Abstract
Background and objectives
Supraventricular arrhythmias are associated with high morbidity and mortality. Nevertheless, this condition has received little attention in patients on hemodialysis. The objective of this study was to analyze the incidence of intradialysis supraventricular arrhythmia and its long–term prognostic value.
Design, setting, participants, & measurements
We designed an observational and prospective study in a cohort of patients on hemodialysis with a 10-year follow-up period. All patients were recruited for study participation and were not recruited for clinical indications. The study population comprised 77 patients (42 men and 35 women; mean age =58±15 years old) with sinus rhythm monitored using a Holter electrocardiogram over six consecutive hemodialysis sessions at recruitment.
Results
Hypertension was present in 68.8% of patients, and diabetes was present in 29.9% of patients. Supraventricular arrhythmias were recorded in 38 patients (49.3%); all of these were short, asymptomatic, and self-limiting. Age (hazard ratio, 1.04 per year; 95% confidence interval, 1.00 to 1.08) and right atrial enlargement (hazard ratio, 4.29; 95% confidence interval, 1.30 to 14.09) were associated with supraventricular arrhythmia in the multivariate analysis. During a median follow-up of 40 months, 57 patients died, and cardiovascular disease was the main cause of death (52.6%). The variables associated with all-cause mortality in the Cox model were age (hazard ratio, 1.04 per year; 95% confidence interval, 1.00 to 1.08), C-reactive protein (hazard ratio, 1.04 per 1 mg/L; 95% confidence interval, 1.00 to 1.08), and supraventricular arrhythmia (hazard ratio, 3.21; 95% confidence interval, 1.29 to 7.96). Patients with supraventricular arrhythmia also had a higher risk of nonfatal cardiovascular events (hazard ratio, 4.32; 95% confidence interval, 2.11 to 8.83) and symptomatic atrial fibrillation during follow-up (hazard ratio, 17.19; 95% confidence interval, 2.03 to 145.15).
Conclusions
The incidence of intradialysis supraventricular arrhythmia was high in our hemodialysis study population. Supraventricular arrhythmias were short, asymptomatic, and self-limiting, and although silent, these arrhythmias were independently associated with mortality and cardiovascular events.
Keywords: Arrhythmias, hemodialysis, atrial fibrillation, mortality, asymptomatic arrhythmias, end-stage renal disease, chronic dialysis, attention, C-Reactive Protein, Cause of Death, Confidence Intervals, diabetes mellitus, Electrocardiography, female, Follow-Up Studies, Heart Atria, Heart Conduction System, humans, hypertension, Incidence, male, Multivariate Analysis, Prevalence, Proportional Hazards Models, Prospective Studies
Introduction
Cardiovascular disease is the main cause of death in patients with ESRD undergoing hemodialysis (1,2). In the US Renal Data System (USRDS) database, two thirds of cardiac deaths are caused by sudden death and arrhythmias, making up >25% of overall mortality (3,4).
Supraventricular arrhythmias (SVAs), especially atrial fibrillation (AF), are an increasingly common problem among patients on hemodialysis (5–8). CKD involves a series of risk factors for this condition, and hemodialysis can favor SVA onset and maintenance (9–11). However, SVAs have received scarce attention in the medical literature, so that the USRDS annual data report called AF “a neglected cardiovascular problem in dialysis patients” (12). Most studies are heterogeneous in terms of the populations studied, the definition of arrhythmias, and the methodology used in diagnosis. These studies merely take into account permanent alterations of rhythm and not the intradialysis paroxysmal episodes that can only be detected using continuous electrocardiographic monitoring. These are the reasons why there is a tremendous variability in the reported incidence and prevalence of SVA (13).
In the general population, the risk of mortality is increased in patients with AF compared with people who remain in sinus rhythm (14). Studies of patients undergoing hemodialysis show a similar prognosis in series including only patients with permanent AF (15,16). However, we know little or nothing about the long–term prognostic role of asymptomatic intradialysis SVA. Recent reports suggest that silent AF is an important risk factor for stroke in the general population (17,18), but relevance of subclinical SVA has not been proven in patients on hemodialysis.
The objective of this study was to analyze the incidence of intradialysis SVA, including asymptomatic arrhythmias, and its long-term association with adverse events in a cohort of patients undergoing hemodialysis.
Materials and Methods
Patients
We performed a prospective observational study. All procedures were conducted within the guidelines of Good Clinical Practice (19), and all patients were recruited for study participation and not for clinical indications, providing their written informed consent for this purpose before any study–specific procedures. A cross–sectional baseline analysis was carried out at recruitment. Follow-up of the patients continued for up to 10 years or until death or renal transplant. All patients were in sinus rhythm and had been on maintenance hemodialysis for at least 6 months. The exclusion criteria were antiarrhythmic therapy, permanent pacemakers, heart transplant, hospitalization, terminal malignancy, residual renal function, inadequate dose of dialysis, and central venous catheters.
Cardiovascular Evaluation
All patients were evaluated by a cardiologist at the beginning of the study, during the recruitment period, and during follow-up. The evaluation involved a clinical assessment, 12-lead electrocardiogram to determine baseline rhythm, color Doppler transthoracic echocardiogram, and Holter electrocardiogram (ECG) monitoring over six consecutive hemodialysis sessions (2 full weeks). Monitoring was started before the beginning of the session and stopped after the end of the dialysis procedure.
SVAs were defined as the association of three or more consecutive supraventricular extrasystoles or flutter or AF (20). The echocardiographic studies were performed in all patients after the midweek dialysis session with patients in their dry weight, thus avoiding the interference of weight gain in the cardiac evaluation. The studies were carried out during the recruitment period by a single cardiologist using the definitions of the American Society of Echocardiography, which were recorded for later viewing and verification (21,22). All were analyzed blind by a cardiologist using a semiautomatic system (SpaceLabs).
Variables Analyzed
The main demographic variables registered included age, sex, etiology of ESRD, hemodialysis vintage, body mass index, previous history of paroxysmal AF, and cardiovascular risk factors. Comorbidity was registered using the Charlson index (23), and the most relevant pharmacologic treatments prescribed were recorded. The serum values of hemoglobin, creatinine, calcium, phosphorus, albumin, cholesterol, triglycerides, C-reactive protein (CRP), and parathyroid hormone were determined during the recruitment period using standardized methods. During the hemodialysis sessions, when the Holter ECG was recorded, we also measured sodium, potassium, magnesium, calcium, and bicarbonate levels at the beginning and after 60, 120, 180, and 240 minutes.
Hemodialysis Characteristics
All patients were undergoing high-flux hemodialysis three times a week with an arteriovenous fistula as vascular access. Dialysate flow was maintained at 500 ml/min, with the temperature between 35.5°C and 36°C. Bicarbonate was used as a buffer, and the electrolyte composition was identical for all patients (sodium: 140 mEq/L; potassium: 1.5 mEq/L; calcium: 3 mEq/L; magnesium: 1 mEq/L; acetate: 4 mEq/L; bicarbonate: 36 mEq/L; and glucose: 150 mg/dl). The duration of the dialysis sessions was 240 minutes adjusted for Kt/V Daugirdas >1.3. We also registered weight gain between dialysis sessions, tolerance, and clinical events and symptoms during the sessions.
Statistical Analyses
Continuous variables were analyzed using the Kolmogorov–Smirnov test to verify a normal distribution. They are described as means±SD or medians and interquartile ranges for non-normal distributions. Variables were compared using the t test or Mann–Whitney test according to data distribution. Categorical variables are expressed as percentages and compared using the chi-squared test. CRP values were observed to follow an exponential curve and underwent logarithmic transformation. Multiple regression analysis and mediation analysis (Sobel test) were performed to investigate the significant factors associated with SVA. Kaplan–Meier survival analysis was performed, and the curves were compared using the log rank test. We performed Cox proportional hazards models to evaluate the relationship between the most relevant clinical variables and mortality. Model 1 was adjusted for age, sex, time on dialysis, coronary artery disease, congestive heart failure, diabetes mellitus, left ventricular mass index, left ventricular ejection fraction, atrial enlargement, CRP, hemoglobin, albumin, and cholesterol, and model 2 was adjusted for variables in model 1 plus SVA. The variables included were statistically significant in the univariate analysis or those with clinical relevance. Independent nephrologists and cardiologists at our center provided a systematic adjudication of outcomes. A P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS 20.0 (IBM SPSS, Chicago, IL).
Results
Baseline Characteristics
Ninety-eight white patients undergoing hemodialysis in our center were initially recruited for study participation. Of these, 85 fulfilled the inclusion criteria. Eight of them were ruled out according to the exclusion criteria (antiarrhythmic treatment [three], permanent pacemaker [one], heart transplant [one], central venous catheter [one], terminal malignancy [one], and hospitalization [one]). The remaining 77 patients (42 men and 35 women) were included in this study (Table 1). The main etiology of ESRD was diabetic nephropathy (22%). Median dialysis vintage was 36 months (interquartile range, 14–105 months).
Table 1.
Baseline characteristics of the study group
| Characteristics | All, n=77 | Non-SVA, n=39 | SVA, n=38 | P Value |
|---|---|---|---|---|
| Age, yr | 58±15 | 52±15 | 63±13 | 0.001 |
| Sex, men/women | 42/35 | 21/18 | 21/17 | 0.56 |
| Dialysis vintage, mo | 36 [14–105] | 30 [18–96] | 36 [11–114] | 0.88 |
| Hypertension, % | 68.8 | 71.8 | 65.8 | 0.56 |
| Diabetes mellitus, % | 29.9 | 38.4 | 21.0 | 0.15 |
| Coronary artery disease, % | 32.5 | 23.1 | 42.1 | 0.08 |
| Congestive heart failure, % | 42.8 | 41.0 | 44.7 | 0.74 |
| Dyslipidemia, % | 32.5 | 30.7 | 34.2 | 0.74 |
| BMI, kg/m2 | 24.5±4 | 25±5 | 24±3 | 0.40 |
| Peripheral vascular disease, % | 26.0 | 28.2 | 23.7 | 0.65 |
| Stroke, % | 7.8 | 7.7 | 7.9 | 0.97 |
| Documented previous AF, % | 10.4 | 2.5 | 18.4 | 0.02 |
| Charlson comorbidity index | 5.5±2.6 | 5.7±2.4 | 5.3±2.9 | 0.54 |
| Hemoglobin, g/dl | 10.5±1.5 | 10.8±1.5 | 10.2±1.5 | 0.09 |
| Serum PTH, pg/ml | 404±370 | 389±378 | 419±367 | 0.34 |
| Cholesterol, mg/dl | 183±35 | 181±41 | 185±28 | 0.58 |
| Triglycerides, mg/dl | 137±50 | 132±52 | 141±48 | 0.47 |
| Albumin, g/dl | 3.6±0.5 | 3.6±0.6 | 3.7±0.5 | 0.78 |
| Log C–reactive protein, mg/L | 0.55±0.39 | 0.44±0.44 | 0.65±0.31 | 0.01 |
| Kt/V Daugirdas | 1.35±0.1 | 1.34±0.09 | 1.36±0.11 | 0.40 |
| Ultrafiltration/session, L | 2.1±0.7 | 2.1±0.6 | 2.1±0.8 | 0.87 |
| LVMI, g/m2 | 140±47 | 132±30 | 148±59 | 0.18 |
| Ejection fraction, % | 60.2±13.0 | 63.4±12.4 | 57.4±13.1 | 0.07 |
| Diastolic dysfunction, % | 50.7 | 53.1 | 48.5 | 0.71 |
| Left ventricular dilation, % | 21.2 | 15.1 | 27.2 | 0.22 |
| Left atrial enlargement, % | 76.2 | 78.1 | 74.1 | 0.71 |
| Right atrial enlargement, % | 31.3 | 17.6 | 45.4 | 0.01 |
| Pulmonary hypertension, % | 18.6 | 16.2 | 21.0 | 0.59 |
| Valvular calcifications, % | 26.6 | 27.2 | 25.8 | 0.89 |
Data are expressed as means±SD, medians [25%–75%], numbers, or percentages. SVA, supraventricular arrhythmia; BMI, body mass index; AF, atrial fibrillation; PTH, parathyroid hormone; LVMI, left ventricular mass index.
Of the 77 patients, 66.7% had left ventricular hypertrophy, 76.2% had left atrial enlargement, and 50.7% had diastolic dysfunction. The echocardiographic results were normal for only four patients (Table 1).
Incidence of SVAs
The intradialysis Holter ECG recordings revealed 84 episodes of arrhythmias in 44 patients (57.1%), resulting in an incidence of 181 arrhythmias per 1000 hemodialysis sessions. SVAs were the most frequent arrhythmias, with a total of 72 episodes in 38 patients (49.3%), compared with 12 episodes of ventricular arrhythmias in ten patients (12.9%). Median duration of SVA was 2.1 seconds (interquartile range, 1.1–5.3 seconds), and only seven arrhythmias (three AFs and four atrial flutters) were sustained. Supraventricular tachycardia was the most frequent arrhythmia (52 episodes in 33 patients) followed by atrial flutter (13 episodes in ten patients) and AF (seven episodes in six patients). All SVAs were asymptomatic and self-limiting, including the longest lasting episodes of flutter and fibrillation.
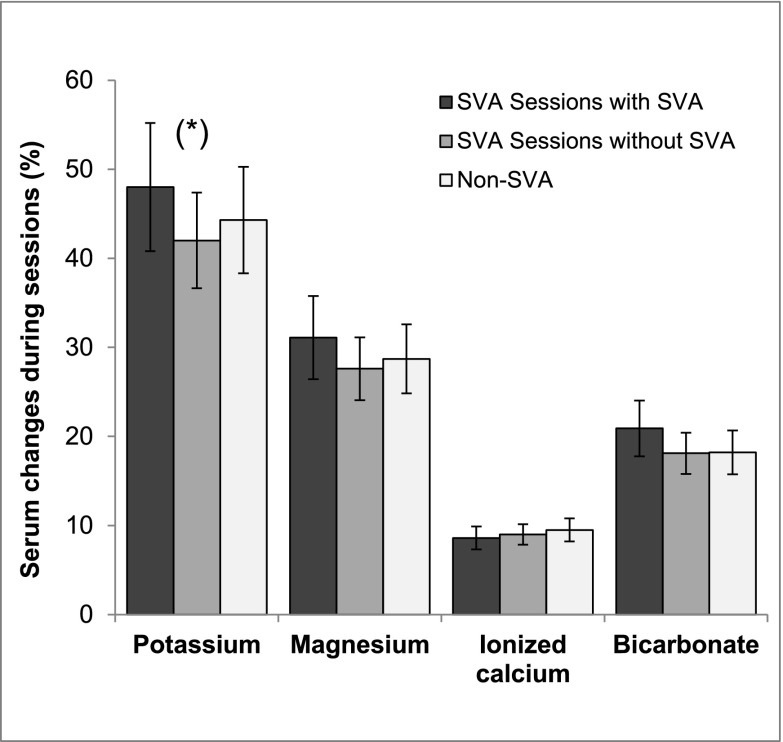
The patients with SVA were older, had a previous history of paroxysmal AF, and had higher CRP levels. Right atrial enlargement was the only echocardiographic finding associated with SVA (Table 1). No differences were found in predialysis serum potassium levels between patients with and without SVA (5.7±0.9 and 5.7±0.7 mEq/L, respectively; P=0.88). However, we observed that patients with SVA did not experience arrhythmias in all of the sessions. Analysis of electrolyte changes during the episodes of SVA showed a greater mean percentage decrease in potassium levels during those sessions (P=0.03). These decreases were no different from those observed in patients without SVA (Figure 1). Logistic regression analysis revealed that only age (hazard ratio [HR], 1.04 per year; 95% confidence interval [95% CI], 1.00 to 1.08) and right atrial enlargement (HR, 4.29; 95% CI, 1.30 to 14.09) were independently associated with the presence of SVA. The Sobel test to determine the role of potassium was 1.21 (P=0.22), showing there was no evidence of mediation.
Figure 1.
Electrolytic changes during hemodialysis sessions (percentages), showing a significantly greater mean percentage decrease in potassium levels during sessions with SVA (P=0.03)*.
The incidence of SVA increased as the dialysis session progressed, with 43% of the SVAs occurring during the last hour. We recorded 32.0±6.1 minutes per patient-session before dialysis and 50.7±7.8 minutes per patient-session after dialysis. No SVAs were observed predialysis, and only four episodes were observed postdialysis. We found no increased incidence of SVA on any specific day of the week.
Analyses of Mortality
Patients were followed up to 10 years, with the median follow-up in the overall group of 40 months (interquartile range, 18–72 months). During this period, 57 patients died, and 15 received transplants. No patients were lost to follow-up. Cardiovascular disease was the main cause of death (53%) followed by infection (28%). It was not possible to determine the cause of death in two patients (one in each group).
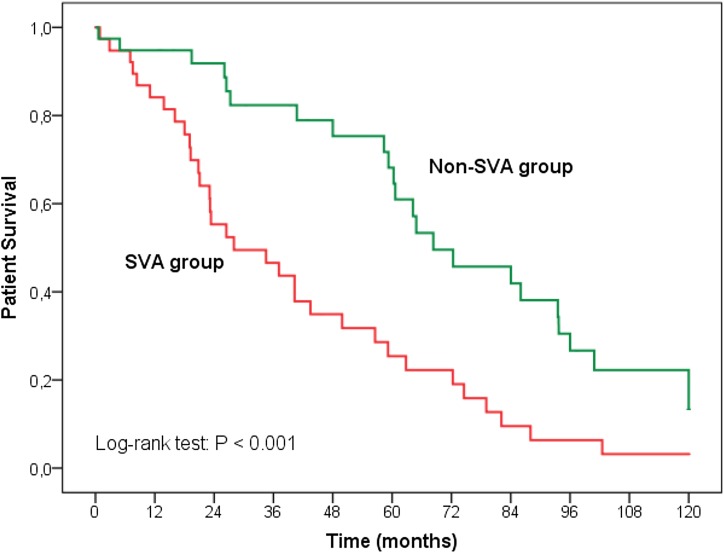
We analyzed patient characteristics according to progress and found no differences in hemodialysis parameters or echocardiographic findings. Age, coronary artery disease, and CRP were associated with overall mortality in the Cox regression analysis (model 1); however, additional adjustment for SVA (model 2) modified these results (Table 2). Age and CRP remained associated with mortality but not coronary artery disease, in which the relationship was attenuated and no longer statistically significant. SVAs were the strongest factor associated with all-cause mortality. We independently analyzed the association between SVA and survival. Mean survival was 68 months for patients without SVA and 28 months for patients with SVA; all-cause mortality was higher among patients with SVA (log rank =12.45; P<0.001). The survival curves show a clear difference between the patients with and without SVA (Figure 2). No significant relationship between duration of arrhythmia and mortality was found.
Table 2.
Adjusted hazard ratios (95% confidence intervals) for all-cause mortality
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, per yr | 1.05 (1.01 to 1.09) | 0.01 | 1.04 (1.00 to 1.08) | 0.04 |
| Coronary artery disease | 2.65 (1.12 to 6.28) | 0.02 | 2.48 (0.99 to 6.15) | 0.06 |
| Log C–reactive protein, per mg/L | 1.04 (1.00 to 1.08) | 0.03 | 1.04 (1.00 to 1.08) | 0.02 |
| Supraventricular arrhythmia | 3.21 (1.29 to 7.96) | 0.01 | ||
Model 1: Cox model adjusted for age, sex, time on dialysis, coronary artery disease, congestive heart failure, diabetes mellitus, left ventricular mass index, left ventricular ejection fraction, atrial enlargement, C-reactive protein, hemoglobin, albumin, and cholesterol. Model 2: Cox model adjusted for covariates in model 1 plus supraventricular arrhythmia. HR, hazard ratio; 95% CI, 95% confidence interval.
Figure 2.
Survival curves for all-cause mortality illustrating survival disadvantage of patients on hemodialysis with supraventricular arrhythmias (SVAs) compared to those without SVAs.
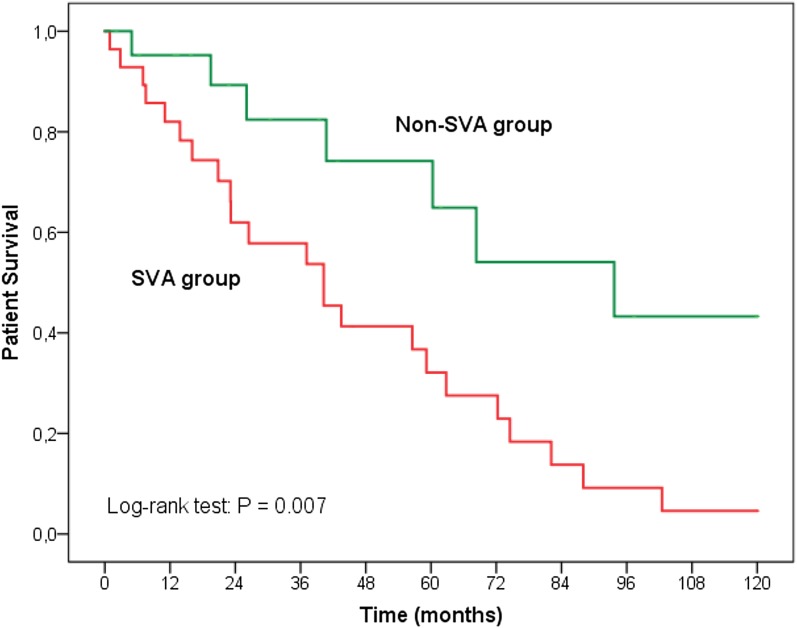
Cardiovascular mortality was higher among patients with SVA than in patients without SVA (log rank =7.36; P<0.01) (Figure 3). SVAs were also the strongest factor associated with cardiovascular mortality after adjusting for defined variables (Table 3). Sudden death was the single most frequent cause of death in the SVA group (34.7% versus 14.3% in non-SVA group; P=0.01). In our series, three patients with SVA (13.1%) and one patient without SVA (14.3%) died from stroke (P=0.93).
Figure 3.
Survival curves for cardiovascular mortality illustrating survival disadvantage of patients on hemodialysis with supraventricular arrhythmias (SVAs) compared to those without SVAs.
Table 3.
Adjusted hazard ratios (95% confidence intervals) for cardiovascular mortality
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, per yr | 1.05 (0.99 to 1.11) | 0.07 | 1.03 (0.97 to 1.09) | 0.30 |
| Coronary artery disease | 4.52 (1.04 to 19.60) | 0.03 | 4.28 (1.02 to 17.88) | 0.04 |
| Log C–reactive protein, per mg/L | 1.01 (1.00 to 1.02) | 0.02 | 1.00 (0.99 to 1.02) | 0.18 |
| Supraventricular arrhythmia | 4.99 (1.21 to 20.47) | 0.01 | ||
Model 1: Cox model adjusted for age, sex, time on dialysis, coronary artery disease, congestive heart failure, diabetes mellitus, left ventricular mass index, left ventricular ejection fraction, atrial enlargement, C-reactive protein, hemoglobin, albumin, and cholesterol. Model 2: Cox model adjusted for covariates in model 1 plus supraventricular arrhythmia. HR, hazard ratio; 95% CI, 95% confidence interval.
Analyses of Cardiovascular Events
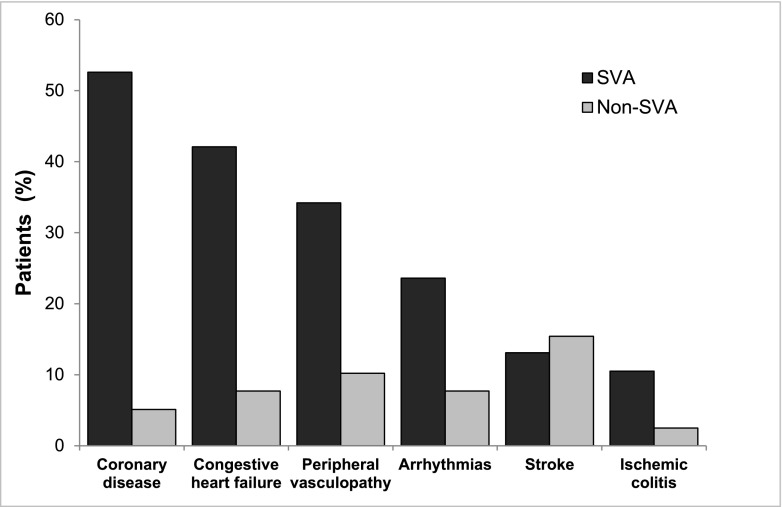
Eighty-seven nonfatal cardiovascular events were recorded in 47 patients (61.0%). Coronary ischemia, congestive heart failure, peripheral vascular disease, and symptomatic arrhythmias were the most frequent events in the SVA group (Figure 4). In the Cox model, SVAs were associated with a higher risk of nonfatal cardiovascular events adjusted for the most relevant clinical variables (HR, 4.32; 95% CI, 2.11 to 8.83).
Figure 4.
Nonfatal cardiovascular events were more frequent in patients who developed supraventricular arrhythmias (SVAs). Coronary disease (52.6% versus 5.1%; P=0.001), congestive heart failure (42.1% versus 7.7%; P=0.001), peripheral vasculopathy (34.2% versus 10.2%; P=0.01), arrhythmias (23.6% versus 7.7%; P=0.04), stroke (13.1% versus 15.4%; P=0.78), and ischemic colitis (10.5% versus 2.5%; P=0.15).
Symptomatic arrhythmias diagnosed during the follow-up were analyzed independently. Eleven patients were diagnosed as having paroxysmal AF. Of these, seven progressed to chronic AF in follow-up. Seven patients had SVA in the Holter ECG performed at baseline, and all of them were treated with antiarrhythmics. An adjusted Cox model revealed that SVAs in the Holter ECG at the start of the study were the major independent predictor of symptomatic AF (HR, 17.19; 95% CI, 2.03 to 145.15).
Discussion
We found a high incidence of intradialysis SVAs, all of which were short, self-limiting, and asymptomatic. These arrhythmias were detected using continuous ECG monitoring in 49.3% of patients. It is difficult to establish a comparison with other series because of the heterogeneous methodology, the different diagnostic procedures, and even the definition of SVA (5–8). Most of these studies only analyzed patients with chronic AF or symptomatic arrhythmias. In our series, nonsustained supraventricular tachycardia was the most frequent arrhythmia, and all episodes were silent. These arrhythmias can only be diagnosed by continuous ECG monitoring methods, such as we used in our study.
Recent attention has centered on the relationship between nonsustained supraventricular tachycardia and AF, especially in patients with structural heart disease, which was the condition affecting most of our patients (24). Vincenti et al. (25) showed how paroxysmal AF episodes in patients on hemodialysis were preceded by a marked increase in supraventricular ectopic beats. We found a strong relationship between SVA in the Holter ECG and symptomatic AF during the follow-up. This finding is consistent with the natural history of this arrhythmia (26). Using continuous ECG monitoring to detect SVAs, which are imperceptible by other methods, enabled us to select a population that was susceptible to paroxysmal or permanent AF.
The patients who developed SVA were older; this is a universal finding in AF in both the general population and patients undergoing hemodialysis (5,7,14,27). Right atrial enlargement was the only echocardiographic finding that differentiated patients with SVA from patients who were free of these arrhythmias. The relationship between the diameter of the right atrium and developing atrial arrhythmias during hemodialysis has received little attention. In their study of 183 patients, Acar et al. (28) showed that age and right atrium enlargement were the only factors related to SVA. Atar et al. (29) reached similar conclusions, although neither group offered a definitive explanation for their findings. As in our study, the population in both cases had been on long-term hemodialysis. Likewise, all of the patients had an arteriovenous fistula as vascular access. In many patients, these fistulas were located in the proximal part of the arm where blood flow is high, which causes chronic volume overload, closely related to atrial remodeling (30). Recent studies highlighted the role of right atrial dilation and remodeling in the development of AF, making it a good predictor of recurrence after cardioversion and pulmonary vein catheter ablation (31,32).
Patients with SVA did not develop arrhythmias in all of the sessions. We observed a higher mean percentage decrease in potassium in those sessions as previously described by other authors (33). Krijthe et al. (34) described the risk of AF in patients with hypokalemia. In this series, low serum potassium levels were no different from those observed in patients without SVA. Taken together, these observations suggest that intradialysis variations in serum potassium levels only play a role in susceptible individuals.
The presence of asymptomatic SVA was associated with significantly higher risk for mortality in our patients. Interest in the long–term prognostic role of SVA in patients undergoing hemodialysis has grown recently. Different studies show that the risk of death is greater in patients with AF than in those who maintained sinus rhythm (5,7,15,35). Vazquez et al. (15) analyzed the prevalence and evolution of AF in 256 patients over a 4-year period. Age and AF were related to mortality as observed in our study. Wizemann et al. (7) and Winkelmayer et al. (5) also showed the association between AF and overall mortality in patients on hemodialysis. Finally, in their meta-analysis, Zimmerman et al. (13) grouped together the main studies on SVA in patients undergoing hemodialysis. Most reported a worse prognosis among patients with SVA. In the studies cited above, diagnosis of AF was made using a 12–lead surface ECG, clinical records, or hospitalization data of the patients. Continuous ECG registers were not carried out in these studies, such as in our series. Therefore, asymptomatic episodes were never registered, and subclinical arrhythmias were not evaluated. In recent years, continuous ECG monitoring has provided useful data about the role of asymptomatic arrhythmias in cryptogenic stroke (17,18). However, the association of subclinical SVA with clinical outcomes has not been studied in patients on hemodialysis up to now. In our study, we observed how, despite its silent behavior, SVAs were strongly associated with long–term all–cause and cardiovascular mortality. Sudden death was the single most frequent cause of death within the group with SVA. We found that 88.8% of the patients who suffered sudden death had developed SVA during the sessions in which Holter ECG tests were carried out. Genovesi et al. (36) showed how AF was associated with a higher risk of sudden death in patients undergoing long-term hemodialysis, such as in our series. Although the relationship between SVA and sudden death is controversial, various studies have shown that both disorders are pathogenically associated (37,38). Silent SVAs probably represent an early and underestimated warning sign associated with an increased risk of mortality. The Monitoring in Dialysis Study, using implantable loop recorders, will be able to provide novel insights in this field (4).
Lastly, we observed a greater risk of cardiovascular events in patients with SVA. This finding has been reported among patients with atrial arrhythmias (14,39). However, we had too few events to examine the association with ischemic or hemorrhagic stroke.
Our study is limited by its small sample size and single-center design. We studied prevalent long–term patients on hemodialysis, all of them white, limiting generalizability to other populations. Nevertheless, it does benefit from a follow-up of up to 10 years. Furthermore, multiple and strict exclusion criteria established at recruitment ensured that the study population was homogeneous. Additionally, all of the cardiovascular evaluations were carried out by the same cardiologist who had proven experience in this field and more specifically, electrophysiology and echocardiography. Likewise, all echocardiographic studies were carried out in the interdialytic period when the patients were euvolemic. We achieved a high level of diagnostic sensitivity in the Holter ECG registers, with each patient analyzed over six complete and consecutive sessions of hemodialysis, resulting in >24 hours of recording per patient. Finally, over these 10 years, some medical practices have changed as a result of new medical advances.
In conclusion, the incidence of intradialytic SVA was high in our patients undergoing hemodialysis. SVAs were short, asymptomatic, and self-limiting, and although silent, these arrhythmias were strongly associated with mortality and cardiovascular events.
Disclosures
The authors did not receive any form of financial support related to this study.
Acknowledgments
The authors thank Thomas O'Boyle for proofreading the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ortiz A, Covic A, Fliser D, Fouque D, Goldsmith D, Kanbay M, Mallamaci F, Massy ZA, Rossignol P, Vanholder R, Wiecek A, Zoccali C, London GM; Board of the EURECA-m Working Group of ERA-EDTA : Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 383: 1831–1843, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Ritz E, Bommer J: Cardiovascular problems on hemodialysis: Current deficits and potential improvement. Clin J Am Soc Nephrol 4[Suppl 1]: S71–S78, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N, Liu J, St Peter W, Guo H, Hu Y, Kats A, Li S, Li S, Maloney J, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L: US Renal Data System 2013 Annual Data Report. Am J Kidney Dis 63[Suppl]: A7, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Charytan DM, Foley R, McCullough PA, Rogers JD, Zimetbaum P, Herzog CA, Tumlin JA; MiD Investigators and Committees : Arrhythmia and sudden death in hemodialysis patients: Protocol and baseline characteristics of the monitoring in dialysis study. Clin J Am Soc Nephrol 11: 721–734, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S: The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol 22: 349–357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, Acquistapace I, Stella A, Bonforte G, DeVecchi A, DeCristofaro V, Buccianti G, Vincenti A: Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis 46: 897–902, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Wizemann V, Tong L, Satayathum S, Disney A, Akiba T, Fissell RB, Kerr PG, Young EW, Robinson BM: Atrial fibrillation in hemodialysis patients: Clinical features and associations with anticoagulant therapy. Kidney Int 77: 1098–1106, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Liao JN, Chao TF, Liu CJ, Wang KL, Chen SJ, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chung FP, Chen TJ, Chen SA: Incidence and risk factors for new-onset atrial fibrillation among patients with end-stage renal disease undergoing renal replacement therapy. Kidney Int 87: 1209–1215, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J: Chronic kidney disease is associated with the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 123: 2946–2953, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal N, Xie D, Tao K, Chen J, Deo R, Horwitz E, Hsu CY, Kallem RK, Keane MG, Lora CM, Raj D, Soliman EZ, Strauss L, Wolf M, Go AS; CRIC Study : Atrial fibrillation and risk of ESRD in adults with CKD. Clin J Am Soc Nephrol 11: 1189–1196, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Severi S, Pogliani D, Fantini G, Fabbrini P, Viganò MR, Galbiati E, Bonforte G, Vincenti A, Stella A, Genovesi S: Alterations of atrial electrophysiology induced by electrolyte variations: Combined computational and P-wave analysis. Europace 12: 842–849, 2010 [DOI] [PubMed] [Google Scholar]
- 12.US Renal Data System : Cardiovascular special studies. USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease; Am J Kidney Dis 47 [Suppl 1]: S173–S184, 2006 [Google Scholar]
- 13.Zimmerman D, Sood MM, Rigatto C, Holden RM, Hiremath S, Clase CM: Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant 27: 3816–3822, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D: Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 98: 946–952, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Vazquez E, Sanchez-Perales C, Garcia-Garcia F, Castellano P, Garcia-Cortes MJ, Liebana A, Lozano C: Atrial fibrillation in incident dialysis patients. Kidney Int 76: 324–330, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Genovesi S, Rossi E, Gallieni M, Stella A, Badiali F, Conte F, Pasquali S, Bertoli S, Ondei P, Bonforte G, Pozzi C, Rebora P, Valsecchi MG, Santoro A: Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant 30: 491–498, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O’Donnell M, Laupacis A, Côté R, Sharma M, Blakely JA, Shuaib A, Hachinski V, Coutts SB, Sahlas DJ, Teal P, Yip S, Spence JD, Buck B, Verreault S, Casaubon LK, Penn A, Selchen D, Jin A, Howse D, Mehdiratta M, Boyle K, Aviv R, Kapral MK, Mamdani M; EMBRACE Investigators and Coordinators : Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 370: 2467–2477, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J; CRYSTAL AF Investigators : Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 370: 2478–2486, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Dixon JR, Jr.: The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur 6: 65–74, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Blomström-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, Campbell WB, Haines DE, Kuck KH, Lerman BB, Miller DD, Shaeffer CW Jr., Stevenson WG, Tomaselli GF, Antman EM, Smith SC Jr., Alpert JS, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Hiratzka LF, Hunt SA, Jacobs AK, Russell RO Jr., Priori SG, Blanc JJ, Budaj A, Burgos EF, Cowie M, Deckers JW, Garcia MA, Klein WW, Lekakis J, Lindahl B, Mazzotta G, Morais JC, Oto A, Smiseth O, Trappe HJ; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines. Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias : ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation 108: 1871–1909, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC Jr., Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO; American College of Cardiology; American Heart Association; American Society of Echocardiography : ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 108: 1146–1162, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography : Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Binici Z, Intzilakis T, Nielsen OW, Køber L, Sajadieh A: Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation 121: 1904–1911, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Vincenti A, Passini E, Fabbrini P, Luise MC, Severi S, Genovesi S: Recurrent intradialytic paroxysmal atrial fibrillation: Hypotheses on onset mechanisms based on clinical data and computational analysis. Europace 16: 396–404, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Brigadeau F, Lacroix D: [Natural history and outcomes of atrial fibrillation]. Rev Prat 63: 193–197, 2013 [PubMed] [Google Scholar]
- 27.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE: Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285: 2370–2375, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Acar G, Akçay A, Doğan E, Işik IO, Sökmen A, Sökmen G, Sayarlioğlu H, Köroğlu S, Nacar AB, Tuncer C: The prevalence and predictors of atrial fibrillation in hemodialysis patients. Turk Kardiyol Dern Ars 38: 8–13, 2010 [PubMed] [Google Scholar]
- 29.Atar I, Konaş D, Açikel S, Külah E, Atar A, Bozbaş H, Gülmez O, Sezer S, Yildirir A, Ozdemir N, Müderrisoğlu H, Ozin B: Frequency of atrial fibrillation and factors related to its development in dialysis patients. Int J Cardiol 106: 47–51, 2006 [DOI] [PubMed] [Google Scholar]
- 30.De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC: Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res 89: 754–765, 2011 [DOI] [PubMed]
- 31.Luong C, Thompson DJ, Bennett M, Gin K, Jue J, Barnes ME, Colley P, Tsang TS: Right atrial volume is superior to left atrial volume for prediction of atrial fibrillation recurrence after direct current cardioversion. Can J Cardiol 31: 29–35, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Akutsu Y, Kaneko K, Kodama Y, Suyama J, Li HL, Hamazaki Y, Tanno K, Gokan T, Kobayashi Y: Association between left and right atrial remodeling with atrial fibrillation recurrence after pulmonary vein catheter ablation in patients with paroxysmal atrial fibrillation: A pilot study. Circ Cardiovasc Imaging 4: 524–531, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Korzets A, Ori Y, Herman M: Serum potassium levels and atrial fibrillation in haemodialysis patients. Nephrol Dial Transplant 16: 1090–1091, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Krijthe BP, Heeringa J, Kors JA, Hofman A, Franco OH, Witteman JC, Stricker BH: Serum potassium levels and the risk of atrial fibrillation: The Rotterdam Study. Int J Cardiol 168: 5411–5415, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Fujii H, Kim JI, Yoshiya K, Nishi S, Fukagawa M: Clinical characteristics and cardiovascular outcomes of hemodialysis patients with atrial fibrillation: A prospective follow-up study. Am J Nephrol 34: 126–134, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Genovesi S, Valsecchi MG, Rossi E, Pogliani D, Acquistapace I, De Cristofaro V, Stella A, Vincenti A: Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transplant 24: 2529–2536, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Chen LY, Sotoodehnia N, Bůžková P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag S, Konety S, Folsom AR, Siscovick D, Alonso A: Atrial fibrillation and the risk of sudden cardiac death: The atherosclerosis risk in communities study and cardiovascular health study. JAMA Intern Med 173: 29–35, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denker S, Lehmann M, Mahmud R, Gilbert C, Akhtar M: Facilitation of ventricular tachycardia induction with abrupt changes in ventricular cycle length. Am J Cardiol 53: 508–515, 1984 [DOI] [PubMed] [Google Scholar]
- 39.Conen D, Chae CU, Glynn RJ, Tedrow UB, Everett BM, Buring JE, Albert CM: Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA 305: 2080–2087, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]