Abstract
Background and objectives
Vancomycin has been in use for more than half a century, but whether it is truly nephrotoxic and to what extent are still highly controversial. The objective of this study was to determine the risk of AKI attributable to intravenous vancomycin.
Design, setting, participants, & measurements
We conducted a systematic review of randomized, controlled trials and cohort studies that compared patients treated with intravenous vancomycin with a control group of patients given a comparator nonglycopeptide antibiotic and in which kidney function or kidney injury outcomes were reported. PubMed and Cochrane Library were searched from 1990 to September of 2015. Two reviewers extracted data and assessed study risk of bias, and one reviewer adjudicated the assessments. A meta-analysis was conducted on seven randomized, controlled trials (total of 4033 patients).
Results
Moderate quality evidence suggested that vancomycin treatment is associated with a higher risk of AKI, with a relative risk of 2.45 (95% confidence interval, 1.69 to 3.55). The risk of kidney injury was similar in patients treated for skin and soft tissue infections compared with those treated for nosocomial pneumonia and other complicated infections. There was an uncertain risk of reporting bias, because kidney function was not a prespecified outcome in any of the trials. The preponderance of evidence was judged to be indirect, because the majority of studies compared vancomycin specifically with linezolid.
Conclusions
Our findings suggest that there is a measurable risk of AKI associated with vancomycin, but the strength of the evidence is moderate. A randomized, controlled trial designed to study kidney function as an outcome would be needed to draw unequivocal conclusions.
Keywords: Glycopeptides, chronic renal insufficiency, meta-analysis, acute kidney injury, Anti-Bacterial Agents, Cohort Studies, Control Groups, Cross Infection, Humans, Libraries, Linezolid, Pneumonia, PubMed, Randomized Controlled Trials as Topic, Risk Assessment, Soft Tissue Infections, Vancomycin
Introduction
Vancomycin is a tricyclic glycopeptide antibiotic that was first approved in 1958. Early preparations extracted using picric acid precipitation were only about 70% pure (1), and initial reports of adverse effects were attributed to these impurities. The frequency of nephrotoxicity even in these early reports has been questioned, because it was largely on the basis of 20 patients reported between 1950 and 1980 (1,2). Moreover, many cases occurred with the concurrent use of vancomycin and aminoglycosides, a combination that has been anecdotally reported to be particularly nephrotoxic. Current preparations purified by HPLC are 90%–95% pure (3). Therefore, the degree to which modern preparations of parenteral vancomycin cause kidney injury is unknown.
Vancomycin given to rats at high doses has been shown by some investigators to cause significant kidney injury (4), but many others have found no effect on kidney function (5,6) and/or only subtle histologic and biochemical changes (6,7). Combining vancomycin with an aminoglycoside potentiates the nephrotoxic effect of the latter agent (5). The mechanism of increased nephrotoxicity of aminoglycosides is thought to be related to increased binding to renal brush border membrane in the presence of vancomycin (8). Moreover, endotoxin has been shown to act as an amplification factor for experimental nephrotoxicity due to gentamicin plus vancomycin (9). The mechanism of vancomycin–induced tubular toxicity has been proposed to be due to free radical–mediated injury (4). However, the majority of cases of vancomycin-associated AKI in humans in whom a kidney biopsy was performed have shown acute interstitial nephritis (10–18), whereas acute tubular injury is rarely reported (19–21). Because drug––induced acute interstitial nephritis is usually due to a hypersensitivity reaction and idiosyncratic, it is not dose dependent, and it is not a true toxic effect.
The recent literature is replete with clinical studies pertaining to vancomycin nephrotoxicity (22–29), but the vast majority are observational and suffer from three major flaws. First, most observational studies lack a control group (22–29). Only patients exposed to vancomycin are studied, and variables that predict AKI are identified. Because patients treated with vancomycin frequently have comorbidities (such as sepsis and multiorgan failure) or receive other tests or therapies that may independently cause AKI (such as radiocontrast studies or other nephrotoxic antibiotics), this study design does not assess the attributable risk from vancomycin itself.
Second, blood concentrations of vancomycin are often used as a surrogate of drug exposure, and the association of higher vancomycin levels with AKI is taken as evidence of causation (22–26,29). However, this logic ignores the possibility of reverse causation; 75%–90% of vancomycin is eliminated unchanged in the urine by glomerular filtration, and therefore, blood vancomycin levels are highly sensitive to the GFR. Baseline CKD or AKI from any other etiology would cause higher drug levels and lead to an erroneous conclusion. A related fallacy in the literature is to conflate vancomycin-associated AKI with vancomycin nephrotoxicity. The assumption that a higher dose or blood level of vancomycin should increase the risk of AKI is unproven.
Third, even in observational studies that include a control group, it is difficult to completely eliminate confounding by indication. Vancomycin was, for a long time, considered to be the drug of choice for critically ill patients with suspected or known methicillin–resistant Staphylococcus aureus and coagulase-negative staphylococci, and such patients often develop multifactorial AKI.
Among nephrologists, infectious diseases specialists, and pharmacists, there seems to be a wide range of beliefs regarding the propensity of vancomycin to cause AKI (or lack thereof). We, therefore, undertook to perform a systematic review of the available literature to answer the following question: what is the risk of AKI that can be attributed to exposure to parenteral vancomycin? To avoid the aforementioned pitfalls, we confined our review to cohort studies and interventional trials that compared vancomycin with a control group treated with a comparator antibiotic.
Materials and Methods
The protocol for this systematic review was prospectively registered on the International Prospective Register of Systematic Reviews (CRD42015026078).
Data Sources and Searches
We searched PubMed and the Cochrane Library to identify interventional trials, cohort studies, and systematic reviews that compared vancomycin with a comparator antibiotic or evaluated the risk of AKI with vancomycin using a broad search strategy. Because concerns about the purity of vancomycin preparations extended to the early 1980s, we restricted our search to publications from 1990 to September of 2015. For example, PubMed was searched by Medical Subject Heading terms using the following query: ((“1990/01/01”[Date - Publication]: “3000”[Date - Publication])) AND vancomycin AND (acute kidney injury OR linezolid OR daptomycin OR ceftaroline OR tigecycline) AND (comparative study OR clinical trial OR meta-analysis). Bibliographies of included publications were then scanned to identify additional relevant references.
Study Selection
Two reviewers independently screened the article title and abstracts to identify clinical trials or comparative cohort studies that compared patients treated with intravenous vancomycin with a control group of patients given a comparator nonglycopeptide antibiotic for similar indications. Then, both reviewers separately read the full text of each article. Studies were included if they reported data on kidney injury outcomes in both the vancomycin and control groups, such as the post–treatment serum creatinine or change in serum creatinine from baseline, AKI, or adverse events coded as kidney injury or kidney impairment. Studies were excluded if they were reviews or meta-analyses, duplicate studies or subanalyses of another included study, observational studies that had no comparator group, case-control studies, case reports or case series, trials that were not randomized and controlled, studies of oral vancomycin or a prophylactic vancomycin regimen, studies in which a known nephrotoxin was prospectively assigned to one arm, studies in which the outcome in vancomycin-treated patients was reported only in aggregate with patients treated with other antibiotics, pediatric studies, and studies not reported in the English language.
Data Extraction and Quality Assessment
Two reviewers independently extracted data and assessed the risk of bias in each study. Any disagreements were discussed with a third reviewer and resolved by consensus. The Newcastle–Ottawa Scales were used to assess risk of bias for the cohort studies (30), and the Cochrane Risk of Bias Assessment Tool was used for the randomized, controlled trials (RCTs) (31).
Data Analyses
A single dichotomous outcome measure of kidney injury was selected for each study and reported as a risk ratio (RR). For studies that reported multiple outcomes related to kidney injury, outcomes that were quantitatively measured (e.g., a threshold value or fold change in serum creatinine) were selected preferentially over qualitative outcomes (e.g., adverse event coded as renal impairment). Outcomes that were recorded without assignment of causality (i.e., adverse event, all cause) were considered to be more objective and selected preferentially over outcomes that required a subjective assessment of causality (i.e., a drug–related adverse event). For subjective measures of differing degrees of kidney injury, the criterion representing more severe kidney injury was selected preferentially (e.g., acute kidney failure instead of serum creatinine increase). For objective measures, the criterion that identified the most events was selected.
Results of the included cohort studies were summarized qualitatively. Meta-analysis of the included RCTs was performed using RevMan 5.3 software (Cochrane Collaboration, Oxford, United Kingdom). Study heterogeneity was judged to be low on the basis of visual inspection of the forest plot, chi-squared test, and I2 statistic (<50%) (32). A fixed effects model and the Mantel–Haenszel method were, therefore, used to determine the pooled relative risk. A random effects model was also used in a sensitivity analysis. Publication bias was assessed by examining the funnel plot. Confidence in the results was assessed using the Grading of Recommendations Assessment, Development and Evaluation method (33).
Role of the Funding Source
This study received no specific external funding.
Results
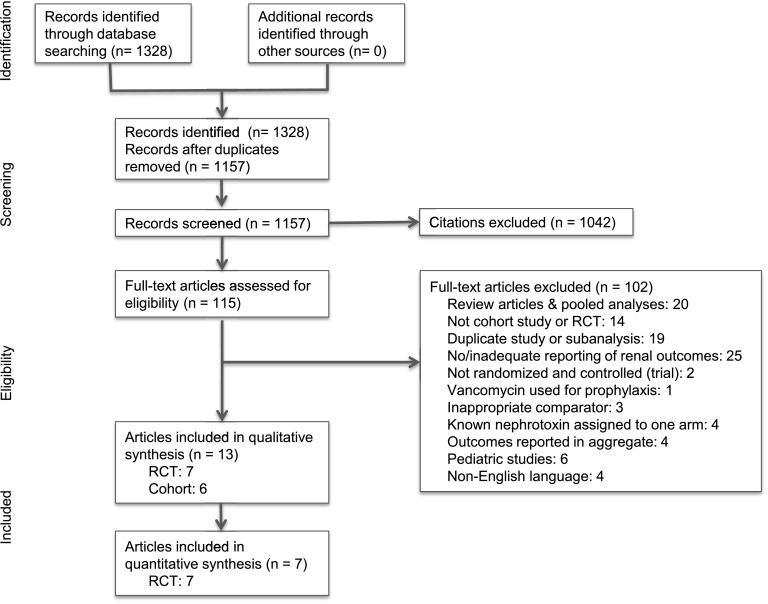
We screened 1328 titles and abstracts and reviewed 115 full-text articles, of which 101 were excluded for the reasons shown in Figure 1.
Figure 1.
Summary of evidence search and selection. RCT, randomized, controlled trial.
Table 1 shows details of the 13 articles that were included in the final analysis. Of these, six were observational cohort studies, all of which compared vancomycin with linezolid. One study included only patients with baseline renal impairment and was, therefore, not felt to be generalizable to the overall population that is likely to be exposed to vancomycin (34). Five of the six studies made no attempt to exclude patients with AKI caused by other etiologies rather than by the antibiotic exposure. The one study that did excluded patients who had received intravenous contrast dye within the previous 7 days (35). None of the studies matched vancomycin and control groups by baseline severity of illness or renal function. In only two studies was multivariable analysis performed to adjust for baseline kidney function, severity of illness (Acute Physiology, Age, Chronic Health Evaluation II score), and other potential confounders (35,36). In the other four studies, multivariable adjustment either was not performed at all (34,37) or was performed only on nonrenal primary outcomes (38,39). One study was considered to have inadequate duration of follow-up (34), and in one, the duration was not reported (37). All six studies failed to report data on the adequacy of follow-up. In addition to problems associated with risk of bias, one study tested for 16 different renal outcomes without correcting for multiple comparisons (36).
Table 1.
Details of studies included in this analysis
| Study and Year (Reference) | Type | Patient Population and/or Specific Organism | Comparator Antibiotic | Most Relevant Outcome to Kidney Injury | Relative Risk Ratio (95% CI) | Total No. of Patients |
|---|---|---|---|---|---|---|
| Wunderink et al. 2003 (55) | RCT, DB | Nosocomial pneumonia | Linezolid and aztreonam | Drug-related SAE: “kidney failure” | 2.13 (0.19 to 23.32) | 623 |
| Jaksic et al. 2006 (56) | RCT, DB | Febrile, neutropenic patients with cancer | Linezolid | “Renal failure”, all cause | 3.03 (1.12 to 8.23) | 603 |
| Kohno et al. 2007 (40) | RCT, OL | Nosocomial PNA, cSSSI, or sepsis with MRSA | Linezolid | Treatment-related AE: “renal function abnormal” | 9.80 (1.18 to 81.71) | 151 |
| Lin et al. 2008 (43) | RCT, OL | Hospitalized patients with PNA or cSSSI | Linezolid | “Acute kidney failure” | 0.33 (0.01 to 8.05) | 142 |
| Wilcox et al. 2009 (41) | RCT, OL | Catheter-related bacteremia | Linezolid | “Kidney failure, acute” | 3.00 (0.82 to 10.99) | 726 |
| Corrado et al. 2010 (44) | RCT, DB | cSSSI | Ceftaroline | Serum creatinine ≥1.5 mg/dl and ≥50% increase | 2.38 (0.92 to 6.14) | 1340 |
| Wunderink et al. 2012 (42) | RCT, DB | Nosocomial PNA with MRSA | Linezolid | 0.5-mg/dl Increase in serum creatinine level if normal at baseline or 50% increase if abnormal at baseline | 2.16 (1.29 to 3.60) | 448 |
| Lodise et al. 2008 (35) | Cohort | Hospitalized patients | Linezolid | Serum creatinine increase of 0.5 mg/dl or 50%, whichever was greater, on at least 2 consecutive d | 1.63 (0.51 to 5.20)a | 265 |
| Rodriguez Colomo et al. 2011 (34) | Cohort | ICU patients with renal failure and known or suspected MR-GPC | Linezolid | Requirement for RRT | 0.33 (0.11 to 0.96) | 147 |
| Chan et al. 2011 (38) | Cohort | VAP with MRSA | Linezolid | Serum creatinine increase of 0.5 mg/dl or 50%, whichever was greater, on at least 2 consecutive d | 1.26 (0.15 to 10.76) | 113 |
| Davies et al. 2013 (36) | Cohort | Surgical ICU patients | Linezolid | “Injury” or more severe level of RIFLE criteria | 1.43 (0.91 to 2.26) | 545 |
| Fujii et al. 2013 (37) | Cohort | Hospitalized patients | Linezolid | Renal dysfunction was defined on the basis of the progression of CKD after treatment | 1.78 (1.03 to 3.05) | 270 |
| Peyrani et al. 2014 (39) | Cohort | VAP with MRSA | Linezolid | Serum creatinine increase of 0.5 mg/dl or 50%, whichever was greater, on at least two consecutive measurements | 1.37 (0.65 to 2.90) | 188 |
RIFLE criteria from ref. 57. 95% CI, 95% confidence interval; RCT, randomized, controlled trial; DB, double blinded; SAE, serious adverse event; OL, open label; PNA, pneumonia; cSSSI, complicated skin and skin structure infection; MRSA, methicillin–resistant Staphylococcus aureus; AE, adverse event; ICU, intensive care unit; MR-GPC, methicillin–resistant gram–positive cocci; VAP, ventilator-associated pneumonia.
For vancomycin dose <4 g/d.
Because all of the cohort studies were judged to have moderate or high risk of bias (Table 2), a qualitative synthesis was performed. The study with the least risk of bias by Lodise et al. (35) compared hospitalized patients treated with vancomycin at standard dose (<4 g/d) or high dose (≥4 g/d) and linezolid. They found a significant difference in the risk of nephrotoxicity defined as a serum creatinine increase of 0.5 mg/dl or 50% between the control linezolid group (6.7%), the low–dose vancomycin group (10.9%), and the high–dose vancomycin group (34.6%; P=0.001). Pairwise comparisons were performed only on the Kaplan–Meier time to nephrotoxicity analysis and showed significant differences between linezolid and high-dose vancomycin and between standard dose and high-dose vancomycin but not between linezolid and standard dose vancomycin. Of the remaining studies, three showed no difference in renal outcomes between vancomycin- and linezolid-treated groups (36,38,39). Rodriguez Colomo et al. (34) showed a lower rate of RRT in vancomycin- compared with linezolid-treated patients, but all patients had preexisting renal disease; also, the distribution of renal function was not balanced between the two groups. The study by Fujii et al. (37) showed an odds ratio of 1.78 (95% confidence interval [95% CI], 1.03 to 3.05) for developing renal dysfunction, but this was defined too vaguely (“progression of chronic kidney disease after treatment”) and therefore, subject to observer bias in the outcome assessment. Overall, the cohort studies did not provide strong evidence for an association of vancomycin with kidney injury.
Table 2.
Risk of bias in included cohort studies
| Study and Year (Reference) | Representative Cohort | Selection of Nonexposed | Exposure Ascertained | Not Present at Outset | Comparability | Outcome Assessment | Duration of Follow-Up | Adequacy of Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Lodise et al. 2008 (35) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| Davies et al. 2013 (36) | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 |
| Chan et al. 2011 (38) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| Peyrani et al. 2014 (39) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| Fujii et al. 2013 (37) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rodriguez Colomo et al. 2011 (34) | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
The Newcastle–Ottawa Scales were used to assess risk of bias for the cohort studies. Each domain was rated on a scale of zero or one star, except comparability, which can be awarded up to two stars. 0, High or unclear risk of bias; 1 or 2, low risk of bias.
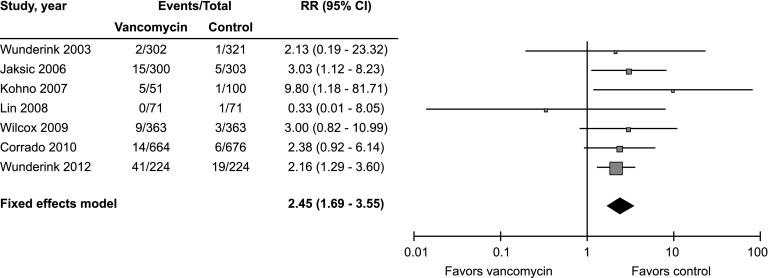
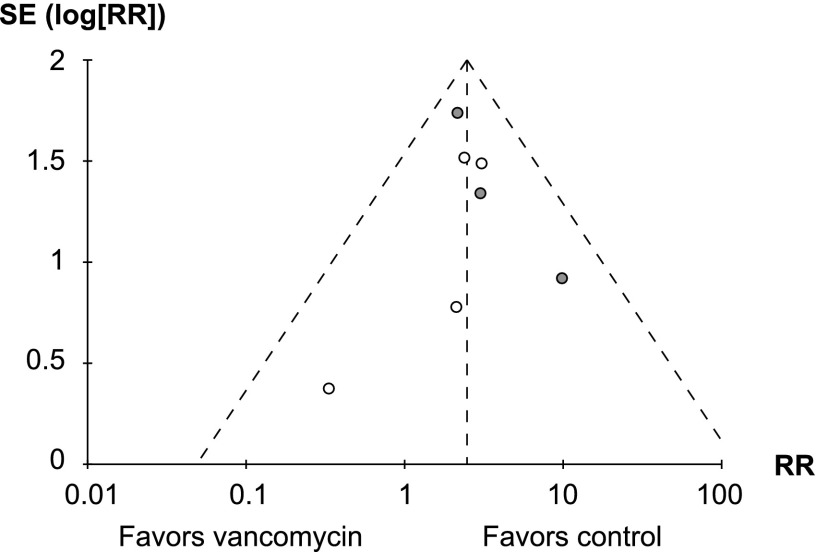
Seven RCTs were included in the analysis (Table 3). Of these, six compared vancomycin with linezolid, and one compared it with ceftaroline. A major limitation is that none of these studies were originally designed to compare the nephrotoxicity of vancomycin with that of the comparator antibiotic, and therefore, none prespecified any measure of kidney injury as a primary or secondary outcome; also, all but one failed to report whether baseline renal function was comparable between the groups. For these reasons, none of the trials were considered to be at low risk of bias. Three studies were judged to be at high risk of bias: two because they had an open label design and allowed but did not report the rate of aminoglycoside use (40,41), and one because there was a substantial imbalance between the groups in the proportion of patients with renal failure at baseline (42). A meta-analysis with a fixed effects model including all seven RCTs (n=4033) showed a higher risk for kidney injury in vancomycin-treated compared with control patients (RR, 2.45; 95% CI, 1.69 to 3.55; P<0.001) (Figure 2). There was minimal heterogeneity between the studies (chi square=3.67; P=0.72 and I2=0%), and six of seven studies showed a greater risk of kidney injury in the vancomycin group. An analysis performed with a random effects model showed very similar results (RR, 2.42; 95% CI, 1.66 to 3.52). Inspection of the funnel plot (Figure 3) showed no strong evidence of publication bias. A secondary pooled analysis that excluded the three RCTs with the highest risk of bias also showed a lower but still significantly increased risk of kidney injury with vancomycin (RR, 2.37; 95% CI, 1.26 to 4.46; P<0.01; n=2708).
Table 3.
Risk of bias in included randomized, controlled trials
| Study and Year (Reference) | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias; AKI) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias |
|---|---|---|---|---|---|---|---|
| Wunderink et al. 2003 (55) | U | U | L | L | L | U | U |
| Corrado et al. 2010 (44) | U | L | L | L | U | U | U |
| Lin et al. 2008 (43) | U | U | L | L | L | U | U |
| Jaksic et al. 2006 (56) | U | U | U | U | L | U | U |
| Wunderink et al. 2012 (42) | U | U | L | L | U | U | H |
| Kohno et al. 2007 (40) | U | H | H | H | L | U | U |
| Wilcox et al. 2009 (41) | U | H | H | H | L | U | U |
The Cochrane Risk of Bias Assessment Tool was used to assess risk of bias for the randomized, controlled trials. H, high risk of bias; L, low risk of bias; U, uncertain risk of bias.
Figure 2.
Forest plot of included randomized, controlled trials. Risk ratios (RRs) and 95% confidence intervals (95% CIs) are shown for each study and the pooled analysis using a fixed effects model and the Mantel–Haenszel method. RR>1.0 means that the risk of kidney injury in the vancomycin group is greater than that in the control group.
Figure 3.
Funnel plot of included randomized, controlled trials. Risk ratio (RR) is plotted against SEM. Dotted lines indicate the pooled effect estimate and 95% confidence intervals. Studies with high risk of bias are shown as gray circles, and studies with uncertain risk of bias are shown as white circles.
Three of the seven RCTs included a significant proportion of patients (ranging from 29% to 100%) with skin and soft tissue infections (SSTIs) as the sole indication for vancomycin (40,43,44). Current guidelines recommend targeting higher trough levels for patients with complicated infections but not for those with SSTIs (45). Nevertheless, excluding these three studies did not alter the magnitude of the effect (RR, 2.40; 95% CI, 1.57 to 3.67).
Overall, the strength of evidence that vancomycin is associated with higher risk of kidney injury was judged to be moderate (Supplemental Table 1). The attributable risk percentage, (RR−1)/RR, which represents the proportion of patients with AKI attributed to use of vancomycin as opposed to the comparator drug, calculated using an RR of 2.45, was 59%.
Discussion
Our review found moderate strength evidence that vancomycin is associated with higher risk of kidney injury. The estimated magnitude of the effect was modest, with an RR of 2.45. Nevertheless, it suggests that, in patients being treated with intravenous vancomycin who develop AKI, more than one half of cases can be attributable to vancomycin (attributable risk percentage of 59%). This is substantial but still much lower than the risk of kidney injury associated with well recognized nephrotoxins, such as aminoglycosides (RR, 8–10) (46) and conventional (nonliposomal) amphotericin B (RR, 4–10) (47,48).
Our study has many strengths. Most importantly, by limiting the review to cohort studies and RCTs that compared vancomycin with a non-nephrotoxic antibiotic, we were able to determine the relative risk of kidney injury from vancomycin and minimize (cohort studies) or eliminate (RCTs) bias due to confounding by indication. The strongest evidence derived from the seven RCTs, because they showed minimal heterogeneity, and all but one of them found a higher risk of kidney injury from vancomycin.
A number of limitations deserve mention. None of the included RCTs were designed to address the risk of kidney injury; all of them were primarily designed to test antibiotic efficacy in the treatment of infection. Thus, in none of the studies were patients in the two arms matched for baseline renal function, other risk factors for AKI, or concurrent exposure to other nephrotoxins; additionally, in most cases, there was no confirmation that these parameters were evenly distributed between the arms. Also, renal end points were not prespecified and in some cases, were not strictly defined, and therefore, we had to rely on qualitative assessments recorded in the safety data.
A corollary of this is that our meta-analyses included effect estimates derived from a wide range of renal end points ranging in severity from a serum creatinine rise above the upper limit of normal to the requirement for RRT. To justify a pooled analysis, we assumed that the relative risk of kidney injury from vancomycin treatment was uniform along the entire continuum of renal end points. In other words, if vancomycin increased the risk of a small rise in serum creatinine by a certain amount, the risk of severe kidney failure, although much lower in absolute terms, would also be increased by vancomycin with approximately the same relative magnitude. Our finding of fairly homogeneous effects (Figure 2) seems to confirm that this was a valid approach.
Our analysis predominantly included studies that compared the risk of kidney injury associated with vancomycin with that associated with linezolid. Although linezolid has not been reported to be associated with kidney injury, any unanticipated effect of linezolid on kidney function could have biased our estimate of the risk attributable to vancomycin.
Our analysis was primarily confined to patients treated with standard doses of vancomycin. Therefore, this study does not address the toxicity of high-dose vancomycin. Because the minimum inhibitory concentration of vancomycin for methicillin–resistant Staphylococcus aureus has increased in recent years, expert guidelines have recommended targeting higher–trough plasma vancomycin concentrations between 15 and 20 mg/L for complicated infections, which could necessitate use of high doses, and therefore, this is an important issue to address. One cohort study did include and separately analyze 26 patients who received ≥4 g/d vancomycin, and it found a higher risk of kidney injury compared with patients who received standard doses (35).
Our study also does not address the question of whether high blood concentrations of vancomycin predispose to kidney injury. Most of the RCTs used pharmacists to determine dosing according to kidney function and maintain blood levels in a range consistent with local prescribing practices, but the target ranges were not reported in any of these studies. We did not include any analyses that examined the association between achieved blood vancomycin concentrations and kidney injury, because renal function is itself the major determinant of vancomycin clearance and hence, blood levels; therefore, we consider such study designs to be fundamentally flawed. Three of the seven RCTs included a significant proportion of patients with complicated SSTIs that might have been treated to lower target trough levels of vancomycin. Nevertheless, excluding these studies did not alter the magnitude of the effect.
This analysis excluded studies comparing vancomycin with other glycopeptides because of the possibility that nephrotoxicity might be a class effect. Telavancin use is associated with a high risk of AKI (49), and teicoplanin may also cause AKI (50), although the risk seems to be lower than that of vancomycin (51,52). This led to the exclusion of a couple of large, good–quality RCTs conducted recently comparing vancomycin with oritavancin (53) and dalbavancin (54). In the dalbavancin trial in particular, there was a large number of end points allowing an accurate estimate of the magnitude of the effect (54). In this study, 119 of 638 patients assigned to the vancomycin arm had an increase in serum creatinine above the upper limit of normal versus 94 of 644 patients assigned to dalbavancin (RR, 1.28; 95% CI, 1.00 to 1.64).
This study also does not address the risk of kidney injury associated with specific combinations of vancomycin with other known nephrotoxins. Specifically, there is widespread belief that the combination of vancomycin with aminoglycosides is particularly nephrotoxic. We excluded studies in which one or both study arms were assigned a known nephrotoxic antibiotic to minimize study heterogeneity.
Finally, our study did not distinguish between different pathologic manifestations of kidney injury. Therefore, we were unable to determine if the association of vancomycin is with acute interstitial nephritis, acute tubular necrosis, or both. On the basis of published case reports, however, it seems likely to be both.
In summary, our review found moderate strength evidence that intravenous vancomycin treatment is associated with a higher risk of kidney injury, but the magnitude of the effect is small. We did not identify any studies with low risk of bias. More studies are needed, particularly RCTs with prespecified renal safety end points, including newer and more sensitive kidney injury biomarkers, to confirm these findings and test whether high-dose vancomycin is associated with even greater risk of kidney injury.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Rachel Vukas (Dykes Library, University of Kansas Medical Center) for help performing the literature search.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Vancomycin and the Risk of AKI: Now Clearer than Mississippi Mud,” on pages 2101–2103.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05920616/-/DCSupplemental.
References
- 1.Bailie GR, Neal D: Vancomycin ototoxicity and nephrotoxicity. A review. Med Toxicol 3: 376–386, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Cheung RP, DiPiro JT: Vancomycin: An update. Pharmacotherapy 6: 153–169, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Nambiar S, Madurawe RD, Zuk SM, Khan SR, Ellison CD, Faustino PJ, Mans DJ, Trehy ML, Hadwiger ME, Boyne MT 2nd , Biswas K, Cox EM: Product quality of parenteral vancomycin products in the United States. Antimicrob Agents Chemother 56: 2819–2823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino Y, Takemura S, Minamiyama Y, Hirohashi K, Ogino T, Inoue M, Okada S, Kinoshita H: Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic Res 37: 373–379, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Wood CA, Kohlhepp SJ, Kohnen PW, Houghton DC, Gilbert DN: Vancomycin enhancement of experimental tobramycin nephrotoxicity. Antimicrob Agents Chemother 30: 20–24, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronoff GR, Sloan RS, Dinwiddie CB Jr. , Glant MD, Fineberg NS, Luft FC: Effects of vancomycin on renal function in rats. Antimicrob Agents Chemother 19: 306–308, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marre R, Schulz E, Anders T, Sack K: Renal tolerance and pharmacokinetics of vancomycin in rats. J Antimicrob Chemother 14: 253–260, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Yano Y, Hiraoka A, Oguma T: Enhancement of tobramycin binding to rat renal brush border membrane by vancomycin. J Pharmacol Exp Ther 274: 695–699, 1995 [PubMed] [Google Scholar]
- 9.Ngeleka M, Beauchamp D, Tardif D, Auclair P, Gourde P, Bergeron MG: Endotoxin increases the nephrotoxic potential of gentamicin and vancomycin plus gentamicin. J Infect Dis 161: 721–727, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg ES, Robbins N, Lenci M: Vancomycin and interstitial nephritis. Ann Intern Med 95: 658, 1981 [DOI] [PubMed] [Google Scholar]
- 11.Bergman MM, Glew RH, Ebert TH: Acute interstitial nephritis associated with vancomycin therapy. Arch Intern Med 148: 2139–2140, 1988 [PubMed] [Google Scholar]
- 12.Codding CE, Ramseyer L, Allon M, Pitha J, Rodriguez M: Tubulointerstitial nephritis due to vancomycin. Am J Kidney Dis 14: 512–515, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Wai AO, Lo AM, Abdo A, Marra F: Vancomycin-induced acute interstitial nephritis. Ann Pharmacother 32: 1160–1164, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Hsu SI: Biopsy-proved acute tubulointerstitial nephritis and toxic epidermal necrolysis associated with vancomycin. Pharmacotherapy 21: 1233–1239, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Hong S, Valderrama E, Mattana J, Shah HH, Wagner JD, Esposito M, Singhal PC: Vancomycin-induced acute granulomatous interstitial nephritis: Therapeutic options. Am J Med Sci 334: 296–300, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Salazar MN, Matthews M, Posadas A, Ehsan M, Graeber C: Biopsy proven interstitial nephritis following treatment with vancomycin: A case report. Conn Med 74: 139–141, 2010 [PubMed] [Google Scholar]
- 17.O’Meara P, Borici-Mazi R, Morton AR, Ellis AK: DRESS with delayed onset acute interstitial nephritis and profound refractory eosinophilia secondary to Vancomycin. Allergy Asthma Clin Immunol 7: 16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Htike NL, Santoro J, Gilbert B, Elfenbein IB, Teehan G: Biopsy-proven vancomycin-associated interstitial nephritis and acute tubular necrosis. Clin Exp Nephrol 16: 320–324, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Wicklow BA, Ogborn MR, Gibson IW, Blydt-Hansen TD: Biopsy-proven acute tubular necrosis in a child attributed to vancomycin intoxication. Pediatr Nephrol 21: 1194–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Shah-Khan F, Scheetz MH, Ghossein C: Biopsy-proven acute tubular necrosis due to vancomycin toxicity. Int J Nephrol 2011: 436856, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belen C, Budhiraja P, Bracamonte E, Popovtzer M: Biopsy-proven acute tubular necrosis associated with vancomycin in an adult patient. Ren Fail 34: 502–505, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH: A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther 29: 1107–1115, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL: Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49: 507–514, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, Mauldin PD: Relationship between vancomycin trough concentrations and nephrotoxicity: A prospective multicenter trial. Antimicrob Agents Chemother 55: 5475–5479, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rostas SE, Kubiak DW, Calderwood MS: High-dose intravenous vancomycin therapy and the risk of nephrotoxicity. Clin Ther 36: 1098–1101, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Hanrahan TP, Harlow G, Hutchinson J, Dulhunty JM, Lipman J, Whitehouse T, Roberts JA: Vancomycin-associated nephrotoxicity in the critically ill: A retrospective multivariate regression analysis*. Crit Care Med 42: 2527–2536, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Cappelletty D, Jablonski A, Jung R: Risk factors for acute kidney injury in adult patients receiving vancomycin. Clin Drug Investig 34: 189–193, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Meaney CJ, Hynicka LM, Tsoukleris MG: Vancomycin-associated nephrotoxicity in adult medicine patients: Incidence, outcomes, and risk factors. Pharmacotherapy 34: 653–661, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Yin Y, Liu XZ, Yao HJ, Li LX, Chen JH, Chen T, Lu XT, Bu SH, Zhang J: Retrospective analysis of vancomycin nephrotoxicity in elderly Chinese patients. Pharmacology 95: 279–284, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 1, 2016
- 31.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group : The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343: d5928, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557–560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ: GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64: 383–394, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez Colomo O, Álvarez Lerma F, González Pérez MI, Sirvent JM, García Simón M; Study Group of Infection in Critical Patients : Impact of administration of vancomycin or linezolid to critically ill patients with impaired renal function. Eur J Clin Microbiol Infect Dis 30: 635–643, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Lodise TP, Lomaestro B, Graves J, Drusano GL: Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 52: 1330–1336, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies SW, Guidry CA, Petroze RT, Hranjec T, Sawyer RG: Vancomycin and nephrotoxicity: Just another myth? J Trauma Acute Care Surg 75: 830–835, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujii S, Takahashi S, Makino S, Kunimoto Y, Nakata H, Noda N, Sakurai K, Miyamoto A: Impact of vancomycin or linezolid therapy on development of renal dysfunction and thrombocytopenia in Japanese patients. Chemotherapy 59: 319–324, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Chan JD, Pham TN, Wong J, Hessel M, Cuschieri J, Neff M, Dellit TH: Clinical outcomes of linezolid vs vancomycin in methicillin-resistant Staphylococcus aureus ventilator-associated pneumonia: Retrospective analysis. J Intensive Care Med 26: 385–391, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Peyrani P, Wiemken TL, Kelley R, Zervos MJ, Kett DH, File TM Jr. , Stein GE, Ford KD, Scerpella EG, Welch V, Ramirez JA; IMPACT-HAP Study Group : Higher clinical success in patients with ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus treated with linezolid compared with vancomycin: Results from the IMPACT-HAP study. Crit Care 18: R118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohno S, Yamaguchi K, Aikawa N, Sumiyama Y, Odagiri S, Aoki N, Niki Y, Watanabe S, Furue M, Ito T, Croos-Dabrera R, Tack KJ: Linezolid versus vancomycin for the treatment of infections caused by methicillin-resistant Staphylococcus aureus in Japan. J Antimicrob Chemother 60: 1361–1369, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Wilcox MH, Tack KJ, Bouza E, Herr DL, Ruf BR, Ijzerman MM, Croos-Dabrera RV, Kunkel MJ, Knirsch C: Complicated skin and skin-structure infections and catheter-related bloodstream infections: Noninferiority of linezolid in a phase 3 study. Clin Infect Dis 48: 203–212, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J: Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: A randomized, controlled study. Clin Infect Dis 54: 621–629, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Lin DF, Zhang YY, Wu JF, Wang F, Zheng JC, Miao JZ, Zheng LY, Sheng RY, Zhou X, Shen HH, Ijzerman MM, Croos-Dabrera RV, Sheng W: Linezolid for the treatment of infections caused by Gram-positive pathogens in China. Int J Antimicrob Agents 32: 241–249, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Corrado ML: Integrated safety summary of CANVAS 1 and 2 trials: Phase III, randomized, double-blind studies evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 65[Suppl 4]: iv67–iv71, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr. , Craig WA, Billeter M, Dalovisio JR, Levine DP: Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 29: 1275–1279, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Smith CR, Moore RD, Lietman PS: Studies of risk factors for aminoglycoside nephrotoxicity. Am J Kidney Dis 8: 308–313, 1986 [DOI] [PubMed] [Google Scholar]
- 47.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B; Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group : Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347: 408–415, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Walsh TJ, Teppler H, Donowitz GR, Maertens JA, Baden LR, Dmoszynska A, Cornely OA, Bourque MR, Lupinacci RJ, Sable CA, dePauw BE: Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med 351: 1391–1402, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Theravance Biopharma Antibiotics I: Vibativ (Package Insert), 2016. Available at: http://vibativ.com/pdf/PrescribingInformation.pdf. Accessed January 1, 2016
- 50.Frye RF, Job ML, Dretler RH, Rosenbaum BJ: Teicoplanin nephrotoxicity: First case report. Pharmacotherapy 12: 240–242, 1992 [PubMed] [Google Scholar]
- 51.Yoshiyama Y, Yazaki T, Kanke M, Beauchamp D: Nephrotoxicity of teicoplanin in rats. Jpn J Antibiot 53: 660–666, 2000 [PubMed] [Google Scholar]
- 52.Cavalcanti AB, Goncalves AR, Almeida CS, Bugano DD, Silva E: Teicoplanin versus vancomycin for proven or suspected infection. Cochrane Database Syst Rev 6: CD007022, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Corey GR, Kabler H, Mehra P, Gupta S, Overcash JS, Porwal A, Giordano P, Lucasti C, Perez A, Good S, Jiang H, Moeck G, O’Riordan W; SOLO I Investigators : Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med 370: 2180–2190, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW: Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 370: 2169–2179, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Wunderink RG, Cammarata SK, Oliphant TH, Kollef MH; Linezolid Nosocomial Pneumonia Study Group : Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia. Clin Ther 25: 980–992, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Jaksic B, Martinelli G, Perez-Oteyza J, Hartman CS, Leonard LB, Tack KJ: Efficacy and safety of linezolid compared with vancomycin in a randomized, double-blind study of febrile neutropenic patients with cancer. Clin Infect Dis 42: 597–607, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.