Abstract
Cryptosporidiosis, caused by the apicomplexan parasite Cryptosporidium parvum, is a diarrheal disease that has produced a large global burden in mortality and morbidity in humans and livestock. There are currently no consistently effective parasite-specific pharmaceuticals available for this disease. Bumped kinase inhibitors (BKIs) specific for parasite calcium-dependent protein kinases (CDPKs) have been shown to reduce infection in several parasites having medical and veterinary importance, including Toxoplasma gondii, Plasmodium falciparum, and C. parvum. In the present study, BKIs were screened for efficacy against C. parvum infection in the neonatal mouse model. Three BKIs were then selected for safety and clinical efficacy evaluation in the calf model for cryptosporidiosis. Significant BKI treatment effects were observed for virtually all clinical and parasitological scoring parameters, including diarrhea severity, oocyst shedding, and overall health. These results provide proof of concept for BKIs as therapeutic drug leads in an animal model for human cryptosporidiosis.
Keywords: Cryptosporidium, cryptosporidiosis, bumped kinase inhibitor, animal model, therapeutic, CpCDPK-1, clinical evaluation, oocyst shedding
Cryptosporidial infections (including those due to Cryptosporidium parvum and Cryptosporidium hominis) are among the most prevalent causes of diarrhea in humans and agriculturally important livestock species worldwide [1–5]. Infection in humans may be anthroponotic, as occurs with C. hominis, or zoonotic, as occurs for some C. parvum infections acquired from calves [6]. In immunocompromised individuals, such as those with AIDS, cryptosporidial diarrhea may become persistent and life threatening [7, 8]. In immunocompetent individuals, Cryptosporidium infection causes watery diarrhea, often continuing beyond 10 days from the onset [7]. Cryptosporidium is a major cause of diarrhea in children in developing countries and is associated with stunting, irreversibly impaired cognitive development, and decreased fitness [9–11]. With improved diagnostic data from the Global Enteric Multicenter Study (GEMS), Cryptosporidium infections are now linked to approximately 25% of moderate-to-severe diarrheal episodes in children aged <2 years in resource-limited countries, second only to rotavirus [3]. Cryptosporidium was the only pathogen significantly associated with diarrheal mortality among toddlers aged 12–23 months in resource-limited settings worldwide [3]. The negative sequelae of cryptosporidiosis are debilitating, extending beyond simple uncomplicated enteric disease, and affect the most vulnerable human populations worldwide.
Despite the disproportionately large burden of disease attributable to this parasite, there are currently no approved vaccines available and no parasite-specific drugs to treat the disease. Nitazoxanide is the only Food and Drug Administration–approved drug, albeit marginally effective, for treating cryptosporidiosis in humans and has no or variable therapeutic or prophylactic efficacy against C. parvum in calves [4, 12, 13]. Treatment with nitazoxanide actually prolonged and exacerbated diarrhea in some Cryptosporidium-infected calves [12]. In humans, nitazoxanide was shown in a randomized trial to reduce the duration of cryptosporidial diarrhea by approximately 2 days in immunocompetent individuals [14]. However, nitazoxanide has limited efficacy in immunocompromised individuals and is associated with an unacceptably high relapse rate [15]. Further, the mechanism of action of nitazoxanide against Cryptosporidium is unknown, and it is speculated that it may act via a nonspecific immunomodulatory effect [7, 16]. In malnourished children, a survival advantage was shown with nitazoxanide treatment, but the response rate was poor [7, 8]. Furthermore, randomized trials show no benefit with nitazoxanide treatment in patients with AIDS [8, 17]. More-effective parasite-specific drugs are clearly needed for treating cryptosporidiosis.
In calves, C. parvum is considered to be among the 4 leading infectious causes of diarrhea in calves <1 month of age [4, 18]. The ubiquity of C. parvum throughout the world and its economic impact on production have been clearly established and represent important public health and animal welfare concerns [4, 18]. There are presently no consistently effective drugs available for treatment of C. parvum infection in livestock [4, 19]. Halofuginone is approved for prevention of Cryptosporidium infection in calves in Europe but not in the United States because its efficacy is controversial and it has potential toxicity liabilities [4, 19, 20]. Paromomycin efficacy in infected calves is variable, but the drug has proven to be a useful positive control in rodent models [4, 21].
Most recently, the calcium-dependent protein kinase 1 of C. parvum (CpCDPK1) has shown promising potential as a drug target in treatment of cryptosporidiosis, as well as other diseases caused by apicomplexan parasites [22–26]. Apicomplexan CDPK homologs have crucial roles in host cell invasion, micronemal secretion, and gliding motility [23, 27–30]. The C. parvum CDPK1 differs from mammalian protein kinases in having a glycine gatekeeper residue in the adenosine triphosphate–binding pocket, making CDPK1 a high-affinity target for highly selective bumped kinase inhibitors (BKIs). BKIs project a bulky aromatic substituent (a “bump”) from the C-3 position into the hydrophobic pocket next to the gatekeeper residue [22]. Large numbers of BKIs have been synthesized, differing mainly by R1 and R2 groups displayed from a few core scaffolds, which selectively target CDPK orthologs of multiple apicomplexan parasites [22, 25, 26, 31]. Treatment with BKI-1294 reduced C. parvum infection in vitro and eliminated infection in 6 of 7 immunodeficient mice [23]. In a preliminary report using a semiquantitative method to measure oocyst output, treatment of C. parvum–infected calves with BKI-1294 commencing 1 hour after challenge reduced oocyst shedding; however, the effect on diarrhea was inconclusive, and it was determined that further studies were needed [24].
The present study assessed the efficacy of 3 BKIs in neonatal calves experimentally infected with C. parvum, using defined clinical and parasitological outcome parameters. Neonatal calves provide an excellent animal model for human cryptosporidiosis. Calves are most likely to become infected with C. parvum in the first 4 weeks of life, a period during which they are functional monogastrics [18, 32]. They develop clinical illness and profuse diarrhea following experimental infection, with intestinal infection sites and lesions being virtually identical to what is observed in humans [18, 33]. Significant treatment effects were observed with each BKI for virtually all parameters, including diarrhea severity, oocyst shedding, and clinical health. These results provide proof of concept for BKIs as potential dual-use therapeutic drug leads in a reliable animal model closely simulating human cryptosporidiosis, as well as in an agriculturally important livestock species.
MATERIALS AND METHODS
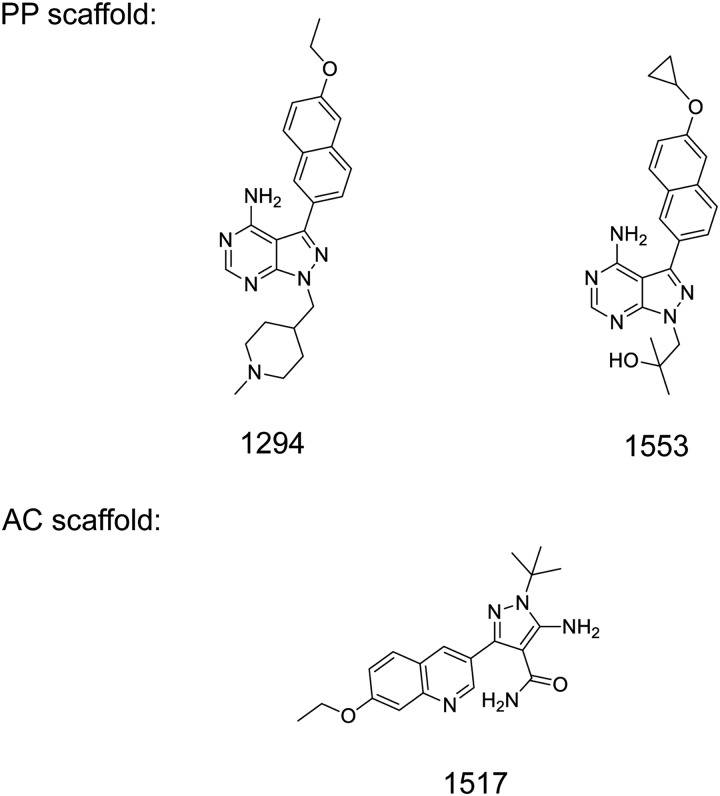
The propagation of C. parvum (Iowa isolate) in calves and oocyst purification [34–37], synthesis of BKIs [22, 25, 27, 38], in vitro CpCDPK1 enzyme assay [22, 25, 38], in vitro determination of C. parvum BKI sensitivity [22, 25, 38, 39], neonatal mouse [36, 40] and calf models of C. parvum infection [37, 41–43], and pharmacologic measurement of BKI plasma and stool levels and plasma protein binding have all been previously described [27, 44]. BKI-1294 and BKI-1553 were synthesized on the pyrazolo [2,3-d] pyrimidine scaffold, while BKI-1517 was synthesized on a 5-aminopyrazole-4-carboxamide scaffold [25] (Figure 1). Previously published details of these methods are specified in the Supplementary Materials.
Figure 1.
Chemical structures of bumped kinase inhibitor 1294 (BKI-1294), BKI-1553, and BKI-1517. Abbreviations: AC, 5-aminopyrazole-4-carboxamide; PP, pyrazolo [2,3-d] pyrimidine.
RESULTS
BKIs Are Effective in Reducing C. parvum Infection Levels in the Neonatal Mouse Model
Previous experiments have shown that BKI-1294 can cure C. parvum infection in immunodeficient SCID-beige mice [23]. To rapidly and economically screen prospective BKIs for evaluation in the calf model, newborn SPF ICR mice were infected with C. parvum and treated orally with individual BKIs. Three of the most efficacious BKIs were identified using this model. BKI-1294, BKI-1517, and BKI-1553 significantly reduced infection levels as compared to controls (P < .0001; Supplementary Table 1). Percentage reductions in the infection levels ranged from 37% to 56%, with BKI-1294 being the most efficacious. These compounds were highly efficient at inhibiting the CpCDPK1 enzyme and C. parvum growth in vitro (Supplementary Table 1). The pharmacological data for these BKIs in adult BALB/c mice indicate that BKI-1517 and BKI-1553 have improved systemic peak plasma concentrations and plasma exposures (measured as the area under the curve) as compared to BKI-1294 (Supplementary Table 1). BKI-1553 is more tightly bound to plasma proteins than the other 2 BKIs. BKI-1553 had the best systemic exposure (>4-fold greater than for BKI-1517), largely because BKI-1517 is cleared more rapidly. However, in this mouse model, the fecal concentrations of BKI-1294 were superior to those of BKI-1517 and BKI-1553. Based on these results, testing in the calf model of cryptosporidiosis was deemed appropriate, and we chose to begin with BKI-1294.
BKI-1294 Every Other Day Yields a Marginal Clinical Response in C. parvum–Infected Calves Despite a Significant Reduction in Parasite Propagation
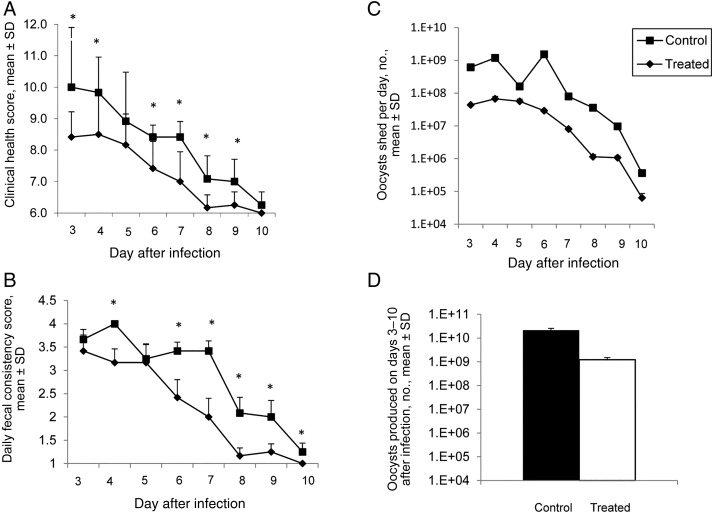
Allometric modeling of BKI-1294 was used to determine the dosage for calves. In brief, oral clearance values in mice (1.4 mL/min) and rats (2.8 mL/min) were determined for BKI-1294. The clearance in these 2 species was then used to extrapolate the oral clearance rate in calves, using the equation CL = 8.6*BW0.4933. We estimated that a plasma value of >1 µM would be necessary for efficacy. By extrapolating from the volume of distribution measured in mice and rats, the volume of distribution in calves was predicted. This led to the calculation that a 10-mg/kg dose given every other day would give plasma values of >1 µM for the duration of the experiment. Thus, calves received 1 dose of 10 mg/kg orally on days 2, 4, 6, and 8 days after infection. BKI-1294 significantly improved overall clinical health scores on days 3, 4, and 6–9 after therapy began (P < .05; Figure 2A) and for the duration of the trial when compared to controls (P < .001 for days 3–10 after infection; data not shown). In addition, total urine output by BKI-1294–treated calves (44.6 L) was significantly greater than that for controls (33.5 L), consistent with less dehydration in treated calves. None of the calves gained weight; however, treated calves lost less weight by the end of the trial (decreases, 3.6% for the BKI-1294 group and 7.2% for controls). Fecal consistency was the main parameter that differed between treated and control calves; treated calves had significantly firmer stools on days 4 and 6–10 after infection (P < .05; Figure 2B). The mean total fecal volume for the duration of the trial was lower in treated calves as compared to controls, but the difference was not significant, owing to skewing by large standard deviations (data not shown). This observation was unfortunate as fecal volume is an important parameter in evaluating diarrhea severity. In contrast, BKI-1294–treated calves shed significantly fewer oocysts daily on all days after infection, starting the first day after therapy (P < .005; Figure 2C), and there was a 1.24 log10 (94%) reduction in the mean total oocyst count for the duration of the trial when compared to controls (2.16 × 1010 vs 1.23 × 109; P < .05; Figure 2D), indicating a substantial impact on parasite propagation.
Figure 2.
Bumped kinase inhibitor 1294 (BKI-1294) treatment every other day at 10 mg/kg produces a marginal clinical response in Cryptosporidium parvum–infected calves despite a significant reduction in parasite propagation. A, Daily clinical health scores were significantly better in treated calves on days 3, 4, and 6–9 after infection (asterisks), compared with values for control calves (P < .05). B, Daily fecal consistency scores were significantly better in treated calves on days 4 and 6–10 after infection (asterisks), compared with values for control calves (P < .05). C, Daily oocyst shedding was significantly reduced for all days after infection in treated calves as compared to control calves (P < .005). D, The total number of oocysts shed over the duration of the trial was significantly reduced in treated calves as compared to control calves (P < .05). Data are for 6 control calves and 6 BKI-1294–treated calves. Scores for clinical health, fecal consistency, and oocyst counts (daily and total) were examined for significant differences by the 1-tailed Student t test. Abbreviation: SD, standard deviation.
The calf pharmacokinetic data indicated that systemic and stool BKI-1294 levels on the second day after dosing were subtherapeutic (ie, <1 µM, the 90% effective concentration), essentially zero. We thus changed the BKI regimen to dosing at 12-hour intervals for subsequent calf experiments.
Receipt of BKIs Twice Daily Improves Clinical Health and Reduces Diarrhea Severity and Parasite Propagation in Calves Having an Established C. parvum Infection
This experimental design evaluated BKI-1294, BKI-1517, and BKI-1553 in repeat cohorts comprising 2 treated and 2 control calves, resulting in 6 calves in each treatment group and 18 controls. Clinical health, diarrhea, and parasitological outcomes were significantly improved by administering BKIs every 12 hours for 5 days starting on day 2 after infection.
Improvement in Clinical Health
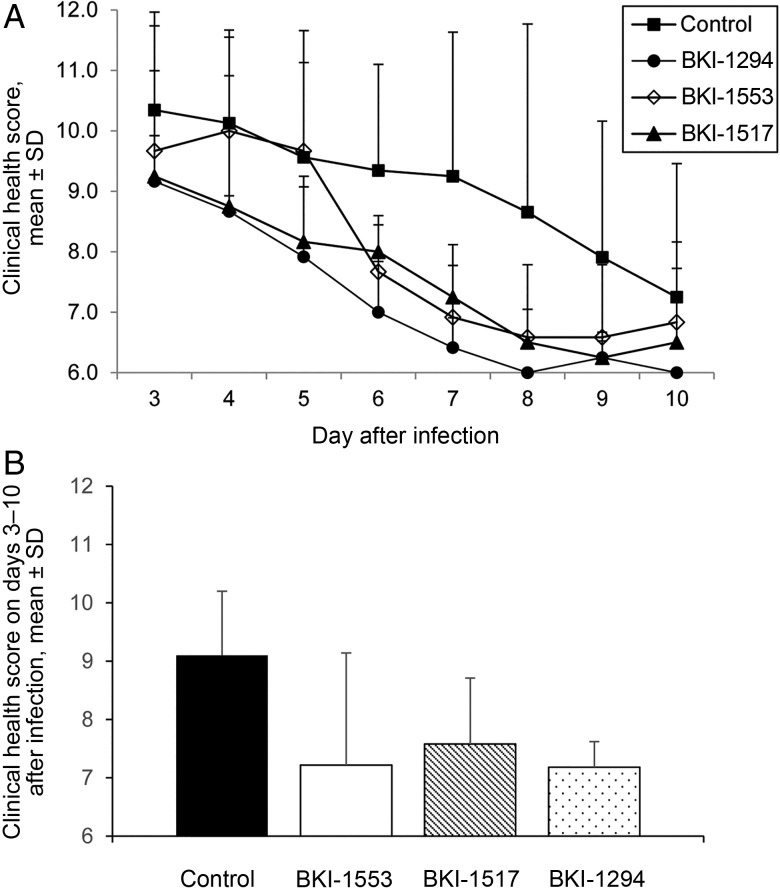
BKI-1294–treated calves had significantly better mean daily clinical health scores on days 4–9 after infection (P < .05), starting 2 days after therapy began, compared with controls (Figure 3A). The other BKI-treated calves had significantly better mean daily clinical health scores for fewer days than controls (P < .05 for days 5–9 after infection for BKI-1517 recipients, and P < .05 for days 6 and 7 after infection for BKI-1553 recipients; Figure 3A). When the 3 BKI-treated calf groups were compared to each other, BKI-1294–treated calves had significantly better mean daily clinical health scores than BKI-1553–treated calves on days 4 and 5 after infection (P < .05) and BKI-1517–treated calves on days 6–8 after infection (P < .05). There were no significant differences between mean daily clinical health scores for BKI-1553– and BKI-1517–treated calves. For mean clinical health scores for the duration of the trial, all 3 BKI-treated calf groups had significantly better scores than controls (P < .005). The mean clinical health score for the duration of the trial for BKI-1294–treated calves was significantly better than for BKI-1553–treated calves (P < .05) but not BKI-1517–treated calves (Figure 3B). Collectively, these data indicate that all 3 BKIs helped alleviate the clinical symptoms of cryptosporidiosis, with BKI-1294 and BKI-1517 being somewhat more effective than BKI-1553. None of the calves, treated or control, gained weight by the end of the trial (day 10 after infection); however, each of the 3 BKI-treated calf groups lost less weight than the control calves, although the differences were not significantly different (decreases, 3.7% for the BKI-1294–treated group, 3.2% for the BKI-1517–treated group, 0.5% for the BKI-1553–treated group, and 5.5% for controls). There was no significant difference in total urine output in treated versus control calves (data not shown).
Figure 3.
Bumped kinase inhibitors (BKIs) administered twice daily at 5 mg/kg improve clinical health scores in calves having an established C. parvum infection, both daily and for the duration of the trial. A, Daily clinical health scores were significantly better in BKI-treated calves as compared to control calves on days 6 and 7 after infection (for BKI-1553; P < .05); days 5–7 and 9 after infection (for BKI-1517; P < .05); and days 4–9 after infection (for BKI-1294; P < .05). BKI-1294 treatment produced better clinical health scores than BKI-1553 on days 4 and 5 after infection (P < .05) and BKI-1517 on days 6–8 after infection (P < .05). No significant differences were seen between BKI-1553 and BKI-1517. B, Mean clinical health scores for the duration of the trial were significantly better in BKI-1294–, BKI-1517–, and BKI-1553–treated calves, compared with control calves (P < .005). The mean clinical health score for the duration of the trial was significantly better in BKI-1294–treated calves as compared to BKI-1553–treated calves (P < .05). Data are for 18 control calves and 6 treated calves per BKI. Clinical health scores were examined for significant differences by the 1-tailed Student t test. Control, n = 18 calves. Abbreviation: SD, standard deviation.
Reduction of Diarrhea Severity
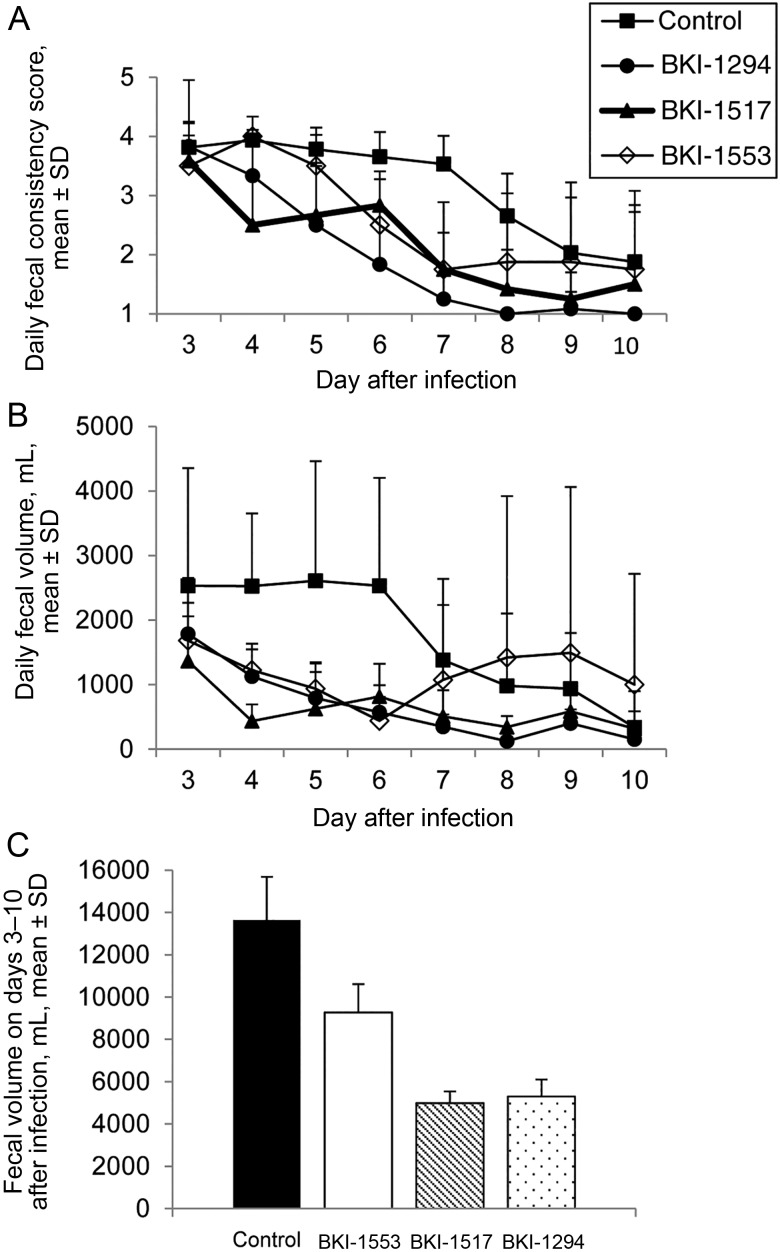
Of the primary clinical parameters evaluated, fecal consistency had the most variability; therefore, this parameter was analyzed separately. Fecal consistency scores provide one index of the duration and severity of diarrhea caused by C. parvum. The duration of diarrhea appeared to be similar for all BKI-treated calves (3–4 days), reflecting a reduced duration as compared to that for control calves, which all remained diarrheic or had loose stools to the end of the experiment (10 days after infection). Diarrhea was less severe in calves treated with all BKIs, compared with controls. BKI-1294 and BKI-1517 had the greatest effect in reducing the severity of diarrhea, with fecal consistency scores significantly better than those for control on days 4–10 (P < .05) and 4–9 (P < .005) after infection, respectively; scores among BKI-1553 recipients were significantly better than those for controls on days 6–8 after infection (P < .005; Figure 4A). Overall, BKI-1294 had the greatest effect in improving fecal consistency scores (Figure 4A).
Figure 4.
Bumped kinase inhibitor (BKI) treatments reduce diarrhea severity in calves with an established Cryptosporidium parvum infection. A, Daily fecal consistency scores were significantly better for BKI-treated calves, compared with those for control calves, on days 6–8 (for BKI-1553; P < .005), 4–9 (for BKI-1517; P < .005), and 4–10 (for BKI-1294; P < .05) after infection. BKI-1294 treatment resulted in better daily fecal consistency scores than BKI-1553 on days 4, 5, 8, and 9 after infection (P < .05) and BKI-1517 on days 6–8 after infection (P < .05). BKI-1517 treatment resulted in better daily fecal consistency scores than BKI-1553 on days 4, 5, and 9 after infection (P < .05). B, Daily fecal volume was significantly reduced in all BKI-treated calves, compared with control calves. BKI-1294 had the greatest effect on reducing diarrhea volume (on days 4–8 after infection; P < .05), followed by BKI-1517 and BKI-1553 (on days 4–6 after infection; P < .05). C, Total fecal volume for the duration of the trial was significantly reduced in calves treated with BKI-1517 or BKI-1294 (P < .05) but not those treated with BKI-1553, compared with control calves. Data are for 18 control calves and 6 calves per BKI. Fecal consistency scores and fecal volumes (daily and total for duration of the trial) were examined for significant differences by the 1-tailed Student t test. Abbreviation: SD, standard deviation.
There was also a clear reduction in diarrhea severity in BKI-treated calves based on daily fecal volumes. Even though all calves (treated and control) developed diarrhea, treated calves had a significantly lower daily volume from the onset of diarrhea (on day 4 after infection) until days 6–8 after infection (Figure 4B). Paralleling fecal consistency scores, BKI-1294 had the greatest effect in reducing daily diarrhea volume (P < .05 for days 4–8 after infection), followed by 1517 and 1553 (P < .05 for days 4–6 after infection), compared with controls (Figure 4B). Additionally, total fecal volume for the duration of the trial was significantly lower in calves treated with BKI-1517 or BKI-1294 (P < .05) but not with BKI-1553, compared with controls (Figure 4C).
Reduction of Fecal Oocyst Shedding
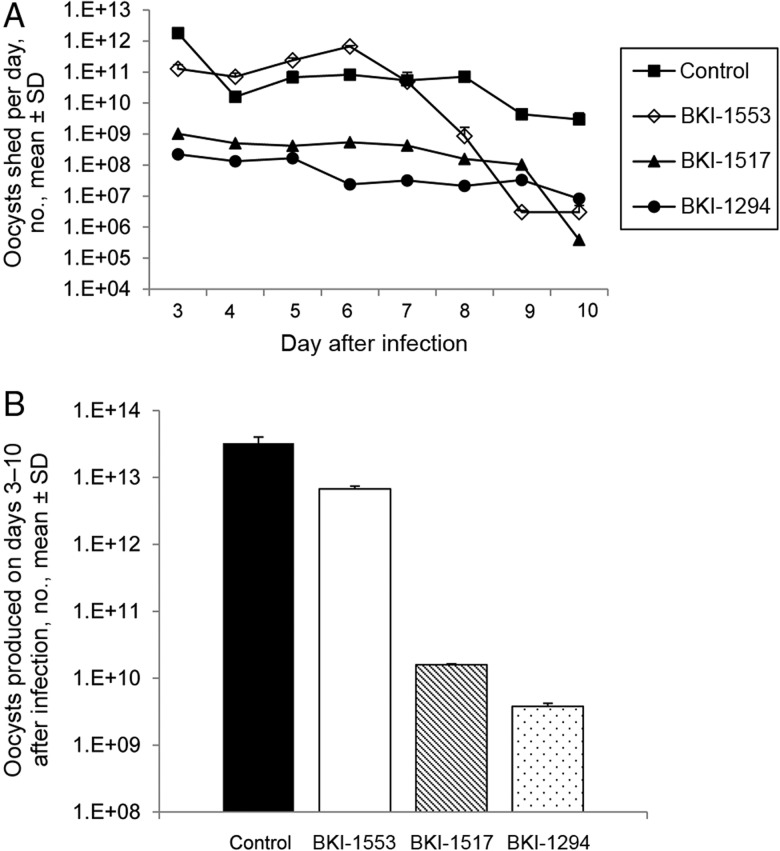
The mean daily number of oocysts shed was significantly lower for calves treated with BKI-1294 or BKI-1517 on all days after therapy began (ie, days 3–10 after infection; P < .05), compared with controls (Figure 5A). The mean daily number of oocysts shed for BKI-1553–treated calves was significantly lower on days 3 and 8–10 after infection (P < .05; Figure 5A). Of the 3 BKIs tested, BKI-1294 was the most effective in reducing oocyst shedding, notably during the peak shedding period (ie, days 3–7 after infection; P < .005). For the mean total oocyst number shed over the duration of the trial, there was a 3.93 log10 (99.9%) reduction in BKI-1294–treated calves and a 3.31 log10 (99.9%) reduction in 1517-treated calves, compared with controls (P < .05; Figure 5B), indicating a dramatic impact on parasite propagation, with BKI-1294 having a greater effect than BKI-1517 (P < .005; Figure 5B).
Figure 5.
Bumped kinase inhibitor (BKI) treatments reduce fecal oocyst shedding in calves having an established Cryptosporidium parvum infection. A, Daily oocyst shedding was significantly reduced in BKI-1294– and BKI-1517–treated calves on days 3–10 after infection (P < .05), compared with values for control calves. Daily oocyst shedding was significantly reduced in BKI-1553–treated calves on days 3 and 8–10 after infection (P < .05). B, The total number of oocysts shed over the duration of the trial was significantly reduced in BKI-1294–treated calves and BKI-1517–treated calves but not BKI-1553–treated calves, compared with control calves (P < .05), with BKI-1294 having a greater effect than BKI-1517 (P < .005). Data are for 18 control calves and 6 calves per BKI. Oocyst counts (daily and total) were examined for significant differences by the 1-tailed Student t test. Abbreviation: SD, standard deviation.
Pharmacokinetics
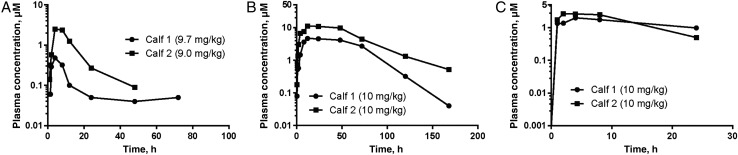
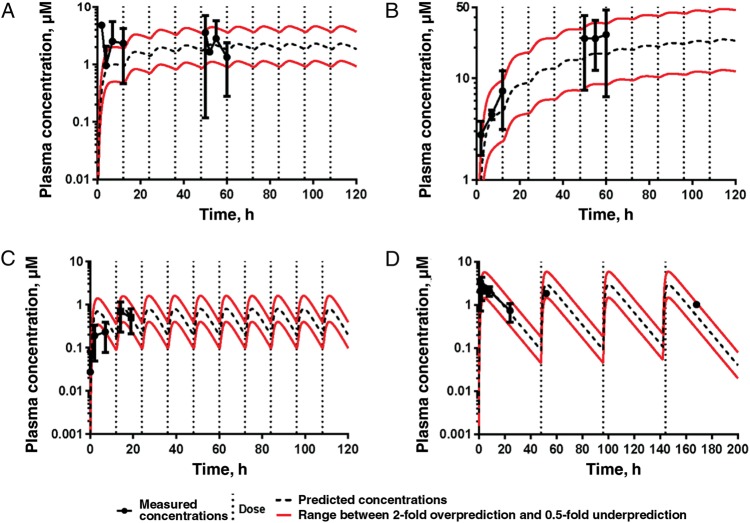
Detailed single-dose pharmacokinetics of plasma levels were obtained for all 3 compounds administered at 9–9.7 mg/kg (BKI-1517) or 10 mg/kg (BKI-1553 and BKI-1294) in uninfected newborn calves (Figure 6). All 3 BKIs gave significant exposures, but BKI-1517 appeared to give more variable exposure than BKI-1294 and BKI-1553. Both plasma and stool BKI values were obtained during the treatment course. Stool values were measured in 24-hour pooled stool specimens and did not vary substantially during the 5 days of dosing, so they are presented as a single mean value (±SD; Supplementary Table 2). Plasma levels during the experiment were mapped onto values predicted from the single-dose pharmacokinetics and fell within 2-fold of the predicted values (Figure 7). Plasma values of BKI-1553 were clearly the highest and appeared to build over the course of the experiment. Plasma values of BKI-1294 were the lowest. This was also the case for stool levels (Supplementary Table 2).
Figure 6.
Two uninfected calves received 1 oral dose of bumped kinase inhibitor 1517 (BKI-1517; A), 2 received 1 oral dose of BKI-1553 (B), and 2 received 1 oral dose of BKI-1294 (C). Noncompartmental analysis of the average measured BKI plasma concentrations was performed with Phoenix WinNonlin.
Figure 7.
Phoenix WinNonlin was used to predict the plasma concentrations of bumped kinase inhibitors (BKIs) after delivery of multiple doses of BKI-1517 (10 mg/kg orally twice daily for 5 days; 10 total doses; A), BKI-1553 (5 mg/kg orally twice daily for 5 days; 10 total doses; B), and BKI-1294 (5 mg/kg orally twice daily for 5 days [10 total doses; C] or 10 mg/kg orally every other day for 8 days [4 total doses; D]). The dot and whisker plots represent average measured plasma BKI concentrations ± SDs. The only concentrations not predicted well were the BKI-1517 concentrations after the first dose (A). However, the concentrations after the fifth dose were predicted.
DISCUSSION
Globally, Cryptosporidium species are one of the most widespread causative agents of diarrhea in humans and livestock [1, 3, 4]. C. parvum and C. hominis are the most important species affecting humans [45], especially in resource-limited countries [3]. There is a clear need to develop effective, parasite-specific, and safe products for the control of cryptosporidiosis. BKIs targeting CpCDPK1 have been shown to inhibit Cryptosporidium infection in vitro and in immunodeficient mice [23], but until the present report, they have not been shown to help ameliorate the clinical disease cryptosporidiosis. BKIs do not significantly inhibit mammalian protein kinases and are nontoxic to the host [22, 23]. For the present study, BKIs were first chosen from a panel of >500 BKIs on the basis of their anti-CpCDPK1 activity, pharmacodynamic/pharmacokinetic properties, bioavailability, nontoxicity, and ability to inhibit C. parvum growth in vitro. BKIs were prescreened in the neonatal mouse infection model, previously shown to have positive predictive value for efficacy in calves [43, 46]. Finally, 3 BKIs (BKI-1294, BKI-1517, and BKI-1553) were evaluated in the calf model of cryptosporidiosis, to ascertain the ability to decrease or eliminate infection and reduce clinical symptoms.
Based on its superior efficacy in mice, we chose to begin calf model studies with BKI-1294, using a dosage derived from allometric modeling (once daily every other day). While treated calves had better clinical health scores, firmer stools, and a 94% reduction in oocyst shedding as compared to controls, no significant reduction in diarrhea was observed, based on fecal volumes. Lendner et al also reported a good parasitological outcome in calves treated with BKI-1294 at 10 mg/kg every other day, but calves in that preliminary report developed few clinical symptoms to evaluate conclusively [24]. Subsequent pharmacokinetic data from calves in the present report indicate that the allometrically determined dosage we (and Lendner et al [24]) used was suboptimal, with stool and plasma BKI levels that were essentially zero on the second day after each dosing. Given these pharmacokinetics data and considering the accelerated gastrointestinal transit time resulting from diarrhea, we hypothesized that more-frequent dosing would result in better treatment outcomes. We thus opted to repeat evaluation of BKI-1294, along with BKI-1553 and BKI-1517, with doses administered at 12-hour intervals.
Clinical health, diarrhea, and parasitological outcomes were significantly improved by administering BKIs every 12 hours for 5 days. All 3 BKIs helped alleviate but did not eliminate the clinical symptoms of cryptosporidiosis, with BKI-1294 and BKI-1517 being somewhat more effective than BKI-1553. Based on firmer fecal consistency and reduced fecal volume, diarrhea was also less severe in calves treated with any of the 3 BKIs, with BKI-1294 and BKI-1517 having the greatest efficacy. Paralleling fecal consistency outcomes, BKI-1294 and BKI-1517 also had the greatest effect in reducing both daily and total fecal volume. All 3 BKIs significantly inhibited parasite shedding based on daily fecal oocyst counts. BKI-1294 was the most effective in reducing oocyst shedding, both daily and total, followed by BKI-1517. Epidemiologically, a reduction in oocyst shedding is an important treatment outcome to decrease the environmental parasite burden and thus potential exposure to infectious organisms in both agricultural and urban settings, where drinking and recreational water use are often implicated in outbreaks [47, 48].
Overall, BKI-1294 appeared to be the most efficacious, clinically and parasitologically, although BKI-1517 was similar or closely behind BKI-1294 for some outcomes. However, it would not have been easy to predict this outcome on the basis of the pharmacokinetic parameters measured. Plasma exposure for BKI-1517 and BKI-1553 was much higher than for BKI-1294. It could be hypothesized that stool levels might be more important than systemic levels for treating cryptosporidiosis, as the organism resides just beneath the plasma membrane on the luminal side of the intestinal epithelium and the infectious sporozoite and merozoite stages targeted by BKIs are extracellular [2]. However, BKI-1294 appeared to yield the lowest stool levels of all 3 BKIs (Supplementary Table 2). It is possible that stool levels may not accurately reflect intraepithelial or intraluminal levels. Total stool levels, as we measured them, could be especially misleading if a substantial quantity of the BKI was bound to stool elements or insoluble. In addition, BKI levels in the terminal small intestine, cecum, and ascending colon probably drive clearance of the majority of the parasites in the gastrointestinal tract and lead to improvement in clinical symptoms. These parameters are not necessarily captured by total stool levels. Thus, further studies are required to understand the pharmacodynamics of good treatment outcomes with BKIs in Cryptosporidium infection.
While BKI-1294 appeared to be the lead BKI in the present study, we later discovered that it has potent hERG inhibitory activity, associated with long Q-T syndrome (cardiotoxicity) in humans [49, 50]. This is likely not a significant liability in agriculturally important animals, including calves, but hERG inhibitory activity eliminated BKI-1294 as our clinical lead for human applications. We used iterative design to direct us away from hERG inhibitory activity and other potential safety liabilities to yield BKI-1517 and BKI-1553 [50]. Both compounds have no hERG inhibitory activity at a concentration of >30 µM (as reported by Vidadala et al for BKI-1553 [50] and as we found for BK-1517 [unpublished data]). Subsequent studies will examine whether BKI-1517, BKI-1553, and other newly identified BKIs will be effective at a dosage similar to that used for nitazoxanide in humans (ie, every 12 hours for 3 days), which is presently the only Food and Drug Administration–approved drug for cryptosporidiosis in humans, although it is marginally effective [15].
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Geno DeHostos and Robert Choy (PATH Drug Solutions), Alejandro Castellanos-Gonzalez and A. Clinton White (University of Texas Medical Branch, Galveston), and Nina Isoheranan (University of Washington, Seattle), for helpful consultation; and Ashley Wilke, Linzey Leinart, Karina Paredes, and Emily Williams (University of Arizona, Tucson), for excellent technical assistance.
Financial support. This work was supported by the Public Health Service, National Institutes of Health, Bethesda (grants R01 AI 111341 and R01 HD 080670), the US Department of Agriculture (grant 2014-67015-22106), and PATH Drug Solutions (grant DFI 1850-02-405099).
Potential conflicts of interest. W. C. V. V. discloses that he is the president of ParaTheraTech, a company involved in developing BKIs for use in animal health. However, W. C. V. V. did not perform the neonatal mouse or calf experiments or analyze the data from these models for the present publication. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Tzipori S, Widmer G. A hundred-year retrospective on cryptosporidiosis. Trends Parasitol 2008; 24:184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fayer R. The general biology of Cryptosporidium. In: Fayer R, Xiao L, eds. Cryptosporidium and cryptosporidiosis. 2nd ed Boca Raton: CRC Press, 2008:1–42. [Google Scholar]
- 3.Kotloff KL, Nataro JP, Blackwelder WC et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt CR, Riggs MW, Fayer R. Cryptosporidiosis in neonatal calves. Vet Clin North Am Food Anim Pract 2010; 26:89–103. [DOI] [PubMed] [Google Scholar]
- 5.Shirley D-AT, Moonah SN, Kotloff KL. Burden of disease from Cryptosporidiosis. Curr Opin Infect Dis 2012; 25:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter PR, Thompson RCA. The zoonotic transmission of Giardia and Cryptosporidium. Int J Parasitol 2005; 35:1181–90. [DOI] [PubMed] [Google Scholar]
- 7.White AC. Cryptosporidiosis (Cryptosporidium species). In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 8th ed Philadelphia: Elsevier/Saunders, 2014:3173–83. [Google Scholar]
- 8.Amadi B, Mwiya M, Sianongo S et al. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: a randomised controlled trial. BMC Infect Dis 2009; 9:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol 1998; 148:497–506. [DOI] [PubMed] [Google Scholar]
- 10.Agnew DG, Lima AAM, Newman RD et al. Cryptosporidiosis in Northeastern Brazilian children: association with increased diarrhea morbidity. J Infect Dis 1998; 177:754–60. [DOI] [PubMed] [Google Scholar]
- 11.Mondal D, Haque R, Sack RB, Kirkpatrick BD, Petri WA. Attribution of malnutrition to cause-specific diarrheal illness: evidence from a prospective study of preschool children in Mirpur, Dhaka, Bangladesh. Am J Trop Med Hyg 2009; 80:824–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Schnyder M, Kohler L, Hemphill A, Deplazes P. Prophylactic and therapeutic efficacy of nitazoxanide against Cryptosporidium parvum in experimentally challenged neonatal calves. Vet Parasitol 2009; 160:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ollivett TL, Nydam DV, Bowman DD et al. Effect of nitazoxanide on cryptosporidiosis in experimentally infected neonatal dairy calves. J Dairy Sci 2009; 92:1643–8. [DOI] [PubMed] [Google Scholar]
- 14.Cabada MM, White AC. Treatment of cryptosporidiosis: do we know what we think we know? Curr Opin Infect Dis 2010; 23:494–9. [DOI] [PubMed] [Google Scholar]
- 15.Checkley W, White AC, Jaganath D et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect Dis 2015; 15:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu G. Biochemistry. In: Fayer R, Xiao L, eds. Cryptosporidium and cryptosporidiosis. 2nd ed Boca Raton: CRC Press, 2008:57–77. [Google Scholar]
- 17.Amadi B, Mwiya M, Musuku J et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 2002; 360:1375–80. [DOI] [PubMed] [Google Scholar]
- 18.Santin M, Trout JM. Livestock. In: Fayer R, Xiao L, eds. Cryptosporidium and cryptosporidiosis. 2nd ed Boca Raton: CRC Press, 2008:452–4. [Google Scholar]
- 19.Stockdale HD, Spencer JA, Blagburn BL. Prophylaxis and chemotherapy. In: Fayer R, Xiao L, eds. Cryptosporidium and cryptosporidiosis. 2nd ed Boca Raton: CRC Press, 2008:255–87. [Google Scholar]
- 20.Silverlås C, Björkman C, Egenvall A. Systematic review and meta-analyses of the effects of halofuginone against calf cryptosporidiosis. Prev Vet Med 2009; 91:73–84. [DOI] [PubMed] [Google Scholar]
- 21.Gorla SK, McNair NN, Yang G et al. Validation of IMP dehydrogenase inhibitors in a mouse model of cryptosporidiosis. Antimicrob Agents Chemother 2014; 58:1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy RC, Ojo KK, Larson ET et al. Discovery of potent and selective inhibitors of CDPK1 from C. parvum and T. gondii. ACS Med Chem Lett 2010; 1:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellanos-Gonzalez A, White AC, Ojo KK et al. A novel calcium-dependent protein kinase inhibitor as a lead compound for treating cryptosporidiosis. J Infect Dis 2013; 208:1342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lendner M, Böttcher D, Delling C, Ojo KK, Van Voorhis WC, Daugschies A. A novel CDPK1 inhibitor—a potential treatment for cryptosporidiosis in calves? Parasitol Res 2015; 114:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Ojo KK, Vidadala R et al. Potent and selective inhibitors of CDPK1 from T. gondii and C. parvum based on a 5-aminopyrazole-4-carboxamide scaffold. ACS Med Chem Lett 2014; 5:40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W, Ojo KK, Zhang Z et al. SAR studies of 5-aminopyrazole-4-carboxamide analogues as potent and selective inhibitors of Toxoplasma gondii CDPK1. ACS Med Chem Lett 2015; 6:1184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojo KK, Pfander C, Mueller NR et al. Transmission of malaria to mosquitoes blocked by bumped kinase inhibitors. J Clin Invest 2012; 122:2301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doggett JS, Ojo KK, Fan E, Maly DJ, Van Voorhis WC. Bumped kinase inhibitor 1294 treats established Toxoplasma gondii infection. Antimicrob Agents Chemother 2014; 58:3547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidadala RSR, Ojo KK, Johnson SM et al. Development of potent and selective Plasmodium falciparum calcium-dependent protein kinase 4 (PfCDPK4) inhibitors that block the transmission of malaria to mosquitoes. Eur J Med Chem 2014; 74:562–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedroni MJ, Vidadala RSR, Choi R et al. Bumped kinase inhibitor prohibits egression in Babesia bovis. Vet Parasitol 2016; 215:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keyloun KR, Reid MC, Choi R et al. The gatekeeper residue and beyond: homologous calcium dependent protein kinases as drug development targets for veterinarian Apicomplexa parasites. Parasitol 2014; 141:1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinrichs AJ. Rumen development in the dairy calf. Adv Dairy Technol 2005; 17:179–88. [Google Scholar]
- 33.Cirle A, Guerrant RL. Clinical disease and pathology. In: Fayer R, Xiao L, eds. Cryptosporidium and cryptosporidiosis. 2nd ed Boca Raton: CRC Press, 2008:235–53. [Google Scholar]
- 34.Riggs MW, Perryman LE. Infectivity and neutralization of Cryptosporidium parvum sporozoites. Infect Immun 1987; 55:2081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arrowood MJ, Donaldson K. Improved purification methods for calf-derived Cryptosporidium parvum oocysts using discontinuous sucrose and cesium chloride gradients. J Eukaryot Microbiol 1996; 43:89S. [DOI] [PubMed] [Google Scholar]
- 36.Riggs MW, Stone AL, Yount PA, Langer RC, Arrowood MJ, Bentley DL. Protective monoclonal antibody defines a circumsporozoite-like glycoprotein exoantigen of Cryptosporidium parvum sporozoites and merozoites. J Immunol 1997; 158:1787–95. [PubMed] [Google Scholar]
- 37.Imboden M, Schaefer DA, Bremel RD, Homan EJ, Riggs MW. Antibody fusions reduce onset of experimental Cryptosporidium parvum infection in calves. Vet Parasitol 2012; 188:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson SM, Murphy RC, Geiger JA et al. Development of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitors with potent anti-Toxoplasma activity. J Med Chem 2012; 55:2416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinayak S, Pawlowic MC, Sateriale A et al. Genetic modification of the diarrheal pathogen Cryptosporidium parvum. Nature 2015; 523:477–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaefer DA, Auerbach-Dixon BA, Riggs MW. Characterization and formulation of multiple epitope-specific neutralizing monoclonal antibodies for passive immunization against cryptosporidiosis. Infect Immun 2000; 68:2608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guy RA, Payment P, Krull UJ, Horgen PA. Real-time PCR for quantification of Giardia and Cryptosporidium in environmental water samples and sewage. Appl Environ Microbiol 2003; 69:5178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedraza-Díaz S, Amar C, McLauchlin J. The identification and characterisation of an unusual genotype of Cryptosporidium from human faeces as Cryptosporidium meleagridis. FEMS Microbiol Lett 2000; 189:189–94. [DOI] [PubMed] [Google Scholar]
- 43.Perryman LE, Kapil SJ, Jones ML, Hunt EL. Protection of calves against cryptosporidiosis with immune bovine colostrum induced by a Cryptosporidium parvum recombinant protein. Vaccine 1999; 17:2142–9. [DOI] [PubMed] [Google Scholar]
- 44.Tatipaka HB, Gillespie JR, Chatterjee AK et al. Substituted 2-phenyl-imidazopyridines: a new class of drug leads for human African trypanosomiasis. J Med Chem 2014; 57:828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao L, Ryan UM. Cryptosporidiosis: an update in molecular epidemiology. Curr Opin Infect Dis 2004; 17:483–90. [DOI] [PubMed] [Google Scholar]
- 46.Imboden M, Riggs MW, Schaefer DA, Homan EJ, Bremel RD. Antibodies fused to innate immune molecules reduce initiation of Cryptosporidium parvum infection in mice. Antimicrob Agents Chemother 2010; 54:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med 1995; 332:855–9. [DOI] [PubMed] [Google Scholar]
- 48.Zambriski JA, Nydam DV, Bowman DD et al. Description of fecal shedding of Cryptosporidium parvum oocysts in experimentally challenged dairy calves. Parasitol Res 2013; 112:1247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas D, Karle CA, Kiehn J. The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications. Curr Pharm Des 2006; 12:2271–83. [DOI] [PubMed] [Google Scholar]
- 50.Vidadala RSR, Rivas KL, Ojo KK et al. Development of an orally available and central nervous system (CNS)-penetrant Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitor with minimal human ether-à-go-go-related gene (hERG) activity for the treatment of toxoplasmosis. J Med Chem 2016; 59:6531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.