Abstract
Paraproteins are monoclonal Igs or their components (light or heavy chains) that are produced by a clonal population of mature B cells, most commonly plasma cells. These paraproteins or monoclonal proteins are secreted into the blood and subsequently filtered by the glomerulus before entering into urine, where they can cause various types of kidney disease, including both glomerular and tubulointerstitial injuries. Furthermore, a monoclonal protein that causes a specific glomerular or tubulointerstitial lesion in a human can reproducibly cause the same pathology when injected into an animal, supporting unique paraprotein characteristics. This Moving Points in Nephrology will provide an update for the Clinical Journal of the American Society of Nephrology readership on some of the clinically relevant kidney lesions associated with monoclonal paraprotein production and the pathophysiology underlying these kidney lesions.
Keywords: monoclonal paraprotein, kidney, amyloidosis, light chains, cast nephropathy, Kidney Diseases, Myeloma Proteins, Paraproteins, Social Behavior, multiple myeloma M-proteins
Introduction
Onconephrology has become an important subspecialty area within the field of nephrology (1–3). Although several cancers can directly injure the kidney through tissue invasion or indirectly promote renal damage via paraneoplastic effects, paraprotein–related kidney diseases are an especially important and interesting complication of certain malignancies (4,5). Paraproteins or monoclonal proteins (M proteins) are classically defined as either monoclonal Igs or light chains (rarely heavy chains) produced by a clonal population of mature B cells, typically plasma cells or B lymphocytes (6). The source of these M proteins can be an overtly malignant clone of plasma cells, like that seen with multiple myeloma (MM), or a plasmacytoma, lymphoproliferative disorders (chronic lymphocytic leukemia, B and T cell lymphomas, and Waldenstrom macroglobulinemia), or a monoclonal gammopathy of undetermined significance (MGUS), which is characterized by a lower clonal cell burden, less paraprotein production, and absence of end organ injury (6).
Monoclonal paraproteins are secreted into the bloodstream and subsequently filtered at the glomerulus, where they are often measurable in the urine (6). Because the kidney receives 20% of the cardiac output, it is not surprising that clonal disorders producing paraproteins are associated with various forms of kidney injury (Table 1). Moreover, all segments of the nephron may be affected, including the glomerulus as well as the proximal and distal tubular segments (Figure 1). Injury tends to be specific to the secreted paraprotein and varies depending on the M protein site of attack. That is to say that an M protein that causes a particular glomerular lesion (for example, amyloidosis in a human with an underlying paraprotein–producing disease) also causes the same glomerular pathology when injected into an animal (7,8). The same holds true for other paraprotein–related kidney lesions, such as light–chain deposition disease (LCDD) and myeloma cast nephropathy.
Table 1.
Paraprotein–related kidney disease
| Glomerular disease |
| AL amyloidosis |
| Light–, heavy–, or light/heavy–chain deposition disease |
| Cryoglobulinemic glomerulopathy |
| Waldenstrom macroglobulinemic glomerulopathy |
| Immunotactoid glomerulopathy |
| Fibrillary glomerulopathy |
| Proliferative GN with monoclonal Ig deposits |
| C3 glomerulopathy with monoclonal gammopathy |
| Tubulointerstitial disease |
| Proximal tubulopathy/Fanconi syndrome |
| Cast nephropathy |
| Uric acid nephropathy |
| Nephrocalcinosis (calcium phosphate deposition) |
| Direct tissue invasion |
| Lymphomatous infiltration |
| Plasma cell infiltration |
| Vascular disease |
| Thrombotic microangiopathy |
| Crystalglobulin-induced nephropathy |
AL, amyloid light chain.
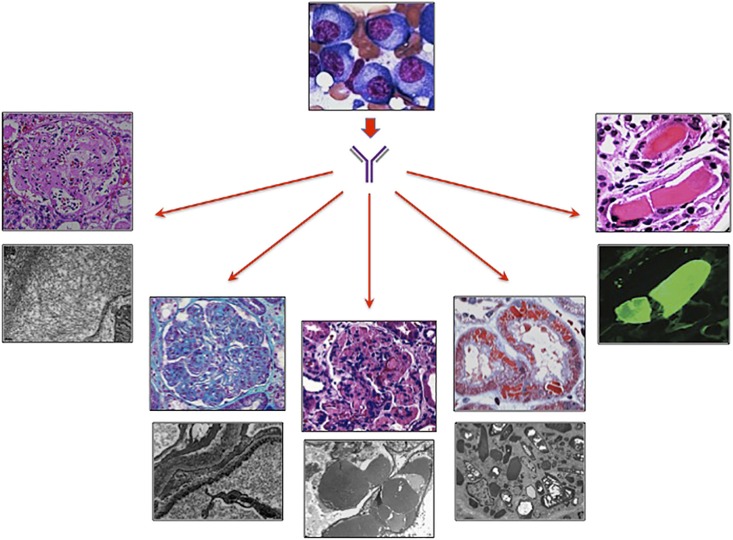
Figure 1.
Paraprotein–induced kidney disease. A clone of mature B cells (plasma cells) produces monoclonal paraproteins (Igs or light chains) that cause various kidney lesions. From left to right are light-chain (AL) amyloidosis on light microscopy (LM) and fibrils on electron microscopy (EM), light–chain deposition disease on LM and granular deposits on EM, type 2 cryoglobulinemic GN on LM and organized deposits within capillary loops on EM, light–chain proximal tubulopathy on LM and cytoplasmic crystals on EM, and cast nephropathy on LM and λ-positive cast staining with immunofluorescence. Images were provided by Vivette D'Agati (College of Physicians and Surgeons, Columbia University, New York, NY),Glen Markowitz (College of Physicians and Surgeons, Columbia University, New York, NY), and Gilbert Moeckel (Yale University School of Medicine, New Haven, CT).
This Moving Points in Nephrology feature focuses on paraprotein–related kidney diseases. The American Society of Nephrology Onco-Nephrology Forum group and other leaders in the cancer-kidney disease area collaborated to write a compilation of articles covering some of the key aspects of paraprotein–related kidney disease. As guest coeditors, it was our pleasure to work with this group of experts to put together a series of articles covering this clinically important area. Included in this compendium are the following topics: (1) glomerular diseases associated with paraprotein production, (2) light chain–derived cast nephropathy occurring in the setting of MM, (3) the entity of monoclonal gammopathy of renal significance (MGRS) and its importance for nephrologists, and (4) the mechanism by which certain paraproteins induce kidney injury and the various types of renal pathology that occur with them.
The first article in this Moving Points feature discusses the glomerular lesions encountered with monoclonal paraprotein production. Lam et al. (9) describe some of the more common glomerular pathology, such as light-chain (AL) amyloidosis, monoclonal Ig deposition disease (such as LCDD and/or heavy–chain deposition disease), and immunotactoid and fibrillary GN that develops in the setting of M protein–producing clinical conditions. Other less common glomerulopathies covered include proliferative GN with monoclonal Ig deposits, C3 glomerulopathy with monoclonal gammopathy, and cryoglobulinemic GN. The authors review the clinical and pathologic characteristics of some of the paraprotein–related glomerular lesions. For example, the M protein may cause glomerular amyloid deposition, which results in an acellular lesion characterized by Congo red–positive staining, apple green birefringence, and 8- to 10-nm haphazard fibrils. In some patients, monoclonal light or heavy chains (or both together) may deposit in basement membranes and mesangium in a granular (powdery) pattern resulting in monoclonal Ig deposition disease characterized by Congo red–negative nodular glomerulosclerosis, of which LCDD is the most common. A proliferative lesion akin to membranoproliferative pattern of injury may develop from monoclonal paraproteins as seen with proliferative GN with monoclonal Ig deposits and C3 glomerulopathy, whereas cryoglobulin plugs may distend capillary loops in those with cryoglobulinemic GN.
Cohen et al. (10) discuss the evaluation of MM cast nephropathy and the appropriate approach to therapy in current times. The authors note that AKI complicates MM in approximately 50% of patients and that cast nephropathy is the most common cause. Because AKI is associated with significant morbidity and mortality and limits therapeutic options in patients with MM, finding a therapy that reduces the occurrence of cast nephropathy would go a long way in improving patient outcomes. Treatment of myeloma using new chemotherapeutic agents that rapidly and effectively lower serum free light chains is now viewed as the first step in a renoprotective approach. Extracorporeal removal of toxic light chains is appealing as an adjunct to the currently available chemotherapeutic agents. Therapeutic plasma exchange has not consistently shown any additive benefit to myeloma therapy. Despite apparent efficacy in uncontrolled studies, high-cutoff hemodialysis was not effective in a recently completed randomized trial, and the onconephrology community continues to wait in anticipation for results from additional randomized trials to understand whether there is additional benefit of high-cutoff hemodialysis when added to newer chemotherapeutic agents in modifying cast nephropathy outcomes.
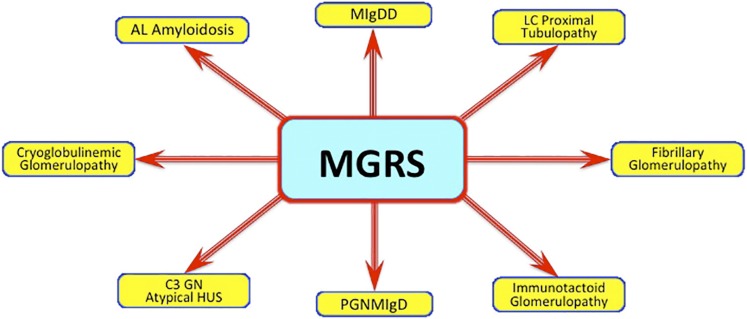
Leung et al. (11) note that MGUS is a biomarker of Ig–producing clonal cells. In their paper, they define the entity of MGRS for the reader and distinguish it from MGUS, asymptomatic (formerly smoldering) myeloma, and full-blown MM. Traditionally, MGUS has been considered a relatively benign entity with low likelihood of progressing to myeloma. From the hematologic standpoint, the small plasma cell clone responsible for MGUS may not progress to myeloma but nonetheless, may still promote kidney injury. In fact, some of the MGUS cases occur in the setting of low–grade B cell lymphoma and/or lymphoplasmacytic lymphoma or chronic lymphocytic leukemia. As seen in Figure 2, there is a wide array of kidney lesions that can develop with MGUS, making MGRS the more appropriate descriptor. In this piece, the authors review the types of renal lesions seen with MGRS, the clinical characteristics observed with paraprotein–related kidney disease, and the best approach to accurately diagnosing the entity and formulating an appropriate treatment plan.
Figure 2.
Monoclonal gammopathy of renal significance (MGRS). Monoclonal paraproteins, despite not meeting criteria of multiple myeloma, can result in a wide variety of kidney lesions. AL, amyloid light chain; HUS, hemolytic uremic syndrome; LC, light chain; MIgDD, monoclonal Ig deposition disease; PGNMIgD, proliferative GN with monoclonal Ig disease.
Finally, Sanders et al. (12) review the mechanisms underlying paraprotein injury in the various kidney compartments. The renal handling of paraproteins is one determinant of the type of kidney injury. To this point, intact monoclonal Igs are not freely filtered at the glomerulus because of their size, thereby interacting with resident glomerular cells to alter their biology and promote various types of glomerular injury. Free monoclonal light chains, however, are small low molecular weight proteins that are readily filtered at the glomerulus and subsequently endocytosed by proximal tubular cells. In the setting of monoclonal light–chain overproduction as occurs with MM, the proximal tubular cell capacity to process all of the free light chains is overwhelmed, causing overflow proteinuria. Both proximal tubular cell injury (light–chain proximal tubulopathy) and downstream tubular cell injury (cast nephropathy) can and do result. In addition to this differential renal handling of paraproteins, the authors point out that the molecular diversity of the secreted M protein (with distinct physiochemical properties) also determines the type of pathologic lesion that develops within the kidney. Finally, the authors also emphasize that host factors, such as urinary pH, sodium chloride concentration, and tissue factors, contribute to the type and severity of renal lesion that develops from exposure to the monoclonal light chain.
It is clear that M proteins are associated with a wide variety of kidney lesions that can involve the entire nephron depending on renal handling, unique paraprotein properties, and host characteristics. We hope that this Moving Points feature covering the topic of paraprotein–associated kidney disease, the proverbial attack of the killer M proteins, will update nephrologists on this important clinical topic in the rapidly growing and ever-expanding area of onconephrology.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Perazella MA, Berns JS, Rosner MH: Cancer and the kidney: The growth of onco-nephrology. Adv Chronic Kidney Dis 21: 4–6, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Finkel KW, Howard SC: Onco-nephrology: An invitation to a new field. J Clin Oncol 32: 2389–2390, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Berns JS, Rosner MH: Onco-nephrology: What the nephrologist needs to know about cancer and the kidney. Clin J Am Soc Nephrol 7: 1691, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Campbell GA, Hu D, Okusa MD: Acute kidney injury in the cancer patient. Adv Chronic Kidney Dis 21: 64–71, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Hutchison CA, Batuman V, Behrens J, Bridoux F, Sirac C, Dispenzieri A, Herrera GA, Lachmann H, Sanders PW; International Kidney and Monoclonal Gammopathy Research Group: The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol 8: 43–51, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook L, Macdonald DH: Management of paraproteinaemia. Postgrad Med J 83: 217–223, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders PW: Mechanisms of light chain injury along the tubular nephron. J Am Soc Nephrol 23: 1777–1781, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Sanders PW: Renal involvement in plasma cell dyscrasias. Curr Opin Nephrol Hypertens 2: 246–252, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Motwani SS, Herlitz L, Monga D, Jhaveri KD, Lam AQ; for the American Society of Nephrology Onco-Nephrology Forum: Glomerular diseases associated with paraproteinemias. Clin J Am Soc Nephrol 11: 2260–2272, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkel KW, Cohen EP, Shirali A, Abudayyeh A; for the American Society of Nephrology Onco-Nephrology Forum: Paraprotein–related kidney disease: Evaluation and treatment of myeloma cast nephropathy. Clin J Am Soc Nephrol 11: 2273–2279, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosner MH, Edeani A, Yanagita M, Glezerman IG, Leung N; for the American Society of Nephrology Onco-Nephrology Forum: Paraprotein–related kidney disease: diagnosing and treating monoclonal gammopathy of renal significance. Clin J Am Soc Nephrol 11: 2280–2287, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doshi M, Lahoti A, Danesh FR, Batuman V, Sanders PW; on behalf of the American Society of Nephrology Onco-Nephrology Forum: Paraprotein–related kidney disease: Kidney injury from paraproteins—What determines the site of injury? Clin J Am Soc Nephrol 11: 2288–2294, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]