Abstract
Background and objectives
Elevated levels of urinary kidney injury molecule-1 and neutrophil gelatinase–associated lipocalin are associated with negative outcomes in CKD. Our study aimed to explore the prognostic accuracy of blood levels of kidney injury molecule-1 and neutrophil gelatinase–associated lipocalin for progression to ESRD, major adverse cardiovascular events, and death in a large cohort of adult patients with all–cause nondialysis–dependent CKD stages 3–5. We considered whether these factors improve prediction in relation to traditional biomarkers and clinical parameters.
Design, setting, participants, & measurements
Kidney injury molecule-1 and neutrophil gelatinase–associated lipocalin were measured on baseline plasma samples from 1982 patients who were recruited to the Chronic Renal Insufficiency Standards Implementation Study between the start of June of 2002 and the start of June of 2013. Associations with study end points were assessed using Cox regression models, receiver operator characteristic curve analyses, and reclassification statistics.
Results
Over a median follow-up of 29.5 months (interquartile range, 14.9–53.5), 21.6% of patients progressed to ESRD, 27% died, and 6.6% suffered a major adverse cardiovascular event. Higher blood levels of kidney injury molecule-1 and neutrophil gelatinase–associated lipocalin were independently associated with a greater risk for ESRD (hazard ratio, 1.25; 95% confidence interval, 1.10 to 1.43; P<0.001 and hazard ratio, 1.35; 95% confidence interval, 1.14 to 1.59; P≤0.001, respectively, per 1 SD higher biomarker concentration). There was no association with risk for cardiovascular events or death. The addition of biomarkers to our baseline risk model of traditional clinical characteristics and laboratory parameters did not significantly improve model discrimination or risk reclassification.
Conclusions
In patients with moderate to severe CKD, kidney injury molecule-1 and neutrophil gelatinase–associated lipocalin blood levels are independent risk factors for progression to ESRD. Additional studies are needed to establish the utility and cost-effectiveness of these novel biomarkers in the clinical setting.
Keywords: KIM-1; NGAL; biomarkers; risk prediction; clinical outcomes; Acute-Phase Proteins; Adult; Biomarkers; follow-up studies; humans; Kidney Failure, Chronic; Lipocalins; Proto-Oncogene Proteins; ROC Curve; renal dialysis; renal insufficiency, chronic; risk factors; LCN2 protein, human
Introduction
CKD is associated with high rates of cardiovascular disease and death (1–3). Accurately predicting an individual’s risk for adverse outcomes in CKD remains a challenge (4). Over the past decade, research interest has focused on the identification of novel biomarkers to improve risk prediction in this population. Among different candidate biomarkers, kidney injury molecule-1 (KIM-1) and neutrophil gelatinase–associated lipocalin (NGAL) have been identified as markers of renal injury and promising biomarkers for investigation in this population (5).
KIM-1 is a glycoprotein localized to the apical membrane of the proximal tubule. It is undetectable in the normal kidney but upregulated in response to ischemic or toxic insults (6). In AKI, urinary KIM-1 has been associated with increased risk for death and requirement for dialysis (7). Blood levels of KIM-1 have been shown to correlate with prevalent CKD (8) and predict progression of kidney disease in patients with type 1 diabetes (9).
NGAL is upregulated and secreted in response to kidney injury (10). Urinary NGAL levels are a sensitive early marker of AKI (11,12). Numerous studies in general CKD populations and disease-specific subgroups have shown associations between higher urinary and blood NGAL levels and progression of kidney disease (13–18), with urinary levels having been assessed in a large cohort of >3000 patients and blood levels having been assessed in smaller series. Any improvements in the prediction of renal outcomes with urinary NGAL have been modest (18,19).
KIM-1 and NGAL are promising novel biomarkers of renal tubular injury. To our knowledge, no studies have investigated blood levels of KIM-1 and NGAL as independent risk factors for progression, major adverse cardiovascular events, and death in a large diverse cohort of patients with nondialysis-dependent CKD. We aimed to evaluate the usefulness of these biomarkers for risk prediction in our population both as a supplement to and compared with existing, commonly used biomarkers (such as creatinine and urinary protein) and clinical covariates (such as age and BP), thereby determining both the additive value of these markers and whether they could be used as a replacement to traditional risk factors.
Materials and Methods
Study Design and Patient Population
The Chronic Renal Insufficiency Standards Implementation Study (CRISIS) is a prospective observational study of outcome in nondialysis kidney disease. The study was started in 2002 and enrolled over 3000 patients between 2002 and 2015. Details of the CRISIS cohort have been published previously (20–23). In brief, all patients ages 18 years old or over who are referred to our renal center (Salford Royal National Health Service Foundation Trust) for management of CKD and have an eGFR<60 ml/min per 1.73 m2 but no immediate requirement for dialysis are eligible for recruitment. Patients are managed in accordance with standard clinical practice guidelines and followed until death or initiation of RRT (dialysis or transplantation). Demographic data are recorded at baseline and annually during study follow-up. Blood samples are drawn for standard clinical tests, and additional samples are immediately centrifuged and stored at −80°C for subsequent biomarker analyses; 2000 baseline patient plasma samples were available for this study. Eighteen patients were excluded because of incomplete baseline data, leaving 1982 samples for the analyses.
The regional ethics committee approved the study, and all enrolled patients provide written informed consent (study approval 05/Q1404/187).
Biomarker Analyses
All biomarker analyses were performed on baseline plasma samples. KIM-1 and NGAL were quantified one time with the MESO QuickPlex SQ 120 Automate using electrochemiluminescence from Mesoscale Discovery Systems (Rockville, MD). Using internal standards provided by the manufacturer, measured intra–assay variation coefficients (VCs) were 3.0% and 2.5% for KIM-1 concentrations of 78.1 and 312.5 pg/ml, respectively (n=34). Measured intra–assay VCs were 5.60% and 3.90% for NGAL concentrations of 625 and 2500 pg/ml, respectively (n=34). At the same concentrations, interassay VCs were 5.45% and 3.02% for KIM-1, respectively, and 3.90% and 5.90% for NGAL, respectively (n=17). eGFR was calculated from Isotope Dilution Mass Spectrometry calibrated creatinine measurements using the four–variable Modified Diet in Renal Disease study equation (24).
Study End Points
The primary study end point was progression to ESRD. ESRD was defined as either initiation of RRT (dialysis or transplantation) or eGFR<9 ml/min per 1.73 m2 (the mean level of kidney function at which dialysis is initiated in the United Kingdom) (25). Secondary end points were all-cause mortality and the occurrence of major adverse cardiovascular events before ESRD. Cardiovascular events were defined as nonfatal stroke or myocardial infarction, incident coronary angiogram plus angioplasty or stenting, or coronary artery bypass graft surgery. For patients not meeting study end points, data were censored at last clinic visit or May 30, 2015.
Date of death was obtained from the Office of National Statistics. Date of initiation of RRT was obtained from centralized electronic patient records. Date and type of patient–reported cardiovascular event were verified against patient records by a study coordinator blinded to the biochemical results.
Statistical Analyses
Baseline patient characteristics are presented as means±SD for normally distributed data and otherwise, medians ± interquartile ranges (IQRs). Descriptive statistics were used for univariate comparisons. Correlations analyses were performed using Spearman rank.
The association between KIM-1, NGAL, and the study end points was assessed using Cox proportional hazards regression analysis. The distributions of NGAL and KIM-1 were both skewed and therefore, transformed on a natural logarithmic scale for the survival analysis. The biomarkers were analyzed as continuous variables, with hazard ratio (HR) reported per SD higher log–transformed biomarker concentration.
For each of the study end points, all available and potentially significant covariates were first analyzed in univariate form. These included baseline demographic factors, such as age, sex, smoking history, BP, prior cardiovascular events, heart failure, and diabetes. Laboratory parameters, including eGFR, urinary protein excretion, phosphate, calcium, parathyroid hormone, C-reactive protein, albumin, and hemoglobin, were also analyzed in univariate form. All covariates attaining a significance of P<0.05 on unadjusted analysis were retained in the multivariate model for the respective study end point. To investigate the association between KIM-1, NGAL, and the study end points, these biomarkers were added to the baseline multivariate risk model. Results are presented as HRs with corresponding 95% confidence intervals (95% CIs). The goodness of model fit was assessed by the Akaike information criterion (AIC) statistic. To best account for the competing risk of death in the ESRD analysis, data were censored at both end points.
Receiver operator characteristic (ROC) analysis was used to assess the discriminant accuracy of the different models. area under the curve (AUC) comparisons were performed according to the nonparametric approach proposed by DeLong et al. (26). To further support the ROC curve analyses, reclassification statistics were used to quantify the net reclassification improvement (NRI) for the end point of ESRD. For the categorical NRI, risk cutoffs were set according to the event rate in our population, and sensitivity analyses were performed to assess the appropriateness of these values.
In all cases, P<0.05 was used to define statistical significance. Analyses were performed using SPSS, version 20.0 (IBM SPSS, Chicago, IL) licensed to the University of Manchester and R, version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) PredictABEL and PROC packages (27,28).
Results
Study Population
Baseline demographic data stratified according to CKD stage are presented in Table 1. The mean age was 64.5±14.7 years old, 62% of the cohort were men, 96% were white, and 31% were diabetic. The mean eGFR was 33±17 ml/min per 1.73 m2; 49.4% of patients had CKD stage 3, 37.1% of patients had CKD stage 4, and 13.4% of patients had CKD stage 5.
Table 1.
Characteristics of study population at baseline stratified according to stage of CKD
| Variables | All Patients | CKD Stage | P Value for Comparison across Stages of CKD | ||
|---|---|---|---|---|---|
| 3, eGFR=30–59 ml/min per 1.73 m2 | 4, eGFR=15–29 ml/min per 1.73 m2 | 5, eGFR<15 ml/min per 1.73 m2 | |||
| No. of patients (%) | 1982 | 980 (49.4) | 736 (37.1) | 266 (13.4) | |
| Age, yr (SD) | 64.5 (14.7) | 62.89 (14.6) | 66.5 (14.7) | 64.9 (14.9) | <0.001 |
| Men (%) | 1235 (62.3) | 631 (64.4) | 442 (60.1) | 162 (60.9) | 0.16 |
| Diabetes (%) | 622 (31.4) | 274 (28) | 262 (35.6) | 86 (32.3) | 0.003 |
| Current or former smoker (%) | 1261 (63.6) | 608 (62) | 491 (66.7) | 162 (60.9) | 0.08 |
| Prior cardiovascular event (%) | 495 (25.0) | 216 (22) | 212 (28.7) | 68 (25.6) | <0.01 |
| Heart failure at baseline (%) | 369 (18.6) | 147 (15) | 169 (23) | 53 (19.9) | <0.001 |
| Mean systolic BP, mmHg | 138 (20.6) | 136 (19.9) | 139 (21) | 141 (21.3) | 0.004 |
| Primary renal disease (%) | |||||
| Diabetes | 310 (15.6) | 114 (11.6) | 145 (19.7) | 51 (19.2) | |
| Hypertension | 261 (13.1) | 132 (13.5) | 99 (13.5) | 30 (11.3) | |
| Renovascular disease | 251 (12.7) | 116 (11.8) | 101 (13.7) | 34 (12.8) | |
| GN | 317 (16.0) | 187 (19.1) | 95 (12.9) | 35 (13.2) | |
| Adult polycystic kidney disease | 107 (5.4) | 47 (4.8) | 35 (4.8) | 25 (9.4) | |
| Other | 453 (22.8) | 232 (23.6) | 158 (21.5) | 63 (23.6) | |
| Unknown | 283 (14.3) | 152 (15.5) | 103 (14.0) | 28 (10.5) | |
| Laboratory results | |||||
| Creatinine, mg/dl | 2.13 (1.57–3.11) | 1.57 (1.31–1.83) | 2.75 (2.38–3.26) | 4.9 (4.28–5.79) | <0.001 |
| MDRD eGFR, ml/min per 1.73 m2 | 32.6 (17.0) | 46 (13.8) | 22.4 (4.24) | 11.5 (2.5) | <0.001 |
| Phosphate, mg/dl | 3.57 (0.87) | 3.22 (0.62) | 3.65 (0.69) | 4.68 (1.11) | <0.001 |
| Corrected calcium, mg/dl | 9.07 (0.58) | 9.14 (0.51) | 9.03 (0.59) | 8.92 (0.74) | <0.001 |
| Albumin, g/dl | 4.30 (0.38) | 4.34 (0.35) | 4.28 (0.4) | 4.24 (0.41) | <0.001 |
| Hemoglobin, g/dl | 12.4 (1.7) | 12.9 (1.7) | 12.0 (1.6) | 11.6 (1.5) | <0.001 |
| Parathyroid hormone, pg/dl | 71.0 (40–130) | 47 (30–72) | 99 (62–160) | 188 (114–308) | <0.001 |
| C-reactive protein, mg/L | 3.1 (1.3–7.2) | 2.7 (1.2–6.3) | 3.5 (1.4–8.1) | 3.6 (1.35–9.0) | 0.003 |
| Urinary protein, g/24 h | 0.17 (0.07–0.56) | 0.11 (0.06–0.33) | 0.2 (0.08–0.58) | 0.54 (0.24–1.19) | <0.001 |
| KIM-1, pg/ml | 357.1 (233.7–558.6) | 276.0 (186.2–429.6) | 408.6 (285.9–632.7) | 478.8 (365.4–736.2) | <0.001 |
| NGAL, ng/ml | 221.3 (144.5–335.3) | 152.1 (107.0–207.9) | 282.7 (212.5–381.1) | 456.3 (343.3–569.2) | <0.001 |
| Outcomes | |||||
| Death before ESRD (%) | 543 (27.3) | 235 (24.0) | 259 (35.2) | 49 (18.4) | <0.001 |
| ESRD (%) | 427 (21.6) | 52 (5.3) | 180 (24.5) | 195 (73.3) | <0.001 |
| Cardiovascular events during follow-up (%) | 130 (6.6) | 60 (6.1) | 54 (7.3) | 16 (6) | 0.56 |
| Follow-up time, mo | 29.5 (14.9–53.5) | 36.1 (19.0–60.4) | 29.3 (17.6–49.7) | 11.6 (5.04–25.3) | |
Cardiovascular event defined as nonfatal stroke or myocardial infarction, incident coronary angiogram plus angioplasty or stenting, or coronary artery bypass graft surgery. Heart failure defined as left ventricular ejection fraction ≤50%, diastolic dysfunction on echocardiogram, or a clinical diagnosis of heart failure with no other alternative cause for symptoms. Systolic BP is the mean of two clinic readings taken at study baseline. Comparison across groups was made with chi-squared tests for categorical variables and ANOVA or Kruskal–Wallis tests for continuous variables. Conversion factors: creatinine millimoles per liter to milligrams per deciliter, divide by 88.4; phosphate millimoles per milliliter to milligrams per deciliter, divide by 0.3229; and calcium millimoles per liter to milligrams per deciliter, divide by 0.2495. MDRD, Modified Diet in Renal Disease; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase–associated lipocalin.
NGAL and KIM-1 Results and Correlations
The median level of NGAL was 221.2 ng/ml (IQR, 144.5–335.3), and the median level of KIM-1 was 357.1 pg/ml (IQR, 233.7–558.6); levels were higher in patients with more advanced CKD. There was a significant correlation between the two biomarkers (Rho=0.50; P<0.001).
There were positive correlations between NGAL and eGFR (Rho=−0.74; P<0.001), phosphate (Rho=0.41; P<0.001), and parathyroid hormone (Rho=0.53; P<0.001), and there was an inverse correlation with hemoglobin (Rho=−0.38; P<0.001). There was a positive correlation between KIM-1 and urinary protein excretion (Rho=0.34; P<0.001), and there were inverse correlations with hemoglobin (Rho=−0.36; P<0.001) and albumin (Rho=−0.31; P<0.001).
Associations between Biomarkers and Study End Points
The median study follow-up was 29.5 months (IQR, 14.9–53.5). As shown in Table 1, 21.6% (n=427) of the patients progressed to ESRD, 27% (n=543) of the cohort died, and 6.6% (n=130) suffered a cardiovascular event during follow-up. Results of the unadjusted analyses for each of the end points are summarized in Table 2.
Table 2.
Results of unadjusted analyses for all available covariates and study end points
| Variable | ESRD | All-Cause Mortality | Nonfatal Cardiovascular Events | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, per 5 yr | 0.90 (0.86 to 0.90) | <0.001 | 1.47 (1.40 to 1.54) | <0.001 | 1.02 (1.10 to 1.22) | <0.001 |
| Men | 0.94 (0.77 to 1.15) | 0.55 | 1.15 (1.05 to 1.25) | 0.003 | 1.36 (0.94 to 1.97) | 0.10 |
| Diabetes | 1.04 (0.84 to 1.28) | 0.73 | 1.71 (1.44 to 2.03) | <0.001 | 1.66 (1.17 to 2.35) | <0.001 |
| Smoker | 1.01 (0.83 to 1.22) | 0.95 | 1.77 (1.47 to 2.14) | <0.001 | 1.07 (0.75 to 1.54) | 0.69 |
| Prior cardiovascular event | 0.79 (0.63 to 1.01) | 0.06 | 2.35 (1.98 to 2.78) | <0.001 | 4.41 (3.11 to 6.24) | <0.001 |
| Heart failure at baseline | 1.05 (0.92 to 1.19) | 0.46 | 2.69 (2.24 to 3.24) | <0.001 | 1.52 (1.26 to 1.83) | <0.001 |
| Mean systolic BP, per 5 mmHg | 1.05 (1.00 to 1.05) | 0.002 | 1.05 (1.00 to 1.05) | <0.01 | 1.00 (0.95 to 1.05) | 0.72 |
| Baseline eGFR, per 5 ml/min per 1.73 m2 | 0.50 (0.44 to 0.53) | <0.001 | 0.86 (0.86 to 0.90) | <0.001 | 0.95 (0.90 to 0.95) | 0.02 |
| Urinary protein excretion, per 0.1 g/24 h | 1.04 (1.04 to 1.05) | <0.001 | 1.01 (0.99 to 1.06) | 0.26 | 1.01 (0.99 to 1.03) | 0.49 |
| Phosphate, per 0.5 mg/dl | 1.64 (1.57 to 1.70) | <0.001 | 1.16 (1.09 to 1.22) | <0.001 | 1.09 (0.98 to 1.20) | 0.11 |
| Corrected calcium, per 0.5 mg/dl | 0.83 (0.75 to 0.92) | <0.001 | 0.99 (0.92 to 1.08) | 0.89 | 0.90 (0.76 to 1.06) | 0.21 |
| Parathyroid hormone, per 10 pg/ml | 1.10 (1.00 to 1.10) | <0.001 | 1.00 (1.00 to 1.10) | <0.001 | 1.00 (1.00 to 1.10) | 0.004 |
| Albumin, per 0.1 g/dl | 0.91 (0.89 to 0.93) | <0.001 | 0.91 (0.89 to 0.93) | <0.001 | 0.94 (0.90 to 0.97) | 0.001 |
| Hemoglobin, per 1 g/dl | 0.66 (0.66 to 0.74) | <0.001 | 0.82 (0.74 to 0.82) | <0.001 | 0.90 (0.82 to 1.00) | 0.12 |
| C-reactive protein, per 1 mg/L | 1.00 (0.99 to 1.01) | 0.81 | 1.01 (1.00 to 1.01) | <0.001 | 1.01 (0.99 to 1.01) | 0.07 |
| Ln KIM-1, per 1 SD | 2.03 (1.86 to 2.21) | <0.001 | 1.33 (1.22 to 1.44) | <0.001 | 1.20 (1.01 to 1.43) | 0.03 |
| Ln NGAL, per 1 SD | 3.50 (3.11 to 3.93) | <0.001 | 1.50 (1.36 to 1.64) | <0.001 | 1.16 (0.96 to 1.39) | 0.12 |
HR, hazard ratio; 95% CI, 95% confidence interval; Ln, natural logarithm; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase–associated lipocalin.
Association with ESRD.
In our study, 427 (21.6%) patients reached the end point of ESRD (395 on either dialysis or transplantation and 32 patients with eGFR<9 ml/min per 1.73 m2) in a median time of 53.4 months (IQR, 22.8–101.1).
Both KIM-1 and NGAL were associated with ESRD on adjusted multivariate analyses, with an HR of 1.25 (95% CI, 1.10 to 1.43; P≤0.001) for KIM-1 and an HR of 1.35 (95% CI, 1.14 to 1.59; P<0.001) for NGAL. When KIM-1 and NGAL were each added alone to the baseline multivariate model, the HRs were 1.31 (95% CI, 1.16 to 1.49; P<0.001) for KIM-1 and 1.49 (95% CI, 1.27 to 1.74; P<0.001) for NGAL. Other factors associated with the ESRD end point were eGFR, urinary protein excretion, hemoglobin, phosphate, and age. Full details of the Cox regression models, including the corresponding HRs for all of the traditional risk factors, are presented in Table 3.
Table 3.
Results of multivariate analysis showing association between variables and ESRD
| Variable | Model 1 (Baseline Covariates) | Model 2 (Baseline Covariates Plus Biomarkers) | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, per 5 yr | 0.86 (0.82 to 0.90) | <0.001 | 0.86 (0.82 to 0.86) | <0.001 |
| Mean systolic BP, per 5 mmHg | 1.05 (1.00 to 1.05) | 0.05 | 1.00 (0.95 to 1.05) | 0.39 |
| Baseline eGFR, per 5 ml/min per 1.73 m2 | 0.56 (0.53 to 0.59) | <0.001 | 0.62 (0.56 to 0.66) | <0.001 |
| Urinary protein, per 0.1 g/24 h | 1.03 (1.02 to 1.04) | <0.001 | 1.03 (1.01 to 1.04) | <0.001 |
| Hemoglobin, per 1 g/dl | 0.90 (0.82 to 0.90) | 0.002 | 0.90 (0.82 to 0.90) | 0.03 |
| Albumin, per 0.1 g/dl | 0.99 (0.96 to 1.02) | 0.54 | 1.01 (0.98 to 1.04) | 0.70 |
| Phosphate, per 0.5 mg/dl | 1.12 (1.06 to 1.18) | <0.001 | 1.01 (1.03 to 1.16) | 0.002 |
| Corrected calcium, per 0.5 mg/dl | 1.01 (1.06 to 1.16) | 0.76 | 0.96 (0.89 to 1.05) | 0.41 |
| Parathyroid hormone, per 10 pg/ml | 1.00 (1.00 to 1.10) | <0.001 | 1.00 (1.00 to 1.00) | 0.001 |
| Ln KIM-1, per SD | 1.25 (1.10 to 1.43) | <0.001 | ||
| Ln NGAL, per SD | 1.35 (1.14 to 1.59) | <0.001 | ||
| c Statistic for model | 0.90 (0.89 to 0.92) | 0.91 (0.89 to 0.92) | ||
HR, hazard ratio; 95% CI, 95% confidence interval; Ln, natural logarithm; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase–associated lipocalin.
We considered the associations between the biomarkers and ESRD according to different stages of baseline CKD. For KIM-1, the most significant association was seen in CKD stage 3, with an adjusted HR of 2.39 (95% CI, 1.69 to 3.41; P<0.001) compared with an adjusted HR of 1.05 (95% CI, 0.84 to 1.32; P<0.02) in CKD stage 5. It was only in CKD stage 3 that a significant association was observed between NGAL and ESRD (adjusted HR, 1.72; 95% CI, 1.04 to 2.86; P=0.04 in stage 3 compared with HR, 1.30; 95% CI, 0.99 to 1.70; P=0.06 in stage 5) (Table 4).
Table 4.
Multivariate analysis for ESRD stratified according to stage of CKD at baseline
| Variable | CKD Stage 3, n=980 | CKD Stage 4, n=736 | CKD Stage 5, n=266 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, per 5 yr | 0.66 (0.56 to 0.73) | <0.001 | 0.82 (0.77 to 0.86) | <0.001 | 0.95 (0.86 to 0.95) | 0.01 |
| Mean systolic BP, per 5 mmHg | 0.95 (0.90 to 1.10) | 0.92 | 1.00 (0.95 to 1.05) | 0.54 | 1.00 (0.95 to 1.05) | 0.96 |
| Baseline eGFR, per 5 ml/min per 1.73 m2 | 0.77 (0.66 to 0.95) | <0.01 | 0.56 (0.47 to 0.69) | <0.001 | 0.25 (0.17 to 0.35) | <0.001 |
| Urinary protein, per 0.1 g/24 h | 0.99 (0.95 to 1.02) | 0.44 | 1.03 (1.02 to 1.06) | <0.001 | 1.03 (1.01 to 1.05) | 0.001 |
| Hemoglobin, per 1 g/dl | 1.10 (0.90 to 1.34) | 0.62 | 1.00 (0.90 to 1.10) | 0.97 | 0.82 (0.74 to 0.90) | 0.002 |
| Albumin, per 0.1 g/dl | 0.87 (0.79 to 0.95) | 0.002 | 0.96 (0.92 to 1.01) | 0.05 | 1.04 (0.99 to 1.09) | 0.10 |
| Phosphate, per 0.5 mg/dl | 1.17 (0.93 to 1.47) | 0.17 | 1.06 (0.95 to 1.19) | 0.30 | 0.99 (0.93 to 1.07) | 0.97 |
| Corrected calcium, per 0.5 mg/dl | 0.81 (0.60 to 1.09) | 0.16 | 0.84 (0.73 to 0.96) | 0.01 | 1.05 (0.94 to 1.18) | 0.37 |
| Parathyroid hormone, per 10 pg/ml | 1.10 (0.90 to 1.21) | 0.18 | 1.00 (1.00 to 1.00) | 0.03 | 1.00 (1.00 to 1.00) | 0.08 |
| Ln KIM-1, 1-SD increase | 2.39 (1.69 to 3.41) | <0.001 | 1.26 (1.04 to 1.52) | 0.02 | 1.05 (0.84 to 1.32) | 0.66 |
| Ln NGAL, 1-SD increase | 1.72 (1.04 to 2.86) | 0.04 | 1.25 (0.99 to 1.56) | 0.06 | 1.30 (0.99 to 1.70) | 0.06 |
| c Statistic for baseline model | 0.87 (0.83 to 0.92) | 0.83 (0.79 to 0.86) | 0.77 (0.71 to 0.83) | |||
| c Statistic for baseline plus biomarkers model | 0.89 (0.85 to 0.93) | 0.83 (0.79 to 0.86) | 0.79 (0.73 to 0.85) | |||
HR, hazard ratio; 95% CI, 95% confidence interval; Ln, natural logarithm; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase–associated lipocalin.
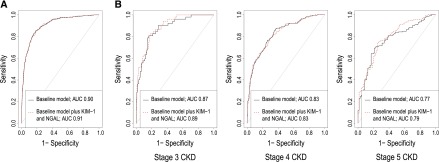
We performed ROC curve analyses to assess the prognostic significance of NGAL and KIM-1 for the end point of ESRD. For the whole cohort, discrimination and fit of the baseline multivariate model were very good, with a c statistic of 0.90 (95% CI, 0.89 to 0.92) and an AIC of 1252.7. A negligible improvement was seen with the addition of NGAL and KIM-1 (c statistic =0.91; 95% CI, 0.89 to 0.92; AIC=1241.4; P=0.88 for difference between AUCs). When we considered the prognostic accuracy of KIM-1 and NGAL for progression to ESRD at different stages of CKD, there was no significant difference between the AUCs of the baseline and biomarker models at any stage. ROC curves for the baseline and biomarker models are shown in Figure 1.
Figure 1.
(A) Receiver operator characteristic (ROC) curve for progression to ESRD for baseline multivariate model and biomarkers models showing minimal improvement in model discrimination following the addition of the KIM-1 and NGAL. (B) ROC curves for progression stratified for CKD stage. AUC, area under the curve; KIM-1, either kidney injury molecule-1; NGAL, neutrophil gelatinase–associated lipocalin.
When reclassification was considered and risk cutoff values were set to reflect the rate of progression to ESRD in our population (<20%, 20%–40%, and >40%), there was no significant improvement in the three-category NRI (0.01; 95% CI, −0.02 to 0.03; P=0.55; event NRI =1.2%; nonevent NRI =0.06%), and there was a small change in the category free NRI (0.32; 95% CI, 0.22 to 0.43; P<0.001). When we considered reclassification in only those with CKD stage 3 at baseline and set risk cutoff values to reflect progression in this subpopulation (<5%, 5%–10%, and >10%), there was no significant change in the three-category NRI. The category free NRI was 0.64 (95% CI, 0.37 to 0.91; P<0.001), greater than that observed across the whole population. Results are shown in Supplemental Table 1.
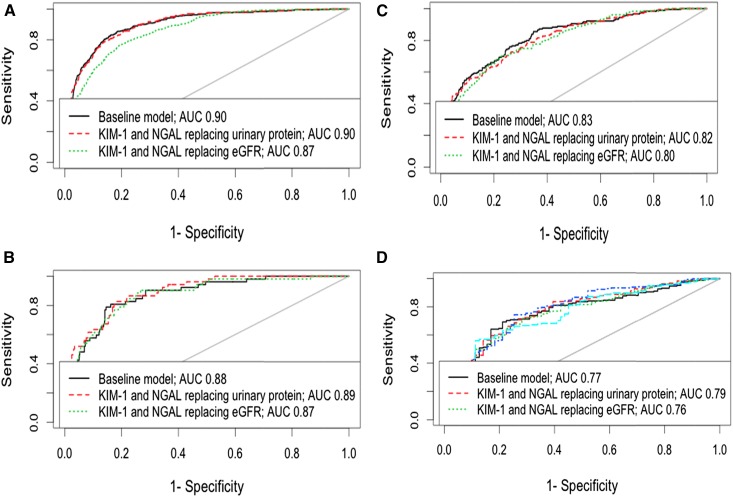
We considered the performance of these novel biomarkers as a replacement for the traditional risk factors in our baseline multivariate risk model (Table 2) rather than as an addition to those factors. The discrimination and fit of the model containing just KIM-1 and NGAL were significantly poorer than in the traditional risk factors model (c statistic =0.79; 95% CI, 0.77 to 0.81 for the stand–alone biomarkers model compared with c statistic =0.90; 95% CI, 0.89 to 0.92 for the traditional risk factors model; P<0.001 for comparison between the two models). We then substituted KIM-1 and NGAL for urinary protein excretion and eGFR in the baseline multivariate model. When the biomarkers were substituted for urinary protein, model discrimination was unchanged, with marginal improvement seen in the CKD stages 3 and 5 subsets. When substituted individually for proteinuria, KIM-1 showed better discrimination than NGAL (data not shown). When NGAL and KIM-1 were substituted for eGFR, model discrimination fell. These findings are summarized in Figure 2.
Figure 2.
Receiver operator characteristic (ROC) curves showing effect of substituting proteinuria in the multivariate risk model for either kidney injury molecule-1 (KIM-1) and neutrophil gelatinase–associated lipocalin (NGAL) in turn or both biomarkers together in our whole study population and according to stage of CKD. Receiver operator characteristic (ROC) curves demonstrating a fall in model discrimination when kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) are substituted for eGFR and no change in discrimination when they are substituted for proteinuria in the multivariate risk model. (A) All CKD stages (B) CKD stage 3 (C) CKD stage 4 (D) CKD stage 5. AUC, area under the curve.
Association with Death.
On unadjusted analysis, both KIM-1 and NGAL were associated with risk for death, with HRs of 1.33 (95% CI, 1.22 to 1.44; P<0.001) and 1.50 (95% CI, 1.36 to 1.64; P<0.001), respectively. There was no significant association between KIM-1 or NGAL and death in the adjusted multivariate model. Factors that were significantly associated with death on this analysis were increasing age, diabetes, smoking, a history of prior cardiovascular events, and heart failure. Significant biochemical parameters included albumin, phosphate, and C-reactive protein (Supplemental Table 2).
Association with Major Adverse Cardiovascular Events.
We considered nonfatal cardiovascular events. There was an association between KIM-1 and cardiovascular events on unadjusted analysis (HR, 1.20; 95% CI, 1.01 to 1.43; P=0.03). On multivariate analysis, no significant association remained. There was no association between NGAL and cardiovascular events on unadjusted analysis. Significant parameters in the multivariate model included a history of prior cardiovascular events and serum albumin (Supplemental Table 3).
Discussion
Our study looked at a large, diverse population of patients with CKD stages 3–5 and showed that increased blood levels of KIM-1 and NGAL are both independent risk factors for progression to ESRD in this population. There was no association with major cardiovascular events or death in our analysis. Urinary KIM-1 has been associated with adverse outcomes in AKI (7) and shown to be elevated in both the blood and urine in CKD (8). Blood levels of KIM-1 are an early marker of progressive kidney disease in patients with type 1 diabetes (8,9). KIM-1 is a specific marker of renal tubular injury (6), and tissue levels associate with the extent of inflammation and fibrosis in biopsy studies (29). In AKI, KIM-1 facilitates the clearance of apoptopic debris from the tubular lumen (6). The implications of long-term expression of KIM-1 in CKD and the role that this may play in processes of continued renal injury remain as yet undefined.
NGAL is predictive of AKI and associated with adverse outcomes in this condition (7,12). NGAL is expressed in the tubular epithelium and released from damaged cells in response to injury (30). It seems to be a key mediator of tubular damage and progressive renal injury, with overexpression able to identify those at risk of rapid progression of CKD (31). Numerous prospective studies have shown NGAL to associate with outcomes of progressive renal disease and death in CKD populations (14–17,19,32). Consistent with the published literature, increased blood levels of NGAL were associated with risk for progression to ESRD in our study.
When we considered model discrimination, in all cases, our base models performed well, leaving little room for additional improvement. When NGAL and KIM-1 were added to our baseline model of traditional risk predictors, there was no significant improvement in model discrimination. Two previous studies have examined the performance of NGAL in risk prediction models. Despite both showing that urinary NGAL is an independent risk factor for progression, significant improvements in the prediction of outcome events were not seen in either study (18,19). When NGAL and KIM-1 alone were compared directly with the model of traditional risk predictors in our study, they did not perform as well as these standard clinical parameters. However, there was no adverse effect on discrimination when they replaced proteinuria in the multivariate model, although there was an adverse effect when eGFR was replaced.
When we considered progression to ESRD for different baseline stages of CKD, the effect of KIM-1 and NGAL was most significant in CKD stage 3 compared with in stages 4 and 5, suggesting that these biomarkers may be of greater utility in earlier renal disease. Furthermore, when we considered risk reclassification, there was a small improvement in the category free NRI in the CKD stage 3 subgroup compared with in the whole population. The significance of the reclassification findings is not entirely clear but may indicate a greater benefit from using these biomarkers in earlier kidney disease when, arguably, progression is less easy to predict. This is an area that merits additional study.
Our study has a number of strengths, including a large population of patients with all-cause CKD and lengthy follow-up periods. Nonetheless, it is important to recognize the limitations of our analysis. The CRISIS cohort is a clearly defined study of referred patients with CKD. Our findings may not be applicable to a nonreferred CKD population or those with earlier stages of disease. As with all observational studies, there is the potential for misclassification of events that rely on patient self-reporting. Our cardiovascular event rate was low, potentially limiting our analysis in reference to this end point. Power calculations were not performed before undertaking the study; this may have resulted in errors in the estimation of model effects, and therefore, these results should be considered preliminary and hypothesis generating. Biomarkers were analyzed on baseline plasma samples, and associations with longitudinal clinical outcomes were investigated; in such an analysis, no account can be made of changes in biomarker levels over time.
Despite the limitations of our data, we feel that this study adds to the literature.
We have shown that blood values of KIM-1 and NGAL are independent risk factors for progression in CKD. This adds to the evidence from analysis of these markers in urine and smaller studies of blood levels to suggest that they may have a role to play in the stratification of risk in patients with CKD. Nevertheless, we found that the improvement that they bring to the prediction of renal end points in our population of patients with advanced CKD is limited. KIM-1 and NGAL are both tubular markers and as such, may not be critical factors for the ESRD outcomes studied. In themselves, KIM-1 and NGAL do not outperform standard, cheaper clinical and biologic parameters and add little to model discrimination or risk reclassification. This raises the importance of finding the right clinical application for these biomarkers and others, so that the additional cost of testing is offset by the value added to clinical decision making. Focusing the investigation of biomarkers in areas of CKD where traditional risk factors provide a less distinct assessment of future clinical risk (for example, in early stage disease) may establish more important predictive roles. NGAL and KIM-1 both have shown great promise in the AKI setting, and this should not be overlooked (8,11,12). Ultimately, prospective testing of the usefulness of these biomarkers in the setting of a clinical trial with a combined cost–effectiveness analysis may be needed to define their value in the most appropriate renal population.
Disclosures
We can confirm that no financial support was received for these studies.
Supplementary Material
Acknowledgments
The authors thank Sister Beverly Lane for assistance in relation to data and sample collection and Dr. Robert Oliver, Dr. Kirk Siddals, and Mrs. Julie Hudson for assistance in relation to sample storage and transfer.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02670316/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Tangri N, Kitsios GD, Inker LA, Griffith J, Naimark DM, Walker S, Rigatto C, Uhlig K, Kent DM, Levey AS: Risk prediction models for patients with chronic kidney disease: A systematic review. Ann Intern Med 158: 596–603, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE: Biomarkers in chronic kidney disease: A review. Kidney Int 80: 806–821, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Bonventre JV: Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol Dial Transplant 24: 3265–3268, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, MacKinnon RW, Li L, Balakrishnan VS, Pereira BJG, Bonventre JV, Jaber BL: Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol 18: 904–912, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee M, Briskin R, De Jager PL, Ngo TT, Radlinski M, Dear JW, Park KB, Betensky R, Krolewski AS, Bonventre JV: Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol 25: 2177–2186, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowak N, Skupien J, Niewczas MA, Yamanouchi M, Major M, Croall S, Smiles A, Warram JH, Bonventre JV, Krolewski AS: Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int 89: 459–467, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Soto K, Papoila AL, Coelho S, Bennett M, Ma Q, Rodrigues B, Fidalgo P, Frade F, Devarajan P: Plasma NGAL for the diagnosis of AKI in patients admitted from the emergency department setting. Clin J Am Soc Nephrol 8: 2053–2063, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Fazio MR, Nicocia G, Buemi M: Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res 32: 91–98, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M: Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol 27: 373–378, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M: Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 4: 337–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding H, He Y, Li K, Yang J, Li X, Lu R, Gao W: Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin Immunol 123: 227–234, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Peters HPE, Waanders F, Meijer E, van den Brand J, Steenbergen EJ, van Goor H, Wetzels JFM: High urinary excretion of kidney injury molecule-1 is an independent predictor of end-stage renal disease in patients with IgA nephropathy. Nephrol Dial Transplant 26: 3581–3588, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Liu KD, Yang W, Anderson AH, Feldman HI, Demirjian S, Hamano T, He J, Lash J, Lustigova E, Rosas SE, Simonson MS, Tao K, Hsu C-Y; Chronic Renal Insufficiency Cohort (CRIC) study investigators : Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int 83: 909–914, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith ER, Lee D, Cai MM, Tomlinson LA, Ford ML, McMahon LP, Holt SG: Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric Stages 3 and 4 chronic kidney disease (CKD). Nephrol Dial Transplant 28: 1569–1579, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Ritchie J, Rainone F, Green D, Alderson H, Chiu D, Middleton R, O’Donoghue D, Kalra PA: Extreme elevations in blood pressure and all-cause mortality in a referred CKD population: Results from the CRISIS Study. Int J Hypertens 2013: 597906, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoefield RA, Kalra PA, Baker P, Lane B, New JP, O’Donoghue DJ, Foley RN, Middleton RJ: Factors associated with kidney disease progression and mortality in a referred CKD population. Am J Kidney Dis 56: 1072–1081, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Hoefield RA, Kalra PA, Lane B, O’Donoghue DJ, Foley RN, Middleton RJ: Associations of baseline characteristics with evolution of eGFR in a referred chronic kidney disease cohort. QJM 106: 915–924, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Eddington H, Hoefield R, Sinha S, Chrysochou C, Lane B, Foley RN, Hegarty J, New J, O’Donoghue DJ, Middleton RJ, Kalra PA: Serum phosphate and mortality in patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 2251–2257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Gilg J, Pruthi R, Fogarty D: UK Renal Registry 17th Annual Report: Chapter 1 UK renal replacement therapy incidence in 2013: National and centre-specific analyses. Nephron 129[Suppl 1]: 1–29, 2015 [DOI] [PubMed] [Google Scholar]
- 26.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: 837–845, 1988 [PubMed] [Google Scholar]
- 27.Kundu S, Aulchenko YS, van Duijn CM, Janssens ACJW: PredictABEL: An R package for the assessment of risk prediction models. Eur J Epidemiol 26: 261–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M: pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12: 77, 2011 [DOI] [PMC free article] [PubMed]
- 29.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA: Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 212: 209–217, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Mori K, Nakao K: Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int 71: 967–970, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F: Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest 120: 4065–4076, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu KD, Yang W, Go AS, Anderson AH, Feldman HI, Fischer MJ, He J, Kallem RR, Kusek JW, Master SR, Miller ER 3rd, Rosas SE, Steigerwalt S, Tao K, Weir MR, Hsu C-Y; CRIC Study Investigators : Urine neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease and death in CKD: Results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 65: 267–274, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.