The data presented in this study help clarify seemingly discrepant findings in the field involving two of the common mouse strains used to study inflammation, particularly regarding the roles of PECAM and CD99 in leukocyte transendothelial migration.
Keywords: CD99, diapedesis, inflammation, intravital microscopy, platelet/endothelial cell adhesion molecule
Abstract
Leukocyte transendothelial migration (TEM) is an essential component of the inflammatory response. In vitro studies with human cells have demonstrated that platelet/endothelial cell adhesion molecule (PECAM) functions upstream of CD99 during TEM; however, results in vivo with mice have been apparently contradictory. In this study we use four-dimensional (4D) intravital microscopy to demonstrate that the site and order of function of PECAM and CD99 in vivo are dependent on the strain of mice. In FVB/n mice, PECAM functions upstream of CD99, as in human cells in vitro, and blocking antibodies against either molecule arrest neutrophils before they traverse the endothelium. However, in C57BL/6 mice, PECAM and CD99 appear to function at a different step, as the same antibodies arrest leukocyte migration through the endothelial basement membrane. These results are the first direct comparison of PECAM and CD99 function in different murine strains as well as the first demonstration of the sequential function of PECAM and CD99 in vivo.
NEW & NOTEWORTHY
The data presented in this study help clarify seemingly discrepant findings in the field involving two of the common mouse strains used to study inflammation, particularly regarding the roles of PECAM and CD99 in leukocyte transendothelial migration.
inflammation is part of the host's immune response to tissue damage. It involves the local delivery of leukocytes to sites of damage via a series of adhesive interactions with the endothelial cells (ECs) lining the postcapillary venules. The committed step of this process is transendothelial migration (TEM), or diapedesis, during which the leukocytes squeeze between adjacent ECs or traverse the body of an intact EC. Platelet/EC adhesion molecule (PECAM) and CD99 are two junctional adhesion molecules known to be critical for TEM (reviewed in Refs. 13 and 19). Human ECs in vitro have been used to show that PECAM functions upstream of CD99 to regulate leukocyte TEM; inhibiting PECAM function arrests leukocytes along the apical surface of the endothelium, whereas blocking CD99 arrests cells partway through EC junctions (16, 23, 34).
In contrast to in vitro studies with human cells, in vivo studies of PECAM and CD99 in diapedesis have provided disparate results. PECAM blockade in vivo has been shown to inhibit leukocyte transmigration in all mouse strains tested except C57BL/6 mice and to arrest leukocytes along the endothelium in FVB/n mice, replicating findings in human cells in vitro. In contrast, in PECAM-deficient C57BL/6 mice, there is a transient defect in crossing the endothelial basement membrane (5, 9, 38) or no detectable effect on transmigration, depending on the time of observation and the inflammatory model (9, 22, 26).
Much less is known about the role of CD99 than PECAM (reviewed in Ref. 13) in TEM in vivo. A recent study from our laboratory demonstrated, using high-resolution three-dimensional (3D) confocal microscopy, that when CD99 function is blocked in FVB/n mice, leukocytes are arrested partway through EC junctions, as seen in vitro (34). Conversely, in C57BL/6 mice, it has been reported that blocking CD99 function arrests neutrophils along the basement membrane in the cremaster circulation (5).
The reason for the discordance of the aforementioned studies in unknown. However, the site of blockade by anti-PECAM and anti-CD99 in these strains has not been compared head-to-head in the same laboratory. The exact function of PECAM and CD99 in vivo may be dependent on the murine strain, variations in the animal housing facilities, and/or the model used to study inflammation. Technical limitations of conventional imaging and resolution of the z-axis have hindered many of these studies. Furthermore, all these conclusions regarding the site of function of these proteins have been based on snapshots of end-point assays. On the basis of the differences obtained by various investigators using the two strains in different inflammatory models, we hypothesized that the differences were intrinsic to the mouse strains and that the site of leukocyte arrest in the presence of blockade of PECAM or CD99 would differ between FVB/n and C57BL/6 mouse strains when compared side-by-side in the same model.
In this report we utilized the state-of-the-art technology of four-dimensional (4D) intravital microscopy (IVM) (21, 37) to study leukocyte diapedesis in real time in vivo and, for the first time, directly compared the function of PECAM and CD99 in FVB/n and C57BL/6 mice. We found that, in FVB/n mice, inhibiting PECAM function apically arrests, whereas inhibiting CD99 function blocks leukocytes partway through endothelial junctions, similar to findings in human cells in vitro. Conversely, in C57BL/6 mice, blocking antibodies against PECAM and CD99 arrest PMN migration between the abluminal surface of the endothelium and the subendothelial basement membrane.
MATERIALS AND METHODS
Animals.
All protocols involving mice were reviewed and approved by the Institutional Animal Care and Use Committee at Northwestern University (US Public Health Service assurance no. A328301). Mice were housed in the institutional animal facility operated by the Center for Comparative Medicine at Northwestern University. Animals were fed a standard chow diet and bred and maintained according to standard American Association for Accreditation of Laboratory Animal Care methods. All experiments in this study used 8- to 12-wk-old mice. The C57BL/6 LysM-eGFP mouse strain (obtained from Dr. Paul Kubes, Calgary, AB, Canada) is described elsewhere (10). Briefly, the myelomonocytic cells of these mice were rendered fluorescent due to the expression of enhanced green fluorescent protein (eGFP) under the lysozyme M (LysM) locus. The FVB/n LysM-eGFP strain was derived by backcrossing C57BL/6 LysM-eGFP mice into wild-type FVB/n mice (Jackson Laboratory, Bar Harbor, ME) for nine generations. Carriers of the LysM-eGFP allele were identified in each backcross by analysis of a blood smear using fluorescence microscopy.
Reagents.
Rat anti-mouse PECAM antibody clone 390 (nonblocking) was purchased from EMD Millipore (Billerica, MA). Armenian hamster anti-mouse PECAM antibody (clone 2H8, blocking) (22) and rat anti-mouse CD99 antibody (clone 3F11, blocking) (34) have been previously described and were purified from culture supernatant using protein G-Sepharose (GE Healthcare, Barrington, IL) affinity chromatography according to standard methods (6, 34). Rabbit anti-mouse collagen IV, rat anti-mouse myeloid-related protein 14 (MRP14), and rabbit anti-mouse α-smooth muscle actin (α-SMA) were purchased from Abcam (Cambridge, MA). Allophycocyanin (APC) rat anti-mouse lymphocyte antigen 6 complex G6D (Ly6G, clone 1A8), APC rat IgG2a, FITC rat anti-mouse CD31 (clone 390), and FITC rat IgG2b were purchased from BioLegend (San Diego, CA). Control rat IgG, goat anti-Armenian hamster IgG, goat anti-rat IgG conjugated to Alexa Fluor 488, and goat anti-rabbit IgG conjugated to Alexa Fluor 647 were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Rat anti-mouse PECAM antibody (clone 390), rat anti-mouse CD99 antibody (clone 3F11), and goat anti-Armenian hamster IgG were directly conjugated with DyLight 550 (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's guidelines. Recombinant mouse interleukin-1β (IL-1β) was purchased from R & D Systems (Minneapolis, MN). Perfusion buffer was prepared by supplementation of Tyrode's salts (Sigma-Aldrich, St. Louis, MO) with 1 g/l sodium bicarbonate (Thermo Fisher Scientific) and adjustment of the pH of the warmed (35°C) solution to 7.4. Ketamine and xylazine were purchased from Henry Schein (Dublin, OH) and Akorn (Decatur, IL), respectively.
IVM of the mouse cremaster.
Preparation of the cremaster muscle for visualization using fluorescence microscopy was essentially as described previously (29–31, 37, 38). Briefly, LysM-eGFP male mice were injected intraperitoneally with 100 μg of antibody (anti-PECAM clone 2H8, anti-CD99 clone 3F11, or control nonspecific rat IgG) in 500 μl of PBS. Immediately after delivery of blocking or control antibody, 150 μl of PBS containing 100 mg of fluorescently conjugated anti-PECAM antibody (clone 390) and 5 ng of mouse IL-1β were injected intrascrotally. Before the surgery, the mice were anesthetized by intraperitoneal injection of ketamine and xylazine (100 mg/kg and 10 mg/kg body wt, respectively). Supplementary doses of anesthetics (∼25 μl containing 25 mg/ml ketamine and 1.25 mg/ml xylazine) were administered upon positive response to a foot pad pinch (typically every 30–45 min). Hair on the scrotum was removed with the depilatory gel Nair.
The mouse was then secured in a supine position on a Plexiglas platform containing an internal heating pad set to ∼37°C. The mouse was secured such that its lower legs straddled the edge of a glass window in the platform upon which a quartz pedestal embedded in silicone was secured. The scrotum was extended using a threaded needle and secured to the platform. Under a dissecting microscope, a ∼1-cm incision was made along the left ventral side of the scrotum. The cremaster muscle and encompassed testicle were exteriorized across the quartz pedestal and secured using suture needles bent into an “L” shape. The tissue was hydrated continuously with warmed perfusion buffer consisting of Tyrode's salts and 1 g/l sodium bicarbonate (pH 7.4). Forceps were used to carefully remove connective tissue from the cremaster muscle. The muscle was opened using microsurgery scissors to make a single distal-to-proximal incision that avoided severing as many large vessels as possible. The bleeding from small vessels was allowed to clot naturally; large vessels were closed by pinching the vessel near the opening with fine forceps or pinning the vessel to the silicone pedestal. The epididymis and associated testicle were freed from the cremaster muscle using microsurgery scissors and returned to the inguinal canal. However, in some mice, a large vessel connected these organs to the muscle. In these mice, this vessel and tissues were left connected but secured out of the viewing field to avoid dramatic tissue damage and blood flow anomalies. The muscle was spread across the quartz pedestal and pinned along its periphery. The platform and secured mouse were then transferred to the microscope for visualization.
The tissue was visualized using an UltraVIEW VoX imaging system equipped with a Yokogawa CSU-1 spinning disk and a ×20 water-immersion objective (1.00 numerical aperture). Images were collected using Volocity software (Perkin Elmer, Naperville, IL) and analyzed using ImageJ (25). Warmed perfusion buffer was set up to continuously drain down the objective onto the tissue at a flow rate of 1 ml/min, so that it would remain hydrated during the visualization. Fields containing postcapillary venules with normal flow were identified using bright-field illumination. Ideal fields were those containing a relatively straight 30- to 50-μm postcapillary venule with robust steady flow. Three separate sets of images were collected for each field. First, a 3D stack in the red (PECAM) channel was captured to determine the vessel dimensions. Then, in the green (PMN, LysM-eGFP) channel, a 60-s single-plane recording (1 frame/s) was captured near the middle of the vessel to determine the rolling velocity and flux. Finally, a 30- to 60-min recording (4 frames/s) of both channels in 3D was captured to quantify TEM. After the recordings were collected, the field was examined again using bright-field illumination to check for changes in flow rate. Fields that had stopped or slowed flow during the acquisition or that appeared to have changed upon bright-field illumination after the acquisition were not analyzed.
Image processing and data collection.
The number of adherent leukocytes was calculated from the 60-s recording. Adherent neutrophils were identified as those that did not move more than one cell diameter (∼5 μm) for ≥30 s during the recording (27, 28). Although the recording captured only one plane (through the middle of the vessel), adherent and rolling neutrophils were easily identified in the adjacent, slightly out-of-focus planes by adjustment of the contrast levels. The total number of adherent neutrophils per vessel surface area was calculated using the vessel dimensions and modeling the vessel as a cylinder with open ends (vessel surface area = πdl, where d is average vessel diameter and l is vessel length). Rolling velocity was calculated by manual tracking of the distance individual neutrophils moved frame-by-frame for those that rolled uninterrupted for ≥10 frames; rolling velocity was only calculated from measurements collected while the neutrophil was actively rolling. Rolling flux was calculated by counting the number of neutrophils that rolled past an arbitrary point in the vessel during the 60-s recording and dividing by the total number (rollers and those free in the bloodstream) that passed the same point. TEM was calculated from the long 4D recordings. TEM events were defined as those in which the neutrophil traversed from clearly inside to clearly outside the vessel and separated from it.
Croton oil dermatitis model and immunofluorescence staining of whole-mounted cremaster muscles.
Mice were subjected to croton oil-induced inflammation essentially as described previously (22, 34). Briefly, age- and sex-matched mice were injected intraperitoneally with 100 μg of the blocking antibody against PECAM (clone 2H8) or CD99 (clone 3F11) or control rat nonspecific IgG. After 1 h, 20 μl of 0.9% croton oil in a 4:1 solution of acetone-olive oil (carrier) were applied to both sides of the right ear of each mouse. The contralateral ear was treated with carrier only. After 5 h, the animals were euthanized, and Nair was used to remove hair from the ears. The ears were removed and placed in 4% formaldehyde in PBS for 30 min; then the two leaflets of each ear were mechanically separated and returned to the fixative overnight. The leaflets were permeabilized and blocked in PBS containing 0.3% Triton X-100, 1% BSA, and 1% goat serum overnight at 4°C. The leaflets were then incubated with primary antibodies [10 μg/ml anti-PECAM (clone 2H8), 1 μg/ml anti-MRP14, and a 1:1,000 dilution of anti-collagen IV original stock] overnight at 4°C in the permeabilization/blocking buffer. The leaflets were then washed in PBS and incubated with secondary antibodies (10 μg/ml each of goat anti-rat IgG-Alexa Fluor 488, goat anti-rabbit IgG-Alexa Fluor 647, and goat anti-Armenian hamster IgG-DyLight 550) in permeabilization/blocking buffer for 4 h at room temperature. After extensive washing in PBS, the leaflets were mounted on slides using FluorSave (EMD Millipore). Images were collected as described above, except a ×40 oil-immersion lens (1.00 numerical aperture) was used. Ears that received carrier alone were examined to ensure that the inflammatory stimulus was active and specific. For the inflamed ears, at least eight fields per ear, which typically corresponded to >100 neutrophils counted per mouse, were recorded.
For examination of pericyte density around postcapillary venules, experiments were performed as described for 4D IVM; however, cremaster tissue was removed and stained with anti-PECAM (clone 2H8) and anti-α-SMA following the protocol described above for the ears.
Fluorescence-activated cell sorting.
Fluorescence-activated cell sorting (FACS) analysis was performed as previously described (34). Briefly, mouse leukocytes were isolated from peripheral whole blood from 10-wk-old sex-matched mice. Blood was collected via cardiac puncture. Red blood cells were lysed using Pharm Lyse (BD Biosciences, San Jose, CA), and Fc receptors were blocked using anti-mouse CD16/32 (eBioscience, San Diego, CA). Cells were labeled with APC-rat anti-mouse Ly6G (Gr-1, clone 1A8), propidium iodide (PI), FITC-rat anti-mouse PECAM (clone 390), and DyLight 550-rat anti-mouse CD99 (clone 3F11) or isotype controls. Cells were then analyzed at the Northwestern University Robert H. Lurie Comprehensive Cancer Center Flow Cytometry Core Facility using LSRFortessa (BD Biosciences). Total leukocytes were gated according to forward/side scatter. Live neutrophils were identified as the Ly6G+/PI− population and then analyzed for their expression of PECAM and CD99.
Statistical analysis.
For the IVM experiments, data were collected from at least three mice for each condition. For the croton oil dermatitis experiments, data represent at least four mice collected from two or three separate experiments. Each experiment contained the appropriate control antibody/strain combination. Data are means and SD (for IVM experiments) or SE (for croton oil experiments). Groups with statistically significant differences were identified using an unpaired two-way ANOVA. Student's t-test was then used to identify which differences were statistically significant and included Bonferroni's correction to account for multiple comparisons.
RESULTS
4D intravital imaging of leukocyte TEM in vivo.
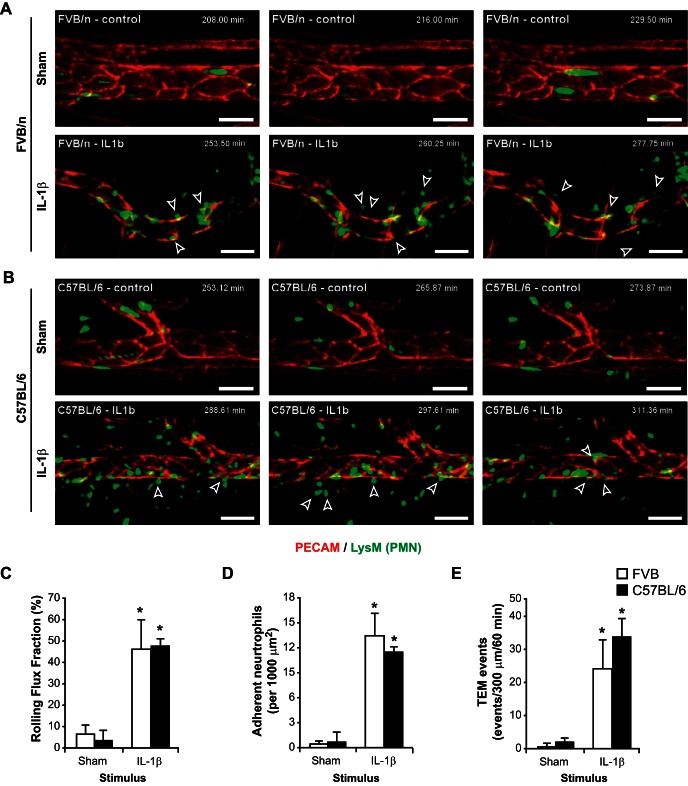
In an effort to properly investigate the molecular mechanisms governing leukocyte diapedesis in vivo with superb spatiotemporal resolution, we used 4D IVM. To validate our model system, FVB/n LysM-eGFP mice received a local intrascrotal cytokine injection of IL-1β or PBS (sham control) along with a DyLight 550-conjugated nonblocking anti-PECAM antibody (clone 390). IL-1β was chosen as an optimal stimulus because of a previously published report that IL-1β is a PECAM-dependent stimulus in C57BL/6 mice (36, 38). After 3 h, the cremaster muscle was exteriorized, prepared, and imaged. After testing multiple time points (data not shown), we empirically chose 3–5 h poststimulation as an optimal time period to study the inflammatory response in our model system. At this acute time point, the predominant effector leukocyte subtype being recruited to sites of inflammation is the mature neutrophil granulocyte; although the LysM promoter is also expressed in monocytes, albeit weakly, this time course is too short to observe significant recruitment of monocytes (10, 37). Compared with sham control, IL-1β activation significantly increased the rolling flux fraction (7.1 ± 3.7% and 46.2 ± 13.9% for sham and IL-1β, respectively, n = 3 for both groups) and number of adherent neutrophils (0.5 ± 0.3 and 13.6 ± 2.6 neutrophils/100 μm2 for sham and IL-1β, respectively, n = 3 for both groups) in postcapillary venules of FVB/n mice (Fig. 1, A, C, and D). These respective changes in leukocyte rolling and adhesion are consistent with previous reports demonstrating proinflammatory changes in the murine cremaster tissue (3, 12, 14, 27, 30). Furthermore, IL-1β activation induced a dramatic increase in the number of neutrophils transmigrating from venules compared with unstimulated tissues [0.5 ± 0.7 and 24.3 ± 8.5 events·300 μm−1·60 min−1 in sham and IL-1β, respectively, n = 3 for both groups; Fig. 1, A and E; also see Supplemental Movies 1A (Sham) and 1B (IL-1β) in Supplemental Material for this article available online at the Journal website]. Using our 4D IVM system, we were able to observe, in real time, the interactions between the transmigrating leukocytes and the ECs comprising the vasculature. Complete/successful TEM was determined by the movement of a neutrophil from the luminal surface of the venule, through the EC (either paracellularly or transcellularly), beyond the abluminal surface. This was verified by examination of the videos from all directions and verification of the movement of PMNs away from the vessel into the interstitial tissue (with a clear separation of the neutrophil and EC markers).
Fig. 1.
Development and analysis of a 4-dimensional (4D) intravital microscopy system for analysis of leukocyte transendothelial migration (TEM) in vivo in real time. A: to induce leukocyte recruitment in cremasteric postcapillary venules, FVB/n LysM-eGFP mice were locally injected intrascrotally with DyLight 550-conjugated anti-PECAM (clone 390) MAb and with IL-1β (50 ng) or PBS alone (Sham). After 3–5 h, the cremaster muscle was surgically exteriorized and imaged using a spinning-disk confocal laser microscope at a rate of 4 frames/min (see Supplemental Movies 1A and 1B). Open arrows denote leukocytes that undergo TEM within a few minutes of the time point. B: experiments identical to those described in A were performed with C57BL/6 LysM-eGFP mice (see Supplemental Movies 1C and 1D). Scale bar = 50 μm. Time stamp denotes time after intrascrotal cytokine stimulation. C–E: quantification of leukocyte rolling flux fraction, adhesion, and TEM events in IL-1β-stimulated or sham-treated FVB/n and C57BL/6 mice. Error bars denote SD; data are representative of recordings from ≥3–4 mice per group. *P < 0.05.
Identical experiments in C57BL/6 LysM-eGFP mice demonstrated a similar increase in neutrophil rolling flux fraction (3.5 ± 4.5% and 47.9 ± 3.0% for sham and IL-1β, respectively, n = 3 for both groups) and adhesion (0.7 ± 1.1 and 11.7 ± 0.4 neutrophils/100 μm2 for sham and IL-1β, respectively, n = 3 for both groups; Fig. 1, B–D). Similarly, a significant increase in neutrophil extravasation was appreciated in IL-1β-treated animals compared with sham controls (2.0 ± 1.4 vs. 33.7 ± 5.7 events·300 μm−1·60 min−1, n = 4 for both groups; Fig. 1, B and E; also see Supplemental Movies 1C and 1D).
Collectively, these data show that, in our 4D IVM system, we are able to induce robust inflammation in FVB/n and C57BL/6 mice in response to local cytokine administration and to image this phenomenon in vivo in real time.
PECAM and CD99 are critical for leukocyte extravasation in vivo in FVB/n and C57BL/6 mice.
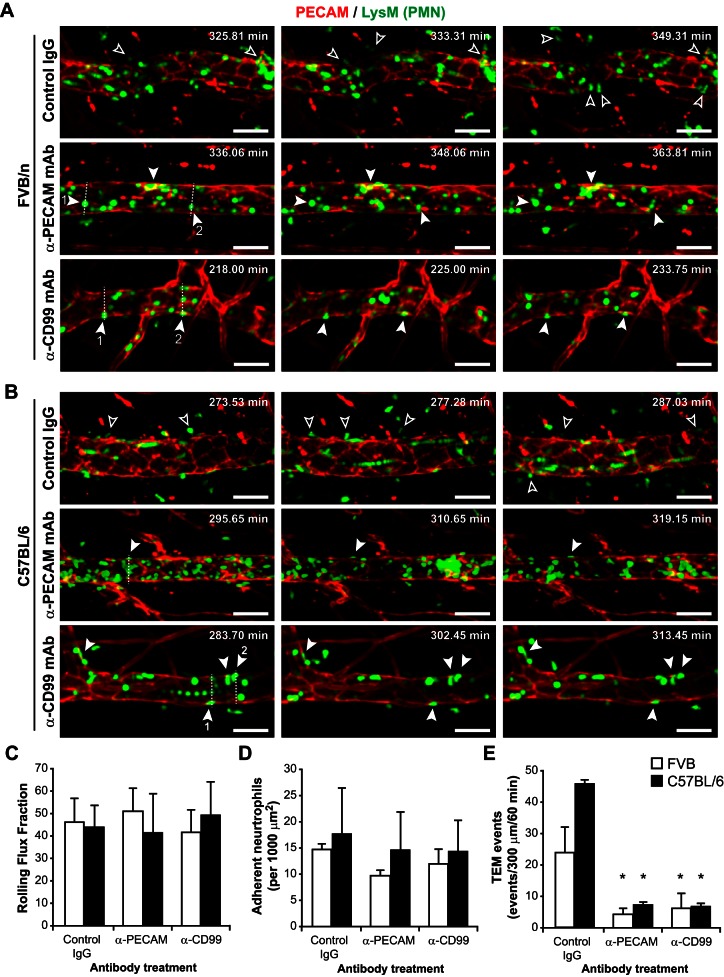
Having verified our model system, we wanted to determine the function of PECAM and CD99 in FVB/n and C57BL/6 mice. To test this, FVB/n LysM-eGFP mice were pretreated with blocking anti-PECAM MAb (clone 2H8), blocking anti-CD99 MAb (clone 3F11), or control IgG before local treatment with IL-1β (Fig. 2A). In accordance with the literature (5, 7), blocking PECAM (7) or CD99 (5) function in vivo had no effect on leukocyte rolling flux fraction (46 ± 14%, 51 ± 8%, and 41 ± 6% for control IgG, anti-PECAM, and anti-CD99, respectively, n = 4 for all measurements) or adhesion (14.7 ± 1.0, 9.6 ± 1.0, and 11.9 ± 2.7 neutrophils/100 μm2 for IgG, anti-PECAM, and anti-CD99, respectively, n = 4 for all measurements; Fig. 2, C and D). However, as expected, anti-PECAM and anti-CD99 treatment significantly attenuated leukocyte transmigration in FVB/n mice [24.0 ± 7.9, 4.6 ± 1.6, and 6.4 ± 4.5 events·300 μm−1·60 min−1 for IgG (n = 3), anti-PECAM (n = 4), and anti-CD99 (n = 4), respectively; Fig. 2E; see also Supplemental Movies 2A (control IgG), 2B (anti-PECAM), and 2C (anti-CD99)].
Fig. 2.
PECAM and CD99 are critical for different steps of leukocyte extravasation in FVB/n and C57BL/6 mice. A: FVB/n LysM-eGFP mice were pretreated with blocking anti-PECAM (clone 2H8), anti-CD99 (clone 3F11), or control IgG (3 mg Ab/kg mouse) prior to local cytokine stimulation with IL-1β and 4D intravital imaging (see Supplemental Movies 2A, 2B, and 2C). Open arrows denote leukocytes that undergo TEM within a few minutes of the time point; filled arrows denote leukocytes that appear to be blocked at the TEM step; dashed lines indicate position of the orthogonal images in Fig. 3. B: experiments identical to those described in A were performed with C57BL/6 LysM-eGFP mice (see Supplemental Movies 2D, 2E, and 2F). Scale bar = 50 μm. Time stamp denotes time after intrascrotal cytokine stimulation. C–E: quantification of leukocyte rolling flux fraction, adhesion, and TEM events in IL-1β-stimulated FVB/n and C57BL/6 mice in the presence of anti-PECAM, anti-CD99, or control IgG. Error bars denote SD; data are representative of recordings from ≥3–5 mice per group. *P < 0.05. Difference in TEM between strains for the control group was not statistically significant (P = 0.133).
Similar experiments were carried out using C57BL/6 LysM-eGFP mice (Fig. 2B). As in the FVB/n strain, neither anti-PECAM nor anti-CD99 had an effect on leukocyte rolling flux fraction [44 ± 9%, 41 ± 17%, and 49 ± 15% for control IgG (n = 5), anti-PECAM (n = 4), and anti-CD99 (n = 5), respectively] or adhesion [17.6 ± 8.8, 14.5 ± 7.3, and 14.2 ± 6.0 neutrophils/100 μm2 for IgG (n = 5), anti-PECAM (n = 4), and anti-CD99 (n = 5), respectively], whereas leukocyte extravasation was dramatically decreased [45.7 ± 1.9, 7.3 ± 5.0, and 6.9 ± 1.9 events·300 μm−1·60 min−1 for IgG (n = 4), anti-PECAM (n = 3), and anti-CD99 (n = 4), respectively; Fig. 2, B–E; also see Supplemental Movies 2D (control IgG), 2E (anti-PECAM), and 2F (anti-CD99)].
These data demonstrate that both PECAM and CD99 are required for complete leukocyte extravasation in the FVB/n and C57BL/6 murine cremaster vasculature in response to IL-1β activation.
PECAM and CD99 function at subsequent steps of diapedesis in FVB/n mice.
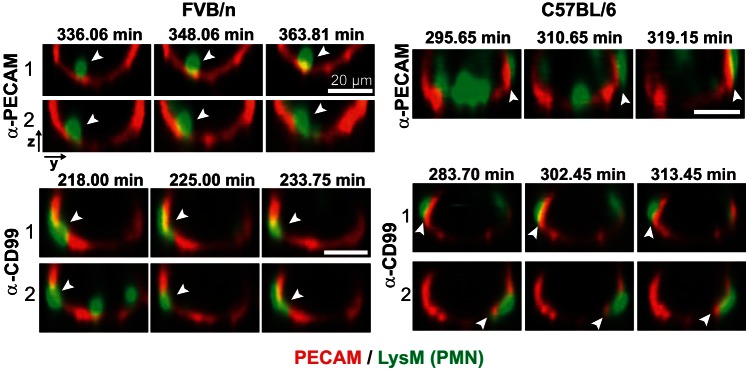
Having confirmed that both PECAM and CD99 are required for leukocyte extravasation in FVB/n mice, we sought to determine where, specifically, these molecules were functioning. Further analysis of our 4D videos revealed that, in FVB/n mice pretreated with anti-PECAM MAb, leukocytes, once they adhered, appeared to crawl intravascularly along clearly demarcated endothelial junctions (see Supplemental Movie 2B). This behavior mimics that previously reported when live-cell imaging was used to study the role of PECAM in vitro (24). The specific level of blockade was evaluated by examining the events from an orthogonal axis and determining the position of the leukocytes in relation to the endothelium and the degree of colocalization. In FVB/n mice under anti-PECAM blockade, many leukocytes were determined to be arrested along the apical surface of the endothelium, as appreciated by minimal colocalization between junctional and leukocyte staining (Fig. 3).
Fig. 3.
Orthogonal views of arrested leukocytes during PECAM and CD99 blockade in vivo in real time. Time-lapse recordings shown in Fig. 2 were examined to identify leukocytes that appeared arrested for long durations. Images show orthogonal views taken along the dashed lines in Fig. 2 for FVB/n or C57BL/6 mice treated with anti-PECAM or anti-CD99 MAb. Arrows denote leukocytes that appear to be arrested apically or partially in the junction (for FVB/n mice treated with anti-PECAM and anti-CD99 MAb, respectively) or beneath the endothelium (for both MAb treatments in C57BL/6 mice). Scale bar = 20 μm. Time stamp denotes time after intrascrotal cytokine stimulation.
We next investigated the site of blockade of anti-CD99 treatment in FVB/n mice. As described above, the response of FVB/n mice pretreated with anti-CD99 MAb to IL-1β stimulation was recorded via 4D IVM. It was intriguing to find that leukocytes blocked by anti-CD99 appeared stuck partway through the endothelium. Orthogonal views demonstrated that portions of the leukocytes were always visible above and below the level of the endothelium (Fig. 3). It was also intriguing that this was not a static process but, rather, a dynamic blockade: many of leukocytes arrested by anti-CD99, although stuck partway through endothelial junctions, appeared to be actively probing the perivascular space with pseudopods, as if attempting to transmigrate; however, a trailing portion of the cell body remained attached to the apical surface (see Supplemental Movie 2C).
From these data, we concluded that, in the FVB/n strain, inhibition of PECAM function arrests leukocytes at the apical level of the endothelium, whereas inhibition of CD99 resulted in arrest of leukocytes partway through endothelial junctions. Thus, in FVB/n mice, PECAM and CD99 block TEM in a manner similar to that previously reported for human cells in vitro (16, 23, 34).
PECAM and CD99 function past the level of the endothelium in C57BL/6 mice.
We next wanted to investigate the level at which PECAM and CD99 function in C57BL/6 mice and directly compare that with our observations in FVB/n mice. In C57BL/6 mice, blocking PECAM (see Supplemental Movie 2E) or CD99 (see Supplemental Movie 2F) resulted in an identical phenotype: neutrophils were able to completely traverse the endothelium; however, they became arrested along the back side of the EC. While we do not have a marker for the basement membrane in the 4D IVM system, we inferred that these cells were arrested at the level of the basement membrane. This was best appreciated by reviewing the orthogonal and longitudinal views of the live images in concert over time. In the orthogonal views, a portion of the arrested PMNs was never seen with a portion of its cytoplasm on the apical surface of the endothelium, and a significant portion was always seen clearly below the level of the endothelium (Fig. 3). Moreover, there is an appreciable morphological difference between neutrophils arrested partway through the endothelium and those blocked along the basement membrane. Neutrophils arrested on the basement membrane appear to have a much flatter phenotype than those within the vessel lumen or actively undergoing TEM while maintaining close juxtaposition to the abluminal surface of the postcapillary venules. Furthermore, these neutrophils are able to locomote along the back side of venules, crossing multiple individual ECs, which could only be achieved if these neutrophils fully traversed the endothelial barrier without any remaining attachment to the endothelial junctions (see Supplemental Movies 2E and 2F).
From these observations, we suspected that, in C57BL/6 mice, PECAM and CD99 function at the same level of leukocyte extravasation, specifically, at the level of the basement membrane, and not at the level of the endothelium, as previously suggested by electron micrographs (5, 9, 32).
Whole mount ex vivo confirmation of the site of anti-PECAM and anti-CD99 blockade.
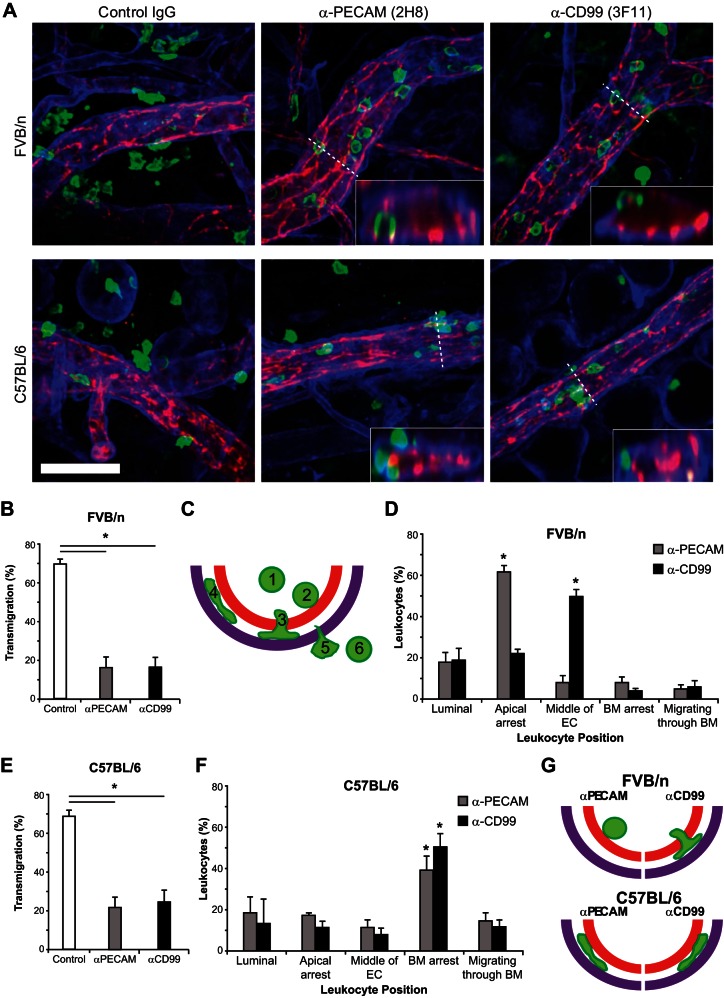
As mentioned above, attempts to fluorescently label various components of the basement membrane with intrascrotally or intravenously administered antibody for 4D IVM were unsuccessful. Therefore, to identify specifically where PECAM and CD99 antibodies arrest PMNs in FVB/n and C57BL/6 mice, we used ex vivo immunofluorescence staining of whole mount tissues to discern the level of leukocyte blockade in relation to the endothelium as well as the basement membrane. Furthermore, we wanted to test our findings in a second vascular bed, so we utilized the croton oil dermatitis model of inflammation. FVB/n or C57BL/6 wild-type mice pretreated with anti-PECAM, anti-CD99, or control IgG were stimulated with croton oil, and 3D confocal imaging of fixed ear tissue was performed as previously described (34, 35) (Fig. 4A). In both strains, anti-PECAM and anti-CD99 treatment significantly attenuated leukocyte extravasation compared with control [FVB/n: 70 ± 2%, 16 ± 5%, and 17 ± 5% for control IgG, anti-PECAM, and anti-CD99, respectively (Fig. 4B); C57BL/6: 77 ± 3%, 14 ± 7%, and 14 ± 7% for control IgG, anti-PECAM, and anti-CD99, respectively (Fig. 4E); n = 4 mice, with ≥8 fields quantified per mouse; Fig. 4, B, D, E, and F]. To identify specifically the step at which anti-PECAM and anti-CD99 were functioning to block extravasation, we analyzed orthogonal views to differentiate where neutrophils were being arrested (Fig. 4A, insets). Neutrophils were scored, on the basis of their position relative to the endothelium and basement membrane, as being in one of six positions: 1) in the vessel lumen not in contact with the endothelium, 2) apically arrested along the endothelium, 3) arrested partway through the ECs, 4) arrested along the basement membrane, 5) actively migrating through the basement membrane, or 6) fully extravasated (schematically represented in Fig. 4C).
Fig. 4.
PECAM and CD99 function past the level of the endothelium in C57BL/6 mice. A: wild-type FVB/n and C57BL/6 mice were pretreated with control IgG, anti-PECAM, or anti-CD99 (3 mg/kg) prior to ear stimulation with croton oil (1% solution, 20 μl/ear) or carrier alone. After 4 h, ear tissue was collected and immunohistochemical staining was performed for PECAM [endothelial cells (ECs), red], myeloid-related protein 14 (neutrophils, green), and collagen IV [basement membrane (BM), purple]. 3-Dimensional confocal images were acquired from each sample and subsequently analyzed. Insets: z-orthogonal view along the indicated line to demonstrate leukocyte arrest in relation to the endothelium and basement membrane at 1.5 times the xy image. Scale bar = 50 μm. B: quantification of findings in A (percent leukocyte extravasation within 50 μm of postcapillary venules in FVB/n mice). C: schematic of quantification of site of leukocyte blockade. Leukocytes were scored as being in 1 of 6 positions: 1) luminal, 2) apically arrested, 3) arrested partway through the endothelium, 4) arrested at the level of the basement membrane, 5) actively migrating through the basement membrane, or 6) fully extravasated. D: quantification of the site of leukocyte arrest in FVB/n mice. E: quantification of findings in A (percent leukocyte extravasation within 50 μm of postcapillary venules in C57BL/6 mice). F: quantification of the site of leukocyte arrest in C57BL/6 mice. G: schematic model of anti-PECAM and anti-CD99 arrest in FVB/n and C57BL/6 mice. One hundred to 200 cells were analyzed per ear. Carrier-alone stimulation resulted in minimal leukocyte recruitment (data not shown). Total leukocytes per field of view, vessel length, and vessel diameter were equivalent for all treatment groups (data not shown). Data are representative of recordings from ≥4 mice per group with ≥8 images quantified per mouse. *P < 0.05.
In FVB/n mice, anti-PECAM arrested the majority of the cells along the apical surface of the endothelium, whereas anti-CD99 arrested the majority of leukocytes partway through endothelial junctions [18 ± 5% and 19 ± 6% (luminal), 62 ± 3% and 22 ± 2% (apical), 8 ± 3% and 50 ± 4% (middle), 8 ± 3% and 4 ± 1% (basement membrane arrest), and 5 ± 2% and 6 ± 3% (migrating basement membrane) for anti-PECAM and anti-CD99, respectively; Fig. 4D], replicating the phenotype observed when blocking PECAM or CD99 in the cremaster and in vitro in human cells (16, 20, 23).
Conversely, in C57BL/6 mice, anti-PECAM and anti-CD99 treatment blocked the majority of the leukocytes along the basement membrane [as labeled with anti-collagen IV; 18 ± 8% and 13 ± 12% (luminal), 17 ± 1% and 11 ± 3% (apical), 11 ± 4% and 8 ± 3% (middle), 39 ± 7% and 50 ± 7% (basement membrane arrest), and 14 ± 4% and 17 ± 3% (migrating basement membrane) for anti-PECAM and anti-CD99, respectively; Fig. 4F]. These data support the phenotype that was observed using 4D IVM (Fig. 2) and confirm our initial impressions that, in C57BL/6 mice, anti-PECAM or anti-CD99 interferes with leukocyte extravasation through the basement membrane.
Taken together with our intravital studies, these data show that, in FVB/n mice, anti-PECAM arrests leukocytes along the apical endothelium and anti-CD99 blocks leukocytes partway through junctions. Conversely, in C57BL/6 mice, anti-PECAM and anti-CD99 block leukocytes along the basement membrane, past the level of the endothelium (see schematic model, Fig. 4G).
Protein expression levels and pericyte morphology are similar between strains.
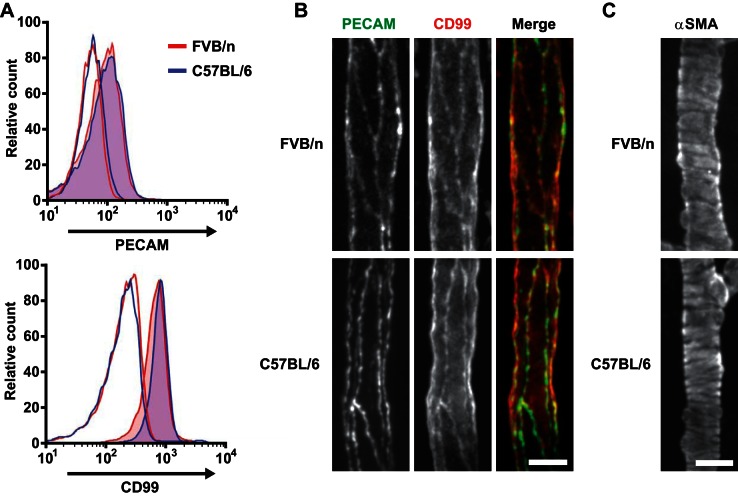
Having demonstrated that PECAM and CD99 function at different steps of leukocyte extravasation in FVB/n and C57BL/6 mice, we next sought to test possible explanations for these findings. FACS analysis was performed to explore whether differences in expression levels of PECAM or CD99 on neutrophils could contribute to the different sites of blockade. Analysis of the PECAM and CD99 surface expression in neutrophils from FVB/n and C57BL/6 mice showed no significant difference in expression of these proteins between the strains (Fig. 5A; n = 4 for each strain). Because PECAM and CD99 expression on ECs could similarly explain the differential site of arrest, we examined the expression of these proteins on the endothelium. Cremaster muscles from wild-type FVB/n and C57BL/6 mice were inflamed and prepared as described for 4D IVM. At 30 min before image acquisition, the mice were injected intravenously with fluorophore-conjugated antibodies against PECAM and CD99. Visualization of the tissue revealed identical expression levels and morphology between the two strains; however, in both strains, CD99 was expressed more diffusely on the EC surface (Fig. 5B). These findings indicate that although the locations at which PECAM and CD99 function differ between the two strains, PECAM and CD99 expression levels were similar on ECs, as well as neutrophils, in FVB/n and C57BL/6 mice.
Fig. 5.
PECAM and CD99 expression are not different between FVB/n and C57BL/6 mice. A: peripheral blood leukocytes were prepared from FVB/n and C57BL/6 mice and labeled with antibodies against lymphocyte antigen 6 complex G6D [Ly6G (Gr-1)], PECAM, and CD99 or isotype controls. Neutrophils were identified by expression of Gr-1 and examined for expression of PECAM and CD99 (filled curves) compared with isotype controls (open curves) for each strain. Four mice were examined for each group; representative plots are shown. B: cremaster muscles of FVB/n or C57BL/6 mice were inflamed with IL-1β and prepared for 4D intravital imaging, except fluorescence-conjugated antibodies against PECAM and CD99 were injected intravenously 30 min before visualization. C: mouse cremaster muscles were prepared as described in B, except tissue was fixed, removed, and processed for immunofluorescence to detect α-smooth muscle actin (α-SMA). Scale bar = 25 μm.
Another plausible cause for the observed phenotypes could be differences in pericyte investment around postcapillary venules between the two strains. Pericyte density and remodeling are known to influence leukocyte extravasation; foci of low basement membrane expression corresponding to areas of low pericyte density are preferentially used by transmigrating neutrophils (33). To test this hypothesis, immunohistochemical staining for α-SMA, a known pericyte marker (21, 33), was performed on excised cremaster muscles from FVB/n and C57BL/6 mice that had been inflamed and prepared for 4D IVM as described above. FVB/n and C57BL/6 mice displayed similar pericyte density and morphology along postcapillary venules (Fig 5C). However, it is still possible that functional differences in pericytes between the two strains could account for the variation in leukocyte emigration. For example, characteristics such as the type, density, and conformation of adhesion receptors on pericytes for neutrophils could differ and warrant further investigation.
From these data, we conclude that there is no significant difference in expression levels of PECAM or CD99 between FVB/n and C57BL/6 mouse strains, nor are there differences in pericyte density. Thus these characteristics do not explain the different effects of PECAM and CD99 blockade on TEM between FVB/n and C57BL/6 mice.
DISCUSSION
PECAM and CD99 have been known to be critical for leukocyte extravasation in vivo for some time (reviewed in Ref. 17); however, this is a complicated, multistep process that involves transmigration across not only the endothelium, but also the basement membrane. There have been discrepancies between studies demonstrating the exact level of function of PECAM and CD99 in relation to the endothelium and basement membrane. In this report we harnessed the technology of 4D IVM to study the step in TEM at which PECAM and CD99 function in vivo in real time. We demonstrate that the role of PECAM and CD99 in leukocyte transmigration is dependent on the murine strain being studied.
The results of these studies explain the apparent inconsistencies among previous studies and largely confirm all previous conclusions. In the commonly used C57BL/6 strain, blocking PECAM or CD99 inhibits migration through the basement membrane, not the endothelium. In contrast, FVB/n mice more accurately replicate results obtained in vitro with human cells, in which blocking PECAM function arrests leukocytes on the apical surface of the endothelium, while blocking CD99 function arrests them partway through endothelial junctions at a subsequent step of TEM (see model, Fig. 4G) (16, 20, 23). Our in vivo studies reported here were limited to PMN interactions in acute inflammatory settings. Based on previous studies (22, 26), we predict that monocytes would behave similarly.
While we can safely conclude that, in C57BL/6 mice, PECAM is required for efficient PMN migration through the basement membrane, we cannot make similar deductions regarding the function of PECAM at the level of the basement membrane in FVB/n mice, given that PMNs are arrested upstream in these animals. Domain mapping in vitro has previously demonstrated that domain 6 of PECAM mediates heterophilic aggregation with heparin sulfate proteoglycans and migration across the basement membrane, whereas domain 1 mediates homophilic adhesion and, thus, TEM (15). In vivo it has been shown that heterophilic PECAM interactions that upregulate α6β1-integrin are critical for leukocyte migration through the basement membrane (7). Given this, it is highly conceivable that, in C57BL/6 and FVB/n strains, PECAM mediates leukocyte migration through the basement membrane via heterophilic interactions with basement membrane components bearing heparin sulfate moieties.
As with PECAM function in FVB/n mice, it is possible that CD99 not only functions at the level of the endothelium, but also mediates basement membrane migration in this strain. While several heterophilic ligands for PECAM have been identified, no heterophilic binding partners of CD99 have been identified. It has been reported that CD99 mediates spontaneous erythrocyte rosetting on T cells, but its ligand is unknown (11). CD99 is a unique 32-kDa type 1 transmembrane protein that is extensively O-glycosylated, with carbohydrate side chains responsible for 44% of its molecular weight (1, 4). Unlike PECAM, poliovirus receptor, or junctional adhesion molecule (JAM)-A, CD99 is not a member of the Ig superfamily. We know that homophilic interaction between leukocyte and endothelial CD99 is required for leukocyte TEM and that antibody blockade of either side prevents this process. In C57BL/6 mice, we know that CD99 functions at the level of the basement membrane as well (5). Given that CD99 staining is enriched primarily along the endothelium in vivo, and not the basement membrane or pericyte sheath, it is most likely that leukocyte CD99 interacts with currently unknown substrates in the basement membrane.
Interestingly, there were fewer overall transmigration events in the FVB/n than C57BL/6 group (∼30% and ∼45% fewer in the experiments in Figs. 1 and 2, respectively). However, these differences were found not to be significant (P > 0.13). Although this difference was not statistically significant, this finding is in line with previously published reports that have described the C57BL/6 strain as a hyperinflammatory mouse model (26), which partially explains its popularity in the field of immunology.
An additional question that arises from these studies is as follows: Why do C57BL/6 mice not require PECAM or CD99 for TEM, only for traversing the basement membrane? A previous study from our laboratory used quantitative trait loci (QTL) mapping between C57BL/6 and FVB/n PECAM−/− mice to identify a 35.8-Mb locus on murine chromosome 2 associated with PECAM-independent inflammation in the thioglycollate peritonitis model (26). Within this QTL locus were multiple genes, notably COX-1 (Ptgs1) and PGE2 synthases 1 and 2 (Ptges and Ptges2). Evaluation of prostaglandins between murine strains in vivo has proven functionally difficult. Importantly, neither Pecam1 nor Cd99 is located in regions that were identified as statistically different between the genomes of the two strains. Furthermore, analysis of the available mouse genomic DNA databases for PECAM and CD99 does not indicate any known polymorphisms or differences within the coding regions between the FVB/n and C57BL/6 strains. This suggests that the observations reported here are not likely due to sequence differences or expression levels for PECAM and CD99. However, it is still possible that slight variations of the alleles below the detection limit for QTL analysis confer phenotypically significant effects or that these proteins are differentially regulated between the two strains via posttranslational modifications.
Recent studies have shown that genetic ablation of endothelial transient receptor potential cation channel 6 (TRPC6)-mediated calcium signaling in C57BL/6 mice, which is downstream of PECAM, arrests leukocytes apically along the endothelium (35). Similarly, in a genetically mixed strain of mice, endothelium-specific knockout of soluble adenylyl cyclase, which is downstream of CD99, blocks leukocytes partway through endothelial junctions (34). These observations suggest that although C57BL/6 mice do not require PECAM or CD99 function at the level of the endothelium, the downstream signaling pathways mediated by these proteins are critical for leukocyte diapedesis. It is possible that C57BL/6 mice have alternative mechanisms, such as via prostaglandin receptors or adhesion molecules, to activate these signaling pathways. A large number of additional EC molecules, for example, CD47, the JAM family, intercellular adhesion molecule (ICAM)-1, and vascular cell adhesion molecule-1 (reviewed in Ref. 18), that could possibly contribute to these differences between FVB/n and C57BL/6 mice are also involved in leukocyte TEM. Perhaps one of these other adhesion/signaling molecules is expressed at different levels between the two strains and, via its downstream effector signaling mechanisms, is able to compensate for the need for PECAM and CD99 in C57BL/6 mice. For example, ICAM-1 interacts with ezrin, which was recently reported to be critical for CD99 signaling, as well as mediating cortactin signaling, which, similar to PECAM, is Src-dependent (reviewed in Ref. 2; also see Refs. 8 and 34).
It is possible that the molecular machinery underlying TEM is dependent not only on the strain of mouse or species of animal, but also on the vascular bed, leukocyte subpopulation, and inflammatory stimulus in question. Until the intricate intracellular signaling pathways of the EC molecules participating in leukocyte transmigration are completely mapped, our understanding of these phenotypic differences between C57BL/6 and FVB/n mice will be incomplete. Experiments addressing these downstream intracellular signaling mechanisms are beyond the scope of this study; work is underway to address these hypotheses.
In summary, we used 4D IVM to study leukocyte recruitment and extravasation in vivo in real time and, for the first time, to directly compare the function of PECAM and CD99 in FVB/n and C57BL/6 mice. We found that, in FVB/n mice, PECAM and CD99 function at the level of the endothelium, similar to human PMNs in vitro. In the C57BL/6 strain, antibodies against PECAM or CD99 do not inhibit TEM but, rather, impair migration through the basement membrane. The exact reason for this phenotype remains unknown. It is possible that C57BL/6 mice do indeed normally utilize PECAM or CD99 for TEM; however, they possess the ability to override this necessity in the face of antibody blockade or genetic absence. Future studies will investigate the mechanism by which C57BL/6 mice are able to circumvent the functional blockade of anti-PECAM and anti-CD99 during diapedesis. These findings may help further our understanding of the downstream, intracellular signaling pathways mediated by PECAM and CD99.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL-046849 and R37 HL-04774 to W. A. Muller and F30 HL-116100 to R. L. Watson and by the Northwestern University Flow Cytometry Core Facility, supported by National Cancer Institute Cancer Center Support Grant CA-060553. Publication costs for this article were paid in part by support from the Sydney and Bess Eisenberg Memorial Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S., R.L.W., and W.A.M. developed the concept and designed the research; D.S. and R.L.W. performed the experiments; D.S. and R.L.W. analyzed the data; D.S. and R.L.W. interpreted the results of the experiments; D.S. and R.L.W. prepared the figures; D.S. and R.L.W. drafted the manuscript; D.S., R.L.W., and W.A.M. edited and revised the manuscript; D.S., R.L.W., and W.A.M. approved the final version of the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Paul Kubes for supplying the LysM-eGFP mice, Dr. Clifford Carpenter for assistance in purifying some of the antibodies, Begum Kutay and Dr. Alexei Mikhailov for technical guidance and assistance in establishing these methods, and Drs. Ryan Winger and Evan Weber for thoughtful discussions regarding this work.
REFERENCES
- 1.Aubrit F, Gelin C, Pham D, Raynal B, Bernard A. The biochemical characterization of E2, a T cell surface molecule involved in rosettes. Eur J Immunol 19: 1431–1436, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Auerbach SD, Yang L, Luscinskas FW. Endothelial ICAM-1 functions in adhesion and signaling during leukocyte recruitment. In: Adhesion Molecules: Function and Inhibition, edited by Ley K. Basel: Birkhauser, 2007, p. 99–116. [Google Scholar]
- 3.Azcutia V, Routledge M, Williams MR, Newton G, Frazier WA, Manica A, Croce KJ, Parkos CA, Schmider AB, Turman MV, Soberman RJ, Luscinskas FW. CD47 plays a critical role in T-cell recruitment by regulation of LFA-1 and VLA-4 integrin adhesive functions. Mol Biol Cell 24: 3358–3368, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banting GS, Pym B, Darling SM, Goodfellow PN. The MIC2 gene product: epitope mapping and structural prediction analysis define an integral membrane protein. Mol Immunol 26: 181–188, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Bixel MG, Li H, Petri B, Khandoga AG, Khandoga A, Zarbock A, Wolburg-Buchholz K, Wolburg H, Sorokin L, Zeuschner D, Maerz S, Butz S, Krombach F, Vestweber D. CD99 and CD99L2 act at the same site as, but independently of, PECAM-1 during leukocyte diapedesis. Blood 116: 1172–1184, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Bogen S, Pak J, Garifallou M, Deng X, Muller WA. Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J Exp Med 179: 1059–1064, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dangerfield J, Larbi KY, Huang MT, Dewar A, Nourshargh S. PECAM-1 (CD31) homophilic interaction up-regulates α6β1 on transmigrated neutrophils in vivo and plays a functional role in the ability of α6β1-integrins to mediate leukocyte migration through the perivascular basement membrane. J Exp Med 196: 1201–1211, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasgupta B, Muller WA. Endothelial Src kinase regulates membrane recycling from the lateral border recycling compartment during leukocyte transendothelial migration. Eur J Immunol 38: 3499–3507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol 162: 3022–3030, 1999. [PubMed] [Google Scholar]
- 10.Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 96: 719–726, 2000. [PubMed] [Google Scholar]
- 11.Gelin C, Aubrit F, Phalipon A, Raynal B, Cole S, Kaczorek M, Bernard A. The E2 antigen, a 32 kd glycoprotein involved in T-cell adhesion processes, is the MIC2 gene product. EMBO J 8: 3253–3259, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang MT, Larbi KY, Scheiermann C, Woodfin A, Gerwin N, Haskard DO, Nourshargh S. ICAM-2 mediates neutrophil transmigration in vivo: evidence for stimulus specificity and a role in PECAM-1-independent transmigration. Blood 107: 4721–4727, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7: 678–689, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Ley K, Mestas J, Pospieszalska MK, Sundd P, Groisman A, Zarbock A. Intravital microscopic investigation of leukocyte interactions with the blood vessel wall. Methods Enzymol 445: 255–279, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med 182: 1337–1343, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J Immunol 178: 1136–1143, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Muller WA. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol 184: 886–896, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol 6: 323–344, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller WA. The regulation of transendothelial migration: new knowledge and new questions. Cardiovasc Res 107: 310–320, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med 178: 449–460, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D'Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med 209: 1219–1234, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol 173: 6403–6408, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol 3: 143–150, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol 5: 393–400, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seidman MA, Chew TW, Schenkel AR, Muller WA. PECAM-independent thioglycollate peritonitis is associated with a locus on murine chromosome 2. PLos One 4: e4316, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumagin R, Kuebel JM, Sarelius IH. Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1. Am J Physiol Cell Physiol 301: C804–C813, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol 295: H969–H977, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumagin R, Sarelius IH. A role for ICAM-1 in maintenance of leukocyte-endothelial cell rolling interactions in inflamed arterioles. Am J Physiol Heart Circ Physiol 293: H2786–H2798, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Sumagin R, Sarelius IH. TNF-α activation of arterioles and venules alters distribution and levels of ICAM-1 and affects leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol 291: H2116–H2125, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson RD, Noble KE, Larbi KY, Dewar A, Duncan GS, Mak TW, Nourshargh S. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood 97: 1854–1860, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Wakelin MW, Sanz MJ, Dewar A, Albelda SM, Larkin SW, Boughton-Smith N, Williams TJ, Nourshargh S. An anti-platelet/endothelial cell adhesion molecule-1 antibody inhibits leukocyte extravasation from mesenteric microvessels in vivo by blocking the passage through basement membrane. J Exp Med 184: 229–239, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med 203: 1519–1532, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson RL, Buck J, Levin LR, Winger RC, Wang J, Arase H, Muller WA. Endothelial CD99 signals through soluble adenylyl cyclase and PKA to regulate leukocyte transendothelial migration. J Exp Med 212: 1021–1041, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber EW, Han F, Tauseef M, Birnbaumer L, Mehta D, Muller WA. TRPC6 is the endothelial calcium channel that regulates leukocyte transendothelial migration during the inflammatory response. J Exp Med 212: 1883–1899, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodfin A, Reichel CA, Khandoga A, Corada M, Voisin MB, Scheiermann C, Haskard DO, Dejana E, Krombach F, Nourshargh S. JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood 110: 1848–1856, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 12: 761–769, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A and PECAM-1. Blood 113: 6246–6257, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.