Using a mouse model of total parenteral nutrition (TPN), this study shows that enteral nutrient deprivation leads to the permeation of TPN-derived nutrients into the gut lumen, where they are preferentially utilized by Enterobacteriaceae. This provides insight into the mechanisms behind the loss of epithelial barrier function and subsequent complications seen in the TPN-dependent state. Dysbiosis related to TPN may be exacerbated by an altered intestinal metabolome.
Keywords: total parenteral nutrition, microbiota, metabolome, nutrient foraging, epithelial barrier function, NanoSIMS
Abstract
Total parenteral nutrition (TPN) leads to a shift in small intestinal microbiota with a characteristic dominance of Proteobacteria. This study examined how metabolomic changes within the small bowel support an altered microbial community in enterally deprived mice. C57BL/6 mice were given TPN or enteral chow. Metabolomic analysis of jejunal contents was performed by liquid chromatography/mass spectrometry (LC/MS). In some experiments, leucine in TPN was partly substituted with [13C]leucine. Additionally, jejunal contents from TPN-dependent and enterally fed mice were gavaged into germ-free mice to reveal whether the TPN phenotype was transferrable. Small bowel contents of TPN mice maintained an amino acid composition similar to that of the TPN solution. Mass spectrometry analysis of small bowel contents of TPN-dependent mice showed increased concentration of 13C compared with fed mice receiving saline enriched with [13C]leucine. [13C]leucine added to the serosal side of Ussing chambers showed rapid permeation across TPN-dependent jejunum, suggesting increased transmucosal passage. Single-cell analysis by fluorescence in situ hybridization (FISH)-NanoSIMS demonstrated uptake of [13C]leucine by TPN-associated bacteria, with preferential uptake by Enterobacteriaceae. Gavage of small bowel effluent from TPN mice into germ-free, fed mice resulted in a trend toward the proinflammatory TPN phenotype with loss of epithelial barrier function. TPN dependence leads to increased permeation of TPN-derived nutrients into the small intestinal lumen, where they are predominately utilized by Enterobacteriaceae. The altered metabolomic composition of the intestinal lumen during TPN promotes dysbiosis.
NEW & NOTEWORTHY
Using a mouse model of total parenteral nutrition (TPN), this study shows that enteral nutrient deprivation leads to the permeation of TPN-derived nutrients into the gut lumen, where they are preferentially utilized by Enterobacteriaceae. This provides insight into the mechanisms behind the loss of epithelial barrier function and subsequent complications seen in the TPN-dependent state. Dysbiosis related to TPN may be exacerbated by an altered intestinal metabolome.
for patients undergoing periods of complete bowel rest, or deprivation of enteral nutrition due to a nonfunctional gastrointestinal tract, total parenteral nutrition (TPN) provides critical support (3, 13). Each year, ∼350,000 patients in the U.S. rely on this therapy (34). While life sustaining, TPN has been associated with adverse consequences. This includes mucosal atrophy and loss of epithelial barrier function (EBF) leading to increased systemic infectious complications (7, 18, 20, 44a). The theory of gut-origin sepsis has been discussed since the 1940s to explain these infectious complications (11). In short, the theory states that, in times of stress and starvation, epithelial barrier dysfunction allows enteric bacteria and/or their by-products to translocate, leading to systemic infection or multisystem organ dysfunction.
To examine the gut origin of sepsis theory, a mouse TPN model has been used (15, 16, 28, 40, 51). In this model, TPN dependence leads to a proinflammatory state within the intestinal mucosa. This is associated with elevated mucosa-derived inflammatory cytokines including tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) (16, 17). These changes then drive mucosal atrophy and a loss of EBF (12). Epithelial barrier dysfunction is commonly attributed to enterocyte malnutrition. This concept has led to administration of enteral and parenteral glutamine, the preferred energy source of enterocytes, to maintain barrier function (32, 45). The clinical effectiveness of this strategy is variable, however, with evidence of increased mortality in critically ill patients (22). As further evidence that enterocyte malnutrition alone is not responsible for EBF loss with TPN, mucosal atrophy is not reversed by ensuring complete nutritional needs via TPN (24). Enterocytes are able to obtain nutrition basally from an intravenous (IV) source (39), and basally fed cultured enterocytes show increased proliferation compared with those receiving only apical nutrition (33). These data suggest that enterocytes are able to adequately utilize IV nutrition. There is an emerging theory for epithelial cell dependence on luminal nutrients that focuses on the role of intestinal microbiota.
The intestinal epithelium serves not only as the site of nutrient absorption, but also as the interface between the host and its resident microbiota. This microbial community is critical in the development of gut immune health (29), and it is highly sensitive to changes in environmental factors such as diet (10). Alterations in the composition of enteral nutrition may impact the microbial population of the gastrointestinal tract and, subsequently, host pathology (47, 48). With complete enteral nutrient deprivation, striking shifts occur in the composition of the small and large bowel microbiota (38), moving from a Firmicutes-dominated population to a less diverse Proteobacteria-rich mucosa-associated community (21, 23, 31). However, a causal relationship between the altered microbiota and decreased EBF has not been demonstrated. In addition, a critical question remains as to how these bacteria forage for nutrients in a state of complete enteral nutrient deprivation.
The present study examined the interactions between the intestinal microbiota and the metabolomic constituents in the TPN-dependent setting. We demonstrate TPN-associated changes in the luminal metabolome, characterized by an amino acid composition closely resembling that of TPN solution. Importantly, we further demonstrate that the observed shift in microbial community composition may be due to selective utilization of TPN-derived amino acids by Proteobacteria. Finally, we show that TPN-derived enteric contents produce a similar proinflammatory phenotype in fed germ-free mice as conventional TPN-dependent mice.
METHODS
Animals and housing.
C57BL/6J male, specific pathogen-free mice (10–12 wk old; Jackson Laboratory, Bar Harbor, ME; n = 6 for each experimental group) were maintained under temperature-, humidity-, and light-controlled conditions. Mice were initially fed ad libitum with standard mouse chow and water. During the administration of IV solutions, mice were singly housed in metabolic cages to prevent coprophagia. Colonization studies were performed with 10- to 12-wk-old germ-free (GF) C57BL/6J mice from a breeding colony established at the University of Michigan germ-free core facility. Mice were housed in sterile isolators with irradiated food, bedding, and water for the duration of the experiments. All animal protocols were approved by the University Committee on the Use and Care of Animals of the University of Michigan (no. PRO00003986).
Operative procedures and TPN.
Mice underwent jugular venous cannulation and TPN administration as previously described (26). After 24 h, mice were randomized into two groups: 1) enterally fed conventional mice (Fed group) that received IV crystalloid solution at 0.2 ml/h and standard laboratory mouse chow; and 2) TPN-dependent conventional mice (TPN group) that were deprived of all enteric nutrition and received TPN at 0.5 ml/h. Groups were maintained isonitrogenous and isocaloric. All animals were killed at 7 days with CO2.
Effluent gavage in germ-free mice.
Small bowel effluent from TPN and Fed mice was sterilely extracted under an oxygen-free nitrogen hood. This was performed by removing the entire small intestine from ligament of Treitz to the terminal ileum and extracting enteric contents. This was immediately gavaged (200 μl intragastric) into enterally fed GF mice under otherwise sterile conditions. Each treatment group was housed in separate sterile isolators. These mice were allowed a standard germ-free diet and euthanized after 7 days for investigation of the inflammatory state of the small bowel.
Untargeted metabolomic interrogation.
To better understand the interaction between the intestinal luminal environment and the host, small bowel effluent from TPN, Fed, and GF mice underwent untargeted liquid chromatography-mass spectrometric (LC/MS) analysis. A small portion of each sample was weighed in a microtube. Samples were than dried under the stream of nitrogen for 5 h, resuspended in 100 μl of water, and placed on ice. Extraction buffer was prepared by mixing 50 ml of methanol:chloroform:water (8:1:1) with 0.45 ml of 2 mg/ml solution of recovery standards (mixture of 13C-labeled amino acids). Extraction buffer was prechilled at −20°C for 30 min and added to each sample at a ratio of 10 μl/mg wet wt. One stainless steel bead was then placed in each test tube and samples were homogenized for 2 min at top speed. Extracts were clarified by centrifugation at 4,750 rpm for 10 min (4°C). Aliquots (200 μl) were dried under the stream of nitrogen, resuspended in 100 μl of water containing injection standards (tBoc-l-alanine, tBoc-l-asparagine, tBoc-l-phenylalanine), clarified by centrifugation at 12,000 rpm for 20 min (4°C) and transferred to HPLC glass vials and moved to the instrument for LC/MS analysis as previously described (30).
Stable isotope-labeled leucine administration.
Stable isotope-labeled l-leucine (2-13C, 99% CLM-2014-1, Cambridge Isotope Laboratories, Andover, MA) was added to a specially formulated TPN (Health Dimensions, Ann Arbor, MI). To maintain an isocaloric and isonitrogenous state, a 50% reduction of leucine in the amine solution was replaced with targeted isotope. Stable isotope-labeled TPN was administered in standard fashion and given either throughout the entire period of enteral deprivation (6 days) or only during the last 2 days of infusion. A group of Fed mice were allowed enteral chow but underwent jugular venous cannulation and were given an IV saline solution with identical concentrations of [13C]leucine either throughout or only during the last 2 days of the experiment.
Isotope collection and analysis.
Serum, small bowel effluent, and bile samples were collected after euthanasia. Microbial biomass was extracted by transferring small bowel effluent into 50 ml of wash buffer [50 mM sodium phosphate buffer (pH 8), 0.1% Tween 80]. The solution was shaken at 100 oscillations/min for 20 min on a reciprocating horizontal platform at room temperature and subsequently stood upright for 15 min to allow large debris to settle. The supernatant was transferred to a clean 50-ml conical tube and kept on ice. The pellet was resuspended in wash buffer and cellular debris was allowed to settle three additional times. Supernatants were pooled and bacteria were collected by centrifugation at 12,000 g for 15 min at room temperature. After 20 min of sonication at 4°C, samples were treated with sarkosyl + Triton X-100 (Sigma-Aldrich; 1.5% + 2%) (42). Samples were then centrifuged at 16,000 g for 15 min, and all pellets were stored at −80 C prior to 13C mass isotopomer flux analysis.
13C mass isotopomer flux analysis.
Aliquots (100 μl) of each sample were extracted with 150 μl of extraction solvent (methanol:chloroform:water) then centrifuged at 15,000 g for 5 min. Supernatant containing metabolites were transferred to autosampler vials and dried at 45°C by a vacuum centrifuge. Dried samples are derivatized with 40 μl of the 20 mg/ml methoxyamine (37°C and 60 min) and 40 μl of BSTFA (70°C for 30 min). Derivatized samples were analyzed by GCMS on an Agilent J&W DB-5 column (250 μm × 0.25 μm × 30 m) under the following GCMS conditions: Temperature program 2 min hold at 70°C, then 70–300°C at 25°C/min, then hold 300°C for 2 min; flow rate 1.1 ml/min; injector temperature 250°C, 1 μl injection at 1:10 split ratio. Data were processed by MassHunter workstation software, version B.06. Isotope enrichment values were normalized to harvested sample mass.
Epithelial barrier function measurement.
Transepithelial resistance (TER) of full-thickness jejunum (0.3 cm2), 6 cm distal to the ligament of Treitz, was assessed by using modified Ussing chambers (Physiologic Instruments, San Diego, CA) as previously described (50). Intestinal permeability was assessed with fluorescein isothiocyanate (FITC)-dextran (4,000 kDa at 50 mg ml−1; Sigma-Aldrich, St. Louis, MO) (5). FITC-dextran (150 μl) was added to the mucosal compartment after equilibration, and 500 μl was removed from the serosal compartment after 60, 90, and 120 min.
For acute amino acid leakage experiments, tissue was mounted in the Ussing chamber such that the transepithelial current was applied in a serosa-to-mucosa direction. [13C]leucine was dissolved in the Krebs buffer of the serosal compartment (15 mM), and 500 μl was removed from the mucosal compartment at 10, 30, 60, and 90 min and snap frozen for mass isotopomer measurement. Results were expressed as the ratio of [13C]leucine to [12C]leucine to account for [12C]leucine derived from sloughed enterocytes during the Ussing chamber experiment.
Fluorescence in situ hybridization of small bowel microbiota.
Flushed luminal contents were fixed with 4% formaldehyde for 4 h, sonicated to disrupt cell aggregates, and used for fluorescence in situ hybridization (FISH) and nano-scale resolution secondary ion mass spectrometry (NanoSIMS) imaging. FISH was performed with fluorescently labeled rRNA-targeted oligonucleotide probes specific for all Bacteria (S-d-Bact-0338-a-A-18-Cy5, 5′-GCT GCC TCC CGT AGG AGT-3′; S-*-BactP-0338-a-A-18-Cy5, 5′-GCA GCC ACC CGT AGG TGT-3′; S-*-BactV-0338-a-A-18-Cy5, 5′-GCT GCC ACC CGT AGG TGT-3′) or Enterobacteriaceae (S-*-EBAC-1790-a-A-18-Cy3, 5′-CGT GTT TGC ACA GTG CTG-3′), using a standard protocol (9). To evaluate potential nonspecific FISH probe binding, parallel samples were hybridized with the reverse complement of the bacterial probe (NONEUB-5′-ACTCCTACGGGAGGCAGC-3′). Hybridized samples were imaged and marked on an epifluorescence laser microdissection microscope (LMD, Leica LMD 7000) as previously described (2).
NanoSIMS imaging of small bowel microbiota.
NanoSIMS measurements were performed on an NS50L (Cameca). Data were recorded as images by scanning a finely focused Cs+ primary ion beam (∼80 nm spot size with 2 pA beam current) and detection of negative secondary ions and secondary electrons. Recorded images had a 512 × 512 pixel resolution and a field-of-view ranging from 60 × 60 to 70 × 70 μm2. Analysis areas were presputtered to establish a Cs+ dose density of 8E14 or 2E16 atoms/cm2. All images were recorded with a dwell time of 5–10 ms/pixel per cycle.
NanoSIMS images were processed by using the WinImage software package (Cameca). Cells were identified in drift-corrected, stack-accumulated NanoSIMS images and manually verified with aligned FISH images. 13C/(12C+13C) isotope fractions, designated as atom percent (at%) 13C throughout the text, were calculated from dead time- and QSA-corrected 12C2- and 12C13C− signal intensities. Summary statistics from each region of interest were calculated for single-cell analysis. Individual cells were considered significantly enriched in 13C if the mean cellular at% 13C was five standard deviations above the mean at% 13C of the unlabeled control cells from the gut lumen and if the measurement error (1σ, Poisson) was smaller than the difference between the at% of the labeled cell and the mean at% of unlabeled control cells.
Cytokine profiling.
Cytokine profiles were measured in tissue lysates MILLIPLEX MAP multiplex kits (Millipore, Billerica, MA) (17). Mucosal scrapings from 1-cm jejunal segments were used to carry out protein purification and cytokine profiling per manufacturer instructions.
Immunofluorescence imaging.
To further study EBF, immunofluorescence microscopy of targeted tight and adherens junctions was performed as previously described (16). Primary antibodies were rat monoclonal anti-E-cadherin 1:100, rabbit polyclonal anti-zonula occludens-1 (ZO-1) 1:100, and mouse monoclonal anti-occludin 1:100 (Invitrogen, Carlsbad, CA). Fluorescence was analyzed with an Olympus BX-51 upright light and fluorescence microscope. Images were stacked by using Nikon image software and processed with Adobe Photoshop CC.
Statistical analysis.
All results are expressed as mean ± standard deviation unless otherwise specified. Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA). Comparison between two groups used the two-tailed, unpaired Student's t-test. Comparison between more than two groups used analysis of variance (ANOVA). A P value of < 0.05 was considered significant.
Principal components analysis.
Principal components analysis (PCA) was used to process untargeted LC/MS metabolomics data. Data analysis was performed using Find by Formula algorithm of the Agilent MassHunter Qualitative analysis software. Library for the analysis was constructed partially from the authentic standards and partially by creating “Known Unknowns” from mass spectral features consistently present in a large proportion of the samples. Molecular features were identified by Molecular Feature Extractor (Agilent MassHunter component) and processed using in-house software. To evaluate possible biological signal PCA and multiple group t-test were performed using all features (except standards) present in >70% of the samples. Statistical analysis was performed using R (cran.r-project.org) and was done separately for positive and negative mode data.
RESULTS
Survival.
All animals survived for 7 days postintervention until experimental end points were reached.
Metabolomic composition of small bowel effluent.
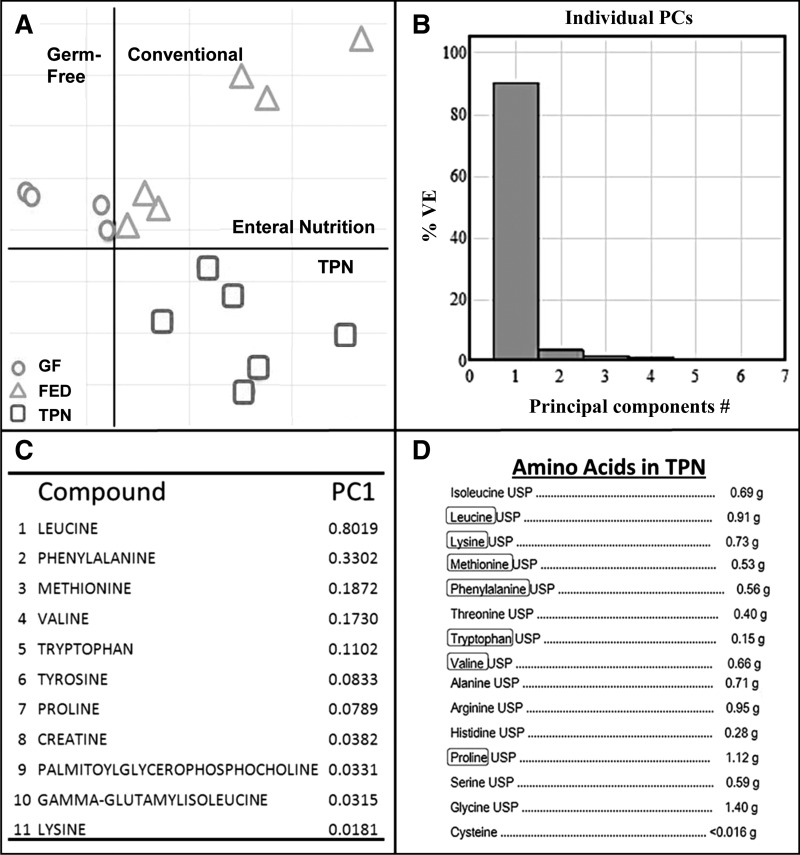
We first asked what metabolomic components were present in the intestinal lumina of mice with enteral nutrient deprivation. Untargeted metabolomics was performed with small bowel effluents from TPN, Fed, and GF mice by using LC/MS. PCA of mass spectrometric data demonstrated significant separation of experimental groups (Fig. 1A).
Fig. 1.
Metabolomic composition of small bowel effluent. A: weighted principal coordinate analysis of metabolite composition of small bowel samples from total parenteral nutrition (TPN), enterally fed (Fed), and germ-free (GF) mice. B: first principal component (PC; x-axis) and second principal component (y-axis) account for 90.1 and 5.4% of overall differences, respectively. C: list of identifiable compounds making up PC1 as determined by untargeted metabolomic analysis via LC/MS. D: crystalline amino acid (AA) composition of the TPN formulation administered to TPN-dependent mice. Circled AAs are those that comprise PC1 of analyzed enteral contents. VE, variance explained; USP, United States Pharmacopeia.
The first principal component (PC1) accounted for 90% of differences between the three study groups (Fig. 1B). The presence of bacteria (TPN and Fed) led to a significant increase in several luminal amino acids compared with GF mice (Table 1). Seven of the first 10 compounds identified in PC1 (Fig. 1C) were amino acids, including increases in leucine, phenylalanine, methionine, valine, tryptophan, tyrosine, and proline (note that leucine and isoleucine coelute and have identical masses and are reported only as “leucine”). Interestingly, these amino acids were significantly increased in Fed mice compared with GF mice despite both groups receiving identical chow. Even higher concentrations of these amino acids were observed in TPN mice despite a complete lack of enteral nutrient delivery. Each of the amino acids identified in PC1 matched those supplied in the TPN solution in crystalline form. The amino acid that contributed most to the first and the second PC (i.e., observed differences in metabolite composition) was leucine, an essential amino acid that was present in a relatively high concentration in the TPN formulation used in our TPN model (Fig. 1D).
Table 1.
Untargeted metabolomics results
| Compound | PC1 | P Value | FED/GF | TPN/GF | TPN/FED |
|---|---|---|---|---|---|
| Leucine | 0.802 | 0.008 | 2.705 | 2.856 | 1.056 |
| Phenylalanine | 0.330 | 0.008 | 2.645 | 3.062 | 1.158 |
| Methionine | 0.187 | 0.025 | 3.460 | 3.966 | 1.146 |
| Valine | 0.172 | 0.008 | 2.311 | 6.672 | 2.887 |
| Tryptophan | 0.110 | <0.001 | 3.084 | 4.889 | 1.161 |
| Tyrosine | 0.083 | 0.027 | 2.022 | 2.299 | 1.137 |
| Proline | 0.079 | 0.031 | 2.261 | 2.626 | 1.161 |
| Creatine | 0.038 | 0.018 | 4.368 | 8.579 | 1.964 |
| Palmitoyl-glycerophosphocholine | 0.033 | 0.022 | 3.066 | 9.765 | 3.184 |
| γ−Glutamylisoleucine | 0.032 | 0.032 | 4.773 | 9.376 | 1.964 |
Fold change in abundance of each compound is identified in the first principal component (PC1) of LC/MS results between small bowel effluent from fed germ-free (GF), enterally fed conventional (Fed), and TPN-dependent conventional mice (TPN). P values were determined via ANOVA.
Nutrient substrate from the TPN solution is found in the intestinal lumen.
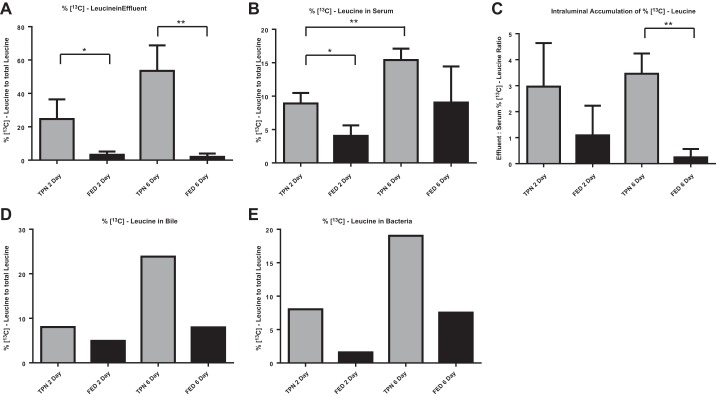
As the TPN mice had no enteral source of amino acids, we hypothesized that the leucine detected in the lumen derived directly from the TPN solution through leakage into the small bowel lumen. To confirm this, we targeted leucine, which was the most significant contributor to PC1, for subsequent studies. Isotope-labeled leucine ([13C]leucine) was added to the TPN (consisting of a 50% substitution of nonisotope leucine) and administered IV during a 6-day period of enteral deprivation. Fed control mice were allowed an enteral diet during [13C]leucine IV infusion. A significantly higher concentration of [13C]leucine (expressed as the % [13C]leucine to total leucine) was present in the small bowel effluent after 6 days of TPN compared with enteral feeding (Fig. 2A). In fact, in TPN-dependent mice, 50% of luminal leucine was 13C labeled after 6 days, the same proportion as in the TPN solution, which demonstrated that the TPN solution is the main source of these luminal amino acids.
Fig. 2.
TPN-derived leucine accumulates in the small bowel and is incorporated into luminal bacteria. 13C mass isotopomer flux analysis of small bowel effluent (A), serum (B), bile (D), and small bowel intraluminal bacterial biomass (E) from fed and TPN-dependent mice. C: ratio of [13C]-leucine concentration in small bowel effluent to serum in fed and TPN-dependent mice. Intravenous infusions were supplemented with [13C]leucine for the entire 6 days of treatment or for the final 2 days only. Bile and bacteria samples were pooled prior to analysis. *P < 0.05, **P < 0.01.
These findings suggested a transepithelial permeation of leucine with TPN administration. To determine whether other sources of leucine were contributing the elevated luminal levels, serum (Fig. 2B) and biliary (Fig. 2D) levels of [13C]leucine were measured. Biliary and serum [13C]leucine levels trended toward isotopic enrichment in TPN-dependent mice compared with enterally fed mice. Possibly, enterally absorbed [12C]leucine acted to dilute IV [13C]leucine in the Fed group. The increased ratio of effluent to serum [13C]leucine in TPN vs. Fed mice suggested that leucine was preferentially concentrated in the intestinal lumen with TPN dependence (Fig. 2C).
TPN-derived leucine becomes incorporated into luminal bacteria.
We next examined the content of [13C]leucine in harvested luminal bacteria. The enrichment of [13C]leucine in bacterial biomass doubled from 2 to 6 days of infusion, suggesting progressive bacterial access to and uptake of TPN-derived leucine with time (Fig. 2E). Furthermore, [13C]leucine enrichment was significantly higher in bacteria derived from TPN mice than in bacteria derived from Fed mice. These findings support the hypothesis that enteral deprivation drives epithelial barrier breakdown with subsequent permeation of TPN-derived amino acids from the serum into the intestinal lumen. The elevated levels of 13C in bacterial isolates demonstrate that the intestinal microbiota utilize TPN-derived amino acids as a direct nutrient source, which has not been previously recognized.
Acute transepithelial amino acid leakage.
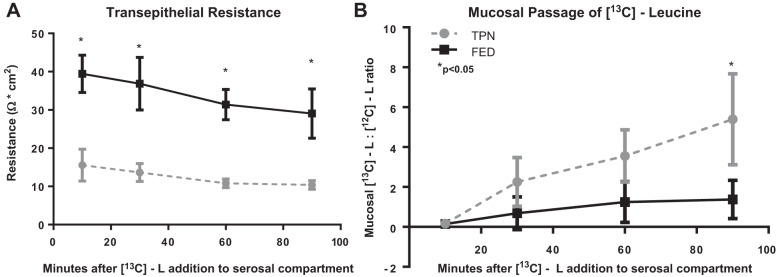
To further demonstrate a transepithelial leakage of amino acids rather than enterocyte uptake and subsequent sloughing, jejunal tissue derived from mice receiving TPN or saline without any enrichment with [13C]leucine was mounted in Ussing chambers with current directed toward the mucosal compartment. [13C]leucine was added to the serosal compartment, and transepithelial movement was assessed by measuring mucosal [13C]leucine. The TER of jejunum from TPN mice was significantly lower than that of Fed mice at all time points, with a parallel gradual decrease in TER over time (Fig. 3A). The relative concentration of [13C]leucine progressively rose in the mucosal compartment and was significantly greater by 90 min (Fig. 3B).
Fig. 3.
Acute intraluminal passage of stable isotope-labeled leucine in TPN-dependent mice. A: transepithelial resistance of fed and TPN-dependent jejunum. B: 13C mass isotopomer flux analysis of consecutive samples derived from the mucosal compartment after addition of [13C]leucine to the serosal compartment. *P < 0.05.
Imaging of TPN-derived [13C]leucine utilization by gut microbiota at the single cell level.
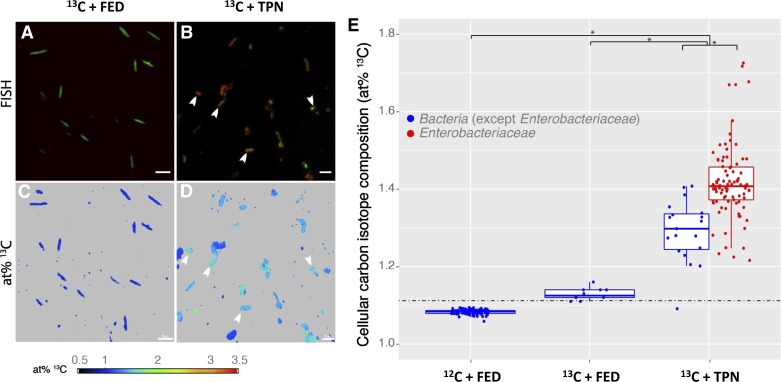
We next examined whether the incorporation of leucine by luminal bacteria correlated with previously reported TPN-associated microbial changes (31). Thus Enterobacteriaceae were specifically targeted, as they are the dominant Proteobacteria known to bloom in small intestine during TPN (31). FISH was combined with NanoSIMS to visualize utilization of [13C]leucine by bacterial cells in the small bowel effluent. In Fed (13C leucine IV + Fed) and control effluent samples (mice not receiving [13C]leucine; 12C + Fed), Enterobacteriaceae were rarely detected and were below the quantification limit of FISH (<0.5% relative abundance) (Fig. 4A). In contrast, many Enterobacteriaceae were detected in the luminal content from 13C leucine + TPN mice and comprised 81% of all bacterial cells (Fig. 4B).
Fig. 4.
NanoSIMS/FISH analysis of small bowel microbiota from enterally fed and TPN-dependent mice receiving intravenous [13C]leucine. A and B: small bowel effluents were hybridized with FISH probes targeting all Bacteria (EUB338 I–III, green) and Enterobacteriaceae (Ebac1790, red). NanoSIMS atom % (at%) 13C distribution maps of the same fields of view for fed (C) and TPN-dependent (D) samples show Enterobacteriaceae significantly enriched in 13C (white arrows) compared with non-Enterobacteriaceae cells from fed mice (scale bar = 5 μm). E: summary of cellular carbon isotope composition from single cells. The dashed line indicates the threshold value for considering a cell 13C enriched over cells from 12C+Fed control effluent (mean + 5 SD). The at% threshold for calling a cell enriched in 13C was +5 standard deviations of the mean 13C isotopic composition of cells from control 12C+Fed mice (1.122 at% 13C; 104 cells analyzed). Asterisks indicate significant differences between treatments and/or target populations (Tukey post hoc test, P < 0.001).
NanoSIMS imaging was performed on representative fields of view to quantify the uptake of [13C]leucine from the administered TPN by individual bacterial cells. Quantification of the 13C labeling of individual cells revealed that 90% of the cells in the 13C+Fed mice were enriched in 13C (12 cells analyzed; Fig. 4, C and E, middle column). Similarly, 99% of the cells in the luminal contents from 13C+TPN mice were enriched in 13C (108 cells measured; Fig. 4, D and E, right column). Statistical analysis showed that treatment (i.e., 13C+TPN, 13C + Fed, and 12C + Fed) as well as target group (i.e., Enterobacteriaceae vs. other Bacteria) were significant factors determining at% 13C (ANOVA, P < 0.001). The enrichment in 13C was greater in cells from 13C+TPN mice than either 13C + Fed or 12C + Fed controls (Tukey post hoc test, P < 0.001). The difference in 13C enrichment between the 13C+Fed and 12C+Fed controls, though elevated, was not significant (Tukey post hoc test, P = 0.08), which may be due to too few cells measured in the 13C + Fed samples. The marked difference observed in the degree of 13C labeling of cells between the 13C + Fed and 13C + TPN mice emphasizes the change in EBF and the permeation of TPN-derived leucine into the small bowel lumen. Additionally, in the TPN mice, Enterobacteriaceae were significantly more enriched in 13C than non-Enterobacteriaceae cells (using a universal FISH probe for all Bacteria; Tukey post hoc test, P < 0.001; Fig. 4E, right column). This difference highlights the ability of TPN-derived nutrients to support the previously reported metabolically selective activity of Enterobacteriaceae and possibly other Proteobacteria members (46).
TPN-derived small bowel effluent drives a proinflammatory response in GF mice.
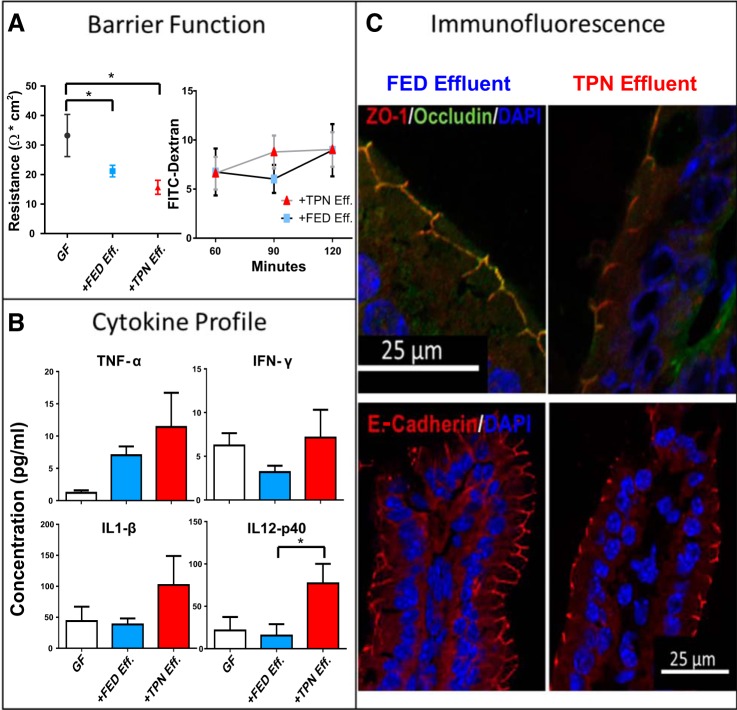
Because of the differences in luminal metabolome and microbiome compositions between GF and the Fed and TPN mouse groups, we then asked whether transplantation of small bowel content from TPN into GF mice could invoke the proinflammatory state and associated loss of EBF seen with TPN dependence. Intestinal effluent was harvested under microaerophilic conditions from TPN and Fed mice, and then sterilely gavaged into a group of GF mice. Gavaged mice were examined after 1 wk of sterile feeding. The introduction of bacteria significantly decreased TER compared with nongavaged GF mice (Fig. 5A), supporting recent reports of the importance of enteral bacteria in modulating barrier function (43). Furthermore, TER decreased slightly more, albeit not significantly (P = 0.057), in GF mice receiving TPN small bowel content compared with GF mice receiving Fed mice small bowel content.
Fig. 5.
TPN-derived small bowel contents induce inflammatory changes and loss of epithelial barrier function (EBF) in axenic mice. Effluent from TPN-dependent (TPN Eff.) and enterally fed (Fed Eff.) small bowel was gavaged into germ-free mice (GF). A: EBF was examined using two methods, transepithelial resistance of full-thickness jejunal specimens (Ω*cm2, left) and permeation of tracer molecule FITC-dextran (4KD, right). B: small bowel mucosal cytokine results are measured with using a multiplex protein assay (Millipore, Billerica, MA). C: representative images of immunofluorescence staining of small bowel tight and adhesion junction proteins was performed and costained with a DAPI nuclear counterstain. TER, transepithelial resistance; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-12p40, interleukin 12p140; ZO-1, zonula occludens 1. *P < 0.05.
Immunofluorescence staining was performed to evaluate junctional proteins in the gavaged mice. Representative images of ZO-1, occludin, and E-cadherin are shown in Fig. 5C. The mice gavaged with Fed effluent displayed a colocalization of ZO-1 and occludin at the cell membrane along intercellular junctions. There was a decline in the intensity of this staining in the TPN effluent-gavaged mice, indicating a decrease in localized tight junction expression with exposure to TPN effluent. This pattern was seen on slides stained for E-cadherin as well. Small bowel mucosal morphology was not significantly altered (crypt depth and villus height of TPN-gavaged and Fed-gavaged mice were 48 ± 2 vs. 55 ± 4 μm; P = 0.06 and 135 ± 5 vs. 151 ± 7 μm; P = 0.06, respectively). These histological trends are similar to those previously found with conventional TPN-dependent mice (16). TPN effluent also drove a trend toward higher immune activity, with increases in inflammatory cytokines. Although only IL12p40 was statistically significant, TPN-gavaged GF mice trended toward higher levels in several other proinflammatory cytokines (Fig. 5B; full list of cytokines in Table 2). These findings demonstrate that intestinal contents from TPN-dependent mice, including TPN-associated microbiota, recapitulate the TPN phenotype in fed GF mice.
Table 2.
Cyotokine expression in germ-free mice gavaged with small bowel effluent from enterally fed or TPN-dependent mice
| Cytokine | FED Effluent | TPN Effluent | P Value |
|---|---|---|---|
| IFN-γ | 3.189 ± 0.731 | 7.143 ± 3.178 | 0.122 |
| TNF-α | 7.030 ± 1.357 | 11.41 ± 5.292 | 0.218 |
| IL-1β | 38.36 ± 9.960 | 102.1 ± 46.76 | 0.101 |
| IL-6 | 4.002 ± 1.132 | 4.827 ± 1.018 | 0.298 |
| IL-12p40 | 15.50 ± 13.40 | 77.20 ± 22.97 | 0.018* |
| IL-12p70 | 3.983 ± 2.023 | 20.96 ± 11.90 | 0.09 |
| IL-17 | 1.172 ± 0.253 | 1.566 ± 0.340 | 0.184 |
| IL-13 | 13.44 ± 5.183 | 22.84 ± 12.19 | 0.244 |
| IL-4 | 1.122 ± 0.064 | 1.746 ± 0.428 | 0.086 |
| IL-10 | 13.34 ± 1.074 | 25.33 ± 5.978 | 0.034* |
Values are expressed as concentration (pg/ml); n = 6 for each group.
P < 0.05.
DISCUSSION
Intestinal inflammation and dysfunctional EBF have been associated with TPN administration. However, the precise mechanism that drives these changes is unknown. A concurrent change in the intestinal microbial community to a less diverse population has been reported, characterized by a loss of Firmicutes and a relative expansion of Proteobacteria (14, 21, 23, 31, 37). Whether the altered microbiota results in or is the product of inflammation has never been answered. In this study, we demonstrated several novel findings. First, we showed that the route of nutrient administration results in a significant change in the metabolomic composition of the intestinal lumen. In the absence of enteral nutrients, a time-dependent change in intestinal permeability occurred that allowed passage of leucine and other TPN nutrients to efflux directly into the gut lumen. This was associated with an alteration in nutrient foraging by luminal bacteria. This is consistent with the work of others, which has shown that altered nutrient composition within the intestinal lumen influences the relative dominance of microbiota based on their specific metabolic potential (19). Using a [13C]leucine isotope tracer in TPN, we demonstrated a significant uptake of IV-delivered leucine by small bowel bacteria in the TPN-dependent mice. By using a novel FISH-NanoSIMS approach, we further show that primarily Enterobacteriaceae (phylum Proteobacteria), the dominant bacterial family in the small bowel lumen of TPN mice, have a selective advantage by metabolizing leucine. Foraging of IV-delivered leucine by this family of bacteria was significantly increased compared with other bacteria present in the lumen and far higher than that seen in enterally fed groups. Finally, we demonstrated that the introduction of small bowel effluent from TPN mice into GF mice led to a phenotype similar to that observed in TPN-dependent mice. Taking these findings together, this study indicates that the pathophysiology of the loss of EBF in the state of complete TPN dependence may be driven by a complex interaction between an altered metabolome and the luminal microbiota.
These data are consistent with the previously identified loss of EBF in the TPN-dependent setting, predominantly due to a proinflammatory response via a Myd88-dependent pathway (31). This study is the first to indicate a potentially causal relationship in that the transfer of enteral effluent from TPN-dependent mice resulted in EBF disturbances in previously germ-free mice. This study supports the theory that a major source of the adverse outcomes with enteral deprivation and TPN dependence may be epithelial barrier dysfunction driven by altered luminal microbiota. The microbiota forages substrates originating from the host serum, including the TPN-derived nutrients, which are increasingly accessible to the gut microbiota as breakdown of EBF occurs. As the microbial population shifts, the invasive nature of Proteobacteria may be responsible for, or promote, epithelial barrier breakdown. Alternatively, the loss of EBF-promoting populations dependent on enteral nutrition may be responsible for epithelial breakdown. Future work should investigate the specific virulence factors of expanded bacterial strains in the TPN-dependent setting to understand specifically how these bacteria induce or contribute to barrier breakdown (1). Once the inflammatory bacterial community is established and EBF is broken down, the leak of TPN-derived nutrients from the serum into the intestinal lumen serves to provide nutrients to those bacteria, which in turn appear to preferentially forage on nutrients that are being intravenously supplied to sustain the host. This results in a self-perpetuating phenomenon. A schematic representation of these changes is summarized in Fig. 6.
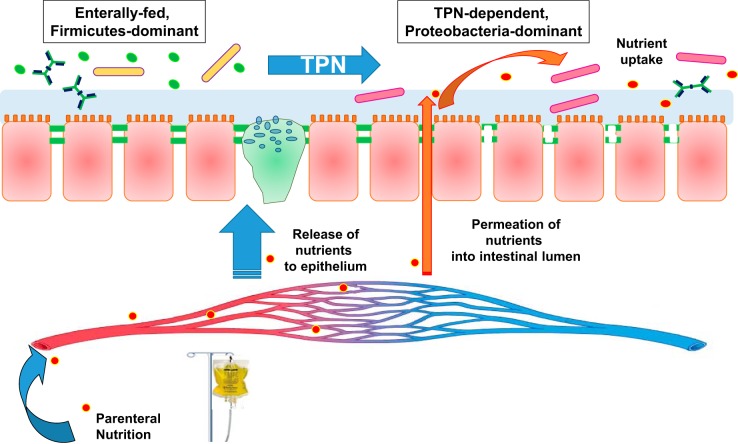
Fig. 6.
Summary of effects of TPN on the luminal metabolome and microbiome. With TPN dependence, an altered microbiome is associated with a loss of epithelial barrier function. TPN-derived serum nutrients are supplied to the epithelium while also permeating into the intestinal lumen via a transepithelial route, further supporting the altered intraluminal bacterial population.
With further breakdown of EBF, bacteria may translocate, possibly resulting in the increased incidence of infectious complications associated with TPN dependence (18). The detrimental impact of the loss of EBF has been well described in animal and human models alike (25–27). Although it is thought that changes in barrier function occur in humans, far less is known about alterations in barrier function with enteral nutrient deprivation on a clinical level (4, 7, 8, 40, 44, 49). Recent human studies, however, have suggested a similar pathophysiology, as enteral nutrient deprivation led to changes in the microbiota of the small bowel as well as to a loss of bacterial diversity in humans (36). Using a set of matched human small bowel specimens, whereby one segment received enteral nutrients and the other was enterally deprived, a loss of barrier function was found in the unfed intestine, as well as an increase in the abundance of TNF-α and Toll-like receptor 4 (35). The findings of the current mouse model may have significant translational applications. For instance, future work might investigate the permeation of other IV-delivered medications into the gut lumen of unfed patients and potential effects these substrates might have on intestinal microbiota and subsequent clinical outcomes.
The present study had some limitations. First, we investigated a single amino acid in the current study: leucine. Future studies looking at alteration of other TPN-derived nutrients may offer better insight into the nutrient requirements of this altered microbiome. Furthermore, we targeted only one Proteobacteria family in this study. While prior studies have identified Enterobacteriaceae as the dominant Proteobacteria in the TPN-dependent state, it is quite possible that other microbial populations may have relevance to other physiological changes in this TPN model. As we saw an expansion of Bacteroides and Akkermansia (to a lesser extent) in our TPN model (12), such organisms may also have important relevance to the pathophysiology of TPN-associated adverse clinical events. Finally, this study was limited by the lack of a viable model of germ-free TPN-dependent mice. Unfortunately, sterile vascular cannulation without introduction of bacteria was not possible; therefore, fed mice were used as the sole germ-free control group.
Concluding Remarks
This study provides support to the theory that the changes in the intestinal environment and microbiota are responsible for the decline in EBF seen with TPN administration. Through untargeted metabolomics and targeted stable isotope labeling analysis, we demonstrated a previously unrecognized mechanistic explanation as to how potentially harmful microorganisms exploit TPN-derived nutrients in the intestinal lumen in a state of complete host enteral nutrient deprivation. This could explain the clinical finding that patients receiving TPN have a higher perioperative complication rate. In the future, therapies directed toward maintenance of a EBF-promoting intestinal microbiota via modulation of the luminal metabolome may lead to a decrease in infectious complications in TPN-dependent patients.
GRANTS
This research was supported in part by NIH 2R01AI-44076 (to D. H. Teitelbaum), the Michigan Regional Comprehensive Resource Core (R24 DK097153), the Austrian Science Fund (FWF, P26127-B20), the Vienna Science and Technology Fund (WWTF, LS12-001), and the Michigan Nutrition Obesity Research Center (P30 DK089503).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author (s).
AUTHOR CONTRIBUTIONS
M.W.R., F.R.D., Y.F., C.F.B., and D.H.T. conception and design of research; M.W.R., F.R.D., A.L., and B.H. performed experiments; M.W.R., F.R.D., Y.F., S.R., C.R., A.S., A.L., B.H., D.B., and D.H.T. analyzed data; M.W.R., F.R.D., Y.F., S.R., C.R., A.S., A.L., B.H., D.B., C.F.B., and D.H.T. interpreted results of experiments; M.W.R., F.R.D., B.H., and D.B. prepared figures; M.W.R., F.R.D., B.H., and D.B. drafted manuscript; M.W.R., F.R.D., A.S., A.L., B.H., D.B., C.F.B., and D.H.T. edited and revised manuscript; M.W.R., F.R.D., Y.F., S.R., C.R., A.S., A.L., B.H., D.B., C.F.B., and D.H.T. approved final version of manuscript.
REFERENCES
- 1.Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, Poroyko V, Liu DC, Zaborina O, Alverdy JC. Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Ann Surg 255: 386–393, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry D, Stecher B, Schintlmeister A, Reichert J, Brugiroux S, Wild B, Wanek W, Richter A, Rauch I, Decker T, Loy A, Wagner M. Host-compound foraging by intestinal microbiota revealed by single-cell stable isotope probing. Proc Natl Acad Sci USA 110: 4720–4725, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F, Espen. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr 28: 378–386, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, Eckhert CD, Hollander D, Gornbein J, Kopple JD, Vijayaroghavan SR. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr 19: 453–460, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Bucker R, Krug SM, Rosenthal R, Gunzel D, Fromm A, Zeitz M, Chakraborty T, Fromm M, Epple HJ, Schulzke JD. Aerolysin from Aeromonas hydrophila perturbs tight junction integrity and cell lesion repair in intestinal epithelial HT-29/B6 cells. J Infect Dis 204: 1283–1292, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, Vlasselaers D, Debaveye Y, Desmet L, Dubois J, Van Assche A, Vanderheyden S, Wilmer A, Van den Berghe G. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 365: 506–517, 2011. [DOI] [PubMed] [Google Scholar]
- 8.D'Antiga L, Dhawan A, Davenport M, Mieli-Vergani G, Bjarnason I. Intestinal absorption and permeability in paediatric short-bowel syndrome: a pilot study. J Pediatr Gastroenterol Nutr 29: 588–593, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Daims H, Stoecker K, Wagner M. Fluorescence in situ hybridization for the detection of prokaryotes. In: Advanced Methods in Molecular Microbial Ecology, edited by Osborn AM, Smith CJ. Abingdon, UK: Bios-Garland, p. 213–239, 2005. [Google Scholar]
- 10.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon 10: 350–356, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demehri FR, Barrett M, Ralls MW, Miyasaka EA, Feng Y, Teitelbaum DH. Intestinal epithelial cell apoptosis and loss of barrier function in the setting of altered microbiota with enteral nutrient deprivation. Front Cell Infect Microbiol 3: 105, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duro D, Kamin D, Duggan C. Overview of pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr 47, Suppl 1: S33–S36, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Engstrand Lilja H, Wefer H, Nystrom N, Finkel Y, Engstrand L. Intestinal dysbiosis in children with short bowel syndrome is associated with impaired outcome. Microbiome 3: 18, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y, McDunn JE, Teitelbaum DH. Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition: a potential mechanism for the development of intestinal mucosal atrophy. Am J Physiol Gastrointest Liver Physiol 298: G833–G841, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Sun X, Yang H, Teitelbaum D. Dissociation of E-cadherin and β-catenin in a mouse model of total parenteral nutrition: a mechanism for the loss of epithelial cell proliferation and villus atrophy. J Physiol 587: 641–654, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, Teitelbaum DH. Tumour necrosis factor-α-induced loss of intestinal barrier function requires TNFR1 and TNFR2 signaling in a mouse model of total parenteral nutrition. J Physiol 591: 3709–3723, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, Debaveye Y, Vlasselaers D, Desmet L, Casaer MP, Garcia Guerra G, Hanot J, Joffe A, Tibboel D, Joosten K, Van den Berghe G. Early versus late parenteral nutrition in critically ill children. N Engl J Med 374: 1111–1122, 2016. [DOI] [PubMed] [Google Scholar]
- 19.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3: 289–306, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gogos CA, Kalfarentzos F. Total parenteral nutrition and immune system activity: a review. Nutrition 11: 339–344, 1995. [PubMed] [Google Scholar]
- 21.Heneghan AF, Pierre JF, Tandee K, Shanmuganayagam D, Wang X, Reed JD, Steele JL, Kudsk KA. Parenteral nutrition decreases paneth cell function and intestinal bactericidal activity while increasing susceptibility to bacterial enteroinvasion. JPEN J Parenter Enteral Nutr 38: 817–824, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heyland D, Wischmeyer PE, Day AG; Canadian Clinical Care Trials Group. Glutamine and antioxidants in critically ill patients. N Engl J Med 369: 484–485, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Hodin CM, Visschers RG, Rensen SS, Boonen B, Olde Damink SW, Lenaerts K, Buurman WA. Total parenteral nutrition induces a shift in the firmicutes to bacteroidetes ratio in association with paneth cell activation in rats. J Nutr 142: 2141–2147, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Inoue Y, Grant JP, Snyder PJ. Effect of glutamine-supplemented total parenteral nutrition on recovery of the small intestine after starvation atrophy. JPEN J Parenter Enteral Nutr 17: 165–170, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Kiristioglu I, Antony P, Fan Y, Forbush B, Mosley RL, Yang H, Teitelbaum DH. Total parenteral nutrition-associated changes in mouse intestinal intraepithelial lymphocytes. Dig Dis Sci 47: 1147–1157, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Kiristioglu I, Teitelbaum DH. Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. J Surg Res 79: 91–96, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, Kuhl MR, Brown RO. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg 215: 503–511; discussion 511–503, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langekamp-Henken B. Effect of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma 39: 44–51, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Maranduba CM, De Castro SB, de Souza GT, Rossato C, da Guia FC, Valente MA, Rettore JV, Maranduba CP, de Souza CM, do Carmo AM, Macedo GC, Silva FS. Intestinal microbiota as modulators of the immune system and neuroimmune system: impact on the host health and homeostasis. J Immunol Res 2015: 931574, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonnell SR, Hwang SR, Rolland D, Murga-Zamalloa C, Basrur V, Conlon KP, Fermin D, Wolfe T, Raskind A, Ruan C, Jiang JK, Thomas CJ, Hogaboam CM, Burant CF, Elenitoba-Johnson KS, Lim MS. Integrated phosphoproteomic and metabolomic profiling reveals NPM-ALK-mediated phosphorylation of PKM2 and metabolic reprogramming in anaplastic large cell lymphoma. Blood 122: 958–968, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyasaka EA, Feng Y, Poroyko V, Falkowski NR, Erb-Downward J, Gillilland MG 3rd, Mason KL, Huffnagle GB, Teitelbaum DH. Total parenteral nutrition-associated lamina propria inflammation in mice is mediated by a MyD88-dependent mechanism. J Immunol 190: 6607–6615, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med 30: 2022–2029, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Perdikis DA, Basson MD. Basal nutrition promotes human intestinal epithelial (Caco-2) proliferation, brush border enzyme activity, and motility. Crit Care Med 25: 159–165, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Pfuntner A, Wier LM, Stocks C. Most frequent procedures performed in U. S. hospitals, 2010: statistical brief #149. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality, 2006. [PubMed] [Google Scholar]
- 35.Ralls MW, Demehri FR, Feng Y, Woods Ignatoski KM, Teitelbaum DH. Enteral nutrient deprivation in patients leads to a loss of intestinal epithelial barrier function. Surgery 157: 732–742, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ralls MW, Miyasaka E, Teitelbaum DH. Intestinal microbial diversity and perioperative complications. JPEN J Parenter Enteral Nutr 38: 392–399, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiga H, Kajiura T, Shinozaki J, Takagi S, Kinouchi Y, Takahashi S, Negoro K, Endo K, Kakuta Y, Suzuki M, Shimosegawa T. Changes of faecal microbiota in patients with Crohn's disease treated with an elemental diet and total parenteral nutrition. Dig Liver Dis 44: 736–742, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Sonoyama K, Fujiwara R, Takemura N, Ogasawara T, Watanabe J, Ito H, Morita T. Response of gut microbiota to fasting and hibernation in Syrian hamsters. Appl Environ Microbiol 75: 6451–6456, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Souba WW, Scott TE, Wilmore DW. Intestinal consumption of intravenously administered fuels. JPEN J Parenter Enteral Nutr 9: 18–22, 1985. [DOI] [PubMed] [Google Scholar]
- 40.Sun X, Yang H, Nose K, Nose S, Haxhija EQ, Koga H, Feng Y, Teitelbaum DH. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol 294: G139–G147, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Tao H, Liu W, Simmons BN, Harris HK, Cox TC, Massiah MA. Purifying natively folded proteins from inclusion bodies using sarkosyl, Triton X-100, and CHAPS. BioTechniques 48: 61–64, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 141: 769–776, 2011. [DOI] [PubMed] [Google Scholar]
- 44.van der Hulst RR, von Meyenfeldt MF, van Kreel BK, Thunnissen FB, Brummer RJ, Arends JW, Soeters PB. Gut permeability, intestinal morphology, and nutritional depletion. Nutrition 14: 1–6, 1998. [DOI] [PubMed] [Google Scholar]
- 44a.Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med 325: 525–532, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Wang B, Wu G, Zhou Z, Dai Z, Sun Y, Ji Y, Li W, Wang W, Liu C, Han F, Wu Z. Glutamine and intestinal barrier function. Amino Acids 47: 2143–2154, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Baumler AJ. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339: 708–711, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong JM, Esfahani A, Singh N, Villa CR, Mirrahimi A, Jenkins DJ, Kendall CW. Gut microbiota, diet, and heart disease. J AOAC Int 95: 24–30, 2012. [DOI] [PubMed] [Google Scholar]
- 48.Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy E, Star J, Weljie AM, Flint HJ, Metz DC, Bennett MJ, Li H, Bushman FD, Lewis JD. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 65: 63–72, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Crit Care Med 31: 1118–1125, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Yang H, Kiristioglu I, Fan Y, Forbush B, Bishop DK, Antony PA, Zhou H, Teitelbaum DH. Interferon-gamma expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann Surg 236: 226–234, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang H, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-gamma evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol 284: G629–G637, 2003. [DOI] [PubMed] [Google Scholar]