The intracardiac nervous system represents the final common pathway for autonomic control of pacemaker function. Here we establish the isolated zebrafish heart as a viable model for investigating this system and characterize a neurally mediated shift in pacemaker locus from the sinoatrial to the atrioventricular region.
Keywords: intracardiac nervous system, sinoatrial node, atrioventricular node, parasympathetic, sympathetic
Abstract
The cardiac pacemaker sets the heart's primary rate, with pacemaker discharge controlled by the autonomic nervous system through intracardiac ganglia. A fundamental issue in understanding the relationship between neural activity and cardiac chronotropy is the identification of neuronal populations that control pacemaker cells. To date, most studies of neurocardiac control have been done in mammalian species, where neurons are embedded in and distributed throughout the heart, so they are largely inaccessible for whole-organ, integrative studies. Here, we establish the isolated, innervated zebrafish heart as a novel alternative model for studies of autonomic control of heart rate. Stimulation of individual cardiac vagosympathetic nerve trunks evoked bradycardia (parasympathetic activation) and tachycardia (sympathetic activation). Simultaneous stimulation of both vagosympathetic nerve trunks evoked a summative effect. Effects of nerve stimulation were mimicked by direct application of cholinergic and adrenergic agents. Optical mapping of electrical activity confirmed the sinoatrial region as the site of origin of normal pacemaker activity and identified a secondary pacemaker in the atrioventricular region. Strong vagosympathetic nerve stimulation resulted in a shift in the origin of initial excitation from the sinoatrial pacemaker to the atrioventricular pacemaker. Putative pacemaker cells in the sinoatrial and atrioventricular regions expressed adrenergic β2 and cholinergic muscarinic type 2 receptors. Collectively, we have demonstrated that the zebrafish heart contains the accepted hallmarks of vertebrate cardiac control, establishing this preparation as a viable model for studies of integrative physiological control of cardiac function by intracardiac neurons.
NEW & NOTEWORTHY
The intracardiac nervous system represents the final common pathway for autonomic control of pacemaker function. Here we establish the isolated zebrafish heart as a viable model for investigating this system and characterize a neurally mediated shift in pacemaker locus from the sinoatrial to the atrioventricular region.
an essential aspect of cardiac function is the ability to adjust the output of the heart to meet the continually varying metabolic demands of the body. To match these demands, the autonomic nervous system (ANS) alters pacemaker rate and myocardial contractility via reflexes acting through the intracardiac nervous system (ICNS). The sympathetic and parasympathetic limbs of the ANS dually innervate the vertebrate heart (21, 42). Reflex output from autonomic centers in the central nervous system are carried by cardiac rami of the vagosympathetic nerve trunks (VSN) to the ICNS, which represents the final common pathway for neuronal control of cardiac function.
Our current understanding of the mechanisms of intracardiac integration of autonomic inputs and the details of the anatomical and functional connections between the ICNS and cardiac effectors is incomplete. In particular, the relationship between the pattern of innervation of pacemaker cells by the ICNS and the responses of these cells to activation of this system is not clear. In mammalian hearts a major difficulty in studying the role of function-specific components of the ICNS, such as those involved in controlling rate, is that this system is deeply embedded in, and distributed throughout, the walls of both atria and ventricles. These components are therefore largely inaccessible for integrative studies. One solution to this problem is the use of reduced experimental preparations representing a subset of the ICNS, such as isolated tissues containing neurons in ganglionated plexi, to study properties of control circuits in vitro (4, 55). This approach, however, eliminates intracardiac neuronal connections, so only limited conclusions may be drawn about the roles of localized circuitry in controlling overall cardiac function.
The isolated whole zebrafish heart has several advantages over traditional mammalian models for investigating the organization and function of the ICNS, and we have employed this model in the present study. The initiation of excitation by a discrete group of sinoatrial pacemaker cells, and the propagation of this signal through the atrial and ventricular myocardium, are strikingly similar from the two-chambered hearts of zebrafish to the four-chambered hearts of adult mammals (7, 18). Recent reports (12, 17, 20, 41, 48, 60) have established the zebrafish heart as a powerful tool for studying cardiac electrophysiology, with the potential to provide broad insights into cardiovascular function. In a previous study (57), we described the basic structure of the ICNS in zebrafish, which is consistent with that of humans and other mammalian models (28, 33, 37, 43, 44). The isolated zebrafish heart thus has technical advantages that may be exploited to better understand the role of the ICNS in modulating chronotropy (57).
Axons entering the zebrafish heart from the cardiac rami of the VSN form terminals close to putative pacemaker cells that express hyperpolarization-activated, cyclic nucleotide-gated channel 4 (HCN4) in the sinoatrial region (SAR). In zebrafish and mammalian species, HCN4 channels contribute a major portion of the diastolic depolarizing current in cardiac pacemaker cells (28, 33, 37, 43, 44). Immunohistochemical evidence also shows that juxta-pacemaker terminals contain the neurotransmitters acetylcholine (ACh; parasympathetic, cardio-inhibitory) and norepinephrine (NE; sympathetic, cardio-excitatory), providing the neuroeffector substrate for bidrectional autonomic control of pacemaker rate (57). At the atrioventricular region (AVR) a subpopulation of HCN4-expressing cells has been identified (57). This anatomical finding is consistent with optogenetic evidence (6) that the zebrafish heart may contain a secondary pacemaker in this region, a situation similar to that in the mammalian heart (6, 22, 49, 52). An important advantage of the zebrafish heart is that the majority of the neurons within the ICNS are located in the basal regions of the sinoatrial valves, are visible using standard microscopical techniques, and are accessible for electrophysiological analyses (57).
In the present study, we establish the isolated, innervated zebrafish heart as a novel model for studies of autonomic control of heart rate. We have taken a combined electrophysiological, pharmacological, and anatomical approach to investigate the integrative neural control of cardiac pacemaker function. Electrocardiogram (ECG) recordings, combined with extrinsic nerve stimulation or pharmacological activation, demonstrated that discrete neural pathways modulate heart rate through adrenergic and cholinergic mechanisms. Optical mapping of electrical activity confirmed the location of a secondary pacemaker in the AVR, as well as modulation of both SAR and AVR pacemaker function by neural stimulation. Finally, immunohistochemical methods characterized the relationship of muscarinic cholinergic (M2R) and β2-adrenergic receptors (β2-AR) to putative pacemaker cells. The results of this study establish the isolated zebrafish heart as a tractable preparation to investigate autonomic control of pacemaker function for improved understanding of integrative cardiovascular physiology.
METHODS
Animals.
A total of 96 adult, AB strain zebrafish (12–18 mo postfertilization; 35 ± 8 mm standard body length, 693 ± 154 mg wet weight) of both sexes were used in this study. Animals were housed in 3- to 10-liter tanks (AquaticHabitats, Apopka, FL) at 28.5°C on a 14:10-h light-dark cycle and fed commercial pellet food (BrineShrimp Direct, Ogden, UT) twice a day. Filtered, conditioned water was continuously supplied from a recirculating system. Experimental procedures were approved by the Dalhousie University Committee on Laboratory Animals, and conformed to Canadian Council on Animal Care Guidelines.
Heart isolation.
Zebrafish were anaesthetized in a buffered solution (pH 7.2) of tricaine (MS-222; 1.5 mM; Sigma-Aldrich, Oakville, ON, Canada) in tank water (28.5°C) until opercular respiratory movements ceased and the animals lacked response to a fin pinch with forceps. A ventral midline incision was made through the body wall to expose the heart, and a block of tissue encompassing the ventral aorta [containing the branchiocardiac nerve trunk, (BCT)], ventricle, atrium, sinus venosus and ducts of Cuvier (containing the cardiac vagosympathetic rami) was then removed for whole mount immunohistochemistry or in vitro recordings. After each in vitro experiment, hearts were photographed, blotted, and weighed to determine if there was a relationship between heart size and heart rate. A relative index of the overall size of the heart was derived from brightfield images of whole hearts by measuring the total area enclosed by the atrium and ventricle.
Heart viability.
In preliminary experiments, to control for possible deterioration of function in the isolated hearts over the duration of our experiments, we monitored heart rate and responses to VSN stimulation in sample hearts for up to 6 h, a period longer than any experiments in this study.
Whole heart ECG recordings and VSN stimulation.
Isolated hearts (n = 24) were pinned through the ventral aorta and walls of the ducts of Cuvier to the Sylgard rubber (Dow Corning, Midland, MI) bottom of a 5-ml chamber and perfused with zebrafish saline (composition in mM: 124.1 NaCl, 5.1 KCl, 2.9 Na2HPO4, 1.9 MgSO4-7H2O, 1.4 CaCl2-2H2O, and 11.9 NaHCO3, pH 7.2; aerated with room air; 25°C) at 10 ml/min. Approximately 1 mm of the left and right cardiac rami of the vagosympathetic trunks was exposed in the walls of the ducts of Cuvier. Vagosympathetic trunks were stimulated individually or simultaneously with bipolar wire electrodes attached to constant-current isolation units (PSIU6; Grass Instruments, Quincy, MA) driven by a stimulator (Grass S88) delivering trains of rectangular pulses (pulse duration: 0.5 ms; train duration: 10 s, pulse frequency: 1–20 Hz). In all experiments, a standard stimulus current of 300 μA was used. This current was chosen as optimal for this preparation as it was double the intensity (140 μA) that reliably activated all axons in the vagi in preliminary experiments measuring compound action potentials. ECG signals were recorded from the surface of the atrium and ventricle via bipolar suction electrodes, differentially amplified (total gain 1,000–10,000) and stored on a personal computer after analog-digital conversion (Digidata 1322A; Axon Instruments, Foster City, CA). To quantify cardiac responses, time between adjacent R waves of the ECG (R-R interval) and latency between atrial and ventricular R waves within individual cardiac cycles (atrioventricular delay) were processed using Axoscope software (Axon Instruments). To control for the possibility of electrotonic spread of current from the site of the nerve stimulating electrodes to active cardiac tissue, in some preparations the electrodes were placed on the duct wall away from the nerve (n = 6) or moved off the nerve into the bath (n = 6); repeated stimulation with the same parameters had no effect on heart rate.
Isolated ventricular pacemaker recordings.
To investigate the function of putative pacemaker cells at the AVR, ECG signals were recorded first from intact hearts (n = 6), followed by isolation of the ventricle along with a small margin of atrial tissue proximal to the atrioventricular valve (<500 μm from atrioventricular valves). Subsequent ECG recordings were made from the dissociated ventricular and atrial tissues (as per Whole heart ECG recordings and VSN stimulation). We were unable to access the intracardiac nerves in the walls of the isolated ventricle to study the direct effects of nerve stimulation on ventricular rate, so we used cholinergic and adrenergic agents to evaluate the potential for neural control in this tissue (see Pharmacological agents).
BCT stimulation.
To evaluate the effects of stimulating the BCT, a bipolar electrode was placed over the nerve as it coursed along the ventral aorta between the origins of the afferent arteries supplying the fourth gill arches and the bulbus arteriosus. Perfusion and stimulation parameters were similar to those for vagosympathetic trunk stimulation. To control for electrotonic current spread, the stimulating electrode was moved away from the nerve and placed on the ventral aorta between the afferent vessels supplying the third and fourth gill arches; repeated stimulation in this region had no effect on heart rate (n = 3).
Pharmacological agents.
Nicotine (agonist of excitatory nicotinic receptors on the membrane of cholinergic intracardiac neurons), muscarine (agonist of postjunctional M2Rs on cardiac effector cells), and isoproterenol (agonist of postjunctional β-ARs on cardiac effector cells) were dissolved in saline (all 1 mM; Sigma Aldrich) on the day of the experiment. Agonists were delivered to the bath directly above the tissue in 200-μl boluses through a micropipette via a calibrated syringe attached to a screw-drive microinjector (IM-4B; Narishige, East Meadow, NY). As a control, 200-μl boluses of saline were delivered in the same manner and did not elicit chronotropic responses. Hexamethonium (a nondepolarizing nicotinic receptor blocker of nictonic acetylcholine receptors on parasympathetic and sympathetic preganglionic neurons) and atropine (postjunctional M2R blocker; 10 μM each; Sigma Aldrich) and timolol (postjunctional β-AR blocker; 100 μM; Sigma Aldrich) were dissolved in saline and perfused continuously starting 15 min before recording.
Voltage optical mapping.
Voltage changes in isolated hearts (n = 6) or AVR-ventricular tissue (n = 5) were optically mapped to determine the normal time course and spread of cardiac excitation from the pacemaker initiation site, to assess whether shifts in the point of initiation occurred with vagal nerve stimulation, and to establish the characteristics of AVR-initiated ventricular activity. Tissues were isolated and pinned to the Sylgard rubber bottom of a 3-ml chamber filled with perfusate and allowed to equilibrate for 30 min (aerated with room air; 25°C; as per Whole heart ECG recordings and VSN stimulation). During this time, the heart was exposed to a voltage-sensitive dye (10 μM di-4-ANBDQPQ; Ref. 38; University of Connecticut Health Center, Farmington, CT) in the perfusate for 10 min, and then washed with fresh saline containing an excitation-contraction uncoupler [10 μM (±)-blebbistatin; Cayman Chemical, Ann Arbor, MI] to eliminate motion artifacts during optical recordings. Blebbistatin was present for the duration of the experiment. In a subset of hearts (n = 3), simultaneous stimulation of both cardiac vagal nerve rami (15 Hz; described above) was performed to test for changes in the pacemaker initiation site.
Preparations were epi-illuminated through a compound microscope with a red light-emitting diode (CBT-90-R; Luminus Devices, Billerica, MA) directed through a 640 ± 10 nm band-pass filter (D640/20X; Chroma Technology, Bellows Falls, VT) and reflected by a 660-nm dichroic mirror (FF660; Semrock, Rochester, NY) on to the preparation. Emitted fluorescence was collected with a ×5, 0.15 NA objective (Plan; Zeiss Canada, Mississauga, ON, Canada) or a ×10, 0.25 NA objective (EA10; Olympus Canada, Richmond Hill, ON, Canada) through a 700-nm long-pass filter (HQ700LP, Chroma) and captured by a 128 × 128 pixel, 16-bit electron-multiplying charge-coupled device camera (Cascade 128+; Photometrics, Tucson, AZ) at 511 frames/s. Previous investigations of the penetrance of 640 ± 20 nm light into myocardium has shown 50% intensity at a depth of 1 mm (T. A. Quinn, unpublished observations). At a depth of 200–400 μm, which is the thickness of the zebrafish tissue preparations used in this study, light intensity is ∼70–80%, resulting in excitation and collection of voltage signals from the entire transmural thickness of the heart.
The camera was controlled and signals were acquired using MultiRecorder software (S. Luther and J. Schröder-Schetelig, Max Planck Institute for Dynamics and Self-Organization, Göttingen, Germany; http://www.bmp.ds.mpg.de/multirecorder.html). As all recordings contained some degree of readout noise, pretreatment activation patterns were mapped from signal-averaged values taken from 10 consecutive beats immediately before introduction of the stimuli. Isochronal activation maps were generated using an activation threshold of 60% of action potential amplitude. Atrioventricular delay was estimated as the time from arrival of the excitation wavefront at the atrioventricular junction to the first occurrence of depolarization in ventricular tissue. All optical data were analyzed with custom routines written in Matlab (The MathWorks, Natick, MA).
Pacemaker cell immunohistochemistry.
Hearts (n = 16) were fixed in a modified periodate-lysine-paraformaldehyde fixative (1.3 mM paraformaldehyde, 250 μM l-lysine and10 mM sodium metaperiodate) (39) in phosphate-buffered saline (PBS). Whole hearts were labeled with antibodies against HCN4 (1:50; APC-052; Alomone Laboratories, Jerusalem, Israel) (60) and the transcription factor, Islet-1 (Isl1; 1:100; Developmental Studies Hybridoma Bank, Iowa City, IA) to detect putative pacemaker cells as previously described (60). To reveal the presence of autonomic receptors associated with these cells, we used antibodies against M2R (1:100; M9558; Sigma-Aldrich) and β2-AR (1:50; sc569 clone H20; Santa Cruz Biotechnology, Dallas, TX) (2) and tyrosine hydroxylase (TH; 1:100; 22941; Immunostar, Hudson, WI). To image β2-AR immunoreactivity, a biotin-avidin fluorophore amplification process modified from Ampatzis and Dermon (2) was used. Whole-mount tissue samples were first processed with the β2-AR primary antibody as previously described (57) and then incubated in a biotinylated anti-rabbit antibody (1:100; Vector Laboratories, Burlington, ON, Canada) for 2 days at 4°C. Tissues were then rinsed in PBS, incubated with fluorophore-conjugated streptavidin (Rhodamine B; S871; Life Technologies), and processed for either anti-TH or anti-β2-AR antibodies raised in mouse. After exposure to these primary antibodies, preparations were incubated with appropriate secondary antibodies conjugated to AlexaFluor 488, 555, or 647 fluorophores (Life Technologies, Burlington, ON, Canada), washed in PBS, and placed in Scale CUBIC-1 clearing solution (58) overnight at room temperature with gentle agitation. Processed specimens were examined as whole mounts using an LSM 510 or LSM 710 confocal microscope (Zeiss). Confocal image stacks were captured using Zeiss Zen2009 software. Omission controls for the antibody against M2R were run as previously described for the HCN4 and Isl11 antibodies (57, 60). Omission of either primary or secondary antibodies from the incubation protocol resulted in absence of immunoreactivity. Omission controls for the antibody against β2-AR were performed by omitting 1) the β2-AR primary antibody, 2) the biotinylated secondary, and 3) the rhodamine-conjugated streptavidin, or both 2 and 3. In all cases omission of antibody or fluorophore-conjugated streptavidin resulted in an absence of immunoreactivity.
The relative distribution of β2-AR and M2R were estimated from digital images of the atrium and ventricle of six specimens. In images from each specimen, five regions were selected at random from both the atrium and ventricle; these regions measured 10 × 10 μm (100 μm2 each, 500 μm2 total area per chamber). To estimate relative receptor density, the number of immunoreactive punctata, which represent clusters of postsynaptic receptors, was counted using ImageJ software (50).
Statistical analyses.
All data are presented as means ± SE. Data were analyzed in SPSS (IBM Analytics, Armonk, NY) using paired Student's t-test, one-way ANOVA with Tukey's post hoc analysis, or regression analysis. P values ≤ 0.05 were considered to indicate significant differences between means.
RESULTS
Viability of heart function in vitro.
In preliminary trials, mean initial heart rate (HR) in the bath was 99 ± 2 beats/min (range 76–129; n = 8) and varied by <10% in all specimens over a 6-h period. VSN stimulations performed periodically throughout these trials gave chronotropic responses similar to those measured immediately after isolation, indicating retained functionality. Neither heart area (9.6 ± 0.4 mm2; range 7–12) nor mass (6.3 ± 0.5 mg; range 5–8) was significantly correlated with rate (r < 0.05).
Electrical stimulation of cardiac rami of vagosympathetic trunks.
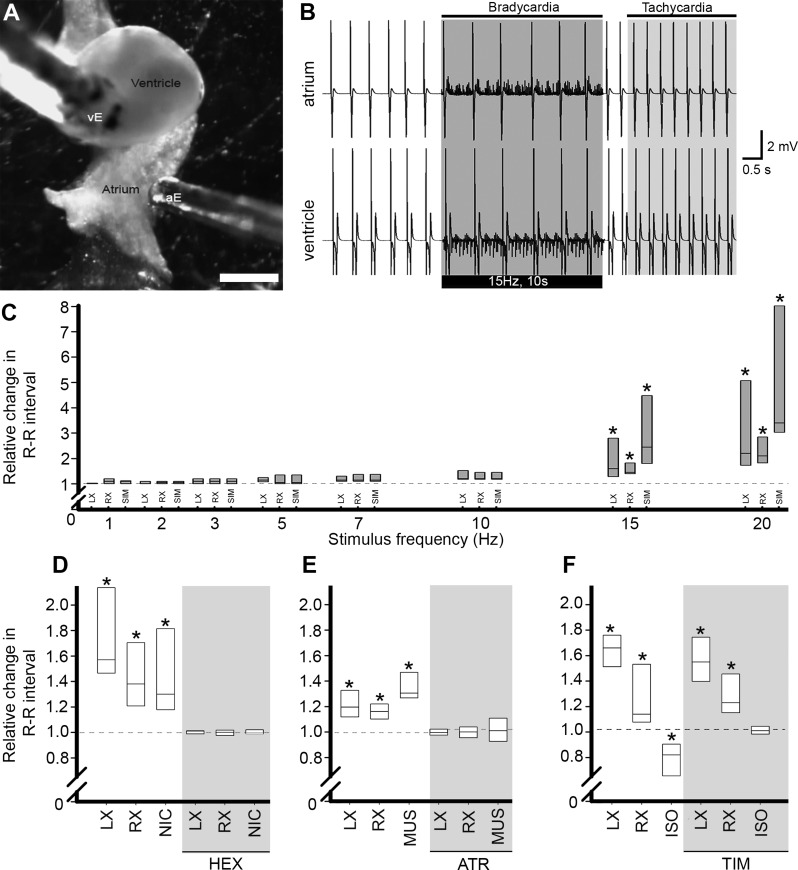
In all preparations, VSN stimulation at a frequency ≥ 15 Hz significantly prolonged the R-R interval (bradycardia), starting with the beat immediately after the initiation of the stimulus and lasting for the duration of the stimulus train (Fig. 1). At 15 Hz, stimulation of either the right or left cardiac VSN evoked an R-R interval increase of 1.7 ± 0.2 or 1.9 ± 0.3 times the prestimulus value, respectively. At a stimulation frequency of 20 Hz, the evoked bradycardia was highly variable, most strikingly resulting in the cessation of beating for the duration of the stimulus period in some hearts (in these cases, the R-R interval was taken as the period of stimulation). Given that 15-Hz stimulation caused a significant change in R-R interval without cardiac standstill in any specimen, this frequency was used to quantify effects of cardiac VSN activation in subsequent experiments (except for voltage optical mapping experiments). It was found that in all experiments, atrioventricular delay was unaltered by VSN stimulation at this level. In 15 of the 24 hearts tested, simultaneous VSN stimulation resulted in a decrease in R-R interval (tachycardia) following cessation of stimulation. This effect had a variable poststimulus latency to onset (5.1 ± 0.8 s) and duration (11.0 ± 1.8 s), after which the R-R interval returned to the prestimulus control value (Fig. 1B).
Fig. 1.
Chronotropic responses to vagosympathetic nerve stimulation and autonomic agents. A: in vitro view of the heart showing positions of bipolar electrodes recording atrial (aE) and ventricular (vE) surface electrocardiograms (ECG). Scale bar = 1 mm. B: typical ECG record from atrium (top) and ventricle (bottom) in the same heart before, during and after simultaneous stimulation of cardiac vagosympathetic nerve trunks (VSN; bar below trace). Elongation of R-R interval (bradycardia) during the stimulus period was followed by post-stimulus reduction of R-R interval (tachycardia) relative to prestimulus interval. C: proportional change in R-R interval in response to stimulation of the left (LX) or right (RX) VSN, or simultaneous stimulation of both nerves (SIM). *P < 0.05 vs. control by one-way ANOVA with Tukey's post hoc test; n = 8 for each. Dashed lines represent preintervention R-R interval. D–F: effects of cholinergic and adrenergic agents on R-R interval. D: R-R interval increased in response to LX and RX VSN stimulation and local application of nicotine (NIC). Effects of VSN stimulation and NIC application were eliminated during exposure to hexamethonium (HEX, grey shading). E: response to VSN stimulation and local application of muscarine (MUS) were eliminated by atropine (ATR, grey shading). F: isoproterenol (ISO) decreased R-R interval. Exposure to timolol (TIM, grey shading) blocked ISO response without affecting VSN-induced responses. *P < 0.05 vs. control by one-way ANOVA with Tukey's post hoc test; n = 8 for each of LX/RX/SIM and n = 6 for each of NIC/MUS/ISO.
Effects of cholinergic and adrenergic agents on R-R interval.
Application of nicotine (n = 6) significantly prolonged R-R interval to 1.5 ± 0.1 times the control value (NIC; Fig. 1D), a response qualitatively similar to that evoked by single VSN stimulation at 15 Hz. Both the nicotinic effect and that of VSN stimulation were eliminated by addition of hexamethonium (HEX; Fig. 1D, shaded). Application of muscarine (n = 6) also caused a significant increase in R-R interval to 1.4 ± 0.1 times control (MUS; Fig. 1E). Neither nicotine nor muscarine alone evoked a biphasic response (data not shown), as was observed with VSN stimulation. Perfusion of atropine blocked effects of both muscarine and VSN stimulation (ATR; Fig. 1E, shaded).
Application of isoproterenol (n = 6) caused a significant decrease in R-R interval to 0.8 ± 0.1 times the preapplication value (ISO; Fig. 1F). This response was blocked by timolol, which had no effect on the initial increase in R-R interval with VSN stimulation but did block the poststimulation decrease in R-R interval (TIM; Fig. 1F, shaded). It was found that in all experiments, atrioventricular delay was unaltered by autonomic agents.
Autonomic effects on AVR activity.
Atrioventricular delay (Fig. 2A; 52.0 ± 1.8 ms) was highly correlated with prestimulus HR (r = 0.98) but was unchanged from pretrial values during VSN stimulation or the application of cholinergic or adrenergic agonists (data not shown).
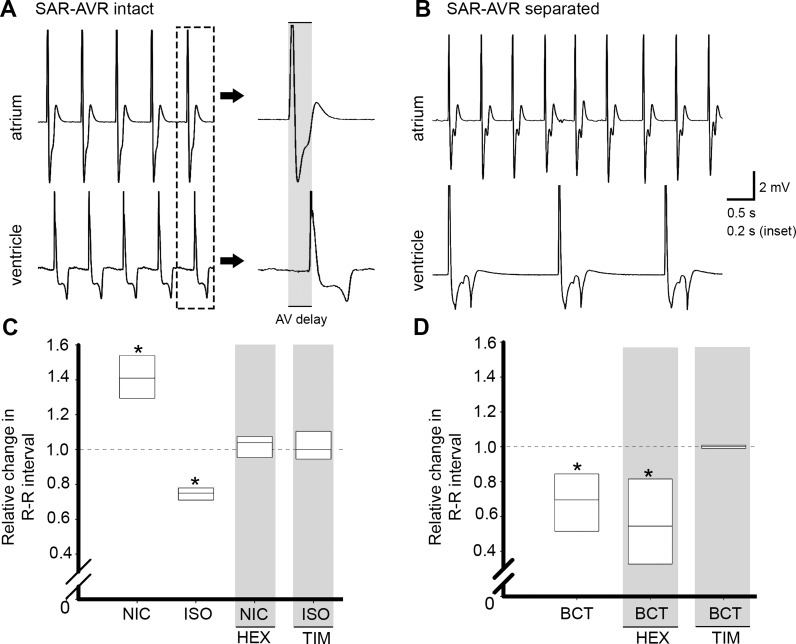
Fig. 2.
Atrioventricular dissociation, modulation of AVR activity, and stimulation of branchiocardiac nerve trunk. A: sample atrial (top trace) and ventricular (bottom trace) ECG traces in intact heart. Inset: atrioventricular delay in one cardiac cycle at a faster recording speed. B: surgical dissociation of AVR from the SAR caused desynchronization of the chambers and a slowed ventricular rate. C: nicotine (NIC) increased the ventricular R-R interval; this response was blocked by hexamethonium (HEX, grey shading). Isoproterenol (ISO) caused the ventricular R-R interval to decrease; this effect was blocked by timolol (TIM, grey shading). D: electrical stimulation of the branchiocardiac nerve trunk (BCT) in the intact heart reduced R-R interval. This response was eliminated by timolol (TIM, grey shading) but not hexamethonium (HEX, grey shading). *P < 0.05 vs. control by paired Student's t-test; n = 6 for each group.
In the whole heart, depolarization of the ventricle was driven by depolarization of the atrium (Fig. 2A). Following isolation of the ventricle from the atrium, the atrial rate was unchanged, but the mean rate of ventricular contraction fell to 0.3 ± 0.1 times the atrial rate (1 ventricular beat to ∼3–4 atrial beats; Fig. 2B). Application of NIC to the isolated ventricle significantly increased R-R interval compared with the prenicotine interval (Fig. 2C), an effect that was blocked by HEX (Fig. 2C, shaded). Isoproterenol exposure evoked a significant decrease in the ventricular R-R interval, an effect that was blocked by TIM (Fig. 2C).
Effect of BCT stimulation.
Electrical stimulation of the BCT in the whole heart resulted in a significant decrease in R-R interval to 0.7 ± 0.1 times control (Fig. 2D). Perfusion of TIM during BCT stimulation eliminated this response, while perfusion of HEX had no effect (Fig. 2D, shaded). Stimulation of the BCT had no effect on the R-R interval in the isolated ventricle.
Imaging of the origin and spread of cardiac excitation.
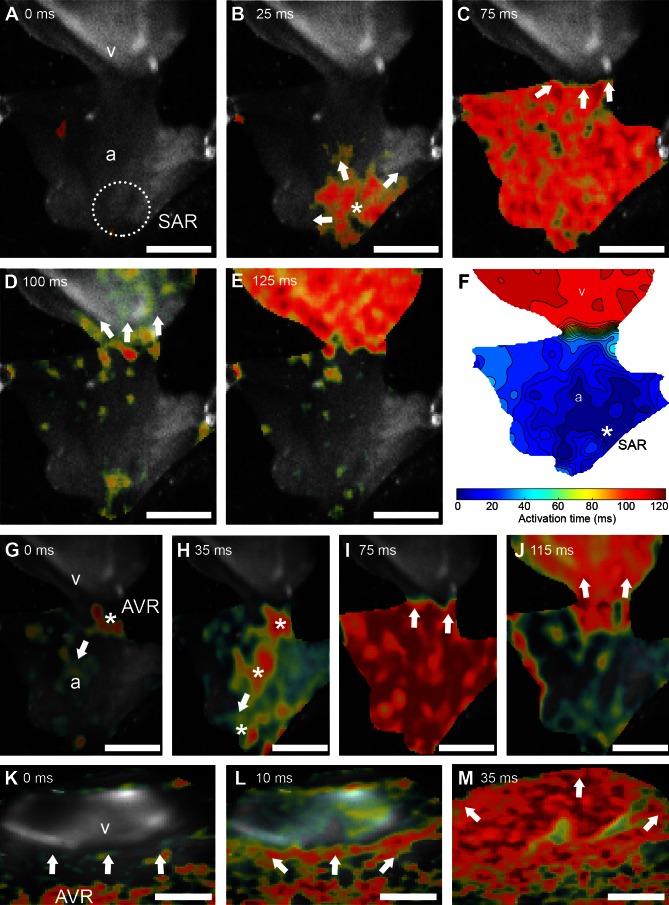
In all hearts sampled (n = 6), the initial site of depolarization of each heart beat was within the SAR (Fig. 3B, asterisk; and Supplemental Movie S1; Supplemental Material for this article is available online at the Journal website). The wave of activation propagated through the atrial wall in a broad sweep toward the AVR. Mean time to full depolarization of the atrium was 59 ± 5 ms (n = 6) while mean atrioventricular delay was 55 ± 2 ms. The atrium began to repolarize after this delay (mean atrial action potential duration was 138 ± 10 ms), while the ventricle was still depolarizing. Complete depolarization of the ventricle took 80 ± 8 ms from the invasion of excitation. Mean ventricular action potential duration was 188 ± 7 ms. Mean total duration from initial depolarization of the atrium to full repolarization of the ventricle was 355 ± 4 ms, followed by a delay of 256 ± 8 ms before the next cycle.
Fig. 3.
Optical mapping of origin and spread of excitation in atrium and ventricle. A–E: sequence of images showing progression of depolarization from the region of origin in the SAR (* in A) into the atrial wall (a in B and C) across the atrioventricular junction (D) and into the ventricle (v in E). Arrows indicate the direction of propagation of the main excitation wavefront. F: isochronal map (isochrones = 8 ms) summarizing the regional spread of electrical activity from the site of origin (*) in the SAR, across the atrium, and into the ventricle. Color scale represents time to activation from initial excitation. G–J: sequence of images showing progression of depolarization during simultaneous stimulation of both cardiac VSN rami, demonstrating a shift in the site of origin toward the AVR. Arrows indicate direction of propagation. The site of first depolarization occurred at the AVR (* in G), followed by multiple foci outside of the SAR (* in H) which depolarized the atrium (I). After a delay at the AVR (I), the depolarizing activity invaded the ventricle (J). K–M: sequence of images showing progression of depolarization from the AVR into the ventricle after surgical isolation from the atrium (K). The main excitation wavefront initiated in the AVR (L) and propagated through the ventricle (M). Scale bars = 1 mm (A–J) and 50 μm (K–M).
Simultaneous VSN stimulation of both cardiac rami at 20 Hz (n = 5) induced a change in the primary initiation site of electrical activity from the SAR to the AVR (Fig. 3, G–J, and Supplemental Movie S2). In this case, the earliest depolarization occurred in the AVR, along with multiple foci outside of the SAR, which passed in a retrograde direction through the atrium to the SAR, depolarizing the atrium. Following depolarization of the atrium, delay of the depolarizing wave front at the AVR (74 ± 5 ms) was significantly increased compared with pretreatment values, before exciting the ventricle. In these hearts, time from initial atrial depolarization to completion of ventricular repolarization (396 ± 3 ms) was also significantly increased compared with pretreatment values.
Following surgical isolation of the AVR and ventricle from the SAR and atrium, the site of origin of electrical activity was in the AVR (Fig. 3, K–M). Propagation of the AVR-initiated depolarizing wave through the ventricle took significantly longer (97 ± 5 ms) than the ventricular propagation time of a SAR-initiated depolarizing wave in the whole heart.
Anatomical identification of putative pacemaker cells in the SAR and AVR.
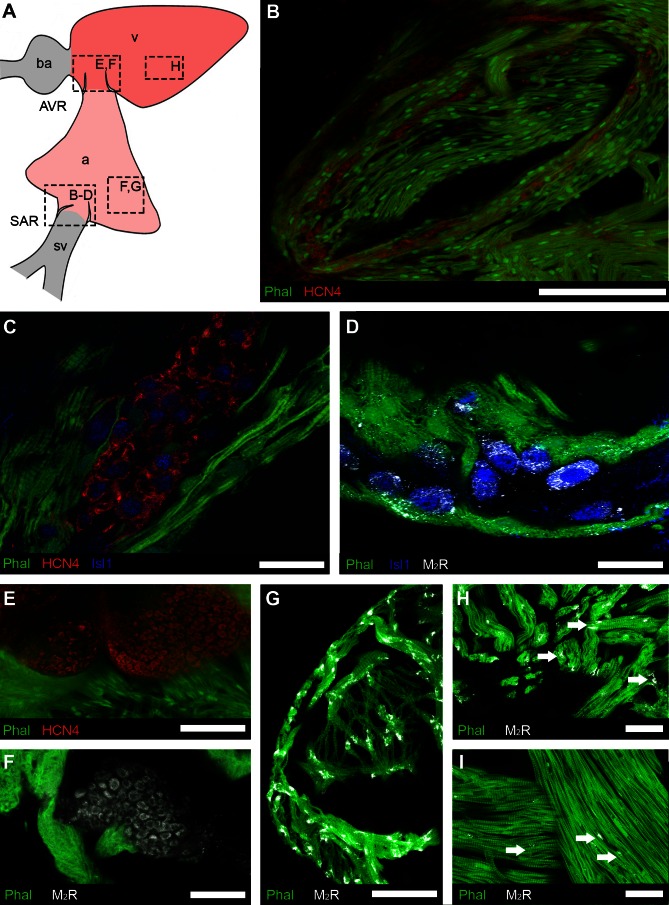
HCN4- and Isl1-immunoreactivity (IR) was used to distinguish putative pacemaker cells from the surrounding cardiomyocytes (n = 12). In the SAR (Fig. 4A), cells expressing HCN4-IR formed multiple clusters that congregated toward the basal portions of the sinoatrial valves (Fig. 4B), corresponding with the region of initial cardiac cycle excitation (Fig. 3). Within a cluster, the HCN4-positive cells appeared to be contiguous, displacing most of the local myocytes to the cluster margin (Fig. 4C).
Fig. 4.
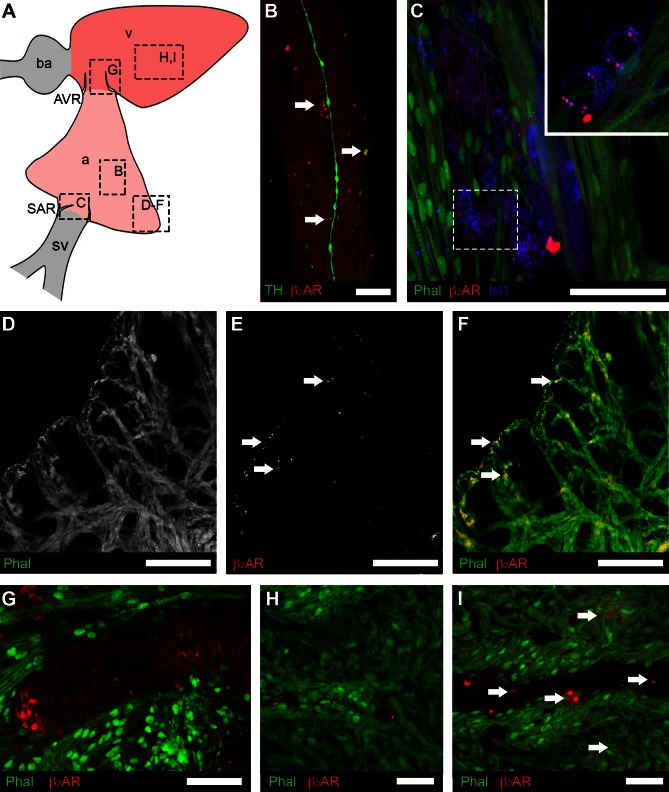
Regional distribution of putative pacemaker cells and muscarinic receptors detected by immunohistochemistry. A: schematic showing the cardiac regions: atrium, a; atrioventricular region, AVR; bulbus arteriosus, ba; sinoatrial region, SAR; sinus venosus, sv; ventricle, v. Boxes indicate the locations of images in B–H. B–D: organization of putative pacemakers and associated muscarinic receptors in SAR. B: low-magnification overview shows HCN4-immunoreactive (-IR) cells (red) embedded in musculature (Phal, green) surrounding the sinoatrial valves. C: islet-1 (Isl1, blue) co-localized with cells expressing HCN4. D: type 2 muscarinic receptors (M2R) appeared to be associated with the membrane of Isl1-IR cells. E: putative pacemaker cells expressing HCN4 were present in AVR, embedded in musculature (Phal) of atrioventricular valves. F: type 2 muscarinic receptors (M2R) associated with the membrane of cells of similar morphology to HCN4-IR cells in the AVR. G–I: M2R were more plentiful in the compact and trabecular myocardium (Phal) of the atrium (G and H; arrows) than in the ventricular trabecular myocardium (I; arrows). Scale bars = 200 μm (B), 40 μm (C), 20 μm (D), and 50 μm (E–I).
The population of Isl1-positive cells in the SAR completely overlapped the population of HCN4-IR cells (Fig. 4C). Cells expressing both markers had spindle-shaped or oval cell bodies, with relatively large oval nuclei. Isl1-IR was more prominent in the cytoplasm, while HCN4-IR appeared to be localized to either the cytoplasm or the cell membrane. Phalloidin labeling was restricted to cardiac myocytes, which predominantly lacked the HCN4 label.
As the antibodies against HCN4, M2R, and β2-AR were generated in the same host species, it was not possible to investigate coexpression of these markers. Instead we used the Isl1 antibody to identify putative pacemaker cells to determine the colocalization patterns of these receptors. In the SAR, all Isl1-IR cells displayed punctate M2R-IR, which was more heavily concentrated on parts of the cell distal to the nucleus (Fig. 4D). M2R-positive punctata were also present on myocytes adjacent to Isl1-IR cells (Fig. 4D) but were sparser than the M2R-IR associated with putative pacemaker cells.
In the AVR, a compact group of HCN4-positive cells was located in tissue of the atrioventricular valves (Fig. 4E). In contrast to HCN4-positive cells in the SAR, cells in the AVR were smaller, were polygonal in shape, and did not coexpress Isl1-IR. Thus, we could not use the combination of Isl1- and M2R-IR to determine whether these receptors were associated with putative pacemaker cells in the AVR (as performed for the SAR). We found that combining phalloidin labeling of local myocytes with M2R-IR demonstrated that these receptors were present on cell-like structures that were phalloidin-negative (Fig. 4F) and in the same location as cells labeled with HCN4 in other specimens (Fig. 4E).
M2R-IR was also associated with the myocardium of both the atrium and ventricle (Fig. 4, G–I). In a survey of the relative density of receptors in these regions, the atrial myocardium had a significantly higher density (5 ± 1 M2R-IR clusters per 100 μm2) than did the ventricular myocardium (2 ± 1 clusters per 100 μm2; P < 0.01).
In the SAR, all Isl1-positive cells displayed punctate β2-AR-IR, distributed evenly over the cell (Fig. 5C). β2-AR-positive punctata were also associated with myocytes proximal to Isl1-IR cells (Fig. 5, D–F), but myocyte-associated β2-AR-IR was sparser than that associated with putative pacemaker cells.
Fig. 5.
Regional distribution of β2-adrenergic receptors (β2-AR) associated with putative pacemaker cells and myocardium. A: schematic showing the cardiac regions; boxes indicate the locations of images in B–I. B: β2-AR (red) were detected adjacent to TH-positive innervation (green) in the atrial wall. C: in the sinoatrial region Isl1 (blue) was colocalized with cells expressing β2-AR. Inset shows a single confocal section from boxed region at a higher magnification. D–F: β2-AR-IR was detected in the compact and trabecular myocardium (Phal) of the atrium (E and F; arrows). G: β2-AR-IR associated with the membrane of cells of similar morphology to HCN4-IR cells in the AVR. H and I: β2-AR-IR was more plentiful in the compact myocardium (Phal) of the ventricle than in the atrium, especially proximate to coronary vasculature (I). Scale bars represent 10 μm (B), 40 μm (C), 20 μm (D–F), and 20 μm (G–I).
In the AVR, β2-ARs were present on cell-like structures that were phalloidin-negative (Fig. 4F) and in the same location as cells labeled with HCN4 in other specimens (Fig. 4E). β2-AR-IR was also associated with the myocardium of both the atrium and ventricle (Fig. 4, G–I). In a survey of the relative density of receptors in these regions, the ventricular myocardium had a significantly higher density of β2-AR-IR (8 ± 2 β2-AR-IR clusters per 100 μm2), than did the atrial myocardium (2 ± 1 clusters per 100 μm2; P < 0.01).
DISCUSSION
In this study we have established the viability of the isolated zebrafish heart as a novel model for studies of autonomic control of chronotropy. Reduced in vitro preparations of mammalian hearts including a functional pacemaker have previously been used for similar studies. However, autonomic elements in these preparations generally have compromised connectivity, thus limiting the capability for full identification of neural mechanisms involved in such functions as pacemaker control. Although the small size of the zebrafish heart presents its own technical challenges, its size also permits visualization and accessibility of the entire ICNS in the whole organ, which has not previously been possible in mammalian models.
Receptor-mediated mechanisms of chronotropic modulation.
In the isolated zebrafish heart, the general function of autonomic control appears to be similar to that observed in the mammalian heart. The cholinergic agents NIC and MUS had parasympathomimetic effects, evoking an increase in R-R interval (the same general chronotropic effect as VSN stimulation). NIC, presumably mimicking the effect of ACh released from vagal terminals, which have previously been shown to contain choline acetyltransferase (the synthetic enzyme of this transmitter) (57), likely activated intracardiac neurons that projected to pacemaker cells. Exposure to the nicotinic receptor antagonist HEX eliminated the responses to both VSN stimulation and NIC, confirming the role of nicotinic cholinergic neurotransmission within the ICNS in VSN-induced bradycardia. MUS acts at cholinergic receptors on pacemaker cells, prolonging the duration of diastolic depolarization and slowing discharge rate (19). Exposure to the muscarinic antagonist ATR eliminated the bradycardia in response to VSN stimulation and MUS, providing functional confirmation that muscarinic receptors play a role in neurogenic bradycardia in the zebrafish heart. The β-adrenergic agent ISO, on the other hand, had an overall sympathomimetic effect, decreasing the R-R interval, while the β-adrenergic antagonist TIM blocked both the poststimulus tachycardia and the response to ISO, suggesting a robust role for adrenergic control of pacemaker discharge rate.
In the present study, the R-R interval response to combined VSN stimulation was summative, supporting the idea that each nerve activated different groups of postganglionic “rate-control” neurons with minimal interactions within the ICNS. This provides functional support both for our previous anatomical evidence in zebrafish (57) and for the anatomical and functional evidence in the mammalian heart that axons of the extrinsic nerves likely target different subpopulations of cardio-inhibitory intracardiac neurons with parallel chronotropic functions (1, 3, 8, 9, 16, 32).
In approximately two-thirds of the hearts tested in this study, VSN stimulation at an intensity that evoked significant bradycardia also elicited a variable poststimulus tachycardia. A biphasic response to vagal stimulation has also been reported in the mammalian heart (53). The presence of sympathetic postganglionic axons in cardiac vagal rami is conserved from zebrafish to mammals (42), and coactivation of these axons, along with vagal preganglionic parasympathetic axons, during VSN stimulation is the most likely explanation for the biphasic response. In the mammalian heart there is partial or complete persistence of the tachycardic portion of the response to vagal stimulation in the presence of β-adrenergic antagonists, which has been proposed to result from corelease of cardio-acceleratory peptides (23, 53). As this response was entirely eliminated by β-blockade in our study, peptide release seems an unlikely cause for the poststimulus tachycardia we observed in the zebrafish heart.
Properties and neural control of pacemaker loci.
In the present study, the origin of myocyte electrical activity was located within the SAR, in agreement with previous functional (24, 34, 46, 49, 51, 60, 61) and anatomical (57, 60) data. The mean time for propagation of atrial excitation, as well as the atrioventricular delay, corresponded with the delay calculated from ECG-derived data and with values previously reported in other optical imaging studies of the zebrafish heart (34, 35). The observed location of the initiation and spread of excitation thus conformed with the recognized pattern of normal heart activation in this species, and established a functional baseline for mapping the effects of VSN stimulation.
In the mammalian heart, it has been suggested that vagal input not only causes bradycardia but also influences the operational pacemaker site. Previous work has shown that vagal drive can result in a shift of the pacemaker site within the sinoatrial node and into the atrial wall and that under certain conditions the atrioventricular junction can become the dominant pacemaker site (10, 11, 26, 52). In the zebrafish heart, strong simultaneous VSN stimulation (20 Hz) both induced bradycardia and altered the pattern of initiation and propagation of electrical activity, in a similar manner to that observed in the mammalian heart. In our experiments, the site of first depolarization occurred at the AVR, followed by multiple foci outside of the SAR, which depolarized the rest of the atrium. Following atrial depolarization, atrioventricular delay was significantly increased compared with prestimulus values, before the ventricle was excited. In mammalian hearts, where epicardial light penetration is a limiting factor for complete transmural imaging, the phenomenon of reentry or transmural breakthrough could offer an explanation for such a shift. As we were collecting signals from the entire thickness of the tissue in our experiments, however, reentry or transmural breakthrough would have been visualized in our recordings, so this was not likely a factor in the present study. Thus, in both zebrafish and mammalian hearts, when vagal tone is elevated and bradycardia occurs, there may be an accompanying shift of the primary pacemaker locus from the SAR to a set of pacemaker cells with a lower intrinsic rate near the sinoatrial node or at the atrioventricular junction. This is the first report of a neurally mediated shift in pacemaker locus in the zebrafish heart and indicates that this powerful system for cardiac control is conserved from fish to mammals.
In the mammalian heart, atrioventricular delay is highly sensitive to autonomic modulation. Yet in the current study it was found that atrioventricular delay was unaltered by VSN stimulation at 15 Hz or by autonomic agents. While the lack of autonomic modulation of conduction may be a zebrafish-specific phenomenon, previous work has shown a high variability in atrioventricular delay in the zebrafish (34). It could therefore be possible that such variability masks any subtle changes in atrioventricular delay occurring during autonomic modulation, warranting future investigation.
In mammals, the existence of a primary pacemaker near the sinoatrial junction and one or more subsidiary pacemakers within the atrial wall is well established (28, 37). In our study the pacemaker at the AVR was also capable of functioning independently after isolation from the atrium to drive spontaneous ventricular excitation at a rate approximately one-third that of the atrial pacemaker. Optical mapping in the isolated ventricle showed that the initial depolarization occurred in the AVR close to the site of HCN4-labeled cells and that the activation waveform spread into the ventricular myocardium, establishing the presence of a second major pacemaker locus in the AVR of the zebrafish heart.
The discharge rate of the mammalian atrioventricular pacemaker is modulated by the ANS in a manner similar to autonomic control of the sinoatrial pacemaker (22, 52). In previous work, we have shown that the zebrafish AVR is innervated by extrinsic axons from the VSN, as well as by a population of intracardiac neurons proximal to the atrioventricular valves (57). In the isolated zebrafish ventricle, the general function of the atrioventricular pacemaker and its autonomic control appeared to be similar to that of the atrioventricular node in mammalian hearts. NIC had a parasympathomimetic effect, evoking an increase in R-R interval, while ISO had a sympathomimetic effect, decreasing the interval.
Influence of the BCT on heart rate.
In mammals, cardiac output and lung ventilation can be highly correlated to maximize oxygen uptake (59). In many fish there is also a close coordination between cardiac output and gill ventilation frequency, partly driven by oxygen chemoreceptors located in the gills (29, 54), which send afferent inputs to branchio-cardiac reflexes through the central nervous system. In the zebrafish heart, the BCT courses along the wall of the ventral aorta and bulbus arteriosus to enter the wall of the ventricle (57). Although its ultimate origin and destinations have not been traced (57), it is possible that the BCT may represent a cardiorespiratory pathway that modulates or augments the operation of centrally mediated branchio-cardiac reflexes. When the BCT was electrically stimulated in the whole heart, the net result was an increase in HR. That this response was eliminated in the presence of TIM, but was unaffected by HEX, indicates that these responses likely represent a direct effect of release of adrenergic neurotransmitters from axon terminals at the pacemaker cells. These results bear out speculation (57) that this nerve may represent an alternative input pathway for sympathetic chronotropic control in the zebrafish.
Anatomical relationship of M2Rs and ARs with putative pacemaker cells.
The role of M2Rs in inhibiting cardiac pacemaker discharge is ubiquitous in vertebrates (15). In the atria of various mammalian species, including humans, it has been reported that M2R represent the predominant subtype (15, 27, 45). In these species, the density of parasympathetic innervation is greatest in the SAR and AVR, with receptors mediating negative chronotropic effects by altering pacemaker cell depolarization, potentially through direct inhibition of HCN4 channels (19).
In the present study, considering that the discharge rates of both the SAR and AVR pacemakers were presumably reduced by ACh released from nerve terminals during VST stimulation and by MUS application and that these responses were blocked by ATR, we sought to determine the relationship of M2R with putative pacemaker cells in the zebrafish heart. In the SAR, there was a high degree of M2R expression associated with the membrane of Isl1-IR cells. In the AVR, the M2R antibody labeled cells were in the same region and had the same apparent morphology as those expressing HCN4. In the atrial myocardium, M2R label was also associated with myocytes, although label intensity was less than that on the putative pacemaker cells.
β2-ARs are G-protein-coupled receptors that have been shown to mediate positive inotropy and chronotropy in the mammalian heart (2, 14, 40). In the cat and guinea pig, it was shown that although β1-ARs were the dominant receptor subtype, β2-ARs comprised ∼25% of the β-ARs in the atria (13, 40). Given higher densities near the SAR in that study, it was postulated that β2-ARs might exert a stronger influence on chronotropy than inotropy (13, 25, 40). Previous work in zebrafish has shown that β-ARs are expressed in the zebrafish heart and exert positive chronotropic effects, although their distribution was not described (56).
In the present study, NE released from nerve terminals and application of ISO acted to increase the discharge rates of both the SAR and AVR pacemaker. As both of these responses were blocked by TIM, we also investigated the relationship of β2-ARs with putative pacemaker cells in the zebrafish heart. Within the SAR, Isl1-IR cells had β2-AR-IR in apparent association with the cell membrane. In the AVR, there was a population of cells in a cluster associated with the bases of the atrioventricular valves that expressed β2-AR-IR in the same location as HCN4- and M2R-positive, phalloidin-negative cells. In the atrial myocardium, β2-AR-IR was associated with myocytes, although immunoreactivity was sparser than on the putative pacemaker cells. It should be noted that although the adrenergic agents used in this study act across the spectrum of β-AR subtypes and that β2-ARs represent only a portion of ARs, to our knowledge there are no well-characterized antibodies for other subtypes of β-AR for use in zebrafish. Therefore, further investigations of the roles of other β-AR subtypes in this system should be undertaken, but confirmation of the anatomical distribution of these receptors must await the availability of suitable antibodies.
In mammalian literature, there is considerable debate concerning the roles of the “membrane clock” (driven by the funny current, which is mediated by HCN channels) and the “calcium clock” (driven by spontaneous calcium release from the sarcoplasmic reticulum activating the sodium-calcium exchanger) in the generation and regulation of HR (30, 31). Previous studies have shown the involvement of both calcium clock (36) and membrane clock mechanisms (35, 62) in determining HR in the zebrafish. In the slo mo zebrafish mutant, which has a defect in the HCN4 channel, a significant decrease in HR was observed (62), which is similar to that observed with HCN4 channel antagonists (34). Although the expression of HCN4 channels in the cell membrane and the transcription factor Isl1 in the cytoplasm have been previously used as anatomical markers for pacemaker cells (33, 60, 63), complete identification of this cell population in the zebrafish heart awaits direct electrophysiological evidence. Given that the population of HCN4-positive cells labeled in the zebrafish heart appeared in the appropriate locations and also displayed intense β2-AR and M2R immunoreactivity that correlated with the chronotropic responses to autonomic activation, it is highly probable that these cells are, in fact, pacemakers.
Perspectives and Significance
The traditional view of a reciprocal and antagonistic system of parasympathetic (inhibitory) and sympathetic (excitatory) innervation, with limited interactions at the level of the heart, is giving way to the recognition of the ICNS as a major site of integration of neural control of cardiac effectors (5, 47). We have characterized the properties of neural control of the sinoatrial and atrioventricular pacemakers, as well as the chronotropic responses to stimulation of the cardiac vagal rami and to exogenous pharmacological agents, in the isolated, innervated zebrafish heart. Furthermore, we have shown that putative pacemaker cells in both the SAR and AVR express M2Rs, and β2-ARs. In studying pacemaker function in the zebrafish heart, we have made a first step toward understanding the integrative autonomic control of heart rate. It is now possible to utilize this model to identify specific relationships between subpopulations of intracardiac neurons and their targeted effectors to discover basic mechanisms of neural integration involved in modulating cardiac chronotropy.
GRANTS
The Natural Sciences and Engineering Council (NSERC) of Canada supported this work through Discovery Grants (27140, to R. P. Croll; RGPIN-2015-04759, to T. A. Quinn). M. R. Stoyek was a funded by a NSERC Post-Graduate Research Scholarship. This work was also supported in part by the Dalhousie University Faculty of Medicine Research Fund (to F. M. Smith).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.R.S., R.P.C., and F.M.S. conception and design of research; M.R.S. and T.A.Q. performed experiments; M.R.S. and T.A.Q. analyzed data; M.R.S., T.A.Q., R.P.C., and F.M.S. interpreted results of experiments; M.R.S. prepared figures; M.R.S., R.P.C., and F.M.S. drafted manuscript; M.R.S., T.A.Q., R.P.C., and F.M.S. edited and revised manuscript; M.R.S., T.A.Q., R.P.C., and F.M.S. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Akiyama T, Yamazaki T. Effects of right and left vagal stimulation on left ventricular acetylcholine levels in the cat. Acta Physiol Scand 172: 11–16, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Ampatzis K, Dermon CR. Regional distribution and cellular localization of β2-adrenoceptors in the adult zebrafish brain (Danio rerio). J Comp Neurol 518: 1418–1441, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol Heart Circ Physiol 251: H764–H773, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Ardell JL, Cardinal R, Beaumont E, Vermeulen M, Smith FM, Armour JA. Chronic spinal cord stimulation modifies intrinsic cardiac synaptic efficacy in the suppression of atrial fibrillation. Auton Neurosci 186: 38–44, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armour JA. Potential clinical relevance of the “little brain” on the mammalian heart. Exp Physiol 93: 165–176, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Arrenberg AB, Stainier DY, Baier H, Huisken J. Optogenetic control of cardiac function. Science 330: 971–974, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Baruscotti M, Robinson RB. Electrophysiology and pacemaker function of the developing sinoatrial node. Am J Physiol Heart Circ Physiol 293: H2613–H2623, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Blinder KJ, Johnson TA, Massari VJ. Negative inotropic vagal preganglionic neurons in the nucleus ambiguus of the cat: neuroanatomical comparison with negative chronotropic neurons utilizing dual retrograde tracers. Brain Res 804: 325–330, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Blinder K, Moore C, Johnson T, John Massari V. Central control of atrio-ventricular conduction and left ventricular contractility in the cat heart: Synaptic interactions of vagal preganglionic neurons in the nucleus ambiguus with neuropeptideY-immunoreactive nerve terminals. Auton Neurosci 131: 57–64, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Boineau JP, Canavan TE, Schuessler RB, Cain ME, Corr PB, Cox JL. Demonstration of a widely distributed atrial pacemaker complex in the human heart. Circulation 77: 1221–1237, 1988. [DOI] [PubMed] [Google Scholar]
- 11.Bouman LN, Gerlings ED, Biersteker PA, Bonke FI. Pacemaker shift in the sino-atrial node during vagal stimulation. Pflügers Arch 302: 255–267, 1968. [DOI] [PubMed] [Google Scholar]
- 12.Briggs JP. The zebrafish: a new model organism for integrative physiology. Am J Physiol Regul Integr Comp Physiol 282: R3–R9, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Brodde OE. β-Adrenoceptors. In: Receptor Pharmacology and Function, edited by Williams M, Glennon RA, Timmermans PB. New York: Marcel Dekker, New York, 1989, p. 207–255. [Google Scholar]
- 14.Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev 51: 651–689, 1999. [PubMed] [Google Scholar]
- 15.Caulfield MP. Muscarinic receptors: characterization, coupling, and function. Pharmacol Ther 58: 319–379, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Z, Zhang H, Guo SZ, Wurster R, Gozal D. Differential control over postganglionic neurons in rat cardiac ganglia by NA and DmnX neurons: anatomical evidence. Am J Physiol Regul Integr Comp Physiol 286: R625–R633, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Chi NC, Shaw RM, Jungblut B, Huisken J, Ferrer T, Arnaout R, Scott I, Beis D, Xiao T, Baier H, Jan LY, Tristani-Firouzi M, Stainier DY. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol 6: e109, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christoffels VM, Smits GJ, Kispert A, Moorman AF. Development of the pacemaker tissues of the heart. Circ Res 106: 240–254, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Dhein S, Van Koppen CJ, Brodde OE. Muscarinic receptors in the mammalian heart. Pharmacol Res 44: 161–182, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Dvornikov AV, Dewan S, Alekhina OV, Pickett FB, Tombe PP. Novel approaches to determine contractile function of the isolated adult zebrafish ventricular cardiac myocyte. J Physiol 592: 1949–1956, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrell AP, Jones DG. The heart. In: Fish Physiology, edited by Hoar WS, Randall DJ, Farrell AP. Toronto, Canada: Academic, vol. XII, p 1–88, 1992. [Google Scholar]
- 22.Goldberg JM, Geesbreght JM, Randall WC, Brynjolfsson G. Sympathetically induced pacemaker shifts following sinus node excision. Am J Physiol 224: 1468–1474, 1973. [DOI] [PubMed] [Google Scholar]
- 23.Hancock J, Hoover D. Capsaicin-evoked bradycardia in anesthetized guinea pigs is mediated by endogenous tachykinins. Regul Pept 147: 19–24, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Haverinen J, Vornanen M. Temperature acclimation modifies sinoatrial pacemaker mechanism of the rainbow trout heart. Am J Physiol Regul Integr Comp Physiol 292: R1023–R1032, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Hedberg A, Minneman KP, Molinoff PB. Differential distribution of beta-1 and beta 2 adrenergic receptors in cat and guinea pig heart. J Pharmacol Exper Ther 212: 503–508, 1980. [PubMed] [Google Scholar]
- 26.Hucker W, Nikolski V, Efimov I. Autonomic control and innervation of the atrioventricular junctional pacemaker. Heart Rhythm 4: 1326–1335, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Hulme EC, Birdsall NJM, Buckley NJ. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol 30: 633–673, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Irisawa H. Comparative physiology of the cardiac pacemaker mechanism. Physiol Rev 58: 461–498, 1978. [DOI] [PubMed] [Google Scholar]
- 29.Jonz MG, Fearon IM, Nurse CA. Neuroepithelial oxygen chemoreceptors of the zebrafish gill. J Physiol 560: 737–752, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol 47: 157–170, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 106: 659–673, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang S, Zieske H, Levy M. Insignificant bilateral convergence of preganglionic vagal fibers on postganglionic neurons to the canine heart. Circ Res 67: 556–563, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Li N, Csepe TA, Hansen BJ, Dobrzynski H, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Rosen MR, Biesiadecki BJ, Fedorov VV. Molecular mapping of sinoatrial node HCN channel expression in the human heart. Circ Arrhythm Electrophysiol 8: 1219–1227, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin E, Ribeiro A, Ding W, Hove-Madsen L, Sarunic MV, Beg MF, Tibbits GF. Optical mapping of the electrical activity of isolated adult zebrafish hearts: acute effects of temperature. Am J Physiol Regul Integr Comp Physiol 306: R823–R836, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin E, Craig C, Lamothe M, Sarunic MV, Beg MF, Tibbits GF. Construction and use of a zebrafish heart voltage and calcium optical mapping system, with integrated electrocardiogram and programmable electrical stimulation. Am J Physiol Regul Integr Comp Physiol 308: R755–R768, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llach A, Molina CE, Alvarez-Lacalle E, Tort L, Benitez R, Hove-Madsen L. Detection, properties, and frequency of local calcium release from the sarcoplasmic reticulum in teleost cardiomyocytes. PLoS One 6: e23708, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mangoni M, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev 88 919–982, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Matiukas A, Mitrea BG, Qin M, Pertsov AM, Shvedko AG, Warren MD, Zaitsev AV, Wuskell JP, Wei MD, Watras J, Loew LM. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm 4: 1441–1451, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative a new fixative for immunoelectron microscopy. J Histochem Cytochem 22: 1077–1083, 1974. [DOI] [PubMed] [Google Scholar]
- 40.McDevitt DG. In vivo studies on the function of cardiac β-adrenoceptors in man. Eur J Heart 10, Suppl B: 22–28, 1989. [DOI] [PubMed] [Google Scholar]
- 41.Nemtsas P, Wettwer E, Christ T, Weidinger G, Ravens U. Adult zebrafish heart as a model for human heart? An electrophysiological study. J Mol Cell Cardiol 48: 161–171, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson S. Comparative anatomy of the autonomic nervous system. Auton Neurosci 165: 3–9, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Pauza DH, Saburkina I, Rysevaite K, Inokaitis H, Jokubauskas M, Jalife J, Pauziene N. Neuroanatomy of the murine cardiac conduction system. A combined stereomicroscopic and fluorescence immunohistochemical study. Auton Neurosci 176: 32–47, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Pauza DH, Rysevaite K, Inokaitis H, Jokubauskas M, Pauza AG, Brack KE, Pauziene N. Innervation of sinoatrial nodal cardiomyocytes in mouse. A combined approach using immunofluorescent and electron microscopy. J Mol Cell Cardiol 75: 188–197, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Peralta EG, Ashkenazi A, Winslow JW, Smith DH, Ramachandran J, Capon DJ. Distinct primary structures, ligand-binding properties, and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J 6: 3923–3929, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pieperhoff S, Wilson K, Baily J, de Mora K, Maqsood S, Vass S, Taylor J, Del-Pozo J, MacRae CA, Mullins JJ, Denvir MA. Heart on a plate: histological and functional assessment of isolated adult zebrafish hearts maintained in culture. PLoS One 9: e96771, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, Shivkumar K. Myocardial infarction induces structural and functional remodeling of the intrinsic cardiac nervous system. J Physiol 594: 321–341, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rider SA, Tucker CS, del-Pozo J, Rose KN, MacRae CA, Bailey MA, Mullins JJ. Techniques for the in vivo assessment of cardo-renal function in zebrafish (Danio rerio) larvae. J Physiol 590: 1803–1809, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saito T, Tenma K. Effects of left and right vagal stimulation on excitation and conduction of the carp heart (Cyprinus carpio). J Comp Physiol 111: 39–53, 1976. [Google Scholar]
- 50.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sedmera D, Reckova M, deAlmeida A, Sedmerova M, Biermann M, Volejnik J, Sarre A, Raddatz E, McCarthy RA, Gourdie RG, Thompson RP. Functional and morphological evidence for a ventricular conduction system in zebrafish and Xenopus hearts. Am J Physiol Heart Circ Physiol 284: H1152–H1160, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Shibata N, Inada S, Mitsui K, Honjo H, Yamamoto M, Niwa R, Boyett MR, Kodama I. Pacemaker shift in the rabbit sinoatrial node in response to vagal nerve stimulation. Exp Physiol 86: 177–184, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Shvilkin A, Danilo P, Chevalier P, Chang F, Cohen I, Rosen M. Vagal release of vasoactive intestinal peptide can promote vagotonic tachycardia in the isolated innervated rat heart. Cardiovasc Res 28: 1769–1773, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Smith FM, Jones D. Localization of receptors causing hypoxic bradycardia in trout (Salmo gairdneri). Can J Zool 56: 1260–1265, 1978. [Google Scholar]
- 55.Smith FM. Extrinsic inputs to intrinsic neurons in the porcine heart in vitro. Am J Physiol Regul Integr Comp Physiol 276: R455–R467, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Steele SL, Yang X, Debiais-Thibaud M, Schwerte T, Pelster B, Ekker M, Tiberi M, Perry SF. In vivo and in vitro assessment of β-adrenergic receptors in larval zebrafish (Danio rerio). J Exp Biol 214: 1445–1457, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Stoyek MR, Croll RP, Smith FM. Intrinsic and extrinsic innervation of the heart in zebrafish (Danio rerio). J Comp Neurol 523: 1683–1700, 2015. [DOI] [PubMed] [Google Scholar]
- 58.Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM, Yokoyama C, Onoe H, Eguchi M, Yamaguchi S, Abe T, Kiyonari H, Shimizu Y, Miyawaki A, Yokota H, Ueda HR. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157: 726–739, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Taylor EW, Jordan D, Coote JH. Central control of the cardiovascular and respiratory systems and their interactions in vertebrates. Physiol Rev 79: 855–916, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Tessadori F, van Weerd JH, Burkhard SB, Verkerk AO, de Pater E, Boukens BJ, Vink A, Christoffels VM, Bakkers J. Identification and functional characterization of cardiac pacemaker cells in zebrafish. PLoS One 7: e47644, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsutsui H, Higashijima SI, Miyawaki A, Okamura Y. Visualizing voltage dynamics in zebrafish heart. J Physiol 588: 2017–2021, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warren KS, Baker K, Fishman MC. The slo mo mutation reduces pacemaker current and heart rate in adult zebrafish. Am J Physiol Heart Circ Physiol 281: H1711–H1719, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Weinberger F, Mehrkens D, Frederich FW, Stubbendorff M, Hua X, Muller JC, Schrepfer S, Evans SM, Carrier L, Eschenhagen T. Localization of Islet-1 positive cells in the healthy and infarcted adult murine heart. Circ Res 110: 1303–1310, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.