Alterations in de novo lipogenesis in T cells can modulate their procolitogenic potential. In this study, we show that stearoyl-CoA desaturase-1 (SCD1)-deficient T cells exhibit an altered cytokine expression profile and membrane fatty acid composition. Using the murine T-cell adoptive transfer model of chronic colitis, we demonstrate that Scd1KO CD4+CD25− T cells induce aggravated and accelerated colitis in the recipients, compared with mice receiving wild-type (WT) CD4+CD25− T cells.
Keywords: SCD1, MUFA, membrane fluidity, intestinal inflammation, colitis
Abstract
Stearoyl-CoA desaturase-1 (SCD1) is a lipogenic enzyme involved in the de novo biosynthesis of oleate (C18:1, n9), a major fatty acid in the phospholipids of lipid bilayers of cell membranes. Accordingly, Scd1KO mice display substantially reduced oleate in cell membranes. An altered SCD1 level was observed during intestinal inflammation; however, its role in modulating inflammatory bowel disease remains elusive. Herein, we investigated the colitogenic capacity of Scd1KO effector T cells by employing the adoptive T-cell transfer colitis model. Splenic effector T cells (CD4+CD25−) from age- and sex-matched wild-type (WT) and Scd1KO mice were isolated by FACS and intraperitoneally administered to Rag1KO mice, which were monitored for the development of colitis. At day 60 postcell transfer, Rag1KO mice that received Scd1KO CD4+CD25− T cells displayed accelerated and exacerbated colitis than mice receiving WT CD4+CD25− T cells. Intriguingly, Scd1KO CD4+CD25− T cells display augmented inflammatory cytokine profile and cellular membrane fluidity with a concomitant increase in proinflammatory saturated fatty acids, which we postulate to potentially underlie their augmented colitogenic potential.
NEW & NOTEWORTHY
Alterations in de novo lipogenesis in T cells can modulate their procolitogenic potential. In this study, we show that stearoyl-CoA desaturase-1 (SCD1)-deficient T cells exhibit an altered cytokine expression profile and membrane fatty acid composition. Using the murine T-cell adoptive transfer model of chronic colitis, we demonstrate that Scd1KO CD4+CD25− T cells induce aggravated and accelerated colitis in the recipients, compared with mice receiving wild-type (WT) CD4+CD25− T cells.
lipids constitute a major category of dietary nutrients, which are essential to maintain diverse physiological functions at the cellular and molecular levels, including functions of the cellular membrane, signal transduction, and protein modification. The major de novo synthesized lipotoxic saturated fatty acids (SFA) are palmitic acid (C16:0) and stearic acid (C18:0), which are converted into the less lipotoxic monounsaturated fatty acid (MUFA) palmitoleate (C16: 1 n7) and oleate (C18:1 n9). The addition of a double bond is catalyzed by the hepatic microsomal, lipogenic rate-limiting enzyme stearoyl-CoA desaturase-1 (SCD1). SCD1, also known as Δ-9 fatty acid desaturase, controls the levels of cellular SFA/MUFA ratio by catalyzing biosynthesis of MUFA from dietary or endogenously synthesized SFA precursors (22, 30).
SCD1 deficiency is well documented to potentiate a hypermetabolic state, protect against diet-induced obesity, and attenuate adipose tissue inflammation (29). Disturbances in cellular SFA/MUFA content may result in adverse metabolic and systemic effects such as inflammation and cellular stress. Indeed, the loss of SCD1 expression in mice resulted in impaired sebaceous glandular function, development of alopecia, augmented skin inflammation, and stress (47). One previous report implicated that SCD1 deficiency may increase susceptibility to intestinal inflammation (9), but this finding was disputed in a later study (34). Another study reported reduced SCD1 expression in the rectal mucosa from active ulcerative colitis patients (6). To date, the role of SCD1 in intestinal inflammation is still not completely understood.
Several recent studies have begun to demonstrate the importance of endogenous fatty acid synthesis pathway and their effects on the metabolic programming of immune cells, particularly T cells (2). Effector T cells play a pathogenic role in multiple inflammatory and autoimmune conditions and thus represent a highly attractive therapeutic targets in inflammatory bowel diseases (IBD) and autoimmune diseases. Berod et al. (3) have shown that T-cell development into either Th17 or Treg subsets can be therapeutically skewed by suppressing the glycolytic-lipogenic pathways in T cells via pharmacological inhibition of acetyl-coA carboxylase 1 (ACC1)-mediated de novo fatty acid synthesis. Although SCD1 was demonstrated to mediate critical de novo lipogenesis of MUFA in proliferating T cells (46), the question of whether loss of SCD1 may exert lasting effects on the immunological function of T cells remains unknown.
In this study, we sought to investigate the consequence of SCD1 deficiency on the activity and function of T cells. By employing the adoptive transfer model of T cell-mediated colitis, we demonstrated that Scd1KO effector T cells display enhanced colitogenic potential than their wild-type (WT) counterparts. Accordingly, recipient Rag1KO mice that received Scd1KO CD4+CD25− T cells exhibited accelerated and more severe colitis compared with mice receiving WT CD4+CD25− T cells. We further showed that SCD1 deficiency significantly alters cellular membrane fluidity and also potentiates the proinflammatory state of CD4+CD25− T cells. The accumulation of the proinflammatory SFA in Scd1KO T cells was found to be strikingly elevated, which we postulate to underlie their augmented colitogenic properties. Altogether, our study highlights the importance of SCD1 in modulating the immune functions of T cells, whereas its deficiency could potentiate its colitogenic potential in a murine model of IBD.
MATERIALS AND METHODS
Reagents.
Hexadecyltrimethylammonium bromide, hydrogen peroxide, concanavalin A, carboxyfluorescein succinimidyl ester (CFSE), Histopaque-1077, ammonium ferrothiocyanate, dipalmitoylphosphatidyl choline (DPPC), RNAlater, TRI Reagent, and FITC-dextran were purchased from Sigma (St. Louis, MO). Guaiacol (2-methoxyphenol) was obtained from Alfa Aesar (Ward Hill, MA). Duoset ELISA kits for mouse lipocalin 2 (Lcn2), keratinocyte-derived chemokine CXCL1 (KC), and soluble IL-1Ra were procured from R&D Systems (Minneapolis, MN). SYBR Green mix was procured from Quanta Biosciences (Gaithersburg, MD). PE-conjugated anti-CD4 and BB515-conjugated anti-CD25 antibodies for cell sorting were purchased from BD Biosciences (San Jose, CA). Mouse CD4+ T-cell isolation kit was purchased from STEMCELL Technologies (Vancouver, Canada). Lipid quantification kits were procured from Randox Laboratories (Crumlin, UK). The Amplex Red cholesterol assay kit was purchased from Molecular Probes (Eugene, OR). All other fine chemicals used in present study were reagent grade and procured from Sigma.
Mice.
Stearoyl-CoA desaturase-1 deficient (Scd1KO) and recombination activating gene 1-deficient (Rag1KO) mice, both mice on the C57BL/6 background, were purchased from Jackson Laboratories. Mice were bred with WT C57BL/6 mice and the resulting offspring were crossed to generate homozygous Scd1KO, Rag1KO mice, and WT littermates. Mice were maintained in the animal facility at the Pennsylvania State University. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at The Pennsylvania State University.
Whole blood and serum collection.
Blood from 8-wk-old female mice was collected into EDTA-containing Vacuette (Greiner bio-one) via nonterminal retro-orbital bleeding. Anticoagulated blood was layered onto Histopaque-1077 (Sigma) and the separation of PBMCs and red blood cells (RBCs) fractions was performed according to the manufacturer's protocol. PBMC and RBC fractions were collected into separate tubes, centrifuged, washed and resuspended with PBS to be used immediately or stored at −80°C until further analysis. For serum collection, the retro-orbital blood was collected in BD microtainer (BD Biosciences) without anticoagulant. Hemolysis-free sera were obtained after centrifugation and stored at −80°C until further analysis.
Osmotic fragility test.
Anticoagulated blood (10 μl) was added into a series of tubes with an increasing concentration of buffered salt solution [pH 7.4; NaCl (%) 0, 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.70, and 0.90; 200-μl final volume]. The tubes were gently mixed and incubated for 30 min at 25°C. Samples were centrifuged at 1,500 g for 10 min and transferred into a 96-well plate. The degree of RBC hemolysis was evaluated spectrophotometrically at 540 nm on a Biotek Eon microplate spectrophotometer.
Preparation of RBC ghosts.
RBC membranes (ghost) were prepared as described by Hanahan and Ekholm (19). Briefly, isolated RBCs were resuspended and mixed gently in isotonic Tris·HCl buffer (0.172 M, pH 7.6, 4°C). The cell suspension was centrifuged at 1000 g for 10 min at 4°C and the supernatant was discarded. RBCs were hemolyzed in hypotonic Tris·HCl buffer (0.011 M, pH 7.6, 4°C) and allowed to stand for 5 min before centrifugation at 20,000 g for 20 min at 4°C. The supernatant was carefully removed by pipetting without disrupting the RBC membranes. Membranes were repeatedly washed with 0.011 M Tris·HCl buffer as above until their color turned to creamy white. The supernatant was removed and RBC ghost were stored in −80°C until analysis.
Quantification of cellular cholesterol.
Cholesterol levels in the RBC ghost was determined by using the Amplex Red cholesterol assay kit (Molecular Probes) according to the manufacturer's protocol. For measurement of total cholesterol, samples were added to the reaction buffer containing 150 μM Amplex red reagent, 1.0 U/ml HRP, 1.0 U/ml cholesterol oxidase, and 0.1 μM cholesterol esterase. Samples were incubated at 37°C for 30 min and fluorescence readings were measured (excitation 530 nm; emission 590 nm) using Biotek Synergy Mx microplate reader. Concentration of cholesterol was determined using a standard curve (0–8 μg/ml) after correcting for background fluorescence.
Measurement of cellular phospholipid.
Total lipid extraction was performed by conventional extraction methods devised by Folch et al. (18). Briefly, RBC ghosts were dissolved in chloroform:methanol (2:1) to a 20 times volume of the pellet, respectively. The samples were agitated for 20 min on an orbital shaker at room temperature and then washed with 0.2 volume of 0.9% NaCl solution. The mixture was centrifuged at 2,000 rpm for 10 min to separate the two phases. The upper aqueous layer was discarded by siphoning, whereas the lower chloroform phase containing lipids were evaporated under nitrogen stream. The levels of phospholipid were determined by colorimetric method as described in Stewart (55). Briefly, the dried lipid extract was dissolved in 2.0 ml chloroform, added to 2.0 ml of ammonium ferrothiocyanate (N/10) in a test tube, and mixed for 1 min on a rotamixer. Following phase separation, the lower chloroform phase was removed and the optical density was measured at 488 nm. The average outer diameter was plotted in a calibration curve prepared by using dipalmitoyllecithin (DPPC) as standard.
Sorting of CD4+CD25− T cells.
For the isolation of T cells, spleens harvested from 8-wk-old female mice were crushed using frosted glass slides and washed with FACS buffer (1× PBS with 2% FBS). Cells were pooled from n = 4 mice. After the cells were pelleted, RBCs were removed using RBC lysis buffer (Sigma) according to the manufacturer's protocol. The splenocytes were collected, washed, resuspended in 2 ml FACS buffer, and filtered through 70-μm nylon cell strainer. Mouse CD4+ T-cell isolation kit (STEMCELL Technologies) was used to enrich for the total CD4+ T cell fraction. Next, the cells were incubated with PE-conjugate rat anti-mouse CD4 and BB515-conjugated rat anti-mouse CD25 antibodies (BD Biosciences). CD4+CD25− T cells were sorted to >99.5% purity using Beckman Coulter MoFlo Astrios cell sorter and used immediately or stored at −800C until further analysis. The procedures above following splenic cell isolation are performed at 4°C or on ice to ensure cell viability.
T cell proliferation assay.
Splenic CD4+CD25− T cells from age- and gender-matched WT and Scd1KO mice were isolated by using mouse CD4+ T-cell isolation kit according to manufacturer's protocol. Cells were stained with 0.5 μM CFSE dye for 7 min in dark at 37°C. Cells were washed three times with PBS containing 5% FBS and then cultured in the presence of concanavalin A (5 ng/ml) for 5 days. Proliferation of CFSE-stained T cells were assayed by quantifying degree of CFSE dilution in proliferated cells via flow cytometry. Data were presented in histogram showing CFSE-labeled T cells.
Fatty acid analysis of cell membranes.
Fatty acids were extracted from RBC pellets with hexane:isopropanol according to Hara and Radin (20). Fatty acid methyl esters were prepared using a dual methylation procedure via base-catalyzed transmethylation with sodium methoxide followed by acid methylation with methanolic HCL according to Kramer et al. (26). White blood cells were lyophilized and directly methylated using the same procedure. Fatty acid methyl esters were quantified by gas chromatography using an Agilent 6890A gas chromatograph (Agilent Technologies, Palo Alto, CA) equipped with a fused-silica capillary column [SP-2560; 100 m × 0.25 mm (inner diameter) with 0.2-μm film thickness; Supelco, Bellefonte, PA] and a flame ionization detector with hydrogen as the carrier gas. The temperature program was 70°C for 4 min, 8°C/min to 110°C, 5°C/min to 170°C for 10 min, and 4°C/min to 215°C for 23 min. Inlet and detector temperatures were 250°C with a 25:1 split ratio. Gas constant flows were held at Hydrogen carrier 1 ml/min and detector hydrogen 25 ml/min, airflow 400 ml/min, and nitrogen plus carrier at 40 ml/min. Fatty acid peaks were identified in the gas chromatographic analysis using pure methyl ester standards (GLC 780, 566, and 461; NuChek Prep, Elysian, MN). An equal weight reference standard (GLC 74; NuChek Prep) was used to determine correction factors for individual fatty acids.
Adoptive T-cell transfer model of colitis.
Sorted splenic CD4+CD25− T cells from female WT or Scd1KO mice were adoptively transferred (5 × 105 cells/mouse) into 4-wk-old female Rag1KO mice (n = 4) via intraperitoneal injection. Recipient mice were maintained for 60 days and the development of colitis was determined by monitoring for body weight, stool consistency, and fecal occult blood.
Colonic myeloperoxidase assay.
Myeloperoxidase (MPO) assay was performed according to the previously described method (53). Briefly, frozen or fresh colon tissue (50 mg/ml) was homogenized in 0.5% hexadecyltrimethylammonium bromide in 50 mM potassium phosphate buffer (pH 6.0), freeze-thawed three times, sonicated, and centrifuged (10,000 g, 4°C), and the clear supernatants were collected. The reaction was initiated by adding final concentrations of 50 mM guaiacol and 0.002% H2O2 to the clear supernatant prepared in 96-well plate (Corning). The change in absorbance at 470 nm was measured over a period of 10 min at 1-min intervals. One unit of MPO activity is defined as the amount that increases absorbance reading by 1.0 per min at 25°C, calculated from the initial rate of reaction using guaiacol as the substrate.
Enzyme-linked immunosorbent assay.
Fecal Lcn2, serum Lcn2, KC, and sIL-1Ra were measured by enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's protocol. Frozen or freshly collected feces were reconstituted (100 mg/ml) in PBS containing 0.1% Tween 20, vortexed for 30 min, and centrifuged (10,000 g, 4°C) to collect clear supernatants for fecal Lcn2 quantification as described previously (8).
Systemic immunoreactivity to flagellin and lipopolysaccharide.
To monitor the systemic immunoreactivity to flagellin and lipopolysaccharide (LPS), serum samples were diluted (1:200) and analyzed by ELISA as described by Ziegler et al. (62).
In vivo epithelial barrier permeability.
FITC-labeled dextran method to assess epithelial barrier was performed as described previously (58). Briefly, 8-wk-old female Scd1KO mice and WT littermates were fasted and deprived of water for 3 h and then gavaged with 4 kDa FITC-dextran (0.6 mg/g body wt). After 3 h, blood was collected retro-orbitally and the fluorescence intensity of each hemolysis-free serum was measured (excitation 490 nm; emission 520 nm) using Biotek Synergy Mx microplate reader.
Quantification of serum total iron.
Serum total iron was analyzed as previously described (59, 60). Briefly, serum proteins were precipitated to release all protein-bound iron by mixing the serum with equal volume of protein precipitation solution (0.1 mg/ml hydrochloric acid, 1.0 mol/l trichloroacetic acid, and 30 ml/l thioglycolic acid). Samples were centrifuged at 6,200 g for 15 min at room temperature. Supernatants were collected and the mixed with equal volume of chromogen solution (1.5 M ferrozine, 1.5 M sodium acetate). Sample absorbance was measured at 562 nm. Total iron levels were estimated using a standard curve generated by using the iron atomic absorption standard (RICCA Chemical).
Colon ex vivo culture.
Two-centimeter sections of mice proximal colon were collected and cultured in serum-free DMEM media (Sigma) supplemented with 1% penicillin-streptomycin (Sigma). After two washes with sterile PBS, the colon was transferred to 12-well culture plate (Corning) containing 1 ml of serum-free DMEM media with 1% penicillin-streptomycin and incubated for 24 h at 37°C in CO2 incubator. The cultures were then centrifuged (10,000g; 4°C) to collect clear supernatant for ELISA.
Quantitative RT-PCR.
Mouse distal colons were collected in RNAlater and stored in −80°C. Total colonic RNA was extracted using TRI Reagent according to the manufacturer's protocol. Purified RNA was used to synthesize cDNA for quantitative (q)RT-PCR using SYBR Green mix according to manufacturer's protocol. qRT-PCR was performed in StepOnePlus instrument (Life technologies, Grand Island, NY). The sequence of primers used for qRT-PCR were (sense and antisense, respectively) as follows: Lcn2 5′-AAGGCAGCTTTACGATGTACAGC-3′ and 5′-CTTGCACATTGTAGCTGTGTACC-3′; Tnfα 5′-ACTCCAGGCGGTGCCTATGT-3′ and 5′-AGTGTGAGGGTCTGGGCCAT-3′; IL-10 5′ATTTGAATTCCCTGGGTGAGAAG-3′ and 5′-CACAGGGGAGAAATCGATGACA-3′; iNOS 5′-TTTGCTTCCATGCTAATGC-GAAAG-3′ and 5′-GCTCTGTTGAGGTCTAAAGGCTCCG-3′; Ifnγ 5′-TCAAGTGGCATAGATGTGGAAGAA-3′ and 5′-TGGCTCTGCAGGATTTTCATG-3′; and 36B4 5′-TCCAGGCTTTGGGCATCA and 5′-CTTTATTCAGCTGCACATCACTCAGA-3′. The thermal profile for the reaction was initial denaturation at 95°C for 10 min and 40 cycles of denaturation (95°C for 15 s) and annealing and extension (60°C for 1 min). Relative fold difference between groups was calculated using comparative Ct (2−ΔΔCt) method. Results obtained were normalized with the housekeeping 36B4 gene.
Histology.
Colons were washed with cold PBS followed by fixation in 10% buffered formalin (Fisher Scientific) for 24 h at room temperature and then transferred into 70% ethanol and stored at room temperature. Paraffin embedding, slides preparation and hematoxylin and eosin (H&E) staining were performed at Animal Diagnostics Laboratories at The Pennsylvania State University using standard protocols. H&E-stained colon sections were graded in a blinded fashion using scoring system for transmural inflammation modified from Erben et al. (15) for the severity (0–4) and extent (0–3) of inflammatory cell infiltrates and epithelial hyperplasia (0–4).
Statistical analysis.
All values in the results are expressed as mean ± SE. Statistical analysis for significance between two groups were determined using Student's t-test (unpaired, two-tailed) with *P < 0.05 considered as significant. Graph Pad Prism 6.0 software was used to calculate statistical significance.
RESULTS
Mice deficient in SCD1 do not display overt intestinal inflammation.
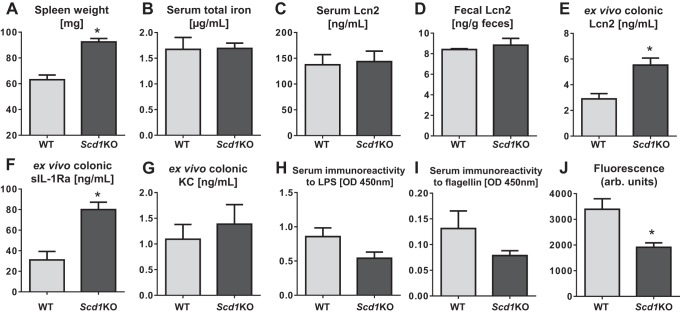
The deficiency of SCD1 in mice was implicated to exacerbate dextran sulfate sodium (DSS)-induced acute colitis in one previous study (9). However, a later study found that the susceptibility of Scd1KO mice to DSS-induced colitis is due to their increased water intake and thus DSS (34); their colitis-prone phenotype was abrogated upon adjusting for their DSS intake. Interestingly, we observed that untreated Scd1KO mice displayed an enlarged spleen (Fig. 1A), reminiscent of splenomegaly typically associated with either anemia, infection, or inflammation. Yet, the serum total iron levels were comparable between Scd1KO and WT mice (Fig. 1B), suggesting that the splenomegaly in Scd1KO mice may not be due to iron-deficient anemia. We also did not observe any overt colitis nor any difference in the basal levels of serum and fecal Lcn2 (a sensitive marker of inflammation) (8) between Scd1KO and WT mice (Fig. 1, C and D). Histological analysis of spleen sections indicated an increased activity of extra-hematopoietic erythropoiesis in Scd1KO mice (data not shown), but this may not be necessarily indicative of overt inflammation in these mice.
Fig. 1.
Scd1KO mice display splenomegaly and altered colonic expression of inflammatory markers. Eight-week-old female Scd1KO mice and their wild-type (WT) littermates (n = 5) were analyzed for spleen weight (A), serum total iron (B), serum Lcn2 (C), and fecal Lcn2 (D). Colonic sections from these mice were cultured in incomplete DMEM media for 24 h and then measured for secretion of lipocalin 2 (Lcn2; E), sIL-1Ra (F), and keratinocyte-derived chemokine CXCL1 (KC; G) into the culture media. To assess dissemination of gut contents, serum samples were analyzed for serum immunoreactivity to LPS (H) and flagellin (I). To assess gut barrier function, mice were fasted for 3 h and then gavaged with FITC-dextran (4 kDa). Mice were bled 3 h later, and serum concentration (J) of FITC were determined by fluorimetry, and represented as arbitrary fluorescence units. Results expressed as mean ± SE. *P < 0.05, unpaired t-test.
As a test to assess intestinal inflammation, we isolated colon sections from Scd1KO mice and WT littermates and cultured them ex vivo in incomplete DMEM for 24 h. We observed elevated levels of inflammatory marker Lcn2 and anti-inflammatory soluble interleukin 1 receptor antagonist (sIL-1Ra) secreted into the culture media with Scd1KO colon compared with WT colon (Fig. 1, E and F), although we did not observe difference in the levels of secreted keratinocyte-derived chemokine CXCL1 (KC; a proinflammatory marker) (Fig. 1G). Despite so, we did not observe any indication of overt intestinal inflammation at the histological level (data not shown). The elevated colonic Lcn2 and sIL-1Ra may instead reflect a mechanism to dampen the inflammatory signal or other perturbations in the gut, which then prompted us to examine whether there is any impairment in the gut barrier function.
To assess intestinal permeability to inflammation-promoting bacterial products, we next measured for their serum immunoreactivity to bacterial flagellin and LPS as a means of measuring dissemination of gut contents. We observed a decreasing trend, yet not significant, in the serum immunoreactivity to flagellin and LPS between Scd1KO and WT mice (Fig. 1, H and I), suggesting that SCD1 deficiency may not impair intestinal permeability. To further examine the gut barrier function, we orally administered FITC-dextran into mice and observed a significant reduction in the translocation of the dye in Scd1KO mice compared with WT littermates (Fig. 1J), thus confirming that the gut barrier in Scd1KO mice are not impaired. In accord with our previous study, we noted that Scd1KO mice do not appear to be susceptible to colitis at baseline nor upon treatment with IL-10 neutralization antibody (47).
Adoptive transfer of Scd1KO CD4+CD25− T cells induces an accelerated colitis in Rag1KO mice.
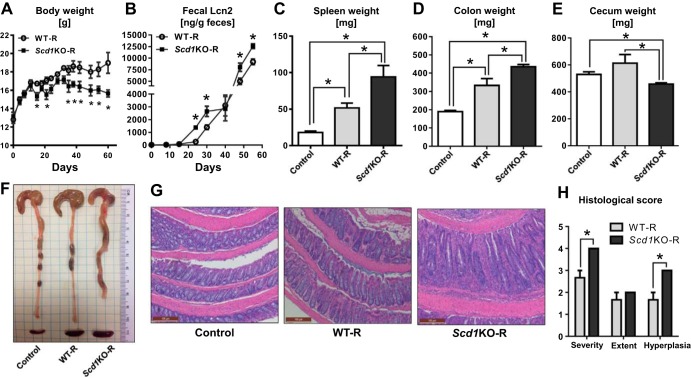
Although SCD1 deficiency is not associated with development of spontaneous colitis, our observation of splenomegaly in Scd1KO mice encouraged us to further examine whether deficiency in SCD1 may alter the functions of lymphopoietic cells, particularly T cells. Since proliferating T cells upregulate the expression of SCD1 (46), we asked whether the loss of SCD1 could influence the function of T cells and thus their inflammatory potential. Accordingly, we sorted CD4+CD25− T cells from Scd1KO mice or WT littermates by flow cytometry and adoptively transferred them into Rag1KO recipient mice. Intriguingly, Rag1KO mice receiving Scd1KO CD4+CD25− T cells (Scd1KO-R) were observed to display diarrhea and loose stools at day 20 onwards postcell transfer, whereas mice receiving WT CD4+CD25− T cells (WT-R) developed similar symptoms much later after day 35 postcell transfer (data not shown). The alterations in the body weights of these mice were variable, although we noted that the loss in body weight occurred much earlier in Scd1KO-R mice (observed at day 15 and 35) than WT-R mice (day 40) (Fig. 2A). The accelerated onset of colitis in Scd1KO-R mice was further reflected in the elevation of the inflammatory marker fecal Lcn2 (8) at earlier time points compared with WT-R mice (Fig. 2B). Mice were maintained until day 60 postcell transfer, a time point at which both Scd1KO-R and WT-R mice developed colitis. Both Scd1KO-R and WT-R mice displayed splenomegaly (compared with the small spleen distinctive of Rag1KO mice); splenomegaly in Scd1KO-R was notably more prominent than WT-R mice (Fig. 2C). In addition, Scd1KO-R mice also displayed colomegaly and shrunken ceca compared with WT-R mice (Fig. 2, D–F). At the histological level, the colon from Scd1KO-R mice displayed severe inflammation, colonic hyperplasia, and thickening of the mucosa compared with the colon from WT-R mice (Fig. 2, G and H).
Fig. 2.
Adoptive transfer of Scd1KO CD4+CD25− T cells accelerated colitis in Rag1KO recipient mice. Splenic CD4+CD25− T cells from 4 wk old female WT and Scd1KO mice (pooled from n = 4) were adoptively transferred (5.0 × 105 cells/mouse) intraperitoneally into 4 wk old female Rag1KO mice (n = 4) and monitored for 60 days. At day 60 postcell transfer, mice were euthanized and the following colitis parameters were analyzed body weights (A), fecal Lcn2 (B), spleen weight (C), colon weight (D), cecum weight (E), gross colon (F), and hematoxylin and eosin (H&E)-stained colons (G) at ×100 magnification and their corresponding (H) histological scores for the severity and extent of inflammatory cell infiltrates, and epithelial hyperplasia. Control, vehicle-treated Rag1KO mice; WT-R, Rag1KO mice receiving WT CD4+CD25− T cells; Scd1KO-R, Rag1KO mice receiving Scd1KO CD4+CD25− T cells. Results expressed as mean ± SE. *P < 0.05, unpaired t-test.
SCD1 deficiency augments systemic and colonic inflammation induced by adoptively transferred CD4+CD25− T cells.
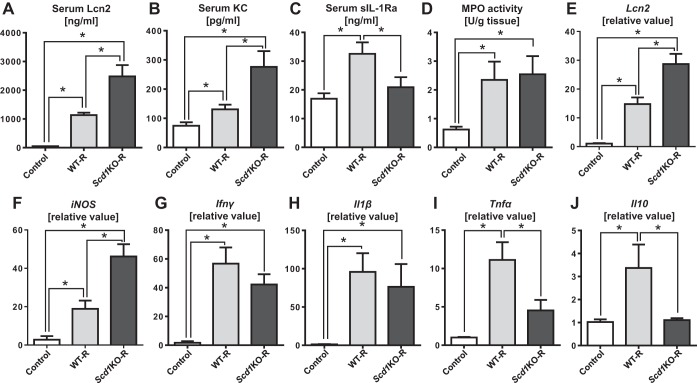
To assess colitis severity, we evaluated the levels of serum markers that correlates with the severity of inflammation. Scd1KO-R mice display elevated levels of serum Lcn2 and KC compared with WT-R mice (Fig. 3, A and B). In addition, the serum levels of the anti-inflammatory sIL-1Ra was observed to be reduced Scd1KO-R mice, but elevated in WT-R mice (Fig. 3C). We did not observe difference in the colonic MPO levels between Scd1KO-R and WT-R mice (Fig. 3D), suggesting that the endogenous neutrophil response (Rag1KO mice) may not be contributing to the augmented colitis in Scd1KO-R mice. We next assessed the mRNA expression of inflammatory markers in the colons and observed elevated expression of proinflammatory genes such as Lcn2 and iNOS in Scd1KO-R mice (Fig. 3, E and F). The mRNA level of Ifnγ and Il1β were comparable between Scd1KO-R and WT-R mice, although they were notably elevated compared with noncolitic control mice (Fig. 3, G and H). The levels of colonic Tnfα and Il10 mRNA were elevated only in WT-R, but inexplicably reduced in Scd1KO-R at levels that are more comparable to noncolitic control mice (Fig. 3, I and J).
Fig. 3.
Scd1KO CD4+CD25− T cells induces exacerbated systemic and colonic inflammation upon adoptive transfer into Rag1KO mice. WT or Scd1KO CD4+CD25− T cells (5.0 × 105 cells/mouse; pooled from n = 4 donor mice) was adoptively transferred into 4-wk-old female Rag1KO mice (n = 4). Recipient mice were euthanized at day 60 postcell transfer and then analyzed for serum Lcn2 (A), serum KC (B), serum sIL-1Ra (C), and colonic MPO (D) activity. Quantitative RT-PCR analysis was used to quantify mRNA expression of Lcn2 (E), iNOS (F), Ifnγ (G), Il1β (H), Tnfα (I), and Il10 (J). mRNA values are represented as fold-change normalized to 36B4 housekeeping gene. Control, vehicle-treated Rag1KO mice; WT-R, Rag1KO mice receiving WT CD4+CD25− T cells; Scd1KO-R, Rag1KO mice receiving Scd1KO CD4+CD25− T cells. Results expressed as mean ± SE. *P < 0.05, unpaired t-test.
Scd1KO CD4+CD25− T cells displayed an altered cytokine expression profile and impaired proliferation capacity.
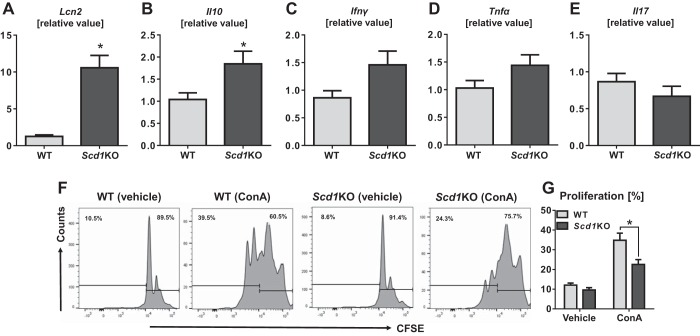
Herein, we observed that Scd1KO CD4+CD25− T cells are more colitogenic than their WT counterparts in the adoptive transfer model of T-cell-mediated colitis. To investigate the colitogenic properties of Scd1KO CD4+CD25− T cells, we again sorted them from Scd1KO mice and WT littermates to be analyzed for cytokine expression profile. Compared with WT, Scd1KO CD4+CD25− T cells display significantly augmented mRNA levels of inflammatory marker Lcn2 and anti-inflammatory Il10 genes (Fig. 4, A and B). Scd1KO CD4+CD25− T cells also exhibited increasing trend of elevated Ifnγ and Tnfα mRNA levels than WT CD4+CD25− T cells (Fig. 4, C and D). However, no difference was observed in mRNA level of Il17 between Scd1KO and WT CD4+CD25− T cells (Fig. 4E). We also assessed the proliferation capacity of CFSE-stained splenic CD4+CD25− T cells upon stimulation with concanavalin A (ConA). Both Scd1KO and WT CD4+CD25− T-cell proliferated upon ConA stimulation; however, the proliferative capacity was notably blunted in Scd1KO T cells (Fig. 4, F and G). Our findings are consistent with the notion that SCD1 plays a key role in cell proliferation, as its pharmacological inhibition have been demonstrated to impede proliferation of cancer cells in various studies (48–50). Yet in this study, we observed Scd1KO CD4+CD25− T cells to be colitogenic despite their impaired proliferative capacity.
Fig. 4.
Scd1KO CD4+CD25− T cells display increased expression of proinflammatory genes, but impaired proliferation. Splenic CD4+CD25− T cells from 4 wk old female WT and Scd1KO mice (pooled from n = 4 mice) were isolated by flow cytometer and subjected to qRT-PCR analysis for mRNA expression of (A) Lcn2, (B) Il10, (C) Ifnγ, (D) Tnfα, and (E) Il17. mRNA values are represented as fold change normalized to 36B4 housekeeping gene. For proliferation assay, T cells were stained with CFSE and cultured for five days in the presence of 5 ng/ml concanavalin A (ConA). (F) Representative histograms depicts the percentage of nonproliferating (vehicle-treated) and proliferating (ConA-treated) T cells. (G) Bar graph represents the average proliferative capacity of Scd1KO and WT T cell (n = 4) assessed in the assay. Results expressed as mean ± SE. *P < 0.05, unpaired t-test.
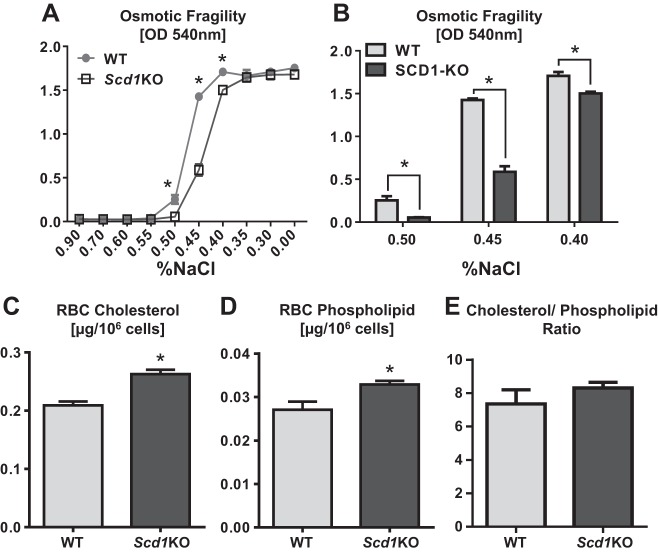
Scd1KO RBCs displayed altered augmented resistance to osmotic stress.
To provide further insights on how SCD1 deficiency could alter cellular properties, we employed RBCs that are a widely used model cell type to study cellular membrane integrity. Briefly, we treated freshly isolated Scd1KO and WT to increasing doses of osmotic stress, ranging from 0.9 to 0.0% NaCl. Intriguingly, Scd1KO RBCs exhibited a significantly increased resistance to osmotic hemolysis than WT RBCs (Fig. 5A). The augmented ability of Scd1KO RBCs to withstand osmotic stress than WT RBCs was most pronounced when cells were treated with 0.50, 0.45, and 0.40% NaCl in the order of increasing osmotic pressure (Fig. 5B). Intriguingly, we observed a significant higher levels of cholesterol and phospholipid in the membranes of Scd1KO RBCs (Fig, 5, C and D), suggesting that their enhanced ability to withstand osmotic pressure could be due to altered membrane fluidity. Yet, the ratio of membrane cholesterol-to-phospholipids was comparable between the two groups (Fig. 5E).
Fig. 5.
SCD1 deficiency augments cellular membrane integrity. Uncoagulated red blood cells (RBCs) from 4-wk-old female Scd1KO mice and WT littermates were collected and the RBCs (A) subjected to increasing osmotic pressure (from 0.90 to 0.00% NaCl). B: bar graphs represent osmotic fragility test of Scd1KO and WT RBCs in 0.50, 0.45, and 0.40% NaCl. Uncoagulated RBCs from 4-wk-old female Scd1KO and WT littermates (n = 4) were collected and prepared as RBCs ghost to be analyzed for membrane cholesterol (C), phospholipid (D), and cholesterol-to-phospholipid ratio (E). Assays are performed in triplicates and representative of 2 independent experiments. Results expressed as mean ± SE. *P < 0.05, unpaired t-test.
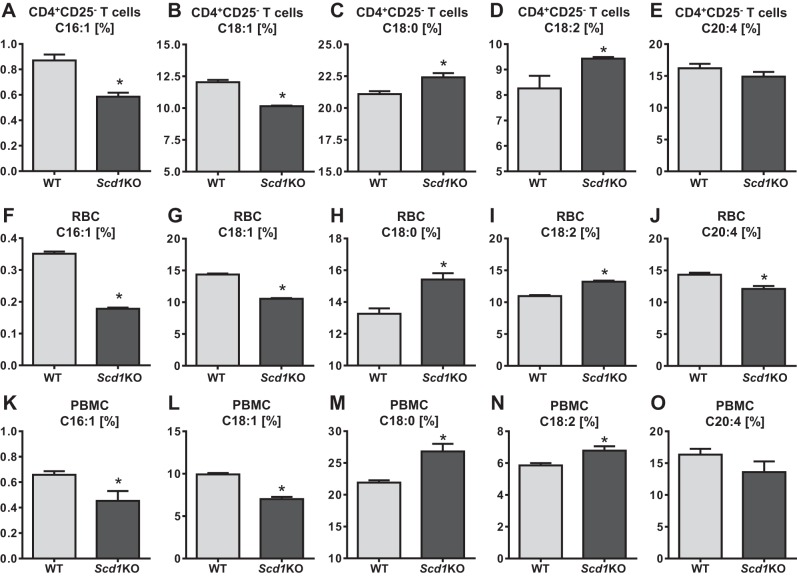
Loss of SCD1 alters the fatty acid composition of CD4+CD25− T cells and RBCs.
The observation that Scd1KO CD4+CD25− T cells are more colitogenic than WT T cells prompted us to investigate the extent to which SCD1 deficiency alters the cellular lipid profile. Accordingly, we collected the fractions of CD4+CD25− T cells, RBCs and PBMCs and analyzed their fatty acid composition via gas chromatography. Notably, a significant decrease in palmitoleate (C16:1) and oleate (C18:1) as observed in the CD4+CD25− T cells, RBCs, and PBMCs from Scd1KO mice than their WT littermates (Fig. 6, A–B, F–G, and K–L). Concurrently, we observed a significant elevation in stearate (C18:0) and linoleic acid (C18:2) in all three Scd1KO cell fractions (Fig. 6, C–D, H–I, and M–N). These alterations are characteristics of SCD1 deficiency as its activity is required to convert C18:0 to C18:1 (major product) and C16:1 (minor product). More importantly, we found that levels of MUFA was significantly reduced whereas the proinflammatory SFA was significantly elevated in Scd1KO CD4+CD25− T cells, which may help to explain their heightened inflammatory cytokine profile. We also observed a significant reduction in the levels of arachidonic acid (C20:4) only in the RBC fraction but not in the CD4+CD25− T cells and PBMC fractions of Scd1KO mice (Fig. 6, E, J, and O).
Fig. 6.
Scd1KO CD4+CD25− T cells, RBCs, and peripheral blood mononuclear cells (PBMCs) exhibited altered fatty acid composition. CD4+CD25− T cells, RBCs and PBMCs from 4-wk-old female Scd1KO mice and WT littermates (n = 4) were analyzed for their lipid composition via gas chromatography. Fatty acid compositions are expressed as percentage of the total fatty acids analyzed. Bar graph represents fatty acid profile of CD4+CD25− T cells: C16:1 (A), C18:1 (B), C18:0 (C), C18:2 (D), and C20:4 (E). Bar graph represents fatty acid profile of RBCs: C16:1 (F), C18:1 (G), C18:0 (H), C18:2 (I), and C20:4 (J). Bar graph represents fatty acid profile of PBMCs: C16:1 (K), C18:1 (L), C18:0 (M), C18:2 (N), and C20:4 (O). Results expressed as mean ± SE. *P < 0.05, unpaired t-test.
DISCUSSION
SCD1 plays an indispensable role in de novo lipogenesis of MUFA from SFA precursors. Since its discovery in the 1980s (10, 41), many subsequent studies have established the adverse role of SCD1 in promoting hepatic steatosis, hypertriglyceridemia, insulin resistance, and obesity (1, 5, 11, 37, 38, 51). Accordingly, mice deficient in SCD1 are demonstrated to be protected against diet-induced and leptin deficiency-induced obesity (12, 43, 47) which generated much interest to inhibit SCD1 activity as a strategy to treat metabolic syndrome. However, its inhibition is not without side effects since SCD1 activity is required to maintain cellular, tissue, and whole body lipid metabolism. Therefore, the present study sought to elucidate the consequence of SCD1 deficiency on effector T cells in the adoptive transfer model of chronic colitis.
The loss of SCD1 may protect against diet-induced obesity (43, 47), yet its deficiency disrupts key steps in de novo fatty acid synthesis and resulted in the accumulation of SFA, which are the substrates for SCD1. Pancreatic β-cells, for instance, are particularly susceptible to SFA-induced lipotoxicity (14, 33, 36), which is augmented upon SCD1 deficiency (7, 21, 24). Furthermore, the proinflammatory properties of SFA have also been implicated in various complications observed in Scd1KO mice including severe alopecia, sebaceous gland hypoplasia, hepatic endoplasmic reticulum (ER) stress, increased skin barrier permeability, dermatitis, and skin inflammation (4, 17, 27, 35, 40, 47). SCD1 deficiency-induced SFA accumulation has also been reported to potentiate macrophage inflammation, endothelial cell dysfunction, and atherosclerosis (5, 35, 44). The observation that SCD1 inhibition could aggravate LPS/Toll-like receptor-1 (TLR4)-driven proinflammatory responses in macrophages (5) further highlights the tight interplay between lipid metabolism and immune responses (25).
One study reported that Scd1KO mice are more susceptible to DSS-induced colitis (9). However, this finding was confounded by the higher water intake by Scd1KO mice, especially since DSS are administered via drinking water. After the adjustment for DSS/water intake, Scd1KO mice was demonstrated to develop colitis that is comparable to WT mice (34). In our previous study, we also reported that Scd1KO mice are resistant to the IL-10 receptor neutralization-induced colitis model (52). In this study, we did not observe any impairment in intestinal permeability of Scd1KO mice, yet these mice are known to display defective skin barrier (4). We also did not observe clinical symptoms of overt gut inflammation in Scd1KO mice, in spite of their enhanced susceptibility to skin inflammation and dermatitis (27). We envision that further studies with other models of experimental colitis may be required to clarify on the debate whether SCD1 deficiency plays a role in influencing the risk and susceptibility to colitis.
The expression of SCD1 was initially thought to be absent in T cells (57), yet a later study discovered a significant upregulation of SCD1 in proliferating T cells (46). Since proliferating T cells display higher SFA and MUFA content than resting T cells (46), SCD1 may play essential role in mediating the balance between cellular ratio of SFA/MUFA. In this study, we observed that Scd1KO CD4+CD25− T cells indeed have elevated SFA/MUFA ratio, implicating that SCD1 deficiency exerts lasting consequence on T cells beyond their proliferative phase. Intriguingly, the transfer of Scd1KO CD4+CD25− T cells induced a more robust chronic colitis in Rag1KO mice than the transfer of WT CD4+CD25− T cells. We postulate that the increase of proinflammatory SFA content may underlie the heightened inflammatory cytokine profile of Scd1KO CD4+CD25− T cells observed in this study. Parallel to this notion, several studies have demonstrated that SFA and PUFA treatments on T cells can modulate their cytokine responses including IL-10, IFNγ, TNFα, IL-1β, IL-6, IL-8 and IL-2 (16, 54, 56). Furthermore, the altered cellular SFA/PUFA balance was also implicated to augment the pathogenicity of Th17 cells via modulation of RORγt (a master regulator of Th17 differentiation) (55). It is not completely understood how changes in the endogenous fatty acid composition could affect T-cell function, although it is possible that such alterations may have downstream effects on the production of proinflammatory lipid mediators that potentially skew the pathogenicity of T cells (32, 61). Additionally, we also cannot rule out that the altered proinflammatory eicosanoid secretion (32) may also contribute to the colitis-prone phenotype of Scd1KO CD4+CD25− T cells.
Nonetheless, our study is in accord with the emerging concept that de novo lipogenesis greatly influences T cell differentiation and function. The prevailing paradigm of SCD1 de novo lipogenesis illustrates a sequential process (42): 1) acetyl-coA carboxylase 1 (ACC1) converts acetyl-CoA into malonyl-CoA; 2) malonyl-CoA is converted by fatty acid synthase (FASN) into palmitate, which can be further elongated into stearate; 3) both palmitate and stearate are then utilized by SCD1 to generate palmitoleate and oleate, respectively; and 4) MUFA generated from SCD1 are used for the synthesis of phospholipids, triglycerides, and cholesteryl esters in the cell. Intriguingly, the loss of ACC1 was shown to impair the peripheral persistence and proliferation of CD8+ T cells (28). More relevant to our present study, the inhibition of ACC1 has been demonstrated to block de novo fatty acid synthesis in CD4+ T cells, which skew T cells to differentiate into Treg rather than Th17 subsets (3). These studies exemplify the notion that the metabolic programming and requirement of T cells dictate their differentiation, especially since effector T cells depend more on glycolytic-lipogenic pathway than regulatory T cells (31). The consideration that ACC1 and SCD1 are involved in the same glycolytic-lipogenic metabolic pathway in T cells (3) further raises the prospect that SCD1 may also be involved in modulating T cell differentiation. This view is supported by studies elucidating that SCD1 could regulate ACC1 activity via AMP-activated protein kinase (AMPK) (13, 23, 48), although it is possible that SCD1 could also directly influence cellular differentiation (45) independently of ACC1. Despite so, we noted that further in-depth studies are required to investigate if enhanced predisposition of Scd1KO CD4+CD25− T cells to differentiate into proinflammatory subsets could, in part, explain its colitis-prone phenotype.
Altogether, our findings highlight the importance of SCD1-mediated de novo lipogenesis in preserving the homeostatic membrane lipid composition of effector T cells and thus modulating their function. Even though oleate could be acquired from the diet, it was demonstrated that dietary sources were insufficient to correct the oleate deficiency due to loss of SCD1 activity (39). The inability of the SCD1-sufficient (Rag1KO) recipient mice to mitigate the augmented colitogenic potential of adoptively transferred Scd1KO effector T cells, as observed in this study, emphasizes the disparity between endogenous and exogenous lipid sources (39). In this regard, further studies are thus warranted to provide mechanistic insight on the role SCD1 and de novo lipogenesis in the metabolic programming in T cells.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-097865 and The Pennsylvania State University, a Child Health and Human Development Seed Grant (to M. Vijay-Kumar), and National Institute of Allergy and Infectious Diseases Grant T32-AI-074551 (to B. S. Yeoh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.S.Y. and M.V.-K. conception and design of research; B.S.Y., P.S., V.S., X.X., and Y.Y. performed experiments; B.S.Y., P.S., V.S., X.X., Y.Y., M.J.K., K.J.H., B.J., and M.V.-K. analyzed data; B.S.Y., J.K.V., M.J.K., K.J.H., B.J., and M.V.-K. interpreted results of experiments; B.S.Y., P.S., and M.V.-K. prepared figures; B .S.Y., P.S., and M.V.-K. drafted manuscript; B.S.Y., P.S., J.K.V., B.J., and M.V.-K. edited and revised manuscript; B.S.Y., P.S., V.S., X.X., Y.Y., J.K.V., M.J.K., K.J.H., B.J., and M.V.-K. approved final version of manuscript.
REFERENCES
- 1.Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, Ntambi JM. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res 43: 1899–1907, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Barbi J, Pardoll D, Pan F. Metabolic control of the Treg/Th17 axis. Immunol Rev 252: 52–77, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bahre H, Tschirner SK, Gorinski N, Gohmert M, Mayer CT, Huehn J, Ponimaskin E, Abraham WR, Muller R, Lochner M, Sparwasser T. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med 20: 1327–1333, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Binczek E, Jenke B, Holz B, Gunter RH, Thevis M, Stoffel W. Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1−/−) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol Chem 388: 405–418, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen T, Zhu X, Duong MN, Wibley AL, Shah R, Davis MA, Kelley K, Wilson MD, Kent C, Parks JS, Rudel LL. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation 118: 1467–1475, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bueno-Hernandez N, Dominguez-Lopez A, Barreto-Zuniga R, Sanchez Munoz F, Yamamoto-Furusho JK. Quantification of low expressed SCD1 gene in colonic mucosa from patients with active ulcerative colitis. Inflamm Bowel Dis 17: E155, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Busch AK, Gurisik E, Cordery DV, Sudlow M, Denyer GS, Laybutt DR, Hughes WE, Biden TJ. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic beta-cells from lipoapoptosis. Diabetes 54: 2917–2924, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One 7: e44328, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Shah YM, Morimura K, Krausz KW, Miyazaki M, Richardson TA, Morgan ET, Ntambi JM, Idle JR, Gonzalez FJ. Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab 7: 135–147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christy RJ, Yang VW, Ntambi JM, Geiman DE, Landschulz WH, Friedman AD, Nakabeppu Y, Kelly TJ, Lane MD. Differentiation-induced gene expression in 3T3–L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev 3: 1323–1335, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol 26: 6786–6798, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297: 240–243, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA 101: 6409–6414, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology 144: 4154–4163, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Erben U, Loddenkemper C, Doerfel K, Spieckermann S, Haller D, Heimesaat MM, Zeitz M, Siegmund B, Kuhl AA. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol 7: 4557–4576, 2014. [PMC free article] [PubMed] [Google Scholar]
- 16.Fernanda Cury-Boaventura M, Cristine Kanunfre C, Gorjao R, Martins de Lima T, Curi R. Mechanisms involved in Jurkat cell death induced by oleic and linoleic acids. Clin Nutr 25: 1004–1014, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Flowers MT, Keller MP, Choi Y, Lan H, Kendziorski C, Ntambi JM, Attie AD. Liver gene expression analysis reveals endoplasmic reticulum stress and metabolic dysfunction in SCD1-deficient mice fed a very low-fat diet. Physiol Genomics 33: 361–372, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 19.Hanahan DJ, Ekholm JE. The preparation of red cell ghosts (membranes). Methods Enzymol 31: 168–172, 1974. [DOI] [PubMed] [Google Scholar]
- 20.Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90: 420–426, 1978. [DOI] [PubMed] [Google Scholar]
- 21.Hellemans KH, Hannaert JC, Denys B, Steffensen KR, Raemdonck C, Martens GA, Van Veldhoven PP, Gustafsson JA, Pipeleers D. Susceptibility of pancreatic beta cells to fatty acids is regulated by LXR/PPARalpha-dependent stearoyl-coenzyme A desaturase. PLoS One 4: e7266, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodson L, Fielding BA. Stearoyl-CoA desaturase: rogue or innocent bystander? Prog Lipid Res 52: 15–42, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Huang GM, Jiang QH, Cai C, Qu M, Shen W. SCD1 negatively regulates autophagy-induced cell death in human hepatocellular carcinoma through inactivation of the AMPK signaling pathway. Cancer Lett 358: 180–190, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Janikiewicz J, Hanzelka K, Dziewulska A, Kozinski K, Dobrzyn P, Bernas T, Dobrzyn A. Inhibition of SCD1 impairs palmitate-derived autophagy at the step of autophagosome-lysosome fusion in pancreatic beta-cells. J Lipid Res 56: 1901–1911, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koberlin MS, Snijder B, Heinz LX, Baumann CL, Fauster A, Vladimer GI, Gavin AC, Superti-Furga GA. Conserved circular network of coregulated lipids modulates innate immune responses. Cell 162: 170–183, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer JK, Fellner V, Dugan ME, Sauer FD, Mossoba MM, Yurawecz MP. Evaluating acid and base catalysts in the methylation of milk and rumen fatty acids with special emphasis on conjugated dienes and total trans fatty acids. Lipids 32: 1219–1228, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Krugner-Higby L, Brown R, Rassette M, Behr M, Okwumabua O, Cook M, Bell C, Flowers MT, Ntambi J, Gendron A. Ulcerative dermatitis in C57BL/6 mice lacking stearoyl CoA desaturase 1. Comp Med 62: 257–263, 2012. [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J, Walsh MC, Hoehn KL, James DE, Wherry EJ, Choi Y. Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity. J Immunol 192: 3190–3199, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Miyazaki M, Flowers MT, Sampath H, Zhao M, Chu K, Paton CM, Joo DS, Ntambi JM. Loss of stearoyl-CoA desaturase-1 attenuates adipocyte inflammation: effects of adipocyte-derived oleate. Arterioscler Thromb Vasc Biol 30: 31–38, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Strable MS, Ntambi JM. Stearoyl CoA desaturase 1: role in cellular inflammation and stress. Adv Nutr 2: 15–22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lochner M, Berod L, Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol 36: 81–91, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Lone AM, Tasken K. Proinflammatory and immunoregulatory roles of eicosanoids in T cells. Front Immunol 4: 130, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 51: 1437–1442, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald ML, Bissada N, Vallance BA, Hayden MR. Absence of stearoyl-CoA desaturase-1 does not promote DSS-induced acute colitis. Biochim Biophys Acta 1791: 1166–1172, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonald ML, van Eck M, Hildebrand RB, Wong BW, Bissada N, Ruddle P, Kontush A, Hussein H, Pouladi MA, Chapman MJ, Fievet C, van Berkel TJ, Staels B, McManus BM, Hayden MR. Despite antiatherogenic metabolic characteristics, SCD1-deficient mice have increased inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol 29: 341–347, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 52: 726–733, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki M, Dobrzyn A, Sampath H, Lee SH, Man WC, Chu K, Peters JM, Gonzalez FJ, Ntambi JM. Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferator-activated receptor-alpha. J Biol Chem 279: 35017–35024, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab 6: 484–496, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Miyazaki M, Kim YC, Ntambi JM. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res 42: 1018–1024, 2001. [PubMed] [Google Scholar]
- 40.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr 131: 2260–2268, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Ntambi JM, Buhrow SA, Kaestner KH, Christy RJ, Sibley E, Kelly TJ Jr, Lane MD. Differentiation-induced gene expression in 3T3–L1 preadipocytes Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem 263: 17291–17300, 1988. [PubMed] [Google Scholar]
- 42.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res 43: 91–104, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA 99: 11482–11486, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peter A, Weigert C, Staiger H, Rittig K, Cegan A, Lutz P, Machicao F, Haring HU, Schleicher E. Induction of stearoyl-CoA desaturase protects human arterial endothelial cells against lipotoxicity. Am J Physiol Endocrinol Metab 295: E339–E349, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Rahimi Y, Mehdizadeh A, Nozad Charoudeh H, Nouri M, Valaei K, Fayezi S, Darabi M. Hepatocyte differentiation of human induced pluripotent stem cells is modulated by stearoyl-CoA desaturase 1 activity. Dev Growth Differ 57: 667–674, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Robichaud PP, Boulay K, Munganyiki JE, Surette ME. Fatty acid remodeling in cellular glycerophospholipids following the activation of human T cells. J Lipid Res 54: 2665–2677, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampath H, Flowers MT, Liu X, Paton CM, Sullivan R, Chu K, Zhao M, Ntambi JM. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J Biol Chem 284: 19961–19973, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scaglia N, Chisholm JW, Igal RA. Inhibition of stearoylCoA desaturase-1 inactivates acetyl-CoA carboxylase and impairs proliferation in cancer cells: role of AMPK. PLoS One 4: e6812, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scaglia N, Igal RA. Inhibition of stearoyl-CoA desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int J Oncol 33: 839–850, 2008. [PubMed] [Google Scholar]
- 50.Scaglia N, Igal RA. Stearoyl-CoA desaturase is involved in the control of proliferation, anchorage-independent growth, and survival in human transformed cells. J Biol Chem 280: 25339–25349, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Singh V, Chassaing B, Zhang L, San Yeoh B, Xiao X, Kumar M, Baker MT, Cai J, Walker R, Borkowski K, Harvatine KJ, Singh N, Shearer GC, Ntambi JM, Joe B, Patterson AD, Gewirtz AT, Vijay-Kumar M. Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab 22: 983–996, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh V, Kumar M, San Yeoh B, Xiao X, Saha P, Kennett MJ, Vijay-Kumar M. Inhibition of interleukin-10 signaling induces microbiota-dependent chronic colitis in apolipoprotein e deficient Mice. Inflamm Bowel Dis 22: 841–852, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh V, Yeoh BS, Xiao X, Kumar M, Bachman M, Borregaard N, Joe B, Vijay-Kumar M. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E coli survival in the inflamed gut. Nat Commun 6: 7113, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stentz FB, Kitabchi AE. Palmitic acid-induced activation of human T-lymphocytes and aortic endothelial cells with production of insulin receptors, reactive oxygen species, cytokines, and lipid peroxidation. Biochem Biophys Res Commun 346: 721–726, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem 104: 10–14, 1980. [DOI] [PubMed] [Google Scholar]
- 56.Szamel M, Rehermann B, Krebs B, Kurrle R, Resch K. Activation signals in human lymphocytes. Incorporation of polyunsaturated fatty acids into plasma membrane phospholipids regulates IL-2 synthesis via sustained activation of protein kinase C. J Immunol 143: 2806–2813, 1989. [PubMed] [Google Scholar]
- 57.Tebbey PW, Buttke TM. Stearoyl-CoA desaturase gene expression in lymphocytes. Biochem Biophys Res Commun 186: 531–536, 1992. [DOI] [PubMed] [Google Scholar]
- 58.Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest 117: 3909–3921, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walmsley TA, George PM, Fowler RT. Colorimetric measurement of iron in plasma samples anticoagulated with EDTA. J Clin Pathol 45: 151–154, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao X, Yeoh BS, Saha P, Olvera RA, Singh V, Vijay-Kumar M. Lipocalin 2 alleviates iron toxicity by facilitating hypoferremia of inflammation and limiting catalytic iron generation. Biometals 29: 451–465, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med 15: 633–640, 2009. [DOI] [PubMed] [Google Scholar]
- 62.Ziegler TR, Luo M, Estivariz CF, Moore DA, Sitaraman SV 3rd, Hao L, Bazargan N, Klapproth JM, Tian J, Galloway JR, Leader LM, Jones DP, Gewirtz AT. Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. Am J Physiol Regul Integr Comp Physiol 294: R402–R410, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]