Abstract
Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylcholine (OxPAPC) attenuates agonist-induced endothelial cell (EC) permeability and increases pulmonary endothelial barrier function via enhancement of both the peripheral actin cytoskeleton and cell junctions mediated by Rac1 and Cdc42 GTPases. This study evaluated the role for the multifunctional Rac1/Cdc42 effector and regulator, IQ domain containing GTPase-activating protein (IQGAP1), as a molecular transducer of the OxPAPC-mediated EC barrier-enhancing signal. IQGAP1 knockdown in endothelial cells by gene-specific small-interfering RNA abolished OxPAPC-induced enlargement of VE-cadherin-positive adherens junctions, suppressed peripheral accumulation of actin polymerization regulators, namely cortactin, neural Wiskott-Aldrich syndrome protein (N-WASP), and actin-related protein 3, and attenuated remodeling of the peripheral actin cytoskeleton. Inhibition of OxPAPC-induced barrier enhancement by IQGAP1 knockdown was due to suppressed Rac1 and Cdc42 activation. Expression of an IQGAP1 truncated mutant showed that the GTPase regulatory domain of IQGAP1 was essential for the OxPAPC-induced membrane localization of cortactin, adherens junction proteins VE-cadherin and p120-catenin, as well as for EC permeability response. IQGAP1 knockdown attenuated the protective effect of OxPAPC against thrombin-induced cell contraction, cell junction disruption, and EC permeability. These results demonstrate for the first time the role of IQGAP1 as a critical transducer of OxPAPC-induced Rac1/Cdc42 signaling to the actin cytoskeleton and adherens junctions, which promotes cortical cytoskeletal remodeling and EC barrier-protective effects of oxidized phospholipids.
Keywords: phosphatidylcholine, vascular biology, signal transduction, lung, endothelial cells, IQ domain containing guanosine 5′-triphosphatase-activating protein, guanosine 5′-triphosphatase, permeability, cytoskeleton, oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylcholine
uncontrolled vascular leak worsens lung dysfunction and perpetuates tissue inflammation. Thus, stimulation of cellular mechanisms leading to restoration or enhancement of vascular endothelial barrier is a critical step in suppression of ongoing acute lung injury (ALI) and its devastating complication, acute respiratory distress syndrome (1, 53). Pathological insults, such as infection, trauma, or mechanical stress, trigger recruitment of neutrophils and other inflammatory cells, induction of proinflammatory cytokines, and subsequent generation of reactive oxygen intermediates that affect a variety of cell types and contribute to the induction and perpetuation of ALI (23, 33).
Cell membrane phospholipids and phospholipids present in circulating lipoproteins undergo oxidation by lipoxygenases or reactive oxygen and nitrogen species as result of ventilator-induced lung injury, trauma, or septic inflammation (18, 28, 31, 36, 42, 55). Oxidative modification of one of the major plasma membrane phospholipids, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC), forms a heterogeneous group of compounds with modified residues at the sn-2 position of the phospholipid. Products of PAPC oxidation (OxPAPC) have been shown to attenuate lipopolysaccharide (LPS)-unmethylated cytosine-guanine dinucleotide (CpG)-triggered inflammatory cascade mediated by Toll-like receptor (TLR) 4 and TLR9 receptors (17, 34). Furthermore, it was shown that OxPAPC also attenuates LPS-induced TNF-α production and inhibits the CpG-triggered inflammatory cascade by interfering with TLR9-mediated NF-κB activation (34), in addition to downregulation of innate immune response (34, 35, 38, 41) and even protection against LPS-mediated lethal shock (17).
OxPAPC is also a potent barrier-protective agent that increases pulmonary endothelial cell (EC) barrier properties and attenuates EC hyperpermeability caused by vasoactive and inflammatory mediators (2, 7, 13, 35, 37). Barrier-enhancing effects of OxPAPC on vascular endothelium are mediated by activation of small GTPases Rap1, Rac1, and Cdc42 (2, 16). In turn, Rac1 and Cdc42 stimulate dynamic remodeling of the peripheral cytoskeleton and enhancement of junction protein complexes, which are the two major features of vascular endothelial barrier restoration after exposure to barrier-disruptive agonists (14, 32, 49).
Emerging evidence suggests a critical importance of local regulation of RhoA and Rac1 GTPases at the cell cortical compartment for coordinated control of EC permeability response and rapid barrier recovery (39, 50, 58). Local regulation of Rac1/RhoA activity is also essential for the maintenance of endothelial and epithelial monolayer integrity, cell polarity, and fine control of peripheral cytoskeletal dynamics (22, 43, 59).
Rho-dependent mechanisms of EC permeability involve actomyosin-dependent, force-induced disruption of cell junctions, disassembly of adherens junction protein complexes, and reduced association of a RhoA negative regulator, p190RhoGAP, to adherens junctions via binding to p120-catenin (47). Together, these changes propagate barrier-disruptive Rho signaling at cell peripheral compartments.
IQ domain containing GTPase-activating protein (IQGAP1) is both a Rac1/Cdc42 effector and regulator with multiple functions. IQGAP1 is a plausible mediator of OxPAPC barrier-protective effects because it controls actin polymerization, cytoskeletal dynamics, and assembly of cell junctions via interactions with Rac1 and Cdc42 (57). IQGAP1 contains a calponin homology domain, a Ca2+/calmodulin binding IQ domain, a RasGAP-related domain (GRD) that binds and activates Rac1 and Cdc42, and a RasGAP COOH-terminus domain (20). As an adaptor protein, IQGAP1 interacts with several other proteins, including β-catenin, E-cadherin, actin filaments, and microtubule-associated plus end tracking proteins (54, 57).
However, a role of IQGAP1 in OxPAPC-mediated endothelial barrier enhancement and EC protection against Rho-mediated barrier disruption has not yet been defined. This study investigated involvement of IQGAP1 in the local control of Rac1/Cdc42 signaling and the enhancement of cortical cytoskeleton and adherens junctions in OxPAPC-stimulated pulmonary endothelial cells. We also elucidated the role of IQGAP1 in the OxPAPC-induced downregulation of barrier-disruptive RhoA signaling and the preservation of EC barrier.
MATERIALS AND METHODS
Cell culture and reagents.
Human pulmonary artery endothelial cells (HPAEC) were obtained from Lonza (East Rutherford, NJ) and used for experiments at passages 5–7. Rac1, Cdc42, p120-catenin, N-WASP, and p21Arc antibodies were purchased from BD Transduction Laboratories (San Diego, CA); RhoA, IQGAP1, cMyc-tag, and VE-cadherin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA); cortactin and phospho-Y421 cortactin antibodies were from Millipore (Billerica, MA); phospho-myosin light chain (MLC) and phospho-p21 protein associate kinase (PAK1) antibodies were obtained from Cell Signaling (Beverly, MA). Texas Red-conjugated phalloidin and Alexa Fluor 488 were purchased form Molecular Probes (Eugene, OR). Unless otherwise specified, all biochemical reagents, including β-actin antibody, were obtained from Sigma (St. Louis, MO).
Small-interfering RNA and DNA transfections.
HPAEC were treated with predesigned standard-purity IQGAP1-specific small-interfering RNA (siRNA) sets purchased from Dharmacon (Lafayette, CO). Transfection of EC with siRNA was performed as previously described (8). Nonspecific nontargeting RNA was used as a control. After 72 h of transfection, cells were used for experiments or harvested for Western blot verification of specific protein depletion. Plasmids encoding Myc-tagged full length IQGAP1 and IQGAP1-ΔGRD mutant encoding an IQGAP1 isoform lacking Rac1/Cdc42 binding domain (19) were kindly provided by D. Sacks (National Institutes of Health, Bethesda, MD) and used for transient transfections according to the protocols described previously (6, 8). After 24 h of transfection cells were treated with the agonist of interest and used for experiments.
Analysis of EC permeability.
The cellular barrier properties were analyzed by measurements of transendothelial electrical resistance (TER) across confluent human pulmonary artery endothelial monolayers using an electrical cell-substrate impedance-sensing system (Applied Biophysics, Troy, NY) as previously described (4, 6). Endothelial permeability to macromolecules was monitored by express permeability testing assay (XPerT) recently developed by our group (24) and now available from Millipore (catalog no. 17-10398; Vascular Permeability Imaging Assay). This assay is based on high-affinity binding of cell-impermeable avidin-conjugated FITC-labeled tracer to the biotinylated extracellular matrix proteins immobilized on the bottom of culture dishes covered with EC monolayers. FITC-avidin binding to the matrix-coated culture dish bottoms increases if the EC barrier is compromised by treatment with a barrier-disruptive agonist. For the permeability assays in the 96-well plates performed in this study, cells were seeded on biotinylated gelatin-coated 96-well plates (3 × 104 cells/well) and grown for 48–72 h before testing. After cell stimulation with OxPAPC, FITC-avidin solution at a final concentration of 25 μg/ml was added directly to the culture medium for 3 min before termination of the experiment. Unbound FITC-avidin was washed out with 200 μl phosphate-buffered saline (PBS), pH 7.4, 37°C (2 cycles, 10 s each). Finally, 100 μl of PBS were added to each well, and the fluorescence of matrix-bound FITC-avidin was measured on a Victor X5 Multilabel Plate Reader (PerkinElmer, Waltham, MA) using an excitation wavelength of 485 nm and emission wavelength of 535 nm.
Glutathione S-transferase-IQGAP1 pulldown assay.
Glutathione S-transferase (GST)-tagged IQGAP1 in pGEX vector was used for bacterial expression in BL21-AI Escherichia coli strain. GST-fusion protein was isolated (52) using glutathione resin (Clontech Laboratories, Mountain View, CA) and stored as 50% glycerol slurry. After stimulation with OxPAPC, endothelial monolayers were washed with PBS and incubated on ice for 15 min with lyses buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 1% Triton X-100, and 10% glycerol). Lysate was clarified by centrifugation and incubated with glutathione resin loaded with GST-IQGAP1 (2 h, 4°C). Next, resin was collected by centrifugation and washed three times with lysis buffer, and the amount of Rac and Cdc42 bound to IQGAP1 beads was evaluated for Western blot analysis.
Rac1/Cdc42 activation assay.
Rac1 and Cdc42 activation was evaluated in pulldown assays using agarose beads with immobilized PAK1-PBD (2). Briefly, after stimulation, cell lysates were collected and GTP-bound Rac1 and Cdc42 were captured using pull-down assays with immobilized PAK1-PBD agarose. The levels of activated Rac1 and Cdc42 proteins as well as total Rac1 and Cdc42 content were evaluated by Western blot analysis.
Immunofluorescence and live cell imaging.
Endothelial cells plated on glass cover slips were treated with the agonist of interest, fixed in 3.7% formaldehyde solution in PBS for 10 min at 4°C, washed three times with PBS, permeabilized with 0.1% Triton X-100 in PBS-Tween (PBST) for 30 min at room temperature, and blocked with 2% BSA in PBST for 30 min. Incubations with antibodies to IQGAP1 were performed in blocking solution (2% BSA in PBST) for 1 h at room temperature followed by staining with Alexa 488-conjugated secondary antibodies. Actin filaments were stained with Texas Red-conjugated phalloidin. After immunostaining, slides were analyzed using a Nikon video imaging system (Nikon Instech) as described elsewhere (6, 11). For live imaging of green fluorescent protein (GFP)-cortactin, cells were plated on MatTek dishes (MatTek, Ashland, MA) and transfected with GFP-cortactin plasmid. Time lapse images were acquired with a 100× numeric aperture 1.45 oil objective in a 3I Marianas Yokogawa-type Spinning Disk Confocal system equipped with a CO2 chamber and a heated stage as we have previously described (14). Quantification of thrombin-induced gap formation by EC monolayers was performed as described elsewhere (3, 6, 12) using MetaVue 4.6 software (Universal Imaging, Downingtown, PA). The gap formation was expressed as a ratio of the gap area to the area of the whole image. The values were statistically processed using Sigma Plot 7.1 (SPSS Science, Chicago, IL) software. For each experimental condition at least 10 microscopic fields in each independent experiment were analyzed.
Differential protein fractionation and immunoblotting.
In subcellular fractionation studies, after agonist stimulation cells were washed in cold PBS, and cytosolic and membrane fractions were isolated using a subcellular protein fractionation kit (Thermo Fisher Scientific, Rockford, IL) according to the manufacturer's protocol. For analysis of protein phosphorylation profiles, cells were stimulated, then lysed, and protein extracts were separated by SDS-PAGE, transferred to polyvinylidene fluoride membrane, and probed with specific antibodies. Equal protein loading was verified by reprobing membranes with antibody to β-actin or the specific protein of interest.
Statistical analysis.
Results are expressed as means ± SD of three to five independent experiments. Stimulated samples were compared with controls by unpaired Student's t-tests. For multiple-group comparisons, a one-way analysis of variance, followed by the post hoc Fisher's test, were used. P < 0.05 was considered statistically significant.
RESULTS
IQGAP1 mediates OxPAPC-induced EC barrier enhancement and peripheral cytoskeleton remodeling.
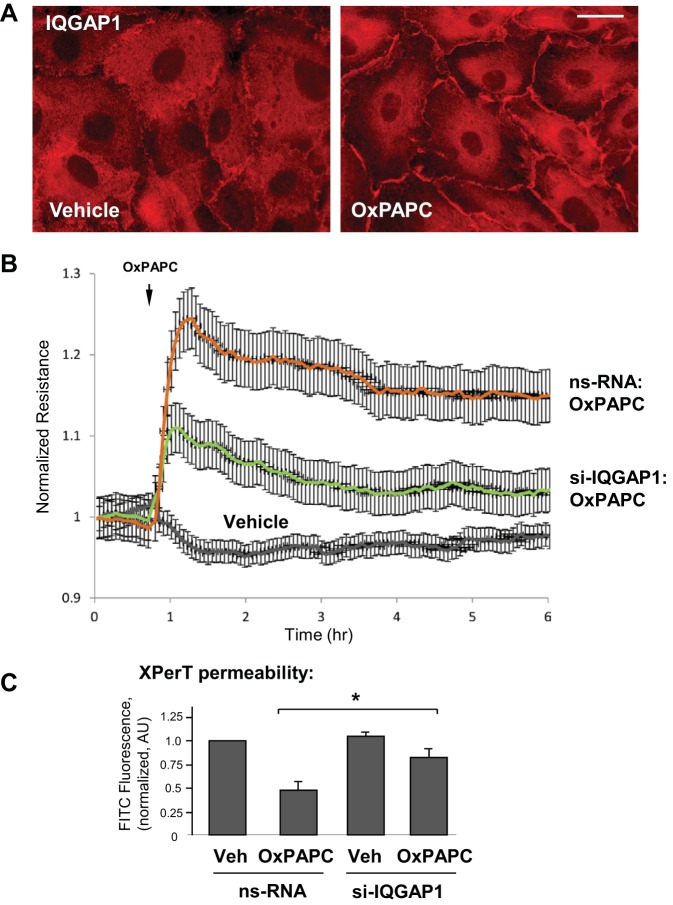
A functional role for IQGAP1 in OxPAPC-induced cytoskeletal remodeling and barrier regulation was studied in HPAEC. Stimulation with OxPAPC induced IQGAP1 accumulation at the cell periphery (Fig. 1A). OxPAPC enhanced the EC barrier response, which was monitored by an increase in the TER. This effect of OxPAPC was considerably attenuated by knockdown of IQGAP1 using siRNA (Fig. 1B).
Fig. 1.
IQ domain containing GTPase-activating protein (IQGAP1) mediates endothelial cell (EC) barrier enhancement caused by oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylcholine (OxPAPC). A: pulmonary EC were stimulated with OxPAPC (10 μg/ml, 15 min) followed by immunofluorescence staining for IQGAP1. Bar = 10 μm. Shown are representative results of 3 independent experiments. B: human pulmonary artery endothelial cells (HPAEC) grown on microelectrodes were transfected with 100 nM IQGAP1-specific small-interfering RNA (siRNA) or nonspecific RNA (ns-RNA) for 72 h and stimulated with OxPAPC. Measurements of transendothelial resistance were performed over time. Shown are normalized average transendothelial electrical resistance (TER) values ± SE from 3 independent readings in 1 experiment; the data are representative of 3 independent experiments. C: EC were grown in 96-well plates coated with biotinylated gelatin (0.25 mg/ml). The cells were transfected with nonspecific or IQGAP1-specific siRNA and stimulated with OxPAPC for 30 min, followed by addition of FITC-avidin (25 μg/ml, 3 min). Unbound FITC-avidin was removed, and FITC fluorescence was measured; n = 4 experiments, *P < 0.05.
The role of IQGAP1 in OxPAPC-induced EC barrier enhancement was additionally examined by analysis of EC monolayer permeability for macromolecules (XPerT assay) with FITC-labeled avidin used as a tracer (24). Nearly confluent EC monolayers grown on biotinylated gelatin and transfected with nonspecific or IQGAP1-specific siRNA were treated with vehicle or OxPAPC, followed by brief incubation with FITC-avidin tracer. After the unbound FITC-avidin was washed off the retained FITC fluorescence on the bottom of plates was measured using a microplate fluorimeter. The bar graph (Fig. 1C) represents a quantitative analysis of permeability in the nearly confluent control and OxPAPC-stimulated EC monolayers. The OxPAPC-induced barrier-enhancing effect was significantly suppressed in EC monolayers with IQGAP1 knockdown.
OxPAPC treatment induced a time-dependent remodeling of the actin cytoskeleton characterized by the disappearance of central stress fibers and substantial accumulation of F-actin at the cell periphery (Fig. 2A, left). These events were accompanied by a dramatic enlargement of VE-cadherin-positive adherens junctions (Fig. 2A, middle). Merged images show the time-dependent colocalization of VE-cadherin with cortical actin (merged F-actin and VE-cadherin staining in Fig. 2A, right), which reached maximal levels by 30–60 min. Knockdown of IQGAP1 using siRNA essentially abolished OxPAPC-induced colocalization of F-actin and VE-cadherin at the cell junction area (Fig. 2B). The insets in Fig. 2B depict cell-cell interface areas at higher magnification.
Fig. 2.
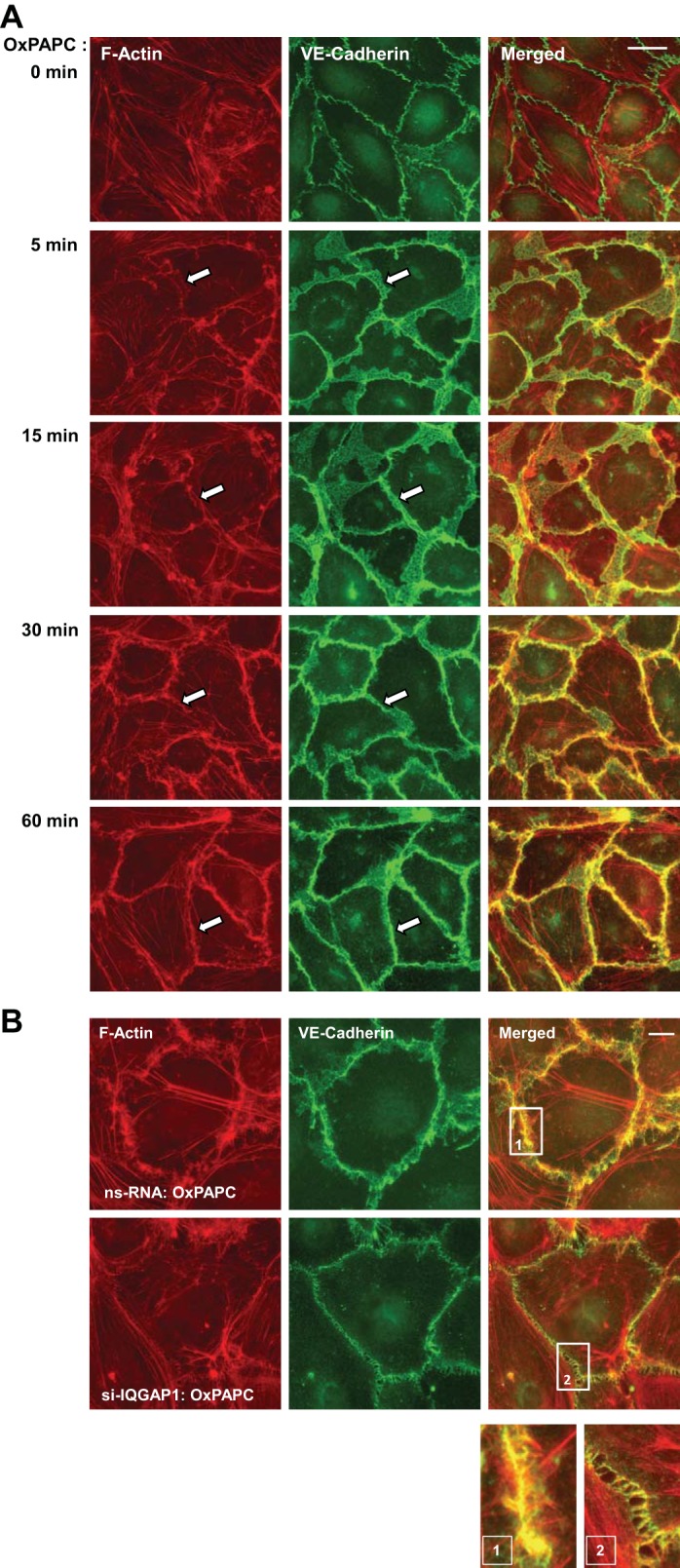
IQGAP1 mediates cytoskeletal remodeling induced by OxPAPC. A: EC were stimulated with OxPAPC (10 μg/ml) for the indicated time periods, followed by double immunofluorescence staining for actin using Texas Red-phalloidin (red) and VE-cadherin (green). Merged images depict areas of protein colocalization, which appear in yellow and marked by arrows. Bar = 10 μm. B: EC transfected with nonspecific or IQGAP1-specific siRNA were stimulated with OxPAPC for 30 min, fixed, permeabilized, and subjected to double staining for actin (red) and VE-cadherin (green). Merged images depict areas of protein colocalization, which appear in yellow. Bar = 5 μm. Higher-magnification insets show details of actin and VE-cadherin localization at the cell cortical areas of control and IQGAP1-depleted EC upon stimulation with OxPAPC. Shown are representative results of 3 independent experiments.
IQGAP1 mediates OxPAPC-induced Rac1/Cdc42 signaling and cytoskeletal dynamics.
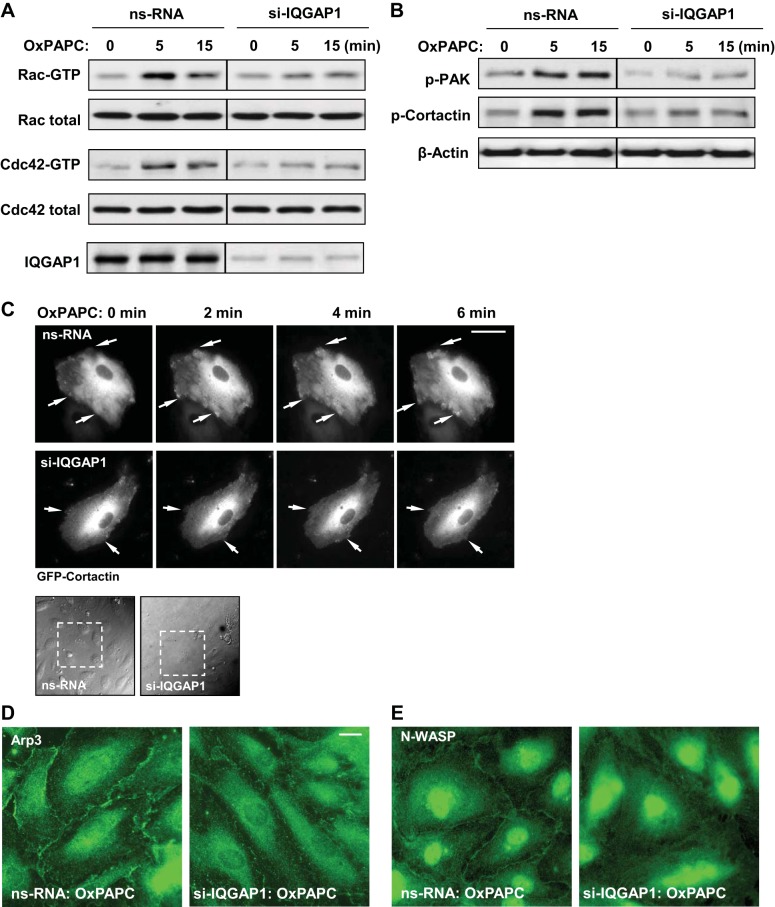
Because IQGAP1 is a multifunctional protein interacting with Rac1 and Cdc42 (57), we tested the role of IQGAP1 in the modulation of OxPAPC-induced Rac1/Cdc42 activation. Rac1/Cdc42-GTP pulldown assays reflecting the levels of activated small GTPases showed that IQGAP1 knockdown attenuated Rac1 and Cdc42 activation by OxPAPC (Fig. 3A).
Fig. 3.
IQGAP1 mediates Rac1/Cdc42-dependent signaling in response to OxPAPC. Lung EC were transfected with 100 nM IQGAP1-specific siRNA or nonspecific RNA for 72 h, followed by OxPAPC (10 μg/ml) stimulation. A: Rac1 and Cdc42 activation was determined by GTPase pulldown assays. The amount of activated Rac1 or Cdc42 was normalized to the total Rac and Cdc42 content, respectively, in EC lysates. siRNA-induced IQGAP1 knockdown was confirmed by probing blots of whole cell lysates with IQGAP1 antibody. Data are representative of 5 independent experiments. B: OxPAPC-induced p21 protein associate kinase (PAK) and cortactin phosphorylation in control and IQGAP1-depleted EC was evaluated by Western blot with phosphospecific antibodies. Equal protein loading was confirmed by probing with β-actin antibody. C: live cell imaging of control and IQGAP1-depleted EC expressing green fluorescent protein (GFP)-cortactin. Snap shots depict OxPAPC-induced cortical dynamics at the cell periphery of control and IQGAP1-depleted cells. Arrows indicate cortactin accumulation and lamellipodia formation at the cell edges. Phase-contrast images (bottom) indicate GFP-cortactin cells within the monolayer that were monitored over time by live microscopy. Bar = 10 μm. Shown are representative results of 3 independent experiments. D and E: OxPAPC-induced peripheral translocation of actin-related protein 3 (Arp3, D) and neural Wiskott-Aldrich syndrome protein (N-WASP, E) was examined in EC cells transfected with nonspecific or IQGAP1-specific siRNA by immunofluorescence staining with the relevant antibodies. Bar = 5 μm.
Cortical actin dynamics is a Rac1/Cdc42-dependent process that requires activation and peripheral accumulation of the regulators of actin polymerization, such as phosphorylated cortactin, Arp2/3, N-WASP, and WASP family Verprolin-homologous protein (21, 29, 56). We have previously reported the essential role of Rac1-dependent PAK1 and cortactin phosphorylation in agonist-induced EC barrier enhancement (5, 9). The current data show that stimulation of EC with OxPAPC (10 μg/ml, 5–15 min) induced phosphorylation of both PAK1 and cortactin, which was inhibited by depletion of IQGAP1 using gene-specific siRNA (Fig. 3B).
IQGAP1 regulation of the OxPAPC-induced cytoskeletal dynamics was further evaluated with live microscopy studies. EC expressing GFP-cortactin were used for analysis. The formation of cortactin-positive lamellipodia-like structures was observed as early as 2 min after OxPAPC treatment and persisted at later time points. IQGAP1 knockdown abolished peripheral cortactin dynamics, reflecting suppression of cytoskeletal remodeling in OxPAPC-stimulated EC with depleted IQGAP1 (Fig. 3C).
IQGAP1 knockdown also abolished peripheral accumulation of other activators of actin polymerization and branching, namely actin-related protein 3 (Arp3) and N-WASP (Fig. 3, D and E). Altogether, these results link the reduced Rac1 and Cdc42 activation and attenuation of OxPAPC-induced cortical cytoskeletal dynamics.
Role of the IQGAP1 GRD domain in OxPAPC-induced EC barrier enhancement.
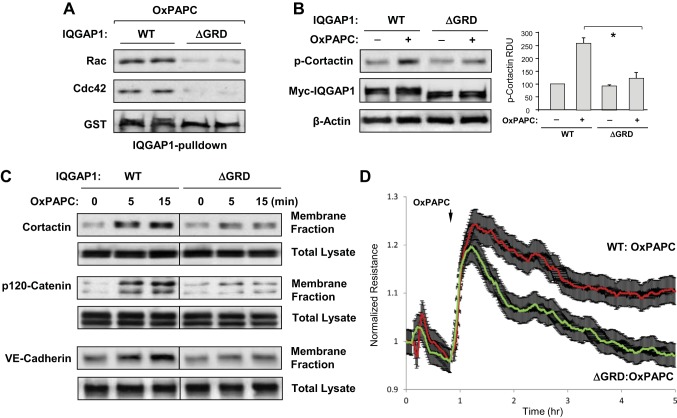
IQGAP1 binds preferentially to activated Rac1 and Cdc42 via Ras GRD flanked by amino acids 1025–1238 (48). Here we used the IQGAP1-ΔGRD deletion mutant to evaluate a functional role of GRD domain in the OxPAPC-induced IQGAP1 interaction with Rac1/Cdc42, and cytoskeletal remodeling. OxPAPC induced interaction of Rac1 and Cdc42 with wild-type IQGAP1, but not with IQGAP1-ΔGRD (Fig. 4A). Similarly, expression of IQGAP1-ΔGRD attenuated OxPAPC-induced phosphorylation of cortactin (Fig. 4B) and accumulation of cortactin and adherens junction proteins p120-catenin and VE-cadherin in the membrane fraction (Fig. 4C).
Fig. 4.
The GTPase regulatory domain (GRD) of IQGAP1 mediates OxPAPC-induced activation of Rac1/Cdc42 signaling and EC barrier enhancement. A: EC were stimulated with OxPAPC (10 μg/ml) for 5 min. EC lysates were incubated with agarose beads conjugated with IQGAP1-wild type (WT) or IQGAP1-ΔGRD. After being washed, immobilized Rac1 and Cdc42 were detected by immunoblotting. Probing the membranes with glutathione S-transferase (GST) antibody was used as a normalization control. Samples are shown in duplicate. B: OxPAPC-induced cortactin phosphorylation in EC expressing full-length IQGAP1 (IQGAP1-WT) or IQGAP1-ΔGRD was evaluated by Western blot with phospho-Y421-cortactin antibody. Expression of recombinant IQGAP1-WT and IQGAP1-ΔGRD was confirmed by probing the membrane with cMyc antibody. Equal protein loading was confirmed by probing for β-actin. Bar graphs depict quantitative analysis of Western blot data; n = 3; *P < 0.05 vs. IQGAP1-WT. C: HPAEC transfected with IQGAP1-WT or IQGAP1-ΔGRD were stimulated with OxPAPC, followed by fractionation assay. After isolation of cytosolic and membrane fractions, cortactin, p120-catenin, and VE-cadherin accumulation in the membrane fraction was monitored by Western blot. Protein content in corresponding total cell lysates was used as a normalization control. D: permeability measurements. EC transfected with IQGAP1-WT or IQGAP1-ΔGRD were stimulated with OxPAPC, and TER was monitored over time. Shown are normalized average TER values ± SE from 3 independent readings in 1 experiment; the data are representative of 4 independent experiments.
A functional role for the IQGAP1 GRD in OxPAPC-mediated EC barrier regulation was tested in a permeability assay using measurements of TER. Compared with OxPAPC-stimulated cells expressing full-length IQGAP1, expression of IQGAP1-ΔGRD mutant lacking Rac1/Cdc42-binding domain attenuated the initial OxPAPC-induced enhancement of EC barrier and suppressed the sustained phase of OxPAPC-induced TER increase (Fig. 4D). These data suggest that expression of IQGAP1-ΔGRD leads to inhibition of cortical dynamics and adherens junction assembly in the pulmonary EC, which results in impaired barrier enhancement caused by OxPAPC.
IQGAP1 mediates OxPAPC protective effect against thrombin-induced EC permeability.
Thrombin is an edemagenic agonist released by activated platelets, which triggers Rho-dependent phosphorylation of myosin light chains leading to activation of actomyosin contraction and EC permeability (11, 25). A role for IQGAP1 in the OxPAPC protective effects against thrombin-induced permeability was evaluated using the XPerT permeability assay (24). For these experiments, agonist-induced changes in fluorescence of FITC-avidin accumulated on the bottoms of 96-well plates covered with EC monolayers were performed using a microplate reader. Protective effects of OxPAPC against thrombin-induced permeability in control cells treated with nonspecific RNA were significantly reduced by IQGAP1 knockdown (Fig. 5A).
Fig. 5.
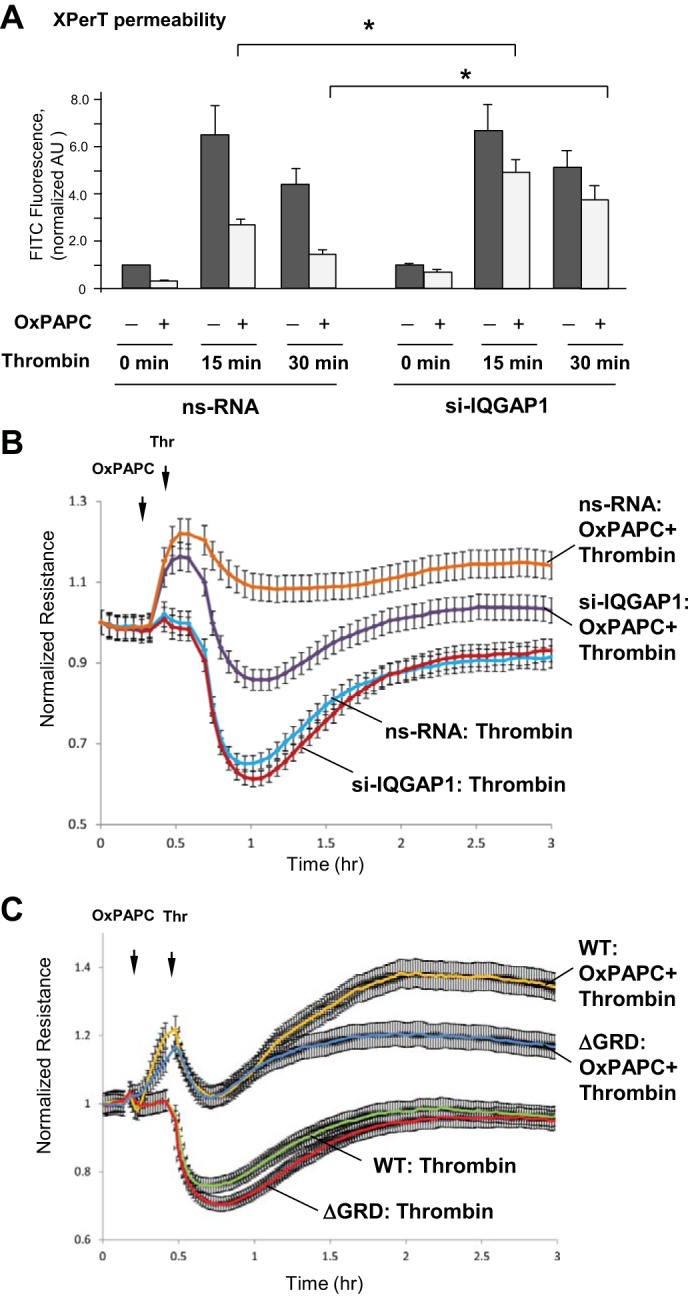
Effect of IQGAP1 depletion or IQGAP1-ΔGRD expression on OxPAPC-mediated protection against thrombin-induced EC hyperpermeability. A: HPAEC grown in 96-well plates coated with biotinylated gelatin (0.25 mg/ml) were transfected with nonspecific RNA or IQGAP1-specific siRNA for 72 h and stimulated with vehicle or OxPAPC (10 μg/ml, 30 min) followed by thrombin (0.3 U/ml) stimulation. FITC-labeled avidin (25 μg/ml) was added for 3 min. Unbound FITC-avidin was removed, and FITC fluorescence was measured; n = 4, *P < 0.05. B and C: EC transfected with IQGAP1-specific or nonspecific siRNA (B), or cells transfected with IQGAP1-WT or IQGAP1-ΔGRD (C), were subjected to thrombin treatment with or without preincubation with OxPAPC, and TER was monitored over time. Shown are normalized average resistance ± SE values from 3 independent readings in 1 experiment; the data are representative of 4 independent experiments.
Effects of IQGAP knockdown or expression of IQGAP1-ΔGRD mutant lacking Rac/Cdc42 binding domain on barrier-protective effects of OxPAPC against thrombin-induced EC permeability were further studied in experiments with TER measurements. TER measurements were performed during the course of experiment, where EC monolayers were first treated with OxPAPC for 15 min followed by thrombin challenge. IQGAP1 knockdown (Fig. 5B) did not affect the TER drop caused by thrombin compared with EC treated with nonspecific siRNA control. However, IQGAP1 knockdown attenuated the peak TER increase caused by addition of OxPAPC and suppressed the protective effect of OxPAPC against thrombin-induced TER decline, which reflects EC barrier dysfunction. In turn, expression of IQGAP1-ΔGRD mutant did not affect the magnitude of TER drop caused by thrombin, but compromised the enhancing effect of OxPAPC on EC barrier recovery after thrombin (Fig. 5C). These data are in accordance with TER data presented in Fig. 4D showing an inhibiting effect of IQGAP1-ΔGRD mutant on the sustainability of OxPAPC-induced EC response.
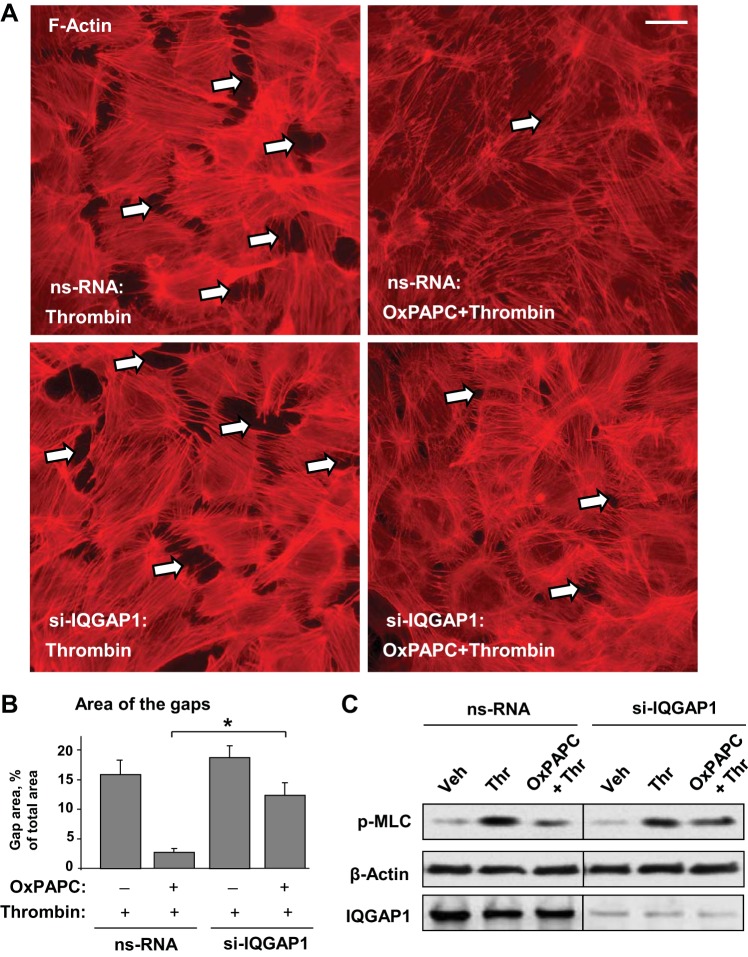
The role of IQGAP1 in OxPAPC-induced preservation of EC monolayer integrity was further evaluated in immunofluorescence studies. Control and IQGAP1-depleted EC were challenged with thrombin with or without OxPAPC pretreatment. Thrombin induced stress fiber formation in both control nonspecific RNA-treated EC and cells with depleted IQGAP1. Whereas OxPAPC attenuated thrombin-induced stress fiber formation and preserved monolayer integrity in thrombin-challenged EC treated with nonspecific RNA, this protective effect of OxPAPC was significantly reduced by knockdown of IQGAP1 (Fig. 6A). Quantitative analysis of gap formation confirmed immunofluorescence results (Fig. 6B).
Fig. 6.
Effect of IQGAP1 depletion on OxPAPC-mediated protection against thrombin-induced EC barrier dysfunction. A: EC transfected with nonspecific RNA or IQGAP1-specific siRNA were subjected to thrombin stimulation (0.3 U/ml, 15 min) with or without OxPAPC pretreatment (10 μg/ml, 30 min). F-actin was visualized by immunofluorescence staining with Texas Red-phalloidin. Paracellular gaps are marked by arrows. Bar = 10 μm. B: bar graphs represent quantitative analysis of gap formation in control and treated HPAEC monolayers. Data are expressed as means ± SD of 3 independent experiments; *P < 0.05. C: phosphorylation of myosin light chain (p-MLC) was detected by Western blot with specific antibody. Probing for β-actin was used as a normalization control. IQGAP1 protein depletion was confirmed by Western blot.
OxPAPC protective effect against thrombin-induced permeability is associated with Rac1-dependent downregulation of the Rho pathway (15). In agreement with this mechanism, IQGAP1 depletion attenuated the inhibitory effect of OxPAPC on thrombin-induced MLC phosphorylation (Fig. 6C).
DISCUSSION
The main finding of this study is the OxPAPC-induced formation of IQGAP1-Rac1-Cdc42 signaling complex regulating assembly of adherens junctions, remodeling of peripheral actin in the endothelial monolayer, and enhancement of the endothelial barrier. Molecular inhibition of IQGAP1 attenuated OxPAPC-induced EC barrier enhancement. On the other hand, IQGAP1 knockdown or expression of an IQGAP1 mutant deficient in binding Rac1/Cdc42 abolished OxPAPC-induced activation of Rac1/Cdc42 and their downstream cytoskeletal targets.
The Rac1/Cdc42 pathway plays a major role in cortical actin remodeling (2), assembly of adherens junctions (44, 51), and functional interactions between cytoskeleton and cell junction protein complexes caused by barrier-enhancing agonists (10, 26, 45). Together, these molecular events define a rapid EC barrier-enhancing response to OxPAPC. However, a distinct feature of OxPAPC effects on pulmonary vascular EC is sustained cortical accumulation of Rac1/Cdc42-regulated activators of peripheral actin polymerization N-WASP, Arp3, and cortactin, cytoskeletal remodeling, and enhancement of cell junctions (Fig. 2 in this study), which were associated with sustained barrier-enhancing EC response. This striking effect of OxPAPC stimulation suggests local regulation of Rac1/Cdc42 signaling responsible for sustained barrier maintenance. The current study shows for the first time the role of IQGAP1 in the OxPAPC-stimulated mechanism of local regulation of Rac1/Cdc42 signaling, peripheral cytoskeletal remodeling, assembly of adherens junctions, and EC barrier enhancement. Of note, OxPAPC treatment enhances the barrier properties in both subconfluent and confluent EC cultures, although in confluent culture the OxPAPC effects are less expressed, since the tight barrier has been already established. From a physiologically relevant perspective, “subconfluent” EC monolayers appear in the injured or inflamed lungs as a consequence of reported endothelial apoptosis or detachment (27), and the IQGAP1-mediated mechanism of EC barrier enhancement is especially important in this situation.
Molecular inhibition of IQGAP1 attenuated OxPAPC-induced accumulation of N-WASP, Arp3, and cortactin at the cell cortical layer that was linked to attenuation of cortical actin rim formation. IQGAP1 serves as an effector of Rac1 and Cdc42. When bound to Rac1/Cdc42, IQGAP1 interacts with activators of actin polymerization (N-WASP, Arp3) (20) and promotes β-catenin VE-cadherin-dependent assembly of adherens junctions (30), which explains the OxPAPC effects observed in this study.
Another interesting question is the mechanism of suppression of OxPAPC-induced Rac1/Cdc42 activation by an IQGAP1-ΔGRD deletion mutant, which acts in a dominant negative fashion and inhibits Rac1/Cdc42 activities despite the presence of endogenous IQGAP1 (48). Previous studies showed that the deletion of any of three IQGAP1 functional domains (COOH-terminus, GRD, or IQ domain) abolished Cdc42 binding to IQGAP1 (46). These findings suggest an allosteric mechanism of IQGAP1 interaction with Cdc42, which may also apply to the IQGAP1 interaction with Rac1. At the same time, the GRD domain in IQGAP1 stabilizes Rac1 and Cdc42 in the GTP-bound active form, which promotes sustained local activation of Rac1 and Cdc42 (40, 57). In our experiments, IQGAP1 knockdown significantly attenuated OxPAPC-induced activation of Rac1 and Cdc42, expression of IQGAP1-ΔGRD attenuated OxPAPC-induced Rac1/Cdc42 targeting to IQGAP1, and both interventions attenuated OxPAPC-induced cytoskeletal remodeling, adherens junction enlargement, and EC barrier enhancement response.
Taken together, these results strongly suggest that IQGAP1 participates in additional Rac1/Cdc42 stimulation induced by OxPAPC by targeting the activated forms of Rac1 and Cdc42 and therefore forming a positive feedback loop of Rac1/Cdc42 signaling. Time-resolved EC permeability assays using TER measurements further support this conclusion and show impairment of sustained barrier enhancement response in EC with IQGAP1 knockdown or IQGAP1-ΔGRD mutant expression. Finally, the results show that the IQGAP1-dependent mechanism of Rac1/Cdc42 activation is important for the OxPAPC protective effects against thrombin-induced EC permeability driven by the Rho/Rho-kinase-dependent pathway characterized in previous studies (2, 7, 15). IQGAP1 knockdown delayed downregulation of RhoA and EC barrier preservation stimulated by OxPAPC.
In summary, this study demonstrates the essential role for IQGAP1 in the peripheral cytoskeletal remodeling and EC barrier enhancement caused by OxPAPC. The results suggest a mechanism of OxPAPC-induced barrier enhancement by regulation of local GTPase signaling via IQGAP1-mediated peripheral capturing of Rac1 and Cdc42. This capturing of activated Rac1 and Cdc42 promotes local regulation of actin polymerization essential for the OxPAPC-induced development of peripheral actin cytoskeletal network and cell-cell junctions leading to sustained EC barrier enhancement.
GRANTS
This work was supported by National Institutes of Health Grants HL-076259, HL-087823, HL-107920, HL-130431, and GM-114171.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.T., X.T., G.G., and N.S. performed experiments; Y.T., X.T., G.G., A.A.B., and K.G.B. analyzed data; Y.T., X.T., G.G., and A.A.B. prepared figures; Y.T., A.A.B., and K.G.B. drafted manuscript; Y.T., X.T., G.G., N.S., D.B.S., A.A.B., and K.G.B. approved final version of manuscript; D.B.S., A.A.B., and K.G.B. edited and revised manuscript; A.A.B. and K.G.B. conception and design of research; A.A.B. and K.G.B. interpreted results of experiments.
ACKNOWLEDGMENTS
We thank Tomomi Ohmura for laboratory assistance.
REFERENCES
- 1.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury, and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res 95: 892–901, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AA. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 290: L540–L548, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Birukova AA, Alekseeva E, Cokic I, Turner CE, Birukov KG. Cross talk between paxillin and Rac is critical for mediation of barrier-protective effects by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 295: L593–L602, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birukova AA, Birukov KG, Smurova K, Adyshev DM, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 18: 1879–1890, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 292: L924–L935, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Birukova AA, Fu P, Xing J, Yakubov B, Cokic I, Birukov KG. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol 298: L837–L848, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birukova AA, Malyukova I, Mikaelyan A, Fu P, Birukov KG. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J Cell Physiol 211: 608–617, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin-β-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 293: L199–L211, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: Role of Rho-dependent mechanisms. J Cell Physiol 201: 55–70, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Birukova AA, Starosta V, Tian X, Higginbotham K, Koroniak L, Berliner JA, Birukov KG. Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Transl Res 161: 495–504, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birukova AA, Tian X, Tian Y, Higginbotham K, Birukov KG. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol Biol Cell 24: 2678–2688, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birukova AA, Zebda N, Cokic I, Fu P, Wu T, Dubrovskyi O, Birukov KG. p190RhoGAP mediates protective effects of oxidized phospholipids in the models of ventilator-induced lung injury. Exp Cell Res 317: 859–872, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birukova AA, Zebda N, Fu P, Poroyko V, Cokic I, Birukov KG. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J Cell Physiol 226: 2052–2062, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature 419: 77–81, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Bochkov VN, Leitinger N, Birukov KG. Role of oxidized phospholipids in acute lung injury. Curr Resp Med Rev 2: 27–37, 2006. [Google Scholar]
- 19.Briggs MW, Li Z, Sacks DB. IQGAP1-mediated stimulation of transcriptional co-activation by beta-catenin is modulated by calmodulin. J Biol Chem 277: 7453–7465, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Brown MD, Sacks DB. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol 16: 242–249, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell 116: 167–179, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Bustos RI, Forget MA, Settleman JE, Hansen SH. Coordination of Rho and Rac GTPase function via p190B RhoGAP. Curr Biol 18: 1606–1611, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J 11: 745–757, 1998. [PubMed] [Google Scholar]
- 24.Dubrovskyi O, Birukova AA, Birukov KG. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest 93: 254–263, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 163: 510–522, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill SE, Rohan M, Mehta S. Role of pulmonary microvascular endothelial cell apoptosis in murine sepsis-induced lung injury in vivo. Respir Res 16: 109, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammerschmidt S, Schiller J, Kuhn H, Meybaum M, Gessner C, Sandvoss T, Arnold K, Wirtz H. Influence of tidal volume on pulmonary NO release, tissue lipid peroxidation and surfactant phospholipids. Biochim Biophys Acta 1639: 17–26, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem 68: 459–486, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol 11: 591–596, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Kalyanaraman B. Nitrated lipids: a class of cell-signaling molecules. Proc Natl Acad Sci USA 101: 11527–11528, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kouklis P, Konstantoulaki M, Vogel S, Broman M, Malik AB. Cdc42 regulates the restoration of endothelial barrier function. Circ Res 94: 159–166, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest 122: 314S–320S, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Ma Z, Li J, Yang L, Mu Y, Xie W, Pitt B, Li S. Inhibition of LPS- and CpG DNA-induced TNF-α response by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 286: L808–L816, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Meliton A, Meng F, Tian Y, Sarich N, Mutlu GM, Birukova AA, Birukov KG. Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am J Physiol Lung Cell Mol Physiol 308: L550–L562, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrow JD, Roberts LJ. The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease. Am J Respir Crit Care Med 166: S25–S30, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, Leitinger N, Garcia JG, Birukov KG. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care 12: R27, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nonas SA, Miller IL, Kawkitinarong K, Garcia JG, Birukov KG. Protective effects of oxidized phospholipids on endotoxin-induced lung injury. Proc Am Thorac Soc 2: A837, 2005. [Google Scholar]
- 39.Noren NK, Arthur WT, Burridge K. Cadherin engagement inhibits RhoA via p190RhoGAP. J Biol Chem 278: 13615–13618, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J Cell Sci 118: 2085–2092, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Oskolkova OV, Afonyushkin T, Preinerstorfer B, Bicker W, von Schlieffen E, Hainzl E, Demyanets S, Schabbauer G, Lindner W, Tselepis AD, Wojta J, Binder BR, Bochkov VN. Oxidized Phospholipids Are More Potent Antagonists of Lipopolysaccharide than Inducers of Inflammation. J Immunol 185: 7706–7712, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, O'Brien K, Geary RL, Heinecke JW. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem 279: 42977–42983, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Popoff MR, Geny B. Multifaceted role of Rho, Rac, Cdc42 and Ras in intercellular junctions, lessons from toxins. Biochim Biophys Acta 1788: 797–812, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Schlegel N, Waschke J. cAMP with other signaling cues converges on Rac1 to stabilize the endothelial barrier- a signaling pathway compromised in inflammation. Cell Tissue Res 355: 587–596, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Singleton PA, Salgia R, Moreno-Vinasco L, Moitra J, Sammani S, Mirzapoiazova T, Garcia JG. CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J Biol Chem 282: 30643–30657, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Sokol SY, Li Z, Sacks DB. The effect of IQGAP1 on Xenopus embryonic ectoderm requires Cdc42. J Biol Chem 276: 48425–48430, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res 87: 243–253, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Swart-Mataraza JM, Li Z, Sacks DB. IQGAP1 is a component of Cdc42 signaling to the cytoskeleton. J Biol Chem 277: 24753–24763, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res 103: 1164–1172, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian Y, Gawlak G, Shah AS, Higginbotham K, Tian X, Kawasaki Y, Akiyama T, Sacks DB, Birukova AA. Hepatocyte growth factor-induced Asef-IQGAP1 complex controls cytoskeletal remodeling and endothelial barrier. J Biol Chem 290: 4097–4109, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann NY Acad Sci 1123: 134–145, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Vikis HG, Guan KL. Glutathione-S-transferase-fusion based assays for studying protein-protein interactions. Methods Mol Biol 261: 175–186, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe T, Noritake J, Kakeno M, Matsui T, Harada T, Wang S, Itoh N, Sato K, Matsuzawa K, Iwamatsu A, Galjart N, Kaibuchi K. Phosphorylation of CLASP2 by GSK-3beta regulates its interaction with IQGAP1, EB1 and microtubules. J Cell Sci 122: 2969–2979, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Waters CM. Reactive oxygen species in mechanotransduction. Am J Physiol Lung Cell Mol Physiol 287: L484–L485, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 20: 6418–6434, 2001. [DOI] [PubMed] [Google Scholar]
- 57.White CD, Erdemir HH, Sacks DB. IQGAP1 and its binding proteins control diverse biological functions. Cell Signal 24: 826–834, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell 127: 1027–1039, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Zegers MM, Friedl P. Rho GTPases in collective cell migration. Small GTPases 5: e28997, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]