Abstract
Background and objectives
A kidney biopsy is preferred for the diagnosis of ANCA-associated vasculitis with renal involvement. The aim of our study was to evaluate the prognostic value of a histopathologic classification scheme recently proposed by an international consortium of renal pathologists in a large Norwegian cohort.
Design, setting, participants, & measurements
Patients diagnosed with ANCA-associated GN were included from the Norwegian Kidney Biopsy Registry 1991–2012. Renal morphology was classified as focal, mixed, crescentic, or sclerotic. Study end point was ESRD. Patients were followed from kidney biopsy to end of 2012.
Results
Two hundred fifty patients with ≥10 glomeruli in the biopsy were included in our study. During a median follow-up of 3.5 years (0.7–7.6), 60 cases of ESRD occurred. Ninety-six (38%) biopsies were classified as focal, 61 (24%) biopsies were classified as mixed, 71 (28%) biopsies were classified as crescentic, and 22 (9%) biopsies were classified as sclerotic; 1- and 5-year cumulative renal survival rates were 96% and 90%, respectively, for the focal class, 86% and 75%, respectively, for the mixed class, 81% and 69%, respectively, for the crescentic class, and 56% and 51%, respectively, for the sclerotic class. By multivariate Cox regression analyses, the sclerotic class had a significantly worse renal prognosis than the focal (hazard ratio, 9.65; 95% confidence interval, 2.38 to 39.16) or combined mixed/crescentic classes (hazard ratio, 3.27; 95% confidence interval, 1.41 to 7.61), but no significant differences in outcome were observed in the crescentic class compared with the mixed class (hazard ratio, 1.13; 95% confidence interval, 0.44 to 2.87) or the combined mixed/crescentic class compared with the focal class (hazard ratio, 1.93; 95% confidence interval, 0.61 to 6.12). Accuracy by receiver operator characteristic curve analysis was estimated to be 0.72 (95% confidence interval, 0.65 to 0.80). In 108 additional patients with three to nine glomeruli in the biopsy, the prognostic value of this classification scheme was unchanged.
Conclusions
The histopathologic classification is a predictor of renal outcome of moderate quality. Merging the mixed and crescentic classes in the future could simplify the scheme.
Keywords: ANCA; classification; vasculitis; renal pathology; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Antibodies, Antineutrophil Cytoplasmic; Biopsy; Follow-Up Studies; glomerulonephritis; Humans; kidney; Kidney Failure, Chronic; Kidney Glomerulus; Prognosis; ROC Curve; Registries; Regression Analysis
Introduction
ANCA-associated vasculitis is associated with substantial morbidity and mortality, even with the latest treatments (1). Renal involvement in the form of a pauci-immune GN (ANCA-GN) is frequently seen in patients with ANCA-associated vasculitis, and it is associated with a more severe prognosis (2–4). Although rapid progressive GN syndrome and a positive ANCA test are diagnostic of ANCA-GN, histologic confirmation from a diagnostic kidney biopsy is still considered the gold standard by which to diagnose ANCA-GN.
The kidney morphology in ANCA-GN also provides useful prognostic information. The proportion of normal glomeruli together with the percentage of globally sclerotic glomeruli are considered to be important histologic indicators of renal prognosis. Furthermore, the quantity of active cellular crescents is another predictor of outcome (5–8). In addition to glomerular changes, tubulointerstitial findings have also been found to be associated with renal prognosis in ANCA-GN (9).
In 2010, Berden et al. (8) proposed a histopathologic classification model for ANCA-GN (ANCA-GN classification). Taking previous findings into account, they proposed four classes: sclerotic (>50% globally sclerotic glomeruli), focal (<50% affected glomeruli), crescentic (>50% active cellular crescents), and mixed (<50% normal/crescentic/sclerotic glomeruli). To our knowledge, this classification model has now been applied to 12 independent patient cohorts (8,10–20). Generally, in univariate analyses, investigators have correlated a favorable prognosis with the focal group and a poor prognosis with the sclerotic group. Regarding renal prognosis in the crescentic versus mixed group, inconsistent results have been reported. Several studies reported a better prognosis with the crescentic class (8,15,18), which has been contradicted by others (10,11,17,20). No group has reported a statistically different risk of ESRD in the mixed versus crescentic group. An important current limitation of the ANCA-GN classification model is its requirement for at least 10 glomeruli to be present in the biopsy core for a valid classification. Its prognostic value in cases with fewer glomeruli, which are common in clinical practice, has not, until now, been examined. Whether the ANCA-GN classification model can be used to derive statistically significant risk factors in multivariate analyses after adjustment for age, sex, ANCA subtype, and eGFR has also not been addressed, principally because of small study cohorts with few end points, which have limited the power of such analyses.
Given that the ANCA-GN classification model had yet to be evaluated in a Scandinavian cohort together with the need to test a large-enough cohort to render multivariate analyses practicable, we decided to apply this model to a cohort of patients diagnosed with ANCA-GN from 1991 to 2012 as identified in the Norwegian Kidney Biopsy Registry (NKBR). We also evaluated the classification model in patients with only three to nine glomeruli present in the kidney biopsy.
Materials and Methods
This study was approved by the Regional Committees for Medical and Health Research Ethics (REC South-East).
Identification of Study Cohort and Baseline Data
All patients in the NKBR diagnosed with kidney biopsy–verified ANCA-GN from 1991 to 2012 were included in our study. All patients had a seropositive test for ANCA, and the GN was pauci-immune. In patients with repeated biopsies, only the initial diagnostic biopsy was included and classified. The histopathologic picture was characterized by varying degrees of normal glomeruli, glomerular fibrinoid necrosis, crescent formation, and sclerosis. L.B., an experienced renal pathologist, was head pathologist at the NKBR for the entire study period and responsible for reviewing, diagnosing, and classifying all of the biopsies sent to the registry.
Baseline data, including age, sex, ANCA type (cytoplasmic ANCA/proteinase 3 ANCA versus perinuclear ANCA/myeloperoxidase ANCA), and eGFR (determined by the Modification of Diet in Renal Disease equation [21]) at the time of diagnosis, were obtained from the NKBR.
Processing, Description, and Diagnosing of the Biopsies in the NKBR
A standard protocol has been followed for processing, describing, and diagnosing all of the biopsies included in our study. Hematoxylin and eosin–, periodic acid–Schiff–, and Jones methenamine silver–stained slides were examined supplemented by Masson trichrome when found necessary.
The biopsies were subject to immunohistochemistry (peroxidase-antiperoxidase method) applying a standard set of antibodies specific for IgG, IgA, IgM, C3, C1q, and κ- and λ-light chains. Transmission electron microscopy was performed when light microscopy and immunohistochemistry rendered ambiguous results.
Positive and negative findings were described in detail in the histology report. Crescents, necrosis, and glomerular sclerosis were quantified and also categorized as either diffuse or focal and either global or segmental (necrosis and sclerosis). The cellular crescent was defined as at least two layers of (proliferating) epithelial and inflammatory cells occupying part or all of the capsular space. The fibrocellular category, according to the ANCA-GN classification model included in the cellular group, comprises crescents with epithelial and inflammatory cells and a variable amount of fibrillary material, amounting to almost complete fibrous replacement of the cellular component.
Scoring of the Patient Cohort According to the ANCA-GN Classification Model
Using the detailed, quantitated, and standardized histopathologic data in the NKBR blinded for clinical findings at biopsy and patient outcome, the renal pathologist scored the study cohort according to the classification model. Patients with incomplete data or less than three glomeruli were excluded. The study cohort was stratified into patients with three to nine and patients with ≥10 glomeruli present in the biopsy.
Definition of the Observation Period
The observation period of this study was defined as the time between the diagnostic kidney biopsy and the first event (ESRD, death, or December 31, 2012). Furthermore, this observation period was stratified into 0–1 year and ≥1 year after kidney biopsy.
Definition and Identification of Primary and Secondary End Points
The primary outcome in this study, ESRD, was defined as the instigation of chronic dialysis therapy or kidney transplantation. Patients with ESRD in the study cohort were identified through records linked with the Norwegian Renal Registry. The secondary outcome, death before initiation of RRT, was identified through records linked with the Population Registry of Norway. Unique 11–digit Norwegian personal identification numbers were used to link the study cohort to the Norwegian Renal Registry and the Population Registry of Norway.
Treatment
Treatment of the individual patients with ANCA-GN in our cohort has not been recorded in the NKBR. From our own clinical practice and previous publications, we know that most patients have received induction therapy consisting of daily oral or intravenous pulse cyclophosphamide in combination with intravenous methylprednison followed by oral corticosteroids (6,22,23). Plasma exchange is used in some patients. After achieving remission, maintenance treatment with low-dose corticosteroids and cyclophosphamide (first part of study period) or azathioprine (later part of study period) was given. In recent years, some patients have received rituximab for both induction and maintenance treatment. At the time of kidney biopsy, the majority of patients have received no or only few days of intravenous methylprednisolone treatment.
Analyses and Statistical Methods
ESRD-free survival at 1 and 5 years of follow-up in the four prognostic groups of the ANCA-GN classification model was measured and compared using Kaplan–Meier statistics and log rank tests. A Cox regression model was used to compare hazard ratios (HRs) of ESRD between the different prognostic groups of the classification model. Unadjusted and adjusted HRs are reported. In multivariate analyses, we first adjusted for sex, age, and ANCA type before readjusting for these criteria plus eGFR. Separate analyses were performed for the 0–1 year and the ≥1 year after kidney biopsy periods. To exclude any selection bias, adjusted HRs for ESRD in the excluded group versus the final study group and the three to nine glomeruli group versus the final study group were calculated. In these analyses, we adjusted for age, sex, ANCA type, initial eGFR, and histologic classification (the latter only in the three to nine versus ≥10 glomeruli group analysis). To test the prognostic capability of the classification model to predict ESRD in the 0–1 year after kidney biopsy observation period, we used receiver operator characteristic (ROC) statistics measuring the area under the curve (AUC). First, we calculated the AUC for the histologic classification model alone. Second, using logistic regression, we created a clinical classification model that included age (years), sex, ANCA type, and eGFR (milliliters per minute per 1.73 m2) and calculated AUC. Third, also using logistic regression, we merged the histologic and clinical classification models and calculated AUC.
The Cumulative 1-Year Risks of Death in the Cohort and Each Prognostic Group of the Classification Model Were Studied Using Kaplan–Meier Statistics
For the comparison of outcomes with this Norwegian study cohort, data from previous studies evaluating the classification model were collected. Studies containing at least 100 patients and reporting 1- and/or 5-year cumulative risk of ESRD were selected. Finally, we analyzed the three to nine glomeruli group. ESRD–free survival rates at 1 and 5 years of follow-up in the four prognostic groups of the classification model were measured and compared using Kaplan–Meier statistics and log rank tests. Results for the three to nine glomeruli group were also compared with those of the ≥10 glomeruli group. Continuous variables are expressed as mean with SD, and categorical variables are expressed as number and percentage. SPSS, version 23 for Windows (IBM SPSS, Chicago, IL) was used for statistical analyses.
Results
Cohort Selection and Characteristics
A total of 445 patients with ANCA-GN diagnosed from 1991 to 2012 in the NKBR were identified; 87 patients were excluded because of missing data or less than or equal to two glomeruli. Three hundred fifty-eight patients (108 patients with three to nine glomeruli and 250 patients with ≥10 glomeruli in the biopsy specimen) were scored according to the ANCA-GN classification model. Baseline characteristics in the final study cohort of 250 patients are shown in Table 1. One hundred twenty-seven patients (51%) were women, the mean cohort age was 59 years old (SD=18), 134 (54%) were cytoplasmic ANCA or proteinase 3 ANCA positive, and the mean eGFR was 35 ml/min per 1.73 m2 (SD=31). Median follow-up length was 3.5 years (25th–75th percentiles =0.7–7.6 years). During the total follow-up period, 60 patients progressed to ESRD. During 198 person-years in the 0–1 year after kidney biopsy observation period, 34 patients progressed to ESRD, and annual risk was 17.2%. In the ≥1 year after kidney biopsy observation period, the number of person-years was 971, and 26 patients progressed to ESRD (mean annual risk =2.7%).
Table 1.
Baseline characteristics stratified for histologic classification
| Characteristic | All, n=250 | Focal, n=96 | Mixed, n=61 | Crescentic, n=71 | Sclerotic, n=22 |
|---|---|---|---|---|---|
| Women (%) | 127 (51) | 41 (43) | 35 (57) | 38 (53) | 13 (59) |
| Age, yr (SD) | 59 (18) | 57 (19) | 63 (13) | 57 (18) | 60 (20) |
| C-ANCA/PR3-ANCA (%) | 134 (54) | 55 (57) | 32 (53) | 42 (59) | 5 (23) |
| eGFR (SD) | 35 (31) | 54 (34) | 27 (18) | 18 (17) | 33 (37) |
eGFR is in milliliters per minute per 1.73 m2. C-ANCA, cytoplasmic ANCA; PR3-ANCA, proteinase 3 ANCA.
Stratification of the Cohort According to the ANCA-GN Classification Model
Ninety-six (38%) cases were classified as focal, 61 (24%) cases were classified as mixed, 71 (28%) cases were classified as crescentic, and 22 (9%) cases were classified as sclerotic. Baseline characteristics stratified per classification model group are shown in Table 1.
One- and 5-Year Cumulative ESRD–Free Survival
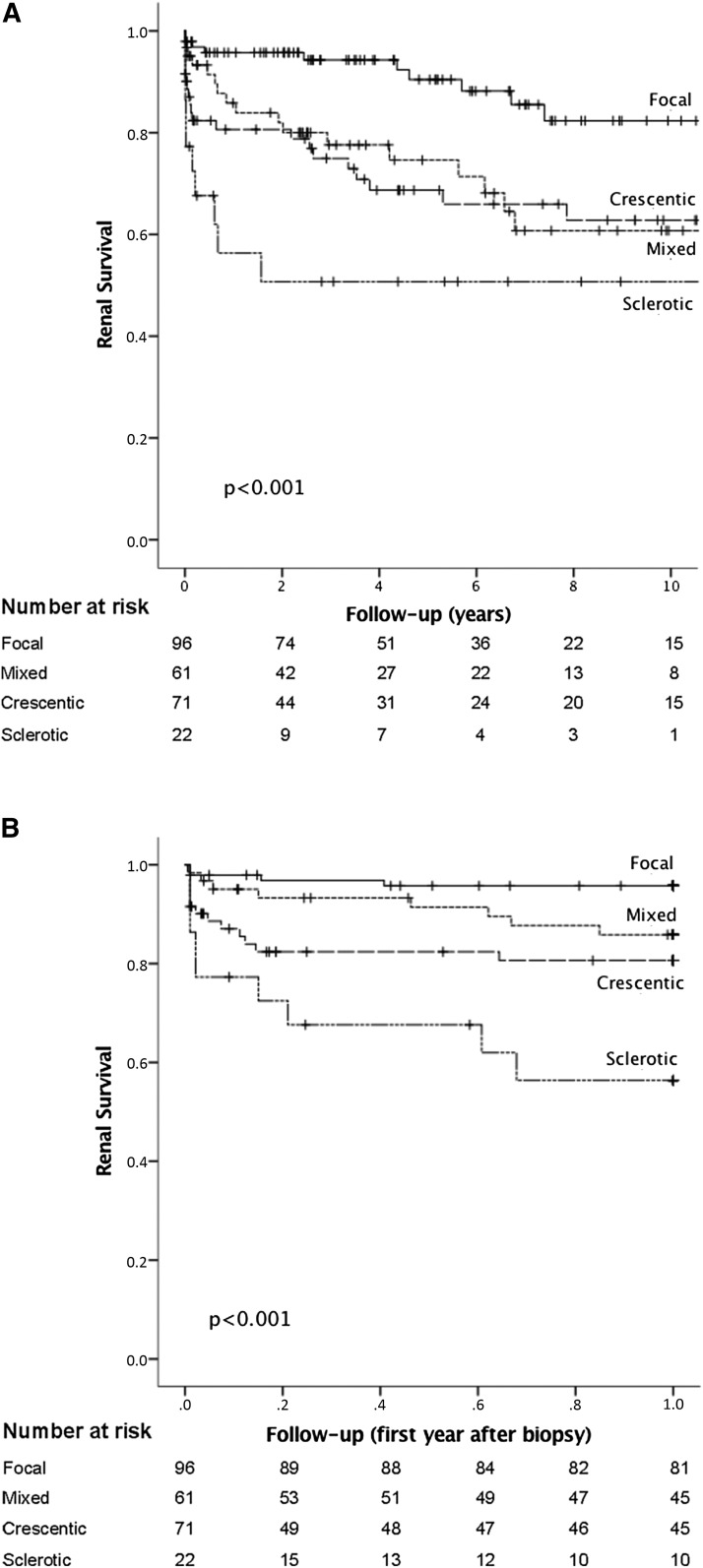
One- and 5-year cumulative ESRD–free survival rates in the study cohort were 86% and 77%, respectively. In the focal group, the equivalent figures were 96% and 90%, respectively. In the mixed group, the equivalent figures were 86% and 75%, respectively. In the crescentic group, the equivalent figures were 81% and 69%, respectively, and in the sclerotic group, the equivalent figures were 56% and 51%, respectively (Figure 1, Table 2).
Figure 1.
Kaplan–Meier plots showing cumulative risk of ESRD in 250 patients with ANCA-GN stratified by the histologic classification model (≥10 glomeruli evaluated). (A) Zero to 10 years after the diagnostic kidney biopsy. (B) Zero to 1 year after the diagnostic kidney biopsy.
Table 2.
Cumulative renal survival stratified for histologic classification
| Characteristic | N | ESRD | 1-yr ESRD-Free Survival, % | 5-yr ESRD-Free Survival, % |
|---|---|---|---|---|
| All | 250 | 60 | 86 | 77 |
| Focal | 96 | 10 | 96 | 90 |
| Mixed | 61 | 17 | 86 | 75 |
| Crescentic | 71 | 23 | 81 | 69 |
| Sclerotic | 22 | 10 | 56 | 51 |
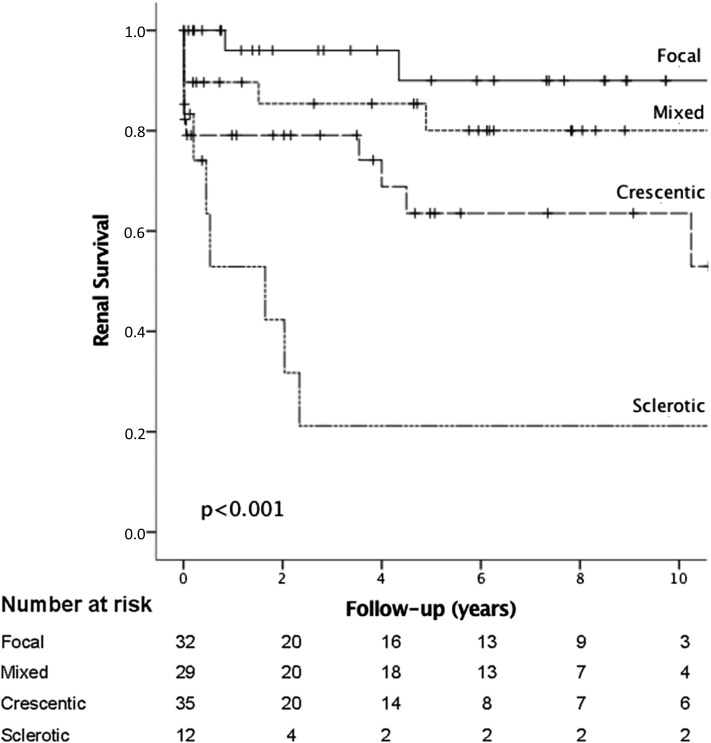
Figure 2.
Kaplan–Meier plots showing cumulative risk of ESRD 0–10 years after the diagnostic kidney biopsy in 108 patients with ANCA-GN stratified by the histologic classification model (three to nine glomeruli evaluated).
Prognostic Performance of the ANCA-GN Classification Model
In univariate analyses (Kaplan–Meier) and as shown in Figure 1, the classification model generated statistically significant predictions for ESRD-free survival in both the total and the 0–1 year after kidney biopsy study periods. However, the difference between the mixed and the crescentic groups was not significant for either the total (P=0.26) or the 0–1 year after kidney biopsy study period (P=0.32). When the classification model score was applied to the ≥1 year after kidney biopsy study period, it could not significantly predict ESRD-free survival. However, a trend toward a better prognosis in the focal group was observed.
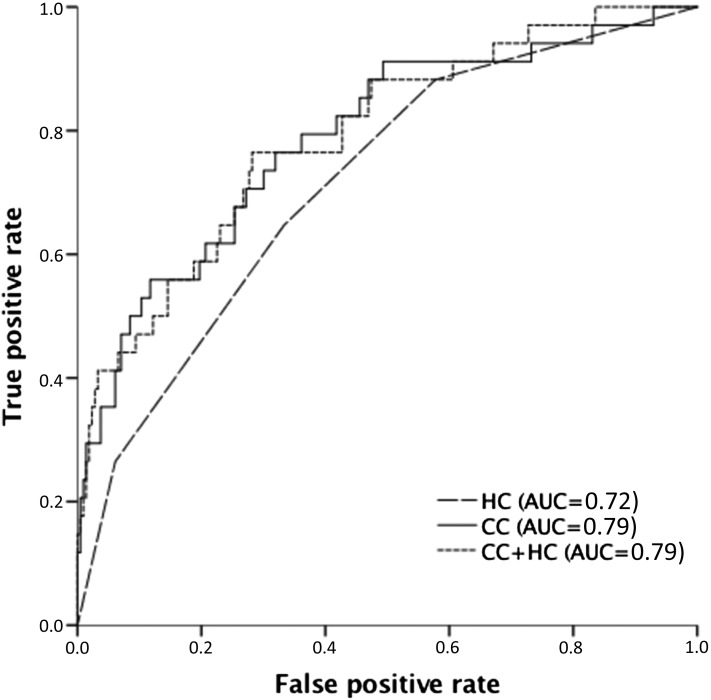
In multivariate analyses (Cox regression) performed on the 0–1 year after kidney biopsy period and with adjustment for age, sex, ANCA type, and eGFR, the HRs of ESRD in the crescentic compared with the mixed group were comparable: HR, 1.13; 95% confidence interval (95% CI), 0.44 to 2.87; P=0.80. To improve statistical power, we, therefore, merged these two groups. As shown in Table 3, the sclerotic group had a significantly higher risk of ESRD than the focal group (adjusted HR, 9.65; 95% CI, 2.38 to 39.16; P=0.002). The sclerotic group also had a significantly higher risk than the mixed/crescentic group (adjusted HR, 3.27; 95% CI, 1.41 to 7.61; P<0.01). There was no statistically higher risk in the mixed/crescentic group versus the focal group (adjusted HR, 1.93; 95% CI, 0.61 to 6.12; P=0.26). Multivariate analyses (Cox regression) performed in the ≥1 year after kidney biopsy period rendered no significant differences. Comparing the excluded patient group and the three to nine glomeruli group with the final study group, no significant differences were observed. As also shown in Figure 3, accuracy as defined by ROC analysis (AUC) was 0.72 (95% CI, 0.65 to 0.80) for the histologic classification model. AUC for the clinical classification model (age, sex, ANCA type, and eGFR) was 0.79 (95% CI, 0.70 to 0.88), and for the combined histologic/clinical model, it was also 0.79 (95% CI, 0.71 to 0.88).
Table 3.
Unadjusted and adjusted hazard ratios for ESRD within the first year after kidney biopsy in the histologic classification groups
| Variable | Unadjusted | Adjusteda | Fully Adjustedb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Focal | 1.0 | — | 1.0 | — | 1.0 | — | |||
| Mixed/crescentic | 4.21 | 1.44 to 12.27 | <0.01 | 4.84 | 1.63 to 14.40 | <0.01 | 1.93 | 0.61 to 6.12 | 0.26 |
| Sclerotic | 11.35 | 3.48 to 37.00 | <0.001 | 12.14 | 3.47 to 42.52 | <0.001 | 9.65 | 2.38 to 39.16 | 0.002 |
| Mixed/crescentic | 1.0 | — | 1.0 | — | 1.0 | — | |||
| Sclerotic | 2.91 | 1.33 to 6.36 | <0.01 | 2.51 | 1.12 to 5.65 | 0.03 | 3.27 | 1.41 to 7.61 | <0.01 |
HR, hazard ratio; 95% CI, 95% confidence interval; —, reference.
Adjusted for sex, age, and ANCA specificity.
Adjusted for sex, age, ANCA specificity, and eGFR.
Figure 3.
Receiver operator characteristic curve comparing the ANCA-GN histologic classification model (HC), a clinical classification model including age (years), sex, ANCA type, and eGFR at the time of biopsy (CC; milliliters per minute per 1.73 m2), and the combined clinical/histologic model (CC + HC). AUC, area under the curve.
One-Year Mortality
Thirty-five (14%) patients died <1 year after receiving a diagnosis of ANCA-GN. Of these, nine were from the focal group (9%), another nine were from the mixed group (15%), 14 died from the crescentic group (20%), and three died from the sclerotic group (14%). The difference in early mortality between the four prognostic groups was not statistically significant (P=0.28).
Comparison of International and Norwegian Findings
Four studies evaluating the ANCA-GN classification model and including at least 100 patients with reports of 1- and/or 5-year renal survival were identified in the literature (8,10,13,20). Findings from these four studies, individually and merged, are shown in Table 4. Mean 1-year renal survival rates in international studies versus the Norwegian study cohort were focal group, 98% versus 96%, respectively; mixed group, 79% versus 86%, respectively; crescentic group, 77% versus 81%, respectively; and sclerotic group, 43% versus 52%, respectively. The equivalent figures for 5-year renal survival were focal group, 94% versus 90%, respectively; mixed group, 70% versus 75%, respectively; crescentic group, 69% versus 69%, respectively; and sclerotic group, 35% versus 51%, respectively (Table 4).
Table 4.
Validation studies for the histologic classification scheme that included ≥100 biopsies
| Characteristic | Berden et al. (8) | Chang et al. (10) | Hilhorst et al. (13) | Tanna et al. (20) | Merged Columns 2–5 | This Study |
|---|---|---|---|---|---|---|
| Patients, n | 100 | 121 | 164 | 104 | 486 | 250 |
| Histopathologic class, n (%) | ||||||
| Focal | 16 (16) | 33 (27) | 81 (49) | 23 (22) | 153 (31) | 96 (38) |
| Mixed | 16 (16) | 24 (20) | 39 (24) | 48 (46) | 127 (26) | 61 (24) |
| Crescentic | 55 (55) | 53 (44) | 43 (26) | 26 (25) | 177 (36) | 71 (28) |
| Sclerotic | 13 (13) | 11 (9) | 1 (1) | 7 (7) | 32 (7) | 22 (9) |
| 1-yr Renal survival, n | ||||||
| Focal | 93 | 100 | — | 100 | 98 | 96 |
| Mixed | 69 | 83 | — | 85 | 79 | 86 |
| Crescentic | 84 | 73 | — | 74 | 77 | 81 |
| Sclerotic | 50 | 29 | — | 50 | 43 | 52 |
| 5-yr Renal survival, n | ||||||
| Focal | 93 | 93 | 91 | 100 | 94 | 90 |
| Mixed | 61 | 72 | 69 | 77 | 70 | 75 |
| Crescentic | 76 | 60 | 64 | 74 | 69 | 69 |
| Sclerotic | 50 | 29 | — | 25 | 35 | 51 |
—, not reported.
Predicting Renal Survival in the Three to Nine Glomeruli Group
As shown in Figure 3, the ANCA-GN classification model was also able to generate a statistically significant prediction of ESRD-free survival in the three to nine glomeruli group. One-year cumulative renal survival rates in the three to nine versus ≥10 glomeruli groups were focal group, 96% versus 96%, respectively; mixed group, 90% versus 86%, respectively; crescentic group, 79% versus 81%, respectively; and sclerotic group, 53% versus 56%, respectively. Five-year cumulative renal survival rates in the three to nine versus ≥10 glomeruli group were focal group, 90% versus 90%, respectively; mixed group, 80% versus 75%, respectively; crescentic group, 64% versus 69%, respectively; and sclerotic group, 21% versus 33%, respectively.
Discussion
In this study using univariate analyses, we show that the classification model used could stratify the risk of ESRD in patients with ANCA-GN, confirming findings in previous studies. Compared with patients with a focal histology, the unadjusted HRs of ESRD 0–1 year after the diagnostic kidney biopsy in patients with ANCA-GN with a mixed/crescentic or sclerotic histology were 4.21 and 11.35, respectively. In multivariate analyses, this result was basically unchanged after adjustment for age, sex, and ANCA specificity. However, when also adjusting for eGFR at the time of kidney biopsy, the HRs were substantially lower (1.93 and 9.65, respectively), with differences between the focal and the mixed/crescentic groups no longer statistically significant (Table 3). A plausible explanation for this lack of statistical significance could be the paucity of end points and therefore, a comparatively wide 95% CI. A further investigation using a larger study cohort or alternatively, a meta-analysis is now needed to establish whether this ANCA-GN prognostic model predicts risk of ESRD independent of age, sex, ANCA specificity, and baseline eGFR.
The result of the ROC analyses, with an AUC value of 0.72, indicates that the histologic classification is a prognostic model of only moderate quality. As shown in Figure 3, the clinical prognostic model including age, sex, ANCA type, and eGFR at time of kidney biopsy yielded a higher AUC value of 0.79. Furthermore, incorporating the histologic classification into this clinical prognostic model did not improve the AUC value (0.79). However, when interpreting these results, it should be taken into account that the study cohort is relatively small with few end points. Development of a prognostic scoring model and defining the role of the histologic classification in it would require international collaboration to achieve a sufficiently large study cohort.
In the first evaluation of the ANCA-GN classification model, Berden et al. (8) found that the prognosis for the crescentic group was better than that for the mixed group; 1-year renal survival was 84% versus 69%, respectively, and 5-year renal survival was 76% versus 61%, respectively. Furthermore, in a Japanese study of 102 patients, a higher percentage of patients classified as mixed developed ESRD compared with those in the crescentic class (44% versus 28%, respectively) (15). Nevertheless, in major validation studies, including this study, the mixed group has a slightly better renal survival than the crescentic group (Table 4). Because there were no statistically significant differences in prognosis between the mixed and crescentic groups in uni- or multivariate analyses, one could consider merging these two histologic classes. Combining these groups would have the additional advantage of simplifying the classification process. If <50% of glomeruli are affected, then the classification is focal. A sclerotic case would arise when >50% of glomeruli are globally sclerotic; all other cases would fall into the mixed/crescentic group.
We have shown that the ANCA-GN classification model predicts renal survival at both 1 and 5 years of follow-up, although this classification is optimal in the 0–1 year after diagnostic kidney biopsy period. The cumulative risk of ESRD was found to be fairly similar in all major studies, which validates histologic classification schemes, including this scheme. One-year risk of ESRD is <5% with a focal histologic pattern, approximately 20% with a mixed/crescentic histologic pattern, and around 50% with a sclerotic histologic pattern. For individual physicians treating patients with ANCA-GN, this is probably the most important prognostic yield from the histologic model.
When presenting the histologic model, Berden et al. (8) suggested that at least 10 whole glomeruli needed to be present for an adequate classification. The reference for this limitation was obtained from “The Banff 97 working classification of renal allograft pathology” (24). To our knowledge, the histologic classification model has never been evaluated in patients with three to nine glomeruli present in the biopsy. Our results show that the model works well with fewer glomeruli. Indeed, the cumulative risk of ESRD deviated little from that found when evaluating patients with ≥10 glomeruli. The requirement of 10 whole glomeruli for adequate classification might, therefore, represent an unnecessary limitation to this histologic prognostic model.
The major strength of this study is the large population–based patient cohort with reliable identification of end points through national registries. Furthermore, multivariate analyses have enabled us to evaluate the classification scheme independent of known confounding and prognostic factors. Lastly, the same nephropathologist using a standardized protocol for describing the biopsies led the histologic section of the NKBR throughout the study period.
We acknowledge that our study has some limitations. The histopathologic scoring was on the basis of data from the initial biopsy interpretation by only one nephropathologist without recourse to any revisions. However, good inter- and intraobserver agreement of the findings (globally sclerosed/crescentic glomeruli) used for classification has previously been shown (25). Furthermore, the distinction of cellular versus fibrous crescent is not strictly identical in the classification model and the NKBR, but we are confident that the great majority of our fibrous crescents are characterized by >90% extracellular matrix, which according to the histologic classification scheme, discriminates cellular from fibrous crescents. Details regarding the treatment of individual patients with ANCA-GN were not available in the NKBR. We have previously shown that the prognosis for our study cohort (22) is equivalent to those reported from other European countries, and it is, therefore, fair to assume that patients received treatment according to European standards.
In summary, we have shown that the histologic classification model is a prognostic tool of moderate quality for predicting renal survival in patients with ANCA-GN. In accordance with several other studies, we found no prognostic difference between the crescentic and mixed cases, which led us to merge these groups. In multivariate analyses, only the sclerotic group had a statistically significant adverse prognosis. Finally, we have shown that the prognostic model is also suitable for patients with only three to nine whole glomeruli available for evaluation.
Disclosures
None.
Acknowledgments
Data linking for the study cohort and the Norwegian Renal Registry was performed by Torbjørn Leivestad.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Jennette JC, Falk RJ: Small-vessel vasculitis. N Engl J Med 337: 1512–1523, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Fauci AS, Haynes BF, Katz P, Wolff SM: Wegener’s granulomatosis: Prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 98: 76–85, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, Rottem M, Fauci AS: Wegener granulomatosis: An analysis of 158 patients. Ann Intern Med 116: 488–498, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Westman KW, Bygren PG, Olsson H, Ranstam J, Wieslander J: Relapse rate, renal survival, and cancer morbidity in patients with Wegener’s granulomatosis or microscopic polyangiitis with renal involvement. J Am Soc Nephrol 9: 842–852, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bajema IM, Hagen EC, Hermans J, Noël LH, Waldherr R, Ferrario F, Van Der Woude FJ, Bruijn JA: Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int 56: 1751–1758, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Aasarød K, Bostad L, Hammerstrøm J, Jørstad S, Iversen BM: Renal histopathology and clinical course in 94 patients with Wegener’s granulomatosis. Nephrol Dial Transplant 16: 953–960, 2001 [DOI] [PubMed] [Google Scholar]
- 7.de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Gaskin G, Rasmussen N, Noël LH, Ferrario F, Waldherr R, Hagen EC, Bruijn JA, Bajema IM: Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: A prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 17: 2264–2274, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Berden AE, Jones RB, Erasmus DD, Walsh M, Noël LH, Ferrario F, Waldherr R, Bruijn JA, Jayne DR, Bajema IM; European Vasculitis Society : Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody-associated glomerulonephritis after rituximab therapy. J Am Soc Nephrol 23: 313–321, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Chang DY, Wu LH, Liu G, Chen M, Kallenberg CG, Zhao MH: Re-evaluation of the histopathologic classification of ANCA-associated glomerulonephritis: A study of 121 patients in a single center. Nephrol Dial Transplant 27: 2343–2349, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Muso E, Endo T, Itabashi M, Kakita H, Iwasaki Y, Tateishi Y, Komiya T, Ihara T, Yumura W, Sugiyama T, Joh K, Suzuki K: Evaluation of the newly proposed simplified histological classification in Japanese cohorts of myeloperoxidase-anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in comparison with other Asian and European cohorts. Clin Exp Nephrol 17: 659–662, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis CL, Manno RL, Havill JP, Racusen LC, Geetha D: Validation of the new classification of pauci-immune glomerulonephritis in a United States cohort and its correlation with renal outcome. BMC Nephrol 14: 210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilhorst M, Wilde B, van Breda Vriesman P, van Paassen P, Cohen Tervaert JW; Limburg Renal Registry : Estimating renal survival using the ANCA-associated GN classification. J Am Soc Nephrol 24: 1371–1375, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unlu M, Kiremitci S, Ensari A, Ozluk Y, Kilicaslan I, Ozdemir BH, Ates D, Ertoy Baydar D, Gonul II, Memis L, Sarsik B, Sen S, Akkaya B, Orhan D, Gonlusen G, Ellidokuz H, Ada S, Cavdar C, Akagun T, Kamali S, Aksu K, Yazisiz V, Paydas S, Soylu A, Sarioglu S: Pauci-immune necrotizing crescentic glomerulonephritis with crescentic and full moon extracapillary proliferation: Clinico-pathologic correlation and follow-up study. Pathol Res Pract 209: 75–82, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Iwakiri T, Fujimoto S, Kitagawa K, Furuichi K, Yamahana J, Matsuura Y, Yamashita A, Uezono S, Shimao Y, Hisanaga S, Tokura T, Wada T, Kitamura K, Asada Y: Validation of a newly proposed histopathological classification in Japanese patients with anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. BMC Nephrol 14: 125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford SL, Polkinghorne KR, Longano A, Dowling J, Dayan S, Kerr PG, Holdsworth SR, Kitching AR, Summers SA: Histopathologic and clinical predictors of kidney outcomes in ANCA-associated vasculitis. Am J Kidney Dis 63: 227–235, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Togashi M, Komatsuda A, Nara M, Omokawa A, Okuyama S, Sawada K, Wakui H: Validation of the 2010 histopathological classification of ANCA-associated glomerulonephritis in a Japanese single-center cohort. Mod Rheumatol 24: 300–303, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Nohr E, Girard L, James M, Benediktsson H: Validation of a histopathologic classification scheme for antineutrophil cytoplasmic antibody-associated glomerulonephritis. Hum Pathol 45: 1423–1429, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Noone DG, Twilt M, Hayes WN, Thorner PS, Benseler S, Laxer RM, Parekh RS, Hebert D: The new histopathologic classification of ANCA-associated GN and its association with renal outcomes in childhood. Clin J Am Soc Nephrol 9: 1684–1691, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanna A, Guarino L, Tam FW, Rodriquez-Cubillo B, Levy JB, Cairns TD, Griffith M, Tarzi RM, Caplin B, Salama AD, Cook T, Pusey CD: Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: Evaluation of the international histological classification and other prognostic factors. Nephrol Dial Transplant 30: 1185–1192, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Sriskandarajah S, Aasarød K, Skrede S, Knoop T, Reisæter AV, Bjørneklett R: Improved prognosis in Norwegian patients with glomerulonephritis associated with anti-neutrophil cytoplasmic antibodies. Nephrol Dial Transplant 30[Suppl 1]: i67–i75, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besada E, Koldingsnes W, Nossent JC: Long-term efficacy and safety of pre-emptive maintenance therapy with rituximab in granulomatosis with polyangiitis: Results from a single centre. Rheumatology (Oxford) 52: 2041–2047, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Marcussen N, Mihatsch MJ, Nadasdy T, Nickerson P, Olsen TS, Papadimitriou JC, Randhawa PS, Rayner DC, Roberts I, Rose S, Rush D, Salinas-Madrigal L, Salomon DR, Sund S, Taskinen E, Trpkov K, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Bajema IM, Hagen EC, Hansen BE, Hermans J, Noël LH, Waldherr R, Ferrario F, van der Woude FJ, Bruijn JA: The renal histopathology in systemic vasculitis: An international survey study of inter- and intra-observer agreement. Nephrol Dial Transplant 11: 1989–1995, 1996 [DOI] [PubMed] [Google Scholar]