Abstract
Diabetic retinopathy (DR) is the leading cause of blindness in the working-age population in developed countries, and its prevalence will increase as the global incidence of diabetes grows exponentially. DR begins with an early nonproliferative stage in which retinal blood vessels and neurons degenerate as a consequence of chronic hyperglycemia, resulting in vasoregression and persistent retinal ischemia, metabolic disequilibrium, and inflammation. This is conducive to overcompensatory pathological neovascularization associated with advanced proliferative DR. Although DR is considered a microvascular complication, the retinal microvasculature is intimately associated with and governed by neurons and glia; neurodegeneration, neuroinflammation, and dysregulation of neurovascular cross talk are responsible in part for vascular abnormalities in both early nonproliferative DR and advanced proliferative DR. Neuronal activity directly regulates microvascular dilation and blood flow in the process of neurovascular coupling. Retinal neurons also secrete guidance cues in response to injury, ischemia, or metabolic stress that may either promote or suppress vascular outgrowth, either alleviating or exacerbating DR, contingent on the stage of disease and retinal microenvironment. Neurodegeneration, impaired neurovascular coupling, and dysregulation of neuronal guidance cues are key events in the pathogenesis of DR, and correcting these events may prevent or delay development of advanced DR. The review discusses the mechanisms of neurovascular cross talk and its dysregulation in DR, and their potential therapeutic implications.
Keywords: angiogenesis, metabolism, retinal degeneration
diabetic retinopathy (DR) is a common and debilitating microvascular complication of both type 1 and type 2 diabetes. DR slowly develops in diabetic patients as a result of many factors, including hyperglycemia, dyslipidemia, metabolic dysregulation, inflammation, and ischemia (59).
The pathophysiology of DR is complex, but it can generally be described as progressing through two stages, early nonproliferative DR and advanced proliferative DR (77). In nonproliferative DR, neurodegeneration, capillary loss, glial activation, impaired vascular regrowth, and increased vascular permeability stress the diabetic retina and compromise visual function (77, 117). Particularly important to nonproliferative DR is diabetic macular edema (DME), which is the leading cause of vision loss in DR (26). In advanced proliferative DR, neovascularization resulting from ischemia often leads to bleeding and/or tractional retinal detachment, which may result in irreversible blindness if left untreated (77).
Current therapeutic approaches for DR target neovascular lesions and macular edema characteristic of advanced disease. The two most common approaches are surgical interventions and intravitreal injection of vascular endothelial growth factor (VEGF)-neutralizing therapies. Both approaches are sight saving, but are also invasive and cost-prohibitive (4). Targeting the upstream events of nonproliferative DR such as neurodegeneration and vasoregression could prevent development of sight-threatening DR and may be less invasive.
THE RETINAL NEUROVASCULAR UNIT
Retinal neurons, glia, microglia, and microvasculature communicate to form the neurovascular unit, which supplies metabolically demanding and sensitive retinal neurons with sufficient oxygen and energy substrates, recycles neurotransmitters, and removes waste (80, 117). This functional and physical association is essential for normal retinal function and allows the neural retina to adapt to varying physiological conditions. In the process of neurovascular coupling, neuronal activity evokes localized reactions, including vasodilation and increased blood flow, to meet the energy demands of neuronal signal transduction and transmission (117).
Organization of Retinal Neurons
Retinal neurons are organized into three layers of nerve cell bodies and two layers of synapses (Fig. 1) (19). The outer nuclear layer contains rod and cone photoreceptor cells responsible for phototransduction, and the inner nuclear layer contains cell bodies of second- and third-order neurons, primarily bipolar, horizontal, and amacrine cells responsible for transmitting visual signals from photoreceptors to the brain (60). The ganglion cell layer contains cell bodies of ganglion cells, displaced amacrine cells, and astrocytes (60).
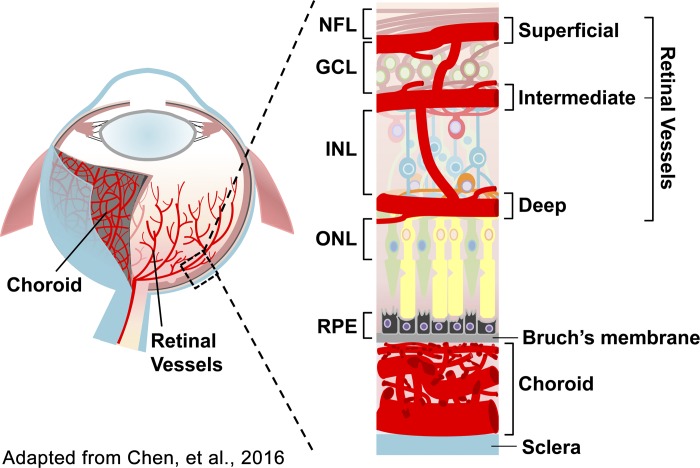
Fig. 1.
Schematic illustration of the ocular vasculature. The outer nuclear layer (ONL) is avascular, relying on the choroidal blood supply. The inner nuclear layer (INL) is vascularized by the deep and intermediate vascular plexuses. The ganglion cell layer (GCL) is vascularized by the intermediate and superficial vascular plexuses. NFL, nerve fiber layer; RPE, retinal pigment epithelium. Adapted from Ref. 19. Copyright 2016 by Springer. Reprinted with permission.
Structure and Regulation of Retinal Vascular Supply
In the human retina, the vasculature consists of a central retinal artery that branches into four intraretinal arterioles that feed into a capillary bed (63). The retinal capillary bed is organized into three layers, the superficial, intermediate, and deep vascular plexuses (Fig. 1) (19). Retinal blood flow is tightly regulated to compensate for varying physiological conditions and changes in neuronal activity. Autoregulation of vascular resistance in both arterioles and capillaries generally compensates for changes in blood pressure, blood gases, or intraocular pressure (63). The arterioles are thought to be primarily responsible for regulating blood flow in response to neuronal activity; under visual stimulus retinal arterioles dilate in response to locally increased neuronal activity, ensuring that actively firing neurons have adequate blood supply (63). There is some evidence that capillaries may also play a role, since activity-regulated changes in retinal blood flow are highly localized in the retina, suggesting that capillaries are also able to respond to neuronal demands (63).
Physical Integration of Retinal Neurons and Vasculature
The outer nuclear layer is avascular in normal physiological conditions, relying on the choroidal blood supply (60). The inner nuclear layer is vascularized by the deep and intermediate vascular plexuses (60). The ganglion cell layer is vascularized by the intermediate and superficial vascular plexuses (60, 108) (Fig. 1). Neurons of the inner nuclear layer and ganglion cell layers are intimately associated with the extensive capillary networks of the inner retina, forming the functional neurovascular unit together with glial cells, perivascular cells, and capillary endothelial cells (108).
Retinal microvessels consist of a basal lamina with a single layer of adherent luminal simple squamous endothelial cells surrounded by pericytes, glial cells, and microglial cells on the external surface (58). Contractile retinal pericytes wrap tightly around the endothelial cells of capillaries and are also physically attached to glial cells (58). Microglial cells have ramified processes that envelop some neurons and synapses in synaptic layers, and also wrap around retinal blood vessels in perivascular forms (58). Microglia interact directly with retinal pericytes and neurons (58).
Regulation and Maintenance of the Neurovascular Unit
Retinal blood vessels, neurons, and glia collectively form the neurovascular unit (Fig. 1), a physical and functional unit for regulation of retinal blood flow and structural support. Cells are in some cases physically associated through tight or gap junctions, or otherwise communicate using chemical messengers. The neurovascular unit is maintained and regulated primarily by endothelial cells, pericytes, neurons, and glial cells. The role of each cell type is discussed below.
Endothelial cells.
Retinal vascular endothelial cells that line the luminal surface of retinal blood vessels are in direct contact with blood, and form a semiselective barrier between the vessel lumen and neural retina (91). Endothelial cells modulate vasodilation and vasoconstriction in response to systemic and localized chemical messengers (40). Tight junctions between endothelial cells regulate vascular permeability, and may become compromised under stress conditions, such as DR, resulting in vascular hyperpermeability (58, 128). Endothelial cell migration and proliferation regulate vascularization during development, revascularization after injury, and pathological neovascularization (40).
Pericytes.
Retinal pericytes play a crucial role in maintenance of the neurovascular unit and blood-retinal barrier by communicating directly with adjacent endothelial cells, glial cells, microglial cells, and neurons (58). Pericytes are generally separated from endothelial cells by the basal lamina, but also make peg-socket contacts with endothelial cells through holes in the basal lamina (58). These contractile cells respond to chemical messengers, and therefore may help regulate blood flow. They help maintain the blood-retinal barrier (6, 58) and participate in maturation and stabilization of endothelial cells.
Neurons.
Second- and third-order neurons are associated with capillaries and regulate retinal blood flow through glial cells and pericytes. Photoreceptors are avascular under normal conditions, relying on the choroidal capillary bed, but regulate neurovascular coupling indirectly through secondary and tertiary neurons (63). Activation of ganglion and amacrine cells by light stimulation evokes intracellular Ca2+ increases in adjacent glial cells, which regulate neurovascular coupling through mechanisms described below (86).
Müller cells.
Müller cells are the most abundant retinal glial cell, and span the width of the neural retina. Müller cell processes are associated with blood vessels and neurons, to maintain the blood-retinal barrier and to provide structural support. They also play a prominent role in neurovascular coupling (80). Müller cells generate increased intracellular Ca2+ in response to neuronal activity, and release vasodilatory epoxyeicosatrienoic acids, vasoconstrictive 20-hydroxyeicosatetraenoic acid, and prostaglandins, which can be either vasoconstrictive or vasodilatory (81). Early studies suggested that Müller cells also regulate neurovascular coupling through potassium siphoning, but these findings have now been refuted (80). Müller cells may also play a role in neuronal glucose metabolism, since an in vitro study suggested that they supply retinal neurons with lactate, although these findings have not been verified in vivo (99). Müller cell gliosis is an early event of DR (25) that may contribute to early microvascular dysfunction.
Astrocytes.
Like Müller cells, astrocytes are glial cells connected to retinal blood vessels and neurons, and play a critical role in maintenance of the blood-retinal barrier, although they are largely restricted to the nerve fiber layer (122). Astrocytes send processes to both retinal blood vessels and neuronal axons provide structural support for the blood-retinal barrier and communicate through gap junctions (122). In the brain, astrocytes regulate neurovascular coupling through mechanisms like those of Müller cells (96), so they may play a similar role in the retina. Astrocytes may also play a role in autoregulation of blood flow, maintaining vascular tone (36).
Microglia.
The retinal microglia function as phagocytic cells, monitor local synaptic activity (111), clear away metabolic products and debris (25), and act as immune sentinels, contributing to immune-related defense mechanisms (67). Microglia secrete anti-inflammatory cytokines in physiological conditions, secrete inflammatory mediators after retinal injury, and hence may affect retinal microvasculature in a paracrine manner (5). Microglia may also directly interact with pericytes (58). The inflammatory response elicited by microglia is beneficial under acute stress such as infection, but may be detrimental under prolonged stress, such as in DR.
NEUROVASCULAR PATHOPHYSIOLOGY OF DR
The diabetic environment is hostile to retinal blood vessels, neurons, and glia and creates a dysfunctional neurovascular unit in both early and late DR. In early nonproliferative DR retinal blood vessels and neurons degenerate (Fig. 2), leading to vascular hyperpermeability that can result in sight-threatening macular edema. Degeneration of retinal blood vessels results in retinal nonperfusion and ischemia, setting the stage for overcompensatory pathological neovascularization in advanced proliferative DR (Fig. 2).
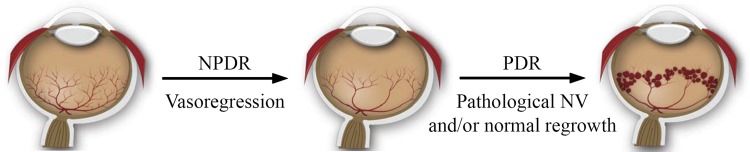
Fig. 2.
Schematic illustration of the development of proliferative diabetic retinopathy (DR) adapted from Ref. 24. Nonproliferative DR (NPDR) is characterized by vasoregression, which results in widespread retinal ischemia. In proliferative DR (PDR), compensatory but ultimately pathological neovascularization occurs in response to retinal ischemia. Neovessels are fragile, prone to leakage, and are often misdirected into the vitreous, later forming fibrosed membranes that may lead to tractional detachments of the retina, resulting in blindness.
Neurodegeneration
Although DR has traditionally been considered a microvascular diabetic complication, recent studies support the notion that retinal neurodegeneration precedes microvascular dysfunction in DR, and may contribute to microvascular abnormalities (117). Diabetic patients without clinically overt microvascular lesions have impaired dark adaptation, contrast sensitivity, and electroretinogram (ERG) responses, suggesting that the neural retina has already begun to degenerate before the development of microvascular lesions (42, 75, 93). ERGs of diabetic patients suggest that neuronal degeneration occurs predominantly in the inner retina, and animal models of experimental diabetes corroborate these findings (75, 93).
Advanced imaging techniques have recently advanced clinical knowledge of neurodegeneration in DR, allowing ophthalmologists to identify early changes in diabetic eyes manifesting before abnormal fundoscopic findings. Optical coherence tomography studies have demonstrated that the nerve fiber, ganglion cell, and inner plexiform layers are thinner in DR patients, also suggesting neuronal loss in the inner retina (121, 123–125). Although thinning of the inner retina is consistently recognized in patient imaging studies, the thickness of the outer retina is controversial, with some groups finding increased thickness and others finding deceased thickness (14, 84). This may be due to the dual presence of both neurodegeneration, which would result in decreased thickness, and macular edema, which would result in increased thickness. These inconsistencies may also be due to differences in equipment and methodology, and improved quantitative analysis may also be helpful in resolving such discrepancies (27).
Within one week of experimental diabetes induction in rats, retinal apoptosis is increased in nonvascular cells, primarily ganglion and amacrine cells (105). Within three months of diabetes, both the ERG response and optokinetic reflex decline in diabetic animals, suggesting functional deficits in the neural retina (57, 92). After 6 to 12 months of experimental diabetes, dependent upon the specific model, all retinal neuronal cell types are affected, and vascular cells, such as endothelial cells and pericytes, also undergo apoptosis (104). Imaging studies in DR patients also suggest that cone loss occurs in advanced DR (72).
Neuronal stress and cell death also lead to glial activation, which occurs early in DR. Acute gliosis benefits the stressed retina, since activated glia and microglia phagocytize apoptotic cells, clear debris and cytotoxins, and secrete neurotrophic factors (25). However, chronic gliosis in DR is detrimental to retinal blood vessels and neurons (25). Activated glia may not properly regulate retinal blood flow or maintain the blood-retinal barrier, and gliotic cells secrete inflammatory cytokines, cytotoxic molecules, and vascular growth factors, perpetuating both microvascular dysfunction and neurodegeneration (25).
Many mechanisms have been proposed for neurodegeneration in DR and are being explored as therapeutic modalities (116). Glutamate, the major excitatory neurotransmitter, accumulates in DR and ultimately results in in excitotoxic cell death (62). Reactive oxygen species are increased in DR, and resultant oxidative stress may be a contributing mechanism to neurodegeneration (116). However, although antioxidants alleviate DR in animal models, clinical trial findings suggest that antioxidants may have limited therapeutic potential for DR and other diabetic complications in humans (30, 61). Neurotrophic factors are decreased in DR, and preliminary studies in animal models and patients suggest that neurotrophic factors are a potential therapeutic modality (116).
Vasoregression
DR advances through two stages: nonproliferative DR, characterized by vasoregression, neurodegeneration vascular hyperpermeability, microaneurysms, and venous beading; and proliferative DR, characterized by pathological neovascularization, which occurs as a compensatory response to widespread ischemia and metabolic insufficiency resulting from vasoregression (Fig. 2) (24, 45). Cotton wool spots, appearing as fluffy white patches on the retina, are caused by infarcts in the ganglion cells resulting from insufficient blood supply (4). Cotton wool spots are generally detected upon fundoscopic examination of the retina, and are an early hallmark of DR (4).
Retinal vasodegeneration, which first affects capillaries, is a mid-early event in nonproliferative DR (45, 119). Retinal endothelial cells undergo mitochondria-mediated apoptotic cell death, which occurs due to metabolic stress, oxidative stress, ischemia, inflammation, and glucose modification of extracellular macromolecules (45). Surrounding neurons and glia may release inflammatory mediators that contribute to endothelial cell dropout (25). Pericytes also undergo apoptotic cell death, in addition to programmed necrotic cell death and pyroptosis (29, 120). Pericyte detachment and migration away from underlying vessels may also contribute to DR-associated loss of pericyte coverage on endothelial cells (97).
Equally important to capillary dropout in early nonproliferative DR is a deficiency in vasoreparative cells such as endothelial progenitor cells (113) and immune cells to reinstate functional vasculature (11). Furthermore, vascular guidance cues secreted by stressed neurons and glia may impair normal revascularization after vascular injury, misdirecting reparative neovessels away from sites of injury and into the vitreous (109).
Vasopermeability
Retinal vascular permeability increases within as little as one week after induction of experimental diabetes, and sight-threatening complications arising from vascular hyperpermeability occur in advanced nonproliferative DR (53). DR-related vascular hyperpermeability is a result of endothelial dropout and dysfunction modulated by inflammatory cytokines, vascular growth factors, and impaired neurovascular coupling (54, 77). Clinically, vascular hyperpermeability manifests in the formation of macular edema, which compromises visual acuity.
Retinal microaneurysms and venous beading occurring as a result of weakened retinal blood vessels are often the first clinically apparent sign of DR, usually observed through fundus imaging (79). Microaneurysms frequently rupture, resulting in formation of dot hemorrhages, and as the blood is cleared away, deposition of hard exudates (79). Retinal microaneurysms may be a contributing factor to diabetic macular edema, discussed below. Retinal microaneurysms and resultant hemorrhages are the target of laser photocoagulation therapy (79).
DME is a major cause of vision loss in DR. In human patients DME is defined as an accumulation of fluid in the outer plexiform and inner nuclear layers, and may impair vision and ultimately progress to blindness if left untreated (26). Macular edema occurs primarily due to breakdown of the blood retinal barrier, and is the primary sight-threatening consequence of hemorrhaged microaneurysms and vascular hyperpermeability (26). Because DME is a leading cause of vision loss in DR, many therapeutic modalities have been explored. DME is responsive to macular laser surgery, micropulse laser therapy, anti-VEGF therapies, intraocular steroids, and vitreoretinal surgery (26). However, these treatment approaches are invasive and costly, so additional therapeutic approaches are a topic of ongoing investigation.
Tight junctions between endothelial cells, comprised of scaffolding, transmembrane, and signaling proteins (43), are compromised in DR, which contributes to blood-retinal barrier breakdown (37). Vascular growth factors and cytokines alter the abundance and organization and integrity of tight junction proteins (37).
The transcytosis of molecules across endothelial cells from the lumen to abluminal regions of retinal vessels via specialized vesicles (115) is suppressed in the barrier endothelium (58, 107). Increases in VEGF as occur in DR can cause increased transcellular permeability independent of tight junction change, suggesting a distinct mechanism of vasopermeability modulated by changes in transcytosis (34). In streptozotocin-induced diabetic rats, blood-retina barrier breakdown is manifested as increased endocytosis of horseradish peroxidase in retinal endothelial cells, but not in the junctional complexes (41).
The extracellular matrix and basal lamina are also compromised in DR (58). Matrix metalloproteinases secreted by pericytes and endothelial cells facilitate breakdown of extracellular matrix components and tight junction proteins in DR (109). Furthermore, advanced glycation end products also disrupt the vascular extracellular matrix (56). Neuronal guidance molecules such as vascular endothelial growth factor and class III semaphorins released by stressed neurons also modulate vascular hyperpermeability in early DR (17).
Impaired Neurovascular Coupling
Retinal neurovascular coupling is impaired in early diabetes. In diabetic patients, flicker-induced vasodilation is impaired, as is hyperoxia-induced vasoconstriction (9, 68, 76), which is likely related to neurodegeneration and changes in retinal neuronal function (117). Advanced imaging studies in DR patients have suggested that retinal blood flow may be impaired or aberrant in DR, suggesting dysregulated neurovascular cross talk (13, 126). Chronic impairment of microvascular responses is a likely contributor to breakdown of the blood-retinal barrier, vasoregression, vascular hyperpermeability, and other microvascular pathologies in DR (117).
Pathological Neovascularization
Pathological neovascularization occurs in advanced end-stage DR and can lead to vision loss if left untreated. In normal conditions, adult retinal blood vessels are quiescent, although endothelial cells maintain their ability to respond to angiogenic signaling in the event of vascular injury such as vasoregression in DR (24, 45). Although normal vascular regrowth is beneficial in nonproliferative DR, pathological neovessels often form in lieu of normal reparative vessels in advanced proliferative DR. Clinically, beading of retinal blood vessels and formation of intraretinal microvascular abnormalities are seen as a precursor to pathological neovascularization.
Neovessels forming as part of pathological neovascularization may be misdirected in the vitreous rather than toward areas of retinal nonperfusion. These vessels are also structurally unsupported, and are therefore prone to rupture; even small changes in blood pressure or blood glucose may lead to rupture and extensive bleeding in the vitreous, potentially causing significant but generally temporary loss of vision (4). In severe cases, neovascular lesions develop a fibrotic change of the neovascular membranes, which may lead to traction retinal detachment and permanent blindness (109).
Neovascularization is a compensatory response to retinal nonperfusion resulting from vasoregression. Ischemic retinal neurons deprived of oxygen and nutrients secrete vascular growth factors, most notably VEGF, to promote neovessel formation (4). Panretinal photocoagulation is the gold standard treatment for neovascularization in proliferative DR (129). In this procedure, laser pulses are applied to the peripheral retina, killing retinal neurons in the peripheral retina, alleviating peripheral ischemia and resultant neovascularization at the expense of peripheral vision (4). However, by preventing sight-threatening neovascularization, panretinal photocoagulation preserves central vision.
Retinal angiogenesis is a tightly regulated process consisting of endothelial cell proliferation, migration, and tube formation in response to vascular growth factors secreted by ischemic/metabolically stressed neuronal, glial, and retinal pigment epithelial cells (109, 128). Vasorepulsive factors may also inhibit vascular growth and, depending on disease stage, can either impair normal vascular regrowth or inhibit pathological neovascularization (109).
ROLE OF NEURONAL AND GLIAL GUIDANCE MOLECULES IN DR
Retinal neurons, glia, and pigment epithelial cells secrete guidance molecules that may either promote or suppress vascular outgrowth and permeability as well as neurite outgrowth and/or neurite growth suppression. These secreted factors are necessary for development and are beneficial under acute stress. However, under the prolonged stress of DR, neuronal/vascular guidance cues may ultimately play a pathological role by promoting pathological neovascularization or misguiding neovessels, impairing normal revascularization after vascular injury. Neuronal guidance cues likely to play significant roles in DR are summarized in Table 1.
Table 1.
Neuronal guidance cues likely to play a significant role in diabetic retinopathy
| Name | Effect | Ref. No. |
|---|---|---|
| VEGF | ↑Neovascularization | 15, 16, 18, 32, 83, 90, 123 |
| ↑Permeability | ||
| PIGF | ↑Neovascularization | 33, 135 |
| Sem3a | ↓Vascular regrowth | 11, 44, 50, 55, 82 |
| ↑Permeability | ||
| ↑Neurodegeneration | ||
| PEDF | ↓Neovascularization | 8, 38, 71, 94, 95, 134 |
| ↓Neurodegeneration | ||
| PDGF | 2, 48, 69, 85, 101, 106 | |
| Norrin | ↑Vascular regrowth | 12, 87–89, 113, 133 |
| ↓Neurodegeneration | ||
| Succinate | ↑Neovascularization | 110 |
DR, diabetic retinopathy; VEGF, vascular endothelial growth factor; PIGF, placental growth factor; PEDF, pigment epithelium-derived factor; PDGF, platelet-derived growth factor; ↑, increase; ↓, decrease.
VEGF
VEGF, a classic modulator of vascular outgrowth, vascular permeability, cell proliferation, and survival is increased in DR (15, 66, 78, 90, 128). VEGF-A is the best characterized of the VEGF family (VEGF-A to -D) in retinopathy, and is generally referred to as VEGF (16, 90). VEGF is expressed in glial, neuronal, and retinal pigment epithelial cells, and is secreted in response to ischemia, starvation, oxidative stress, inflammation, and other stressors (18, 32, 65). VEGF is central to vascular hyperpermeability and pathological neovascularization. VEGF binding to VEGFR2 promotes phosphorylation of tight junction proteins to modulate their degradation and subsequent disruption of endothelial tight junctions and the blood-retinal barrier (3, 46, 118). VEGFR2 also activates ERK1/2 signaling to stimulate endothelial proliferation and FAK, p38 MAPK, and SMAD2/3 signaling to promote endothelial migration (128).
Overexpression of VEGF or administration of exogenous peptide is sufficient to induce retinal neovascularization and vasopermeability, and neutralization of VEGF temporarily alleviates DR and related retinopathies (1, 83, 98, 103). However, anti-VEGF therapy must be administered through frequent intravitreal injection (up to every 8 wk) to treat retinopathy. Furthermore, VEGF modulates survival signaling in retinal blood vessels and neurons (38, 102), and long-term use of anti-VEGF therapy may result in neuronal and vascular degeneration (64, 102).
Despite the drawbacks and potential adverse effects of anti-VEGF therapy, it is highly effective and continues to be a standard of care. The Diabetic Retinopathy Clinical Research Network recently identified that intravitreal VEGF-neutralizing antibodies were at least as effective as panretinal laser photocoagulation, which is currently considered the gold standard for proliferative DR (129). Anti-VEGF therapy is also highly effective in treating DME, preventing accumulation of subretinal fluid and subsequent deterioration of visual acuity (79).
Placental Growth Factor
Placental growth factor (PIGF) is a member of the VEGF family secreted primarily by retinal ganglion cells (33). Both VEGF-A and PIGF activate VEGFR1, so may have some redundant downstream effects (114). However, the spatial and temporal expression pattern of PIGF in retinopathy is distinct from that of VEGF-A: PIGF is localized predominately to the inner retina, and is highly expressed during the vasoobliterative stage of oxygen-induced retinopathy (OIR), whereas VEGF-A is expressed by many retinal cell types during the proliferative stage of OIR (33, 134). Zheng et al. found that blocking PIGF alleviates neovascularization in the OIR model and in the cornea (134). However, Shih et al. found that PIGF activation of VEGFR1 during the vasoobliterative stage of OIR decreases vasodegeneration, suggesting that PIGF may be protective in nonproliferative DR (114). The effect of PIGF should be directly evaluated in diabetic animal models to assess its therapeutic potential for DR.
Semaphorins
Semaphorins regulate neurite outgrowth. They also regulate endothelial cell migration and outgrowth during development and in revascularization of ischemic tissue. Semaphorins are upregulated in DR and related disease models, and dysregulation of retinal semaphorins may play a pathological role in DR.
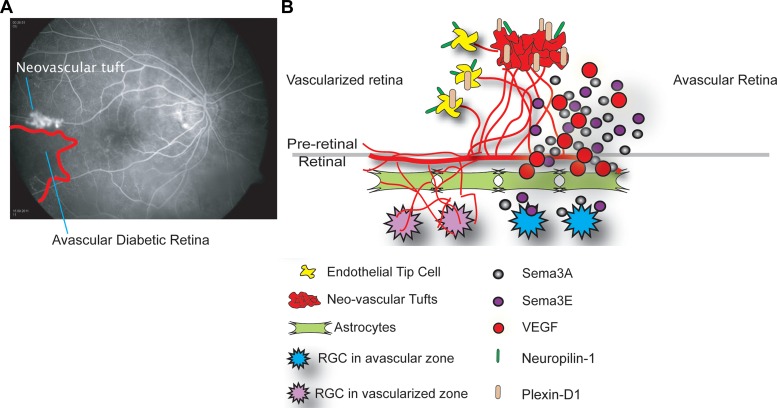
The class III semaphorin SEMA3A (28, 55) is secreted by ischemic retinal ganglion cells in response to IL-1β, and prevents revascularization of ischemic but salvageable neurons after vascular injury (55). SEMA3A binds the neuropilin-1 (NRP1) receptor to mediate endothelial cell cytoskeleton collapse and prevent migration, and can inhibit VEGF-mediated chemotaxis (Fig. 3) (82). SEMA3A also promotes endothelial apoptosis through NRP1 (44). Joyal et al. demonstrated that inhibition of SEMA3A facilitates normal revascularization of the inner retina after vascular injury in OIR mice, alleviating ischemic proliferative retinopathy (Fig. 3) (55). SEMA3A also promotes endothelial apoptosis through NRP1, and exogenous intravitreal injection of SEMA3A during OIR blocks both tuft formation and physiological revascularization (55).
Fig. 3.
Schematic of SEMA3A and SEMA3E signaling from Refs. 39 and 108. A: in the diabetic retina potentially reparative neovessels fail to revascularize the ischemic avascular retina and instead form misdirected neovascular tufts, which is regulated in part by semaphorins. B: semaphorins are secreted by hypoxic retinal ganglion cells (RGCs, shown in blue) in the avascular retina. SEMA3A (gray) represses vascular outgrowth by binding receptor Neuropilin-1 (green) on endothelial tip cells and neovascular tufts, whereas SEMA3E (purple) represses vascular outgrowth by binding receptor Plexin-D1 (beige) on tip cells and neovascular tufts.
Levels of SEMA3A are significantly elevated in the vitreous of patients with diabetic macular edema (17) and proliferative DR (28). In early DR SEMA3A induces vascular hyperpermeability through receptor NRP1, subsequently activating Src kinase and FAK, loosening endothelial tight junctions, and facilitating blood-retinal barrier breakdown (17). SEMA3A is upregulated in DR before VEGF, and neutralization of SEMA3A alleviates vascular hyperpermeability in early DR, at a stage when VEGF neutralization is ineffective (17). In addition, neuron-derived SEMA3A and VEGF provoke microglial chemotaxis through NRP-1 and may thus contribute to the destructive inflammation associated with retinopathy (28). These findings suggest that SEMA3A is a potential therapeutic target for DR and DME, and may be more effective than VEGF neutralization in early disease.
SEMA3A may also promote apoptosis in retinal neurons, contributing to neurodegeneration in retinopathy. Hua et al. demonstrated that a SEMA3A-neutralizing antibody alleviates neurodegeneration in OIR (50). SEMA3A neutralization may be beneficial in retinopathy-associated or other neurodegeneration.
The class III semaphorin SEMA3E also deters vascular growth. SEMA3E secreted by severely ischemic retinal neurons activates endothelial cell Plexin D1 to deter VEGF-induced filipodial projections in the OIR model (Fig. 3) (39, 108). However, in contrast to SEMA3A, SEMA3E normalizes revascularization in OIR, since it selectively suppresses extraretinal vascular outgrowth without affecting normal regeneration of the retinal vasculature (39). SEMA3E is also decreased in the vitreous of DR patients, further suggesting its relevance to DR (39).
Basic research findings suggest semaphorins contribute to neurovascular pathophysiology of DR, and semaphorins remain a topic of intense investigation for DR and other neovascular retinal diseases. Further studies may be directed toward better understanding their mechanisms of action and developing therapeutic interventions that can be brought into clinical use.
Pigment Epithelium-Derived Factor
Pigment epithelium-derived factor (PEDF) secreted by the retinal pigment epithelium, Müller cells, ciliary epithelium, and cornea suppresses both neurodegeneration and pathological neovascularization in retinal disease (8). Retinal PEDF is decreased in DR, and restoration of retinal PEDF levels has therapeutic effects in DR and related disease models, alleviating neurodegeneration, vascular hyperpermeability, and neovascularization (71).
Our group demonstrated that PEDF expression is decreased in the retina of DR models, and that PEDF suppresses hypoxia-induced factor-1-regulated VEGF expression in DR, thereby alleviating vascular hyperpermeability (133). PEDF also suppresses inflammation and neovascularization in the retina and cornea, potentially by inhibiting heterodimerization of Wnt coreceptors, thereby suppressing Wnt signaling, which contributes to retinal neovascularization and vascular hyperpermeability (94, 95). Furthermore, PEDF suppresses mobilization of endothelial progenitor cells in ischemic proliferative retinopathy, decreasing neovascularization (74).
Interestingly, although retinal PEDF is suppressed in DR, circulating serum PEDF is increased in diabetic patients, and high PEDF levels are positively correlated with diabetic complications (51, 52). Systemic elevation of PEDF may reflect a protective response to diabetes (47). Future studies of PEDF will be pointed toward better understanding its mechanisms of action and developing recombinant PEDF peptides for clinical use.
Platelet-Derived Growth Factor
Platelet-derived growth factors (PDGFs-A to -E) function as homodimers with exception of the heterodimer PDGF-AB (2). PDGF secreted by retinal neurons and glia binds tyrosine kinase cell surface receptors on retinal neurons and endothelial cells to initiate Ras and phosphatidylinositol-II pathways, mediating cellular survival, proliferation, and migration (48), necessary for retinal neuronal survival and vascular homeostasis in normal conditions (69, 85). However, PDGF is increased in proliferative DR, and contributes to pathological neovascularization (101). Anti-PDGF therapies alleviate proliferative retinopathy in animal models of DR and in human patients (106), but anti-PDGF therapies may have similar limitations to anti-VEGF therapy, with long-term suppression potentially leading to neuronal and vascular degeneration. Future studies will further evaluate neuroprotective and angiogenic effects of PDGF to better establish its role in the normal and diseased retina and therapeutic potential for early and advanced DR.
Norrin
Norrin is a protein encoded by the Norrin gene. Norrin mutations are associated with a very rare hereditary eye disease with incomplete retinal blood vessel development, X-linked Norrie disease (10, 23). Müller cells are the primary source of norrin in the retina (89, 131). Norrin plays a crucial role in vascular outgrowth and retinal neuronal survival. Norrin-deficient mice fail to develop intraretinal capillaries (100), and overexpression of norrin alleviates both vasoobliteration and neovascularization in OIR (88), suggesting that norrin is essential for vascular development and supports normal revascularization following retinal vascular injury. Norrin-deficient mice develop retinal neurodegeneration, which may be due to primary defects in blood vessels and neurons, or may result later from the lack of oxygen and nutrients associated with an inadequate vascular supply (87, 100). Norrin also protects retinal neurons against excitoxic stress and light damage (12, 88).
Norrin is structurally unrelated to Wnt ligands, but is able to activate canonical Wnt signaling by binding to the frizzled-4 (Fzd-4) receptor, ultimately stabilizing the transcription factor β-catenin and facilitating transcription of Wnt target genes (130). Neutralization of Fzd-4 suppresses the beneficial effects of norrin in retinal blood vessels and neurons, suggesting that norrin-mediated vascular outgrowth and neuroprotection are dependent on Wnt signaling activation (89). Wnt signaling is enhanced in pathological neovascularization in the OIR model, and suppression of Wnt signaling alleviates neovascularization (20). In addition to regulating angiogenesis, norrin and Wnt signaling also control retinal vascular permeability through regulation of tight junction proteins claudin-5 and plasmalemma vesicle-associated protein (21, 112, 127, 135).
Wnt target genes Sox17, angiopoetin-2 (Ang-2), and claudin-5 may be responsible in part for Norrin/Fzd-4-mediated retinal angiogenesis, since exogenous Sox17 alleviates vascular deficiencies in cultured Fzd4−/− endothelial cells in vitro (132), and neutralization of Ang-2 blocks norrin-mediated vascular outgrowth (88). Blocking claudin-5 suppresses Wnt ligand-driven angiogenesis in vitro, and developmental and pathological neovascularization in retinopathy (20). However, overexpressing Sox17 in endothelial cells in vivo cannot rescue the defective angiogenesis in Norrin knockout mice, suggesting that additional factors are likely involved (136). Neuroprotective effects of norrin have been attributed to upregulation of Wnt target genes leukemia inhibitory factor and endothelin 2 and subsequent increases in neurotropic factors (88).
The therapeutic potential of manipulating the norrin pathway for DR is presently unclear. Norrin supports vascular regrowth and neuronal survival under stress conditions, and therefore could protect against neuronal and vascular degeneration in nonproliferative DR. However, the effects of norrin are regulated through Wnt signaling, which contributes to vascular permeability in early DR and pathological neovascularization in advanced proliferative DR (22). Studies in diabetic animal models are warranted to further investigate the therapeutic potential of norrin upregulation (or suppression) in DR.
Neuronal Nutrient Sensing
The primary role of the retinal vasculature is to supply energetically demanding retinal neurons with sufficient oxygen and nutrients for energy production and sensory transmission. The consequences of insufficient oxygen supply, or hypoxia, in driving retinal angiogenesis is well characterized. However, increasing evidence suggests that neuronal nutrient sensing may also dictate angiogenesis.
Acetyl-CoA derived from glucose and lipids is used in the tricarboxylic acid (TCA) cycle to generate reducing equivalents for ATP production. TCA cycle intermediates such as succinate may accumulate under hypoxic conditions when oxygen supplies are insufficient for aerobic respiration. Sapieha et al. found that, in the ischemic retina, succinate levels rise and activate the metabolite-sensing G protein-coupled receptor GPR91 in retinal ganglion cells (110). Activation of GPR91 in hypoxia occurs before stabilization of HIF-1α and upregulates VEGF, stimulating neovascularization (110). Consequently, inhibition of GPR91 in ischemic retinopathy suppresses pathological neovascularization (110). This study provided the first direct evidence that imbalances in neuronal energy metabolism may dictate pathological neovascularization.
Metabolic changes in photoreceptors also impact DR. Du et al. demonstrated that photoreceptors are a primary source of mitochondrial reactive oxygen species in DR, and may increase generation of reactive oxygen species as a result of an altered metabolic state (31). Furthermore, Liu et al. found that inhibition of the visual cycle, which is energetically demanding, alleviates DR, further suggesting that changes in neuronal metabolism may influence DR (70). Preliminary imaging studies in diabetic patients have also demonstrated evidence of metabolic stress in early DR (35).
In addition, during sustained ischemia/metabolic dysregulation, neurons such as retinal ganglion cells (RGCs) go into a state of limited energy consumption and activate pathways of endoplasmic reticulum (ER) stress (11). Under milder conditions of nutrient and oxygen deprivation, RGCs attempt to reinstate local microcirculation and repair damage by recruiting the innate immune system. After acute or short-term injury of retinal ganglion cells, monocyte-derived macrophages act as inflammation-resolving macrophages, suppressing inflammation and regulating recruitment of activated innate immune cells, restoring immune homeostasis (73). Monocyte-derived macrophages also upregulate neurotrophic factors, directly supporting neuronal survival (73).
When ischemia is sustained, RGCs activate an ER stress-related endoribonuclease, IRE-1α that inactivates a series of RNAs, including that of the classical neuronal guidance cue Netrin-1. Under normal physiological conditions, Netrin-1 can activate a program of reparative angiogenesis in microglia to restore retinal vasculature. Hence, under sustained ischemic stress, RGCs suppress the production of factors that stimulate the immune system to repair tissue (11). This might be an organ survival response to neural cells driven beyond a threshold of repair to shunt metabolic stores toward more salvageable zones of the retina (11). It may also be a functional link between metabolic stress and inflammation in the diabetic retina.
The role of altered neuronal energy metabolism in DR is as of yet unknown. However, diabetes is at its core a metabolic disease, and increased levels of circulating glucose and lipids are likely to have profound effects on retinal energy metabolism. It is likely that metabolic changes in retinal neurons are central to the development and progression of DR.
CONCLUSIONS AND FUTURE DIRECTIONS
Surgical intervention and anti-VEGF therapies are the most common clinical interventions for DR, and are generally used to treat advanced disease. Although these treatments are sight saving, they are also invasive and costly. Preventing macular edema, neovascularization, and other sight-threatening microvascular pathologies by targeting upstream events may yield more desirable approaches to treatment. Glycemic control is the most effective and inexpensive intervention to prevent DR and other diabetic complications, but patient compliance may be problematic, and complications may still develop in spite of these preventative measures.
Abundant basic and clinical research findings demonstrate that retinal neurons and glia profoundly influence the microvasculature, and that neuronal degeneration and dysfunction precede development of overt microvascular DR (7, 117). In early DR, stressed retinal neurons exacerbate vasoregression and vascular hyperpermeability through dysregulated neurovascular coupling and secretion of cytotoxic molecules, vasorepulsive factors, and mediators of vascular permeability (117). In advanced DR, neurons exacerbate pathological neovascularization and misdirect outgrowth of reparative neovessels by secreting angiogenic and vasorepulsive factors and exacerbating inflammation (117). Normalizing neurovascular cross talk by directly targeting aberrant signaling is an effective approach to treatment, as demonstrated by the success of anti-VEGF therapy, which neutralizes VEGF secreted, in part, by retinal neurons (129). Alternatively, neuroprotective therapies aimed at preventing neuronal cell death and dysfunction may indirectly normalize neurovascular cross talk. Basic research and preliminary clinical findings suggest neuroprotective therapies hold significant potential for DR (49).
Equally important to additional basic research in understanding the mechanisms of DR is improved clinical management of diabetic patients. Early events such as neurodegeneration are generally not evaluated in routine screenings for DR, so preventative neuroprotective therapies would be difficult to implement under current standards of care. Furthermore, because DR is frequently one of the first complications to develop, ophthalmologists are often the first to detect diabetic vascular changes. In many cases DR is predictive of other complications, so improved communication between ophthalmologists, endocrinologists, and other providers could enhance preventative care and screening.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants T32-EY-005318 (E. P. Moran) and EY-024963 (J. Chen); a Canada Research Chair in Retinal Cell Biology, Wolfe Senior Scholar Award, and Canadian Diabetes Research Foundation Grant OG-3-14-4544-PS (P. Sapieha); NIH Grants EY-024868, EY-017017, EY-0222275, and P91-HD-8655, the Lowy Medical Research Institute, and European Commission FP7 project 305485 PREVENT-ROP (L. E. H. Smith); and NIH Grants EY-012231, EY-018659, EY-019309, and P20-GM-104934 Juvenile Diabetes Research Foundation Grant 2-SRA-2014-147-Q-R (J.-X. Ma).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.P.M., L.E.S., and J.-x.M. conception and design of research; E.P.M. and Z.W. analyzed data; E.P.M. interpreted results of experiments; E.P.M., Z.W., J.C., P.S., and L.E.S. prepared figures; E.P.M., Z.W., J.C., P.S., and L.E.S. drafted manuscript; E.P.M., J.C., P.S., L.E.S., and J.-x.M. edited and revised manuscript; E.P.M., Z.W., J.C., P.S., L.E.S., and J.-x.M. approved final version of manuscript; Z.W. performed experiments.
REFERENCES
- 1.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 92: 10457–10461, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc 81: 1241–1257, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem 274: 23463–23467, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med 366: 1227–1239, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Ardeljan D, Chan CC. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retinal Eye Res 37: 68–89, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature 468: 557–561, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 102: 783–791, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnstable CJ, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retinal Eye Res 23: 561–577, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Bek T, Hajari J, Jeppesen P. Interaction between flicker-induced vasodilatation and pressure autoregulation in early retinopathy of type 2 diabetes. Graef Arch Clin Exp Ophthalmol 246: 763–769, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Berger W, Meindl A, van de Pol TJ, Cremers FP, Ropers HH, Doerner C, Monaco A, Bergen AA, Lebo R, Warburg M. Isolation of a candidate gene for Norrie disease by positional cloning. Nat Genet 1: 199–203, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Binet F, Mawambo G, Sitaras N, Tetreault N, Lapalme E, Favret S, Cerani A, Leboeuf D, Tremblay S, Rezende F, Juan AM, Stahl A, Joyal JS, Milot E, Kaufman RJ, Guimond M, Kennedy TE, Sapieha P. Neuronal ER stress impedes myeloid-cell-induced vascular regeneration through IRE1alpha degradation of netrin-1. Cell Metab 17: 353–371, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Braunger BM, Ohlmann A, Koch M, Tanimoto N, Volz C, Yang Y, Bosl MR, Cvekl A, Jagle H, Seeliger MW, Tamm ER. Constitutive overexpression of Norrin activates Wnt/beta-catenin and endothelin-2 signaling to protect photoreceptors from light damage. Neurobiol Dis 50: 1–12, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Burns SA, Elsner AE, Chui TY, Vannasdale DA Jr, Clark CA, Gast TJ, Malinovsky VE, Phan AD. In vivo adaptive optics microvascular imaging in diabetic patients without clinically severe diabetic retinopathy. Biomed Optics Express 5: 961–974, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrera DeBuc D, Somfai GM. Early detection of retinal thickness changes in diabetes using Optical Coherence Tomography. Int Med J Exp Clin Res 16: MT15–MT21, 2010. [PubMed] [Google Scholar]
- 15.Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retinal Eye Res 49: 67–81, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casini G, Dal Monte M, Fornaciari I, Filippi L, Bagnoli P. The beta-adrenergic system as a possible new target for pharmacologic treatment of neovascular retinal diseases. Prog Retinal Eye Res 42C: 103–129, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Cerani A, Tetreault N, Menard C, Lapalme E, Patel C, Sitaras N, Beaudoin F, Leboeuf D, De Guire V, Binet F, Dejda A, Rezende FA, Miloudi K, Sapieha P. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metab 18: 505–518, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Cervia D, Catalani E, Dal Monte M, Casini G. Vascular endothelial growth factor in the ischemic retina and its regulation by somatostatin. J Neurochem 120: 818–829, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Liu CH, Sapieha P. Retinal Vascular Development. In: Anti-Angiogenic Therapy in Ophthalmology, edited by Cham SA. New York, NY: Springer, 2016, p. 1–19. [Google Scholar]
- 20.Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, Sapieha P, Hua J, Hatton CJ, Juan AM, Aderman CM, Willett KL, Guerin KI, Mammoto A, Campbell M, Smith LE. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation 124: 1871–1881, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Stahl A, Krah NM, Seaward MR, Joyal JS, Juan AM, Hatton CJ, Aderman CM, Dennison RJ, Willett KL, Sapieha P, Smith LE. Retinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout mice. PloS one 7: e30203, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Hu Y, Zhou T, Zhou KK, Mott R, Wu M, Boulton M, Lyons TJ, Gao G, Ma JX. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am J Pathol 175: 2676–2685, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen ZY, Hendriks RW, Jobling MA, Powell JF, Breakefield XO, Sims KB, Craig IW. Isolation and characterization of a candidate gene for Norrie disease. Nat Genet 1: 204–208, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc 4: 1565–1573, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retinal Eye Res 43: 17–75, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology 122: 1375–1394, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Debuc DC, Salinas HM, Ranganathan S, Tatrai E, Gao W, Shen M, Wang J, Somfai GM, Puliafito CA. Improving image segmentation performance and quantitative analysis via a computer-aided grading methodology for optical coherence tomography retinal image analysis. J Biomed Optics 15: 046015, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dejda A, Mawambo G, Cerani A, Miloudi K, Shao Z, Daudelin JF, Boulet S, Oubaha M, Beaudoin F, Akla N, Henriques S, Menard C, Stahl A, Delisle JS, Rezende FA, Labrecque N, Sapieha P. Neuropilin-1 mediates myeloid cell chemoattraction and influences retinal neuroimmune crosstalk. J Clin Invest 124: 4807–4822, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devi TS, Hosoya K, Terasaki T, Singh LP. Critical role of TXNIP in oxidative stress, DNA damage and retinal pericyte apoptosis under high glucose: implications for diabetic retinopathy. Exp Cell Res 319: 1001–1012, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Marco E, Jha JC, Sharma A, Wilkinson-Berka JL, Jandeleit-Dahm KA, de Haan JB. Are reactive oxygen species still the basis for diabetic complications? Clin Sci (Lond) 129: 199–216, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci USA 110: 16586–16591, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Famiglietti EV, Stopa EG, McGookin ED, Song P, LeBlanc V, Streeten BW. Immunocytochemical localization of vascular endothelial growth factor in neurons and glial cells of human retina. Brain Res 969: 195–204, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Feeney SA, Simpson DA, Gardiner TA, Boyle C, Jamison P, Stitt AW. Role of vascular endothelial growth factor and placental growth factors during retinal vascular development and hyaloid regression. Invest Ophthalmol Vis Sci 44: 839–847, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Feng Y, Venema VJ, Venema RC, Tsai N, Behzadian MA, Caldwell RB. VEGF-induced permeability increase is mediated by caveolae. Invest Ophthalmol Vis Sci 40: 157–167, 1999. [PubMed] [Google Scholar]
- 35.Field MG, Elner VM, Puro DG, Feuerman JM, Musch DC, Pop-Busui R, Hackel R, Heckenlively JR, Petty HR. Rapid, noninvasive detection of diabetes-induced retinal metabolic stress. Arch Ophthalmol 126: 934–938, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filosa JA, Morrison HW, Iddings JA, Du W, Kim KJ. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience 323: 96–109, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frey T, Antonetti DA. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antiox Redox Signal 15: 1271–1284, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Fu S, Dong S, Zhu M, Sherry DM, Wang C, You Z, Haigh JJ, Le YZ. Muller glia are a major cellular source of survival signals for retinal neurons in diabetes. Diabetes 64: 3554–3563, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest 121: 1974–1985, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardiner TA, Archer DB, Curtis TM, Stitt AW. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation 14: 25–38, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Gardiner TA, Stitt AW, Archer DB. Retinal vascular endothelial cell endocytosis increases in early diabetes. Lab Invest 72: 439–444, 1995. [PubMed] [Google Scholar]
- 42.Ghirlanda G, Di Leo MA, Caputo S, Cercone S, Greco AV. From functional to microvascular abnormalities in early diabetic retinopathy. Diabetes/Metab Rev 13: 15–35, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol 81: 1–44, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem 282: 26294–26305, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes 60: 9–16, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci 47: 5106–5115, 2006. [DOI] [PubMed] [Google Scholar]
- 47.He X, Cheng R, Benyajati S, Ma JX. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin Sci (Lond) 128: 805–823, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79: 1283–1316, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez C, Simo R. Neuroprotection in diabetic retinopathy. Curr Diabetes Rep 12: 329–337, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Hua N, Liu H, Qian X, Dong L, Wu J, Li X. The effect of semaphorin 3A in the process of apoptosis in oxygen induced retinopathy in rats. Zhonghua Yan Ke Za Zhi 50: 440–447, 2014. [PubMed] [Google Scholar]
- 51.Jenkins A, Zhang SX, Gosmanova A, Aston C, Dashti A, Baker MZ, Lyons T, Ma JX. Increased serum pigment epithelium derived factor levels in Type 2 diabetes patients. Diabetes Res Clin Pract 82: e5–e7, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jenkins AJ, Zhang SX, Rowley KG, Karschimkus CS, Nelson CL, Chung JS, O'Neal DN, Januszewski AS, Croft KD, Mori TA, Dragicevic G, Harper CA, Best JD, Lyons TJ, Ma JX. Increased serum pigment epithelium-derived factor is associated with microvascular complications, vascular stiffness and inflammation in Type 1 diabetes. Diabet Med 24: 1345–1351, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol 158: 147–152, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J 16: 438–440, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Joyal JS, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, Shao Z, Polosa A, Zhu T, Hamel D, Djavari M, Kunik D, Honore JC, Picard E, Zabeida A, Varma DR, Hickson G, Mancini J, Klagsbrun M, Costantino S, Beausejour C, Lachapelle P, Smith LE, Chemtob S, Sapieha P. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood 117: 6024–6035, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kandarakis SA, Piperi C, Topouzis F, Papavassiliou AG. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog Retinal Eye Res 42: 85–102, 2014. [DOI] [PubMed] [Google Scholar]
- 57.Kirwin SJ, Kanaly ST, Hansen CR, Cairns BJ, Ren M, Edelman JL. Retinal gene expression and visually evoked behavior in diabetic long evans rats. Invest Ophthalmol Vis Sci 52: 7654–7663, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retinal Eye Res 34: 19–48, 2013. [DOI] [PubMed] [Google Scholar]
- 59.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 102: 527–532, 1984. [DOI] [PubMed] [Google Scholar]
- 60.Kolb H. Simple anatomy of the retina. In: Webvision: The Organization of the Retina and Visual System, edited by Kolb H, Fernandez E, and Nelson R. Salt Lake City, UT: 1995. [Google Scholar]
- 61.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2007: 43603, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kowluru RA, Engerman RL, Case GL, Kern TS. Retinal glutamate in diabetes and effect of antioxidants. Neurochem Int 38: 385–390, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retinal Eye Res 31: 377–406, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest 122: 4213–4217, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kvanta A. Expression and regulation of vascular endothelial growth factor in choroidal fibroblasts. Curr Eye Res 14: 1015–1020, 1995. [DOI] [PubMed] [Google Scholar]
- 66.Lakshminarayanan S, Antonetti DA, Gardner TW, Tarbell JM. Effect of VEGF on retinal microvascular endothelial hydraulic conductivity: the role of NO. Invest Ophthalmol Vis Sci 41: 4256–4261, 2000. [PubMed] [Google Scholar]
- 67.Langmann T. Microglia activation in retinal degeneration. J Leukocyte Biol 81: 1345–1351, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Lasta M, Pemp B, Schmidl D, Boltz A, Kaya S, Palkovits S, Werkmeister R, Howorka K, Popa-Cherecheanu A, Garhofer G, Schmetterer L. Neurovascular dysfunction precedes neural dysfunction in the retina of patients with type 1 diabetes. Invest Ophthalmol Vis Sci 54: 842–847, 2013. [DOI] [PubMed] [Google Scholar]
- 69.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277: 242–245, 1997. [DOI] [PubMed] [Google Scholar]
- 70.Liu H, Tang J, Du Y, Lee CA, Golczak M, Muthusamy A, Antonetti DA, Veenstra AA, Amengual J, von Lintig J, Palczewski K, Kern TS. Retinylamine Benefits Early Diabetic Retinopathy in Mice. J Biol Chem 290: 21568–21579, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Leo LF, McGregor C, Grivitishvili A, Barnstable CJ, Tombran-Tink J. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol Med 18: 1387–1401, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lombardo M, Parravano M, Lombardo G, Varano M, Boccassini B, Stirpe M, Serrao S. Adaptive optics imaging of parafoveal cones in type 1 diabetes. Retina 34: 546–557, 2014. [DOI] [PubMed] [Google Scholar]
- 73.London A, Itskovich E, Benhar I, Kalchenko V, Mack M, Jung S, Schwartz M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J Exp Med 208: 23–39, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Longeras R, Farjo K, Ihnat M, Ma JX. A PEDF-derived peptide inhibits retinal neovascularization and blocks mobilization of bone marrow-derived endothelial progenitor cells. Exp Diabetes Res 2012: 518426, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lorenzi M, Gerhardinger C. Early cellular and molecular changes induced by diabetes in the retina. Diabetologia 44: 791–804, 2001. [DOI] [PubMed] [Google Scholar]
- 76.Lott ME, Slocomb JE, Shivkumar V, Smith B, Gabbay RA, Quillen D, Gardner TW, Bettermann K. Comparison of retinal vasodilator and constrictor responses in type 2 diabetes. Acta Ophthalmol 90: e434–e441, 2012. [DOI] [PubMed] [Google Scholar]
- 77.Lutty GA. Effects of diabetes on the eye. Invest Ophthalmol Vis Sci 54: ORSF81–ORSF87, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malecaze F, Clamens S, Simorre-Pinatel V, Mathis A, Chollet P, Favard C, Bayard F, Plouet J. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol 112: 1476–1482, 1994. [DOI] [PubMed] [Google Scholar]
- 79.Mathew C, Yunirakasiwi A, Sanjay S. Updates in the management of diabetic macular edema. J Diabetes Res 2015: 794036, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci 27: 2468–2471, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci 26: 2862–2870, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol 146: 233–242, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 120: 106–114, 2013. [DOI] [PubMed] [Google Scholar]
- 84.Min JK, Lee S, Kim JS, Woo JM, Yang HS. Effects of diabetic macular edema on repeatability of retinal nerve fiber layer thickness measurements at the macular and peripapillary area using swept-source optical coherence tomography. Curr Eye Res 1–8, 2016. [DOI] [PubMed] [Google Scholar]
- 85.Mudhar HS, Pollock RA, Wang C, Stiles CD, Richardson WD. PDGF and its receptors in the developing rodent retina and optic nerve. Development 118: 539–552, 1993. [DOI] [PubMed] [Google Scholar]
- 86.Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cereb Blood Flow Metab 33: 1685–1695, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ohlmann A, Scholz M, Goldwich A, Chauhan BK, Hudl K, Ohlmann AV, Zrenner E, Berger W, Cvekl A, Seeliger MW, Tamm ER. Ectopic norrin induces growth of ocular capillaries and restores normal retinal angiogenesis in Norrie disease mutant mice. J Neurosci 25: 1701–1710, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ohlmann A, Seitz R, Braunger B, Seitz D, Bosl MR, Tamm ER. Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice. J Neurosci 30: 183–193, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohlmann A, Tamm ER. Norrin: molecular and functional properties of an angiogenic and neuroprotective growth factor. Prog Retinal Eye Res 31: 243–257, 2012. [DOI] [PubMed] [Google Scholar]
- 90.Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis 38: 258–268, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Pappenheimer JR, Renkin EM, Borrero LM. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol 167: 13–46, 1951. [DOI] [PubMed] [Google Scholar]
- 92.Pardue MT, Barnes CS, Kim MK, Aung MH, Amarnath R, Olson DE, Thule PM. Rodent hyperglycemia-induced inner retinal deficits are mirrored in human diabetes. Trans Vision Sci Technol 3: 6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parisi V, Uccioli L, Parisi L, Colacino G, Manni G, Menzinger G, Bucci MG. Neural conduction in visual pathways in newly-diagnosed IDDM patients. Electroencephalogr Clin Neurophysiol 108: 490–496, 1998. [DOI] [PubMed] [Google Scholar]
- 94.Park K, Jin J, Hu Y, Zhou K, Ma JX. Overexpression of pigment epithelium-derived factor inhibits retinal inflammation and neovascularization. Am J Pathol 178: 688–698, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park K, Lee K, Zhang B, Zhou T, He X, Gao G, Murray AR, Ma JX. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol 31: 3038–3051, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron 71: 782–797, 2011. [DOI] [PubMed] [Google Scholar]
- 97.Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 57: 2495–2502, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA 92: 905–909, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poitry-Yamate CL, Poitry S, Tsacopoulos M. Lactate released by Muller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci 15: 5179–5191, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richter M, Gottanka J, May CA, Welge-Lussen U, Berger W, Lutjen-Drecoll E. Retinal vasculature changes in Norrie disease mice. Invest Ophthalmol Vis Sci 39: 2450–2457, 1998. [PubMed] [Google Scholar]
- 101.Robbins SG, Mixon RN, Wilson DJ, Hart CE, Robertson JE, Westra I, Planck SR, Rosenbaum JT. Platelet-derived growth factor ligands and receptors immunolocalized in proliferative retinal diseases. Invest Ophthalmol Vis Sci 35: 3649–3663, 1994. [PubMed] [Google Scholar]
- 102.Robinson GS, Ju M, Shih SC, Xu X, McMahon G, Caldwell RB, Smith LE. Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. FASEB J 15: 1215–1217, 2001. [DOI] [PubMed] [Google Scholar]
- 103.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci USA 93: 4851–4856, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robinson R, Barathi VA, Chaurasia SS, Wong TY, Kern TS. Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Dis Mod Mech 5: 444–456, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roufail E, Soulis T, Boel E, Cooper ME, Rees S. Depletion of nitric oxide synthase-containing neurons in the diabetic retina: reversal by aminoguanidine. Diabetologia 41: 1419–1425, 1998. [DOI] [PubMed] [Google Scholar]
- 106.Sadiq MA, Hanout M, Sarwar S, Hassan M, Do DV, Nguyen QD, Sepah YJ. Platelet derived growth factor inhibitors: A potential therapeutic approach for ocular neovascularization. Saudi J Ophthalmol 29: 287–291, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sagaties MJ, Raviola G, Schaeffer S, Miller C. The structural basis of the inner blood-retina barrier in the eye of Macaca mulatta. Invest Ophthalmol Vis Sci 28: 2000–2014, 1987. [PubMed] [Google Scholar]
- 108.Sapieha P. Eyeing central neurons in vascular growth and reparative angiogenesis. Blood 120: 2182–2194, 2012. [DOI] [PubMed] [Google Scholar]
- 109.Sapieha P, Hamel D, Shao Z, Rivera JC, Zaniolo K, Joyal JS, Chemtob S. Proliferative retinopathies: angiogenesis that blinds. Int J Biochem Cell Biol 42: 5–12, 2010. [DOI] [PubMed] [Google Scholar]
- 110.Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal JS, Cho JH, Honore JC, Kermorvant-Duchemin E, Varma DR, Tremblay S, Leduc M, Rihakova L, Hardy P, Klein WH, Mu X, Mamer O, Lachapelle P, Di Polo A, Beausejour C, Andelfinger G, Mitchell G, Sennlaub F, Chemtob S. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med 14: 1067–1076, 2008. [DOI] [PubMed] [Google Scholar]
- 111.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74: 691–705, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schafer NF, Luhmann UF, Feil S, Berger W. Differential gene expression in Ndph-knockout mice in retinal development. Invest Ophthalmol Vis Sci 50: 906–916, 2009. [DOI] [PubMed] [Google Scholar]
- 113.Shaw LC, Neu MB, Grant MB. Cell-based therapies for diabetic retinopathy. Curr Diabetes Rep 11: 265–274, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shih SC, Ju M, Liu N, Smith LE. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. J Clin Invest 112: 50–57, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simionescu M, Simionescu N. Endothelial transport of macromolecules: transcytosis and endocytosis. A look from cell biology. Cell Biol Rev 25: 5–78, 1991. [PubMed] [Google Scholar]
- 116.Simo R, Hernandez C, European Consortium for the Early Treatment of Diabetic Retinopathy. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab 25: 23–33, 2014. [DOI] [PubMed] [Google Scholar]
- 117.Stem MS, Gardner TW. Neurodegeneration in the pathogenesis of diabetic retinopathy: molecular mechanisms and therapeutic implications. Curr Med Chem 20: 3241–3250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sundstrom JM, Tash BR, Murakami T, Flanagan JM, Bewley MC, Stanley BA, Gonsar KB, Antonetti DA. Identification and analysis of occludin phosphosites: a combined mass spectrometry and bioinformatics approach. Journal of proteome research 8: 808–817, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tam J, Dhamdhere KP, Tiruveedhula P, Manzanera S, Barez S, Bearse MA Jr, Adams AJ, Roorda A. Disruption of the retinal parafoveal capillary network in type 2 diabetes before the onset of diabetic retinopathy. Invest Ophthalmol Vis Sci 52: 9257–9266, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trudeau K, Molina AJ, Roy S. High glucose induces mitochondrial morphology and metabolic changes in retinal pericytes. Invest Ophthalmol Vis Sci 52: 8657–8664, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Dijk HW, Verbraak FD, Kok PH, Stehouwer M, Garvin MK, Sonka M, DeVries JH, Schlingemann RO, Abramoff MD. Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci 53: 2715–2719, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retinal Eye Res 51: 1–40, 2016. [DOI] [PubMed] [Google Scholar]
- 123.Verbraak FD. Neuroretinal degeneration in relation to vasculopathy in diabetes. Diabetes 63: 3590–3592, 2014. [DOI] [PubMed] [Google Scholar]
- 124.Verma A, Rani PK, Raman R, Pal SS, Laxmi G, Gupta M, Sahu C, Vaitheeswaran K, Sharma T. Is neuronal dysfunction an early sign of diabetic retinopathy? Microperimetry and spectral domain optical coherence tomography (SD-OCT) study in individuals with diabetes, but no diabetic retinopathy. Eye 23: 1824–1830, 2009. [DOI] [PubMed] [Google Scholar]
- 125.Vujosevic S, Midena E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Muller cells alterations. J Diabetes Res 2013: 905058, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y, Fawzi A, Tan O, Gil-Flamer J, Huang D. Retinal blood flow detection in diabetic patients by Doppler Fourier domain optical coherence tomography. Optics Express 17: 4061–4073, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151: 1332–1344, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retinal Eye Res 22: 1–29, 2003. [DOI] [PubMed] [Google Scholar]
- 129.Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL 3rd, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. J Am Med Assoc 314: 2137–2146, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116: 883–895, 2004. [DOI] [PubMed] [Google Scholar]
- 131.Ye X, Smallwood P, Nathans J. Expression of the Norrie disease gene (Ndp) in developing and adult mouse eye, ear, and brain. Gene Expression Patterns 11: 151–155, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 139: 285–298, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang SX, Wang JJ, Gao G, Parke K, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol 37: 1–12, 2006. [DOI] [PubMed] [Google Scholar]
- 134.Zheng Y, Gu Q, Xu X. Inhibition of ocular neovascularization by a novel peptide derived from human placenta growth factor-1. Acta Ophthalmol 90: e512–e523, 2012. [DOI] [PubMed] [Google Scholar]
- 135.Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest 124: 3825–3846, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou Y, Williams J, Smallwood PM, Nathans J. Sox7, Sox17, and Sox18 cooperatively regulate vascular development in the mouse retina. PloS one 10: e0143650, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]