This study demonstrates that redox changes of ryanodine receptor type 2 occur together with Ca2+ calmodulin-dependent kinase II phosphorylation of the sarcoplasmic reticulum Ca2+ channel in hearts subjected to ischemia/reperfusion. Both posttranslational modifications seem to act synergistically in determining reperfusion arrhythmogenesis.
Keywords: arrhythmias, ischemia/reperfusion, ryanodine receptor type 2, redox modifications
Abstract
Previous results from our laboratory showed that phosphorylation of ryanodine receptor 2 (RyR2) by Ca2+ calmodulin-dependent kinase II (CaMKII) was a critical but not the unique event responsible for the production of reperfusion-induced arrhythmogenesis, suggesting the existence of other mechanisms cooperating in an additive way to produce these rhythm alterations. Oxidative stress is a prominent feature of ischemia/reperfusion injury. Both CaMKII and RyR2 are proteins susceptible to alteration by redox modifications. This study was designed to elucidate whether CaMKII and RyR2 redox changes occur during reperfusion and whether these changes are involved in the genesis of arrhythmias. Langendorff-perfused hearts from rats or transgenic mice with genetic ablation of CaMKII phosphorylation site on RyR2 (S2814A) were subjected to ischemia-reperfusion in the presence or absence of a free radical scavenger (mercaptopropionylglycine, MPG) or inhibitors of NADPH oxidase and nitric oxide synthase. Left ventricular contractile parameters and monophasic action potentials were recorded. Oxidation and phosphorylation of CaMKII and RyR2 were assessed. Increased oxidation of CaMKII during reperfusion had no consequences on the level of RyR2 phosphorylation. Avoiding the reperfusion-induced thiol oxidation of RyR2 with MPG produced a reduction in the number of arrhythmias and did not modify the contractile recovery. Conversely, selective prevention of S-nitrosylation and S-glutathionylation of RyR2 was associated with higher numbers of arrhythmias and impaired contractility. In S2814A mice, treatment with MPG further reduced the incidence of arrhythmias. Taken together, the results suggest that redox modification of RyR2 synergistically with CaMKII phosphorylation modulates reperfusion arrhythmias.
NEW & NOTEWORTHY
This study demonstrates that redox changes of ryanodine receptor type 2 occur together with Ca2+ calmodulin-dependent kinase II phosphorylation of the sarcoplasmic reticulum Ca2+ channel in hearts subjected to ischemia/reperfusion. Both posttranslational modifications seem to act synergistically in determining reperfusion arrhythmogenesis.
restoration of coronary flow after a myocardial ischemic event rescues the heart from further damage. However, reperfusion creates a pathophysiological scenario of its own. A contractile dysfunction and myocyte cell death are pathological manifestations of the reperfusion injury, which depend on the duration of ischemia. Even after a brief period of ischemia, which causes no or little tissue damage, life-threatening ventricular arrhythmias can occur (54). Several clinical reports have referred to the presence of arrhythmias during the relief of coronary artery spasm, exercise-induced ischemia, and silent ischemia or after thrombolysis subsequent to acute myocardial infarction (30, 37, 41).
During reperfusion, a great part of the arrhythmic events is thought to be related to an imbalance in ionic currents favoring a depolarizing net inward current (triggered arrhythmias) (3). Early afterdepolarizations occur during the action potential (AP), usually in the setting of prolonged repolarization, and are classically attributed to reactivation of l-type Ca2+ current (ICa) (20, 32). Delayed afterdepolarizations (DADs) occur after completion of AP repolarization and are a consequence of spontaneous sarcoplasmic reticulum (SR) Ca2+ release and activation of a transient inward current (Iti), primarily mediated by the Na+/Ca2+ exchanger (36). This initial stimulus of nonreentrant nature may give rise to episodes of ventricular tachycardia or fibrillation. Previous results from our laboratory showed that, during the early reperfusion of stunned heart, most of the ventricular premature beats (VPBs) detected were triggered by DADs and, as expected, decreased when the SR function was disabled (40). These reperfusion-induced arrhythmias were diminished by 70% in the presence of a Ca2+/calmodulin-dependent protein kinase II (CaMKII) inhibitor and were reduced by 50% in mice lacking the CaMKII phosphorylation site on the ryanodine receptor (RyR2) (40). These results provided compelling evidence for a critical role of CaMKII target proteins, particularly RyR2, in reperfusion arrhythmogenesis. They also suggest that other mechanisms cooperate in an additive way to these rhythm alterations. Reperfusion after ischemia is a condition of oxidative stress in which reactive oxygen and nitrogen species (ROS/RNS) are generated by several different cellular sources, which include the mitochondria, NADPH oxidase (NOX), xanthine oxidase (XO), and nitric oxide synthase (NOS). Redox modifications of both CaMKII and RyR2 have been described in different cardiac diseases, such as heart failure and diabetes (9, 13, 18, 23). However, these changes have been poorly studied in the ischemic heart injury (2).
Oxidation and autophosphorylation of CaMKII are posttranslational modifications resulting in autonomous activation of the enzyme (10). Redox-dependent CaMKII activation has been found to be related to angiotensin II and aldosterone-induced apoptosis (10, 17, 49), electrical remodeling (51), sinus node dysfunction (46), and cardiac glycoside treatment (19), all situations in the course of which life-threatening arrhythmias can occur. Moreover, oxidized CaMKII has been reported to be enhanced in atria from patients with atrial fibrillation (38). Recently, an NO-dependent mechanism for myocardial CaMKII activation has been elucidated. Gutierrez et al. (15) showed that CaMKII activity was enhanced in the presence of NO donors, and Curran et al. (5) extended these findings showing S-nitrosylation of CaMKII in response to β-adrenergic stimulation. These studies reported that the increased NO-dependent CaMKII activity was associated with an increased SR Ca2+ leak. Both oxidation and nitrosylation of CaMKII might occur during ischemia/reperfusion. It is still unknown whether these mechanisms are involved in the activation of CaMKII, the consequent phosphorylation of RyR2, and the generation of arrhythmias observed under this pathological condition.
Extensive information in the literature shows that RyR2 possesses cysteine residues highly susceptible to redox modifications, which have remarkable effects on RyR2 function (18). RyR2 is endogenously S-glutathionylated (43) and S-nitrosylated (55), being both reversible redox modifications. Whereas increases in S-glutathionylation enhanced RyR2-mediated Ca2+ release and decreased RyR2-mediated Ca2+ leak (7, 42), the consequences of S-nitrosylation of RyR2 remain controversial. It has been reported that decreased S-nitrosylation of RyR2 through genetic deletion or pharmacological inhibition of neuronal NOS is able to induce either an increase or a decrease in spontaneous SR Ca2+ release in isolated cardiomyocytes (6, 12, 52). The conflicting results in these and other reports (25, 56) suggest that the impact of S-nitrosylation on RyR2 activity is critically dependent on the underlying cell conditions. Surprisingly, no evidence has been reported about the changes in the redox state of RyR2 during myocardial reperfusion and their possible functional implications. The present study was designed to elucidate the occurrence of CaMKII and RyR2 oxidation in isolated rat hearts subjected to ischemia/reperfusion and their involvement in the genesis of reperfusion arrhythmias.
METHODS
The experiments were performed on male Wistar rats (200–300 g body wt) and transgenic mice (25–30 g) with genetic ablation of the CaMKII site on RyR2 (S2814A) (4), approved by the Institutional Animal Care and Use Committee (IACUC) of the School of Medicine, National University of La Plata, Argentina (Nro T05022014) conforming to the Guide for the Care and Use of Laboratory Animals (NIH, 2011).
Ex Vivo Experiments: Langendorff Perfusion and Experimental Protocol
Animals were anaesthetized by intraperitoneal injection of ketamine and zylazine (70 and 5 mg/kg, respectively). The dose was sufficient to produce a surgical level of anesthesia (loss of pedal withdrawal reflex) without profoundly affecting the cardiovascular function. Central thoracotomy and heart excision were performed immediately after phase III of anesthesia was reached. Isolated rat hearts were perfused at a constant temperature (37°C), heart rate (4 Hz), and flow (14 ml/min) with bicarbonate buffer solution (BBS) composed of (in mM): 128.30 NaCl, 4.70 KCl, 1.35 CaCl2, 20.20 NaHCO3, 0.40 NaH2PO4, 1.10 MgSO4, 11.10 glucose, and 0.04 Na2EDTA. This solution was equilibrated with 95% O2-5% CO2 to give a pH of 7.4, as described (50). Perfusion of the isolated mouse hearts was modified as follows: heart rate 6 Hz, flow 4 ml/min, and 2.5 mM CaCl2 in the perfusate. The mechanical activity of the hearts was assessed by introducing into the left ventricle (LV) a latex balloon connected to a pressure transducer (ADInstruments) and filled with aqueous solution to achieve an LV end-diastolic pressure (LVEDP) of ∼5–10 mmHg. LV contractility was evaluated measuring the developed pressure (LVDP) and the maximal rate of pressure development (+dP/dt) (50).
Epicardial monophasic APs.
Monophasic APs (MAPs) were obtained by using a silver/silver chloride Ag/AgCl electrode opposed to the epicardial surface of the free LV wall as previously described (39). MAP recordings obtained satisfied previously documented criteria of a stable baseline and triangular MAP morphology, rapid upstroke phase, and consistent amplitude (21, 39). Although MAP measurements are local, they were always associated with global changes in contractility. This makes it possible to correlate these electrical events with biochemical changes measured in the whole ventricle. Quantification of VPBs was accomplished by counting the number of extra MAPs that do not follow the basal heart rhythm. VPBs and ventricular tachycardia events (VT) were studied during the first 3 min of reperfusion.
Experimental protocol.
After a 10-min stabilization period, rat/mouse hearts were subjected to 20/15 min of global normothermic ischemia followed by 30 min of reperfusion with BBS. In previous experiments, we have shown that this protocol did not produce irreversible tissue damage (27). When drugs were used, they were perfused 10 min before the onset of ischemia and during the reperfusion period [10 μM apocynin (APO), 100 μM NG-nitro-l-arginine methyl ester (l-NAME), 2 mM mercaptopropionylglycine (MPG), 1 mM 4,5-dihydroxy-1,3-benzenedisulfonic acid (Tiron), and 10 μM S-methyl-l-thiocitrulline (SMTC)]. APO, l-NAME, MPG, and DMSO, used for the dilution of drugs, did not affect basal contractility and MAP duration at 90% of repolarization (MAPD90) (Table 1). A group of hearts perfused but not submitted to ischemia/reperfusion protocol was used as control (Ctrl). For biochemical assays, hearts were freeze clamped at 1 min of reperfusion (28).
Table 1.
Effects of MPG, APO, and l-NAME on basal contractility, relaxation, and MAP duration
| LVDP, mmHg | +dP/dt, mmHg/s | t1/2, ms | MAPD90, ms | |
|---|---|---|---|---|
| Control | 84.6 ± 7.4 | 2761.3 ± 238.5 | 48.8 ± 1.9 | 51.8 ± 5.8 |
| MPG (n = 8) | 92.7 ± 12.3 | 2560.0 ± 144.8 | 54.6 ± 2.8 | 52.5 ± 2.8 |
| Control | 88.8 ± 3.3 | 2696.6 ± 128.3 | 48.4 ± 0.8 | 50.6 ± 6.9 |
| APO (n = 6) | 90.1 ± 5.6 | 2686.7 ± 155.9 | 53.9 ± 2.2 | 55.8 ± 0.7 |
| Control | 96.2 ± 8.0 | 3340.3 ± 243.5 | 49.8 ± 3.9 | 56.0 ± 1.5 |
| l-NAME (n = 9) | 97.5 ± 6.0 | 3389.7 ± 203.4 | 55.8 ± 5.3 | 57.0 ± 0.6 |
Values are means ± SE and were obtained at the end of the stabilization (Control) and at the end of the 10-min period of drug perfusion, prior to ischemia. LVDP, left ventricular developed pressure; +dP/dt, maximal rate of pressure development; t1/2, half relaxation time; MAPD90, monophasic action potential duration at 90% repolarization; MPG, mercaptopropionylglycine; APO, apocynin; l-NAME, NG-nitro-l-arginine methyl ester.
Electrophoresis and Western Blot
Pulverized frozen ventricles were homogenized in four volumes of the following (in mM): 25 NaF, 300 sucrose, 1 EDTA, 30 KH2PO4 (pH 7.0) plus protease inhibitor cocktail. The homogenate was sedimented at 13,000 g for 10 min, and the supernatant was fractionated in small aliquots, frozen in liquid N2, and stored at −80°C. Protein concentration was measured using the Bradford method with bovine serum albumin as the standard. For immunological detection of phosphorylated CaMKII (pCaMKII), phospholamban (PLN), phosphorylated PLN (pThr17-PLN), and GAPDH, 20–50 μg of homogenate protein was electrophoresed per gel lane in 10% acrylamide gels according to Porzio and Pearson (11). For immunological detection of RyR2 and phosphorylated RyR2 (pS2814-RyR2), 100 μg of homogenate protein was electrophoresed per gel lane in 3.5–8.0% gradient acrylamide gels according to Laemmli (11). For immunological detection of the redox state of CaMKII (oxidized CaMKII, ox-CaMKII) and RyR2 (S-glutathionylation and S-nitrosylation), 50 μg of homogenate protein was resuspended in nonreducing loading solution containing 35 mM N-ethylmaleimide (NEM) and separated in 10% acrylamide (ox-CaMKII) and 3.5–8.0% gradient Tris-acetate (RyR2) gels under nonreducing conditions (42). Proteins were transferred to PVDF membranes (Immobilon-P, Millipore). Blots were blocked with Tris-buffered saline (TBS) (50 mM Tris, 150 mM NaCl, pH 7.4) with or without the addition of 0.1% Tween (TBST) and 5% skim milk or albumin, as indicated for each antibody, and then incubated with the following primary antibodies: ox-CaMKII (Millipore, 1:1,000 in 5% milk TBST) and Thr286-phosphorylated CaMKII (Abcam, 1:1,000 in 1% albumin TBST), RyR2 (Santa Cruz Biotechnology, 1:5,000 5% milk TBST), Ser2814-phosphorylated RyR2 (Badrilla, 1:2,500 in 5% milk TBST), Thr17-phosphorylated PLN (Badrilla, 1:2,500 in 5% milk TBS), PLN (Thermo Fisher Scientific, 1:5,000 in 1% milk TBS), GAPDH (Millipore, 1:10,000 in 1% milk TBST), anti-glutathione (GSH) (Virogen, 1:1,000 in 5% albumin TBST), and anti-nitrosocysteine (AG-Scientific, 1:1,000 in 5% albumin TBST). The membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, 1:10,000 in 1% milk TBST) and developed using an enhanced chemiluminescence reagent (Millipore). The signals emitted for chemiluminescence were detected using Chemidoc Imaging system (Bio-Rad) and analyzed with ImageJ software (NIH). The results were normalized to corresponding densitometry signal of unphosphorylated protein or GAPDH.
Determination of NOX Activity
Superoxide production was measured by lucigenin chemiluminescence in SR vesicles. Microsomal fractions enriched in SR vesicles were obtained from frozen hearts homogenized in four volumes of 20 mM MOPS-Tris (pH 6.8) and 300 mM sucrose with protease inhibitors (4 μg/ml leupeptin, 4 μg/ml pepstatin A, 1 mM benzamidine, and 1 mM PMSF). The homogenate was sedimented at 3,800 g for 15 min, and the resulting pellet was rehomogenized as above. The combined supernatants were sedimented at 28,000 g for 15 min. The resulting supernatant was sedimented at 120,000 g for 1 h after addition of solid KCl to a final concentration of 0.65 M. The pellet was resuspended at 10 mg protein/ml in homogenization buffer, fractionated in small aliquots, frozen in liquid N2, and stored at −80°C. SR vesicles (0.2 mg/ml) were incubated in 100 mM MOPS-Tris (pH 7.0), 100 μM NADPH, and 5 μM lucigenin at 25°C. Chemiluminescence was measured in a Berthold FB 12 luminometer and expressed as nanomoles of superoxide anion per milligram of protein per minute (42).
Assessment of Reduced GSH Levels
Total reduced GSH content was determined according to Ellman's method (44). Briefly, pulverized frozen ventricles were treated with four volumes of 10% tricarboxylic acid, placed in an ice water bath for at least 20 min, and then sedimented at 8,500 g for 15 min. The determination of GSH levels in the supernatant was based on the reaction of nonprotein sulfhydryl groups with 5,5′dithiobis 2-nitrobenzoic acid (0.01 M) in 0.4 M Tris buffer pH 8.9 to give a compound that absorbs at 412 nm. GSH levels were expressed as micrograms per gram of tissue.
Detection of ROS Production
ROS generation was determined using dihydroethidium (DHE, Sigma) fluorescence method. DHE produces red fluorescence when oxidized to ethidium bromide, which is then intercalated into DNA. Fresh heart sections were frozen in Tissue Tec optimal cutting temperature compound (Sakura Finetek Europe). With the use of a Leica cryostat, 20-μm transverse sections were cut, mounted on slides (Pearl), and incubated with 10 μM DHE at 37°C for 30 min in a light-protected and humidified chamber. Sections were washed twice with BBS and stored in the dark. Images were acquired using an epifluorescence microscope (Nikon Eclipse E200) with rhodamine filter. The fluorescence intensity per image was quantified using ImageJ analysis software (NIH). Five frozen cardiac sections per animal were analyzed, and 20 photographs were randomly taken for each section with a ×40 objective (26).
RyR2 Free Thiol Content
The content of free thiols in RyR2 was determined using the monobromobimane (mBB, Calbiochem) fluorescence method. Briefly, frozen heart tissue was homogenized in five volumes HEN buffer containing 250 mM Hepes (pH 7.4), 1 mM EDTA, 50 mM neocuproine, 300 mM sucrose, and protease inhibitors (0.5 μg/ml leupeptin, 1.0 μg/ml pepstatin, 1.0 mM phenylmethylsulfonyl fluoride, 1.0 mM benzamidine, and protease inhibitor cocktail). Homogenates were centrifuged at 12,000 g for 10 min, and supernatants were incubated with 4 mM NEM, 10 mM dithiothreitol (DTT) or untreated for 30 min in the dark and at room temperature. Samples were then centrifuged at 40,000 g for 1 h, and pellets were washed and resuspended in HEN buffer. Incubation with 5 mM mBB was performed for 1 h in the dark and at room temperature. To remove unbound mBB, samples were centrifuged at 40,000 g for 1 h, and the pellets were washed, resuspended in HEN buffer, and combined with nonreducing loading buffer with a final concentration of 2 mM NEM. Samples were heated for 20 min at 50°C, and proteins were separated in 3.5–8.0% Tris-acetate gels under nonreducing conditions. Images were acquired using Chemidoc Imaging system (Bio-Rad) and analyzed with ImageJ software (NIH). mBB fluorescence was normalized to RyR2 amount determined using Coomassie blue staining of the gels (12, 47). Treatment with reducing (DTT) and oxidizing (NEM) agents was used to obtain maximum and minimum mBB fluorescence values, respectively, for each intervention.
Statistics
Data are expressed as means ± SE. Statistical significance was determined by Student's t-test for paired or unpaired observations as appropriate and ANOVA when different groups were compared. The Newman-Keuls test was used to examine statistical differences observed with the ANOVA. Numbers of arrhythmias between two different groups were analyzed using the Mann-Whitney test. Values of P < 0.05 were considered significant.
RESULTS
Oxidation of CaMKII During Ischemia/Reperfusion
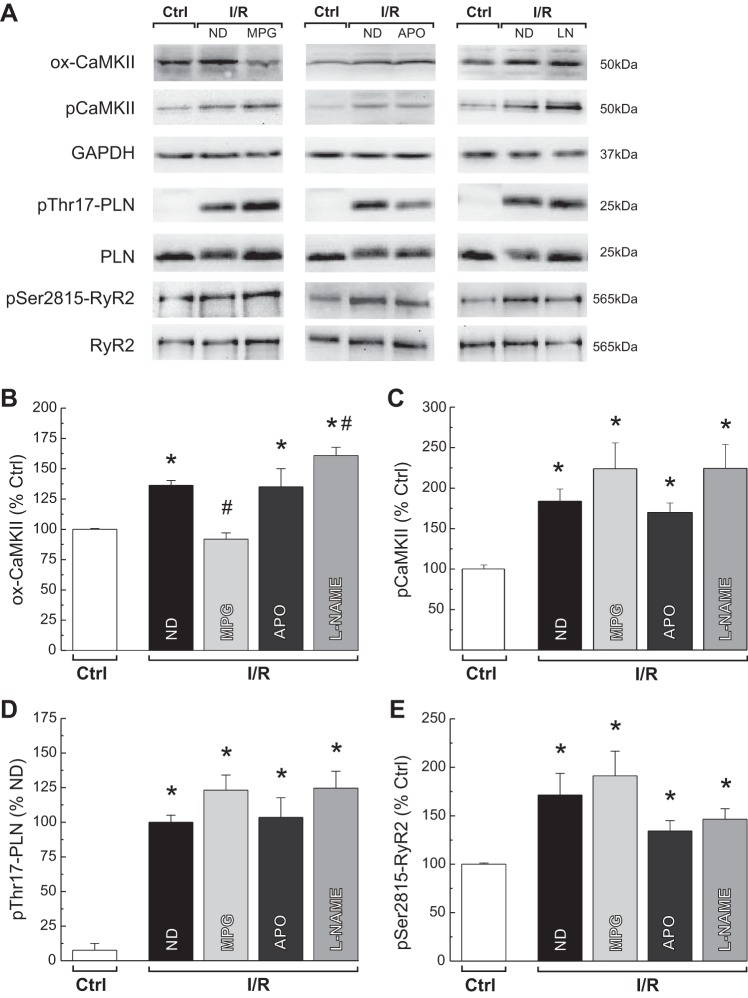
Oxidative stress is a hallmark feature of reperfusion injury, and CaMKII is directly modified by ROS. Therefore, we examined the possible involvement of oxidation-dependent activation of CaMKII in rat hearts subjected to ex vivo ischemia/reperfusion. Oxidation of CaMKII (ox-CaMKII) increased by 36% at the beginning of reperfusion. This enhancement was only abrogated by the free radical scavenger MPG. Neither the NOX inhibitor, APO, nor the general NOS inhibitor (l-NAME) was able to reduce the reperfusion-induced increase in ox-CaMKII (Fig. 1, A and B). To determine the effect of ox-CaMKII, we studied the phosphorylation of three CaMKII targets: the kinase itself, PLN, and RyR2 in the absence and the presence of the different antioxidant treatments (Fig. 1, A–E). The reduction in ox-CaMKII induced by MPG was not associated with a diminished phosphorylation level of CaMKII, Thr17 of PLN, and Ser2815 of RyR2. As expected, the inhibition of NOX and NOS by APO and l-NAME, respectively, did not modify the phosphorylation of CaMKII targets. The use of another free radical scavenger, Tiron, mimicked the effects of MPG on reperfusion-induced ox-CaMKII levels and autophosphorylation of CaMKII. Whereas Tiron significantly reduced ox-CaMKII from 130.9 ± 6.0% to 114.3 ± 2.9% control, no changes in pCaMKII were observed (260.0 ± 25.7% vs. 356.2 ± 66.3% control), n = 5–13. The overall findings suggest that, although ox-CaMKII increases during early reperfusion, it seems not to be necessary to sustain CaMKII activity at least toward its SR substrates. Moreover, the lack of effect of l-NAME on the phosphorylation of CaMKII and its targets indirectly indicates that the described activation by S-nitrosylation of CaMKII (5) is not occurring in our experimental conditions.
Fig. 1.
Reperfusion-induced increase in Ca2+ calmodulin-dependent kinase II (CaMKII) activity is independent of its oxidation state. Typical blots (A) and overall results of experiments showing oxidation (ox-CaMKII, B) and autophosphorylation of CaMKII (pCaMKII, C) and phosphorylation of its 2 substrates, Thr17 site of phospholamban (PLN) (D) and Ser2815 of ryanodine receptor 2 (RyR2) (E), at the onset of ischemia/reperfusion (I/R) in the absence (ND) or presence of different antioxidants are shown. Reperfusion-induced increase in ox-CaMKII was only abolished by mercaptopropionylglycine (MPG) treatment. MPG, apocynin (APO), or NG-nitro-l-arginine methyl ester (l-NAME) (LN) were not able to prevent the CaMKII-dependent increase in protein phosphorylations. The data represent means ± SE (n = 5–13 hearts). *P < 0.05 with respect to control values (Ctrl). #P < 0.05 with respect to reperfusion with no drug ND.
Oxidative/Nitrosative Modifications of RyR2 and Their Impact on Reperfusion Arrhythmias
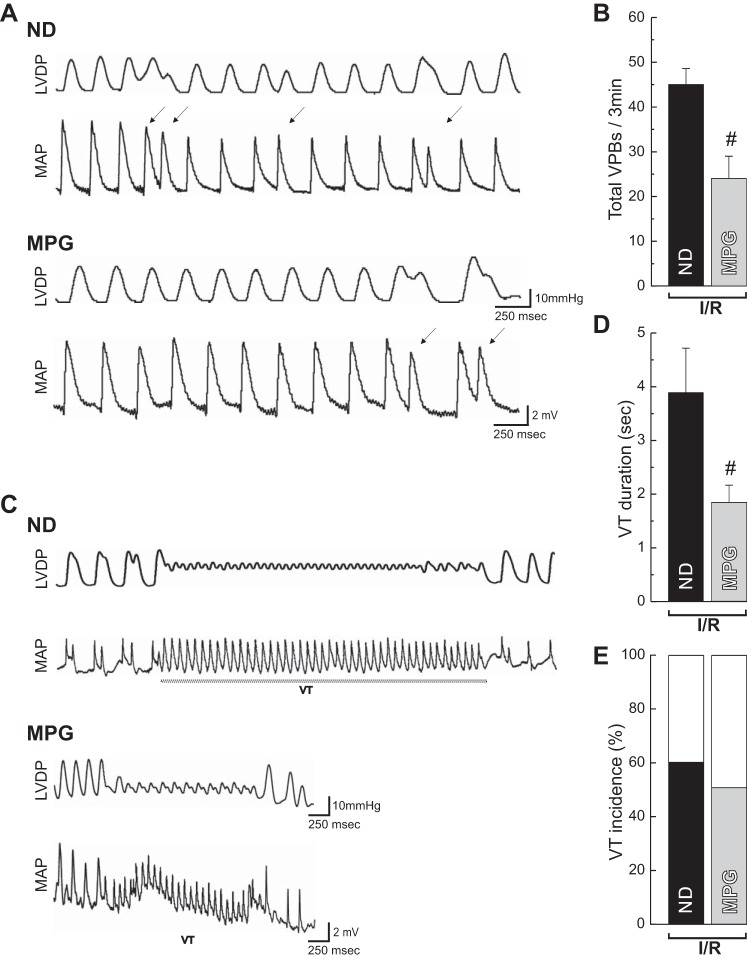
ROS/RNS signaling plays a role in the regulation of RyR2 channel gating by promoting redox posttranslational modifications. It has been shown that oxidative stress developed during the course of different cardiac diseases induces RyR2 SR Ca2+ leak, leading to the appearance of arrhythmias. In these experiments, we particularly examined the S-glutathionylation and S-nitrosylation of the channel in the stunned heart and its correlation with the presence of arrhythmias. The level of these two RyR2 posttranslational modifications increased during early reperfusion, and these changes were prevented when the ischemia/reperfusion protocol was performed in the presence of MPG (Fig. 2, A and B). In addition, MPG decreased the number of VPBs (Fig. 3, A and B) and diminished the average duration of VT, a more severe type of arrhythmia (Fig. 3, C and D), with a tendency to decrease the incidence of these events (Fig. 3E). Similar results were obtained when MPG was given only at reperfusion. Overall, these findings are in line with the widely accepted concept that oxidized RyR2 is prone to arrhythmias.
Fig. 2.
Reperfusion increases the S-glutathionylation and S-nitrosylation of RyR2. Typical blots and overall results of experiments showing the S-glutathionylation (A) and S-nitrosylation (B) of RyR2 in hearts subjected to I/R in the absence (ND) or presence of MPG are shown. Reperfusion-induced increase in S-glutathionylation and S-nitrosylation of RyR2 was prevented by MPG. The data represent means ± SE (n = 7–13 hearts). *P < 0.05 with respect to Ctrl. #P < 0.05 with respect to reperfusion with ND. GSH, glutathione; SNO, nitrosocysteine.
Fig. 3.
MPG reduces the severity of reperfusion arrhythmias. Representative simultaneous recordings of mechanical (left ventricular developed pressure, LVDP) and electrical activity (monophasic action potential, MAP) showing ventricular premature beats (VPBs) (A) and ventricular tachycardia (VT) (C) during the early reperfusion in the absence (ND) or the presence of MPG are shown. Overall results of total VPBs (B) and VT, duration, and incidence (D and E) in the first 3 min of reperfusion are shown. The antioxidant MPG decreased the number of total VPBs (Mann-Whitney test) and the duration of VT episodes. The data represent means ± SE (n = 5–14 hearts). #P < 0.05 with respect to reperfusion with ND.
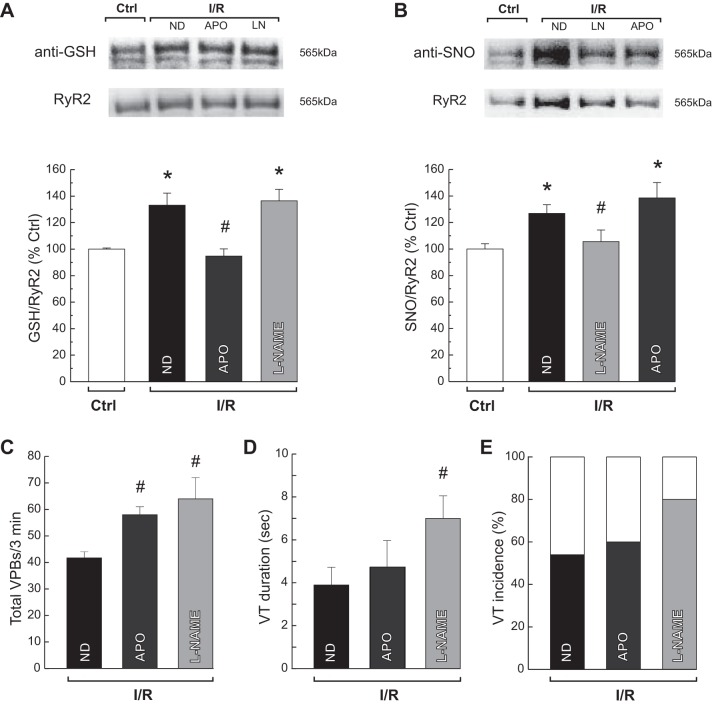
To determine the relative contribution of S-glutathionylation and S-nitrosylation to the generation of arrhythmias, the number of VPBs and the duration and incidence of VTs were evaluated in the presence of APO and l-NAME. The reperfusion-induced increase in NOX activity (from 2.6 ± 0.1 to 4.1 ± 0.4 nmol O2·mg protein−1·min−1, P < 0.05) was diminished to control values by APO (2.1 ± 0.1 nmol O2·mg protein−1·min−1). This treatment selectively suppressed the S-glutathionylation of RyR2 without affecting the level of S-nitrosylation (Fig. 4, A and B). In addition, l-NAME abolished the S-nitrosylation of RyR2 in the absence of changes in S-glutathionylation of RyR2 (Fig. 4, A and B). Neither APO nor l-NAME treatment alleviated arrhythmogenesis. Conversely, the number of VPBs significantly increased (Fig. 4C), whereas VTs episodes showed a trend toward a longer duration and higher incidence with respect to ischemia/reperfusion hearts with no intervention (Fig. 4, D and E). Similarly to l-NAME, treating the hearts with a NOS-1-specific inhibitor, SMTC, increased the number of VPBs at early reperfusion (SMTC: 63 ± 3; l-NAME: 65 ± 6 vs. ND: 45 ± 4, n = 3–8).
Fig. 4.
Inhibition of specific oxidative/nitrosative changes of RyR2 increases reperfusion arrhythmias. Typical blots and overall results of experiments show the reperfusion-induced increase in S-glutathionylation (A), S-nitrosylation of RyR2 (B), total VPBs (C), VT, and duration and incidence (D and E) in the first 3 min of reperfusion in the absence (ND) or the presence of APO and l-NAME. APO selectively abolished the S-glutathionylation of RyR2, and l-NAME did the same with the S-nitrosylation of RyR2. Both drugs augmented the number of VPBs (Mann-Whitney test) and showed a tendency to increase the duration and incidence of VT. The data represent means ± SE (n = 4–11 hearts). *P < 0.05 with respect to I/R. #P < 0.05 with respect to reperfusion with ND.
Oxidative/Nitrosative Modifications of RyR2 and Their Impact on Contractile Recovery
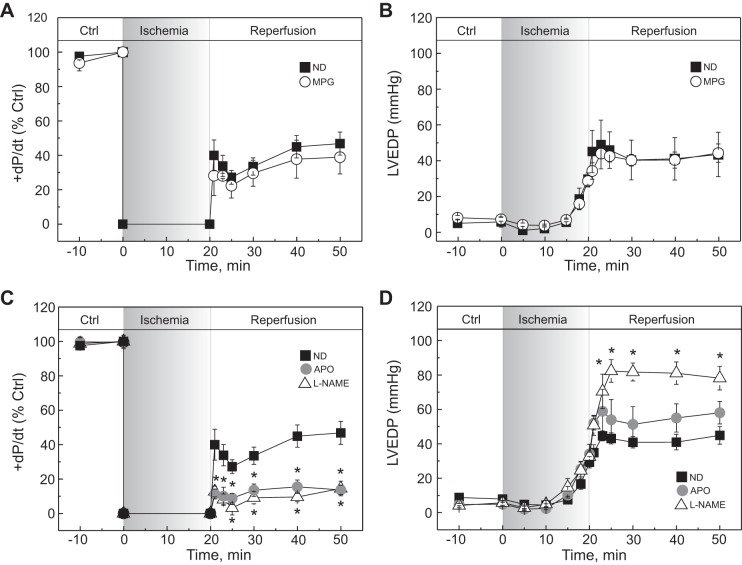
Because arrhythmogenic activity during reperfusion has been linked to SR Ca2+ leak, changes in SR Ca2+ load and therefore in contractility could be expected. The contractile recovery after ischemia was not modified by MPG; +dP/dt and LVEDP attained levels similar to control hearts reperfused in the absence of drugs (Fig. 5, A and B). However, both mechanical parameters were drastically impaired by APO and l-NAME; +dP/dt was significantly reduced, whereas LVEDP was enhanced (Fig. 5, C and D). Therefore, removing S-glutathionylation and/or S-nitrosylation of RyR2 by a reducing agent (MPG) or through selective enzyme inhibitors (APO, l-NAME) seemed to be associated with different effects on contractile recovery after ischemia.
Fig. 5.
Differential effects of the antioxidants on the contractile recovery during reperfusion. Time courses are shown of maximal rate of pressure development (+dP/dt, A and C) and left ventricular end-diastolic pressure (LVEDP) (B and D) of rat hearts submitted to I/R protocols in the absence (ND) or presence of MPG (A and B), APO, and l-NAME (C and D). Hearts treated with MPG showed a similar recovery pattern to ND. The presence of APO or l-NAME deteriorated the contractility with respect to ND and further increased LVEDP. The data represent means ± SE (n = 4–11 hearts). *P < 0.05 with respect to ND.
Redox Balance During Ischemia/Reperfusion
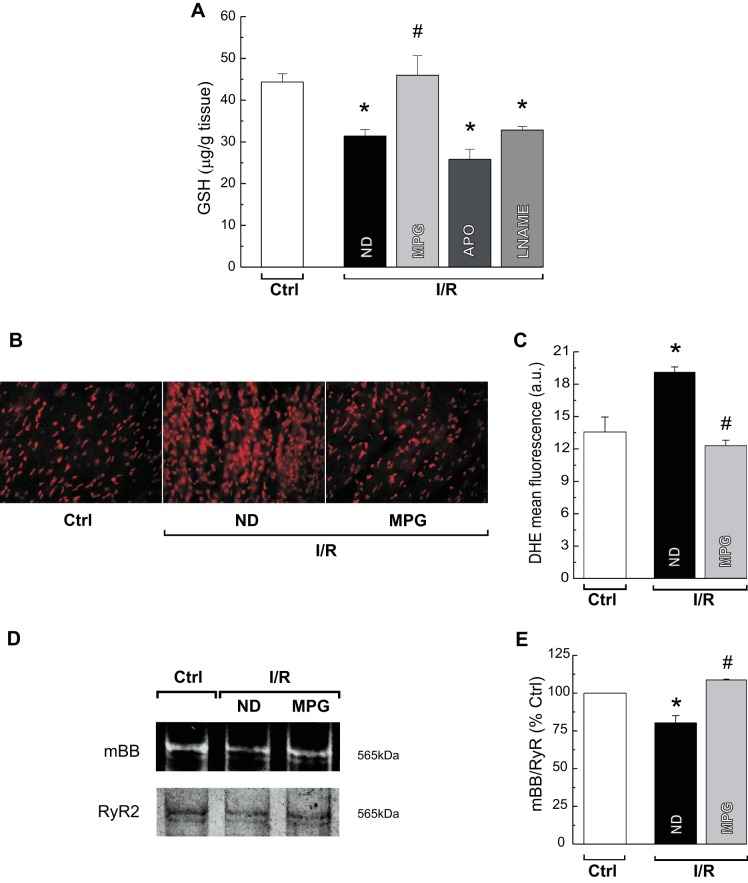
Depending on the pharmacological tool used to prevent the S-glutathionylation and S-nitrosylation, beneficial or deleterious effects on electrical and mechanical activities during reperfusion were observed. To assess whether this discrepancy originates from a different oxidative environment derived from the treatment used, reduced GSH levels were measured in hearts subjected to ischemia/reperfusion in the presence or absence of MPG, APO, or l-NAME. The level of GSH was reduced in ischemia/reperfusion hearts, confirming that oxidative stress is increased by the injury. Treatment with MPG restored GSH to control levels; however, APO or l-NAME did not (Fig. 6A), indicating that only MPG was able to prevent the oxidative stress induced by ischemia/reperfusion. The antioxidant effect of MPG was confirmed by quantifying ROS production using DHE staining. The increased ethidium fluorescence intensity observed in hearts subjected to ischemia/reperfusion was reduced by MPG treatment (Fig. 6, B and C). These changes in ROS production were paralleled by changes in the redox state of RyR2, as determined by the mBB fluorescence method. Ischemia/reperfusion decreased the level of free thiol content on RyR2, consistent with increased oxidation of the SR Ca2+ channel, and the presence of MPG precluded this effect (Fig. 6, D and E). Thus the apparent beneficial or deleterious effects of S-glutathionylation and S-nitrosylation of RyR2 on the electrical and mechanical activity during reperfusion seem to depend on the cellular redox milieu, which is differentially affected by the three antioxidants used.
Fig. 6.
Effects of the antioxidants on reduced GSH content, reactive oxygen species (ROS) formation, and RyR2 free thiol content during reperfusion. Average levels of reduced form of GSH measured in tissue homogenates from Ctrl or I/R rat hearts in the absence (ND) or presence of MPG, APO, or l-NAME are shown. Only MPG treatment restored GSH levels to Ctrl values (A). Representative images (B) and overall results (C) of dihydroethidium (DHE) staining for ROS production in Ctrl and I/R hearts without (ND) or with MPG treatment are shown. MPG reduces ROS production to Ctrl levels. Representative (monobromobimane, mBB) fluorescence intensity and Coomassie blue-stained gels of RyR2 (D) and average results of free thiol content on RyR2 (E) in Ctrl and I/R hearts in the absence (ND) or presence of MPG are shown. Content of free thiols decreases in I/R, and it is restored by treatment with MPG. The data represent means ± SE (n = 3–6 hearts). *P < 0.05 with respect to Ctrl. #P < 0.05 with respect to reperfusion with ND.
Impact of CaMKII-Dependent Phosphorylation and Oxidation of RyR2 on the Propensity for Reperfusion Arrhythmias
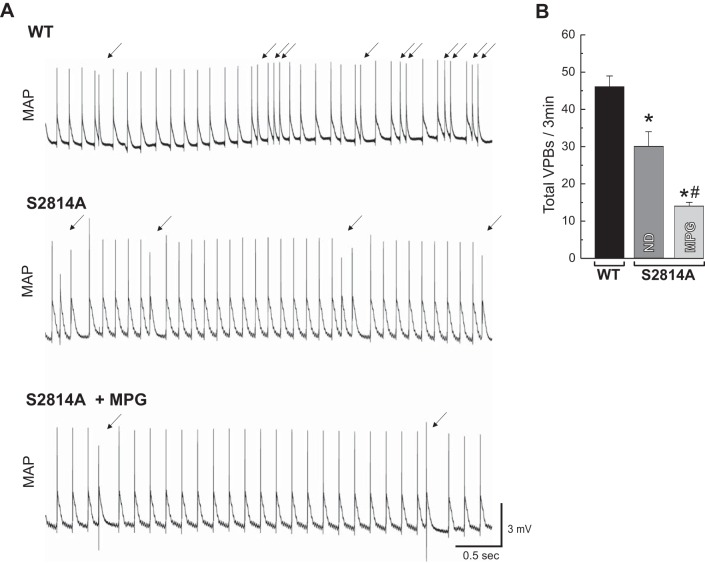
As we previously described, knockin mice lacking the principal CaMKII phosphorylation site in RyR2 (S2814A) showed a marked decrease in reperfusion arrhythmogenesis (40). To test whether antioxidant treatment further reduced the appearance of arrhythmias, S2814A mice were subjected to ischemia/reperfusion in the absence and presence of MPG (Fig. 7). Reduction of cellular oxidative stress by MPG, which avoids the oxidation of RyR2 during reperfusion (Fig. 6, D and E), further diminished the number of VPBs by 50%. These results give support to the concept that both phosphorylation and oxidation of RyR2 are involved in the generation of reperfusion arrhythmias. Furthermore, the results suggest that oxidation of RyR2 contributes to reperfusion arrhythmias, even in the absence of the CaMKII-dependent phosphorylation of RyR2.
Fig. 7.
CaMKII-dependent phosphorylation of RyR2-Ser2814 site and redox changes are critical for generation of reperfusion arrhythmias. Representative recordings of electrical activity (MAP) showing VPBs (arrows) during early reperfusion in WT mice or mice with genetic mutation of Ser2814 site of RyR2 (S2814A) in the absence or presence of MPG (A) and overall results of total VPBs in the first 3 min of reperfusion (B) are shown. There was a significant decrease in VPBs in RyR2-S2814A mice relative to WT, but the treatment with MPG declined them further. The data represent means ± SE (n = 3–8 hearts). *P < 0.05 with respect to WT. #P < 0.05 with respect to S2814A without MPG.
DISCUSSION
Oxidative stress is a prominent feature in the onset and progression of a number of heart diseases, including ischemia/reperfusion injury. It is well known that CaMKII and RyR2 are functionally influenced by redox modifications (9, 13, 18, 23). Our results indicate that both proteins become oxidized following ischemia/reperfusion in the intact heart, but the impact of such alterations on the genesis of arrhythmias and the recovery of contractility are apparently different. Although oxidation of CaMKII seems to have no consequences in terms of the activity of the enzyme and the phosphorylation of two SR target proteins, the redox modifications of RyR2 appear to have both beneficial and detrimental effects. In the present work, we particularly studied the S-glutathionylation and S-nitrosylation of RyR2, two reversible posttranslational modifications of the channel that seem to play a protective role in the arrhythmogenesis and the contractile recovery of the stunned heart.
CaMKII Mechanism of Activation During Ischemia/Reperfusion
Redox-dependent CaMKII activation alters different mechanisms that may contribute to arrhythmia propensity (23). In postischemic reperfusion, a well-known situation of oxidative stress, experiments from our laboratory previously demonstrated that CaMKII-dependent phosphorylation of RyR2 is a crucial factor for triggering arrhythmias (40). The present findings further showed that ox-CaMKII increased during reperfusion by a NOX-uncoupled/NOS-independent mechanism. In this scenario, mitochondria appear as a good candidate to provide the ROS involved in CaMKII oxidation. However, redox-dependent activation of this enzyme did not influence the degree of RyR2 phosphorylation. The lack of relationship between oxidation and activation of CaMKII has also been reported by Bell et al. (2), who showed similar levels of ox-CaMKII in stunned male and female rat hearts associated with different phosphorylation extent of CaMKII and PLN. Taken together, the overall results suggest that ox-CaMKII is not essential to maintain the kinase activity in ischemia/reperfusion at least toward its SR substrates. It is likely that the massive Ca2+ overload at early reperfusion produced by both an uncontrolled Ca2+ influx (34) and SR Ca2+ depletion (48) provides the necessary intracellular Ca2+ to activate and sustain CaMKII activity independently of its oxidation state.
Oxidative/Nitrosative Changes of RyR2 and Arrhythmogenesis During Ischemia/Reperfusion
RyR2 is highly sensitive to redox state attributable to the large number of cysteines that compose the homo-tetramer (18). Depending on the degree of oxidative stress, RyR2 free thiols can be reversibly or irreversibly oxidized (31). Three types of reversible redox modifications have been described for RyR2: disulfide oxidation, S-nitrosylation, and S-glutathionylation. Whereas irreversible oxidation unambiguously increases RyR2 activity (31, 45, 53), the impact of reversible redox changes on the channel function remains controversial (6, 7, 12, 25, 42, 52, 56). It has been suggested that RyR2 intersubunit cross linking or alterations in RyR2 association with auxiliary proteins, as a result of oxidative stress, promote an increase in RyR2 activity and diastolic Ca2+ leak (24, 33). This Ca2+ leak could be the cause of the formation of Ca2+ waves sufficient to induce spontaneous APs and therefore cardiac arrhythmias during reperfusion. Among protein redox modifications, S-nitrosylation and S-glutathionylation have been proposed to conspire to the redox-induced deleterious effects by protecting the reactive thiol groups from further irreversible oxidation (14, 29). In the stunned heart, neither the occurrence of redox changes of RyR2 nor their functional consequences on arrhythmias and contractile dysfunction have been previously described.
In the present study, we found that S-nitrosylation and S-glutathionylation of RyR2 augmented during early reperfusion after ischemia. Prevention of these reversible redox modifications in the reperfusion-induced oxidative environment was associated with a higher number of arrhythmias and an impaired contractile recovery. It is likely that, in this context, S-nitrosylation and S-glutathionylation emerge as protective mechanisms. Our findings are consistent with Cuttler et al. (6), who found that the combination of decreased S-nitrosylation and increased oxidation of RyR2 leads to a striking increase in spontaneous SR Ca2+ release and ventricular arrhythmias, in intact hearts exposed to elevated [Ca2+] and oxidative stress, two prevailing conditions in ischemia/reperfusion. Similar results were described in the failing heart (1, 13).
A different situation occurred when the reperfusion-induced oxidative stress was precluded by the presence of the free radical scavenger, MPG. Although the S-nitrosylation and S-glutathionylation of RyR2 were blunted, the number of VPBs and the duration of VTs decreased. In this environment, both the reversible and the irreversible oxidations of RyR2 might be prevented, turning RyR2 into a less leaky channel and decreasing the severity of arrhythmias. The fact that the content of free thiols on RyR2 in those hearts subjected to ischemia/reperfusion in the presence of MPG was similar to control conditions supports this possibility. The antiarrhythmic effects of MPG treatment were not associated with an enhancement of the contractile recovery after ischemia. This behavior might reflect the capacity of myocyte Ca2+ handling for autoregulation (8). Although the SR Ca2+ content might be transiently higher in the presence of MPG, the compensatory changes in Ca2+ fluxes would restore Ca2+ cycling and steady-state contractility after ischemia. However, this mechanism might be overwhelmed when the severity of reperfusion arrhythmias increased, as in the case of APO and l-NAME. The lack of reversible oxidations, which turns RyR2 hyperactive as a result of irreversible oxidation, could lead to a drastic SR Ca2+ leak, which would deplete the SR, decrease Ca2+ transient, and impair contractility.
Several experiments studying the impact of redox changes on RyR2 activity have been performed in artificial planar bilayer systems or isolated myocytes (1, 16, 47). The novelty of the present findings is that we were able to detect reperfusion-induced redox modifications of RyR2 in the intact ex vivo heart, a model in which the general nitroso/redox balance is better preserved.
Contribution of Nitroso/Redox Imbalance to Ischemia/Reperfusion Arrhythmias in S2814A Mice
Numerous studies have shown that either CaMKII-dependent phosphorylation or oxidation of RyR2 favors the generation of arrhythmias (1, 4). Recently, a potential interaction of the two posttranslational modifications of RyR2 has been described in different animal models (6, 22, 53). In the mdx mouse model of Duchenne muscular dystrophy (22, 53), the oxidation state and the activity of RyR2 were markedly reduced when RyR2 phosphorylation was genetically inhibited. In contrast, the reduced S-nitrosylation of RyR2 caused by inhibition of neuronal NOS increased the oxidation of RyR2 and Ca2+-triggered arrhythmias in intact guinea pig hearts, together with a decrease in the phosphorylation of Ser2814 site of RyR2. A cross talk between ROS/RNS modifications and phosphorylation of RyR2 was not evident in the present experiments of hearts subjected to ischemia/reperfusion. Antioxidant treatments, which modified the redox state of RyR2, altered the propensity to arrhythmias, without affecting the reperfusion-induced increase in CaMKII phosphorylation of RyR2. Moreover, in mice lacking the CaMKII phosphorylation site of RyR2, which showed a decreased number of VPBs compared with wild-type, MPG further reduced the propensity to arrhythmias. Thus, in our experimental conditions, oxidation and phosphorylation seem to act as independent and additive pathways of regulation of RyR2 activity.
In conclusion, our present results provide new insights into the mechanisms underlying the reperfusion arrhythmias. They suggest that, whereas S-nitrosylation and S-glutathionylation of RyR2 restrain the propensity for arrhythmias, other oxidations of RyR2 amplify the arrhythmogenic effect of the CaMKII phosphorylation of the channel. These mechanisms, working in concert, provide the molecular basis for reperfusion arrhythmias, and they must be considered as therapeutic targets in the ischemia/reperfusion injury.
Limitations of This Study
The present data show that nitroso/redox imbalance is an important mechanism that contributes to the triggering of VPBs and VT during the early reperfusion phase and suggest that RyR2 is a putative player in this scenario. We are aware of the fact that a causal relationship between RyR2 redox modification and arrhythmias is difficult to ascertain in the intact heart in which pharmacological interventions are unlikely to have effects limited only to RyR2. Alteration of other redox-sensitive proteins cannot be excluded. In this context, it was recently reported that SERCA2a oxidation plays a significant role in repolarization alternans associated with myocardial infarction (35). Future work is needed to validate the contribution of selective RyR2 redox modifications to reperfusion arrhythmogenesis.
GRANTS
This work was supported by Consejo de Investigaciones Científicas y Técnicas (CONICET), Argentina (PIP no. 0463 to M. Said), by Agencia Nacional de Promoción Científica y Técnica, Argentina (PICT no. 2634 to C. Mundiña-Weilenmann and PICT no. 2073 to L. Vittone) and PICT 0856 to M. Said, by Fondecyt 1110257 and 1130407 to P. Donoso and G. Sanchex, and NIH-NHLBI (HL089598, HL091947, HL117641, HL129570), and the American Heart Association (13EIA14560061) to X. Wehrens.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.B., B.S.R., M.N.D.C., J.I.M., M. Salas, G.S., G.R.S., C.M.-W., and M. Said performed experiments; R.B., B.S.R., M.N.D.C., J.I.M., M. Salas, G.S., G.R.S., C.M.-W., and M. Said analyzed data; R.B., M. Salas, G.R.S., L.V., C.M.-W., and M. Said interpreted results of experiments; R.B., B.S.R., C.M.-W., and M. Said prepared figures; M. Salas, L.V., C.M.-W., and M. Said conception and design of research; M. Salas, G.S., P.D., L.V., X.H.W., C.M.-W., and M. Said edited and revised manuscript; L.V., C.M.-W., and M. Said drafted manuscript; L.V., X.H.W., C.M.-W., and M. Said approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mirta Reynaldo and Mario Perelló for help in the tissue sectioning from the Multidisciplinary Institute of Cell Biology [IMBICE-Argentine Research Council (CONICET) and Scientific Research Commission, Province of Buenos Aires (CIC-PBA)].
REFERENCES
- 1.Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, Carnes CA, Györke S. The relationship between arrhythmogenesis and impaired contractility in heart failure: Role of altered ryanodine receptor function. Cardiovasc Res 90: 493–502, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell JR, Raaijmakers AJ, Curl CL, Reichelt ME, Harding TW, Bei A, Ng DC, Erickson JR, Vila Petroff M, Harrap SB, Delbridge LM. Cardiac CaMKIIδ splice variants exhibit target signaling specificity and confer sex-selective arrhythmogenic actions in the ischemic-reperfused heart. Int J Cardiol 181: 288–296, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet E. Cardiac ionic currents and acute ischemia: From channels to arrhythmias. Physiol Rev 79: 917–1017, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Müller FU, Schmitz W, Schotten U, Anderson ME, Valderrábano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest 119: 1940–1951, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curran J, Tang L, Roof SR, Velmurugan S, Millard A, Shonts S, Wang H, Santiago D, Ahmad U, Perryman M, Bers DM, Mohler PJ, Ziolo MT, Shannon TR. Nitric oxide-dependent activation of CaMKII increases diastolic sarcoplasmic reticulum calcium release in cardiac myocytes in response to adrenergic stimulation. PLoS One 9 e87495, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutler MJ, Plummer BN, Wan X, Sun QA, Hess D, Liu H, Deschenes I, Rosenbaum DS, Stamler JS, Laurita KR. Aberrant S-nitrosylation mediates calcium-triggered ventricular arrhythmia in the intact heart. Proc Natl Acad Sci USA 109: 18186–18191, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donoso P, Finkelstein JP, Montecinos L, Said M, Sánchez G, Vittone L, Bull R. Stimulation of NOX2 in isolated hearts reversibly sensitizes RyR2 channels to activation by cytoplasmic calcium. J Mol Cell Cardiol 68: 38–46, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Eisner DA, Trafford AW, Díaz ME, Overend CL, O'Neill SC. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc Res 38: 589–604, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Erickson JR. Mechanisms of CaMKII activation in the heart. Front Pharmacol 5: 1–5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrero P, Said M, Sánchez G, Vittone L, Valverde C, Donoso P, Mattiazzi A, Mundiña-Weilenmann C. Ca2+/calmodulin kinase II increases ryanodine binding and Ca2+-induced sarcoplasmic reticulum Ca2+ release kinetics during beta-adrenergic stimulation. J Mol Cell Cardiol 43: 281–291, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci USA 104: 20612–20617, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem 285: 28938–28945, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grek CL, Zhang J, Manevich Y, Townsend DM, Tew KD. Causes and consequences of cysteine S-glutathionylation. J Biol Chem 288: 26497–26504, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez DA, Fernandez-Tenorio M, Ogrodnik J, Niggli E. NO-dependent CaMKII activation during β-adrenergic stimulation of cardiac muscle. Cardiovasc Res 100: 392–401, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Hanna AD, Lam A, Thekkedam C, Gallant EM, Beard NA, Dulhunty AF. Cardiac ryanodine receptor activation by a high Ca2+ store load is reversed in a reducing cytoplasmic redox environment. J Cell Sci 127: 4531–4541, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med 17: 1610–1618, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hidalgo C, Donoso P, Carrasco MA. The ryanodine receptors Ca2+ release channels: Cellular redox sensors? IUBMB Life 57: 315–322, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Ho HT, Liu B, Snyder JS, Lou Q, Brundage EA, Velez-Cortes F, Wang H, Ziolo MT, Anderson ME, Sen CK, Wehrens XH, Fedorov VV, Biesiadecki BJ, Hund TJ, Györke S. Ryanodine receptor phosphorylation by oxidized CaMKII contributes to the cardiotoxic effects of cardiac glycosides. Cardiovasc Res 101: 165–174, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.January CT, Riddle JM. Early afterdepolarizations: Mechanism of induction and block. A role for L-type Ca2+ current. Circ Res 64: 977–990, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Knollmann BC, Katchman AN, Franz MR. Monophasic action potential recordings from intact mouse heart: Validation, regional heterogeneity, and relation to refractoriness. J Cardiovasc Electrophysiol 12: 1286–1294, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Kyrychenko S, Poláková E, Kang C, Pocsai K, Ullrich ND, Niggli E, Shirokova N. Hierarchical accumulation of RyR post-translational modifications drives disease progression in dystrophic cardiomyopathy. Cardiovasc Res 15: 666–675, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest 123: 1262–1274, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazurek SR, Bovo E, Zima AV. Regulation of sarcoplasmic reticulum Ca2+ release by cytosolic glutathione in rabbit ventricular myocytes. Free Radic Biol Med 68: 159–167, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Mészáros LG. Suppression of spontaneous calcium release events by nitric oxide in rat ventricular myocytes. J Muscle Res Cell Motil 25: 604–605, 2004. [PubMed] [Google Scholar]
- 26.Mital R, Zhang W, Cai M, Huttinger ZM, Goodman LA, Wheeler DG, Ziolo MT, Dwyer KM, d'Apice AJ, Zweier JL, He G, Cowan PJ, Gumina RJ. Antioxidant network expression abrogates oxidative posttranslational modifications in mice. Am J Physiol Heart Circ Physiol 300: H1960–H1970, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosca SM, Gelpi RJ, Milei J, Fernández Alonso G, Cingolani HE. Is stunning prevented by ischemic preconditioning? Mol Cell Biochem 186: 123–129, 1998. [PubMed] [Google Scholar]
- 28.Mundiña-Weilenmann C, Ferrero P, Said M, Vittone L, Kranias EG, Mattiazzi A. Role of phosphorylation of Thr17 residue of phospholamban in mechanical recovery during hypercapnic acidosis. Cardiovasc Res 66: 114–122, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Murphy E, Kohr M, Sun J, Nguyen T, Steenbergen C. S-nitrosylation: A radical way to protect the heart. J Mol Cell Cardiol 52: 568–577, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narins CR, Zareba W, Moss AJ, Goldstein RE, Hall WJ. Clinical implications of silent versus symptomatic exercise-induced myocardial ischemia in patients with stable coronary disease. J Am Coll Cardiol 29: 756–763, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Niggli E, Ullrich ND, Gutierrez D, Kyrychenko S, Poláková E, Shirokova N. Posttranslational modifications of cardiac ryanodine receptors: Ca2+ signaling and EC-coupling. Biochim Biophys Acta 1833: 866–875, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuss HB, Kääb S, Kass DA, Tomaselli GF, Marbán E. Cellular basis of ventricular arrhythmias and abnormal automaticity in heart failure. Am J Physiol 277: H80–H91, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Oda T, Yang Y, Uchinoumi H, Thomas DD, Chen-Izu Y, Kato T, Yamamoto T, Yano M, Cornea RL, Bers DM. Oxidation of ryanodine receptor (RyR) and calmodulin enhance Ca2+ release and pathologically alter, RyR structure and calmodulin affinity. J Mol Cell Cardiol 85: 240–248, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piper HM, Abdallah Y, Schafer C. The first minutes of reperfusion: A window of opportunity for cardioprotection. Cardiovasc Res 61: 365–371, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Plummer BN, Liu H, Wan X, Deschênes I, Laurita KR. Targeted antioxidant treatment decreases cardiac alternans associated with chronic myocardial infarction. Circ Arrhythm Electrophysiol 8: 165–173, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res 88: 1159–1167, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Pride YB, Appelbaum E, Lord EE, Sloan S, Cannon CP, Sabatine MS, Gibson CM; TIMI Study Group. Relation between myocardial infarct size and ventricular tachyarrhythmia among patients with preserved left ventricular ejection fraction following fibrinolytic therapy for ST-segment elevation myocardial infarction. Am J Cardiol 104: 475–479, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, Neef S, Sowa T, Gao Z, Luczak ED, Stefansdottir H, Behunin AC, Li N, El-Accaoui RN, Yang B, Swaminathan PD, Weiss RM, Wehrens XH, Song LS, Dobrev D, Maier LS, Anderson ME. Oxidized Ca2+/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 128: 1748–1757, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Said M, Becerra R, Palomeque J, Rinaldi G, Kaetzel MA, Diaz-Sylvester PL, Copello JA, Dedman JR, Mundiña-Weilenmann C, Vittone L, Mattiazzi A. Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Heart Circ Physiol 295: H1669–H1683, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Said M, Becerra R, Valverde CA, Kaetzel MA, Dedman JR, Mundiña-Weilenmann C, Wehrens XH, Vittone L, Mattiazzi A. Calcium-calmodulin dependent protein kinase II [CaMKII]: A main signal responsible for early reperfusion arrhythmias. J Mol Cell Cardiol 51: 936–944, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salerno JA, Previtali M, Chimienti M, Klersy C, Bobba P. Vasospasm and ventricular arrhythmias. Ann NY Acad Sci 427: 222–233, 1984. [DOI] [PubMed] [Google Scholar]
- 42.Sánchez G, Escobar M, Pedrozo Z, Macho P, Domenech R, Härtel S, Hidalgo C, Donoso P. Exercise and tachycardia increase NADPH oxidase and ryanodine receptor-2 activity: Possible role in cardioprotection. Cardiovasc Res 77: 80–386, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Sánchez G, Pedrozo Z, Domenech RJ, Hidalgo C, Donoso P. Tachycardia enhances RyR2 S-glutathionylation and RyR2 activity via activation of NAD[P]H oxidase. J Mol Cell Cardiol 39: 982–991, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25: 192–205, 1968. [DOI] [PubMed] [Google Scholar]
- 45.Shao CH, Tian C, Ouyang S, Moore CJ, Alomar F, Nemet I, D'Souza A, Nagai R, Kutty S, Rozanski GJ, Ramanadham S, Singh J, Bidasee KR. Carbonylation induces heterogeneity in cardiac ryanodine receptor function in diabetes mellitus. Mol Pharmacol 82: 383–399, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest 121: 3277–3288, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terentyev D, Györke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Györke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res 103: 1466–1472, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valverde CA, Kornyeyev D, Ferreiro M, Petrosky AD, Mattiazzi A, Escobar AL. Transient Ca2+ depletion of the sarcoplasmic reticulum at the onset of reperfusion. Cardiovasc Res 85: 671–680, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velez Rueda JO, Palomeque J, Mattiazzi A. Early apoptosis in different models of cardiac hypertrophy induced by high renin-angiotensin system activity involves CaMKII. J Appl Physiol 112: 2110–2120, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vittone L, Mundiña-Weilenmann C, Said M, Ferrero P, Mattiazzi A. Time course and mechanisms of phosphorylation of phospholamban residues in ischemia-reperfused rat hearts. Dissociation of phospholamban phosphorylation pathways. J Mol Cell Cardiol 34: 39–50, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species-activated Ca2+/calmodulin kinase IIδ is required for late INa augmentation leading to cellular Na+ and Ca2+ overload. Circ Res 108: 555–565, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Viatchenko-Karpinski S, Sun J, Györke I, Benkusky NA, Kohr MJ, Valdivia HH, Murphy E, Györke S, Ziolo MT. Regulation of myocyte contraction via neuronal nitric oxide synthase: role of ryanodine receptor S-nitrosylation. J Physiol 588: 2905–2917, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q, Wang W, Wang G, Rodney GG, Wehrens XH. Crosstalk between RyR2 oxidation and phosphorylation contributes to cardiac dysfunction in mice with Duchenne muscular dystrophy. J Mol Cell Cardiol 89: 177–184, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wit AL, Janse MJ. Reperfusion arrhythmias and sudden cardiac death: a century of progress toward an understanding of the mechanisms. Circ Res 89: 741–743, 2001. [PubMed] [Google Scholar]
- 55.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel [ryanodine receptor] by poly-S-nitrosylation. Science 279: 234–237, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Ziolo MT, Katoh H, Bers DM. Expression of inducible nitric oxide synthase depresses beta-adrenergic-stimulated calcium release from the sarcoplasmic reticulum in intact ventricular myocytes. Circulation 104: 2961–2966, 2001. [DOI] [PubMed] [Google Scholar]