on the latest developments and scientific advances on the mechanisms underlying organ injury in alcohol abuse. This symposium provided unique, interdisciplinary alcohol research, including several organs, liver, muscle, adipose, and brain, affected by excessive alcohol use.
Keywords: alcohol, circadian clock, muscle atrophy, adipose tissue, endocannabinoids
alcohol is a frequently abused substance that can permeate nearly all tissues in the body, producing distinct effects on different target sites resulting in considerable comorbid consequences. Common pathophysiological mechanisms of alcohol-induced tissue and organ injury are inflammation, increased oxidative stress, abnormal posttranslational modifications of proteins, impaired anabolic signaling, upregulation of catabolic processes, and dysregulation in lipid metabolism and signal transduction pathways (51, 55).
The liver is the primary site of alcohol metabolism, and liver cells are vulnerable to alcohol-induced injury. Although the majority of heavy drinkers will develop liver steatosis, only a minority will progress to alcoholic steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma (6). Several risk factors, such as genetic, metabolic, and environmental, will determine the progression of alcoholic liver disease. Among these factors are alterations in epigenetics (71), dietary lipids (33), protein trafficking (23), mechanisms of autophagy (45), and circadian rhythms (86) as a consequence of chronic and acute alcohol use.
Another consequence of alcohol use disorder is muscle wasting that is most characterized histologically by type II fiber atrophy as a consequence of an imbalance between protein synthesis and protein degradation machinery (63). Several researchers have shown that a decreased rate of protein synthesis seems to be the primary contributor to this condition compared with protein degradation (38, 39, 43, 56, 64).
Acute and chronic alcohol use also promotes an inflammatory milieu in adipose tissue (74), with the latter producing obvious alterations in adipocyte function, resulting in smaller adipocytes (1), adipose redistribution and dyslipidemia (28), and modified adipokine release (84). These alcohol-induced adipocyte dysfunctions suggest a role of alcohol use and the risk of metabolic syndrome.
Finally, alcohol affects neurotransmission within the brain, leading to an imbalance between excitatory and inhibitory synaptic inputs when acutely used (87) and neuroadaptative changes when consumed chronically (48). In addition, alcohol use also modifies the function of many neurotransmitters and modulators, as well as the endogenous opioid and endocannabinoid systems and nicotinic cholinergic transmission (47).
The purpose of this symposium was to promote significance and awareness of a broad range of different organ injuries induced by alcohol that highlight the physiological processes of organ damage. Specifically, it focuses on the alcohol effects in liver, skeletal muscle, adipose tissue, and brain. Shannon Bailey, PhD (University of Alabama) described the role of the hepatocyte circadian rhythm in chronic alcohol-mediated mitochondrial bioenergetic dysfunction in the liver. Charles Lang, PhD (Penn State University) described the mechanisms of alcohol-induced regulation of protein synthesis in cardiac and skeletal muscle. Laura Nagy, PhD (Lerner Research Institute, Cleveland Clinic, Cleveland, OH) described the adipose tissue as a target for excess alcohol consumption. And last, Loren Parsons, PhD (The Scripps Research Institute, La Jolla, CA) described the role of the endocannabinoid system in mediating the neural adaptations to alcohol.
Circadian Clocks and Alcohol-Induced Liver Disease
Alcoholic liver disease.
Heavy alcohol consumption underpins many chronic diseases, including liver and heart disease, cancer, and mental health problems. Alcoholic liver disease is a major cause of morbidity and mortality from heavy alcohol use and is the leading cause of death from chronic alcohol consumption (50). Over two million Americans are estimated to be afflicted with some degree of alcoholic liver disease (ALD), resulting in an average of 20,000 deaths each year (50). Regrettably, there are no effective treatments for any stage of ALD. These grim statistics illuminate the continuing need to improve the scientific and medical understanding of ALD so that more efficacious therapies and strategies can be developed for patients with this serious liver disease, and other related hepatic pathologies.
Chronic consumption of alcohol dramatically alters liver function at multiple levels (e.g., genomic, proteomic, and metabolomic) in all liver cell types. These changes result in a spectrum of liver pathologies ranging from the earliest initiator stage of steatosis (aka fatty liver) to the more serious conditions of alcoholic steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma. The vast majority of heavy alcohol drinkers develop steatosis, which is recognized by many to be the critical first “hit” in the initiation of ALD. Over the years, researchers in many labs have identified a large number of putative metabolic and molecular derangements in the early steatosis stage that contribute to ALD, including glycogen depletion, triglyceride and cholesterol accumulation, redox changes, mitochondrial and oxidative damage, and proteotoxicity, to name just a few. These molecular changes, and others, that occur during steatosis sensitize hepatocytes, the most abundant cells in the liver, to other toxic exposures (e.g., acetaminophen), metabolic stresses (e.g., hypoxia), and inflammatory stimuli [e.g., lipopolysaccharide (LPS)], leading to hepatocyte death.
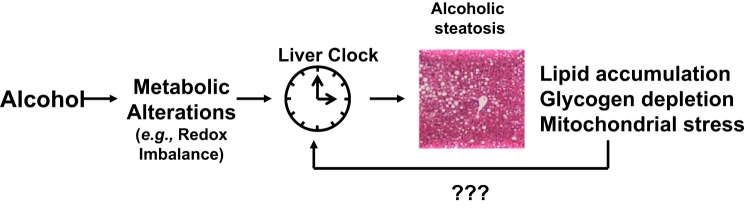
It has become increasing clear that an intrinsic cellular oscillator mechanism, also kmown as the molecular circadian clock, can significantly influence the responsiveness of peripheral tissues like the heart, skeletal muscle, liver, and kidney to toxic insults and stressors in a time-of-day dependent manner. For example, the liver circadian clock is believed to regulate certain aspects of xenobiotic metabolism and susceptibility to acetaminophen (15, 29). Despite this knowledge, there is little information available regarding whether the liver circadian clock is important in alcohol toxicity. Fortunately, though, with the growing interest in the circadian clock and disease pathogenesis, the molecular clock mechanism is now being investigated in the pathobiology of alcohol-induced tissue injuries. Several new studies have shown a role for clocks in alcohol-induced gut leakiness (19, 80–82) and alcoholic liver injury (18, 85, 96). This has led our laboratory and others to propose that alterations to the circadian clock contribute to the metabolic dysfunctions observed in the alcohol-exposed liver (Fig. 1). Before we highlight some of this new work, the following section will provide a brief overview of the molecular circadian clock mechanism.
Fig. 1.
Disruption in the liver circadian clock contributes to alcohol-induced metabolic alterations and liver injury. It is proposed that chronic alcohol-mediated alterations in some yet unknown metabolic process (e.g., change in the cellular redox state) negatively influences the activity of the circadian clock in the liver. This in turn disrupts circadian regulation of various metabolic pathways, leading to lipid accumulation, glycogen depletion, and a state of bioenergetic stress in the liver. The possibility exists that these alcohol-mediated alterations in key energy metabolism pathways feed back and further disrupt clock function in the liver.
Molecular circadian clock.
Many behavioral, physiological, and metabolic functions vary or oscillate over the course of the 24-hour day. With regard to liver metabolism, hepatic glucose and glycogen homeostasis, lipid and cholesterol metabolism, bile acid metabolism, and various aspects of drug biotransformation exhibit diurnal oscillations (83). Similarly, several studies have reported that a significant percentage of the liver transcriptome, proteome, and/or metabolome may change or oscillate throughout the day (17, 49, 66). And, while many metabolic rhythms largely align with external factors, like the sleep/wake and fasting/feeding cycles, it is believed that the molecular circadian clock functions to help coordinate metabolism in a time-of-day-dependent manner. For example, the circadian clock functions to help organisms, tissues, cells, and/or organelles synchronize and/or adapt various metabolic processes to changes occurring in their external environment over the course of the day (21). As such, synchronizing metabolism and maintaining proper physiology is believed to be a fundamental function of circadian clocks.
At the molecular level, the circadian clock is comprised of a core set of proteins that function as a transcriptional-translational feedback loop (58). On the positive arm of the feedback loop are the transcription factors brain and muscle aryl hydrocarbon nuclear translocator-like 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK). These two transcription factors bind to each other and form a BMAL1-CLOCK heterodimer that binds to E-box regions in the promoter of their target genes. Importantly, BMAL1-CLOCK activate the transcription of Period (Per1, 2, and 3) and Cryptochrome (Cry1 and 2) genes, which serve as the negative arm of the clock transcriptional-translational feedback loop. PER and CRY proteins translocate back into the nucleus where they repress their own transcription by interfering with BMAL1-CLOCK activity. Degradation of the proteins that make up the negative arm of the clock (i.e., CRY) by the proteasome is believed to be one mechanism that reactivates BMAL1-CLOCK transcriptional activity, thus maintaining the oscillatory function of the clock and its downstream targets (9, 73). In addition to the primary BMAL1/CLOCK/PER/CRY feedback loop, a secondary feedback loop involving the opposing actions of the nuclear receptors retinoid acid receptor-related orphan receptor (ROR)α, -β, and -γ and REV-ERBα and -β (aka NR1D1 and NR1D2, nuclear receptor subfamily 1, group D, member 1 or 2) controls the rhythmic expression of BMAL1 (24), which is essential for generation of circadian clock-controlled rhythms. Notably, ROR stimulates, whereas REV-ERB represses BMAL1 transcription. The BMAL1-CLOCK heterodimer also activates the transcription of many metabolic genes in a diurnal fashion. Various posttranslational modifications of clock proteins are also important for clock activity, as they provide another layer of regulation of temporal control to this internal oscillator system. These protein modifications can influence clock protein degradation and turnover, cellular localization, nuclear translocation, protein-protein interaction, and DNA binding activity. For example, ADP ribosylation of CLOCK influences the phase or the timing of the clock, and deacetylation of PER2 triggers its own degradation (3, 4). Thus, disruptions in the normal posttranslational modification program of clock proteins could have serious negative effects on the daily coordination of clock-controlled metabolic pathways.
The importance of the circadian clock in regulating and fine-tuning metabolism is exemplified by the fact that mouse models in which clocks are genetically altered (e.g., mutated or genetically ablated) throughout the entire body have significant and profound alterations in behavior, metabolism, and immune function, leading to significant health problems and tissue pathologies (93). Similarly, humans with disrupted circadian systems (e.g., long-term night and/or rotating shift workers) are at higher risk for many cardiovascular disease problems, such as hypertension and dyslipidemia, as well as obesity and diabetes (53). Therefore, the circadian clock is believed to be an important intrinsic cellular mechanism necessary for maintaining cellular and whole body health.
Alcohol and circadian clocks.
Within the alcohol field, the vast amount of research on clocks has largely focused on behavioral and neurobiological aspects of alcohol use. The circadian clock influences alcohol dependence and addictive behaviors (14, 59, 60). Associations also exist between alcohol consumption and clock disruption due to genetic or environmental factors. For example, genetic variants in clock genes are associated with increased alcohol consumption and dependence (20, 36). Long-term rotating shift work increases alcohol consumption (91). Thus, disrupting the clock can promote alcohol preference and consumption. But what about a possible role of circadian clocks in alcohol-induced end-organ diseases like ALD? And what effect does alcohol have on clock function and downstream clock-driven metabolic pathways in the liver? The remainder of this section will be spent summarizing initial results of studies that have begun to address these important questions in this exciting new area of alcohol research.
One of the first questions we asked when we began working in this new research area was: what impact does alcohol have on the circadian clock system in the liver? To address this, we fed a cohort of male C57BL/6J mice the Lieber-DeCarli alcohol and control liquid diets for a period of 5 wk (12:12-h light-dark cycle) to induce steatosis and disruptions in other energy metabolism pathways, including glycogen metabolism and mitochondrial respiration (18). Liver tissue was collected from mice at six different time points during the day (ZT 3, 7, 11, 15, 19, and 23; ZT-0 = lights on/beginning of the inactive/sleep phase; and ZT-12 = lights off/beginning of the active/awake phase of the day) to determine whether alcohol altered diurnal rhythms in clock gene expression. We found that alcohol consumption for 5 wk significantly altered the diurnal expression rhythms of 12 clock genes (18). We observed a significant decrease in the mean expression and amplitude of all clock genes measured in the livers of mice fed alcohol compared with rhythms measured in control-fed mice. Alcohol consumption also disrupted the phase or timing of the peak of clock gene expression in a subset of clock genes. Surprisingly, alcohol had no effect on clock gene expression in the central suprachiasmatic nucleus (SCN) clock, suggesting a liver-specific effect on the clock using this particular alcohol model. Importantly, these findings have been confirmed by others using similar approaches (96), thus strengthening the hypothesis that alcohol-induced disruption in liver clock function may contribute, in part, to metabolic disturbances in the alcohol consumer.
Conclusions and knowledge gaps.
With this new information in hand, the next steps in this important area of research will be the identification of specific metabolic and/or signaling pathways are “misaligned” in the alcohol-exposed liver, thus giving important clues as to which clock-regulated pathways might be implicated in the disease process. Initial work from our studies shows significant alcohol-mediated alterations in the diurnal rhythms of various energy metabolism pathways, including lipid, glycogen, and mitochondrial metabolism genes and proteins (18, 85). For example, alcohol significantly disrupts the tightly controlled diurnal rhythm in hepatic glycogen and rhythms in several key regulatory glycogen metabolism genes and proteins (85). This finding is significant, as glycogen depletion has been reported to underpin hepatocyte death in models of ALD (13). Similarly, Zhou et al. (96) show significant alterations in lipid and bile acid metabolism genes in livers of alcohol-fed mice. Finally, Keshavarzian and colleagues have shown that alcohol-mediated alterations in intestinal cell clocks may participate in the phenomenon of gut leakiness from alcohol consumption (19, 80, 81). Importantly, night shift workers have increased alcohol-induced intestinal permeability (82), suggesting that circadian misalignment may place individuals at increased risk to alcohol hepatotoxicity. Collectively, these recent findings provide support for a role of the circadian clock in alcohol-induced alterations in metabolism and tissue injury. However, this area of alcohol research is still in its infancy, and much more work needs to be done to better define the role of clocks in alcohol hepatotoxicity. A partial list of key research areas that should be investigated to move the field forward is provided in Table 1. Finally, as highlighted in this thematic article, alcohol impacts the whole body; thus, ALD is not just a liver disease but a pathology involving multiple organ systems and whole body physiological disturbances. Thus, the impact alcohol has on clock function in multiple organ systems (gut, brain, adipose, skeletal muscle) should also be strongly considered in when investigating the pathobiology of ALD.
Table 1.
Knowledge gaps related to alcohol, circadian clocks, and alcoholic liver disease
| Alcohol, Circadian Clocks, and Alcoholic Liver Disease |
|---|
|
|
|
|
In summary, several laboratories have shown that alcohol alters the circadian clock in peripheral organs. Moving forward, our mission will be to understand how disruption in this essential and fundamental internal timing system participates in the initiation and progression of liver disease in the alcohol consumer. Improved understanding of interconnected nature of circadian clocks and metabolism will most likely open new avenues and perspectives for treatment of alcoholic liver disease and other alcohol-related pathologies and disorders.
Regulation of Protein Synthesis in Skeletal Muscle by Alcohol
Although the devastating effects of acute and chronic alcohol abuse are particularly well recognized in reference to liver, pancreas, and brain, alcohol also adversely perturbs the structure and function of striated muscle, both skeletal and cardiac. Such changes, over time, can negatively impact both morbidity and mortality in this population. To better focus attention on the cellular mechanisms responsible for alcohol-induced changes in protein balance, this section highlights recent advances specifically related to changes in protein synthetic pathways in skeletal muscle. Those interested in a detailed history of this research field are directed toward earlier comprehensive reviews (63).
Alcohol-induced changes in basal protein synthesis.
Underlying morphometric changes in this disease include a reduction in muscle fiber size, with little or no change in fiber number, with the atrophic response largely restricted to muscles with a predominance of type II fast-twitch fibers (63, 79). There is a general consensus that lean body mass is reduced in rodent models where all groups consume an isocaloric and isonitrogenous diet and which permit stringent control of nutritional variables. Consistent with the proximal weakness seen in humans with alcoholic myopathy, the mass of the gastrocnemius, psoas, and quadriceps, but not soleus, is also decreased. Although we currently lack an explanation, it also appears that the effect of alcohol on skeletal muscle may take several months to manifest, whereas alcohol-induced changes in liver pathology are routinely observed at early time points (e.g., several weeks).
The global rate of protein synthesis, determined in vivo, is reduced 25–35% in gastrocnemius in response to both acute alcohol intoxication (1–4 h) and chronic ingestion (12–24 wk) (42, 77). Furthermore, a comparable alcohol-induced decrease in protein synthesis is detected in both the myofibrillar and sarcoplasmic fractions. No such alcohol-induced changes are routinely observed in the slow-twitch soleus muscle. As will be expanded upon later, the decrease in muscle protein synthesis occurs without a concomitant reduction in the number of ribosomes, suggesting that alcohol impairs translational efficiency. It is also noteworthy that this decrease in muscle protein synthesis is partially reversible when alcohol consumption is eliminated (89), a situation that differs from that seen in other tissues (e.g., ALD).
It would appear that much of alcohol's effect is directly targeted toward muscle and does not require the oxidation of ethanol by the liver, which generates potentially toxic metabolites or the generation of some unidentified secondary mediator from other peripheral organs. For example, alcohol decreases muscle protein synthesis when added to the isolated perfused hindlimb preparation, to the isolated incubated epitrochlearis, or to cultured myocytes (27, 41). Furthermore, at least under acute conditions, inhibition of alcohol dehydrogenase with 4-methylpyrazole does not prevent the alcohol-induced decrease in muscle protein synthesis. However, recent evidence suggests that the central nervous system, possibly via activation of the sympathetic nervous system, may have the ability to inhibit muscle protein synthesis (65).
Of the many hormones and metabolic substrates quantified, one of the most consistent findings is the reduction in the plasma concentration and muscle mRNA content for insulin-like growth factor (IGF)-I in alcohol-fed rodents (38). The underlying mechanism for this decrease is in part due to the development of resistance to the ability of growth hormone to stimulate IGF-I synthesis by both liver and skeletal muscle via activation of the JAK/STAT pathway (40). These data on plasma IGF-I contrast with the lack of change observed for the plasma concentrations for insulin, corticosterone, and testosterone, all of which can potentially influence muscle protein synthesis under certain conditions. Similarly, the alcohol-induced reduction in protein synthesis is independent of the total concentration of amino acids in the blood as well as the prevailing level of leucine and other branched-chain amino acids.
The consensus from several independent laboratories provides compelling evidence that both acute intoxication and chronic alcohol consumption decrease muscle protein synthesis via an mTOR (mammalian target of rapamycin)-dependent mechanism (Fig. 2) (41, 42). This is evidenced by an alcohol-induced decrease in the phosphorylation of native downstream substrates of mTOR, ribosomal S6 kinase-1 (S6K1) and eukaryotic initiation factor 4E-binding protein-1 (4E-BP1). The functional significance of the decrease in S6K1 activity in these conditions is evidenced by the reduced phosphorylation of the ribosomal protein S6, eIF4G, and several other substrates. Moreover, the functional significance of the alcohol-induced decrease in 4E-BP1 phosphorylation is illustrated by the enhanced binding of eIF4E with 4E-BP1 and decreased binding of eIF4E with eIF4G, the latter of which decreases the formation of the active eIF4F complex and cap-dependent translation.
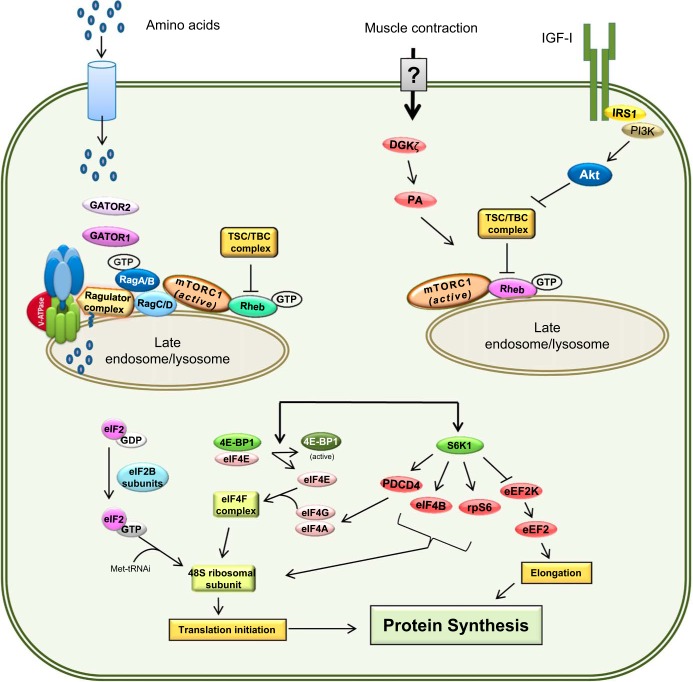
Fig. 2.
Pathways modulating skeletal muscle protein balance. Signals from various anabolic stimuli enter the cell via membrane transporters (i.e., amino acids) or receptors (i.e., IGF-I). Amino acids induce mTOR translocation and activation at the lysosomal surface via the Rag family of proteins and the GTP loading of Rheb. In contrast, signal transduction of IGF-I and insulin binding to their cognate receptors converge at Akt to either activate mTOR at the lysosome via TSC1· TBC complex phosphorylation and subsequent Rheb-GTP loading. Upon lysosomal translocation and activation, mTOR phosphorylates several downstream targets, including 4E-BP1 and S6K1. Phosphorylation of 4E-BP1 and S6K1 enhances translation initiation, while other factors may stimulate protein elongation.
mTOR is partitioned between two active complexes, mTOR complex 1 and 2 (mTORC1 and mTORC2, respectively), both of which consist of multiple core and associated regulatory proteins (79). While mTORC2 is traditionally considered to regulate cytoskeletal organization (but can also have metabolic effects), mTORC1 is generally recognized to govern translational control of protein synthesis. In this regard, neither acute nor chronic alcohol consistently alters the total cellular content of various mTORC1 proteins, including mTOR per se, Raptor, Deptor, PRAS40, or GβL (mLST8) (42). In contrast, when the scaffolding protein Raptor is immunoprecipitated, the extent of the molecular interaction with Deptor is enhanced in muscle from alcohol-fed rats. As Deptor is a known negative regulator of mTOR activity, this alcohol-induced increase in Raptor·Deptor binding is consistent with the observed reduction in protein synthesis. Furthermore, lentiviral shRNA knockdown of Deptor attenuates the alcohol-induced decrease in basal protein synthesis in vitro in C2C12 myocytes and in vivo when electroporated directly into skeletal muscle (32).
Alcohol produces anabolic resistance.
Over the past decade, it has become increasingly evident that alcohol suppresses both basal protein synthesis and the ability of multiple nutrients and hormones that typically have a strong anabolic effect. This phenomenon is referred to as “anabolic resistance” and is equivalent to the insulin resistance observed in diabetic patients. For example, exogenously administered IGF-I, complexed with IGF-binding protein-3 (IGFBP3) to delay the clearance of the peptide hormone from the circulation, to alcohol-fed rats while increasing muscle protein synthesis, fails to fully restore synthetic rates to those seen in muscle from control-fed animals treated with IGF-I (38). Such IGF-I resistance is also seen after acute alcohol intoxication (41), and using this animal model shown to be both alcohol dose and time dependent.
Similarly, alcohol also prevents the ability of the branched-chain amino acid leucine to stimulate mTORC1-mediated increases in muscle protein synthesis (37). In this regard, most in vivo studies on nutrient resistance have been performed using leucine as the anabolic stimulus and models of acute alcohol intoxication. For example, oral gavage of a maximally stimulating dose of leucine increases global muscle protein synthesis 20–25% in the postabsorptive state in control animals, a response that is associated with a concomitant elevation in both S6K1 and 4E-BP1 (37). In contrast, acute alcohol completely blunts this mTOR-dependent increase in muscle protein synthesis. This alcohol-induced leucine resistance cannot be attributed to differences in the prevailing plasma concentration of either leucine or insulin, the latter of which is increased due to leucine-stimulated pancreatic insulin secretion. When S6K1 phosphorylation is used as a proxy for mTORC1 activity, studies have shown this alcohol effect is both dose and time dependent (41), with suppression occurring at blood alcohol levels that occur routinely in humans after consuming two to three standard drinks and persists for at least 8 h after alcohol ingestion. Furthermore, this alcohol-induced leucine resistance is observed in both male and female rats, under both fed and fasted conditions, and occurs regardless of whether alcohol is administered either intraperitoneally or via oral gavage.
Much work has been done on the signaling pathway by which leucine stimulates mTORC1, which clearly implicates the central regulatory role of the Rag (Ras-related GTPases) proteins that are necessary for recruitment of mTORC1 to the lysosomal surface where it interacts with and is activated by Rheb (67). In this regard, there is no difference in the RagA and RagC content of whole muscle homogenates from alcohol- and control-fed animals. However, the extent of binding of RagA to immunoprecipitated Raptor was dramatically reduced by chronic alcohol consumption, suggesting a possible mechanism for the alcohol-induced leucine resistance. Such a role is further supported by data demonstrating that the overexpression of constitutively active RagA/RagC in C2C12 myocytes is capable of partially ameliorating alcohol-induced leucine resistance under in vitro conditions (27).
Finally, muscle contraction and exercise can increase protein synthesis and cause hypertrophy via activation of mTORC1, but likely via a mechanism differing from that used by amino acids (92). Steiner et al. (77) showed that acute alcohol intoxication prior to electrical stimulation of the sciatic nerve essentially blocked the contraction-induced increase in muscle protein synthesis for at least 12 h after alcohol administration and that the suppression was associated with impaired mTORC1 activity. Similarly, acute alcohol intoxication was also capable of reversing the increase in muscle protein synthesis when alcohol was given after muscle stimulation (78). In contrast, chronic alcohol feeding did not impair protein synthesis induced by electrical stimulation or in response to overload-induced muscle hypertrophy (e.g., synergistic muscle ablation) (76). The importance of this difference remains to be determined, but it represents a discordant effect of acute and chronic alcohol abuse which is not seen in response to either hormones (e.g., IGF-I) or nutrients (e.g., leucine).
Alcohol exaggerates catabolic stimuli.
In addition to alcohol minimizing increases in muscle protein synthesis in response to anabolic stimuli, alcohol may also exaggerate catabolic stimuli that decrease muscle protein synthesis. In this regard, two examples will suffice. First, muscle disuse produces atrophy that is mediated by an mTORC1-dependent decrease in protein synthesis. When rats are administered alcohol during a 3-day period of hindlimb immobilization (e.g., casting), the loss of muscle and the decrease in protein synthesis is larger than results from either alcohol or disuse alone (88). In addition, alcohol consumption during the reloading period (e.g., after cast removal) also slows muscle regrowth. An exaggerated alcohol-induced decrement in muscle protein synthesis is also observed in aged (22 mo) compared with adult (8 mo) rats (35).
Conclusions and knowledge gaps.
The protein synthetic response in muscle typically demonstrates a daily oscillatory pattern, increasing after meals and exercise while decreasing during times of fasting and rest. The consensus of the literature indicates that acute alcohol intoxication or long-term consumption of alcohol 1) decreases basal muscle protein synthesis; 2) attenuates or completely inhibits the increase in muscle protein synthesis produced by hormones, nutrients and muscle contraction; and 3) amplifies the decrease in muscle protein synthesis produced by catabolic stimuli. These changes are largely the result of alcohol's ability to alter translational efficiency via modulating mTORC1; but, as mTORC1 serves as a hub for integrating multiple anabolic and catabolic signals, alcohol may indeed cause multiple defects in this signal transduction pathway.
Although much has been learned over the past several years pertaining to the physiological response of alcohol on muscle protein synthesis, considerable gaps in our knowledge exist (Table 2). First, and arguably most important, there is a paucity of literature on the effect of alcohol, either acute or chronic, on muscle protein synthesis in humans. Hence, research needs to be supported that examines synthesis and mTOR signaling (and possibly other pathways) in humans and assesses whether there are sexually dimorphic differences. While the effect of acute alcohol intoxication on basal protein synthesis should be relatively straightforward, eventually studies will need to be performed on patients with a long-term history of excessive alcohol intake. Second, a more complete understanding is needed of why alcoholic myopathy is characterized by the atrophy of predominantly fast-twitch vs. slow-twitch muscles. This will require greater insight into how these muscle types differ with respect to energy production/utilization, oxidative defense mechanisms, and blood flow regulation, to name just a few. On a related issue, although skeletal and cardiac muscles are both striated, the mechanism(s) whereby alcohol impairs protein balance in these two types of muscle should be fully defined. Third, there are numerous recent advances related to the cellular mechanisms mediating nutrient and hormone stimulation of protein synthesis that need to be examined under in vivo conditions to determine their applicability to the atrophic response produced by alcohol. Fourth, currently there are few or no studies using state-of-the-art genomic and proteomic approaches in muscle from alcohol-treated myotubes or animals. Such approaches should reveal new candidate molecules and pathways that are altered by alcohol but are currently unknown or underappreciated. Moreover, these methods can be leveraged to determine whether there are specific proteins or classes of proteins whose rates of synthesis are selectively altered by alcohol. Fifth, alcohol can directly inhibit the activity of selected enzymes. However, whether ethanol and other alcohols can directly impair the intrinsic kinase activity of mTOR under in vitro conditions has not been investigated. Sixth, while the focus of this review has been directed toward the synthetic side of the protein balance equation, there remains considerable controversy as to whether alcohol alters protein degradation in muscle, not only under basal conditions but also in response to various anabolic and catabolic conditions that might occur simultaneously with alcohol consumption. Moreover, as with protein synthesis, initial studies will need to determine whether global rates of protein breakdown are increased and by which pathway, but also whether there are specific proteins in muscle that undergo enhanced rates of proteolysis. Finally, there are essentially no studies that systematically investigate the effect of alcohol on contractile function in skeletal muscle and the causal relationship, if any, to changes in muscle protein balance.
Table 2.
Knowledge gaps related to alcohol and muscle protein balance
| Alcohol and Muscle Protein Balance |
|---|
|
|
|
|
|
|
|
|
In conclusion, estimates vary on the prevalence of alcohol-induced skeletal muscle myopathy, depending on the criteria used for identification, but range from ∼45 to 70% of individuals categorized with long-term alcohol use disorder. As a result, more than five million men and women in the US may be afflicted, making alcoholic myopathy the most frequently occurring form of muscle disease. As the average age of the US population continues to increase, extending the period of alcohol abuse, the frequency of skeletal muscle myopathy will undoubtedly rise, thereby further complicating health care delivery. Hence, a greater appreciation for the underlying mechanisms driving alcoholic myopathy in otherwise healthy individuals and those with existing comorbidities should remain a pillar of the mission of the National Institute on Alcohol Abuse and Alcoholism.
Adipose Tissue as a Target of Excess Alcohol Consumption
Alcohol abuse is a leading cause of morbidity and mortality worldwide, and recent data indicate that ALD affects over 10 million Americans. Epidemiological studies suggest that alcohol consumption also modulates the risk for the development of type 2 diabetes, the most common metabolic disease among older North Americans, with light alcohol consumption decreasing risk and chronic heavy alcohol consumption increasing risk in a J-shaped curve. Chronic, heavy ethanol exposure may result in the development of glucose intolerance and insulin resistance (75).
While the role of adipose tissue in the regulation of energy stores has long been appreciated, it is now clear there is a critical role of adipose tissue in regulating metabolic homeostasis, including the ability to modulate insulin sensitivity in skeletal muscle and liver, contribute to the regulation of inflammatory responses, and regulate appetitive behaviors. Importantly, there is also a growing appreciation that adipose tissue is a specific target of ethanol action and that change in adipose tissue function and metabolism can impact the progression of alcohol-induced liver injury. This section highlights recent advances in our understanding of the mechanisms by which excessive alcohol consumption impacts the metabolic and homeostatic functions of adipose tissue. For more detailed discussion of the impact of ethanol on metabolic pathways, the reader is referred to more comprehensive reviews (75).
Ethanol-induced changes to insulin signaling, glucose homeostasis, and lipid metabolism.
Chronic ethanol feeding to rats results in the development of insulin resistance and decreased glucose uptake into adipose tissue (31). Decreased glucose uptake into adipose tissue after chronic ethanol feeding is associated with impaired insulin receptor-mediated signaling, as well as disruption in the trafficking of the GLUT4-containing vesicles to the plasma membrane (61). Chronic ethanol also reduces the antilipolytic actions of insulin, resulting in an increase in lipolysis (30). Chronic ethanol feeding disrupts cAMP and fibroblast growth factor (FGF)-21 signaling in adipose, which also contributes to abnormal regulation of lipid metabolism (95). These changes in cAMP and insulin signaling likely contribute to an increased availability of free fatty acids to the liver and may serve as substrates for triglyceride synthesis during the development of ethanol-induced hepatic steatosis.
Inflammatory changes in adipose tissue in response to chronic ethanol feeding.
Chronic ethanol feeding to both rats and mice increases the number of macrophages in adipose tissue as well as an increase in the expression of inflammatory cytokines and chemokines (31, 68). This upregulation is reminiscent of the increase in the inflammatory milieu in adipose tissue in models of high-fat diet-induced and genetically induced obesity in rodents. In models of obesity, it is postulated that, as adipocyte size increases due to the accumulation of lipids, adipocytes are increasingly prone to injury and cell death. Macrophages are then recruited to adipose tissue to facilitate the clearance of cellular debris (22). This response results in a chronic, low-grade inflammatory state, termed metaflammation, that is thought to contribute to insulin resistance and metabolic dysfunction associated with obesity (22).
Ethanol metabolism in adipose tissue.
While the inflammatory state of adipose tissue after chronic ethanol consumption is consistent with the metaflammation characteristic of obesity, the development of ethanol-induced inflammation in adipose is not associated with an increase in the size of adipocytes. Therefore, there are likely ethanol-specific mechanisms for increased inflammation in adipose tissue that are independent of adipocyte size.
It is well known that ethanol metabolism in the liver results in oxidative stress, which in turn can contribute to hepatocellular death. While hepatocytes are considered the major site of ethanol metabolism, adipose tissue also expresses alcohol dehydrogenases (94), as well as CYP2E1 (cytochrome P-450 2E1) (68). CYP2E1 expression is predominantly expressed in adipocytes and not stromal vascular cells, and expression is increased after chronic ethanol feeding (68). CYP2E1 expression in response to ethanol is associated with the development of oxidative stress in adipose tissue as well as increases in adipocyte apoptosis and impaired secretion of adiponectin, an important adipokine that has anti-inflammatory and antifibrotic effects (68, 84).
Relation among CYP2E1, adipocyte apoptosis, and inflammation during chronic ethanol exposure.
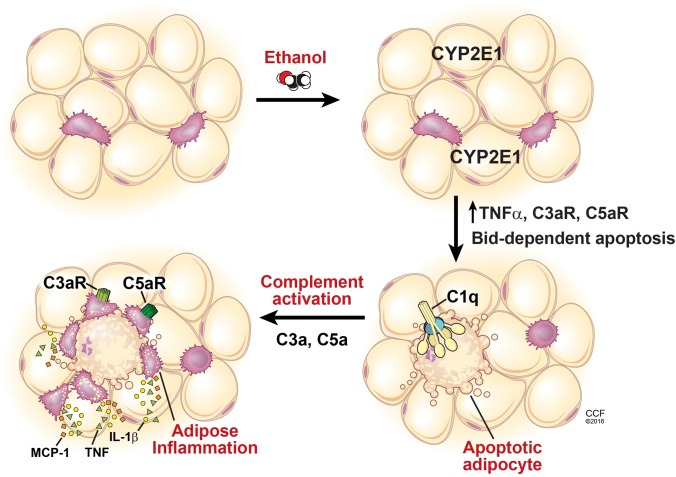
Chronic ethanol feeding to mice increases adipocyte apoptosis as well as inflammation in adipose tissue; these responses are ameliorated in CYP2E1−/− mice, indicating a causal contribution of ethanol metabolism via CYP2E1 in adipose tissue inflammation (68). Importantly, adipocyte apoptosis and adipose inflammation are also prevented in Bid−/− mice, confirming a critical role for apoptosis in the development of adipose tissue inflammation (68) (Fig. 3).
Fig. 3.
Schematic diagram illustrating the role of CYP2E1, Bid-dependent apoptosis, and complement activation in the development of adipose tissue inflammation during chronic ethanol feeding to mice. Chronic ethanol feeding induces the expression of CYP2E1 in adipocytes. CYP2E1 then leads to increased expression of TNFα, which in turn activates BID-dependent apoptosis of adipocytes. C1q, a component of the classical pathway of complement, binds to cell surface markers on apoptotic adipocytes, resulting in complement activation. Complement activation products, interacting with the anaphylatoxin receptors C3a receptor and C5a receptor, further increase cytokine and chemokine production in adipose tissue. The cumulative result of the CYP2E1 Bid-dependent apoptosis C1q-mediated complement activation pathway is the development of adipose tissue inflammation.
Complement activation and ethanol-induced adipose inflammation.
Complement is an important element of the innate immune system and is involved in the clearance of apoptotic bodies via activation of C1q in the classical pathway of complement activation. Making use of C1q−/− mice, a critical role for complement in ethanol-induced adipose inflammation was identified (68). In wild-type mice, chronic ethanol feeding activates complement signaling, as evidenced by the accumulation of C3 cleavage products in adipose tissue. However, when C1q−/− mice are exposed to chronic ethanol, adipocytes still undergo apoptosis, but complement is not activated and inflammation is not observed. These data indicate that complement activation provides a critical link between ethanol-induced adipocyte apoptosis and the initiation of the inflammatory response (68).
Additional pathways for activation of inflammatory responses in adipose tissue are also likely. For example, recent studies have identified a role for LPS, leaking into adipose tissue via the lymphatic system, as a likely pathway toward inflammation in mesenteric adipose tissue (74).
Conclusions and knowledge gaps.
Adipose tissue plays diverse roles in the regulation of metabolism and innate immunity. Chronic ethanol feeding to rats and mice disrupts many of these important regulatory functions, contributing to impaired glucose and lipid homeostasis. Recent data have identified a critical role for CYP2E1 in mediating adipocyte injury in response to heavy ethanol exposure, leading to the recruitment of the complement system and activation of inflammatory responses (Fig. 3). While these insights are exciting, there is much that is still not understood related to the interactions between ethanol and adipose tissue (Table 3). It is critical to keep in mind that there are multiple depots of adipose tissue that can have important localized influence in a number of organs from the liver, to bone, and reproductive organs. At this time, studies related to ethanol have been limited to gonadal, subcutaneous, and mesenteric depots; therefore, further work is necessary. Most importantly, there is little information about the impact of alcohol on adipose tissue in humans; it will be necessary to begin to investigate the impact of both moderate and heavy alcohol consumption on adipose tissue. Changes in the function of adipose tissue could influence not only glucose and lipid metabolism in humans but also appetitive behaviors via alterations in adipokine release and signaling.
Table 3.
Knowledge gaps related to alcohol and adipose tissue
| Alcohol and AdiposeTissue |
|---|
|
|
|
|
|
|
Role of Endocannabinoid System in Mediating Neural Adaptations to Alcohol
Growing evidence points to a prominent influence of the endocannabinoid system (ECS) in the mediation and modulation of alcohol reward and addiction. Endocannabinoids (ECBs) are neuroactive lipids that participate in a range of physiological processes, including reward, motivation, emotional homeostasis, neuroprotection, and synaptic plasticity, contributing to learning and memory (44). At present, the best-characterized ECB ligands are N-arachidonylethanolamide (anandamide, AEA) and 2-arachidonoylglycerol (2-AG), each of which exerts agonist activity at CB1 and CB2 receptors (CB1R and CB2R, respectively). CB1Rs are the most abundant G protein-coupled receptors expressed in the adult brain, with dense expression in each of the interconnected structures involved in reward, where they exert widespread modulatory influences on excitatory and inhibitory signaling in a manner that influences reward processing (72). Due to their lipid nature, AEA and 2-AG are not stored in vesicles, they are primarily synthesized on an “on demand” basis, and their signaling is tightly regulated by enzymatic hydrolysis that provides for their rapid clearance. AEA is primarily catabolized through fatty acid amide hydrolase-1 (FAAH1), and 2-AG is catabolized through monoacylglycerol lipase (MAGL) and to a lesser extent, α,β-hydrolase-6 (ABHD6), cyclooxygenase 2 (COX2), and FAAH1.
Modulation of CB1R signaling potently influences alcohol consumption.
Much of the evidence implicating an ECB role in the motivation for alcohol is based on the influence of CB1R signaling on alcohol consumption. In general, CB1Rs exert a facilitory influence on the rewarding effects of alcohol. Rodent studies employing the conditioned place preference and operant self-administration paradigms demonstrate that CB1 agonists increase the motivational and rewarding effects of alcohol, while diminished CB1R signaling (either genetic receptor deletion or pharmacological inhibition) attenuates these effects of alcohol (54). The CB1 influence on alcohol reward may be mediated in part though modulation of alcohol-induced increases in mesolimbic dopamine signaling, as CB1R antagonism blocks alcohol-induced excitation of ventral tegmental area (VTA) dopamine (DA) cells and associated increases in nucleus accumbens (NAc) DA release. Moreover, alcohol self-administration is reduced by following region-specific CB1R antagonism in the VTA and NAc (2, 10). However, while the rewarding effects of some drugs such as nicotine are critically dependent on the mesolimbic DA system, the motivational and rewarding effects of alcohol are less DA dependent (34), and as such the CB1R modulation of alcohol reward may involving nondopaminergic mechanisms.
Evidence of alcohol-induced alterations in brain ECB formation.
The inhibitory influence of CB1R antagonism on alcohol reward has led to the hypothesis that brain ECB formation is increased by alcohol consumption and that long-term alcohol exposure may induce aberrant ECB signaling. Early studies demonstrated that chronic alcohol exposure increases both AEA and 2-AG formation in human neuroblastoma cells and primary cultures of rodent neurons (69). Subsequent studies evaluated alterations in ECB levels extracted from post mortem brain tissue following acute and chronic alcohol exposure, and while the results clearly demonstrate alcohol-induced alterations in ECB levels in relevant brain regions [such as the NAc, amygdala, and prefrontal cortex (PFC)], substantial inconsistencies among studies make it difficult to draw clear conclusions on the direction of change and regional nature of the effects. This may result from study-specific differences in rat strain, sex, amount of daily alcohol consumption, duration of alcohol exposure, and post-exposure time that was evaluated. Moreover, quantification of tissue ECB content is highly sensitive to the procedures employed for tissue preparation and ECB extraction and analysis (7), and these and other methodological differences likely underlie the many discrepant literature reports on alcohol-induced alterations in brain ECB levels. The effects of acute and chronic alcohol have also been studied using in vivo microdialysis procedures. Voluntary alcohol self-administration robustly increases NAc dialysate 2-AG levels without altering AEA levels, with the rise and fall of dialysate 2-AG content aligning with the pattern of blood alcohol concentrations typically observed following oral consumption (10). Interestingly, forced administration of moderate alcohol doses produces a mild increase in dialysate 2-AG levels while reducing AEA levels in the NAc of alcohol-naïve rats, although higher doses induce a mild increase in dialysate AEA levels (54). Compared with voluntary alcohol self-administration, these findings suggest that the volitional nature of alcohol exposure (e.g., voluntary vs. forced administration) may differentially impact ECB responses. Available evidence also demonstrates that alcohol induces region-specific alterations in brain ECBs, as significant alcohol-induced disruptions in ECB levels are consistently observed in striatal regions while alcohol-induced disruptions in frontal cortical ECB levels are not evident (54).
Chronic alcohol exposure dysregulates brain ECB function.
Neuroadaptations resulting from long-term drug exposure are a major contributing factor to the etiology of drug dependence and addiction, and several observations suggest that ECB signaling is dysregulated following chronic alcohol exposure. For example, chronic alcohol exposure downregulates CB1R expression and function in rodent brain, and several post mortem studies of human brain tissue have demonstrated disrupted CB1R expression in the PFC and ventral striatum of alcohol-dependent individuals (90). In vivo imaging studies have reported decreased CB1R availability in heavy-drinking alcoholics that persist for at least one month of abstinence (11). Although these findings in humans may be related to variants in the genes encoding cannabinoid receptors (see the section on genetic variants below), a common interpretation is that these CB receptor adaptations result in part from prolonged alcohol-induced increases in brain ECB levels, similar to CB receptor downregulation that occurs following long-term exposure to synthetic CB receptor agonists. This interpretation is further supported by evidence of a transient recovery (and perhaps upregulation) of CB1R function during protracted alcohol abstinence (11). Chronic alcohol exposure also disrupts gene expression for the primary ECB clearance enzymes FAAH and MAGL in a manner that is sensitive to the intermittent nature of alcohol exposure and post-alcohol abstinence period (70). Collectively, there is substantial biochemical evidence that long-term alcohol exposure disrupts ECB processing and signaling mechanisms.
Chronic alcohol exposure impairs ECB-mediated synaptic plasticity in brain.
It is well established that ECBs serve as retrograde messengers at neuronal synapses in the central nervous system and contribute to different forms of short- and long-term synaptic plasticity, and growing evidence indicates that acute and repeated alcohol exposure disrupts ECB-mediated synaptic plasticity (57, 72). Short periods of low-frequency stimulation produce a CB1R-dependent long-lasting disinhibition (DLL) of striatal output neurons as a result of reduced synaptic strength at inhibitory synapses. Both acute in vitro alcohol exposure and chronic voluntary alcohol consumption persistently reduce DLL in the dorsolateral striatum. ECB-mediated long-term depression (LTD) at inhibitory striatal synapses is also reduced by acute alcohol, although no significant alcohol effects are evident on LTD of excitatory synapses. Because the dorsal striatum is involved in reward-guided learning and habitual behavior, it is possible that alcohol-induced interference in ECB-LTD contributes to maladaptive habitual behavior associated with addiction. Indeed, recent work demonstrates that CB1R-dependent long-term depression is absent in the dorsolateral striatum of mice following chronic intermittent alcohol exposure (16). This disruption in ECB-mediated plasticity is associated with enhanced neuronal encoding of striatal-based behaviors, which may drive aberrant reward learning and modulation of rewarded behavior in a manner that contributes to the progression of alcoholism.
ECB involvement in craving and drug-seeking (relapse).
Drug exposure produces powerful interoreceptive effects, and memory of these effects increases the likelihood of continued drug use. Over time, these interoreceptive effects become associated with environmental cues to the extent where these drug-associated cues alone can induce craving and thereby propel drug use. Recently developed animal models of drug-seeking demonstrate a robust influence of cannabinoid signaling in the reinstatement of extinguished drug-seeking behaviors, implicating the ECS as a potential therapeutic target for reducing relapse and prolonging abstinence (69). In this regard, detoxified alcoholics present significantly lower baseline plasma AEA levels compared with nondependent social drinkers (46). Furthermore, a significant negative relationship between resting plasma AEA levels and the severity of cue-induced craving was evident in social drinkers but not alcoholics. In social drinkers, alcohol-related cues increased both craving and plasma AEA levels, and the relative magnitude of cue-induced increases in AEA was correlated with the severity of craving. Interestingly, although alcohol-related cues elicited more intense craving in alcoholics than in social drinkers, alcoholics did not present significant cue-induced increases in plasma AEA. These findings suggest that deficits in plasma AEA levels may confer increased susceptibility to alcohol craving, allowing the hypothesis that cue-induced increases in plasma AEA reflect an adaptive response that actually moderates the desire for alcohol in social drinkers, and its absence in alcoholics somehow contributes to excessive craving responses to alcohol-related cues. Further evaluations in humans are clearly needed to investigate these issues.
ECB influence on stress responsivity and affective state.
It is well established that ECB signaling serves a homeostatic role in the constraint of behavioral and physiological responses to stress, including stress-induced hypothalamic-pituitary-adrenal (HPA) axis activation (52). It is thus conceivable that disrupted ECB-mediated plasticity induced by long-term drug exposure contributes to a persistent dysregulation of HPA axis function and increased stress sensitivity associated with addiction. Additionally, because stress exposure is implicated in the development of addiction, it is notable that stress can alter ECB-mediated plasticity in addiction-related brain regions. For example, prolonged stress exposure impairs ECB-mediated DSI (depolarization-induced suppression of inhibition), LTD (long-term depression), and fEPSPs (field excitatory postsynaptic potential) in the NAc, attenuates ECB-mediated DSE and DSI in the paraventicular nucleus (PVN), and enhances 2-AG-mediated DSI in the basolateral amygdala (69, 72). Similarly, establishment of conditioned fear increases the efficacy of DSE (depolarization-induced suppression of excitation) and DSI in the central nucleus of the amygdala. Posttraumatic stress disorder (PTSD) is an anxiety disorder with particularly high prevalence among individuals with alcohol use disorders (AUD). The fear-potentiated startle (FPS) paradigm is often used to model PTSD-like behaviors, and rodent lines selectively bred for high alcohol consumption exhibit significantly greater FPS than corollary lines bred for low alcohol consumption (5), and these rodent lines may thus provide models for characterizing mechanisms contributing to anxiety, AUD, and the intersection of these pathologies. In this regard, pharmacological inhibition of FAAH activity selectively reduces FPS in high-alcohol-preferring, but not low-alcohol-preferring mice (62). However, these effects are evident only following repeated FPS testing and not upon first exposure to the FPS paradigm, suggesting that FAAH inhibition facilitates the extinction of learned fear responses in high-alcohol-preferring mice, consistent with substantial evidence that FAAH inhibition generally accelerates extinction of aversive memories (25). FAAH inhibition also enhances the expression of alcohol-induced conditioned place preference without altering alcohol consumption itself, suggesting that FAAH inhibition influences memory-related processes regulating the expression of conditioned fear and conditioned alcohol reward in animals genetically predisposed toward high alcohol consumption. Acute FAAH inhibition attenuates anxiety-like behavior evident in rats following acute, high-dose alcohol administration (12). Collectively, these findings suggest that FAAH inhibition may be beneficial for reducing alcohol-related anxiety-like behavior, although further investigation is warranted, particularly evaluations of persistent anxiety-like behavior associated with protracted withdrawal in alcohol-dependent subjects.
Genetic variants in the ECS and alcohol use disorder.
Several genetic polymorphisms of ECB-related genes have been linked to alcohol abuse (54, 57). For example, a triplet repeat of varying number (AAT)n in the 3′-flanking region of the CNR1 (CB1) genetic locus is significantly associated with decreased P300 event-related potential amplitudes in subjects with AUD and male alcoholics with a history of attention-deficit/hyperactivity disorder. CNR1-associated SNPs, particularly rs6454674, rs806368, rs1049353, and rs1535355 are also associated with alcohol dependence. The C allele of rs2023239 appears to be associated with greater CB1R binding in the PFC, greater alcohol cue-elicited brain activation in the midbrain and PFC, and greater subjective reward from alcohol consumption. Additionally, four SNPs in or near the CNR1 gene (rs1535225, rs2023239, rs1049353, rs806368), and a triplet repeat in the 3′-flanking region of the CNR1 genetic locus, are each associated with increased impulsivity in a Native American population characterized by a high prevalence of alcoholism and substance abuse. Genetic alterations in FAAH function may also confer susceptibility to problem alcohol and drug use. FAAH expression and function are reduced in the PFC of rats selectively bred for high alcohol preference and consumption, and FAAH deletion results in increased alcohol preference and consumption in mice (54). Animals lacking FAAH are also less sensitive to alcohol-induced motor incoordination and exhibit reduced signs of alcohol withdrawal, leading to the theory that reductions in the aversive effects of alcohol intake confer increased motivation for alcohol consumption in these animals. In humans, a missense SNP in the FAAH DNA sequence (C385A, rs324420) results in reduced FAAH activity and a presumed increase in levels of AEA and other FAAH substrates. Initial reports described a significant association between the C385A polymorphism and problem drug use, although further studies suggest that individuals with this SNP are not at greater risk for alcohol dependence. However, there is evidence that dysregulation of FAAH function through the C385A polymorphism confers increased impulsivity and decreased threat perception that are similar to patterns observed in individuals with high familial risk for alcoholism (26). Moreover, genotype comparisons of the FAAH C385A polymorphism showed that CC carriers were overrepresented among risky drinkers relative to A allele carriers (8).
Conclusions and knowledge gaps.
As described above, substantial evidence demonstrates strong interactive effects between ECB signaling and the behavioral effects produced by alcohol. A number of genetic variants in ECB signaling are associated with both alcohol dependence and psychiatric disturbances that are risk factors for the development of problematic alcohol use. Despite some inconsistencies in the literature, a preponderance of evidence suggests that alcohol exposure increases ECB formation in brain and that chronic alcohol exposure disrupts ECB clearance mechanisms, downregulates cannabinoid receptor expression/influence, and impairs ECB-mediated forms of synaptic plasticity in a manner that persists well into protracted alcohol abstinence. This may be of relevance given that alcohol dependence and protracted withdrawal are characterized by increased prevalence of anxiety, depression, and sensitivity to stressors, and ECB signaling plays a prominent role in the maintenance of affective state and the constraint of stress responsivity. These symptoms of protracted withdrawal play a prominent role in the relapse to heavy alcohol consumption, and if these symptoms derive in part from impaired ECB signaling, then therapeutic approaches aimed at restoring or bolstering cannabinoid signaling may have clinical benefit for alcohol dependence. The use of exogenous CB1R agonists for this purpose would likely be problematic, as these compounds can promote alcohol-seeking behavior, increase the magnitude of excessive alcohol consumption evident following periods of abstinence, and can exacerbate withdrawal-related affective disorders when administered at high doses. However, enhancement of ECB tone through inhibition of ECB clearance may be a viable therapeutic approach in light of evidence that these manipulations constrain stress responses and produce anxiolytic- and antidepressive-like effects. Indeed, rodent studies demonstrate that acute FAAH inhibition ameliorates alcohol withdrawal-related anxiety-like behavior without promoting alcohol-seeking behavior. Importantly, because ECBs are generally produced in response to specific stimuli, the behavioral effects of moderate ECB clearance inhibition may be preferentially evident in limited circumstances such as exposure to stress or drug-associated conditioned cues. ECB clearance inhibition may also facilitate region-specific increases in brain ECB signaling as a result of regionally distinct stimulus-induced ECB production, thereby producing fewer unwanted behavioral effects than those produced by exogenous cannabinoid agonists that induce widespread cannabinoid receptor activation. However, although initial preclinical studies have evaluated the effects of acute FAAH inhibition on alcohol dependence-related behaviors, the effects of long-term FAAH inhibition have not been characterized, and studies in humans are lacking. Moreover, there is sparse knowledge regarding the effects of MAGL inhibition on alcohol dependence-related behaviors. These mechanisms reflect an important area of study involving the ECB system as a viable therapeutic target for alcohol use disorders and alcoholism.
There are a number of additional gaps in our understanding of the ECB influence in AUD and alcoholism. The endocannabinoid system plays a prominent role in brain development, and as such disruptions in ECB function at an early age likely has substantial consequences for adult brain function. However, despite knowledge of particularly disruptive alcohol effects resulting from early life and adolescent exposure, the potential involvement of impaired ECB function in these effects has not been thoroughly investigated. Robust bidirectional interactions between the ECS and sex hormones are recognized, and sex differences in the effects of alcohol are well known, although few studies have characterized a possible role for disrupted ECB function in these effects. There are also substantial limitations in the interpretation and replication of genetic analyses of the ECB influence in alcohol addiction due to the heterogeneity of the populations studied and the phenotypes examined.
SUMMARY
This symposium brought together experts to address the effects of alcohol in skeletal muscle, adipose tissue, liver, and brain in order to promote a broader understanding of the wide range of physiological processes influenced by alcohol use and to emphasize the unique aspects of alcohol in initiating and exacerbating a variety of pathological changes especially in individuals who consume alcohol chronically or to excess.
Dr. Shannon Bailey provided her perspective on the effects of alcohol on circadian clocks, noting that even within the alcohol field, research on clocks has largely focused on behavioral and neurobiological aspects of alcohol use where circadian disruption by alcohol has been shown to influence alcohol dependence and addictive behaviors. Dr. Bailey provided a compelling argument that the alcohol-induced disruption of circadian rhythms causes multiple metabolic and/or signaling pathways to be “misaligned” in the alcohol-exposed liver and that the impact alcohol has on clock function in multiple organ systems (gut, brain, adipose, skeletal muscle) should be strongly considered when investigating the pathobiology of alcoholic liver disease.
Dr. Charles Lang, emphasizing the need for a dynamic balance between synthesis and degradation that is disrupted by alcohol through induction of anabolic resistance leading to muscle wasting, provided further evidence for the unique aspects of alcohol-induced changes. Dr. Lang provided data on the importance of recognizing local (cell- and organ-specific) influences exemplified by the observed alcohol-induced reduction in protein synthesis in fast-twitch skeletal muscle that are not observed in slow-twitch muscle and on the direct effects of alcohol that are independent of liver or the metabolic pathways for alcohol. Finally, Dr. Lang provided a review of the multiple signaling pathways influenced by alcohol and the gaps in our current knowledge of these important pathways and how alcohol leads to an imbalance with consequences for both smooth and skeletal muscle.
Dr. Laura Nagy presented a unique perspective on the underappreciated role of adipose tissue in response to chronic alcohol. Dr. Nagy provided an overview of impaired insulin receptor-mediated signaling leading to increased lipolysis, release of free fatty acids, and increased triglyceride synthesis contributing to the development of ethanol-induced hepatic steatosis. She also examined in detail the processes leading to the low-grade inflammatory state, termed metainflammation, as a contributing cause for insulin resistance and metabolic dysfunction. New data reviewed by Dr. Nagy have indicated CYP2E1, complement factor C1q, and LPS as important players in adipose tissue inflammation. Finally, Dr. Nagy has reiterated the important caveat that the influence of adipose tissue may be variable according to the microenvironment or proximity to various organs.
Dr. Loren Parsons contributed his analysis of alcohol effects in the brain and the important role of the endocannabinoid system in mediating alcohol reward and addiction. By diminishing CB1 receptor signaling, through genetic manipulations in animal experiments or by pharmacological inhibition, the motivational and rewarding effects of alcohol can be attenuated. Dr. Parsons reviewed the evidence for alcohol-induced alterations in brain ECB formation, and the reduction of the CB1 receptor number with chronic alcohol exposure, describing the biochemical evidence that chronic alcohol use dysregulates this system and its downstream signaling mechanisms. Additional data from genetic studies were provided to further support the important role of the ECB system in alcohol seeking and addictive response. Finally the role of FAAH in clearing the ECBs provides a strong scientific rationale for the use of inhibitors of this clearance process as a viable therapeutic approach that may get around issues of increasing alcohol-seeking behavior.
By focusing on their own area of expertise, each of the authors has provided a framework for better understanding of the alcohol research field, and each has identified important gaps in our current knowledge of the effects of alcohol and the mechanisms by which these effects are generated. The descriptions, while not intended to provide a comprehensive treatment of the entire alcohol field, were meant to generate a seed within the research community to recognize that discussion of alcohol-induced changes in physiology should not be limited to only brain and liver. As discussed here, alcohol induces physiologically important changes in liver, brain, adipose tissue, and skeletal muscle; however, it is necessary also to consider the effects of alcohol on the gut, lungs, pancreas, bone, cardiovascular system, and immune system. In short, it is necessary to consider the effects of alcohol in the whole body. Many of the observed effects of alcohol may be mediated by common, often immune-related processes, but often these are also modulated by local, cell- or organ-specific processes that must also be woven into the tapestry to obtain the full picture.
GRANTS
Funding for this conference was made possible (in part) by 1 R13 AA-024974-01 from the National Institute on Alcohol Abuse and Alcoholism. The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the US Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F.M.S.-S., C.H.L., L.E.N., S.M.B., and L.H.P. drafted manuscript; F.M.S.-S., C.H.L., and G.M. edited and revised manuscript; F.M.S.-S., C.H.L., L.E.N., S.M.B., and G.M. approved final version of manuscript; C.H.L., L.E.N., and S.M.B. prepared figures.
ACKNOWLEDGMENTS
We thank the American Physiological Society and the National Institutes of Health grant support.
REFERENCES
- 1.Addolorato G, Capristo E, Marini M, Santini P, Scognamiglio U, Attilia ML, Messineo D, Sasso GF, Gasbarrini G, Ceccanti M. Body composition changes induced by chronic ethanol abuse: evaluation by dual energy X-ray absorptiometry. Am J Gastroenterol 95: 2323–2327, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Jaimes L, Polis I, Parsons LH. Regional influence of cannabinoid CB1 receptors in the regulation of ethanol self-administration by Wistar rats. Open Neuropsychopharmacol 2: 77–85, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134: 317–328, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 142: 943–953, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Barrenha GD, Chester JA. Genetic correlation between innate alcohol preference and fear-potentiated startle in selected mouse lines. Alcohol Clin Exp Res 31: 1081–1088, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Croce L, Sasso F, Pozzato G, Cristianini G, Brandi G. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 41: 845–850, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buczynski MW, Parsons LH. Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Br J Pharmacol 160: 423–442, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhler KM, Huertas E, Echeverry-Alzate V, Gine E, Molto E, Montoliu L, Lopez-Moreno JA. Risky alcohol consumption in young people is associated with the fatty acid amide hydrolase gene polymorphism C385A and affective rating of drug pictures. Mol Genet Genomics 289: 279–289, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SI, Draetta GF, Pagano M. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science 316: 900–904, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci 27: 3695–3702, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceccarini J, Hompes T, Verhaeghen A, Casteels C, Peuskens H, Bormans G, Claes S, Van Laere K. Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J Neurosci 34: 2822–2831, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology 198: 449–460, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham CC, Van Horn CG. Energy availability and alcohol-related liver pathology. Alcohol Res Health 27: 291–299, 2003. [PMC free article] [PubMed] [Google Scholar]
- 14.Damaggio AS, Gorman MR. The circadian timing system in ethanol consumption and dependence. Behav Neurosci 128: 371–386, 2014. [DOI] [PubMed] [Google Scholar]
- 15.DeBruyne JP, Weaver DR, Dallmann R. The hepatic circadian clock modulates xenobiotic metabolism in mice. J Biol Rhythms 29: 277–287, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci USA 110: 14783–14788, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P. Coordination of the transcriptome and metabolome by the circadian clock. Proc Natl Acad Sci USA 109: 5541–5546, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filiano AN, Millender-Swain T, Johnson R Jr, Young ME, Gamble KL, Bailey SM. Chronic ethanol consumption disrupts the core molecular clock and diurnal rhythms of metabolic genes in the liver without affecting the suprachiasmatic nucleus. PloS One 8: e71684, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamble KL, Motsinger-Reif AA, Hida A, Borsetti HM, Servick SV, Ciarleglio CM, Robbins S, Hicks J, Carver K, Hamilton N, Wells N, Summar ML, McMahon DG, Johnson CH. Shift work in nurses: contribution of phenotypes and genotypes to adaptation. PloS One 6: e18395, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamble KL, Young ME. Metabolism as an integral cog in the mammalian circadian clockwork. Crit Rev Biochem Mol Biol 48: 317–331, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Ann Rev Immunol 29: 415–445, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Groebner JL, Tuma PL. The altered hepatic tubulin code in alcoholic liver disease. Biomolecules 5: 2140–2159, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillaumond F, Dardente H, Giguere V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms 20: 391–403, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci 34: 637–644, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry 66: 9–16, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong-Brown LQ, Brown CR, Kazi AA, Navaratnarajah M, Lang CH. Rag GTPases and AMPK/TSC2/Rheb mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. Am J Physiol Cell Physiol 302: C1557–C1565, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarvis CM, Hayman LL, Braun LT, Schwertz DW, Ferrans CE, Piano MR. Cardiovascular risk factors and metabolic syndrome in alcohol- and nicotine-dependent men and women. J Cardiovasc Nurs 22: 429–435, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Johnson BP, Walisser JA, Liu Y, Shen AL, McDearmon EL, Moran SM, McIntosh BE, Vollrath AL, Schook AC, Takahashi JS, Bradfield CA. Hepatocyte circadian clock controls acetaminophen bioactivation through NADPH-cytochrome P450 oxidoreductase. Proc Natl Acad Sci USA 111: 18757–18762, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem 282: 28465–28473, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Kang L, Sebastian BM, Pritchard MT, Pratt BT, Previs SF, Nagy LE. Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines. Alcohol Clin Exp Res 31: 1581–1588, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Kazi AA, Hong-Brown L, Lang SM, Lang CH. Deptor knockdown enhances mTOR activity and protein synthesis in myocytes and ameliorates disuse muscle atrophy. Mol Med 17: 925–936, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirpich IA, Miller ME, Cave MC, Joshi-Barve S, McClain CJ. Alcoholic liver disease: update on the role of dietary fat. Biomolecules 6: 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 13: 3–30, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korzick DH, Sharda DR, Pruznak AM, Lang CH. Aging accentuates alcohol-induced decrease in protein synthesis in gastrocnemius. Am J Physiol Regul Integr Comp Physiol 304: R887–R898, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lonnqvist J, Partonen T. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol Alcohol 45: 303–311, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Lang CH, Frost RA, Deshpande N, Kumar V, Vary TC, Jefferson LS, Kimball SR. Alcohol impairs leucine-mediated phosphorylation of 4E-BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab 285: E1205–E1215, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Lang CH, Frost RA, Svanberg E, Vary TC. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab 286: E916–E926, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Lang CH, Frost RA, Vary TC. Skeletal muscle protein synthesis and degradation exhibit sexual dimorphism after chronic alcohol consumption but not acute intoxication. Am J Physiol Endocrinol Metab 292: E1497–E1506, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Lang CH, Liu X, Nystrom G, Wu D, Cooney RN, Frost RA. Acute effects of growth hormone in alcohol-fed rats. Alcohol Alcohol 35: 148–158, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Lang CH, Pruznak AM, Deshpande N, Palopoli MM, Frost RA, Vary TC. Alcohol intoxication impairs phosphorylation of S6K1 and S6 in skeletal muscle independently of ethanol metabolism. Alcohol Clin Exp Res 28: 1758–1767, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Lang CH, Pruznak AM, Nystrom GJ, Vary TC. Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: comparable effects in young and mature rats. Nutr Metab 6: 4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang CH, Wu D, Frost RA, Jefferson LS, Kimball SR, Vary TC. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol Endocrinol Metab 277: E268–E276, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Lu HC, Mackie K. An Introduction to the endogenous cannabinoid system. Biol Psychiatry 79: 516–525, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y, Cederbaum AI. Autophagy Protects against CYP2E1/Chronic Ethanol-Induced Hepatotoxicity. Biomolecules 5: 2659–2674, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangieri RA, Hong KI, Piomelli D, Sinha R. An endocannabinoid signal associated with desire for alcohol is suppressed in recently abstinent alcoholics. Psychopharmacology 205: 63–72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michalak A, Biala G. Alcohol dependence—neurobiology and treatment. Acta Pol Pharm 73: 3–12, 2016. [PubMed] [Google Scholar]
- 48.Mihic SJ, Harris RA. GABA and the GABAA receptor. Alcohol Health Res World 21: 127–131, 1997. [PMC free article] [PubMed] [Google Scholar]
- 49.Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA 104: 3342–3347, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minino AM, Heron MP, Smith BL. Deaths: preliminary data for 2004. Natl Vital Stat Rep 54: 1–49, 2006. [PubMed] [Google Scholar]
- 51.Molina PE, Gardner JD, Souza-Smith FM, Whitaker AM. Alcohol abuse: critical pathophysiological processes and contribution to disease burden. Physiology (Bethesda) 29: 203–215, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morena M, Patel S, Bains JS, Hill MN. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 41: 80–102, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog brain Res 199: 337–358, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Natividad LA, Maccioni P, Parsons LH, Colombo G. Cannabinoid-alcohol interactions. In: Cannabinoids, Endocannabinoids, and Modulation of Emotion, Memory, and Motivation, edited by Fattore L, Campolongo P. New York: Springer, 2015, p. 363–391. [Google Scholar]
- 55.Osna NA, Kharbanda KK. Multi-organ alcohol-related damage: mechanisms and treatment. Biomolecules 2016. April 15;6(2). pii: E20. doi: 10.3390/biom6020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pacy PJ, Preedy VR, Peters TJ, Read M, Halliday D. The effect of chronic alcohol ingestion on whole body and muscle protein synthesis—a stable isotope study. Alcohol Alcohol 26: 505–513, 1991. [DOI] [PubMed] [Google Scholar]
- 57.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci 16: 579–594, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24: 90–99, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Partonen T. Clock genes in human alcohol abuse and comorbid conditions. Alcohol 49: 359–365, 2015. [DOI] [PubMed] [Google Scholar]
- 60.Perreau-Lenz S, Spanagel R. Clock genes × stress × reward interactions in alcohol and substance use disorders. Alcohol 49: 351–357, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poirier LA, Rachdaoui N, Nagy LE. GLUT4 vesicle trafficking in rat adipocytes after ethanol feeding: regulation by heterotrimeric G-proteins. Biochem J 354: 323–330, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Powers MS, Barrenha GD, Mlinac NS, Barker EL, Chester JA. Effects of the novel endocannabinoid uptake inhibitor, LY2183240, on fear-potentiated startle and alcohol-seeking behaviors in mice selectively bred for high alcohol preference. Psychopharmacology 212: 571–583, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Preedy VR, Adachi J, Ueno Y, Ahmed S, Mantle D, Mullatti N, Rajendram R, Peters TJ. Alcoholic skeletal muscle myopathy: definitions, features, contribution of neuropathy, impact and diagnosis. Eur J Neurol 8: 677–687, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Preedy VR, Keating JW, Peters TJ. The acute effects of ethanol and acetaldehyde on rates of protein synthesis in type I and type II fibre-rich skeletal muscles of the rat. Alcohol Alcohol 27: 241–251, 1992. [PubMed] [Google Scholar]
- 65.Pruznak AM, Nystrom J, Lang CH. Direct central nervous system effect of alcohol alters synthesis and degradation of skeletal muscle protein. Alcohol Alcohol 48: 138–145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O'Neill JS, Wong GK, Chesham J, Odell M, Lilley KS, Kyriacou CP, Hastings MH. Circadian orchestration of the hepatic proteome. Curr Biol 16: 1107–1115, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 351: 53–58, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sebastian BM, Roychowdhury S, Tang H, Hillian AD, Feldstein AE, Stahl GL, Takahashi K, Nagy LE. Identification of a cytochrome P4502E1/Bid/C1q-dependent axis mediating inflammation in adipose tissue after chronic ethanol feeding to mice. J Biol Chem 286: 35989–35997, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Therap 132: 215–241, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serrano A, Rivera P, Pavon FJ, Decara J, Suarez J, Rodriguez de Fonseca F, Parsons LH. Differential effects of single versus repeated alcohol withdrawal on the expression of endocannabinoid system-related genes in the rat amygdala. Alcohol Clin Exp Res 36: 984–994, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shukla SD, Aroor AR, Restrepo R, Kharbanda KK, Ibdah JA. In vivo acute on chronic ethanol effects in liver: a mouse model exhibiting exacerbated injury, altered metabolic and epigenetic responses. Biomolecules 5: 3280–3294, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sidhpura N, Parsons LH. Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. Neuropharmacology 61: 1070–1087, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siepka SM, Yoo SH, Park J, Song W, Kumar V, Hu Y, Lee C, Takahashi JS. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129: 1011–1023, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Souza-Smith FM, Siggins RW, Molina PE. Mesenteric Lymphatic-Perilymphatic Adipose Crosstalk: Role in Alcohol-Induced Perilymphatic Adipose Tissue Inflammation. Alcohol Clin Exp Res 39: 1380–1387, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steiner JL, Crowell KT, Lang CH. Impact of alcohol on glycemic control and insulin action. Biomolecules 5: 2223–2246, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steiner JL, Gordon BS, Lang CH. Moderate alcohol consumption does not impair overload-induced muscle hypertrophy and protein synthesis. Physiol Rep 2015. March;3(3). pii: e12333. doi: 10.14814/phy2.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steiner JL, Lang CH. Alcohol impairs skeletal muscle protein synthesis and mTOR signaling in a time-dependent manner following electrically stimulated muscle contraction. J Appl Physiol 117: 1170–1179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steiner JL, Lang CH. Alcohol intoxication following muscle contraction in mice decreases muscle protein synthesis but not mTOR signal transduction. Alcohol Clin Exp Res 39: 1–10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steiner JL, Lang CH. Dysregulation of skeletal muscle protein metabolism by alcohol. Am J Physiol Endocrinol Metab 308: E699–E712, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, Vitaterna MH, Song S, Turek FW, Keshavarzian A. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PloS One 8: e67102, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swanson G, Forsyth CB, Tang Y, Shaikh M, Zhang L, Turek FW, Keshavarzian A. Role of intestinal circadian genes in alcohol-induced gut leakiness. Alcohol Clin Exp Res 35: 1305–1314, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swanson GR, Gorenz A, Shaikh M, Desai V, Kaminsky T, Van Den Berg J, Murphy T, Raeisi S, Fogg LF, Vitaterna MH, Forsyth C, Turek FW, Burgess HJ, Keshavarzian A. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am J Physiol Gastrointest Liver Physiol 311: G192–G201, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tahara Y, Shibata S. Circadian rhythms of liver physiology and disease: experimental and clinical evidence. Nat Rev Gastroenterol Hepatol 13: 217–226, 2016. [DOI] [PubMed] [Google Scholar]
- 84.Tang H, Sebastian BM, Axhemi A, Chen X, Hillian AD, Jacobsen DW, Nagy LE. Ethanol-induced oxidative stress via the CYP2E1 pathway disrupts adiponectin secretion from adipocytes. Alcohol Clin Exp Res 36: 214–222, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Udoh US, Swain TM, Filiano AN, Gamble KL, Young ME, Bailey SM. Chronic ethanol consumption disrupts diurnal rhythms of hepatic glycogen metabolism in mice. Am J Physiol Gastrointest Liver Physiol 308: G964–G974, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Udoh US, Valcin JA, Gamble KL, Bailey SM. The molecular circadian clock and alcohol-induced liver injury. Biomolecules 5: 2504–2537, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valenzuela CF. Alcohol and neurotransmitter interactions. Alcohol Health Res World 21: 144–148, 1997. [PMC free article] [PubMed] [Google Scholar]
- 88.Vargas R, Lang CH. Alcohol accelerates loss of muscle and impairs recovery of muscle mass resulting from disuse atrophy. Alcohol Clin Exp Res 32: 128–137, 2008. [DOI] [PubMed] [Google Scholar]
- 89.Vary TC, Nairn AC, Lang CH. Restoration of protein synthesis in heart and skeletal muscle after withdrawal of alcohol. Alcohol Clin Exp Res 28: 517–525, 2004. [DOI] [PubMed] [Google Scholar]
- 90.Vinod KY, Kassir SA, Hungund BL, Cooper TB, Mann JJ, Arango V. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J Psychiatric Res 44: 591–597, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Virtanen M, Jokela M, Nyberg ST, Madsen IE, Lallukka T, Ahola K, Alfredsson L, Batty GD, Bjorner JB, Borritz M, Burr H, Casini A, Clays E, De Bacquer D, Dragano N, Erbel R, Ferrie JE, Fransson EI, Hamer M, Heikkila K, Jockel KH, Kittel F, Knutsson A, Koskenvuo M, Ladwig KH, Lunau T, Nielsen ML, Nordin M, Oksanen T, Pejtersen JH, Pentti J, Rugulies R, Salo P, Schupp J, Siegrist J, Singh-Manoux A, Steptoe A, Suominen SB, Theorell T, Vahtera J, Wagner GG, Westerholm PJ, Westerlund H, Kivimaki M. Long working hours and alcohol use: systematic review and meta-analysis of published studies and unpublished individual participant data. Br Med J 350: g7772, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walker DK, Dickinson JM, Timmerman KL, Drummond MJ, Reidy PT, Fry CS, Gundermann DM, Rasmussen BB. Exercise, amino acids, and aging in the control of human muscle protein synthesis. Med Sci Sport Exerc 43: 2249–2258, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging 3: 479–493, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang W, Zhong W, Sun X, Sun Q, Tan X, Li Q, Sun X, Zhou Z. Visceral white adipose tissue is susceptible to alcohol-induced lipodystrophy in rats: role of acetaldehyde. Alcohol Clin Exp Res 39: 416–423, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao C, Liu Y, Xiao J, Liu L, Chen S, Mohammadi M, McClain CJ, Li X, Feng W. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. J Lipid Res 56: 1481–1491, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou P, Ross RA, Pywell CM, Liangpunsakul S, Duffield GE. Disturbances in the murine hepatic circadian clock in alcohol-induced hepatic steatosis. Sci Reports 4: 3725, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]