Abstract
Background. Uptake of human papillomavirus (HPV) vaccine in the United States is slow, and the effectiveness of the vaccine has not been assessed in high-risk adolescent populations.
Methods. We conducted a longitudinal study of 1139 sexually active, inner-city adolescent women receiving the 3-dose quadrivalent (4vHPV) vaccine. Cervical and anal specimens collected semiannually were tested using an L1-specific polymerase chain reaction assay. Postvaccination incidence of 4vHPV vaccine and nonvaccine HPV types, and risk of cervical cytological abnormalities, were assessed in relation to time to completion of all 3 vaccine doses.
Results. Compared to vaccine naive women at enrollment, vaccinated women had significantly lower incidence rate ratios of cervical infection with HPV6/11/16/18 (0.2; 95% confidence interval [CI], .1–.4) and the related types HPV31 and HPV45 (0.4 [95% CI, .2–1.0] and 0.3 [95% CI, .1–.6], respectively), as well as significantly lower incidence rate ratios of anal infection with HPV6/11/16/18 (0.4; 95% CI, .2–.7). Notably, we observed higher risks of cervical HPV6/11/16/18 infection (hazards ratio [HR], 2.9; 95% CI, 1.0–8.0) and associated cytological abnormalities (HR, 4.5; 95% CI, .7–26.0) among women immunized at ≥15 years of age who took ≥12 months (vs <12 months) to complete the 3-dose regimen.
Conclusions. Among adolescents immunized at ≥15 years of age, a longer time to complete the 3-dose schedule was associated with an increased risk of anogenital HPV6/11/16/18 infection and an increased incidence of associated cervical cytological abnormalities.
Keywords: human papillomavirus, cervical neoplasia, vaccination, adolescent medicine, women's health
The quadrivalent human papillomavirus (4vHPV) vaccine has been shown to be highly effective at reducing the incidence of anogenital HPV infections and diseases related to vaccine types (HPV6/11/16/18) [1]. It has also been shown to provide cross-protection for some but not all phylogenetically related high-risk (HR) HPV types [2], with some studies also reporting declines in rates of cervical precancer irrespective of the HPV type detected [3, 4]. The recently approved 9-valent HPV (9vHPV) vaccine promises to provide greater protection by targeting 5 additional HR-HPV types (31/33/45/52/58), increasing coverage to >90% of anogenital cancers [5, 6]. However, the evidence to date from the clinical trials and national vaccination programs involved mostly lower-risk individuals with few sex partners who were vaccinated early and were highly compliant with the 3-dose vaccine schedule [7–9].
In contrast, uptake and completion of the HPV vaccine in the United States has been persistently low [10, 11], with almost half (43%) of adolescent women getting vaccinated after age 14 years [12] and many getting <3 doses [13]. Whereas a reduced-dose regimen has been shown to be as effective among young vaccinees [14], current recommendations are to keep the 3-dose schedule if vaccination is initiated after the 15th birthday [15]. Moreover, the effectiveness of the vaccine may be further diminished by the fact that the efficacy of the vaccine is substantially reduced among populations with a past or present HPV infection [16, 17] and that almost half (42%) of adolescent women in the United States are already sexually active by the 10th grade (ie, age 15–16 years) [18].
Subsequent to the approval and release of the 4vHPV vaccine, we initiated a prospective cohort study of sexually active inner-city, mostly minority, adolescent women attending the largest adolescent-specific primary care facility in the United States—the Mount Sinai Adolescent Health Center (MSAHC) in New York City. This center provides free health services, including HPV vaccination, to adolescents and young adults aged 12–24 years [19, 20]. We present here data on the prevalence and incidence of cervical and anal HPV infection after vaccination and assessed the impact of delayed uptake and completion of the 3-dose 4vHPV vaccine schedule on the risk of HPV infection and associated cervical cytological abnormalities.
METHODS
Study Population
Adolescent women presenting for health services and HPV vaccination were invited to participate in a prospective cohort study with repeated assessment every 6 months for cervical, anal, and oral HPV infection [19, 20]. Women were aged 12–19 years at time of enrollment, were eligible to participate if they had already engaged in vaginal intercourse, and intended to receive or had already received the Food and Drug Administration–approved 4vHPV vaccine (GardasilMerck & Co., Kenilworth, New Jersey). Women pregnant at the time of recruitment or who had terminated a pregnancy within the last 6 weeks were invited to return at a later date after resolution of their pregnancy. Written informed consent was collected from all participants prior to enrollment. The study was approved by the Institutional Review Board at The Icahn School of Medicine at Mount Sinai.
Study Visits and Specimens
Participants received a gynecological examination at each visit, including an assessment of sexual, reproductive, behavioral, and psychosocial history and updated immunizations. A questionnaire was completed by self-report at each visit to assess recent and lifetime vaginal, anal, and oral sexual behaviors; history of sexually transmitted diseases and pregnancy; use of hormonal contraceptives; and condom use.
Specimen collection was performed at each 6-month visit by clinicians following previously described protocols [19]. Briefly, cervical cells were collected using an endocervical Cytobrush placed in PreservCyt transport medium (Hologic, Malborough, Massachusetts). Anal cells were also collected in PreservCyt, using a polyethylene terephthalate swab moistened in tap water. Oral cell samples were collected by oral rinse and gargle using Scope mouthwash (Proctor and Gamble, Cincinnati, Ohio). Specimens were stored at −20°C immediately following collection. The methods for polymerase chain reaction (PCR) detection and typing of HPV DNA have been described in detail elsewhere [21].
Additional cervical Cytobrush specimens were collected for liquid-based Papanicolaou (Pap) cytology at enrollment and at least annually thereafter for all returning subjects. Pap results indicating the presence of atypical squamous cells of undetermined significance (ASCUS), a low-grade squamous intraepithelial lesion (LSIL), a high-grade SIL (HSIL), or atypical squamous cells, cannot rule out HSIL (ASC-H) were reviewed by 2 pathologists (A. H. S. and M. W.) blinded to each subject's HPV status.
Statistical Methods
Characteristics of the study cohort were described using frequency distributions and descriptive statistics with exact confidence intervals (CIs). Type-specific prevalence of cervical and anal HPV was assessed prior to first vaccine dose and after completion of the third vaccine dose. Postvaccination HPV infection incidence was assessed for women who were HPV-type negative at their first 6-month study visit occurring after receipt of the third vaccine dose.
To estimate the incremental effect of vaccination (ie, vaccine effect) on detection of vaccine and nonvaccine HPV types, we fit 2 time-dependent regression models by using a previously described generalized estimating equation (GEE) approach that allowed for estimation of incidence rate ratios for multiple types concurrently [22]. We assessed the association between detection of cervical and anal HPV after vaccination, including all visits that occurred ≥6 months after vaccine completion, assuming a Poisson distribution. Exposure time for detection of HPV after vaccination was estimated from the first 6-month study visit following completion of the 3-dose 4vHPV vaccine schedule. Postvaccine detection rates were then compared to HPV detection prior to receipt of first dose. For vaccine-naive subjects who received the first vaccine dose at enrollment, we assumed a risk period of 6 months prior to their first visit. We also attempted to estimate the effect of the vaccine on incident infections (ie, new HPV infections detected after vaccination) by excluding women who presented with detection of a prevalent HPV infection at their first postvaccination visit. Overdispersion was examined and was adjusted for using robust variance through GEE estimation.
In all models, the association between vaccine exposure and detection of vaccine types and nonvaccine HR-HPV types were modeled concurrently by using interaction terms such that the vaccine effect was allowed to vary by HPV type. The correlations within subjects were adjusted for using exchangeable correlation structure. We mutually adjusted for other significant risk factors and confounders selected using a change-in-point estimate criterion for the vaccine effects [23]. Other HR-HPV types detected (ie, HPV31/33/35/39/45/51/52/56/58/59/66/68) were assessed individually and grouped together to reflect the 9vHPV vaccine formulation or other nonvaccine HR-HPV types [24–26].
We also assessed the association between time to completion of the 3-dose 4vHPV vaccine and incident cervical infection with vaccine types (HPV6/11/16/18) following completion of the 3-dose vaccine regimen, using the Kaplan–Meier procedure and multivariable Cox regression models. Time to event was adjusted by considering the midpoint of the interval preceding incident events. For women who received the vaccine prior to enrolling into the study and outside of the MSAHC, we reviewed the New York Citywide Immunization Registry and recorded dates for vaccine doses. Only women with complete immunization records were included. We used a change-in-point estimate criterion to identify potential confounders for the association between vaccine dose delay and incidence of cervical and anal HPV infection. Analyses were conducted with the Stata 14.0 (StataCorp, College Station, Texas) statistical software package.
RESULTS
The study sample included 1139 adolescent and young adult women. At enrollment, 16.0% of subjects had not received the HPV vaccine, and 58.8% had received all 3 doses (Table 1). Study participants were followed prospectively every 6 months, with a median follow-up of 28.5 months (mean ± SD, 30.9 ± 23.1 months). Half of subjects (50.1%) identified themselves as African American, while 41% did not identify with a particular racial group, most of whom reported being of Hispanic ethnicity, which also represented the majority (59.5%) of study subjects. All subjects reported having initiated vaginal intercourse, one third (31.3%) reported anal intercourse, and most (91.6%) had also engaged in oral-to-genital or oral-to-anal sex. At the time of enrollment, most women (64.7%) had had at least 3 sex partners in their lifetime. The median age at first vaginal intercourse was 15 years (range, 10–19 years), and over half (53.0%) had initiated sexual intercourse ≥1 year before vaccination.
Table 1.
Study Cohort Characteristics at Time of Enrollment
| Characteristics | Study Sample, No. (%) (n = 1139) |
|---|---|
| Age at entry, y | |
| 13–14 | 20 (1.8) |
| 15–16 | 201 (17.7) |
| 17–18 | 540 (47.4) |
| 19–21 | 378 (33.2) |
| Race | |
| Black/African American | 571 (50.1) |
| White | 64 (5.6) |
| Asian | 19 (1.7) |
| Native American/Pacific Islander | 18 (1.6) |
| Unspecified race (Hispanic only) | 467 (41.0) |
| Ethnicitya | |
| Non-Hispanic | 442 (38.8) |
| Hispanic | 678 (59.5) |
| Sexual activity | |
| Male vaginal sex partners, lifetime no. | |
| 1 | 199 (17.5) |
| 2 | 203 (17.8) |
| 3–4 | 334 (29.3) |
| 5–9 | 282 (24.8) |
| ≥10 | 121 (10.6) |
| Age at first vaginal intercourse, ya | |
| <14 | 182 (16.0) |
| 14–15 | 540 (47.4) |
| 16–17 | 355 (31.2) |
| ≥18 | 53 (4.7) |
| Sex partners in past 6 mo, no.a | |
| 0 | 47 (4.1) |
| 1 | 626 (55.0) |
| 2 | 259 (22.7) |
| 3–4 | 156 (13.7) |
| ≥5 | 48 (4.2) |
| Anal intercourse evera | |
| No | 773 (67.9) |
| Yes | 357 (31.3) |
| Anal sex partners, lifetime no.b | |
| 1 | 206 (18.1) |
| ≥2 | 84 (7.4) |
| Age at first anal intercourse, yb | |
| <16 | 57 (5.0) |
| ≥16 | 224 (19.7) |
| Oral sexa | |
| Never | 95 (8.3) |
| Ever (given or received) | 1043 (91.6) |
| HPV vaccine doses received at entry, no. | |
| 0 (vaccine naive) | 182 (16.0) |
| 1 | 143 (12.6) |
| 2 | 144 (12.6) |
| 3 | 670 (58.8) |
| Interval between first sex and vaccinationa | |
| ≥1 y before vaccination | 427 (37.5) |
| Within 1 y of vaccination | 176 (15.5) |
| >1 y after vaccination | 473 (41.5) |
| Age at first HPV vaccine dose, ya | |
| <15 | 482 (42.3) |
| ≥15 | 598 (52.5) |
| Pregnancies, no.a | |
| 0 | 734 (64.4) |
| 1 | 221 (19.4) |
| ≥2 | 87 (7.6) |
| History of STDs | |
| None | 718 (63.0) |
| Any | 421 (37.0) |
Abbreviations: HPV, human papillomavirus; STD, sexually transmitted disease.
a Percentage and total no. may not add up to 100% and 1139, respectively, owing to missing values.
b Total number does not add up to 357, owing to missing values.
Compared to subjects who entered the study vaccine naive (ie, prior to receiving their first dose of the 4vHPV vaccine), women who had received at least 1 dose of vaccine prior to enrollment were significantly more likely to have initiated vaginal intercourse at a younger age, to have had anal sex, or to have used hormonal contraceptives and emergency contraception, but were less likely to have had a prior pregnancy or to have acquired a sexually transmitted disease (including chlamydial infection).
Prevalence and Incidence of Cervical and Anal HPV Infection
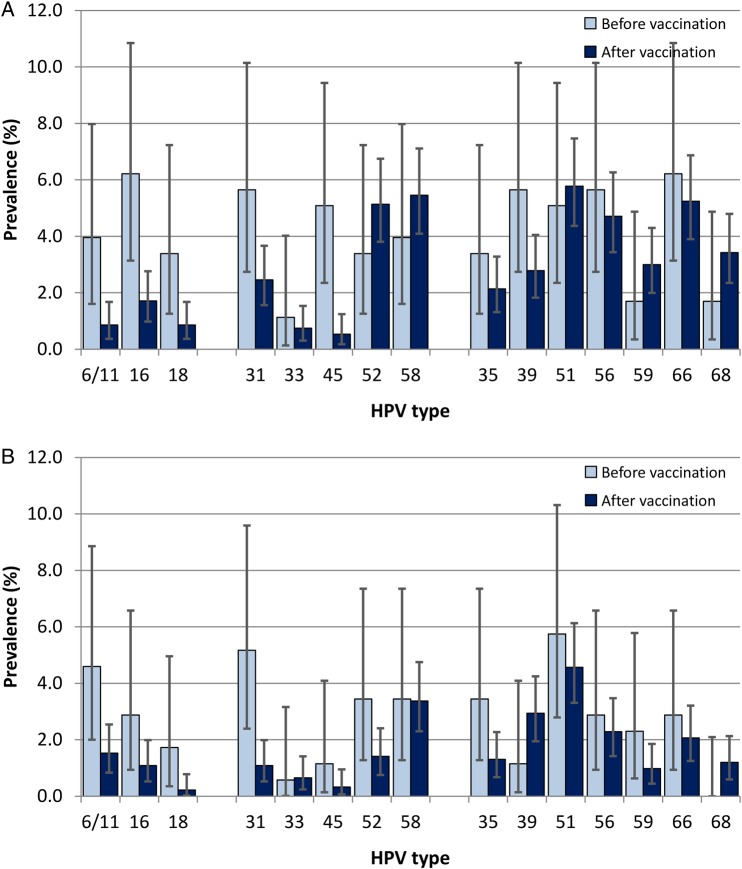
The overall prevalence of any HPV in cervical specimens (including the >40 types tested by our comprehensive assay) was only slightly lower for women who entered the study after having received the 4vHPV vaccine (49.5%), compared to women who were tested prior to vaccination (57.6%), for a difference of 8.1% (95% CI, .2%–16.1%). However, detection of HPV6/11/16/18 was significantly lower after vaccination (3.2%) as compared to before vaccination (12.4%; P < .0001; Figure 1A). When we compared the detection of other HR-HPV types in cervical specimens obtained before versus after 4vHPV vaccination, we found significant declines in the prevalence of HPV16–phylogenetically related type 31 (from 5.7% to 2.5%; P = .0216), and HPV18-related type 45 (from 5.1% to 0.5%; P < .0001). Declines in prevalence among anal specimens after vaccination were also seen for HPV6/11/16/18 (from 8.6% to 2.7%; P = .0001) and HPV31 (from 5.2% to 1.1%; P = .0001; Figure 1B).
Figure 1.
Prevalence of human papillomavirus (HPV) infection at enrollment and at the first 6-month visit after vaccination. Type-specific prevalence and exact 95% confidence intervals for detection of cervical (A) and anal HPV (B) HPV infection among adolescents in specimens obtained before initiation and ≥6 months after completion of the 3-dose HPV vaccine regimen. HPV types 6/11/16/18 are targeted by the quadrivalent vaccine (4vHPV); 31/33/45/52/58, as well as the 4vHPV types, are targeted by the nonavalent vaccine; and high-risk types 35/39/51/56/59/66/68 are not targeted by vaccine.
When we looked at the incidence of HPV6/11/16/18 infection in cervical specimens collected at least 6 months after vaccination, we saw low rates (ie, <10 new infections per 1000 women per year) for HPV6/11/16/18, as well as for HPV31 and HPV33 (Supplementary Figure 1). However, reflecting the continued burden of HPV in this sexually active population, we observed relatively higher rates of infection by other HR-HPV types (range, 13–50 infections per 1000 women-years), with the highest rates observed for HPV52, 39, 51, 56, 66, and 68. Anal HPV incidence rates of <10 infections per 1000 women-years were seen for HPV6/11/16/18 and for HPV31, 33, and 45. In contrast, HPV51 was the most frequent HR-HPV type detected in anal specimens after vaccination, with an estimated rate of 33 new infections per 1000 woman-years. Because of low rates of oral HPV for vaccine and HR-HPV types in our population, we were not able to test for the effect of vaccination on oral HPV prevalence and incidence.
Effect of Vaccination on the Risk of HPV Infection
Table 2 shows the relative effects of vaccination on detecting prevalent and incident cervical and anal infections ≥6 months after vaccination for different HPV types, with mutual adjustment for exposure time, all concurrent types, age, race/ethnicity, lifetime number of (vaginal or anal) sex partners, history of anal sex, recent number of vaginal sex partners, age at first intercourse, and sexual experience at time of vaccination. Compared with vaccine-naive women at enrollment, women vaccinated with the 4vHPV vaccine had significantly lower rates of cervical HPV infection with HPV6/11, 16, 18, and 45. Surprisingly, vaccinated participants had higher relative rates of infection for other HR-HPV types, including HPV52, 59, and 68, although the effects were not significant (ie, P ≥ .05). Similar associations were observed after excluding women who were vaccinated prior to first sexual intercourse, and the significant associations remained (and even strengthened) after excluding women who presented with any HPV infection at their first postvaccination visit (Supplementary Table 1). For anal specimens, the relative rates of infections by HPV6/11/16/18 and 31 were significantly lower for vaccinated individuals (Table 2).
Table 2.
Effect of Human Papillomavirus (HPV) Vaccination on Detection of Cervical and Anal HPV Infection in Adolescent Women
| HPV Type(s) | Cervical HPV Infection |
Anal HPV Infection |
||
|---|---|---|---|---|
| IRR (95% CI) | P Value | IRR (95% CI) | P Value | |
| Any 4vHPV vaccine types | 0.22 (.13–.37) | <.001 | 0.36 (.18–.69) | .002 |
| HPV6/11 | 0.24 (.10–.59) | .002 | 0.37 (.16–.85) | .020 |
| HPV16 | 0.21 (.11–.41) | .000 | 0.33 (.12–.92) | .033 |
| HPV18 | 0.20 (.07–.59) | .003 | 0.33 (.04–2.72) | .303 |
| Added 9vHPV vaccine types | 0.97 (.61–1.54) | .894 | 0.64 (.35–1.19) | .157 |
| HPV31 | 0.44 (.19–1.04) | .062 | 0.21 (.09–.51) | .001 |
| HPV33 | 0.68 (.11–4.18) | .676 | 1.37 (.13–14.4) | .795 |
| HPV45 | 0.26 (.11–.60) | .002 | 0.63 (.14–2.81) | .542 |
| HPV52 | 2.23 (.94–5.26) | .068 | 0.71 (.28–1.81) | .475 |
| HPV58 | 1.49 (.55–4.06) | .433 | 0.96 (.36–2.56) | .937 |
| Other nonvaccine types | 1.18 (.78–1.79) | .438 | 1.18 (.72–1.91) | .516 |
| HPV35 | 1.14 (.45–2.93) | .778 | 0.74 (.29–1.88) | .530 |
| HPV39 | 0.89 (.42–1.86) | .750 | 3.04 (.64–14.3) | .160 |
| HPV51 | 1.25 (.59–2.65) | .559 | 0.80 (.41–1.56) | .515 |
| HPV56 | 0.91 (.44–1.87) | .800 | 1.09 (.40–2.96) | .869 |
| HPV59 | 2.10 (.54–8.25) | .286 | 0.89 (.24–3.34) | .861 |
| HPV66 | 0.90 (.44–1.82) | .764 | 1.10 (.41–2.95) | .848 |
| HPV68 | 4.22 (.83–21.5) | .084 | … | |
Data denote incremental increase in the rate of prevalent and incident HPV detection after vaccination, comparing fully vaccinated women (for at least 6 months) to unvaccinated women. Estimates were derived by generalized estimating equations with Poisson regression with log link and robust variance, mutually adjusting for exposure time, all concurrent types, current age, race/ethnicity, lifetime number of (vaginal or anal) sex partners, history of anal sex, recent number of vaginal sex partners, age at first intercourse, and sexual experience at time of vaccination.
Abbreviations: 4vHPV, quadrivalent HPV vaccine; 9vHPV, nonavalent HPV vaccine; CI, confidence interval; IRR, incidence rate ratio.
Effect of Age at Vaccination and Dose Delay on the Risk of Infection
Despite an active vaccine program with repeated recalls and reminders in place at the MSAHC, delays of ≥12 months between the first and last doses were reported by almost half (43.2%) of study participants. We therefore assessed whether delayed completion of the 3-dose schedule by >6 months from the recommended schedule was associated with increased risk of an incident cervical or anal HPV6/11/16/18 infection. We found moderate but higher risks of incident detection of HPV6/11/16/18 after vaccination in cervical (Figure 2A) and anal (Figure 2B) specimens for women who took ≥12 months (vs <12 months) to complete the 3-dose schedule, if they initiated the vaccine after their 15th birthday.
Figure 2.
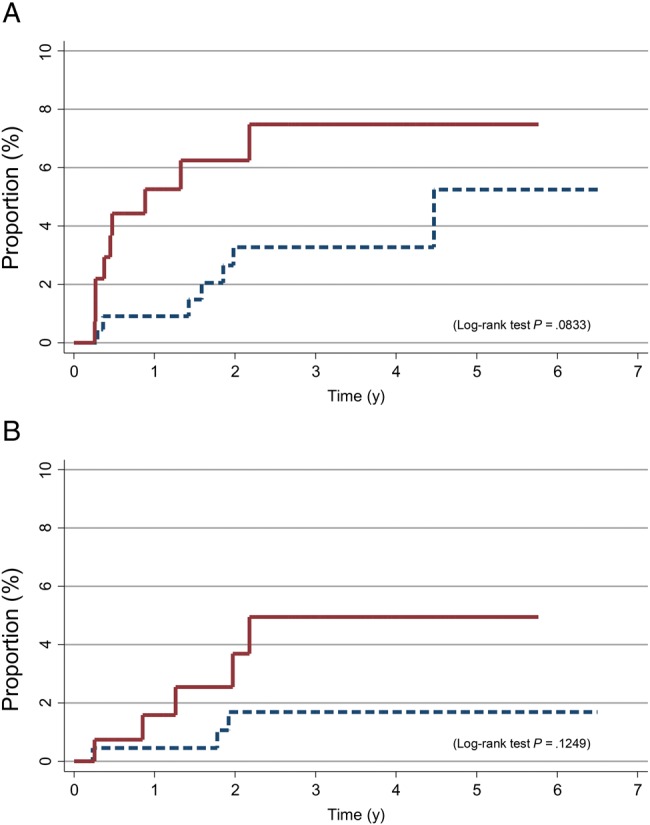
Cumulative proportion of incident human papillomavirus virus (HPV) 6/11/16/18 infection detected in cervical (A) and anal (B) specimens after vaccination among adolescents who initiated the 3-dose HPV vaccination regimen after their 15th birthday, by completion of the regimen in <12 months (dashed lines) or ≥12 months (solid lines).
While we were not able to assess differences between doses 1–2 and 2–3 separately with statistical power, the majority (69.0%) of women who took ≥12 months to complete the 3-dose vaccine schedule were also more likely to delay receipt of their second dose by ≥6 months. We found that women who took ≥12 months to complete the first 2 doses were more likely to acquire an incident HPV6/11/16/18 infection, compared with women who completed all 3 doses within 12 months and even with women who completed the first 2 doses within 12 months but took ≥12 months to complete all 3 doses (Supplementary Figure 2).
The association between dose delay and risk of cervical HPV6/11/16/18 infection appeared to be independent of sexual experience prior to vaccination or other risk factors, as the association remained after multivariate adjustment for age at enrollment, sexual experience at first dose, recent number of sex partners, use of hormonal contraception, and condom use during vaginal sex (Table 3). A nonsignificant increase in hazard risk ratio was also observed for the incidence of anal HPV infection. In contrast, among women aged <15 years when vaccinated, a longer time to completion of the 3-dose vaccine schedule had no association with incident detection of HPV6/11/16/18 in cervical or anal specimens.
Table 3.
Estimated Hazard Rate Ratios (HRRs) of Human Papillomavirus (HPV) Detection, by Age at First Dose and Time to Completion of the 3-Dose Vaccine Regimen
| HPV Characteristic, Time to Completion | HRRa (95% CI), by Age at Vaccination Initiation |
|
|---|---|---|
| ≥15 y | <15 y | |
| Cervical infection | ||
| Due to HPV6/11/16/18 | ||
| <12 mo | 1.0 (reference) | 1.0 (reference) |
| ≥12 mo | 2.9 (1.0–8.0) | 0.8 (.2–4.0) |
| Due to nonvaccine high-risk HPV types | ||
| <12 mo | 1.0 | 1.0 |
| ≥12 mo | 0.9 (.6–1.4) | 0.6 (.4–1.0) |
| Anal infection | ||
| Due to HPV6/11/16/18 | ||
| <12 mo | 1.0 | 1.0 |
| ≥12 mo | 2.2 (.6–8.9) | 0.7 (.2–3.1) |
| Due to nonvaccine high-risk HPV types | ||
| <12 mo | 1.0 | 1.0 |
| ≥12 mo | 0.9 (.6–1.4) | 0.8 (.5–1.2) |
Abbreviation: CI, confidence interval.
a By Cox regression, adjusted for age at enrollment (in years), sexual experience at first dose, recent number of sex partners, use of hormonal contraception, and condom use during vaginal sex.
Effect of Vaccination on the Risk of Cervical Cytological Abnormalities
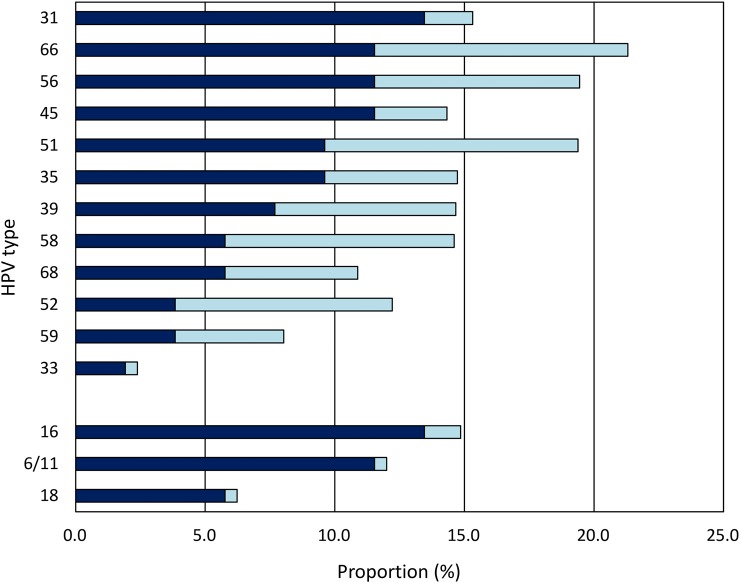
We also assessed the prevalence and incidence of cytological abnormalities in vaccine-naive and vaccinated women. Study subjects were screened annually for cervical lesions, using a liquid-based Pap cytology technique. We found significant differences in the proportion of cervical cytological abnormalities (ASCUS/ASC-H/LSIL/HSIL) positive for HPV6/11/16/18 before versus after vaccination, as well as significant declines in the prevalence of HPV31 and HPV45 (Figure 3).
Figure 3.
Comparison of the proportion of cervical cytologic abnormalities (atypical squamous cells of undetermined significance, a low-grade squamous intraepithelial lesion [SIL], a high-grade SIL [HSIL], or atypical squamous cells, cannot rule out HSIL) detected that were positive for quadrivalent human papillomavirus (HPV) vaccine types (HPV6/11/16/18) and high-risk HPV types (31/33/35/39/45/51/52/56/58/59/66/68) among women tested before HPV vaccination (dark blue bars) and ≥6 months after completing the 3-dose vaccine regimen (light blue bars). *P < .01, **P < .001, and ***P < .0001, by the 2-tailed χ2 test for proportions. This figure is available in black and white in print and in color online.
With respect to the incidence of cervical cytological abnormalities, we found that, whereas no difference in the risk of any cervical abnormality was seen for women aged <15 years versus ≥15 years at time of vaccination overall, restricting to HPV16/18-positive cytological abnormalities did show higher risks for older versus younger vaccinees (Supplementary Figure 3). The risk associations persisted even after adjustment for dose delay, age, vaccine status at entry, sexual experience at time of first dose, detection of HR-HPV types other than HPV16/18 in the cervical specimen, history of chlamydial infection, and history of abnormal Pap smears (Table 4). Older age at vaccination was also significantly associated with the risk of high-grade cervical abnormalities (ASC-H/HSIL), irrespective of HPV type detected, although no association was seen with dose delay.
Table 4.
Effect of Delayed Vaccine Uptake on the Risk of Human Papillomavirus 16/18 (HPV16/18) Positive and High-Grade Cervical Cytological Abnormalities
| Risk Factor | HRRa (95% CI), by HPV16/18 Status and/or Cervical Pap Finding |
|
|---|---|---|
| HPV16/18 Positive, ASCUS + | Any ASC-H/HSIL | |
| Age at first HPV vaccine dose, y | ||
| <15 | 1.0 (reference) | 1.0 (reference) |
| ≥15 | 4.50 (.7–26.0) | 3.80 (1.3–11.0) |
| Time to completion, mob | ||
| <12 | 1.0 (reference) | 1.0 (reference) |
| ≥12 | 2.57 (.9–7.6) | 0.78 (.4–1.7) |
| Interval between first sex and vaccination | ||
| >1 y after vaccination | 1.0 (reference) | 1.0 (reference) |
| ≤1 y before vaccination | 5.09 (1.2–21.4) | 1.38 (.6–3.4) |
| Vaccine doses received at entry, no. | ||
| 0 (vaccine naive) | 1.0 (reference) | 1.0 (reference) |
| ≥1 | 1.43 (.3–6.4) | 1.09 (.4–2.7) |
| Cervical high-risk HPV status | ||
| Negative for non-16/18 HR-HPV type | 1.0 (reference) | 1.0 (reference) |
| Positive for non-16/18 HR-HPV type | 2.80 (1.0–7.8) | 8.02 (3.7–17.5) |
| History of chlamydial infection | ||
| No | 1.0 (reference) | 1.0 (reference) |
| Yes | 1.06 (.4–3.0) | 0.75 (.4–1.5) |
| History of abnormal Pap findings | ||
| No | 1.0 (reference) | 1.0 (reference) |
| Yes | 1.68 (.6–5.1) | 1.19 (.6–2.5) |
| Age (y) | 0.75 (.5–1.1) | 0.59 (.4–.8) |
Abbreviations: ASC-H, atypical squamous cells, cannot rule out high-grade squamous intraepithelial lesion; ASCUS, atypical squamous cells of undetermined significance; CI, confidence interval; HRR, hazard rate ratio; HSIL, high-grade squamous intraepithelial lesion; Pap, Papanicolaou test.
a By time-dependent Cox regression mutually adjusted for all variables listed.
b Time to complete 3-dose HPV vaccine regimen.
DISCUSSION
In this prospective study of high-risk, sexually active, inner-city adolescent women, we show that despite an extremely high prevalence of anogenital HPV infection in unvaccinated subjects, administration of the 4vHPV vaccine was associated with a 60%–80% reduction in detection of cervical and anal HPV types targeted by the vaccine (ie, HPV6/11/16/18). In addition, we saw declines in infection rates for phylogenetically related HR-HPV types 31 and 45 in the cervix and for HPV31 in the anal canal. These findings were in line with observed reductions in population-level prevalence of cervical HPV infection reported following vaccination in US women 14–24 years of age [13]. The reduction in anal HPV detection after vaccination adds to the evidence of the effectiveness of the vaccine [7, 27]. Nevertheless, not all women received equivalent protection. In particular, among adolescents immunized after their 15th birthday, a longer time to compete the 3-dose vaccine schedule was associated with an increased risk of cervical and anal HPV infection.
Although HPV DNA detection is considered an intermediate end point of HPV-associated neoplastic disease, we also assessed the potential impact of vaccination on the risk of cervical cytological abnormalities. We found significant declines in the proportion of cervical samples (ie, ASCUS/LSIL/ASC-H/HSIL) positive for 4vHPV vaccine types and related HR-HPV types 31 and 45 detected after vaccination, compared with before vaccination. In contrast, the prevalence of cytological abnormalities associated with 4vHPV vaccine and related HR-HPV types before and after vaccination remained unchanged. These results differ with findings from a recent study [3] that reported significant declines in the incidence of ASCUS+ findings among vaccinated women, compared with unvaccinated women, followed at other New York City adolescent health facilities. Previous studies have shown significant declines in the incidence of histologically confirmed high-grade cervical lesions regardless of HPV type among vaccinated women [4, 14, 28]. This presumably reflects declines in the incidence of infection with HR-HPV vaccine types (eg, HPV16) [29] responsible for the majority of cervical high-grade disease in young women [30–33].
However, our study also showed that the effect of 4vHPV vaccination was not uniform for all adolescent women. In particular, we observed that older age at vaccination (ie, ≥15 years) was associated with an increased risk of HPV6/11/16/18 infections and associated high-grade cervical cytological abnormalities (ASC-H/HSIL). Recent data indicates that immune responses to the vaccine are greater when initiated before adolescence [34, 35]. In addition, HPV antibody titers measured after HPV vaccination in women aged 16–26 years have been shown to be lower than those in younger women [36–39]. While we did not ascertain prior HPV exposure (eg, by serological analysis) for subjects who entered the study having received at least 1 dose, the associations between the age at vaccination and risk of anogenital HPV6/11/16/18 infection and cervical cytological abnormalities did not appear to be due to differences in sexual experience at time of vaccination. We observed null or even positive associations between the number of recent vaginal sex partners at enrollment (odds ratio [OR], 1.08; 95% CI, .9–1.2), history of anal sex (OR, 1.14; 95% CI, .9–1.5), history of other sexually transmitted diseases (OR, 1.18; 95% CI, .9–1.5), use of hormonal contraception (OR, 1.13; 95% CI, .9–1.4) or condom use (OR, 1.22; 95% CI, .9–1.6), and age at vaccination (ie, vaccination before versus after the 15th birthday). Thus, there is an inherent advantage to immunization in younger women, even if they are at high risk for HPV infection.
In addition to age at vaccination, we also saw significant increases in risks of HPV6/11/16/18 infection among adolescents aged ≥15 years who took longer to complete the 3-dose schedule. The differences did not appear to be associated with sexual history at the time of vaccination. Women who initiated vaccination prior to engaging in sexual intercourse took longer to complete the vaccine (median, 13.2 months) as compared to women who were sexually experienced at the time of the first dose (9.9 months), although this was not significant. In addition, women aged <15 years (vs ≥15 years) at the time of vaccination also took longer to complete the 3-dose schedule (14.3 vs 10.2 months, respectively). However, we were not able to assess the impact of the combined differences in dosing intervals between the first, second, and third doses together. This may be important given evidence from alternate dosing trials, which indicate that a third dose should be administered at least 6 months after the first dose if the interval between the first 2 doses is <5 months [15].
The importance of being compliant with the 3-dose vaccine if immunized after age 14 years would suggest that, in individuals with presumably longer and more extensive prior sexual exposure/infection with HPV, such as those in our study population, there could be some gain in timely vaccination and administration of a booster within 6–12 months because of improved immunogenicity and immune control of existing quiescent or latent HPV infection [34]. Trials of extended dosing schedules (0, 12, and 24 months) have also shown lower antibody titers after vaccination, compared with shorter dosing schedules [34, 36, 37]. In addition, the number of vaccine doses and older age at vaccination have been shown to differentially influence T- and B-cell responses, respectively [34]. As a result, current World Health Organization recommendations are that the 3-dose schedule should be kept if HPV vaccination is initiated after the 15th birthday and that the first and last doses occur no more than 12–15 months apart [6, 15]. Currently, however, there is no evidence to support restarting the doses or revaccinating women with the 9vHPV vaccine if they do not complete the 4vHPV vaccine doses on time [6].
The reported study has strengths and limitations. Among the limitations, it should be noted that because we are specifically studying sexually active adolescent women, our cohort was older than the targeted age for vaccination but covered the age range recommended for catch-up vaccination [6]. In addition, while we attempted to adjust for sexual activity at the time of vaccination as a proxy measure, HPV exposure prior to vaccination was not known for the majority of subjects. Last, given the low incidence of vaccine-type HPV infection after vaccination and the rare occurrence of HSIL, we were not able to confirm the observed associations, using measures of persistent infection or histologically confirmed disease.
Despite these limitations, we find compelling evidence for a real-world benefit of 4vHPV vaccination in sexually active, minority, inner-city adolescent and young adult women against cervical and anal HPV infection with vaccine-targeted types, as well as for HPV31 and HPV45. Nevertheless, we see that delayed completion of the 3-dose vaccine schedule among women immunized at ≥15 years of age is associated with an increased risk of incident infection (or reinfection) with anogenital HPV6/11/16/18 and a risk of associated cytological abnormalities. The implication of these data is that the cohort of women receiving HPV vaccine at older ages will need active screening to prevent cervical cancer.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the participants of this study; the study staff and volunteers Sarah Pickering, Deborah Du, Madelyn Garcia, Natalie Johnson, Stephannie Ratcliff, Emily Bail, Amira El-Behiri, Jessica Kempner, and Devonte Cleghorn, for their time and effort spent enrolling participants and with data entry; Thomas Moody and Kevin Carroll, for the HPV genotyping analyses; Christine Soghomonian, Mindy Ginsberg, Victor Kamensky, and Gregory Rosenblatt, for their assistance with data management; and our study collaborators, Jocelyn M Weiss and Lourdes Oriana Linares, for their help with study management and questionnaire design.
Financial support. This work was supported in part by the National Institute of Allergy and Infectious Diseases (R01 AI072204 to A. D., N. F. S., and R. D. B.), the Icahn School of Medicine at Mount Sinai, and the National Cancer Institute (grant P30 CA013330 to the Albert Einstein Cancer Center).
Potential conflicts of interest. N. F. S. was on advisory boards for Merck, GlaxoSmithKline, and PDS Biotechnology and is an investigator on a research project sponsored in part by Merck's Investigator-Initiated Studies Program. H. D. S. was on an advisory board for GlaxoSmithKline. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Drolet M, Benard E, Boily MC et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malagón T, Drolet M, Boily MC et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:781–9. [DOI] [PubMed] [Google Scholar]
- 3.Hofstetter AM, Ompad DC, Stockwell MS, Rosenthal SL, Soren K. Human Papillomavirus Vaccination and Cervical Cytology Outcomes Among Urban Low-Income Minority Females. JAMA Pediatr 2016; 170:445–52. [DOI] [PubMed] [Google Scholar]
- 4.Munoz N, Kjaer SK, Sigurdsson K et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010; 102:325–39. [DOI] [PubMed] [Google Scholar]
- 5.Joura EA, Giuliano AR, Iversen OE et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372:711–23. [DOI] [PubMed] [Google Scholar]
- 6.Petrosky E, Bocchini JA Jr, Hariri S et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015; 64:300–4. [PMC free article] [PubMed] [Google Scholar]
- 7.Garland SM, Hernandez-Avila M, Wheeler CM et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–43. [DOI] [PubMed] [Google Scholar]
- 8.Vesikari T, Brodszki N, van Damme P et al. A Randomized, Double-Blind, Phase III Study of the Immunogenicity and Safety of a 9-Valent Human Papillomavirus L1 Virus-Like Particle Vaccine (V503) Versus Gardasil(R) in 9–15-Year-Old Girls. Pediatr Infect Dis J 2015; 34:992–8. [DOI] [PubMed] [Google Scholar]
- 9.Paavonen J. Baseline demographic characteristics of subjects enrolled in international quadrivalent HPV (types 6/11/16/18) vaccine clinical trials. Curr Med Res Opin 2008; 24:1623–34. [DOI] [PubMed] [Google Scholar]
- 10.Reagan-Steiner S, Yankey D, Jeyarajah J et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years--United States, 2014. MMWR Morb Mortal Wkly Rep 2015; 64:784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman M, McGrath CJ, Hirth JM, Berenson AB. Age at HPV vaccine initiation and completion among US adolescent girls: trend from 2008 to 2012. Vaccine 2015; 33:585–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stokley S, Jeyarajah J, Yankey D et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014--United States. MMWR Morb Mortal Wkly Rep 2014; 63:620–4. [PMC free article] [PubMed] [Google Scholar]
- 13.Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV After Introduction of the Vaccination Program in the United States. Pediatrics 2016; 137:1–9. [DOI] [PubMed] [Google Scholar]
- 14.Brotherton JML, Malloy M, Budd AC, Saville M, Drennan KT, Gertig DM. Effectiveness of less than three doses of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia when administered using a standard dose spacing schedule: Observational cohort of young women in Australia. Papillomavirus Res 2015; 1:59–73. [Google Scholar]
- 15.Human papillomavirus vaccines: WHO position paper, October 2014—Recommendations. Vaccine 2015; 33:4383–4. [DOI] [PubMed] [Google Scholar]
- 16.Haupt RM, Wheeler CM, Brown DR et al. Impact of an HPV6/11/16/18 L1 virus-like particle vaccine on progression to cervical intraepithelial neoplasia in seropositive women with HPV16/18 infection. Int J Cancer 2011; 129:2632–42. [DOI] [PubMed] [Google Scholar]
- 17.Olsson SE, Kjaer SK, Sigurdsson K et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Human Vaccines 2009; 5:696–704. [DOI] [PubMed] [Google Scholar]
- 18.Kann L, Kinchen S, Shanklin SL et al. Youth risk behavior surveillance--United States, 2013. MMWR Suppl 2014; 63:1–168. [PubMed] [Google Scholar]
- 19.Braun-Courville DK, Schlecht NF, Burk RD et al. Strategies for conducting adolescent health research in the clinical setting: the Mount Sinai Adolescent Health Center HPV experience. J Pediatr Adolesc Gynecol 2014; 27:e103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlecht NF, Burk RD, Nucci-Sack A et al. Cervical, anal and oral HPV in an adolescent inner-city health clinic providing free vaccinations. PLoS One 2012; 7:e37419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castle PE, Schiffman M, Gravitt PE et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol 2002; 68:417–23. [DOI] [PubMed] [Google Scholar]
- 22.Xue X, Gange SJ, Zhong Y et al. Marginal and mixed-effects models in the analysis of human papillomavirus natural history data. Cancer Epidemiol Biomarkers Prev 2010; 19:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenland S, Robins JM. Identifiability, exchangeability, and epidemiological confounding. Int J Epidemiol 1986; 15:413–9. [DOI] [PubMed] [Google Scholar]
- 24.Munoz N, Bosch FX, de Sanjose S et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–27. [DOI] [PubMed] [Google Scholar]
- 25.Clifford GM, Gallus S, Herrero R et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 2005; 366:991–8. [DOI] [PubMed] [Google Scholar]
- 26.Bouvard V, Baan R, Straif K et al. A review of human carcinogens? Part B: biological agents. Lancet Oncol 2009; 10:321–2. [DOI] [PubMed] [Google Scholar]
- 27.Kreimer AR, Gonzalez P, Katki HA et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol 2011; 12:862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith LM, Strumpf EC, Kaufman JS, Lofters A, Schwandt M, Levesque LE. The early benefits of human papillomavirus vaccination on cervical dysplasia and anogenital warts. Pediatrics 2015; 135:e1131–40. [DOI] [PubMed] [Google Scholar]
- 29.Hariri S, Unger ER, Schafer S et al. HPV type attribution in high-grade cervical lesions: assessing the potential benefits of vaccines in a population-based evaluation in the United States. Cancer Epidemiol Biomarkers Prev 2015; 24:393–9. [DOI] [PubMed] [Google Scholar]
- 30.Mesher D, Cuschieri K, Hibbitts S et al. Type-specific HPV prevalence in invasive cervical cancer in the UK prior to national HPV immunisation programme: baseline for monitoring the effects of immunisation. J Clin Pathol 2015; 68:135–40. [DOI] [PubMed] [Google Scholar]
- 31.Brotherton JM, Tabrizi SN, Garland SM. Does HPV type 16 or 18 prevalence in cervical intraepithelial neoplasia grade 3 lesions vary by age? An important issue for postvaccination surveillance. Future Microbiol 2012; 7:193–9. [DOI] [PubMed] [Google Scholar]
- 32.Baandrup L, Munk C, Andersen KK, Junge J, Iftner T, Kjaer SK. HPV16 is associated with younger age in women with cervical intraepithelial neoplasia grade 2 and 3. Gynecol Oncol 2012; 124:281–5. [DOI] [PubMed] [Google Scholar]
- 33.Porras C, Rodriguez AC, Hildesheim A et al. Human papillomavirus types by age in cervical cancer precursors: predominance of human papillomavirus 16 in young women. Cancer Epidemiol Biomarkers Prev 2009; 18:863–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smolen KK, Gelinas L, Franzen L et al. Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine 2012; 30:3572–9. [DOI] [PubMed] [Google Scholar]
- 35.Reisinger KS, Block SL, Lazcano-Ponce E et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–9. [DOI] [PubMed] [Google Scholar]
- 36.Neuzil KM, Canh do G, Thiem VD et al. Immunogenicity and reactogenicity of alternative schedules of HPV vaccine in Vietnam: a cluster randomized noninferiority trial. JAMA 2011; 305:1424–31. [DOI] [PubMed] [Google Scholar]
- 37.Dobson SR, McNeil S, Dionne M et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309:1793–802. [DOI] [PubMed] [Google Scholar]
- 38.Block SL, Nolan T, Sattler C et al. Comparison of the Immunogenicity and Reactogenicity of a Prophylactic Quadrivalent Human Papillomavirus (Types 6, 11, 16, and 18) L1 Virus-Like Particle Vaccine in Male and Female Adolescents and Young Adult Women. Pediatrics 2006; 118:2135–45. [DOI] [PubMed] [Google Scholar]
- 39.Apter D, Wheeler CM, Paavonen J et al. Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin Vaccine Immunol 2015; 22:361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.