Abstract
Background and objectives
Our aim was to use a national electronic AKI alert to define the incidence and outcome of all episodes of community– and hospital–acquired adult AKI.
Design, setting, participants, & measurements
A prospective national cohort study was undertaken in a population of 3.06 million. Data were collected between March of 2015 and August of 2015. All patients with adult (≥18 years of age) AKI were identified to define the incidence and outcome of all episodes of community- and hospital-acquired AKI in adults. Mortality and renal outcomes were assessed at 90 days.
Results
There was a total of 31,601 alerts representing 17,689 incident episodes, giving an incidence of AKI of 577 per 100,000 population. Community-acquired AKI accounted for 49.3% of all incident episodes, and 42% occurred in the context of preexisting CKD (Chronic Kidney Disease Epidemiology Collaboration eGFR); 90-day mortality rate was 25.6%, and 23.7% of episodes progressed to a higher AKI stage than the stage associated with the alert. AKI electronic alert stage and peak AKI stage were associated with mortality, and mortality was significantly higher for hospital-acquired AKI compared with alerts generated in a community setting. Among patients who survived to 90 days after the AKI electronic alert, those who were not hospitalized had a lower rate of renal recovery and a greater likelihood of developing an eGFR<60 ml/min per 1.73 m2 for the first time, which may be indicative of development of de novo CKD.
Conclusions
The reported incidence of AKI is far greater than the previously reported incidence in studies reliant on clinical identification of adult AKI or hospital coding data. Although an electronic alert system is Information Technology driven and therefore, lacks intelligence and clinical context, these data can be used to identify deficiencies in care, guide the development of appropriate intervention strategies, and provide a baseline against which the effectiveness of these interventions may be measured.
Keywords: acute renal failure; clinical epidemiology; clinical nephrology; Epidemiology and outcomes; acute kidney injury; adult; cohort studies; humans; Incidence; Intelligence; kidney; Prospective Studies; Receptor, Epidermal Growth Factor; Surveys and Questionnaires; EGFR protein, human
Introduction
The reported incidence of AKI varies depending on its definition, the clinical setting in which it is detected, and the population studied. The definitions of AKI used in many previous studies in the literature varied, making direct comparison of these difficult. In 2009, the National Confidential Inquiry into Patient Outcome and Death (1) report identified significant deficiencies in the management of AKI in hospitals in the United Kingdom. This led to the development and implementation of strategies, such as the use of electronic results reporting to aid early AKI recognition (2). In response, the Royal College of Physicians, at a consensus conference in the United Kingdom, recommended the adoption of an electronic alert (e-alert) system to aid in the early identification of AKI (3). On the basis of a presumption that early identification may help raise standards of care and improve patient outcomes, an automated real–time e-alert system for AKI on the basis of the Kidney Disease Improving Global Outcomes (KDIGO) change in creatinine diagnostic criteria has been established and implemented nationally across all areas of the National Health Service in Wales. Using a centralized system of data collection, the aim of this study was to provide a comprehensive characterization of the base incidence and the definition of AKI identified by e-alerts (AKI) and its outcome across both primary and secondary care.
Materials and Methods
Setting
The National Health Service in Wales, which serves a population of 3.06 million, is organized into seven local health boards (LHBs) (Supplemental Figure 1). Data were collected from all health boards. The study was approved under Service Evaluation Project Registration.
Development of the Electronic Reporting System
The all Wales Laboratory Information Management System (InterSystems TrakCare Lab) in real time automatically compares measured creatinine values on an individual patient with previous results to generate alerts (Supplemental Figure 2) on the basis of the KDIGO AKI criteria (Supplemental Table 1). The definition of AKI, therefore, relies on creatinine but does not rely on urine output. A summary of the rules is shown in Supplemental Table 2, and each e-alert code together with the comment that accompanies the e-alert are shown in Supplemental Table 3. Any patient presenting with AKI but without a measurement of renal function in the previous 365 days will, therefore, not be included in the study.
Data Collection
Prospective data were collected for all patients with adult (≥18 years of age) AKI in Wales between March of 2015 and August of 2015. Clinical location, patient age, AKI stage, and the rule under which the AKI alert was generated were collected together with all measurements of renal function for up to 90 days after the AKI alert. An incident AKI episode was defined as 90 days (i.e., any AKI e-alert for the same patient within 90 days); the incident alert was not considered a new episode. Peak AKI stage was assigned by comparing the highest serum creatinine (SCr) value during an AKI incident episode with the baseline SCr of the incident alert. To prevent inclusion of patients known to be receiving RRT, alerts transmitted by patients from a renal, renal transplant, or dialysis setting and those by patients who had a previous blood test in a dialysis setting were excluded. All incident patients with AKI alerted for the first time in a nonrenal location before transfer to the regional renal unit.
Incidence rate was calculated using Mid-2013 Office for National Statistics (ONS) Population Estimates. Patients for whom the first e-alert was generated from a creatinine value measured in primary care were classified as primary care AKI. All patients for whom the first alert was issued during a hospital admission and who also had a normal SCr value generated in a hospital setting within the preceding 7 days were defined as patients with hospital-acquired AKI (HA-AKI). Patients alerting in a noninpatient setting (including accident and emergency/acute assessment units) and not alerting in primary care were classified as patients with nonprimary care community–acquired AKI (CA-AKI). Primary care and nonprimary care CA-AKI, therefore, collectively represent CA-AKI.
Hospitalization of CA-AKI was defined as first or second measurement of renal function in an inpatient setting (within 7 days) after the alert. Mortality data were collected from the Welsh Demographic Service. Patients were censored at 1 year for survival analysis. Renal outcome analysis required patients to have 90-day follow-up data available and included only those patients surviving at this time point. Linear regression analysis of renal outcome included surviving and nonsurviving patients. Nonrecovery from an AKI episode was defined as achievement of an SCr value closest to and within 90 days, still consistent with the definition of AKI compared with baseline SCr values. Preexisting CKD was defined as an eGFR (calculated by the Chronic Kidney Disease Epidemiology Collaboration eGFR equation [4]) <60 ml/min per 1.73 m2 derived from the baseline SCr. A worsening eGFR was calculated using the eGFR value closest to and within 90 days and defined by a decline from baseline eGFR of >15% or >5 ml/min per 1.73 m2 (5).
The Welsh Index of Multiple Deprivation (WIMD) is the Welsh Government official measure of relative deprivation. This generates a rank (WIMD score) for 1909 lower superoutput geographic areas (LSOAs) in Wales on the basis of eight domains; income, employment, health, education, access to services, community safety, physical environment, and housing (6). Patients were georeferenced to an LSOA of residence and ranked according to WIMD score. Ranked data were categorized into percentiles, with percentile 1 the most deprived and percentile 100 the least deprived. Patients were aggregated to their geographic area (LSOA of residence), and incidence of AKI was calculated using the total adult population in each LSOA derived from Mid-2013 ONS Population Estimates.
Statistical analysis was carried out using SPSS software, version 20 (IBM SPSS, Chicago, I ); t test was used for analysis of normally distributed data. Categorical data were compared using a Pearson chi–squared test. Multivariate Cox proportional hazard modeling was used to analyze patient survival. P values <0.05 were considered statistically significant.
Validation
The diagnostic accuracy was determined by manually checking baseline creatinine values for a sample of 200 patients distributed across each rule and e-alert code and across two LHBs. All of the e-alerts generated conformed to the mathematic definition of AKI.
When patients known to be on dialysis were not identified as such by the request through the location code, a proportion of patients known to be on dialysis generated an AKI e-alert. This was only applicable to ABS1, ABS2, and DELTA1 codes (Supplemental Table 3). For ABS1 codes, 89% of flagged patients were known to be on dialysis. In total, 105 patients were flagged by this code. These were all excluded from the analysis, and therefore, 11% of the cohort identified by this code (12 patients) with probable AKI were excluded from the overall analysis. For ABS2 code, 26% of patients were patients known to be on dialysis. ABS2 accounted for a total of 562 patients. These have been included in the analysis, and therefore, by extrapolation, 146 patients likely to be on dialysis are included in the analysis. For the DELTA1 code, 60% of those flagged who had a creatinine of >4.5 mg/dl were patients on dialysis. In total, 89 patients were flagged by this code and had a creatinine of >4.5 mg/dl. These were excluded, and therefore, 40% of the cohort identified by this code (36 patients) with probable AKI were excluded. Using these criteria results in a false negative rate of 0.27% (exclusion of patients with AKI) and a false positive rate of 0.83% (inclusion of patients known to be on dialysis).
Results
Incidence and Demographics
We observed a total of 31,601 alerts (Table 1). The majority (62.9%) of patients generated only one alert. Of those patients who triggered multiple e-alerts, 18.5% generated two alerts, 8.3% generated three alerts, 4.2% generated four alerts, 2.1% generated five alerts, and 1.3% generated six alerts, with the remainder generating between seven and 27 alerts. Only 2.8% of incident episodes were the result of a second episode from the same patient.
Table 1.
Incidence/demography of AKI
| Variable | All AKI | |||
|---|---|---|---|---|
| n Per 100,000 population (n) | 577 (17,689) | |||
| AKI severity, % (n) | ||||
| Stage 1 | 78.7 (13,922) | |||
| Stage 2 | 14.3 (2522) | |||
| Stage 3 | 7.0 (1245) | |||
| AKI rule, % (n) | ||||
| Rule 1 | 9.9 (1753) | |||
| Rule 2 | 27.1 (4799) | |||
| Rule 3 | 63.0 (11,137) | |||
| Clinical location, % (n) | ||||
| Hospital | 41.2 (7288) | |||
| Community | 49.3 (8724) |
| All AKI | HA-AKI | CA-AKI | ||
|---|---|---|---|---|
| Health board, n per 100,000 population (n) | ||||
| Abertawe Bro Morgannwg UHB | 549 (2857) | 396.9 | 216.6 | |
| Aneurin Bevan UHB | 550 (3185) | 189.9 | 265.9 | |
| Betsi Cadwaladr UHB | 564 (3906) | 219.1 | 282.2 | |
| Cardiff and Vale UHB | 513 (2457) | 247.7 | 239.1 | |
| Cwm Taf UHB | 814 (2402) | 313.8 | 429.0 | |
| Hywel Dda UHB | 693 (2659) | 258.4 | 392.5 | |
| Powys THB | 60 (80) | 5.3 | 46.0 |
| All AKI | AKI Stage 1 | AKI Stage 2 | AKI Stage 3 | |
|---|---|---|---|---|
| Mean age±SD, yr | 71.1±17.0 | 71.0±17.3 | 71.8±15.9 | 70.5±15.9 |
| Sex, % (n) | ||||
| Men | 46.9 (8285) | 46.1 (6407) | 46.4 (1171) | 56.8 (707) |
| Women | 53.1 (9388) | 53.9 (7499) | 53.6 (1351) | 43.2 (538) |
| Preexisting CKD, % (n) | 41.9 (6877) | 38.5 (5354) | 34.5 (870) | 52.5 (653) |
| Mean baseline SCr, mg/dl | 1.0 | 1.0 | 0.9 | 1.4 |
| Mean baseline eGFR, ml/min per 1.73 m2 | 71.6 | 72.0 | 74.4 | 61.7 |
| Mean alert SCr, mg/dl | 1.8 | 1.5 | 2.1 | 4.7 |
| Mean peak SCr, mg/dl | 2.3 | 1.9 | 2.5 | 5.3 |
Data on patient sex were missing for 16 patients and excluded from analysis of the sex variable. Baseline eGFR data were missing for 24 patients and excluded from analysis of the preexisting CKD variable. HA-AKI, hospital-acquired AKI; CA-AKI, community-acquired AKI; UHB, University Health Board; THB, Teaching Health Board; SCr, serum creatinine.
The alerts generated represent 17,689 episodes of AKI. This translates into an incidence of AKI of 577 per 100,000 population over the 6-month timeframe and 1.2 patients per 100 person-years. The majority (78.7%) of episodes were classified as AKI stage 1 at presentation, with 14.3% classified as AKI stage 2 and 7.0% classified as AKI stage 3; 23.7% of stages 1 and 2 episodes progressed to a higher peak AKI stage relative to the incident AKI alert stage: 15.1% (944) and 9.0% (562) of AKI stage 1 progressed to AKI stages 2 and 3, respectively, and 21.8% (247) of AKI stage 2 progressed to AKI stage 3.
CA-AKI and HA-AKI
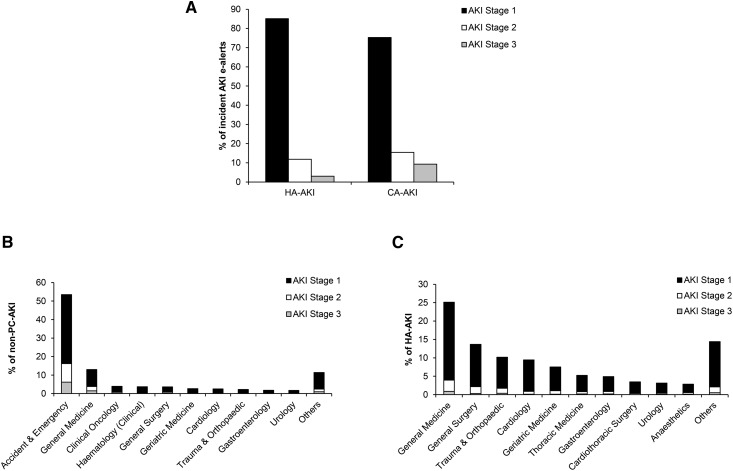
The distribution of e-alerts by the location in which the alert was generated is shown in Figure 1A. CA-AKI and HA-AKI accounted for 49.3% and 41.2% of all alerts, respectively. The remaining 9.5% of alerts were generated in an inpatient setting, but because no results were available for the previous 7 days, it was not possible to confidently classify these as either CA-AKI or HA-AKI. For both AKI in the community and that acquired in hospital, the overwhelming majority was AKI stage 1.
Figure 1.
Source of incident AKI electronic alerts (e-alerts). (A) Distribution of AKI stages for hospital-acquired AKI (HA-AKI) and community-acquired AKI (CA-AKI). (B) Percentage and number of nonprimary care (non-PC) patients with CA-AKI divided according to clinical specialty and AKI stage. Clinical specialty data were missing for 289 patients and excluded from analysis. (C) Percentage and number of patients with HA-AKI divided according to clinical specialty and AKI stage. Clinical specialty data were missing for 692 patients and excluded from analysis.
The distribution of clinical locations for both nonprimary care CA-AKI and HA-AKI alerts, stratified by AKI stage, is shown in Figure 1, B and C (Supplemental Tables 4 and 5). The majority (53%) of AKI acquired in a nonprimary care community setting is first detected in the accident and emergency department. For HA-AKI, the largest single cohort is acquired in a general medical inpatient setting (25%) followed closely by the combination of general surgical and trauma/orthopedics, which accounts for 24% of all HA-AKI.
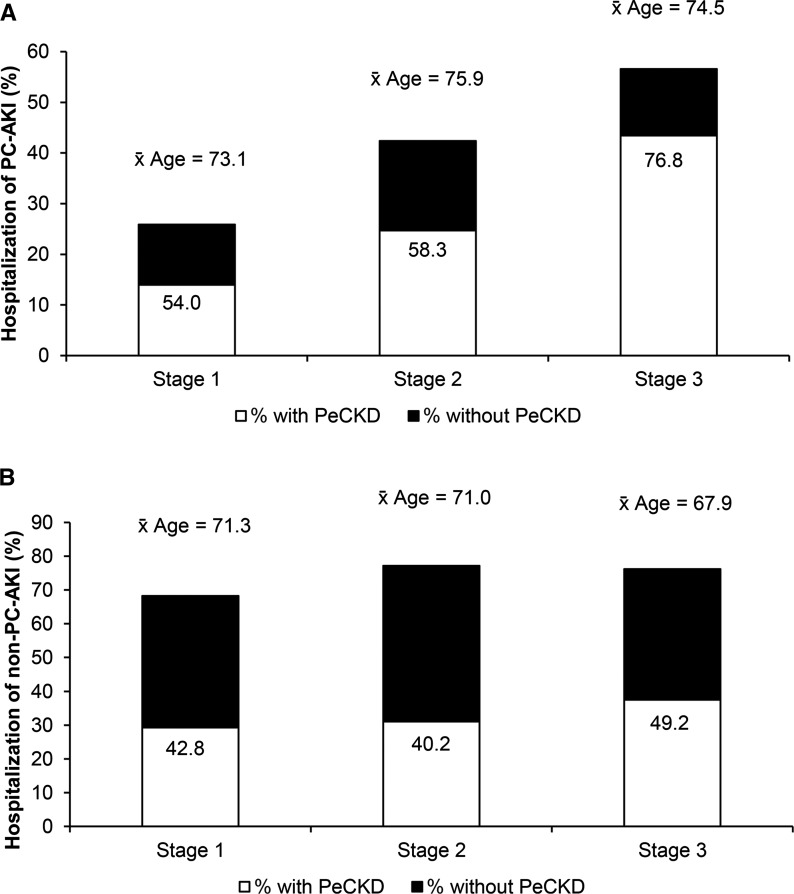
For incident CA-AKI episodes, 30.6% were generated by an alert issued to primary care, which represents 14.6% of all of the incident AKI episodes. The remainder of CA-AKI was accounted for by patients alerting in a noninpatient setting (including accident and emergency/acute assessment units) but excluding primary care. Primary care AKI e-alerts were followed by hospital admission in 31% of patients (Figure 2A). For primary care CA-AKI, admission was associated with greater severity of renal injury, with 26% of patients with AKI stage 1 admitted compared with 42% of patients with AKI stage 2 and 56% of patients with AKI stage 3. Nonprimary care community AKI e-alerts were followed by hospital admission in 71% of patients (Figure 2B). For this group, admission to hospital was not related to AKI severity.
Figure 2.
Differing hospitalization rates for community-acquired AKI subsets. (A) Percentage, average age, and percentage with preexisting CKD (shaded area of each bar) in patients with primary care AKI (PC-AKI) who were hospitalized divided according to AKI stage (total number of patients: stage 1, 1531; stage 2, 255; and stage 3, 175). (B) Percentage, average age, and percentage with preexisting CKD (shaded area of each bar) in patients with nonprimary care AKI (non–PC-AKI) who were hospitalized divided according to AKI stage (total number of patients: stage 1, 3727; stage 2, 889; stage 3, and 542). PeCKD, preexisting CKD.
There was a positive relationship between the time to repeat measurement of renal function and hospitalization, with significantly longer mean times for patients not hospitalized for primary care CA-AKI (7.4±13.8 versus 11.9±14.1 days; P<0.001) and nonprimary care CA-AKI (2.7±7.6 versus 11.1±16.2 days; P<0.001). In nonhospitalized CA-AKI at the time of retesting, 18.2% of patients had additional elevation of SCr (compared with 40.9% of patients with CA-AKI who were hospitalized). Of those patients with CA-AKI not diagnosed in primary care, 19.9% of patients had a measurement of SCr (that did not generate an e-alert) in the preceding 30 days.
Regional Variations
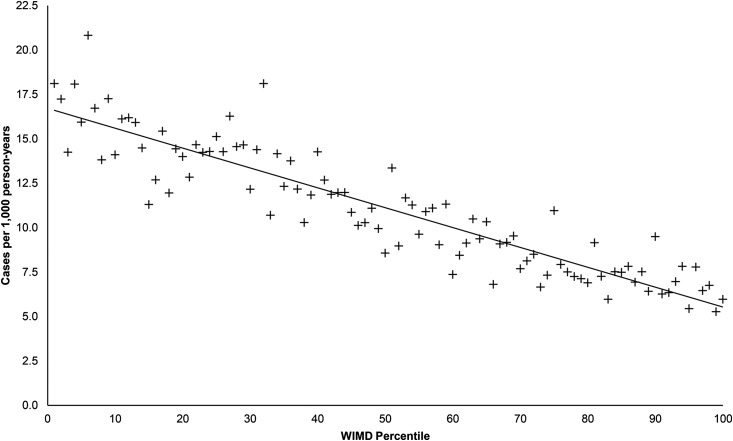
The geographic variation of AKI incidence is shown in Table 1. The low overall incidence in Powys and the higher incidence in Hywel Dda likely reflect the organization of health care, with no secondary care services in Powys. Its population is served predominantly by hospital services in the neighboring Hywel Dda Health Board (a smaller proportion may access hospital service in English hospitals, for which we have no data). The high incidence in Cwm Taf occurs in both hospital– and community–acquired groups. This board serves the most socially deprived population in the principality. The relationship between incidence of AKI and patient socioeconomic status is shown in Figure 3. There was a strong negative correlation between ranking by WIMD score and the incidence of AKI (r=−0.91; 95% confidence interval [95% CI], −0.94 to −0.87; P<0.001).
Figure 3.
Negative association between incidence of AKI and the index of social deprivation. Two hundred twenty-one patients with missing postcode data were excluded from analysis; 121 patients with English postcodes were excluded from analysis. WIMD, Welsh Index of Multiple Deprivation, where percentile 1 is the most deprived and percentile 100 is the least deprived.
Significance of an Episode of AKI
Mortality.
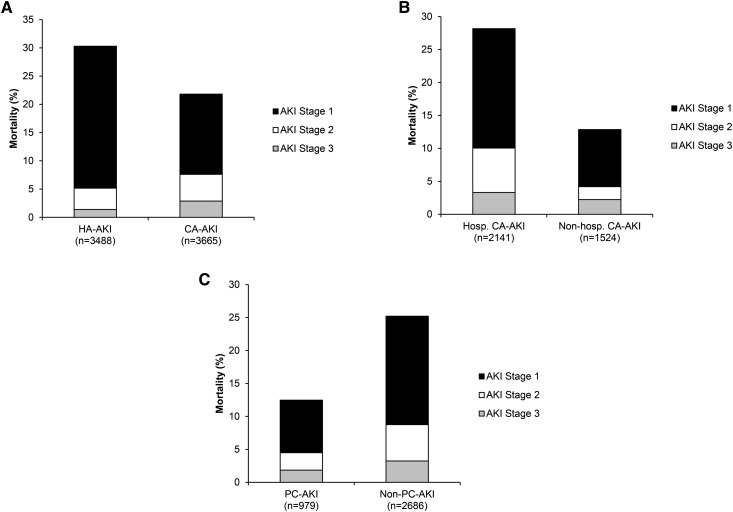
Ninety-day mortality for AKI is shown in Figure 4. Overall 90-day mortality was 25.6%. Mortality was significantly higher (P<0.001) in HA-AKI compared with CA-AKI (Figure 4A). For CA-AKI, mortality (Figure 4, B and C) was significantly higher in the hospitalized cohort (P<0.001) and nonprimary care CA-AKI (P<0.001). Cox regression proportional hazard modeling analysis (with follow-up data up to and including 12 months) showed higher hazards of death associated with older age (hazard ratio [HR], 1.03; 95% CI, 1.03 to 1.03), more severe AKI at presentation (AKI stage 2/3 versus AKI stage 1; HR, 1.43; 95% CI, 1.34 to 1.54), and peak AKI stage (AKI stage 2/3 versus AKI stage 1; HR, 2.36; 95% CI, 2.20 to 2.53). Increased hazards of death were associated with nonprimary care CA-AKI (unadjusted HR, 1.77; 95% CI, 1.59 to 1.97; adjusted HR, 1.65; 95% CI, 1.48 to 1.84; P<0.001) and HA-AKI (unadjusted HR, 2.04; 95% CI, 1.83 to 2.26; adjusted HR, 1.98; 95% CI, 1.78 to 2.19; P<0.001) compared with primary care CA-AKI. For CA-AKI, hospitalization was also associated with increased hazard of death (HR, 1.31; 95% CI, 1.23 to 1.39; P<0.001).
Figure 4.
Differing ninety-day mortality rates associated with incident AKI electronic alerts for clinical location of AKI subsets. (A) Percentage of patients with AKI who died divided according to place of identification of AKI. (B) Percentage of patients with community-acquired AKI (CA-AKI) who died divided according to hospitalization. (C) Percentage of patients with CA-AKI who died divided according to place of identification of AKI. Mortality was significantly higher for all of the admitted groups (P<0.001 compared with nonadmitted groups). Mortality rates were comparable in the admitted nonprimary care CA-AKI and hospital-acquired AKI (HA-AKI) groups, which were significantly higher than in the primary care AKI (PC-AKI) admitted cohort (P<0.01). Numbers of patients with data available are indicated in parentheses on the x axis. Shading indicates the proportion of patients who died by AKI stage. Hosp. CA-AKI, hospitalized community–acquired AKI; non-hosp. CA-AKI, nonhospitalized community–acquired AKI; non–PC-AKI, nonprimary care AKI.
Renal Outcomes.
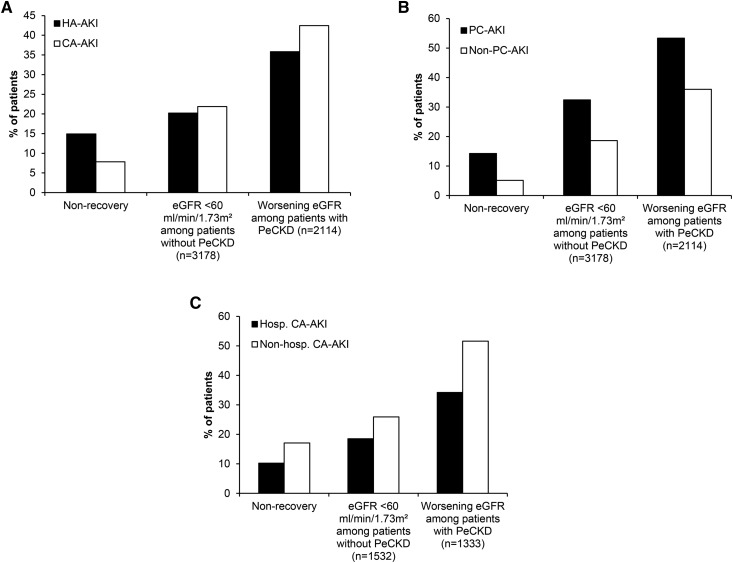
The relationship between the incident AKI e-alert and subsequent renal function is shown in Figure 5. Significantly more patients did not recover their renal function after an episode of HA-AKI compared with CA-AKI (14.6% versus 7.9%; P<0.001). In contrast, more patients with CA-AKI and preexisting CKD were likely to have worsening renal function after the AKI episode than after HA-AKI (42.5% versus 35.9%; P=0.002). For the whole cohort, more severe AKI at presentation (AKI stage 2/3 versus AKI stage 1; HR, 1.82; 95% CI, 1.64 to 2.03) and peak AKI stage (AKI stage 2/3 versus AKI stage 1; HR, 3.98; 95% CI, 3.49 to 4.54) were associated with nonrecovery of renal function.
Figure 5.
Differing renal outcomes associated with AKI electronic alerts for clinical location of AKI subsets. (A) Renal outcome of patients with AKI divided according to place of identification of AKI. Of the patients for whom 90-day follow-up data were available, 1841 (1047, hospital-acquired AKI [HA-AKI]; 794, community-acquired AKI [CA-AKI]) had died within the 90-day follow-up period and were excluded from analysis. (B) Renal outcome of patients with CA-AKI divided according to place of identification of AKI. Of the patients for whom 90-day follow-up data were available, 794 (121, primary care–acquired AKI [PC-AKI]; 673, nonprimary care–acquired AKI [non–PC-AKI]) had died within the 90-day follow-up period and were excluded from analysis. Nonrecovery is expressed as a percentage of the whole cohort and was defined as achievement of a serum creatinine (SCr) value closest to and within 90 days still consistent with the definition of AKI in comparisons to baseline SCr values. (C) Renal outcome of patients with CA-AKI divided according to hospitalization. Of the patients for whom 90-day follow-up data were available, 794 (599, hospitalized community–acquired AKI [Hosp. CA-AKI]; 195, nonhospitalized community–acquired AKI [Non-hosp. CA-AKI]) had died within the 90-day follow-up period and were excluded from analysis.
For CA-AKI picked up in primary care (Figure 5B), nonrecovery of renal function was significantly higher than in nonprimary care CA-AKI (P<0.001). Similarly, AKI detected in primary care was associated with a greater likelihood of developing eGFR<60 ml/min per 1.73 m2 for the first time (P<0.001), and of those patients with preexisting CKD, patients with primary care CA-AKI were significantly more likely to experience a worsening eGFR (P<0.001). The relationship between admission to hospital and renal outcome for all CA-AKI groups is shown in Figure 5C. Hospitalization was associated with better outcome in terms of recovery from the acute episode (P<0.001), a lower proportion of patients developing an eGFR<60 ml/min per 1.73 m2 for the first time, and fewer patients with preexisting CKD experiencing worsening eGFR (P<0.001 for both parameters). By linear regression, better acute outcome adjusted for both incident and peak AKI stages was also associated with hospitalization (HR, 1.23; 95% CI, 1.16 to 1.29; P<0.001).
Discussion
The majority of publications of large series characterizing AKI rely on making and recording an accurate diagnosis of AKI through hospital coding or retrospective review of hospital records (7–10). Although providing essential information on the epidemiology of AKI, there is significant potential for AKI episodes to be missed, resulting in underestimation of the true incidence of AKI. There are publications that have sought to overcome this via a biochemical identification of AKI as a trigger to identify the patients. These are, however, either single–center, hospital–based studies (11,12) or reliant on an e-alert that was not on the basis of an internationally agreed on AKI definition (13). To address this, we used a national dataset to provide a comprehensive characterization of the incidence of electronic AKI alerts and the subsequent clinical course.
The first key finding in this study is the high incidence of AKI. Previous studies have suggested an annual incidence of 200–300 per 100,000 in high-income countries (14). The use of an alert-based system for patient identification, therefore, overcomes systematic under-reporting of AKI associated with previous studies. The study also shows a significant association of AKI with renal function at 90 days after the incident episode. For the whole cohort of >17,000 patients, more than one quarter of the population either developed an eGFR<60 ml/min per 1.73 m2 for the first time, which may be indicative of the development of de novo CKD, or experienced worsening of preexisting CKD after the incident AKI e-alert, which may affect the need to plan for long-term provision of RRT.
In contrast to studies describing HA-AKI, less is known regarding the characterization of CA-AKI. Published studies are mainly reliant on small patient numbers and because of geographic differences in disease patterns, may not be directly applicable to all populations (15–17). The findings in this manuscript are, however, consistent with our previous publications (5,18) and other recent smaller studies from Scotland (19) and Kentucky (20) showing that CA-AKI represents a significant proportion of all AKI. The outcome for CA-AKI defined by an e-alert is better than that for HA-AKI. This needs to be qualified by the observation that a significant proportion of patients with CA-AKI is not admitted to hospital and therefore, is not reported on in the majority of publications that characterize the nature and outcome of CA-AKI.
In this study, there is significant mortality after an AKI e-alert. Mortality is clearly higher in the cohort of patients admitted to the hospital; however, it is of note that, even in patients with CA-AKI who are not admitted to hospital, there is a 90-day mortality of 10%–15%, suggesting that, even in this group for which admission may not be appropriate or desirable, AKI is a marker of frailty. In the surviving patients, it is also of note that nonadmission is associated with a significantly worse renal outcome. Although in some patients, nonadmission may be appropriate and reflect a conscious decision (e.g., in the setting of palliative care), our previous published data (5,18) and the data on time to repeat measurement of renal function in this study suggest that nonadmission is, at least in part, because of lack of recognition of the significance of the alert. Our data are, however, consistent with the recent report by Sawhney et al. (21), in which patients with nonadmitted AKI, while having a lower mortality, were associated with greater nonrecovery of renal function.
On a national level, our data suggest regional variations in the incidence of AKI, with two areas in particular highlighted as outliers. The very small incidence in Powys likely reflects the rural nature of the area, with the population relying on hospitals in neighboring areas. Even accepting this discrepancy, the reported incidence is very low. Access to hospital facilities and renal services has long been established as a factor influencing the reported incidence of CKD (22,23), and it is interesting to speculate that the same may be true in terms of awareness of AKI. The second notable exception in AKI incidence is Cwm Taf. The WIMD (6) is produced at a small area level called LSOA and derived from a broad range of factors; 73 of the 188 LSOAs in this LHB (39%) are among the most deprived one fifth in Wales. The tight association of AKI incidence and WIMD rank across the whole cohort supports the notion that a higher prevalence of AKI is associated with social deprivation, which has been previously described for CKD (24,25). Although beyond the scope of this study, we speculate that this, at least in part, reflects a higher incidence of comorbidities, which are AKI risk factors (26), in areas of social deprivation.
Although this study is, to our knowledge, the first national study using an e-alert–based system to characterize the magnitude and effect of AKI, its findings need to be qualified by its limitations. Because the e-alert system is Information Technology driven, it lacks intelligence, and therefore, there is no clinical context applied. For this reason, the variation in SCr seen in patients on dialysis, unless specifically flagged by location, leads to a number of false positives. To minimize this effect, we have excluded incident patients flagged by two codes (ABS1 and DELTA1), which also excluded some patients with true AKI. The study is also limited in that any patient presenting with AKI but without a measurement of renal function in the previous 365 days will not be included. Using an Information Technology-based approach also precludes inclusion of clinical information, such as patient comorbidity and linkage to primary care datasets, and lacks the details of the cause of AKI, the need for RRT, and the cause of death. It should also be noted that the data collected are for a 6-month period, and therefore, potential seasonal effects on incidence may be lost. Although we have collected data on the development of CKD, this is limited by outcome data to 90 days only, and therefore, longer-term studies of follow-up are needed to truly describe the association with progressive CKD. It should also be noted that the outcomes reported in our study may be influenced by the transmission of the alert, making direct comparison with other studies difficult. Despite these limitations, our study provides the first large–scale description of AKI using a creatinine–based electronic AKI alert.
Disclosures
None.
Supplementary Material
Acknowledgments
The work was carried out under the auspices of the Welsh AKI Steering Group, which is sponsored by the Welsh Renal Clinical Network and the Welsh Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05170516/-/DCSupplemental.
References
- 1.National Confidential Enquiry into Patient Outcome and Death [NCEPOD], 2009. Acute Kidney Injury: Adding Insult to Injury. London, United Kingdom: NCEPOD [Google Scholar]
- 2.Selby NM, Crowley L, Fluck RJ, McIntyre CW, Monaghan J, Lawson N, Kolhe NV: Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol 7: 533–540, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Feehally J, Gilmore I, Barasi S, Bosomworth M, Christie B, Davies A, Dhesi J, Dowdle R, Gibbins C, Gonzalez I, Harding S, Lamont D, Murphy G, Ostermann M, Parr J, Stevens PE: RCPE UK consensus conference statement: Management of acute kidney injury: The role of fluids, e-alerts and biomarkers. J R Coll Physicians Edinb 43: 37–38, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wonnacott A, Meran S, Amphlett B, Talabani B, Phillips A: Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol 9: 1007–1014, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsh Government, 2014. Welsh Index of Multiple Deprivation. Available at: http://gov.wales/statistics-and-research/welsh-index-multiple-deprivation/?lang=en. Accessed March 14, 2016
- 7.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE 2nd, Perkins RM: Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS: Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol 4: 891–898, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS: Community-based incidence of acute renal failure. Kidney Int 72: 208–212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL: Epidemiology and outcomes of acute renal failure in hospitalized patients: A national survey. Clin J Am Soc Nephrol 1: 43–51, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Porter CJ, Juurlink I, Bisset LH, Bavakunji R, Mehta RL, Devonald MA: A real-time electronic alert to improve detection of acute kidney injury in a large teaching hospital. Nephrol Dial Transplant 29: 1888–1893, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Wallace K, Mallard AS, Stratton JD, Johnston PA, Dickinson S, Parry RG: Use of an electronic alert to identify patients with acute kidney injury. Clin Med (Lond) 14: 22–26, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas M, Sitch A, Dowswell G: The initial development and assessment of an automatic alert warning of acute kidney injury. Nephrol Dial Transplant 26: 2161–2168, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Lewington AJ, Cerdá J, Mehta RL: Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int 84: 457–467, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Z, Wang Y, Fan MH: [Comparison of clinical characteristics between hospital-acquired and community-acquired acute renal failure]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 17: 615–618, 2005 [PubMed] [Google Scholar]
- 16.Daher EF, Silva Junior GB, Santos SQ, R Bezerra CC, Diniz EJ, Lima RS, Babosa CA, C Guimarães AA, Mota RM, Abreu KL, Libório AB: Differences in community, hospital and intensive care unit-acquired acute kidney injury: Observational study in a nephrology service of a developing country. Clin Nephrol 78: 449–455, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Obialo CI, Okonofua EC, Tayade AS, Riley LJ: Epidemiology of de novo acute renal failure in hospitalized African Americans: Comparing community-acquired vs hospital-acquired disease. Arch Intern Med 160: 1309–1313, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Talabani B, Zouwail S, Pyart RD, Meran S, Riley SG, Phillips AO: Epidemiology and outcome of community-acquired acute kidney injury. Nephrology (Carlton) 19: 282–287, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Aitken E, Carruthers C, Gall L, Kerr L, Geddes C, Kingsmore D: Acute kidney injury: Outcomes and quality of care. QJM 106: 323–332, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Schissler MM, Zaidi S, Kumar H, Deo D, Brier ME, McLeish KR: Characteristics and outcomes in community-acquired versus hospital-acquired acute kidney injury. Nephrology (Carlton) 18: 183–187, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Sawhney S, Fluck N, Fraser SD, Marks A, Prescott GJ, Roderick PJ, Black C: KDIGO-based acute kidney injury criteria operate differently in hospitals and the community-findings from a large population cohort. Nephrol Dial Transplant 31: 922–929, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig KJ, Riley SG, Thomas B, Penney M, Donovan KL, Phillips AO: The impact of an out-reach clinic on referral of patients with renal impairment. Nephron Clin Pract 101: c168–c173, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Boyle PJ, Kudlac H, Williams AJ: Geographical variation in the referral of patients with chronic end stage renal failure for renal replacement therapy. QJM 89: 151–157, 1996 [DOI] [PubMed] [Google Scholar]
- 24.So BH, Methven S, Hair MD, Jardine AG, MacGregor MS: Socio-economic status influences chronic kidney disease prevalence in primary care: A community-based cross-sectional analysis. Nephrol Dial Transplant 30: 1010–1017, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Hossain MP, Palmer D, Goyder E, El Nahas AM: Social deprivation and prevalence of chronic kidney disease in the UK: Workload implications for primary care. QJM 105: 167–175, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Roberts G, Phillips D, McCarthy R, Bolusani H, Mizen P, Hassan M, Hooper R, Saddler K, Hu M, Lodhi S, Toynton E, Geen J, Lodhi V, Grose C, Phillips A: Acute kidney injury risk assessment at the hospital front door: What is the best measure of risk? Clin Kidney J 8: 673–680, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.