Abstract
Parietal epithelial cell (PEC) response to glomerular injury may underlie a common pathway driving fibrogenesis following podocyte loss that typifies several glomerular disorders. Although the mammalian target of rapamycin (mTOR) pathway is important in cell homeostasis, little is known of the biological role or impact of reducing mTOR activity on PEC response following podocyte depletion, nor in the aging kidney. The purpose of these studies was to determine the impact on PECs of reducing mTOR activity following abrupt experimental depletion in podocyte number, as well as in a model of chronic podocyte loss and sclerosis associated with aging. Podocyte depletion was induced by an anti-podocyte antibody and rapamycin started at day 5 until death at day 14. Reducing mTOR did not lead to a greater reduction in podocyte density, despite greater glomerulosclerosis. However, mTOR inhibition lead to an increase in PEC density and PEC-derived crescent formation. Additionally, markers of epithelial-to-mesenchymal transition (platelet-derived growth factor receptor-β, α-smooth muscle actin, Notch-3) and PEC activation (CD44, collagen IV) were further increased by mTOR reduction. Aged mice treated with rapamycin for 1, 2, and 10 wk before death at 26.5 mo (≈75-yr-old human age) had increased the number of glomeruli with a crescentic appearance. mTOR inhibition at either a high or low level lead to changes in PEC phenotype, indicating PEC morphology is sensitive to changes mediated by global mTOR inhibition.
Keywords: podocyte, glomerulosclerosis, glomerulus, crescent, pS6RP, aging
focal and segmental glomerulosclerosis (FSGS) is characterized by scarring and adhesions within the glomerulus and occurs in response to genetic defects of key podocyte proteins, viral illnesses, drugs, and hyperfiltration (12, 13). Additionally, glomerulosclerosis is a common pathological feature of other proteinuric glomerular diseases, including diabetes, lupus, and IgA nephropathy (12), and in the aged kidney (45). Depletion of podocyte number, and the inability of these terminally differentiated epithelial cells to self-renew, are thought to be a major pathological basis of glomerulosclerosis (21, 47).
Glomerular parietal epithelial cells (PEC) are a monolayer of small flat ciliated cells that line Bowman's capsule and surround the urinary space (46). They form junctions with proximal tubular epithelial cells at the urinary pole, and podocytes at the vascular pole. Functionally, PECs form an impermeable barrier to filtered proteins through tight junctions and are involved in albumin uptake (10, 42). In response to glomerular injury, PECs have the ability to migrate and proliferate (16, 26, 49, 52). There is increasing evidence that the response of PECs to podocyte injury, and/or podocyte depletion, also contributes to glomerular scarring through their deposition of extracellular matrix as they migrate on to the glomerular tuft (15, 50–52).
Under certain circumstance, PECs may provide a progenitor population to replace damaged glomerular cells (46). Appel and colleagues (5) identified transitional cells at the glomerular vascular stalk that exhibit features of both PECs and podocytes and the number of these transitional cells increases in response to glucocorticoids and retinoid treatment in FSGS, as well as in diabetes (4, 61, 62), suggesting that a subset of PECs can change their phenotype in response to glomerular injury. More and more is being deciphered regarding PEC regulation: what signaling pathways are activated and what induces PECs to proliferate at the capsule to become a crescent (34), migrate into the glomerulus to replete podocytes (15), or secrete matrix and contribute to scarring (36).
The mammalian target of rapamycin (mTOR) pathway regulates essential cellular processes, including translation, transcription, and autophagy in response to a wide variety of nutrient cues, including growth factors, amino acids, cellular energy content, and stress (27). mTOR has been particularly well studied in the podocyte. mTOR activity in podocytes is increased in FSGS, diabetic kidney disease, IgA nephropathy, and minimal change disease (22, 28, 54, 58). In experimental crescentic glomerulonephritis (GN), mTOR activity, measured by S6 ribosomal phosphorylation protein (pS6RP) expression was increased in podocytes and PECs early after induction of disease. The mTOR inhibitor everolimus administered early in this model leads to greater glomerular injury, while mTOR inhibition at a later time point reduced the development of crescents (31). Genetic reduction of mTORC1 in podocytes prevented the development of diabetic nephropathy (28), while deletion of the TSC1 gene, a negative regulator of mTORC1, leads to constitutive mTORC1 hyperactivation, resulting in diabetic nephropathy-like changes, as well as crescents in the glomerulus (19). Additionally, podocyte-specific disruption of mTORC1 complex during glomerular development resulted in secondary FSGS (19, 28).
These studies show that the biological role of mTOR in the glomerulus may be context, dose, and cell type dependent. However, the impact of reducing mTOR activity on PEC response following abrupt podocyte depletion, and in the aging kidney, are not well delineated. The purpose of the present study was to determine the impact on PECs of reducing mTOR activity following an abrupt depletion in podocyte number that leads to glomerulosclerosis, as well as in a model of chronic podocyte loss and sclerosis in the aging kidney.
METHODS
Animals
For rapamycin studies, 12 (7 female, 5 male) 10- to 12-wk-old mice were used. For aging studies, 24-mo-old female mice (n = 28) were obtained from the National Institute on Aging (Charles River). By the end of a further 10-wk treatment or control aging, these mice are considered advanced age, equivalent to humans aged 78 yr old (38, 55). Three-month-old female mice (n = 7) served as young control mice. In the aging study, only female animals were studied to exclude sex differences in life span. Mice were housed in the animal care facility of the University of Washington under specific pathogen-free conditions with ad libitum food and water. Animal protocols were approved by the University of Washington Institutional Animal Care and Use Committee (2968-04).
Experimental Acute Podocyte Depletion
Acute podocyte depletion (APD) was induced in mice (n = 8) with a cytotoxic sheep anti-podocyte antibody, as previously described (60, 62). Briefly, two doses of sheep anti-glomerular antibody, at 10 mg/20 g body wt, were dosed via intraperitoneal (IP) injection, 24 h apart. This cytotoxic antibody induces abrupt podocyte depletion, accompanied by glomerulosclerosis, as previously described (15, 43, 62, 63).
Rapamycin Treatment
In the experimental APD group, mice were randomly assigned to rapamycin treatment (LC Laboratories, Woburn, MA) (8 mg/kg IP) or vehicle treatment from day 5 (D5) to D13 and were killed on D14 (n = 4). Rapamycin blood concentration with this dose regimen has been previously described (30). Normal baselines (n = 4) were killed at D1 of the experiment. In the aging mice study, microencapsulated rapamycin (Rapamycin Holdings, San Antonio, TX) at a dose of 14 parts/million was added to standard chow (Purina) 1 wk (n = 7), 2 wk (n = 7), and 10 wk (n = 7) before death at 26.5 mo of age. Control mice (n = 7) received standard chow. Rapamycin blood concentration with both dose regimen has been previously described (9, 30). At death, mice were perfused with 10 ml of ice-cold PBS to remove excess red blood cells. Kidneys were fixed overnight at 4°C in 10% neutral buffered formalin (Globe Scientific, Paramus, NJ), rinsed in 70% ethanol, processed, and embedded in paraffin.
Immunostaining
Immunoperoxidase staining was performed on 4-μm tissue sections from mouse kidney biopsies fixed in formalin and embedded in paraffin. Sections were deparaffinized using Histoclear (National Diagnostics, Atlanta, GA) and rehydrated in a graded series of ethanol.
Antigen retrieval was performed by boiling in 10 mM citric acid buffer (pH 6.0 or 7.0) or in 10 mM EDTA buffer (pH 6.0). Nonspecific antibody binding was blocked using background buster (Accurate Chemical & Scientific, Westbury, NY) for 20 min at room temperature. When biotinylated secondary antibodies were used, endogenous biotin activity was suppressed with an avidin/biotin blocking kit (Vector Labs, Burlingame, CA). Mouse block was used for 20 min before mouse monoclonal antibodies. Antibodies were diluted in 1% IgG-free BSA (Sigma-Aldrich, St. Louis, MO) in PBS and incubated overnight at 4°C. Secondary antibodies and streptavidin conjugates were incubated for 1 h at room temperature.
For immunoperoxidase staining, endogenous peroxidase activity was blocked by incubation in 3% H2O2 for 15 min. After primary antibody incubation and labeling with horseradish peroxidase, immunostaining was visualized by precipitation of diaminobenzidine (Sigma-Aldrich, St. Louis, MO). Periodic acid-Schiff (PAS) staining was performed as a counterstain, and slides were then dehydrated in ethanol and mounted with Histomount (National Diagnostics, Atlanta, GA). The following primary antibodies were used for immunoperoxidase staining: rabbit anti-p57 (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-ki67 (BD Bioscience, San Jose, CA). Single immunofluorescence staining was performed on 4-μm tissue sections from tissue fixed in formalin and embedded in paraffin using the following primary antibodies: rabbit anti-paired box gene 8 (PAX8; Abcam, Cambridge, MA), rabbit anti-Src-suppressed C-kinase substrate (SSeCKS) (Abcam), and rat anti-claudin-1 (Abcam). Staining was detected using Alexa 488-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA).
To discern staining of PECs on Bowman's capsule, double-immunofluorescence staining for mouse anti-α-smooth muscle actin (α-SMA) (Sigma)/rabbit anti-PAX8, rabbit anti-Notch-3/rabbit anti-PAX8, biotinylated goat-anti-collagen IV (Southern Biotech, Birmingham, AL)/rabbit anti-PAX8, rat anti-CD44/rabbit anti-PAX8, rabbit-anti-pS6RP (Abcam)/rabbit anti-PAX8, rabbit anti-platelet-derived growth factor receptor-β (PDGFβR)/rabbit anti-PAX8, and rabbit anti-PDGFβR/biotinylated goat-anti-collagen IV was performed in paraffin-embedded tissue. For podocyte detection, immunofluorescence staining for mouse anti-synaptopodin (Abcam) and rabbit pS6RP double was performed. pS6RP, PAX8, Notch-3, and PDGFβR antibodies were detected using Alexa 488-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch). PAX8 was additionally detected using IgG (Vector Labs), in combination with Alexa 594 conjugated streptavidin (Life Technologies). CD44 was detected using biotinylated anti-rat (Vector Labs) and visualized with Alexa 594-conjugated streptavidin (Life Technologies). Synaptopodin and α-SMA were detected using biotinylated anti-mouse and visualized with Alexa 594-conjugated streptavidin (Life Technologies). Collagen type IV was visualized with Alexa 594-conjugated streptavidin (Life Technologies). Blocking with rabbit IgG Fab fragment (Jackson) fab fragment goat anti-rabbit IgG was performed between anti-rabbit primary antibodies. For all staining, omission of the primary antibody served as negative controls. Bright-field pictures for quantification were acquired with a Leica DMRB microscope (Leica, Wetzlar, Germany).
Images of fluorescent staining were acquired on a Leica TCS SPE II laser scanning confocal microscope.
Assessment of Glomerulosclerosis, Crescents, and Podocyte Depletion
Immunostaining was performed for p57 with PAS counterstaining to measure podocyte density and assess glomerulosclerosis, as previously reported (57, 61–63). In brief, paraffin sections were processed as described above, with antigen retrieval in 1 mM EDTA, pH 8.0. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide, and sections were incubated overnight at 4°C with a primary rabbit anti-p57 (1:800, Santa Cruz Biotechnology), followed by rabbit on rodent HRP-polymer (Biocare Medical). Visualization of immunostaining was by precipitation of diaminobenzidine (Sigma-Aldrich, St. Louis, MO). Counterstaining was performed with PAS by washing slides in fresh 0.5% periodic acid (Sigma-Aldrich) for 8 min; washing for 5 min in double-distilled H2O; sections were incubated for 10 min at room temperature with Schiff's reagent (Sigma-Aldrich); washing two times for 5 min in fresh 0.5% sodium metabisulfate (Sigma-Aldrich); and washing for 5–10 min under running tap water. Slides were dehydrated in ethanol and mounted with Histomount. An average of 40 + 10 glomeruli in each animal was graded, as previously reported. Quantification of podocyte density was performed on p57/PAS-stained sections, as described (57). An average of 37 ± 9 glomeruli was assessed for each animal. The number, diameter of podocytes, and area of glomerular cross section was measured with ImageJ (version 1.48, National Institutes of Health, Bethesda, MD), and scale bars were applied to all pictures using this software. Crescents were identified on PAS-stained sections as glomeruli with two or more layers of cells lining Bowman's capsule excluding the proximal tubular region.
Quantification and Unbiased Stereology Microscopy
Immunostaining was examined on a Lecia DMRB microscope, an EVOS FL Cell imaging system, and Leica TCS SPE II laser scanning confocal microscope.
Quantification of PEC Number
The number of PECs was assessed by PAX8 staining; PECs were identified as cells lining Bowman's capsule that showed strong nuclear staining. An average of 97 ± 11 glomeruli was assessed for each animal. As the number of PECs was biased by changes in glomerular size, we also measured the Bowman's capsule length of each glomerulus. Using an unbiased stereology approach, we then calculated PEC density by dividing the number of PAX8-stained cells in individual glomeruli by the corresponding Bowman's capsule length in that glomerulus.
Quantification of pS6RP, Notch-3, Collagen IV Activity
The area of Bowman's capsule staining positive for pS6RP, Notch-3, and collagen IV staining was calculated using Image J (version 1.48, National Institutes of Health). Briefly, 8-bit images were inverted, and, with the same threshold on maximum entropy, each glomerulus was encircled carefully around Bowman's capsule, and this area was measured. The process was repeated again, measuring only around the glomerular tuft. The area positive for Bowman's capsule minus glomerular tuft was then calculated for 40 glomeruli per animal to give area positive for pS6RP, Notch-3, and collagen IV.
Quantification of PDGFβR, α-SMA, and CD44
To exclude quantifying periglomerular PDGFβR staining, the number of glomeruli positive for PDGFβR on Bowman's capsule was calculated using double immunofluorescence staining for collagen IV to indicate the location of Bowman's capsule. Glomeruli were considered to be positive were PDGFβR staining occurred within the urinary side of collagen IV staining. The number of glomeruli positive for α-SMA staining was calculated using double immunofluorescence staining for PAX8+, and α-SMA with glomeruli considered positive where PAX8+ nuclei occurred within range of α-SMA staining. Forty glomeruli were assessed for the quantification of each antibody. A glomerulus was considered positive if there was at least one cell lining the Bowing's capsule that stained with the respective antibody.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6.0 (La Jolla, CA). A two-tailed unpaired Student's t-test was applied to compare means of groups, and P < 0.05 was considered statistically significant. Data are presented as means ± SD.
RESULTS
APD Is Associated with an Increase in mTOR Activity in PECs
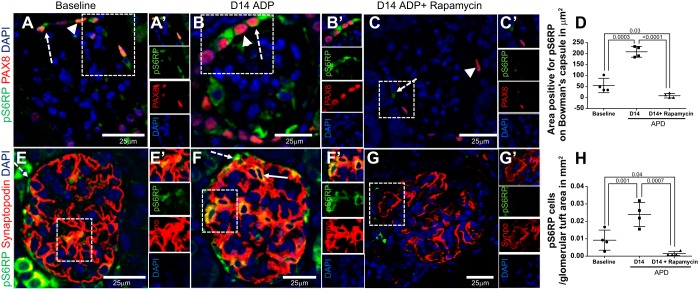
Because mTOR activates protein synthesis through phosphorylation of S6 kinase and pS6RP, pS6RP immunostaining is used as an indicator of mTOR activity (27). Double-staining for pS6RP and PAX8 was performed to quantify the number of PECs with active mTOR (Fig. 1, A–D). pS6RP staining was only occasionally present at baseline in PECs on Bowman's capsule (Fig. 1A), but was higher following the induction of APD [54.97 ± 16.26 (baseline) vs. 207.3 ± 12.33 μm2 of Bowman's capsule pS6RP positive (D14 APD), P = 0.002] (Fig. 1, B and D). When given starting at D5 APD, rapamycin resulted in near complete reduction of mTOR activity throughout the kidney, including reduced pS6RP staining in PECs compared with baseline mice [54.97 ± 16.26 (baseline) vs. 8.27 ± 5.15 μm2 of Bowman's capsule pS6RP positive (D14 APD + rapamycin), P = 0.03] and to D14 APD mice without rapamycin [207.3 ± 12.33 μm2 (D14 APD) vs. 8.27 ± 5.15 μm2 of Bowman's capsule pS6RP positive (D14 APD + rapamycin), P = 0.0001].
Fig. 1.
Acute podocyte depletion (APD) is associated with an increase in mTOR activity that is reduced by rapamycin. A–C: representative high-power (×400) confocal microscopy images of immunofluorescent double staining for the mTOR activity marker pS6RP (green, cytoplasmic, dashed arrow) and paired box protein 8 (PAX8) (red, nuclear, arrowhead) used to identify PECs. Nuclei are stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Scale bars are provided. pS6RP (green) is shown in PECs from 10- to 12-wk-old mice at baseline (A) and day 14 of experimental APD (D14 APD; B), and D14 APD mice given rapamycin 8 mg/kg ip daily, started at D5 to death at D14 (D14 APD + rapamycin; C). The boxes in A–C show a magnified image (A′, B′, C′), and single channels are shown to the right of each image. D: pS6RP expression in PEC is demonstrated. Graph shows that the area positive for PS6RP staining on Bowman's capsule was higher at D14 APD compared with baseline, which was reduced by rapamycin treatment. E–G: images showing high-power (×400) confocal microscopy images of immunofluorescent double staining for pS6RP (green, cytoplasmic, dashed arrow) and synaptopodin (red, cytoplasmic) used to demarcate podocytes within glomerular tuft. Nuclei are stained blue with DAPI. Scale bars are provided. pS6RP (green) is colocalized within podocytes expressing synaptopodin (red) and appears as yellow (solid arrow) at baseline (E) and D14 APD (F), but is reduced in D14 APD mice given rapamycin (G). The boxes in E–G show a magnified image (E′, F′, G′), and single channels are shown to the right of each image. H: graph showing that the number of cells staining positive for pS6RP in the glomerular tuft area was higher at D14 APD compared with baseline and was reduced by rapamycin treatment.
Double-staining for pS6RP and synaptopodin identified mTOR activity in podocytes (Fig. 1, E–H). As expected, the number of cells positive for pS6RP within the glomerular tuft also increased at D14 APD compared with baseline [0.009 ± 0.002 (baseline) vs. 0.026 ± 0.005 cells/mm2 glomerular tuft area (D14 APD), P = 0.04] (Fig. 1, E–G). Rapamycin resulted in a reduction in pS6RP in cells in the glomerular tuft compared with baseline mice [0.009 ± 0.002 (baseline) vs. 0.001 ± 0.0002 cells/mm2 glomerular tuft area (D14 APD + rapamycin), P = 0.04] and compared with D14 APD mice without rapamycin [0.02 ± 0.002 (D14 APD) vs. 0.001 ± 0.0002 cells/mm2 glomerular tuft area (D14 APD + rapamycin), P = 0.007] (Fig. 1, C–F).
These results show that staining for the marker of mTOR activity, pS6RP, increased in PECs and in podocytes in a model of podocyte injury and acute depletion, and that rapamycin reduces pS6RP staining, consistent with reduced mTOR activity in both glomerular epithelial cell types.
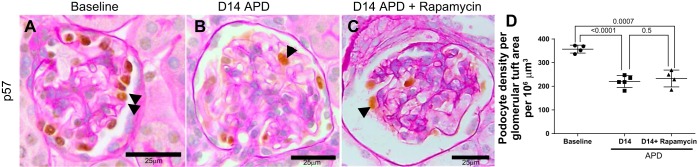
Inhibition of mTOR in a Model of APD Does Not Change Podocyte Density
We next assessed the impact of inhibiting mTOR on podocyte density in this model of APD. Because our laboratory's previous studies showed podocyte depletion and albuminuria was established at D5 APD (15), rapamycin, a specific inhibitor of the mTORC1 pathway, was only started at D5 APD to test the impact on podocyte density at a later time point (D14). Podocyte density, identified by p57 immunostaining, was lower in untreated APD mice [357 ± 16 (baseline) vs. 219 ± 24 podocytes/μm3 (D14 APD), P < 0.05 vs. baseline] (Fig. 2, A–C). Podocyte density did not differ in mice treated with rapamycin at D14 [219 ± 24 (D14 APD) vs. 232 ± 35 podocytes/μm3 (D14 APD + rapamycin), P = 0.9] (Fig. 2D). Rapamycin treatment did not result in any significant change in tuft volume [3,443 ± 257 (D14 APD) vs. 2,815 ± 325 μm2 (D14 APD + rapamycin), P = 0.1].
Fig. 2.
The depletion in podocyte density was not altered by rapamycin in experimental APD. Podocytes were identified by p57 staining (brown nuclear stain, examples indicated by black arrowheads) in sections counterstained with periodic acid Schiff stain from mice at baseline (A), D14 APD (B), and D14 APD + rapamycin (C). Scale bars are provided. Representative images were taken under the same magnification (×400). Podocyte density was lower at D14 of experimental APD, but this was no greater with rapamycin. D: graph showing podocyte density in number of podocyte per 106 mm3 at baseline, D14 APD, and D14 APD + rapamycin. N = 4/group. P < 0.05 for significance, Student's T-test between groups.
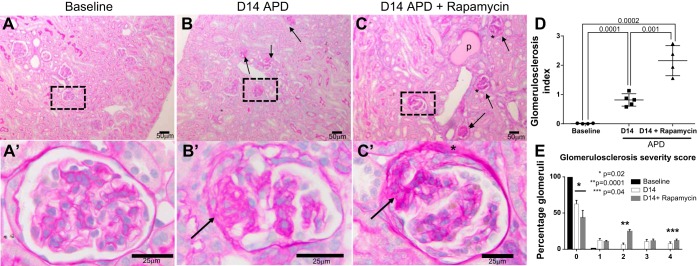
Inhibition of mTOR in APD Results in Higher Glomerulosclerosis
Overall, the number of injured glomeruli was increased in rapamycin-treated mice with crescents, synechiae, and thrombosis of capillaries present (Fig. 3, A–C). The severity of glomerulosclerosis was measured by scoring 55 ± 6 glomeruli on a scale of 0–4.
Fig. 3.
Glomerulosclerosis is higher in rapamycin-treated mice with APD. A–C: representative examples of low-power magnification (×100) periodic acid Schiff stained sections used to quantify glomerulosclerosis in mice at baseline (A, A′), D14 FSGS (B, B′), and D14 FSGS with rapamycin (C, C′), and the results are shown in the graph (D). A′–C′: higher power magnification (×400) of the insets in A–C. Scale bars are provided. Glomerulosclerosis was increased at D14 FSGS compared with baseline and was further increased following rapamycin treatment. Black arrows indicate examples of sclerosed glomeruli demonstrating synechiae and thrombosis. Asterisks indicate glomeruli with crescents. p indicates tubule dilated with proteinaceous material. E: graph of percentage of glomeruli with glomerulosclerosis at baseline, D14 APD, and D14 APD + rapamycin, scored from 0 to 4 by extent of sclerosis, where 0 is without sclerosis. The number of glomeruli with a score of 4 was higher in the rapamycin-treated mice with FSGS. *P = 0.02. **P = 0.0001. ***P = 0.04.
Glomerulosclerosis was higher in mice treated with rapamycin at D14 [0.89 ± 0.07 (D14 APD) vs. 1.23 ± 0.09 (D14 APD + rapamycin), P = 0.04] (Fig. 3D). Moreover, the number of severely damaged glomeruli, i.e., glomeruli with a score of 4, was higher in the rapamycin group (Fig. 3E). These results show that, when mTOR activity was significantly lowered in PECs and in podocytes, glomerulosclerosis was significantly more severe.
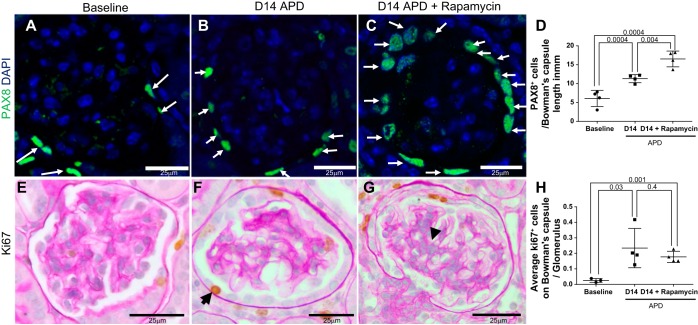
PEC Density Is Increased by mTOR Inhibition in Model of APD
PAX8 staining was used as a marker to identify PECs, from which PEC density was calculated (Fig. 4, A–D). Experimental APD was associated with a higher number of PAX8+ cells on Bowman's capsule at D14 [6.0 ± 1 (baseline) vs. 11.32 ± 0.5 PAX8+ cells/mm Bowman's capsule length (D14 APD), P = 0.001] (Fig. 4, A, B, and D). PEC density was higher in rapamycin-treated APD mice compared with nontreated APD mice at D14 [11.32 ± 0.5 (D14 APD) vs. 16.52 ± 1.0 PAX8+ cells/mm Bowman's capsule length (D14 + rapamycin), P < 0.05] (Fig. 4, A–D). Consistent with this, the number of proliferating cells on Bowman's capsule, measured by ki67 immunostaining, was higher at D14 APD compared with baseline [0.02 ± 0.007 (baseline) vs. +0.23 ± 0.06 ki67+ on Bowman's capsule/glomerulus (D14 APD), P < 0.05] (Fig. 4, E, F, and H).
Fig. 4.
Parietal epithelial cell (PEC) number is increased in rapamycin-treated mice with experimental APD. PECs were identified by paired box gene 8 (PAX8) staining (green, nuclear, white arrows show examples) (A–C) and their density quantitated (D). Compared with baseline (A), the number of cells staining for PAX8 was higher at D14 APD (B). C: PAX8-stained cells were highest in rapamycin-treated APD mice. E–G: proliferating cells were identified by ki67 staining (brown nuclear stain, examples indicated by black arrowheads) in sections counterstained with periodic acid Schiff stain in mice at baseline (E), D14 APD (F), and D14 + rapamycin (G). Scale bars are provided. Representative images were taken under the same magnification (×400). H: graph showing the number of ki67+ cells on Bowman's capsule increased at D14 APD, but was no higher with rapamycin treatment.
However, the number of ki67+ cells/glomerulus on Bowman's capsule did not differ between animals with APD, with and without rapamycin treatment [0.23 ± 0.06 (D14 APD) vs. 0.17 ± 0.01 ki67+ on Bowman's capsule/glomerulus (D14 + rapamycin), P = 0.4] (Fig. 4, F–H).
Rapamycin did not alter Bowman's capsule length following APD [207 ± 10 (D14 APD) vs. 237 ± 20 μm (D14 APD + rapamycin)]. Phosphohistone H3, another proliferation marker, was not different between D14 APD and D14 APD + rapamycin (data not shown).
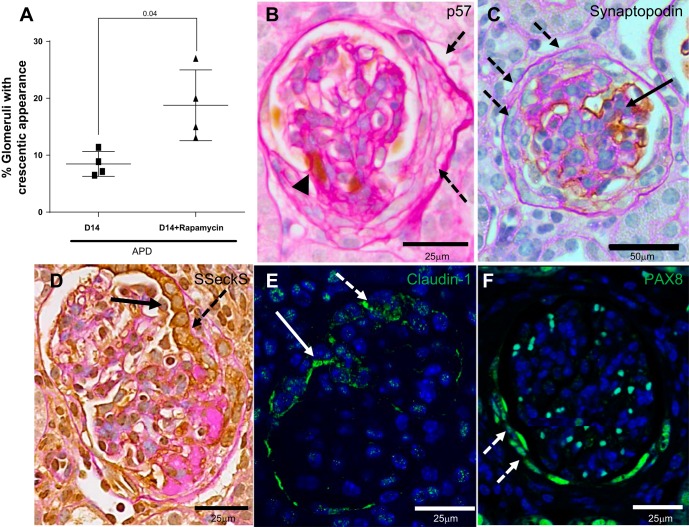
Crescents Increase in Rapamycin-Treated Mice with APD
Cellular crescents were defined as two or more cell layers lining Bowman's capsule. An example is demonstrated in Fig. 5B. In APD mice treated with rapamycin, the percentage of glomeruli with cellular crescents was higher [8.7 ± 1.0 (D14) vs. 18.77 ± 3.1% (D14 APD + rapamycin), P = 0.02] (Fig. 5A). Cells in the crescents did not stain for the podocyte markers p57 and synaptopodin (Fig. 5, B and C). SSeCKS and claudin-1 staining mark PECs (8). In a proportion of glomeruli with crescents, SSeCKS expression remained in the PEC, covering the urinary aspect of the crescent, and its expression was lower in the cuboidal layers of cells below this (Fig. 5D). SSeCKS expression in another proportion of crescents was absent. This pattern of expression was similar for claudin-1 in crescents (Fig. 5E). All crescents stained positive for PEC marker PAX8 (Fig. 5F). There was no difference in the number of p57+ cells present on Bowman's capsule in APD mice with and without rapamycin (data not shown). These results showed that reducing mTOR in a model of APD was accompanied by increased crescents, comprising PECs but not podocytes.
Fig. 5.
Glomerular crescents develop in a subset of glomeruli in APD mice given rapamycin. A: graph showing that the percentage of glomeruli exhibiting crescents, defined as two or more cell layers along Bowman's capsule, was higher in APD treated with rapamycin. B: representative high-power image (×400) of podocytes identified by p57 staining (brown nuclear stain, arrowhead) in sections counterstained with periodic acid Schiff stain from mice D14 APD given rapamycin. Dashed arrows indicates a crescent that is negative for p57. C: podocytes identified by synaptopodin staining (brown nuclear stain, solid arrow) in sections counterstained with periodic acid Schiff stain from mice D14 APD given rapamycin with crescent negative for synaptopodin staining (dashed arrow) but nonaffected area positive for synaptopodin (solid arrow). Representative image is at ×200 magnification. D: PECs identified by Src-suppressed C-kinase substrate (SSeCKS) staining (brown cytoplasmic stain on periodic acid Schiff background) in a glomerulus with a crescent indicated by dashed arrow at high power (×400). In the region of the crescent, PEC cells are cuboidal and stained positive for SSeCKS (solid arrow). E: crescent is positive for PEC marker claudin-1. Representative confocal microscopy image is of a glomeruli at high power (×400) stained for claudin-1 (green, cytoplasmic). Nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Crescent is indicated by dashed white arrow. White solid arrow indicates claudin-1 staining on cytoplasmic border of PEC cell. F: representative confocal microscopy image of a glomerulus at high power (×400) stained for paired box gene 8 (PAX8; green) with DAPI nuclear stain. A crescent, indicated by dashed white arrow, is positive for PEC marker PAX8.
Mesenchymal Markers in PECs Are Higher in APD Mice Given Rapamycin
α-SMA.
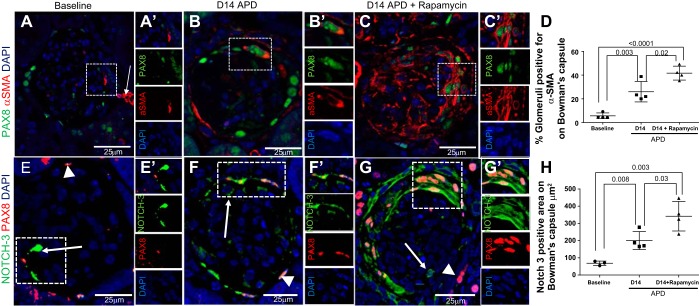
The PECs response to primary podocyte damage includes an increase in mesenchymal marker expression, thought to be part of a maladaptive repair sequence (39, 48). Double immunofluorescence staining was performed on kidney sections for de novo expression of α-SMA and PDGFβR. PAX8 was co-stained with α-SMA so that Bowman's capsule could be identified accurately. At D14 APD, the number of glomeruli with PEC positive for the mesenchymal markers α-SMA was increased [5.6 ± 1.2 (baseline) vs. 26.0 ± 4.3% (D14 APD), P < 0.0001] (Fig. 6, A, B, and D); this was higher in rapamycin-treated mice with ADP compared with untreated mice treated with anti-podocyte antibody [26.0 ± 4.3 (D14 APD) vs. 41.6 ± 2.9% (D14 APD + rapamycin), P = 0.02] (Fig. 6, B–D).
Fig. 6.
Markers for epithelial-to-mesenchymal transition are further increased in PECs in APD mice given rapamycin. A–D: α-SMA and paired box 8 (PAX8) double-staining. Confocal microscopy (magnification ×400) showing immunofluorescent double staining for α-SMA (red, cytoplasmic, arrow) and PAX8 (green, nuclear) used to better demarcate Bowman's capsule. Nuclei are stained blue with 4′,6-diamidino-2-phenylindole (DAPI). The dashed box shows a magnified image (A′), and single channels are shown to the right. Scale bars are provided. D: graph of the quantitation for α-SMA staining. A: α-SMA staining was occasionally detected along Bowman's capsule in a small subset of glomeruli at baseline. White arrow indicates positive control staining in the adjacent vasculature. Dashed box shows a magnified image of PAX8+ cell and adjacent α-SMA cytoplasmic positive Bowman's capsule in A′, and single channels are shown to the right. B: at D14 APD, α-SMA staining was increased along Bowman's capsule. B′: when the inset is viewed under higher power, the results show PAX8+ nuclear staining with α-SMA+ cytoplasmic staining in the same cell. C: α-SMA staining is higher after rapamycin treatment than D14 APD. Area in dashed box shows a crescent with PAX8+ nuclear staining with α-SMA+ cytoplasmic staining in the same cells to indicate a glomerulus positive for α-SMA. This is shown magnified in C′, and single channels are shown to the right. D: graph showing percentage of glomeruli positive for α-SMA along Bowman's capsule was significantly higher at D14 APD + rapamycin compared with baseline and D14 APD. E–H: confocal microscopy showing immunofluorescent double staining for Notch-3 (green, arrow) and PAX8 (green, nuclear, solid arrow) (magnification: ×400). Confocal microscopy (magnification ×400) showing immunofluorescent double staining for Notch-3 (green, cytoplasmic, arrow) and PAX8 (red, nuclear, arrowhead) was used to better demarcate Bowman's capsule. Nuclei are stained blue with DAPI. Scale bars are provided. E: staining for Notch-3 was present on PEC border (arrow) and glomerular tuft (arrowhead) at baseline in many glomeruli. This is shown in E′ and as single-channel images to the right. F and F′: the area positive higher in both PECs (arrow) increased at D14 APD. G: Notch-3 staining was augmented in PECs (arrow) by rapamycin treatment within the crescentic area, as indicated by multilayers of PAX8+ PECs. This is shown in G′ and in single channels to the right. H: graph showing area on Bowman's capsule positive for Notch-3 increased at D14 APD compared with baseline and was significantly higher at D14 APD + rapamycin compared with baseline and D14 APD. Area positive for Notch-3 was calculated in cubic micrometers, as the number of glomeruli positive was high at baseline but area positive appeared increased. N = 4/group. P < 0.05 for significance, Student's T-test between groups.
Notch-3.
Notch-3 signaling is activated in epithelial-mesenchymal transition and is an important signaling pathway for determining cell fate and differentiation (33, 37, 40, 56). Staining for Notch-3 was higher in both PECs at D14 [68.3 ± 7.6 (baseline) vs. 200.0 ± 26.1 μm2 (D14 APD), P = 0.003] (Fig. 6, E and F–H). Notch-3 staining was augmented in PECs by rapamycin treatment [200.0 ± 26.1 (D14 APD) vs. 341.9 ± 43 μm2 (D14 APD + rapamycin), P = 0.008] (Fig. 6, F–H).
PDGRβR.
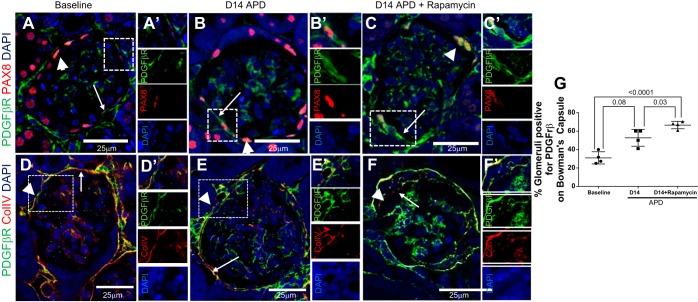
PDGRβR was co-stained with collagen IV so that Bowman's capsule location could be identified accurately. This was particularly important for PDGFβR, as this was strongly staining in the periglomerular region in addition to the expression on Bowman's capsule (3). We defined glomeruli with PDGFβR positive for PECs as PDGFβR signal on the urinary aspect of the collagen IV staining (45). PDGFβR co-staining adjacent to PAX8+ cells on Bowman's capsule was also established (Fig. 7, D and E). The number of glomeruli positive for PDGFβR in PECs at D14 APD was increased [30.9 ± 3.3 (baseline) vs. 52.7 ± 4.7% (D14 APD), P = 0.008] (Fig. 7, A and B) and was higher in rapamycin-treated mice with APD compared with untreated APD mice [52.7 ± 4.7% (D14 APD) vs. 66.6 ± 2.0% (D14 APD + rapamycin), P = 0.03] (Fig. 7, B–D).
Fig. 7.
Marker for epithelial-to-mesenchymal transition PDGFβR is increased in PECs in APD mice given rapamycin. A–C: confocal microscopy showing immunofluorescent double staining for PDGFβR (green, arrowheads) and PAX8 (red, arrows) (magnification: ×400). PAX8 staining was used to demarcate PECs. Scale bars are provided. Nuclei were stained blue with DAPI. A: representative image of glomeruli at baseline showing PDGFβR in PECs. A′: a PEC positive for PDGFβR adjacent to a PAX8+ PEC is seen magnified and is shown as single-channel images below. B: the number of glomeruli positive for PDGFβR increases at D14 APD. The staining for PDGFβR on the same plane as PAX8 is apparent in the dashed box and is shown magnified in B′ and is shown as single-channel images to the right. C: representative image of glomeruli at D14 APD + rapamycin showing PDGFβR in PEC along Bowman's capsule. The staining for PDGFβR along PAX8+ PEC is apparent in the dashed box and is shown magnified in C′ and as single-channel images to the right. Collagen type IV staining was also used to demarcate Bowman's capsule, as there was no antibody cross reactivity. Scale bars are provided. Nuclei were stained blue with DAPI. We defined glomeruli with PDGFβR positive in PECs when there was PDGFβR signal on the urinary aspect of the collagen IV staining. D: representative image of glomeruli at baseline showing PDGFβR in PECs. D′: a PEC positive for PDGFβR but negative for collagen IV is seen magnified and is shown as single channel image below. E: the number of glomeruli positive for PDGFβR increases at D14 APD. The staining for PDGFβR on the urinary side of collagen IV is apparent in the dashed box and is shown magnified in E′ and is shown as single-channel images to the right. F: representative image of glomeruli at D14 APD + rapamycin showing PDGFβR in PEC along Bowman's capsule. The staining for PDGFβR on the urinary side of collagen IV is apparent in the dashed box and is shown magnified in F′ and as single-channel images to the right. G: graph showing percentage of glomeruli positive for PDGFβR along Bowman's capsule at baseline, D14 APD, and D14 APD + rapamycin was significantly higher at D14 APD + rapamycin compared with baseline and D14 APD.
Taken together, these results show that reducing mTOR activity in experimental APD is temporally associated with increased staining for the mesenchymal markers α-SMA, Notch-3, and PDGFβR in PECs.
PEC Activation Is Higher in Rapamycin-Treated APD Mice
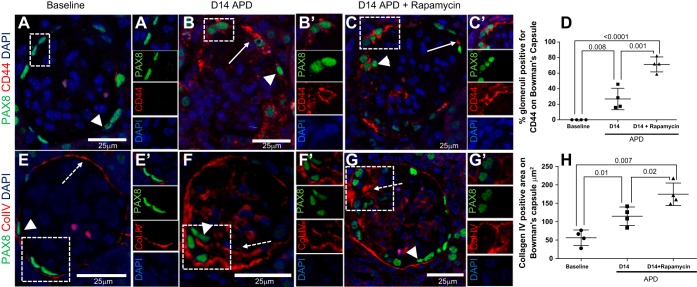
CD44 staining is used as a marker of PEC activation (52). As our laboratory previously reported in this model of APD (7), CD44 staining is increased de novo in PECs (Fig. 8, A, B, and D). The number of glomeruli with PECs staining for CD44 was further increased following rapamycin treatment [26.8 ± 6.8 (D14 APD) vs. 71.2 ± 4.8% (D14 APD + rapamycin), P = 0.008] (Fig. 8, B–D). CD44 staining was present within crescents and synechiae of both groups (Fig. 8, C and D). Collagen IV is a major component of the basement membrane that is deposited by PEC cells with levels increasing in response to glomerular injury (26, 53). As expected, the area positive for collagen IV staining on Bowman's capsule increased following induction of APD [56.4 ± 10.5 (baseline) vs. 114.9 ± 12.5 μm2 (D14 APD), P = 0.01], and this was further increased in mice given rapamycin [114.9 ± 12.5 (D14 APD) vs. 174.6 ± 15.2 μm2 (D14 APD + rapamycin), P = 0.01] (Fig. 8, E–H). Taken together, lowering mTOR was accompanied by PEC activation and increased matrix accumulation in experimental APD.
Fig. 8.
Immunostaining for PEC activation marker CD44 and collagen IV is higher in PECs in APD mice given rapamycin. A–D: confocal microscopy showing immunofluorescent double staining for CD44 (red, solid arrow) and PAX8 (green, arrowheads) (magnification: ×400). PAX8 staining was used to demarcate PECs. Scale bars are provided. Nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). A: representative image of glomeruli at baseline in which CD44 expression in glomeruli is absent. A′: a PEC negative for CD44 is seen magnified and is shown as single-channel images below. B: expression of CD44 increases in PECs at D14 APD, as indicated by solid arrow. PEC surrounded by CD44 is shown magnified in B′ and is shown as single channels below. C: CD44 expression is higher in PECs following rapamycin treatment. The dashed box demonstrating CD44 in PEC on Bowman's capsule is shown in C′ and as single images to the right. D: graph showing percentage of glomeruli positive CD44 at baseline, D14, and D14 + rapamycin, with increasing number of glomeruli positive at D14 APD and again significantly higher at D14 APD + rapamycin. Scale bars are provided. Nuclei were stained blue with DAPI. E–H: confocal microscopy (magnification: ×400) showing double immunofluorescent staining for collagen IV (red, dashed arrow) and PAX8 (arrowhead) expression in glomeruli at baseline (E). Dashed arrow indicates positive staining along Bowman's capsule at baseline, which is delicate and thin, consistent with quiescent PECs. Collagen expression is adjacent to PAX8+ PEC (dashed box) and is shown in E′ and as single-channel images to the right. F: expression of collagen IV increases in PECs at D14 APD, as indicated by arrowhead and dashed arrow. Area in dashed box is shown in F′ and as single-channel images to the right. G: collagen IV expression is higher in PECs following rapamycin treatment. The area positive for collagen IV and PEC in the dashed box is shown in G′ and as single-channel images to the right. H: graph showing area positive along Bowman's capsule for collagen IV increases from baseline compared with D14 and D14 + rapamycin. Scale bars are provided. Nuclei were stained blue with DAPI.
PEC Density and Glomerular Crescents Increase in Aged Kidneys Following mTOR Inhibition
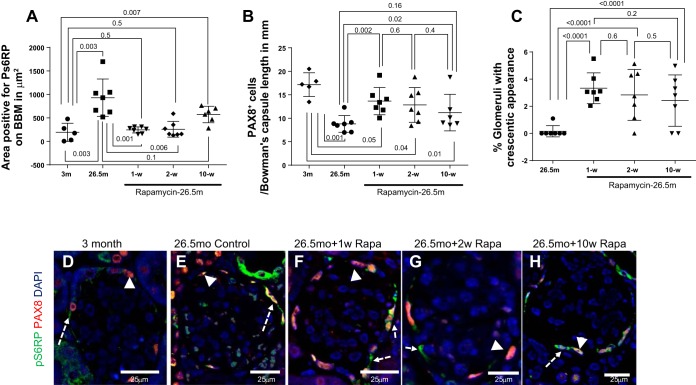
Given the aforementioned findings in an acute model of glomerulosclerosis and podocyte depletion, we next assessed mTOR within PECs in the aged mouse kidney. Because inhibition of mTOR with rapamycin on PEC in an acute model of glomerulosclerosis increased glomeruli with a crescentic appearance in the setting of increased PEC density, we assessed whether inhibition of mTOR with rapamycin in aging would also lead to an increase in glomeruli with sclerosis, crescentic appearance, and an increase in PEC density. Accordingly, 26.5-mo-old mice were treated with microencapsulated rapamycin for 1, 2, or 10 wk before death. The dose of mTOR given was 14 ppm in chow diet, and this has been previously described to result in a moderate reduction (15–30%) in mTOR activity, as measured by the ratio of pS6RP to total S6RP within the kidney, as well as to a 14% increase in maximal lifespan when administered continuously beginning at 18 mo of age (23, 64). Glomerular mTOR activity (measured by pS6RP staining) increased in aged PECs (identified by PAX8 double-staining), with the average area positive for pS6RP on Bowman's capsule border per glomerulus increasing from mice aged 3 mo compared with mice that were 27 mo old [193.2 ± 86.7 (3 mo) vs. 930.2 ± 150.2 μm2 (26.5 mo), P = 0.003] (Fig. 9A). Glomerular mTOR activity was reduced in aged PEC following 1 wk [930.2 ± 150.2 (26.5 mo) vs. 244.7 ± 24.72 μm2 (26.5 mo + 1 wk rapamycin), P = 0.0007] and 2 wk of rapamycin [930.2 ± 150.2 (26.5 mo) vs. 257.8 ± 63.37 μm2 (26.5 mo + 2 wk rapamycin), P = 0.01]. However, the reduction in pS6RP staining was not sustained at 10 wk of rapamycin [930.2 ± 150.2 (26.5 mo) vs. 572.9 ± 71.62 μm2 (26.5 mo + 10 wk rapamycin), P = 0.06] (Fig. 9, A and C–G).
Fig. 9.
Aged mice given rapamycin have an initial reduction in pS6RP expression, and this is associated with an increase in PEC density. A: graph representing the area on Bowman's capsule positive for pS6RP in mice at 3 mo and 26.5 mo, and in mice treated with 1, 2, and 10 wk of rapamycin in diet. pS6RP expression is increased in aged mice, and this is decreased after 1 and 2 wk of microencapsulated rapamycin in chow diet, but is not significantly lower after 10 wk of rapamycin in diet. B: graph demonstrating PEC density in mice at 3 mo and 26.5 mo, and in mice treated with 1, 2, and 10 wk of rapamycin before death at 26.5 mo. As previously reported, PEC density decreases in aged mice, but this decreased was less after rapamycin treatment. C: graph representing the percentage of glomeruli with a crescent, defined as two or more cell layers along Bowman's capsule in PAS-stained slides. In 26.5-mo-old baseline mice, crescents were not detected. Crescents were detected in a small percentage of glomeruli in 26.5-mo-old mice given rapamycin for 1, 2, and 10 wk. D–H: confocal microscopy (magnification: ×400) showing double immunofluorescent staining for PAX8 (red, arrowhead) and pS6RP (green, dashed arrow) expression in Bowman's capsule at 3 mo (D), 26.5 mo (E), 1 wk (F), 2 wk (G), and 10 wk (H) of microencapsulated rapamycin (Rapa) in chow diet.
PEC density is lower in aged mice (45), which we also found in this study [17.16 ± 1.13 (3 mo) vs. 8.79 ± 0.67 PAX8+ cells/mm Bowman's capsule length (26.5 mo), P < 0.001] (Fig. 9, B, D, and E). However, PEC density increased following rapamycin treatment [8.79 ± 0.67 (26.5 mo) vs. 13.65 ± 1.09 (26.5 mo + 1 wk rapamycin) vs. 12.84 ± 1.39 PAX8+ cells/mm Bowman's capsule length (26.5 mo + 2 wk rapamycin), P = 0.002 and P = 0.02, respectively]. However, in mice treated with 10 wk of rapamycin treatment, PEC density was not significantly different from aged mice [8.79 ± 0.67 (26.5 mo) vs. 11.20 ± 1.58 PAX8+ cells/mm Bowman's capsule length (26.5 mo + 2 wk rapamycin), P = 0.1] (Fig. 9, B and H).
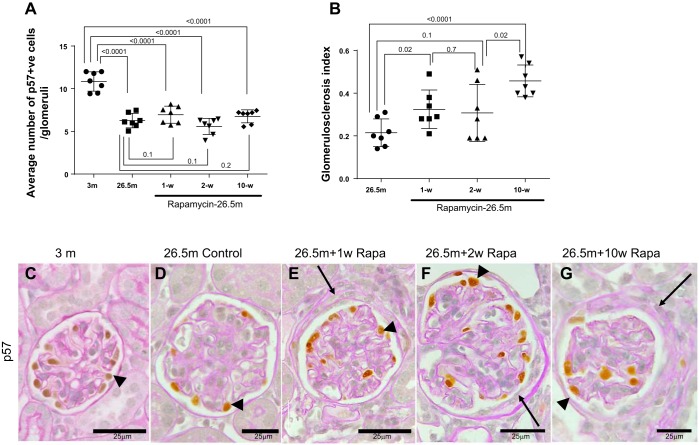
The number of glomeruli with a crescentic appearance as defined by two or more layers of cells on Bowman's capsule was increased in all three groups treated with rapamycin compared with 26.5-mo-old mice not given rapamycin (Fig. 9C and Fig. 10, C–F). Aging is associated with chronic glomerulosclerosis and chronic podocyte depletion. Average podocyte number per glomerulus was lower in 26.5-mo-old control mice compared with 3-mo-old mice (Fig. 10, A, C, and D) [10.85 ± 0.43 (3 mo) vs. 6.27 ± 0.32 p57+ cells/glomerulus (26.5 mo), P < 0.0001]. Additionally, rapamycin treatment for 1, 2, and 10 wk in aged mice resulted in greater sclerosis (Fig. 10B), but podocyte number was not significantly difference from aged mice.
Fig. 10.
Aged mice given rapamycin develop glomerular crescents. A: podocyte number. p57 staining was used to identify podocytes. Graph represents average number of p57 cells per glomerulus in mice at 3 mo, 26.5 mo, and 26.5 mo given microencapsulated rapamycin in their diet for 1 wk, 2 wk, and 10 wk. Podocyte number was lower in 26.5-mo-old mice compared with 3-mo-old mice. Rapamycin did not impact podocyte number in aged mice. B: graph representing the degree of glomerulosclerosis in aged mice at 26.5 mo and after 1, 2, and 10 wk of rapamycin treatment. Rapamycin after 10 wk results in greater glomerulosclerosis, but overall scores are low. C–G: p57-PAS staining. Podocytes identified by p57 staining (brown nuclear stain, black arrowheads) in sections counterstained with periodic acid Schiff stain are from mice at 3 mo (C), 26.5 mo (D), 26.5 mo and 1 wk of rapamycin in diet (E), 26.5 mo and 2 wk of rapamycin in diet (F), and 26.5 mo and 10 wk of rapamycin in diet (G). Scale bars are provided. Black arrows indicate crescentic area along Bowman's capsule. Representative images were taken under the same magnification (×400).
DISCUSSION
In recent years, several studies have suggested that mTOR signaling within the glomerulus has effects on the PEC phenotype (6, 19, 35), and that mTOR activity increases in PECs in response to glomerular injury (22). The purpose of this study was to evaluate the effect of inhibiting mTOR on the PEC response to an experimental model of APD and in the aged kidney.
We first needed to establish glomerular mTOR activity in this model of experimental APD. The pattern of pS6RP staining within the glomerular tuft, together with co-staining for synaptopodin, suggests podocyte expression of active mTOR. This is consistent with other studies that indicate an important role of mTOR in podocyte function in Adriamycin-induced nephropathy, puromycin-induced nephropathy, and crescentic GN models in rats (19, 22, 24, 27, 28, 31). mTOR activity was also increased in PECs in this model of APD. Taken together, our findings that mTOR activation is increased in PECs and glomerular tuft is consistent with mTOR activation as a common reactive mechanism through which glomerular epithelial cells respond to glomerular injury.
To better understand the impact of active mTOR following ADP, we administered rapamycin to lower mTOR signaling only after podocytes were significantly depleted. The results show that, at the concentration given, rapamycin lowered mTOR activity. Our second finding was that glomerulosclerosis was increased in rapamycin-treated APD mice, and that the number of glomeruli with more severe injury was higher at D14 when rapamycin treatment is started at D5. Despite this, podocyte density was similar among APD mice with and without rapamycin. Podocyte density, rather than podocyte number, was calculated to ensure an artificially higher number of podocytes would not be calculated, if rapamycin treatment resulted in changes in glomerular volume. However, there was no change in glomerular tuft volume in this model, either with or without rapamycin, as previously shown (32).
Importantly, we initiated rapamycin treatment on D5, as we had established from previous studies that podocytes were significantly depleted at this time in the current model (15). The timing of rapamycin administration appears to be an important contextual determinant of whether greater damage or improvement occurs in response to injury (44). From our study, inhibiting mTOR following podocyte depletion worsens glomerulosclerosis. Noteworthy is the fact that the higher glomerulosclerosis is not due a greater fall in podocyte number. The lack of a drop in podocyte number in mice treated with rapamycin was, therefore, an unexpected finding, given the augmented glomerular injury.
Given the podocyte results, we focused on PECs as a possible explanation of the findings. Our third finding was that PEC density was higher at D14 in mice with APD treated with rapamycin compared with untreated mice at the same time point. This was despite there being no difference in expression of the proliferation markers ki67 or phosphohistone H3 on Bowman's capsule between D14 APD and D14 APD + rapamycin. This suggest that, if proliferation did occur, then it occurred early on after rapamycin exposure. Consistent with this, changes in PEC density were noted after 1 wk of rapamycin treatment in the aged mice. Of note, PEC density did not increase beyond this after 2 wk of treatment and was no different to similarly aged mice after 10 wk of treatment. To ensure that changes in PEC density was simply not due to alterations in the length of Bowman's capsule, the number of PAX8+ cells/Bowman's capsule length was calculated. In studies on PEC number and density performed by our group, absolute PEC number was lower in aged mice, but PEC density was higher in aged mice due to changes in Bowman's capsule length with age (15). In both experimental groups, we found the percentage of glomeruli with crescents increased when mTOR was reduced, and PEC density increased in rapamycin-treated mice with APD, which was accompanied by an increase in glomerular crescents in this model. Indeed, a major cell type making up the crescents were PECs, identified as PAX8+, podocyte marker negative cells. Genetic manipulation of the mTOR pathway has resulted in crescent formation in other models (31, 35). For example, podocyte-specific deletion of tuberous sclerosis complex, a negative regulator of mTOR signaling resulting in hyperactivated mTOR, resulted in crescentic appearance of glomeruli (35). Conversely, complete inhibition of mTOR signaling by podocyte-specific knock down of raptor and rictor, essential components of mTOR signaling, also resulted in crescent formation within the kidney (19). Micro-RNA-21 activity leads to increased mTOR activity through inhibition of phosphatase and tensin homolog (14) and anti-Mir21 oligonucleotide treatment of an Alport's nephropathy model in mice resulted in reduced number of crescents, with a reduction of pS6RP expression in PECs (unpublished observation; Ref. 18). Finally, anti-neutrophil cytoplasmic antibody, anti-glomerular basement membrane, and IgA-mediated crescentic lesions in humans show increased mTOR signaling, as evidenced by pS6RP within the crescent (35), while there is one case report describing the development of necrotizing GN with crescents in response to late everolimus conversion from calcineurin inhibitor in a transplant patient (41).
Activation of PECs following podocyte depletion is consistent with a pathway by which scarring occurs in the setting of FSGS (21, 51). We noted the increase in CD44 and collagen IV expression with FSGS at D14 was further increased post-rapamycin treatment, consistent with greater injury in the glomerulus. Crescents may be another marker for PEC activation, and, indeed, crescents are recognized to occur in many glomerular diseases outside of anti-neutrophil cytoplasmic antibody and anti-glomerular basement membrane GN, including fibrillary GN, IgA, postinfectious GN, C3 glomerulopathy, lupus, and membranous nephropathy (29).
Our fourth finding was that rapamycin treatment in APD resulted in greater staining for several mesenchymal markers (α-SMA, PDGFβR, Notch-3). We speculate that these findings suggest that mTOR activity in the glomerular tuft in response to APD is needed to keep PECs from overresponding to injury, reducing activation, dedifferentiation, and scarring. This is consistent with other studies, both in animal and humans, that mTOR inhibition leads to greater injury (17). For example, rats treated with rapamycin immediately after adriamycin-induced FSGS developed glomerular necrosis, while a pilot study of rapamycin in FSGS was halted due to worsening proteinuria and decline in glomerular filtration rate (11). mTOR signaling in the glomerulus appears to be part of an important cross-signaling pathway modulating PEC response with regard to number, phenotype, and migration to podocyte injury.
We attempted to further delineate candidate mechanisms of the aforementioned in vivo results by directly applying rapamycin to mouse PECs in culture. However, this maneuver did not affect PEC proliferation, which suggests that direct mTOR inhibition alone was not sufficient to influence PEC proliferation. We can, therefore, only speculate on the role of mTOR on PECs in the present studies. One possibility is because rapamycin causes apoptosis of activated PECs, followed by compensatory proliferation of neighboring PECs, which then form a crescent (49). Second, there is literature linking mTOR with ERK signaling, as both are transduced via the phosphatidylinositol 3-kinase. Previous studies by our group have shown that activated PECs are positive for both pERK and CD44 (15). However, we found no difference in pERK expression in glomeruli or PECs treated with rapamycin (data not shown).
Finally, aging in the kidney is characterized by several morphological features, including progressive glomerulosclerosis and chronic podocyte depletion (25). In the present study, mTOR activity was higher in PECs in 26.5-mo-old mice compared with younger control mice of 3 mo. Mice at 26.5 mo of age correspond to 78-yr-old humans, and changes in PEC morphology in this model have been previously described (45). In the study by Roeder et al. (45), the number of glomeruli positive for pS6RP did not differ between young and old mice significantly. However, by calculating the area positive on Bowman's capsule for pS6RP, which is more sensitive, we now find that mTOR activity in PECs is higher in older mice compared with younger mice. This is not surprising, given changes that occur in the glomeruli with age, including size of glomeruli increasing with age due to compensatory hypertrophy. mTORC1 is thought to be a critical component in the kidney's response to hypertrophy. When pS6RP was rendered inactive by making it unphosphorylatable, compensatory hypertrophy in response to uninephrectomy was prevented (59). Compared with mice aged 26.5 mo, aged-matched mice treated with rapamycin for 1, 2, or 10 wk before death had an increase in the number of glomerular crescents. This is an intriguing finding, especially as there was not a complete reduction in mTOR signaling in PECs within the glomerulus. It indicates that PEC cells are very responsive to changes in mTOR activity in other cells within the kidney.
This study has a number of limitations that must be acknowledged. First, inhibition of the mTOR pathway was through rapamycin. Thus, because a whole body reduction in mTOR activity occurred, it is not possible to delineate the signal from which PEC number was increased. Based on previous studies, we can hypothesize that it is from podocytes, but it could equally be from changes in endothelial cells or even outside the glomerulus. Second, the study is mainly descriptive, limiting the determination of mechanisms by which changes in mTOR activity in the glomerular tuft leads to alterations in PEC phenotype. Finally, the rapamycin dose that was used in the aging study reduced mTOR activity initially: it was not sustained in PEC or glomerular cells, at least as measured by pS6RP expression. Despite these limitations, the current studies in two models suggest that PECs are exquisitely sensitive to mTOR signaling in the setting of podocyte loss or injury. This study adds to a growing body of literature linking mTOR signaling to PEC-derived crescents. Better understanding of how mTOR pathway signaling positively or negatively affects all cells of the glomerulus is key to bringing better therapeutic use of mTOR inhibitors to patients. The remarkable longevity and excellent renal function experienced by some transplant patients who are treated with rapamycin as their immunosuppressant is a clinical holy grail, but understanding why other patients experience new onset proteinuria, decline in glomerular filtration rate, and dedifferentiation of podocytes may lead to methods to circumvent this occurring or predict who is likely to better benefit from agents that affect this pathway (1, 2, 20, 27).
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 5-R01-DK-056799-10, 5-R01-DK-056799-12, and 1-R01-DK-097598-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.A.M., P.S.R., and S.J.S. conception and design of research; B.A.M., D.G.E., J.L., and P.S.R. performed experiments; B.A.M. analyzed data; B.A.M., J.W.P., and S.J.S. interpreted results of experiments; B.A.M. prepared Figs.; B.A.M. drafted manuscript; B.A.M., D.G.E., P.S.R., J.W.P., and S.J.S. edited and revised manuscript; B.A.M., D.G.E., P.S.R., J.W.P., and S.J.S. approved final version of manuscript.
REFERENCES
- 1.Afshinnia F, Vega-Warner V, Killen P. Focal segmental glomerulosclerosis in association with neurofibromatosis type 1: a case report and proposed molecular pathways. Clin Kidney J 6: 208- 210, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akl AI, Adel H, Rahim MA, Wafa EW, Shokeir AA. Outcome of glomerulonephritis in live-donor renal transplant recipients: a single-centre experience. Arab J Urol 13: 295–305, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpers CE, Seifert RA, Hudkins KL, Johnson RJ, Bowen-Pope DF. PDGF-receptor localizes to mesangial, parietal epithelial, and interstitial cells in human and primate kidneys. Kidney Int 43: 286–294, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Andeen NK, Nguyen TQ, Steegh F, Hudkins KL, Najafian B, Alpers CE. The phenotypes of podocytes and parietal epithelial cells may overlap in diabetic nephropathy. Kidney Int 88: 1099–1107, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balbas MD, Burgess MR, Murali R, Wongvipat J, Skaggs BJ, Mundel P, Weins A, Sawyers CL. MAGI-2 scaffold protein is critical for kidney barrier function. Proc Natl Acad Sci U S A 111: 14876–14881, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger K, Bangen JM, Hammerich L, Liedtke C, Floege J, Smeets B, Moeller MJ. Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci U S A 111: 1533–1538, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnworth B, Pippin J, Karna P, Akakura S, Krofft R, Zhang G, Hudkins K, Alpers CE, Smith K, Shankland SJ, Gelman IH, Nelson PJ. SSeCKS sequesters cyclin D1 in glomerular parietal epithelial cells and influences proliferative injury in the glomerulus. Lab Invest 92: 499–510, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Carrano AC, Liu Z, Dillin A, Hunter T. A conserved ubiquitination pathway determines longevity in response to diet restriction. Nature 460: 396–399, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang AM, Ohse T, Krofft RD, Wu JS, Eddy AA, Pippin JW, Shankland SJ. Albumin-induced apoptosis of glomerular parietal epithelial cells is modulated by extracellular signal-regulated kinase 1/2. Nephrol Dial Transplant 27: 1330–1343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho ME, Hurley JK, Kopp JB. Sirolimus therapy of focal segmental glomerulosclerosis is associated with nephrotoxicity. Am J Kidney Dis 49: 310–317, 2007. [DOI] [PubMed] [Google Scholar]
- 12.D'Agati VD. Pathobiology of focal segmental glomerulosclerosis: new developments. Curr Opin Nephrol Hypertens 21: 243–250, 2012. [DOI] [PubMed] [Google Scholar]
- 13.D'Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, Cohen AH, Gipson DS, Gassman JJ, Radeva MK, Moxey-Mims MM, Friedman AL, Kaskel FJ, Trachtman H, Alpers CE, Fogo AB, Greene TH, Nast CC. Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol 8: 399–406, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey N, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. TGFβ-stimulated microRNA-21 utilizes PTEN to orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and matrix expansion. PLoS One 7: e42316, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng DG, Sunseri MW, Kaverina NV, Roeder SS, Pippin JW, Shankland SJ. Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis. Kidney Int 88: 999–1012, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fatima H, Moeller MJ, Smeets B, Yang HC, D'Agati VD, Alpers CE, Fogo AB. Parietal epithelial cell activation marker in early recurrence of FSGS in the transplant. Clin J Am Soc Nephrol 7: 1852–1858, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fervenza FC, Fitzpatrick PM, Mertz J, Erickson SB, Liggett S, Popham S, Wochos DN, Synhavsky A, Hippler S, Larson TS, Bagniewski SM, Velosa JA, Committee MNC. Acute rapamycin nephrotoxicity in native kidneys of patients with chronic glomerulopathies. Nephrol Dial Transplant 19: 1288–1292, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, Xia TH, Liu X, Borza DB, Grafals M, Shankland SJ, Himmelfarb J, Portilla D, Liu S, Chau BN, Duffield JS. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest 125: 141–156, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121: 2197–2209, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González F, Espinoza M, Reynolds E, Herrera P, Espinoza O, Rocca X, Lorca E, Hidalgo J, Roessler E. Effectiveness and cost of replacing a calcineurin inhibitor with sirolimus to slow the course of chronic kidney disease in renal allografts. Transplant Proc 42: 284–287, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Hakroush S, Cebulla A, Schaldecker T, Behr D, Mundel P, Weins A. Extensive podocyte loss triggers a rapid parietal epithelial cell response. J Am Soc Nephrol 25: 927–938, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamatani H, Hiromura K, Sakairi T, Takahashi S, Watanabe M, Maeshima A, Ohse T, Pippin JW, Shankland SJ, Nojima Y. Expression of a novel stress-inducible protein, sestrin 2, in rat glomerular parietal epithelial cells. Am J Physiol Renal Physiol 307: F708–F717, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartleben B, Wanner N, Huber TB. Autophagy in glomerular health and disease. Semin Nephrol 34: 42–52, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O'Connor C, Yang Y, Meadowbrooke C, Chowdhury M, Kikuchi M, Wiggins JE, Wiggins RC. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J Am Soc Nephrol 26: 3162–3178, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holderied A, Romoli S, Eberhard J, Konrad LA, Devarapu SK, Marschner JA, Müller S, Anders HJ. Glomerular parietal epithelial cell activation induces collagen secretion and thickening of Bowman's capsule in diabetes. Lab Invest 95: 273–282, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Inoki K, Huber TB. Mammalian target of rapamycin signaling in the podocyte. Curr Opin Nephrol Hypertens 21: 251–257, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121: 2181–2196, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeanette O, Silva D'Agati. Heptinstall's pathology of the Kidney (7th Ed). Philadelphia, PA: : Lippincott Williams & Wilkins, 2014. [Google Scholar]
- 30.Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, Oh K, Wasko BM, Ramos FJ, Palmiter RD, Rabinovitch PS, Morgan PG, Sedensky MM, Kaeberlein M. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 342: 1524–1528, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurayama R, Ito N, Nishibori Y, Fukuhara D, Akimoto Y, Higashihara E, Ishigaki Y, Sai Y, Miyamoto K, Endou H, Kanai Y, Yan K. Role of amino acid transporter LAT2 in the activation of mTORC1 pathway and the pathogenesis of crescentic glomerulonephritis. Lab Invest 91: 992–1006, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Lane PH, Steffes MW, Mauer SM. Estimation of glomerular volume: a comparison of four methods. Kidney Int 41: 1085–1089, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Lasagni L, Ballerini L, Angelotti ML, Parente E, Sagrinati C, Mazzinghi B, Peired A, Ronconi E, Becherucci F, Bani D, Gacci M, Carini M, Lazzeri E, Romagnani P. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells 28: 1674–1685, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magil AB. Histogenesis of glomerular crescents. Immunohistochemical demonstration of cytokeratin in crescent cells. Am J Pathol 120: 222–229, 1985. [PMC free article] [PubMed] [Google Scholar]
- 35.Mao J, Zeng Z, Xu Z, Li J, Jiang L, Fang Y, Xu X, Hu Z, He W, Yang J, Dai C. Mammalian target of rapamycin complex 1 activation in podocytes promotes cellular crescent formation. Am J Physiol Renal Physiol 307: F1023–F1032, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Moeller MJ, Smeets B. Role of parietal epithelial cells in kidney injury: the case of rapidly progressing glomerulonephritis and focal and segmental glomerulosclerosis. Nephron Exp Nephrol 126: 97, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Murea M, Park JK, Sharma S, Kato H, Gruenwald A, Niranjan T, Si H, Thomas DB, Pullman JM, Melamed ML, Susztak K. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int 78: 514–522, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadon NL. Exploiting the rodent model for studies on the pharmacology of lifespan extension. Aging Cell 5: 9–15, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Naito S, Pippin JW, Shankland SJ. The glomerular parietal epithelial cell's responses are influenced by SM22 alpha levels. BMC Nephrol 15: 174, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med 14: 290–298, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Nowacka-Cieciura E, Perkowska-Ptasińska A, Sulikowska-Rowińska A, Cieciura T, Wazna E, Durlik M. Late conversion to everolimus complicated with necrotizing glomerulonephritis in a renal allograft recipient: case report. Transplant Proc 41: 441–445, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Ohse T, Pippin JW, Chang AM, Krofft RD, Miner JH, Vaughan MR, Shankland SJ. The enigmatic parietal epithelial cell is finally getting noticed: a review. Kidney Int 76: 1225–1238, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ. De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol 298: F702–F711, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rangan GK, Coombes JD. Renoprotective effects of sirolimus in nonimmune initiated focal segmental glomerulosclerosis. Nephrol Dial Transplant 22: 2175–2182, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Roeder SS, Stefanska A, Eng DG, Kaverina N, Sunseri MW, McNicholas BA, Rabinovitch P, Engel FB, Daniel C, Amann K, Lichtnekert J, Pippin JW, Shankland SJ. Changes in glomerular parietal epithelial cells in mouse kidneys with advanced age. Am J Physiol Renal Physiol 309: F164–F178, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shankland SJ, Anders HJ, Romagnani P. Glomerular parietal epithelial cells in kidney physiology, pathology, and repair. Curr Opin Nephrol Hypertens 22: 302–309, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Shankland SJ, Eitner F, Hudkins KL, Goodpaster T, D'Agati V, Alpers CE. Differential expression of cyclin-dependent kinase inhibitors in human glomerular disease: role in podocyte proliferation and maturation. Kidney Int 58: 674–683, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu M, Kondo S, Urushihara M, Takamatsu M, Kanemoto K, Nagata M, Kagami S. Role of integrin-linked kinase in epithelial-mesenchymal transition in crescent formation of experimental glomerulonephritis. Nephrol Dial Transplant 21: 2380–2390, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Sicking EM, Fuss A, Uhlig S, Jirak P, Dijkman H, Wetzels J, Engel DR, Urzynicok T, Heidenreich S, Kriz W, Kurts C, Ostendorf T, Floege J, Smeets B, Moeller MJ. Subtotal ablation of parietal epithelial cells induces crescent formation. J Am Soc Nephrol 23: 629–640, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smeets B, Dijkman HB, Wetzels JF, Steenbergen EJ. Lessons from studies on focal segmental glomerulosclerosis: an important role for parietal epithelial cells? J Pathol 210: 263–272, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Smeets B, Stucker F, Wetzels J, Brocheriou I, Ronco P, Gröne HJ, D'Agati V, Fogo AB, van Kuppevelt TH, Fischer HP, Boor P, Floege J, Ostendorf T, Moeller MJ. Detection of activated parietal epithelial cells on the glomerular tuft distinguishes early focal segmental glomerulosclerosis from minimal change disease. Am J Pathol 184: 3239–3248, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 20: 2604–2615, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taneda S, Hudkins KL, Cui Y, Farr AG, Alpers CE, Segerer S. Growth factor expression in a murine model of cryoglobulinemia. Kidney Int 63: 576–590, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Tian J, Wang Y, Liu X, Zhou X, Li R. Rapamycin ameliorates IgA nephropathy via cell cycle- dependent mechanisms. Exp Biol Med (Maywood) 240: 936–945, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 54: B492–B501, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Ueno T, Kobayashi N, Nakayama M, Takashima Y, Ohse T, Pastan I, Pippin JW, Shankland SJ, Uesugi N, Matsusaka T, Nagata M. Aberrant Notch1-dependent effects on glomerular parietal epithelial cells promotes collapsing focal segmental glomerulosclerosis with progressive podocyte loss. Kidney Int 83: 1065–1075, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venkatareddy M, Wang S, Yang Y, Patel S, Wickman L, Nishizono R, Chowdhury M, Hodgin J, Wiggins PA, Wiggins RC. Estimating podocyte number and density using a single histologic section. J Am Soc Nephrol 25: 1118–1129, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogelbacher R, Wittmann S, Braun A, Daniel C, Hugo C. The mTOR inhibitor everolimus induces proteinuria and renal deterioration in the remnant kidney model in the rat. Transplantation 84: 1492–1499, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Xu J, Chen J, Dong Z, Meyuhas O, Chen JK. Phosphorylation of ribosomal protein S6 mediates compensatory renal hypertrophy. Kidney Int 87: 543–556, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Hansen KM, Pippin JW, Chang AM, Taniguchi Y, Krofft RD, Pickering SG, Liu ZH, Abrass CK, Shankland SJ. De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol 302: F571–F580, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Pippin JW, Krofft RD, Naito S, Liu ZH, Shankland SJ. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Renal Physiol 304: F1375–F1389, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, Pippin JW, Vaughan MR, Krofft RD, Taniguchi Y, Romagnani P, Nelson PJ, Liu ZH, Shankland SJ. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp Nephrol 121: e23–e37, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Yanez D, Floege A, Lichtnekert J, Krofft RD, Liu ZH, Pippin JW, Shankland SJ. ACE- inhibition increases podocyte number in experimental glomerular disease independent of proliferation. J Renin Angiotensin Aldosterone Syst 16: 234–248, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Réndon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci 69: 119–130, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]