Abstract
Atmospheric CO2 is expected to more than double by the end of the century. The resulting changes in ocean chemistry will affect the behaviour, sensory systems and physiology of a range of fish species. Although a number of past studies have examined effects of CO2 in gregarious fishes, most have assessed individuals in social isolation, which can alter individual behaviour and metabolism in social species. Within social groups, a learned familiarity can develop following a prolonged period of interaction between individuals, with fishes preferentially associating with familiar conspecifics because of benefits such as improved social learning and greater foraging opportunities. However, social recognition occurs through detection of shoal-mate cues; hence, it may be disrupted by near-future CO2 conditions. In the present study, we examined the influence of elevated CO2 on shoal familiarity and the metabolic benefits of group living in the gregarious damselfish species the blue-green puller (Chromis viridis). Shoals were acclimated to one of three nominal CO2 treatments: control (450 µatm), mid-CO2 (750 µatm) or high-CO2 (1000 µatm). After a 4–7 day acclimation period, familiarity was examined using a choice test, in which individuals were given the choice to associate with familiar shoal-mates or unfamiliar conspecifics. In control conditions, individuals preferentially associated with familiar shoal-mates. However, this association was lost in both elevated-CO2 treatments. Elevated CO2 did not impact the calming effect of shoaling on metabolism, as measured using an intermittent-flow respirometry methodology for social species following a 17–20 day acclimation period to CO2 treatment. In all CO2 treatments, individuals exhibited a significantly lower metabolic rate when measured in a shoal vs. alone, highlighting the complexity of shoal dynamics and the processes that influence the benefits of shoaling.
Keywords: Calming effect, carbon dioxide, familiarity, respiratory physiology, shoaling, social recognition
Introduction
Atmospheric CO2 has risen to >400 ppm (Dlugokencky and Tans, 2016) because of human activity, higher than any time in the last 800 000 years (Masson-Delmotte et al., 2013). The partial pressure of CO2 (pCO2) in the world's oceans is rising at approximately the same rate as in the atmosphere (Doney et al., 2009; Le Quéré et al., 2013). If current anthropogenic CO2 emissions continue unabated, average CO2 levels in the atmosphere and surface ocean will more than double from present-day levels by the year 2100 (Fabry et al., 2008; Collins et al., 2013). Furthermore, new models indicate that seasonal cycles in ocean pCO2 will be amplified in the future, meaning that marine organisms will experience extended periods of ocean pCO2 in excess of 1000 µatm by the end of this century (McNeil and Sasse, 2016). Rising CO2 levels are predicted to affect a range of behavioural (Briffa et al., 2012; Nagelkerken and Munday, 2016) and physiological processes (Pörtner et al., 2004; Heuer and Grosell, 2014) in marine organisms, with potentially far-reaching effects on marine ecosystems (Wittmann and Pörtner, 2013).
Higher environmental CO2 levels can be a problem for marine organisms because they act to acidify the blood and tissues and thus affect pH-dependent physiological processes (Pörtner et al., 2004). Fish defend against acidosis in a high-CO2 environment by actively regulating acid–base-relevant ions in their blood and tissues (Heuer and Grosell, 2014). Consequently, they are able to maintain a pH suitable for cellular processes, even at very high ambient CO2 levels (Ishimatsu et al., 2008; Esbaugh et al., 2012, 2016). However, this acid–base regulation leads to changes in extracellular ion concentrations that may interfere with the function of neurotransmitter receptors (Nilsson et al., 2012). These neurological changes can lead to altered behaviour and impaired sensory systems. Behavioural effects of exposure to high CO2 include reduced learning ability (Jutfelt et al., 2013; Chivers et al., 2014), altered activity levels (Munday et al., 2010; Ferrari et al., 2011a), higher anxiety (Hamilton et al., 2014), disrupted behavioural lateralization (Domenici et al., 2011; Jutfelt et al., 2013) and reduced predator avoidance behaviour (Dixson et al., 2010; Munday et al., 2010; Ferrari et al., 2011b). Behavioural responses to visual (Ferrari et al., 2012b; Chung et al., 2014), olfactory (Munday et al., 2009b) and auditory cues (Simpson et al., 2011; Rossi et al., 2016) are all affected, although one study found that visual cues were less affected than olfactory preferences at projected near-future CO2 levels (Lönnstedt et al., 2013). Some behavioural traits appear to be unaffected by elevated CO2, particularly foraging behaviour and swimming kinematics (Munday et al., 2009c; Nowicki et al., 2012; Maneja et al., 2015). In addition, some species, such as the Atlantic cod (Gadus morhua), exhibit tolerance to elevated CO2 in terms of behavioural effects (Jutfelt and Hedgärde, 2013, 2015). Even among closely related coral reef fish, there is substantial variability among species in the degree of behavioural effects in response to elevated CO2 (Ferrari et al., 2011a).
The effects of elevated pCO2 and decreased pH on other physiological characteristics are unclear. Theoretically, the energetic cost of increased regulatory mechanisms (such as acid–base balance regulation) should manifest in higher overall energetic needs (Ishimatsu et al., 2008). However, studies measuring standard metabolic rate (SMR; the metabolic rate of a resting, fasting and non-stressed individual; a measure of basic energetic needs) of fishes under elevated pCO2 have found highly variable results (reviewed by Heuer and Grosell, 2014; Lefevre, 2016), reporting increases (Munday et al., 2009a; Enzor et al., 2013), decreases (Rummer et al., 2013) and no effects of pCO2 on SMR (Deigweiher et al., 2008; Melzner et al., 2009; Strobel et al., 2012; Couturier et al., 2013), suggesting that the effects may be species or context specific. However, another important consideration is that, although many studies have examined the effect of pCO2 on the metabolic rate of gregarious fish species (Munday et al., 2009a; Miller et al., 2012; Rummer et al., 2013), all have measured metabolic rate in solitary individuals, which can have effects on the measured metabolic rate because of the stress of isolation (Nadler et al., 2016). Therefore, how social context may modulate the effect of pCO2 on metabolic traits, such as SMR, remains unknown. Recent work found that the immediate social environment can have a significant impact on metabolic rate, with individuals tested in the presence of shoal-mate cues exhibiting a significantly lower minimal measured metabolic rate than individuals tested in social isolation (Nadler et al., 2016). One factor that is likely to contribute to this calming effect is a reduced need for individual vigilance, because animal groups exhibit improved threat detection by having ‘many eyes’ to scan for predators (Roberts, 1996; Ward et al., 2011). Individuals accustomed to a social environment may also exhibit reduced stress when allowed to associate with conspecifics (Hennessy et al., 2009). The importance of these benefits could increase in the presence of environmental stressors, such as rising pCO2, because having a reduced metabolic rate in shoaling conditions could aid in coping with the projected rise in energy demand associated with changing environmental conditions.
Group living is widespread among fish species and carries benefits for individuals with respect to predator avoidance, foraging opportunities and energy use (Shaw, 1978; Krause and Ruxton, 2002). A learned familiarity can be attained following a prolonged period of interaction between social individuals (reviewed by Ward and Hart, 2003), increasing the probability of reciprocal cooperation between members of an animal group (Granroth-Wilding and Magurran, 2013). This greater cooperation can have benefits for a range of fitness-enhancing processes and characteristics, including foraging, social learning, body condition and survival (Seppä et al., 2001; Swaney et al., 2001; Atton et al., 2014). As a result, fish prefer to shoal with familiar conspecifics (e.g. Magurran et al., 1994; Griffiths and Magurran, 1997; Bhat and Magurran, 2006; Edenbrow and Croft, 2012), with individual identification achieved primarily through olfactory stimuli (Partridge and Pitcher, 1980; Brown and Smith, 1994; Ward et al., 2002). As elevated pCO2 is known to impact behavioural traits and sensory abilities necessary for social recognition, the ability to recognize familiar shoal-mates may be compromised in future environmental conditions.
Elevated pCO2 may affect the calming effect and the ability of fish to recognize conspecifics owing to its effects on fish behaviour, sensory abilities or physiology. In the present study, we examined the effect of elevated pCO2 on familiarity and the calming effect in the blue-green puller, Chromis viridis (Cuvier, 1830), a common species of shoaling damselfish. Shoals were acclimated to one of the following three CO2 treatments: control (450 µatm), mid-CO2 (750 µatm) or high-CO2 (1000 µatm). Our first aim was to determine whether elevated pCO2 modulated familiarity, using a choice test in which individuals were given the choice to associate with familiar shoal-mates or unfamiliar conspecifics. Our second aim was to explore whether the calming effect was altered by environmental pCO2, using an intermittent-flow respirometry methodology for social species. We hypothesized that familiarity would be disrupted by elevated pCO2. Given the known benefits of familiarity to shoaling fish (Seppä et al., 2001; Swaney et al., 2001; Atton et al., 2014), we also predicted that the calming effect on the minimal measured metabolic rate would be reduced if familiarity was disrupted at elevated pCO2.
Materials and methods
Fish collection and maintenance
Experiments were conducted at the Lizard Island Research Station in the northern Great Barrier Reef (14°40′08″S; 145°27′34″E). Shoals of C. viridis (standard length, 3.22 ± 0.03 cm; body mass, 1.29 ± 0.04 g; mean values ± SEM) were collected from reefs in the lagoon adjacent to the Lizard Island Research Station using hand nets and barrier nets. Chromis viridis is an abundant, live coral-associated shoaling species found on coral reefs throughout the Indo-Pacific region in groups ranging in size from a few to hundreds of individuals (Randall et al., 1997). Fish were placed into groups composed of eight individuals and housed in replicate 30 litre aquaria in a flow-through seawater system. All experimental shoals were held together for a minimum of 15 days to ensure that they exhibited a uniform degree of familiarity (Ward et al., 2003). Fish were fed to satiation twice daily with INVE Aquaculture pellets and newly hatched Artemia sp.
Carbon dioxide treatments and administration
Shoals were acclimated to one of the following three CO2 treatments: 450 µatm (ambient control), 750 µatm or 1000 µatm (4–7 days for behaviour experiments and 17–20 days for physiology experiments; seawater chemistry summarized in Table 1). These elevated-CO2 treatments were chosen based on the range of CO2 levels projected for the year 2100 (Collins et al., 2013; McNeil and Sasse, 2016). The CO2 administration methodologies followed standard procedures for ocean acidification research (Gattuso et al., 2010). The only deviation from this prescribed methodology was the use of single header tanks for each CO2 treatment (Cornwall and Hurd, 2015), as space limitations in the field prevented us from having multiple header tanks for each CO2 treatment. Seawater was pumped directly from the ocean into each 60 litre header tank. Elevated-CO2 seawater treatments were achieved by dosing CO2 to a set pH, using a pump placed into each header tank through which CO2 was diffused. This pump aided in rapid dissolution of CO2 and vigorous stirring of water in the header tank. A pH controller (Aqua Medic, Germany) attached to each CO2 treatment header tank maintained pH at the desired level. In control header tanks, air was diffused through sump pumps. Equilibrated seawater was then pumped at a rate of ~700 ml/min to each of the replicate 30 litre experimental tanks. For each of these replicate tanks, seawater pHNBS (pH measured on the NBS scale; Mettler Toledo SevenGo Pro) and temperature (Comark C22) were recorded daily. Seawater CO2 was confirmed with in situ CO2 measurements, using a portable CO2 equilibrator and non-dispersive infrared (NDIR) sensor (Vaisala GMP343; Hari et al., 2008; Munday et al., 2014b). For experiment 1, in situ CO2 measurements were conducted once weekly in the control and 1000 µatm treatments to confirm CO2 levels based on pH measurements. During experiment 2, these measurements were conducted on each treatment at least three times weekly, during which CO2 measures were recorded. These measurements are detailed in Table 1 and confirm our calculated pCO2. Salinity was measured by an automated float in the Lizard Island lagoon (Bainbridge, 2015). Water samples were taken twice weekly and analysed for total alkalinity by Gran titration (888 Titrando, Metrohm, Switzerland) to within 1% of certified reference material (Professor A. Dickson, Scripps Institution of Oceanography). Average pCO2 was calculated with the program CO2SYS, from measured pHNBS, temperature, salinity and total alkalinity, using constants from Mehrbach et al. (1973) refitted by Dickson and Millero (1987) and Dickson (1990) for KHSO4.
Table 1:
Summary of seawater chemistry parameters in control and elevated-carbon dioxide treatments for experiments 1 and 2
| Treatment | Experiment no. | Temperature (°C) | Salinity (psu) | pHNBS | Total alkalinity [μmol (kg seawater)−1] | pCO2 (calculated, μatm) | pCO2 (in situ, μatm) |
|---|---|---|---|---|---|---|---|
| Control CO2 | 1 | 28.8 (±0.2) | 35.5 (±0.01) | 8.15 (±0.010) | 2284 (±1) | 442 (±9) | 465 (±13) |
| 2 | 28.9 (±0.3) | 35.0 (±0.03) | 8.13 (±0.002) | 2309 (±8) | 461 (±2) | 449 (±11) | |
| Mid CO2 | 1 | 29.1 (±0.2) | 35.5 (±0.01) | 7.96 (±0.001) | 2285 (±12) | 734 (±5) | – |
| 2 | 29.0 (±0.3) | 35.0 (±0.03) | 7.96 (±0.001) | 2296 (±8) | 753 (±2) | 766 (±11) | |
| High CO2 | 1 | 28.8 (±0.2) | 35.5 (±0.01) | 7.86 (±0.001) | 2296 (±2) | 963 (±7) | 981 (±17) |
| 2 | 28.8 (±0.3) | 35.0 (±0.03) | 7.87 (±0.001) | 2297 (±11) | 952 (±3) | 983 (±15) |
The estimated partial pressure of CO2 (Estimated pCO2) was calculated in the program CO2SYS using the other measured parameters. In situ pCO2 was measured using a portable CO2 equilibrator with non-dispersive infrared (NDIR) sensor. Seawater pH was measured on the NBS (National Bureau of Standards) scale (pHNBS). Error is SEM.
Experiment 1: effect of elevated CO2 on familiarity
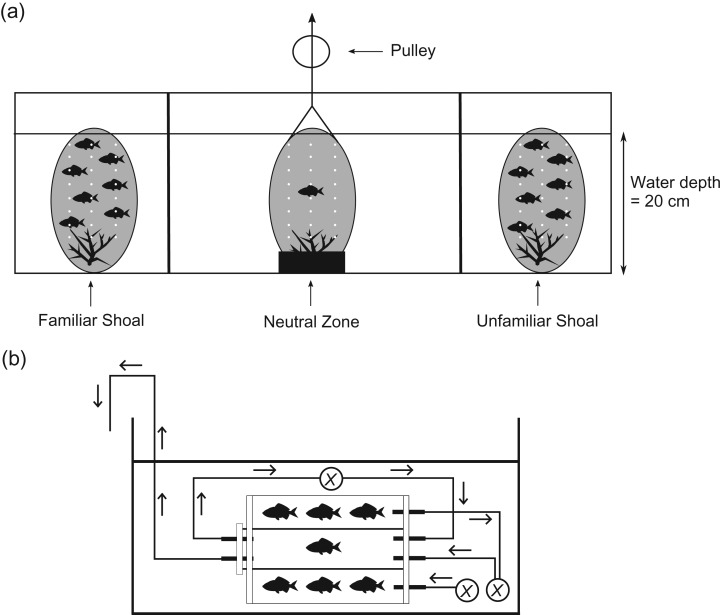
Nine experimental C. viridis shoals, each composed of eight fish, were acclimated to each CO2 treatment for a period of 4–7 days before experimentation. This time period is sufficient for elevated CO2 to induce behavioural changes in reef fishes, and previous studies indicate that longer acclimation periods do not change results (Munday et al., 2013a, 2014a; Welch et al., 2014). Two individuals per group were chosen randomly for testing for shoal association preferences (n = 18 individuals per treatment). These individuals were distinguished from each other and their shoal-mates using unique visible implant elastomer (VIE) tags (Hoey and McCormick, 2006). The VIE tags were administered 24–48 h before placement in the CO2 treatment. Shoaling preference was established using a choice test, using methodology adapted from Griffiths and Magurran (1997). An elongate testing tank (Fig. 1a) was filled to a depth of 20 cm with seawater at the same CO2 level as the relevant treatment. Two 1 litre plastic containers (height, 24 cm × diameter, 10 cm) were placed at each end of the tank, 6 cm from the side-wall. The plastic containers were transparent and made porous to olfactory cues by holes drilled around the circumference (50 5 mm holes per container). Shoals composed of 7 fish of either the familiar or an unfamiliar group were placed in these bottles. The location of the familiar shoal (right or left bottle) was randomized. The shoal used as unfamiliar was also randomized, to ensure that each shoal within a treatment was used as the unfamiliar shoal a uniform number of times and that a different unfamiliar shoal was used when testing each of the two focal fish from a shoal. The focal fish was placed in a clear, porous container in the centre of the tank. This container sat over a small coral shelter, and the bottom 3 cm of the container was opaque to allow the fish to take shelter. All fish were left to acclimate in this container for 15 min, which was a sufficient time period for all fish to calm down after handling. The container surrounding the focal fish was then lifted using a pulley system so that the focal fish would not be disturbed by visual cues of the observer. Trials lasted 15 min and were video-recorded (Canon Powershot D10). Pilot trials were conducted with food colouring to estimate the degree of olfactory cue mixing throughout the choice test tank during the 30 min trial (including both the 15 min acclimation period and the 15 min testing period). While there was olfactory mixing in the neutral zone of the experimental tank (Fig. 1a), no mixing occurred in the shoal association zones within this time frame.
Figure 1:
Schematic diagrams of the two experimental set-ups. (a) Choice test tank used in experiment 1 (90 cm length × 30 cm width × 30 cm height). The dark ovals on either end of the tank represent the shoal holding containers, and the dark oval in the centre of the tank illustrates the container used for the focal fish during the pre-trial acclimation period. White dots represent the porosity of the containers (each container contained 50 5 mm holes). (b) Side view of the respirometry chamber. The experimental set-up was composed of an inner respirometry chamber (length, 13.5 cm; inner diameter, 3.24 cm; volume of chamber and associated gas-impermeable tubing, 100 ml) and an outer shoal-mate holding chamber (length, 12.0 cm; inner diameter, 11.4 cm; volume of chamber, 1.10 litres). Arrows indicate the direction of water flow through tubing. Each X indicates a water pump used for mixing the inner chamber and flushing both chambers. The outer shoal-mate holding chamber was flushed with its own pump. The outflow port for this outer chamber was connected to the flush pump for the inner respirometry chamber, to provide olfactory cues of shoal-mates to the focal individual. In order to ensure proper mixing in the inner respirometry chamber, a pump ran continuously in a closed loop. Deoxygenated water in the inner chamber was discarded during on phases of the flush pump. All focal individuals were tested in both an alone-testing treatment and a shoal-testing treatment (with six shoal-mates).
Using QuickTime Player 7 (v 7.6.6), videos were analysed for the following factors: (i) the proportion of time spent shoaling with each group; (ii) initial shoal choice following removal of the barrier; and (iii) total shoal visits (a proxy for activity, which indicates the number of times that the focal fish traversed the experimental tank). Individuals were said to be shoaling when they were swimming within two body lengths of the shoal (Pitcher and Parrish, 1993). To ensure that focal fish were making an informed choice (e.g. had experienced the sensory cues of both stimulus shoals), they had to visit both shoal preference zones within a trial or they were retested the next day (occurred with 22% of focal fish across CO2 treatment groups). Different unfamiliar shoals were used when retesting to prevent learning of unfamiliar conspecifics. Activity was recorded so that we could confirm that any effect of CO2 on shoal association preferences was not attributable to changes in activity levels between treatments.
Experiment 2: effect of elevated CO2 on the calming effect
Ten experimental shoals were acclimated to each CO2 treatment for a period of 17–20 days. This longer acclimation period was used for this experiment because studies show that metabolism requires a longer period of time to adjust to elevated CO2 treatments (Enzor et al., 2013). One individual per group was chosen randomly for testing (n = 10 individuals per treatment) and was identified using VIE tags (Hoey and McCormick, 2006). The VIE tags were administered 24–48 h before placement in the CO2 treatment.
The calming effect was measured using a previously described intermittent-flow respirometry methodology for social species (Nadler et al., 2016; Fig. 1b). Respirometry is a technique in which oxygen uptake rates are measured as a proxy for aerobic metabolism (Steffensen, 1989; Nelson, 2016). Each respirometry chamber was composed of two cylindrical glass tubes: an inner tube (length, 13.5 cm; inner diameter, 3.24 cm; total volume of chamber and associated gas-impermeable tubing, 100 ml) and an outer tube (length, 12.0 cm; inner diameter, 11.4 cm; total volume of chamber minus volume occupied by inner chamber, 1.10 litres). The outer chamber was affixed to the exterior of the inner chamber and was used to provide visual and olfactory cues of shoal-mates to the focal individual. This larger chamber was aerated with a continuously running flush pump. To provide olfactory cues of shoal-mates to the focal individual, the water leaving the outflow port was attached to the inflow vent for the inner chamber's flush pump. The inner chamber was connected to a recirculating pump (to mix water in the respirometer) and a flushing pump that flushed the chamber with oxygen-saturated water for 3 min between each 9 min measurement period. The water used to flush the chamber between measurement periods was maintained at the same pH and pCO2 as the focal fishes’ treatment. Chambers were immersed in separate, temperature-controlled water baths (29 ± 0.5°C). Temperature was maintained through a combination of air conditioning and controlling ambient water flow to the water bath. The metabolic rate of each focal fish was recorded in an alone-testing treatment (no shoal-mates in the outer chamber) and a group-testing treatment (six shoal-mates in the outer chamber). The order of testing trials (testing of the alone or group treatment first) was randomized. All focal fish were given 48 h between testing trials.
Dissolved oxygen concentration in the inner, focal chamber was measured every 2 s and logged using a Fire-Sting fibre-optic oxygen meter (Pyroscience, Germany), connected to a computer. The oxygen-sensing optode was mounted in the recirculation loop in a flow-through cell, to ensure that flow was sufficient for a fast response time of the sensor (Svendsen et al., 2016). Focal fish were fasted for 24–26 h before experimentation to ensure that they were in a post-absorptive state and were left undisturbed in the respirometers for 17–19 h overnight, as C. viridis is quiescent at night. A dim light remained on through the night in the laboratory to simulate moonlight, allowing the focal fish to see their shoal-mates in group testing trials. Activity was recorded during daylight hours using a webcam (H264 Webcam software) and was measured by counting the number of 180° turns for 10 min/h of testing (from which turns/min was calculated). Activity was recorded to ensure that any measured effects of CO2 on oxygen uptake were not attributable to changes in activity between CO2 treatments. Slopes (s) were calculated from plots of oxygen concentration vs. time using linear least-squares regression (LabChart v6) and converted to the rate of oxygen uptake (; in milligrams of O2 per hour). For all trials, background respiration was measured in empty chambers for three measurement periods both before and after trials. Microbial respiration was then subtracted from all fish respiration measurements, assuming a linear increase in microbial respiration over time (Rodgers et al., 2016).
Once focal individuals had completed both the alone- and group-testing trials, maximum metabolic rate (MMR) was measured in separate trials, so that each individual's aerobic scope (AS) could be calculated. The AS is an individual's aerobic metabolic capacity, which indicates the available energy that an individual has for all aerobic processes beyond basic maintenance (Farrell, 2016). The MMR was measured using the chase protocol, in which individuals are exercised to exhaustion through manual chasing (Roche et al., 2013). Although this method may not always provide the highest estimates of MMR (Roche et al., 2013), it is an accepted and repeatable method for determining a relative value for MMR between individuals. Fish were considered exhausted when they no longer responded to chasing by burst swimming. Fish were then air exposed for 30 s to ensure that they had depleted all endogenous oxygen stores. Individuals were then transferred to their respective respirometry chambers, and oxygen uptake was measured for 8–10 min (this time frame was used to ensure that oxygen saturation in the water remained >80% air saturation; Hughes, 1973). This method elicits anaerobic exercise in individuals, and maximal rates of oxygen uptake were measured during subsequent recovery. The MMR was measured for all fish in an alone-testing treatment. These oxygen uptake slopes were measured at 3 min intervals, with the greatest oxygen uptake during this period taken as MMR.
Three measures of metabolic rate were analysed. First, the minimal measured metabolic rate in fish exposed to each treatment (MRmin) was estimated using the protocol typically employed to measure SMR in the literature. This was accomplished by taking MRmin as the lowest 10th percentile of all measurements (Killen, 2014; Chabot et al., 2016), and comparisons were drawn between individuals tested alone and with a group. Second, routine metabolic rate (RMR; the metabolic rate of an undisturbed animal, including costs of random activity) was calculated as the mean excluding the first 5 h in the respirometer, and differences between fish tested alone (RMRalone) and fish tested in groups (RMRgroup) were assessed (Killen et al., 2011). These 5 h were excluded from RMR calculations because pilot trials determined that in C. viridis takes an average of 5 h to stabilize (SS. Killen, LE. Nadler, MI. McCormick, unpublished data). Third, individuals’ response to stress was also determined by using the first slope (FS) of each alone- and group-testing trial, following transfer to the respirometer. The stress response was calculated in the context of AS (AS = MMR − MRmin), in order to determine the proportion of AS that fish were using in response to stress (the stressor in this case being handling stress during transfer to the respirometer). The initial stress response (ISR) was therefore calculated using the following equation:
The is commonly used as an indicator of stress and reaction to threats, such as predation, because of the previously established link between oxygen uptake and stress hormones, including cortisol and epinephrine, with oxygen uptake increasing as the concentration of stress hormones rises (e.g. Brown et al., 1982; Morgan and Iwama, 1996). In the present study, the stressor was the handling stress induced during transfer to the respirometer and any stress of being in isolation.
Statistical analysis
Statistical analysis was conducted in the R Statistical Environment (v. 3.2.4) using the packages ‘nlme’, ‘multcomp’, ‘lme4’ and ‘car’ (Bates and Maechler, 2009; R Development Core Team, 2015; Pinheiro et al. 2016). For experiment 1, three separate models were conducted, to determine the preference for the familiar shoal within each treatment (as measured by the proportion of time spent with the familiar shoal). As the null hypothesis is 0.5 (which would indicate no preference for either shoal), the deviation from 0.5 for each observation was used as the response variable, and differences in deviation from 0 were assessed in general linear mixed-effects models (LMMs), with shoal number as a random effect (so that each individual was nested within their experimental shoal). Differences in activity (total shoal visits) between treatments were tested using an LMM, with CO2 treatment as a fixed effect and shoal number as a random effect. To ensure that all assumptions were met, homogeneity of variance and normality were assessed through visual inspection of the residual and quantile–quantile (Q-Q) plots, respectively. No transformations were necessary to meet assumptions. Initial shoal choice was tested using an LMM with a binomial distribution, with CO2 treatment as a fixed effect and shoal number as a random effect.
For experiment 2, differences in the MRmin, ISR and activity were analysed using an LMM, with CO2 treatment and testing treatment (alone or group) as fixed effects, body mass as a covariate (to account for differences in size between individuals), and individual as a random effect. In statistical analysis, whole-animal metabolic rate values were used. In figures, metabolic rate measures were mass corrected by plotting the residual values for each measure from the relationship between the logarithm of body mass (in grams) and the logarithm of metabolic rate (in milligrams of O2 per hour). Each residual was added to the fitted value for mass = 1.29 g, the mean mass of all fish used in the study. Significant differences in CO2 treatments (which had three levels) discovered using LMM were investigated further using Tukey's multiple comparisons post hoc tests. Differences in MMR and AS with CO2 treatment were examined using a generalized linear model (GLM), with body mass as a covariate. For these models, assumptions of homogeneity and normality were again checked through visual inspection of residual and Q-Q plots. No transformations were necessary to conform to these assumptions.
Results
Experiment 1: effect of elevated CO2 on social recognition
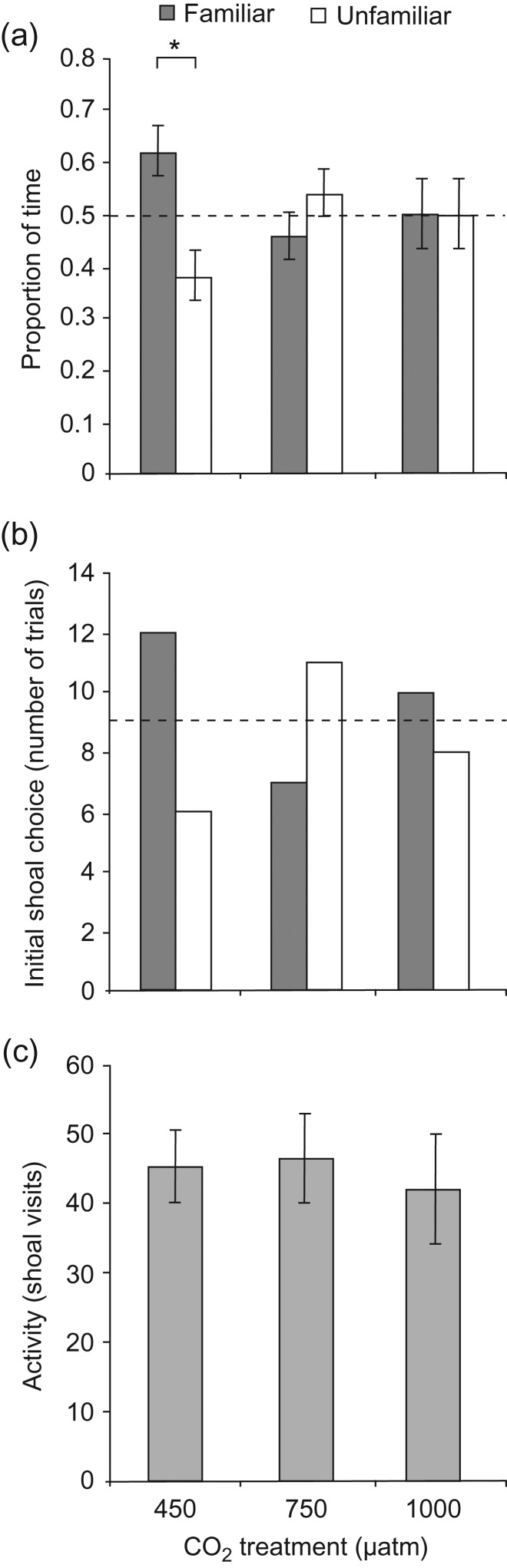
Individuals exhibited a significant preference for the familiar shoal in control conditions, but this preference was lost in both elevated-CO2 treatments (Fig. 2a; 450 µatm, F1,10 = 6.10, P = 0.033; 750 µatm, F1,10 = 0.660, P = 0.438; and 1000 µatm, F1,10 = 0.001, P = 0.991). Trends in initial shoal choice matched those found for shoal preference, but the effect of CO2 treatment on initial shoal choice was not statistically significant (Fig. 2b; χ2 = 0.8103, P = 0.368). Total shoal visits were not significantly different between the CO2 treatments (Fig. 2c; F2,25 = 0.1138, P = 0.893), with individuals exhibiting an overall mean of 44.7 shoal visits per trial (range, 2–133 shoal visits per trial).
Figure 2:
Effect of CO2 on shoal preference and activity. (a) Proportion of time spent with each shoal (familiar and unfamiliar). (b) Initial shoal choice after removal of the barrier (in number of trials). (c) Mean activity per trial (number of shoal visits). Error bars are SEM, and n = 18 for all treatments. Asterisks indicate statistical significance (*P < 0.05).
Experiment 2: effect of elevated CO2 on the calming effect
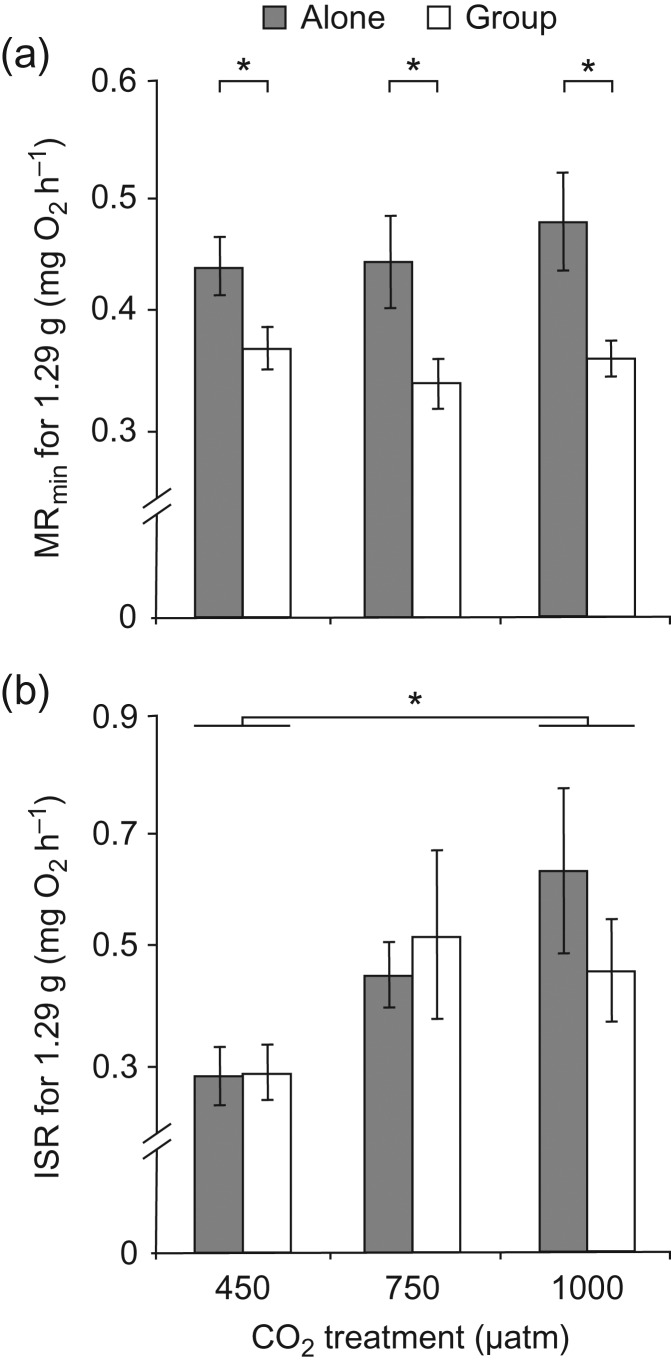
The MRmin tested in a group was significantly lower than MRmin tested alone (Fig. 3a; F1,26 = 29.01, P < 0.001), regardless of CO2 treatment (Fig. 3a; F2,27 = 0.37, P = 0.698), with 26 out of 30 fish tested exhibiting an average reduction in MRmin of 22.8% (the remaining four fish exhibited an average increase in MRmin of 10.5% when tested in a group; these four fish were included in all statistical analyses). The interaction between testing and CO2 treatment was not significant (F2,26 = 0.71, P = 0.501); however, the magnitude of the calming effect was higher in both elevated-CO2 treatments than it was in control conditions (450 µatm, 13.9 ± 5.6%; 750 µatm, 21.4 ± 4.2%; and 1000 µatm, 19.8 ± 7.3%; Fig. 3a). Elevated-CO2 treatments produced a trend towards higher ISR (Fig. 3b; F2,27 = 2.94, P = 0.069), with differences attributable to a significant increase in ISR from the control to the high-CO2 treatment (Tukey's test: 450 vs. 1000 µatm, P = 0.028; for all other comparisons, P > 0.05). The ISR was not affected by testing treatment (F1,26 = 0.27, P = 0.606).
Figure 3:
Effect of CO2 and testing treatment on the minimal metabolic rate (MRmin; in milligrams of O2 per hour; a) and initial stress response (ISR; in milligrams of O2 per hour; b). In these panels, metabolic rate measures were mass corrected by using residuals of the relationship between logarithm of the body mass and logarithm of whole-animal metabolic rate added to the fitted value for mass = 1.29 g, the mean mass of all fish used in the study. Error bars are SEM, and n = 10 for all treatments. Asterisks indicate statistical significance (*P < 0.05). Statistical analysis was conducted on whole-animal metabolic rates, with body mass as a covariate.
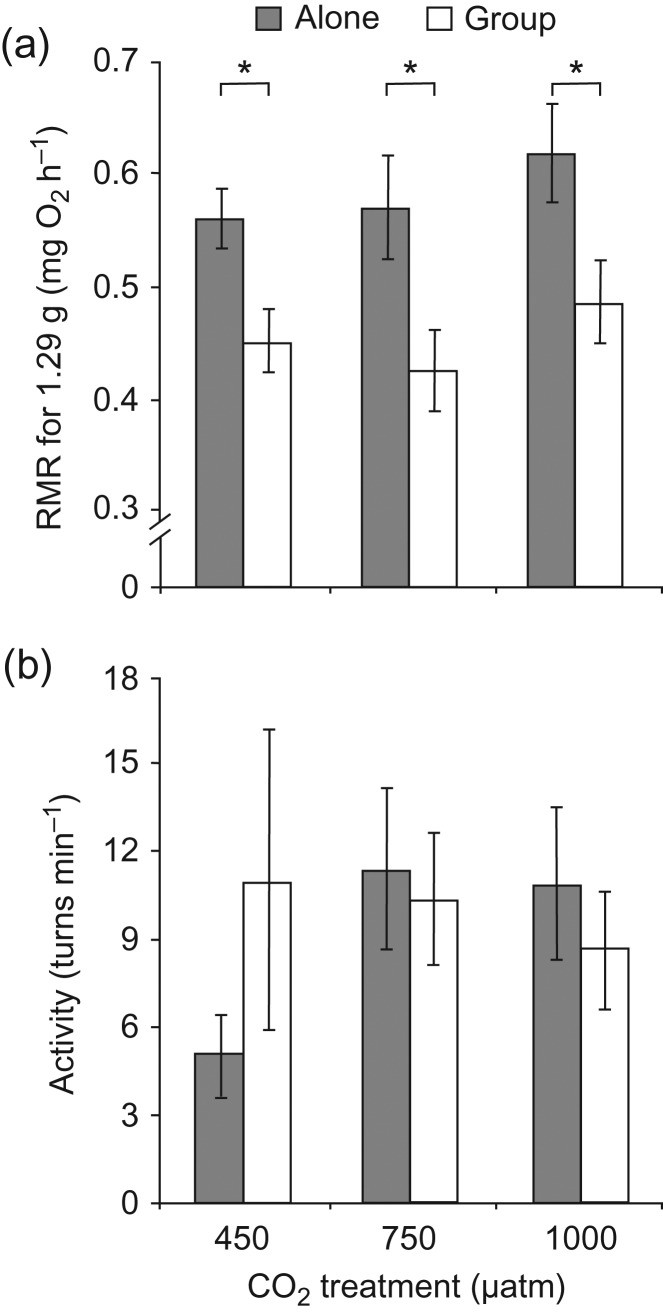
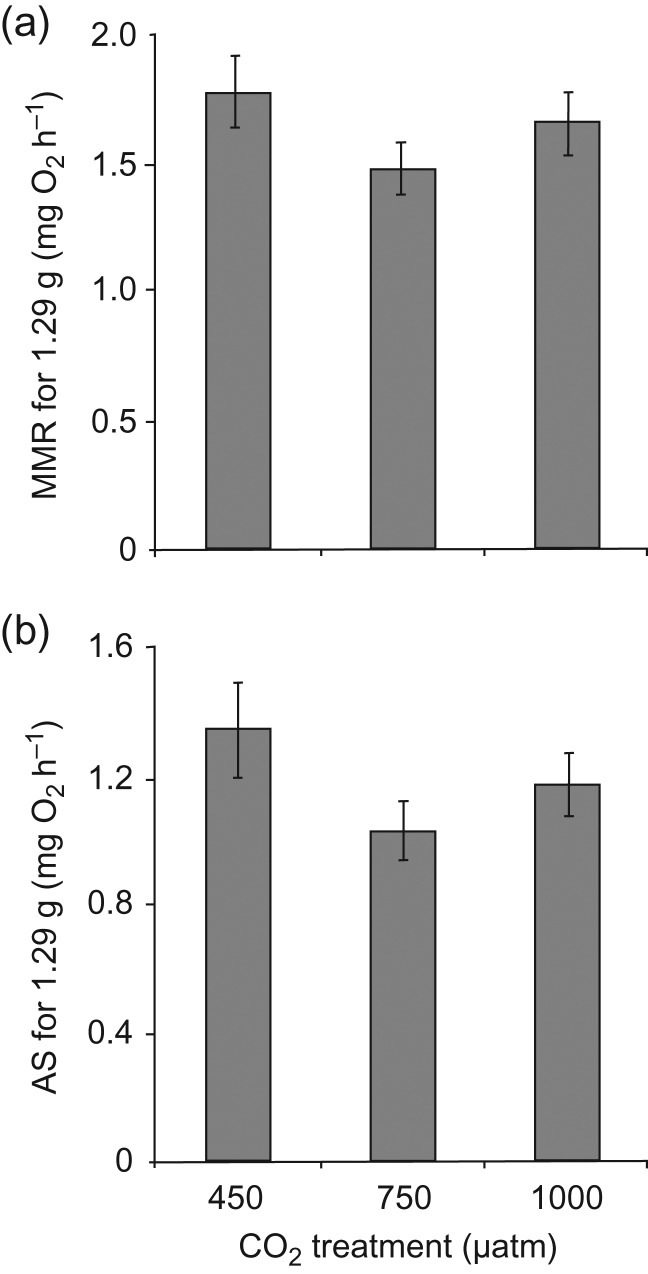
The RMRgroup was significantly lower than RMRalone (Fig. 4a; F1,26 = 33.84, P < 0.001). Respirometry treatment had a comparable effect on RMR in individuals from all CO2 treatments (F2,27 = 0.73, P = 0.490), and there was no significant interaction between testing and CO2 treatment (F2,26 = 0.43, P = 0.714). Neither testing (F1,26 = 0.31, P = 0.583) nor CO2 treatment (F2,27 = 0.32, P = 0.732) exerted a significant effect on activity (Fig. 4b), with individuals exhibiting an overall mean of 9.5 turns/min during daylight hours (range, 0.5–55.7 turns/min). The MMR (F1,27 = 1.15, P = 0.294) and AS (F1,27 = 1.93, P = 0.176) were not significantly different between CO2 treatments (Fig. 5).
Figure 4:
Effect of CO2 and testing treatment on the routine metabolic rate (RMR; in milligrams of O2 per hour; a) and activity (number of 180° turns/min; b). In these panels, metabolic rate measures were mass corrected by using residuals of the relationship between the logarithm of the body mass and logarithm of whole-animal metabolic rate added to the fitted value for mass = 1.29 g, the mean mass of all fish used in the study. Error bars are SEM, and n = 10 for all treatments. Asterisks indicate statistical significance (*P < 0.05). Statistical analysis was conducted on whole-animal metabolic rates, with body mass as a covariate.
Figure 5:
Effect of CO2 on maximal metabolic rate (MMR; in milligrams of O2 per hour; a) and aerobic scope (AS; in milligrams of O2 per hour; b). In these panels, metabolic rate measures were mass corrected by using residuals of the relationship between the logarithm of body mass and logarithm of whole-animal metabolic rate added to the fitted value for mass = 1.29 g, the mean mass of all fish used in the study. Error bars are SEM, and n = 10 for all treatments. Statistical analysis was conducted on whole-animal metabolic rates, with body mass as a covariate.
Discussion
Elevated CO2 disrupted familiarity, but not the calming effect, in C. viridis. As familiarity is important for a range of processes in shoaling fish (Ward and Hart, 2003), many of the benefits of group living may be altered in changing environmental conditions. However, the calming benefit of shoaling on metabolic rate was maintained in high-CO2 conditions, indicating that the benefits of group living on overall metabolic demand will be likely to persist under projected future pCO2.
The loss of familiarity with elevated CO2 could have occurred as a result of a number of possible mechanisms. First, social recognition may have been disrupted if fish lost the sensory abilities necessary for identifying individuals, particularly by olfactory cues (Partridge and Pitcher, 1980; Brown and Smith, 1994; Ward et al., 2002; Munday et al., 2009b). The changes in shoal-mate association found with rising CO2 in the present study are consistent with previous work that tested for preferences between conspecifics from different reefs (home vs. foreign reef site) in the cardinalfish, Cheilodipterus quinquelineatus (Devine et al., 2012). In that study, fish lost the association for conspecifics from their home reef under elevated CO2, suggesting that association preferences generally may be altered. Alternatively, individuals may still be able to recognize familiar shoal-mates, but may simply have lost the preference to shoal with familiar rather than unfamiliar individuals. Many previous studies have established that shoaling fish prefer to group with familiar conspecifics (e.g. Magurran et al., 1994; Griffiths and Magurran, 1997; Bhat and Magurran, 2006; Edenbrow and Croft, 2012), but few have investigated what factors may cause this preference to be lost (Granroth-Wilding and Magurran, 2013). Neural circuitry is likely to contribute to the development of social behaviour and preferences in fish species (Dreosti et al., 2015). As neurotransmitter function may be impaired by elevated pCO2 conditions (Nilsson et al., 2012; Heuer and Grosell, 2014), this effect may account for the loss of preferential association with familiar shoal-mates. In addition, memory and learning play an integral role in familiarity, by allowing individuals to learn about their shoal-mates and remember their identity. Although it is known that learning is interrupted by elevated CO2 (Ferrari et al., 2012a; Chivers et al., 2014), no studies have yet examined effects on fish memory. Nevertheless, a disruption to memory could account for the loss of association preference found here in the high-CO2 treatments.
These mechanisms of familiarity disruption could have a number of ecological implications. If social recognition is disrupted, as a result of either a loss of sensory abilities or a loss of memory, a number of important processes may be affected. First, social learning may be impaired as individuals are unable to distinguish between informed and naïve shoal-mates (Swaney et al., 2001). Second, Galhardo et al. (2012) found that personality traits, such as exploratory behaviour and boldness, decrease in fishes in unfamiliar shoals, suggesting that disruption to social recognition could impact fishes’ personality traits. Third, defensive behaviours may become less effective, as unfamiliar shoals are slower to react to a predator threat than familiar shoals (Griffiths et al., 2004). Alternatively, if only the preference for the familiar shoal is lost, a range of traits related to shoaling dynamics could be impacted. First, shoal fidelity may decrease, because, without the preference for the familiar shoal, the trade-offs of staying with the familiar shoal vs. migrating to a more suitable, unfamiliar shoal may shift (Muleta and Schausberger, 2013). Second, cooperation between shoal-mates may decrease, because individuals’ perception of shoal-mates could shift from that of a collaborator to a competitor in this different social context as the reliability of reciprocal cooperation may be compromised (Granroth-Wilding and Magurran, 2013; Engelmann and Herrmann, 2016).
Given the benefits of familiarity to a range of important shoaling processes, including foraging and social learning (Seppä et al., 2001; Swaney et al., 2001; Atton et al., 2014), we expected the magnitude of the calming effect to suffer under elevated CO2. However, unlike familiarity, the calming effect was maintained, and even enhanced, under high CO2. This surprising result implies that familiarity and the calming effect may rely on different mechanisms. Previous studies have highlighted the central role of olfactory sensing abilities in social recognition of familiar shoal-mates (Partridge and Pitcher, 1980; Brown and Smith, 1994; Ward et al., 2002), which appear to be more vulnerable to the effects of elevated CO2 than the visual system (Lönnstedt et al., 2013). Therefore, unlike familiarity, the calming effect may be able to compensate for olfactory impairments using visual cues, as has previously been found for anti-predator behaviours (Lönnstedt et al., 2013). The importance of shoaling to energy budgets could increase in the presence of environmental stressors, as evidenced by the increasing magnitude of the calming effect with higher pCO2. Any reduction in metabolic demands (like those induced by shoaling) could aid in coping with the projected rise in energy demand associated with changing environmental conditions. In addition, no effect of CO2 was found on any of the metabolic traits measured (including MRmin, RMR, MMR and AS). Although some studies have indicated an effect of CO2 on metabolism, most have not, indicating that the results presented here are consistent with many of the studies in the literature (Lefevre, 2016).
The initial physiological reaction to stress increased with high CO2. This result is consistent with greater incidences of anxious behaviour in fish exposed to elevated CO2 (Hamilton et al., 2014). In social species, such as C. viridis, this amplified stress response could stem from the mechanisms presented above for familiarity. If social recognition or memory were lost, individuals may have perceived their shoal-mates to be unfamiliar, owing to the inability to distinguish between individuals, although this effect was not evident between the alone- and group-testing treatments. Stress hormones, such as cortisol, increase when individuals are exposed to an unfamiliar shoal (Yue et al., 2006), which could account for the greater acute stress response that was measured with high CO2. Conversely, the increased metabolic stress response may have contributed to the loss of preference for familiar shoal-mates. Shoaling motivation increases with stress and predation risk (Croft et al., 2009; Stier et al., 2013); therefore, the desire to shoal may outweigh the strategic choice to shoal with familiar fish in elevated-CO2 conditions. No matter what the underlying mechanism is, these results indicate that shoaling may become even more important in altered environmental conditions, with the potential to be used as a behavioural compensatory mechanism (Connell and Ghedini, 2015).
Overall activity (total shoal visits and number of 180° turns) did not vary in either experiment in response to CO2 treatment, indicating that differences in activity cannot explain the results found. Previous studies have reported a range of findings on the effect of CO2 on activity. For instance, Munday et al. (2014a) reported an increase in the activity of reef fish species, and Regan et al. (2016) found a reduction in the activity of a river catfish species. In contrast, Nowicki et al. (2012) found no effect of elevated CO2 on general activity in clownfish, and Munday et al. (2016) measured no effect in larvae of a pelagic kingfish species. These trends imply that CO2 may have variable effects on activity depending on a range of traits, such as the natural mobility of the study organism, ontogenetic stage and environmental conditions.
As with all ocean acidification research, these results must be viewed in the context in which the study was conducted. This type of study must be conducted in the laboratory in order to expose fish to controlled, elevated-CO2 conditions. Although every effort is made to make these conditions as realistic as possible, the laboratory setting may impart unknown effects on our results. Importantly, fishes will incrementally reach projected CO2 conditions over a period of many decades, so there may be the potential for acclimation or adaptation over this time period (Munday et al., 2013b). A longer exposure period to elevated CO2 might lead to different effects on behaviour. Parental exposure to elevated CO2 does not appear to ameliorate impairments to a number of relevant behavioural traits and sensory systems (Welch et al., 2014), but whether adaptation could reduce the behavioural effects of high CO2 over longer time frames is unknown.
Future research should work to tease apart which mechanism (social recognition, preference for familiarity or memory) is more likely to be causing the effect of CO2 on familiarity. Familiarity is important for many aspects of shoaling dynamics (Swaney et al., 2001; Griffiths et al., 2004), so its disruption may create further carry-over effects on a range of processes. The maintenance of the calming effect in the presence of high CO2, however, highlights the complexity of shoal dynamics and illustrates that many processes, in addition to familiarity, influence the benefits of shoaling.
Acknowledgements
We thank the Lizard Island Research Station staff, Ross Barrett, Paloma Matis, Ana Guerra, Laura Smith, Carly Giosio and Stephen Brown for logistical support and Rhondda Jones for statistical advice. This research was conducted under James Cook University Animal Ethics approval number A2103. We also thank three anonymous reviewers for their valuable feedback on an earlier version of the manuscript. The data from this manuscript are publicly available at the Tropical Data Hub Research Data repository (http://dx.doi.org/10.4225/28/58080af2157bf).
Funding
Funding was provided by an Australian Postgraduate Award, International Postgraduate Research Scholarship, Lizard Island Reef Research Foundation Doctoral Fellowship, Great Barrier Reef Marine Park Authority Science for Management Award and James Cook University Graduate Research Scheme to L.E.N., a NERC Advanced Fellowship (NE/J019100/1) to S.S.K., ARC Future Fellowship to P.L.M., ARC Discovery Grant to M.I.M. and ARC Centre of Excellence for Coral Reef Studies funding to M.I.M. and P.L.M.
References
- Atton N, Galef BJ, Hoppitt W, Webster MM, Laland KN (2014) Familiarity affects social network structure and discovery of prey patch locations in foraging stickleback shoals. Proc Biol Sci 281: 20140579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge S. (2015) Lizard Island Sensor Float 2 Salinity @10.9m. In IMOS FAIMMS Sensor Network data. http://data.aims.gov.au/metadataviewer/faces/view.xhtml?uuid=9be0f96c-e3ee-4e71-9dcf-4199446bf37f.
- Bates D, Maechler M (2009) lme4: linear mixed-effects models using S4 classes. In R package version 3.1-122. https://cran.r-project.org/web/packages/lme4/index.html.
- Bhat A, Magurran AE (2006) Benefits of familiarity persist after prolonged isolation in guppies. J Fish Biol 68: 759–766. [Google Scholar]
- Briffa M, de la Haye K, Munday PL (2012) High CO2 and marine animal behaviour: potential mechanisms and ecological consequences. Mar Pollut Bull 64: 1519–1528. [DOI] [PubMed] [Google Scholar]
- Brown GE, Smith R.JF (1994) Fathead minnows use chemical cues to discriminate natural shoalmates from unfamiliar conspecifics. J Chem Ecol 20: 3051–3061. [DOI] [PubMed] [Google Scholar]
- Brown MR, Fisher LA, Rivier J, Spiess J, Rivier C, Vale W (1982) Corticotropin-releasing factor: effects on the sympathetic nervous system and oxygen consumption. Life Sci 30: 207–210. [DOI] [PubMed] [Google Scholar]
- Chabot D, Steffensen JF, Farrell AP (2016) The determination of standard metabolic rate in fishes. J Fish Biol 88: 81–121. [DOI] [PubMed] [Google Scholar]
- Chivers DP, McCormick MI, Nilsson GE, Munday PL, Watson SA, Meekan MG, Mitchell MD, Corkill KC, Ferrari MC (2014) Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Glob Change Biol 20: 515–522. [DOI] [PubMed] [Google Scholar]
- Chung WS, Marshall NJ, Watson SA, Munday PL, Nilsson GE (2014) Ocean acidification slows retinal function in a damselfish through interference with GABA-A receptors. J Exp Biol 217: 323–326. [DOI] [PubMed] [Google Scholar]
- Collins M, Knuttl R, Arblaster J, Dufresne JL, Flchefet T, Friedlingstein P, Gao X, Gutowski WJ, Johns T, Krinner G et al. (2013) Long-term climate change: projections, commitments and irreversibility In Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM,eds, Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK and New York, NY, USA. [Google Scholar]
- Connell SD, Ghedini G (2015) Resisting regime-shifts: the stabilising effect of compensatory processes. Trends Ecol Evol 30: 513–515. [DOI] [PubMed] [Google Scholar]
- Cornwall CE, Hurd CL (2015) Experimental design in ocean acidification research: problems and solutions. ICES J Mar Sci 73: 572–581. [Google Scholar]
- Couturier CS, Stecyk JA, Rummer JL, Munday PL, Nilsson GE (2013) Species-specific effects of near-future CO2 on the respiratory performance of two tropical prey fish and their predator. Comp Biochem Physiol A Mol Integr Physiol 166: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft DP, Darden SK, Ruxton GD (2009) Predation risk as a driving force for phenotypic assortment: a cross-population comparison. Proc Biol Sci 276: 1899–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deigweiher K, Koschnick N, Pörtner HO, Lucassen M (2008) Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am J Physiol Regul Integr Comp Physiol 295: R1660–R1670. [DOI] [PubMed] [Google Scholar]
- Devine BM, Munday PL, Jones GP (2012) Homing ability of adult cardinalfish is affected by elevated carbon dioxide. Oecologia 168: 269–276. [DOI] [PubMed] [Google Scholar]
- Dickson AG. (1990) Standard potential of the reaction: AgCl(s) + 1/2H2(g) = Ag(s) + HCl (aq), and the standard acidity constant of the ion HSO4− in synthetic sea water from 273.15 to 318.15 K. J Chem Thermodyn 22: 113–127. [Google Scholar]
- Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res 34: 1733–1743. [Google Scholar]
- Dixson DL, Munday PL, Jones GP (2010) Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett 13: 68–75. [DOI] [PubMed] [Google Scholar]
- Dlugokencky E, Tans P (2016) Trends in atmospheric carbon dioxide. www.esrl.noaa.gov/gmd/ccgg/trends/.
- Domenici P, Allan B, McCormick MI, Munday PL (2011) Elevated carbon dioxide affects behavioural lateralization in a coral reef fish. Biol Lett 8: 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Ann Rev Mar Sci 1: 169–192. [DOI] [PubMed] [Google Scholar]
- Dreosti E, Lopes G, Kampff AR, Wilson SW (2015) Development of social behavior in young zebrafish. Front Neural Circuit 9: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenbrow M, Croft DP (2012) Kin and familiarity influence association preferences and aggression in the mangrove killifish Kryptolebias marmoratus. J Fish Biol 80: 503–518. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Herrmann E (2016) Chimpanzees trust their friends. Curr Biol 26: 252–256. [DOI] [PubMed] [Google Scholar]
- Enzor LA, Zippay ML, Place SP (2013) High latitude fish in a high CO2 world: synergistic effects of elevated temperature and carbon dioxide on the metabolic rates of Antarctic notothenioids. Comp Biochem Physiol A Mol Integr Physiol 164: 154–161. [DOI] [PubMed] [Google Scholar]
- Esbaugh AJ, Heuer R, Grosell M (2012) Impacts of ocean acidification on respiratory gas exchange and acid–base balance in a marine teleost, Opsanus beta. J Comp Physiol B 182: 921–934. [DOI] [PubMed] [Google Scholar]
- Esbaugh AJ, Ern R, Nordi WM, Johnson AS (2016) Respiratory plasticity is insufficient to alleviate blood acid–base disturbances after acclimation to ocean acidification in the estuarine red drum, Sciaenops ocellatus. J Comp Physiol B 186: 97–109. [DOI] [PubMed] [Google Scholar]
- Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65: 414–432. [Google Scholar]
- Farrell AP. (2016) Pragmatic perspective on aerobic scope: peaking, plummeting, pejus and apportioning. J Fish Biol 88: 322–343. [DOI] [PubMed] [Google Scholar]
- Ferrari MCO, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP (2011. a) Intrageneric variation in antipredator responses of coral reef fishes affected by ocean acidification: implications for climate change projections on marine communities. Glob Change Biol 17: 2980–2986. [Google Scholar]
- Ferrari MCO, McCormick MI, Munday PL, Meekan MG, Dixson DL, Lonnstedt Ö, Chivers DP (2011. b) Putting prey and predator into the CO2 equation – qualitative and quantitative effects of ocean acidification on predator–prey interactions. Ecol Lett 14: 1143–1148. [DOI] [PubMed] [Google Scholar]
- Ferrari MC, Manassa RP, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP (2012. a) Effects of ocean acidification on learning in coral reef fishes. PLoS One 7: e31478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MCO, McCormick MI, Munday PL, Meekan M, Dixson DL, Lonnstedt O, Chivers DP (2012. b) Effects of ocean acidification on visual risk assessment in coral reef fishes. Funct Ecol 26: 553–558. [Google Scholar]
- Galhardo L, Vitorino A, Oliveira RF (2012) Social familiarity modulates personality trait in a cichlid fish. Biol Lett 8: 936–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattuso JP, Gao K, Lee K, Rost B, Schulz KG (2010) Approaches and Tools to Manipulate the Carbonate Chemistry. Luxembourg, Publications office of the European Union.
- Granroth-Wilding HM, Magurran AE (2013) Asymmetry in pay-off predicts how familiar individuals respond to one another. Biol Lett 9: 20130025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths SW, Magurran AE (1997) Familiarity in schooling fish: how long does it take to acquire. Anim Behav 53: 945–949. [Google Scholar]
- Griffiths SW, Brockmark S, Höjesjö J, Johnsson JI (2004) Coping with divided attention: the advantage of familiarity. Proc Biol Sci 271, 695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton TJ, Holcombe A, Tresguerres M (2014) CO2-induced ocean acidification increases anxiety in rockfish via alteration of GABAA receptor functioning. Proc Biol Sci 281: 20132509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari P, Pumpanen J, Huotari J, Kolari P, Grace J, Vesala T, Ojala A (2008) High-frequency measurements of productivity of planktonic algae using rugged nondispersive infrared carbon dioxide probes. Limnol Oceanogr Methods 6: 347–354. [Google Scholar]
- Hennessy MB, Kaiser S Sachser N (2009) Social buffering of the stress response: diversity, mechanisms, and functions. Front Neuroendocrinol 30: 470–482. [DOI] [PubMed] [Google Scholar]
- Heuer RM, Grosell M (2014) Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am J Physiol Regul Integr Comp Physiol 307: R1061–R1084. [DOI] [PubMed] [Google Scholar]
- Hoey AS, McCormick MI (2006) Effects of subcutaneous fluorescent tags on the growth and survival of a newly settled coral reef fish, Pomacentrus amboinensis (Pomacentridae). Proceedings of the 10th International Coral Reefs Symposium 2006: 420–425.
- Hughes GM. (1973) Respiratory responses to hypoxia in fish. Am Zool 13: 475–489. [Google Scholar]
- Ishimatsu A, Hayashi M, Kikkawa T (2008) Fishes in high-CO2, acidified oceans. Mar Ecol Prog Ser 373: 295–302. [Google Scholar]
- Jutfelt F, Hedgärde M (2013) Atlantic cod actively avoid CO2 and predator odour, even after long-term CO2 exposure. Front Zool 10: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutfelt F, Hedgärde M (2015) Juvenile Atlantic cod behavior appears robust to near-future CO2 levels. Front Zool 12: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutfelt F, Bresolin de Souza K, Vuylsteke A, Sturve J (2013) Behavioural disturbances in a temperate fish exposed to sustained high-CO2 levels. PLoS One 8: e65825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen SS, Marras S, McKenzie DJ (2011) Fuel, fasting, fear: routine metabolic rate and food deprivation exert synergistic effects on risk-taking in individual juvenile European sea bass. J Anim Ecol 80: 1024–1033. [DOI] [PubMed] [Google Scholar]
- Killen SS. (2014) Growth trajectory influences temperature preference in fish through an effect on metabolic rate. J Anim Ecol 83: 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, Ruxton GD (2002) Living in Groups. Oxford University Press, Oxford. [Google Scholar]
- Lefevre S. (2016) Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO2 and their interaction. Conserv Physiol 4: cow009; doi:10.1093/conphys/cow009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Quéré C, Peters GP, Andres RJ, Andrew RM, Boden T, Ciais P, Friedlingstein P, Houghton RA, Marland G, Moriarty R et al. (2013). Global carbon budget 2013. Earth Sys Sci Data Disc 6: 689–760. [Google Scholar]
- Lönnstedt OM, Munday PL, McCormick MI, Ferrari MCO, Chivers DP (2013) Ocean acidification and responses to predators: can sensory redundancy reduce the apparent impacts of elevated CO2 on fish. Ecol Evol 3: 3565–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil BI, Sasse TP (2016) Future ocean hypercapnia driven by anthropogenic amplification of the natural CO2 cycle. Nature 529: 383–386. [DOI] [PubMed] [Google Scholar]
- Magurran AE, Seghers BH, Shaw PW, Carvalho GR (1994) Schooling preferences for familiar fish in the guppy, Poecilia reticulata. J Fish Biol 45: 401–406. [Google Scholar]
- Maneja RH, Frommel AY, Browman HI, Geffen AJ, Folkvord A, Piatkowski U, Durif CMF, Bjelland R, Skiftesvik AB, Clemmesen C (2015) The swimming kinematics and foraging behavior of larval Atlantic herring (Clupea harengus L.) are unaffected by elevated pCO2 . J Exp Mar Biol Ecol 466: 42–48. [Google Scholar]
- Masson-Delmotte V, Schulz M, Abe-Ouchi A, Beer J, Ganopolski A, González Rouco JF, Jansen E, Lambeck K, Luterbacher J, Naish T et al. (2013) Information from Paleoclimate Archives In Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, eds, Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK and New York, NY, USA. [Google Scholar]
- Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM (1973) Measurements of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18: 897–907. [Google Scholar]
- Melzner F, Göbel S, Langenbuch M, Gutowska MA, Pörtner HO, Lucassen M (2009) Swimming performance in Atlantic Cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater PCO2 . Aquat Toxicol 92: 30–37. [DOI] [PubMed] [Google Scholar]
- Miller GM, Watson S, Donelson JM, McCormick MI, Munday PL (2012) Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat Clim Change 2: 858–861. [Google Scholar]
- Morgan JD, Iwama GK (1996) Cortisol-induced changes in oxygen consumption and ionic regulation in coastal cutthroat trout (Oncorhynchus clarki clarki) parr. Fish Physiol Biochem 15: 385–394. [DOI] [PubMed] [Google Scholar]
- Muleta MG, Schausberger P (2013) Smells familiar: group-joining decisions of predatory mites are mediated by olfactory cues of social familiarity. Anim Behav 86: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Crawley NE, Nilsson GE (2009. a) Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar Ecol Prog Ser 388: 235–242. [Google Scholar]
- Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Doving KB (2009. b) Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci USA 106: 1848–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Donelson JM, Dixson DL, Endo GG (2009. c). Effects of ocean acidification on the early life history of a tropical marine fish. Proc Biol Sci 276: 3275–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP (2010) Replenishment of fish populations is threatened by ocean acidification. Proc Natl Acad Sci USA 107: 12930–12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Pratchett MS, Dixson DL, Donelson JM, Endo GGK, Reynolds AD, Knuckey R (2013. a) Elevated CO2 affects the behavior of an ecologically and economically important coral reef fish. Mar Biol 160: 2137–2144. [Google Scholar]
- Munday PL, Warner RR, Monro K, Pandolfi JM, Marshall DJ (2013. b) Predicting evolutionary responses to climate change in the sea. Ecol Lett 16: 1488–1500. [DOI] [PubMed] [Google Scholar]
- Munday PL, Cheal AJ, Dixson DL, Rummer JL, Fabricius KE (2014. a) Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat Clim Change 4: 487–492. [Google Scholar]
- Munday PL, Watson S-A, Chung W-S, Marshall NJ, Nilsson GE (2014. b) Response to ‘The importance of accurate CO2 dosing and measurement in ocean acidification studies’. J Expl Biol 217: 1828–1829. [DOI] [PubMed] [Google Scholar]
- Munday PL, Watson S-A, Parsons DM, King A, Barr NG, McLeod IM, Allan BJM Pether SMJ (2016) Effects of elevated CO2 on early life history development of the yellowtail kingfish, Seriola lalandi, a large pelagic fish. ICES J Mar Sci 73: 641–649. [Google Scholar]
- Nadler LE, Killen SS, McClure EC, Munday PL, McCormick MI (2016) Shoaling reduces metabolic rate in a gregarious coral reef fish species. J Exp Biol 219: 2802–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerken I, Munday PL (2016) Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob Chang Biol 22: 974–989. [DOI] [PubMed] [Google Scholar]
- Nelson JA. (2016) Oxygen consumption rate v. rate of energy utilization of fishes: a comparison and brief history of the two measurements. J Fish Biol 88: 10–25. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Dixson DL, Domenici P, McCormick MI, Serenson C, Watson S-A, Munday PL (2012) Near-future carbon dioxide levels alter fish behavior by interfering with neurotransmitter function. Nat Clim Change 2: 201–204. [Google Scholar]
- Nowicki JP, Miller GM, Munday PL (2012) Interactive effects of elevated temperature and CO2 on foraging behavior of juvenile coral reef fish. J Exp Mar Biol Ecol 412: 46–51. [Google Scholar]
- Partridge BL, Pitcher TJ (1980) The sensory basis of fish schools: relative roles of lateral line and vision. J Comp Phys A 135: 315–325. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team (2016) nlme: linear and nonlinear mixed effects models. In R package version 3.1-122. https://cran.r-project.org/web/packages/nlme/index.html.
- Pitcher TJ, Parrish JK (1993) The functions of shoaling behaviour. In TJ Pitcher 2nd ed, The behaviour of teleost fishes, Chapman and Hall, London, p. 363–439. 715 p. [Google Scholar]
- Pörtner HO, Langenbuch M, Reipschläger A (2004) Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J Oceanogr 60: 705–718. [Google Scholar]
- R Development Core Team (2015) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Randall JE, Allen GR, Steene RC (1997) Fishes of the Great Barrier Reef and Coral Sea. Honolulu, University of Hawaii Press. [Google Scholar]
- Regan MD, Turko AJ, Heras J, Andersen MK, Lefevre S, Wang T, Bayley M, Brauner CJ, Huong do TT, Phuong NT et al. (2016) Ambient CO2, fish behaviour and altered GABAergic neurotransmission: exploring the mechanism of CO2-altered behaviour by taking a hypercapnia dweller down to low CO2 levels. J Exp Biol 219: 109–118. [DOI] [PubMed] [Google Scholar]
- Roberts G. (1996) Why individual vigilance declines as group size increases. Anim Behav 51: 1077–1086. [Google Scholar]
- Roche DG, Binning SA, Bosiger Y, Johansen JL, Rummer JL (2013) Finding the best estimates of metabolic rates in a coral reef fish. J Exp Biol 216: 2103–2110. [DOI] [PubMed] [Google Scholar]
- Rodgers GG, Tenzing P, Clark TD (2016) Experimental methods in aquatic respirometry: the importance of mixing devices and accounting for background respiration. J Fish Biol 88: 65–80. [DOI] [PubMed] [Google Scholar]
- Rossi T, Nagelkerken I, Pistevos JC, Connell SD (2016) Lost at sea: ocean acidification undermines larval fish orientation via altered hearing and marine soundscape modification. Biol Lett 12: 20150937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummer JL, Stecyk JAW, Couturier CS, Watson SA, Nilsson GE, Munday PL (2013) Elevated CO2 enhances aerobic scope of a coral reef fish. Conserv Physiol 1: cot023; doi:10.1093/conphys/cot023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä T, Laurila A, Peuhkuri N, Piironen J, Lower N (2001) Early familiarity has fitness consequences for Arctic char (Salvelinus alpinus) juveniles. Can J Fish Aquat Sci 58: 1380–1385. [Google Scholar]
- Shaw E. (1978) Schooling fishes: the school, a truly egalitarian form of organization in which all members of the group are alike in influence, offers substantial benefits to its participants. Amer Sci 66: 166–175. [Google Scholar]
- Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY (2011) Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol Lett 7: 917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen JF. (1989) Some errors in respirometry of aquatic breathers: how to avoid and correct for them. Fish Physiol Biochem 6: 49–59. [DOI] [PubMed] [Google Scholar]
- Stier AC, Geange SW, Bolker BM (2013) Predator density and competition modify the benefits of group formation in a shoaling reef fish. Oikos 122: 171–178. [Google Scholar]
- Strobel A, Bennecke S, Leo E, Mintenbeck K, Pörtner HO, Mark FC (2012). Metabolic shifts in the Antarctic fish Notothenia rossii in response to rising temperature and PCO2 . Front Zool 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen MB, Bushnell PG, Steffensen JF (2016) Design and setup of intermittent-flow respirometry system for aquatic organisms. J Fish Biol 88: 26–50. [DOI] [PubMed] [Google Scholar]
- Swaney W, Kendal J, Capon H, Brown C, Laland KN (2001) Familiarity facilitates social learning of foraging behaviour in the guppy. Anim Behav 62: 591–598. [Google Scholar]
- Ward AJW, Hart PJB (2003) The effects of kin and familiarity on interactions between fish. Fish Fish 4: 348–358. [Google Scholar]
- Ward AJW, Axford S, Krause J (2002) Mixed-species shoaling in fish: the sensory mechanisms and costs of shoal choice. Behav Ecol Sociobiol 52: 182–187. [Google Scholar]
- Ward AJW, Axford S, Krause J (2003) Cross-species familiarity in shoaling fishes. Proc Biol Sci 270: 1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AJW, Herbert-Read JE, Sumpter DJT, Krause J (2011) Fast and accurate decisions through collective vigilance in fish shoals. Proc Natl Acad Sci USA 108: 2312–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MJ, Watson S, Welsh JQ, McCormick MI, Munday PL (2014) Effects of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nat Clim Change 4: 1086–1089. [Google Scholar]
- Wittmann AC, Pörtner HO (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Change 3: 995–1001. [Google Scholar]
- Yue S, Duncan IJH, Moccia RD (2006) Do differences in conspecific body size induce social stress in domestic rainbow trout. Env Biol Fish 76: 425–431. [Google Scholar]