Abstract
It is not well understood how monosodium glutamate (MSG) affects gastrointestinal physiology, especially regarding the absorption and the subsequent transport of dietary lipids into lymph. Thus far, there is little information about how the ingestion of MSG affects the lipid lipolysis, uptake, intracellular esterification, and formation and secretion of chylomicrons. Using lymph fistula rats treated with the infusion of a 2% MSG solution before a continuous infusion of triglyceride, we show that MSG causes a significant decrease in both triglyceride and cholesterol secretion into lymph. Intriguingly, the diminished lymphatic transport of triglyceride and cholesterol was not caused by an accumulation of these labeled lipids in the intestinal lumen or in the intestinal mucosa. Rather, it is a result of increased portal transport in the animals fed acutely the lipid plus 2% MSG in the lipid emulsion. This is a first demonstration of MSG on intestinal lymphatic transport of lipids.
Keywords: chylomicron, triacylglycerol metabolism, cholesterol, enterocytes, portal transport, monosodium glutamate
monosodium l-glutamate (MSG) is a sodium salt of glutamic acid. MSG has long been used as a flavor enhancer in a variety of foods. l-Glutamate is a common amino acid; it is an important component of many proteins and peptides and is found in most human tissues, including muscle, brain, and kidneys. It is a key molecule in cellular metabolism and a major neurotransmitter in the brain.
In food, free l-glutamate elicits the fifth taste of umami and is present in a variety of fresh unprocessed foods such as fish, meat, cheese, and some vegetables (5). In human breast milk, glutamate in free form is the most abundant amino acid and accounts for more than 50% of the total free amino acid content, which is nearly 10 times higher than the levels found in cow's milk (11).
When MSG reaches the small intestine, the glutamate moiety is rapidly utilized for the production of ATP and other amino acids (including aspartic acid, alanine, proline, ornithine, citrulline, and arginine) (19). It has been suggested that chronic ingestion of dietary MSG may result in excess weight gain and obesity among other metabolic abnormalities; however, it has recently been shown that physiologically relevant doses of MSG fed chronically to mice do not induce obesity, dyslipidemia, or insulin resistance (12). In two animal models, neonatal pigs and rats, dietary MSG has been shown to enhance intestinal antioxidative capacity and reduce hepatic lipid content (9, 13, 14).
The underlying physiological effects of MSG on intestinal lipid absorption are unknown and may be an important mechanism through which dietary MSG impacts metabolism. Lipid absorption begins with the digestion of dietary triacylglycerol (TAG) in the lumen of the small intestine to form 2-monoacylglycerol (2-MAG) and fatty acid, which are then incorporated in bile salt mixed micelles. The mixed micelles deliver these digestion products to enterocytes for uptake. There, 2-MAG and fatty acid are reesterified to form TAG, which is then incorporated into chylomicrons to be carried by the lymphatic system. Short-chain fatty acids bypass this lymphatic absorption and immediately pass into the portal circulation. We hypothesized that MSG may alter these processes involved in lipid lipolysis, uptake, intracellular esterification, and formation and secretion of chylomicrons in the intestine. For this study, we used the lymph fistula mouse model to directly measure any alteration in dietary TAG absorption caused by the inclusion of MSG in a lipid meal. The lymph fistula model has significant advantages including the following: 1) it is an in vivo model, with intact circulatory, neural, and hormonal connections to the intestine; 2) the ability to measure the dynamic changes in lipid output continuously during a lipid infusion; 3) both chylomicron formation and lymph flow are not altered by anesthesia because the mice are conscious during lipid infusion; 4) the lymph we collect reflects what is secreted by the intestine in response to dietary lipid and is not obscured by lipolysis or modification that occur in the plasma compartment; and 5) mice are fed exclusively by a duodenal infusion tube to control and quantify the exact amount of lipid administered, without the confounding effects of gastric emptying.
MATERIALS AND METHODS
Animals.
Male adult Sprague Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN) and were acclimated in the animal facility at the University of Cincinnati Medical Center for 2 wk before experiments. All animal procedures were performed in accordance with approval by the University of Cincinnati Internal Animal Care and Use Committee and in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Portal vein cannulation.
The animals were fasted overnight. The animals were anesthetized with isoflurane and surgically prepared (clipped and scrubbed). A ventral midline incision was made to expose the abdominal viscera to allow the placement of a tube into the portal vein via the ileocolic vein. This placement did not occlude the flow of blood. The cannula used was Micro-Renathane type MRE-033 (OD 0.033 inches, ID 0.014 inches) manufactured by Braintree Scientific (Braintree, MA). The incision was closed by first closing the muscle layer followed by the skin layer. The portal vein cannula was heparinized, closed with a knot, and placed inside the peritoneal cavity. It was exteriorized at the time of lymphatic surgery to allow blood withdrawal.
Analgesic (Buprenex) was given to alleviate pain associated with surgery. The animals were observed for 3 days and allowed access to chow. Only animals with body weight that returned to the approximate level of the presurgical weight, with no apparent blood loss attributable to portal vein cannulation, were used for lymphatic cannulation (described in Lymph cannulation and duodenal intubation). Animals not meeting these recovery criteria were euthanized. Portal blood flow was periodically sampled (100 μl) by a syringe inserted into the cannula.
Lymph cannulation and duodenal intubation.
Rats were fasted overnight. Preoperative analgesic (Buprenex) was administered, and the animals were anesthetized with isoflurane and surgically prepared (clipped and scrubbed). The major lymphatic duct was cannulated according to the procedure described by Bollman, Cain, and Grindlay (1) with slight modifications. Instead of suture, a drop of tissue glue was used to secure the lymphatic cannula. A soft silicone tube was introduced about 1 cm into the duodenum through the fundal incision in the stomach. The duodenal infusion tube was secured in place through a transmural suture, and the fundal incision was closed by purse string suture and sealed by a drop of tissue glue. Following surgery, the animals were infused via the duodenal tube with a saline solution containing 5% glucose. The animals were allowed to recover overnight in restraining cages (Bollman cages) that were kept warm in a temperature-regulated chamber (∼30°C). Although the animals were restrained, they had considerable freedom to move backward, forward, and sideways. Analgesics were provided to alleviate pain.
Infusion of radiolabeled lipid emulsion.
In this study, rats had only lymphatic cannulation plus duodenal intubation. Two groups of animals were studied, saline control animals (n = 13) and the animals infused intraduodenally with 2% MSG in saline overnight (n = 15). In both groups of animals, the infusion was replaced the next morning with a continuous infusion of a lipid emulsion containing the following: 40 μmol triolein (labeled with 3.0 nCi/pmol glycerol tri[9,10(n)-3H] oleate), plus 7.8 μmol cholesterol (CHOL) labeled with [24-14C]CHOL, 2.5 nCi/μmol (Perkin Elmer), 7.8 μmol egg phosphatidylcholine (PC; Sigma, St. Louis, MO), and 57 μmol of sodium taurocholate (Sigma) sonicated in 3 ml of PBS with or without 2% MSG added (pH 6.4). In short, all animals received an overnight duodenal infusion of either saline or 2% MSG, followed by a continuous 6-h infusion of lipid into the duodenum. This study design allows us to examine the impact of MSG on lipid absorption post-MSG treatment. All infusate lipids and components were purchased from Sigma and were used without further purification. During experiments, the lipid emulsions were infused at a constant rate intraduodenally at 3 ml/h for 6 h. The lipid emulsion remained stable during the study, and samples of the infusate were taken both at the beginning and the end of the study; the radioactivity varied <2%.
Collection of lymph.
Fasting lymph was collected on ice for 2 h before lipid infusion, and, during the continuous lipid infusion, lymph samples were collected hourly for 6 h in microcentrifuge tubes on ice. After a brief centrifugation to eliminate the white blood cells, the remaining lymph was collected. Each lymph sample contained 10% by volume of an antiproteolytic cocktail (0.25 M EDTA, 0.80 mg/ml aprotinin) and 80 U/ml heparin. At the end of the infusion period, the rats were anesthetized with isofluorane and killed by exsanguination.
Collection of luminal samples.
Immediately after death, the small intestine was divided into four equal-length segments, and the contents of each were eluted three times with 5-ml aliquots of 10 mM sodium deoxycholate. The aliquots of the intestinal contents were added to scintillation fluid, and radioactivity was determined by scintillation counting. The stomach and colon were excised separately, with care to prevent leakage of luminal contents, and put into stoppered Erlenmeyer flasks. After being washed three times with 5 ml of 10 mM sodium taurocholate solution, the luminal washings were combined, and aliquots of the stomach and the colonic contents were used for liquid scintillation counting. The luminal washings from the various parts of the gastrointestinal tract represent lipids that were infused but not yet absorbed by the gastrointestinal tract.
Mucosal recovery.
Mucosa from the four intestinal segments were scraped with glass slides, and the lipids were extracted with chloroform/methanol (2:1, vol/vol) according to the procedure of Folch et al. (4). Aliquots were taken for radioactivity determination.
Radioactivity determination.
Radioactivity was measured in an aqueous miscible scintillant (Poly-Flour; Packard, Downers Grove, IL). The samples were counted for 10 min in a liquid scintillation spectrometer (LKB model 1209, Rackbeta). Samples were corrected for quenching by reference to a series of 3H standards that had been progressively quenched.
Gene expression.
Duodenal, jejunal, and ileal samples were collected from rats after infusion of either MSG or saline for gene expression measurement. Total RNA was extracted using RNeasy isolation Kit (Qiagen, Valencia, CA) and converted into complementary DNA (cDNA) using iScript (Bio-Rad, Hercules, CA). The cDNA from these samples was processed for quantitative PCR analysis. Quantitative real-time PCR was performed on a Bio-Rad iCycler system. The following primers were used: CD36, forward ACGACTGCAGGTCAACATACTGGT, reverse TGGTCCCAGTCTCATTTAGCCACA; DGAT2, forward CAAGAAGTTCCCTGGCATAA, reverse GTATACCTCATTCTCTCCAAAGG; monoacylglycerol acyl transferase 2 (MGAT2), forward GAGGTTCCGCATCTACAAAC, reverse GCCGTCTTTATCGACATTCC; microsomal triglyceride transfer protein (MTP), forward TCAGGTGCTGGGTGTCACTTCAAA, reverse ATTACTCCTGCCACTTGCTTCCCA; FABP2, forward AAGGAATAGGCCAGCTTCTTG, reverse CAGTGAGTGAGCCTGCATTAT; cyclophilin A, forward CGACTGTGGACAGCTCTAAT, reverse CCTGAGCTACAGAAGGAATG.
Statistical analysis.
Data were first analyzed using SPSS GLM repeated measures with treatment as between-subject factor and lymph flow rate with time as within-subject factor. There were significant effects of both time and treatment on a number of the parameters we determined in the lymph and significant interaction between time and treatment. The particular parameter at each particular time point was further analyzed using t-test with Levene's test for equality of variance. **P < 0.01; ***P < 0.001.
RESULTS
Lymphatic radioactive triolein and CHOL output.
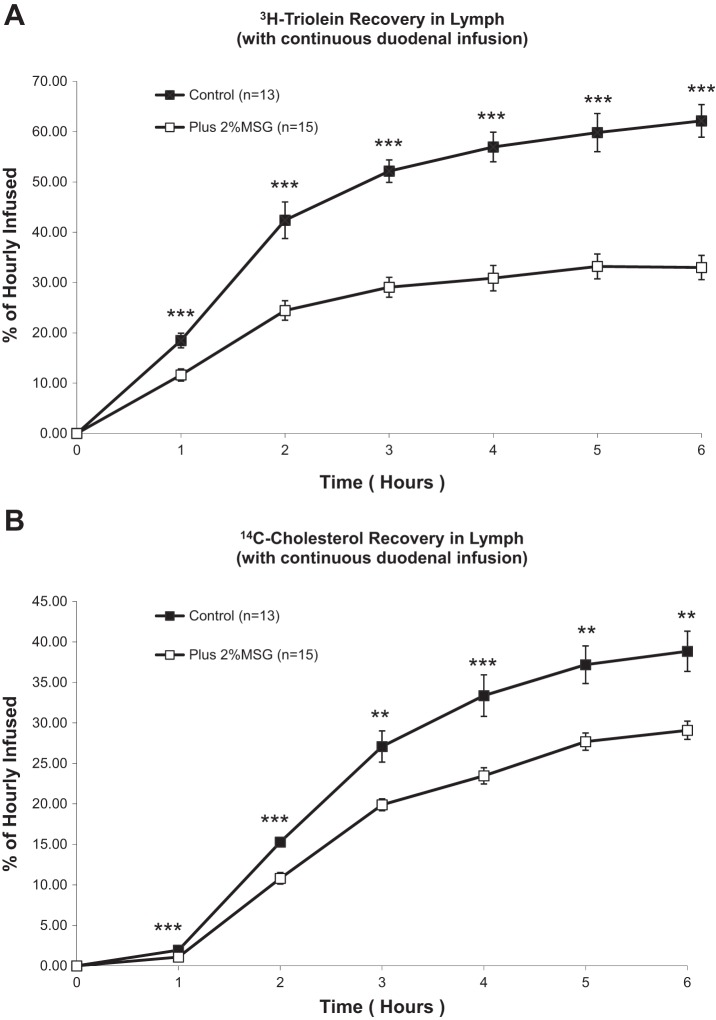
Rats that received 2% MSG before the continuous duodenal infusion of a labeled triolein emulsion had greatly depressed lymphatic transport of radioactive triglyceride (TG) (Fig. 1A). The differences between the control and the MSG groups are significant at each hour during the 6-h study, and the difference becomes greater at the later time points. There were also significant effects of MSG treatment on the recovery rate of CHOL in lymph (Fig. 1B). 14C-CHOL transport increased as a result of the lipid infusion in both groups, but treatment with MSG significantly blunted this increase, mirroring the effect of TG secretion into lymph in Fig. 1A. As with the TG secretion rates, the difference was significant from hours 1–6 of lipid infusion.
Fig. 1.
A: lymphatic transport of radioactive triolein in lymph fistula rats infused intraduodenally with a lipid emulsion with or without 2% monosodium glutamate (MSG) added. Hourly output values are expressed as a percentage of the hour-infused radioactive triolein. B: lymphatic transport of radioactive cholesterol in lymph fistula rats infused intraduodenally with a lipid emulsion with or without 2% MSG added. Hourly output values are expressed as a percentage of the hour-infused radioactive triolein. **P < 0.01; ***P < 0.001.
As shown in Fig. 2, the lymph flow rates were not significantly different between rats treated with MSG or saline before the lipid infusion; therefore the change in TG and CHOL output is not due to a change in lymph flow or intestinal fluid balance.
Fig. 2.
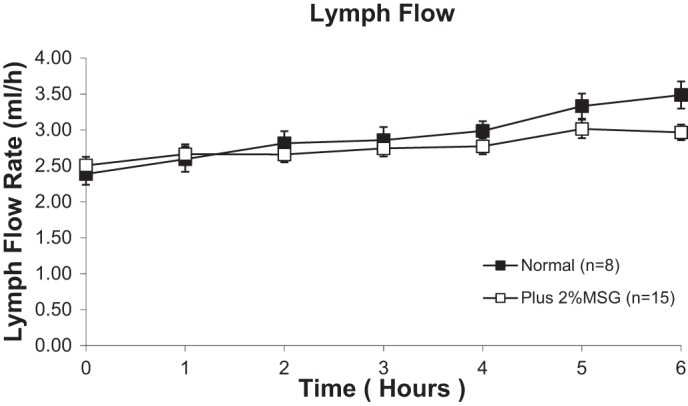
Lymph flow rate in lymph fistula rats infused intraduodenally with a lipid emulsion with or without 2% MSG added. There were no statistically significant differences between rats, as determined by 2-way repeated-measures ANOVA. Values are means ± SE.
Luminal and mucosal recoveries of 3H-triolein and 14C -CHOL.
The lower lymphatic TG and CHOL outputs could be a result of differences in the uptake of both TG and CHOL from the intestinal lumen. After lipid infusion and lymph collection, the contents of both the lumen and the intestinal mucosa [divided in four equal length intestinal segments (L1–L4)] were collected. As shown in Fig. 3A, there were no significant differences in the total luminal recovery of 3H-TG between the control and the MSG-treated rats. However, we did find a small but significant decrease in the recovery of TG in the most distal segment (L4), coupled with a trend toward an increase in luminal TG in the MSG-treated rats, although this did not ultimately lead to a statistically significant increase in total luminal TG recovery in the MSG-treated rats. It is possible that the enterocytes were not as efficient in packaging the absorbed lipid into chylomicrons. If so, then one would expect more radioactive TG accumulating in the intestinal mucosa. As shown in Fig. 3B, this may be the case. We isolated mucosa from rats after lymph collection and measured the accumulation of radioactive TG from the four equal-length intestinal segments (M1 being most proximal and M4 being most distal). Most of the radioactive TG was absorbed by the first and second segments of the small intestine in both groups of animals. Supporting the luminal data, very little of the radioactive TG was absorbed by the lower segments of the small intestine. Again, we see a trend toward decreased mucosal TG accumulation in the MSG-treated rats.
Fig. 3.
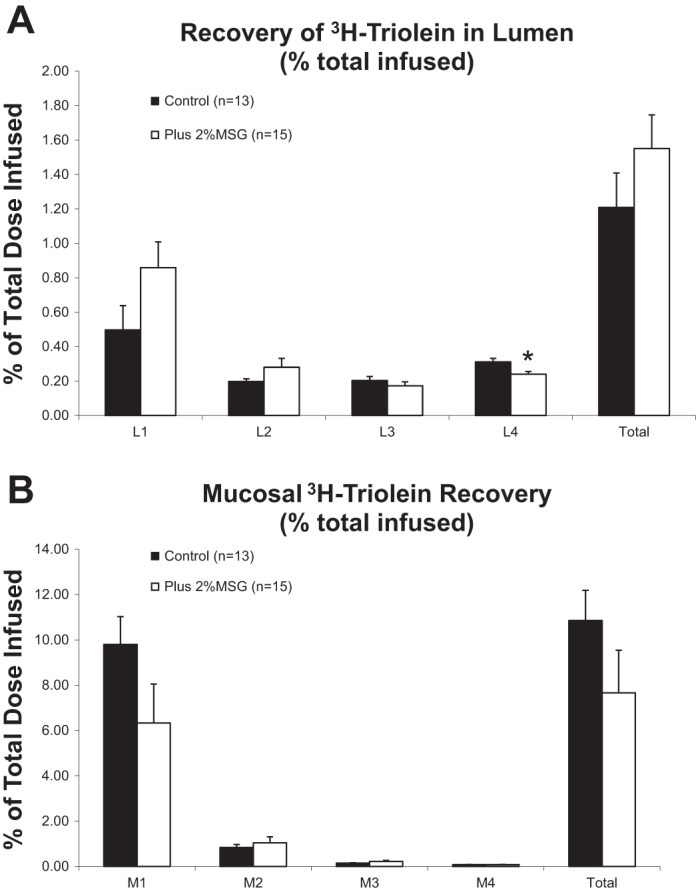
A: 3H-triolein recovery rate in lumen as total and each part of the lumen were analyzed using t-test with Levene's test for equality of variance. L1–L4 represent the 4 equal-length segments from the proximal (L1) to the distal (L4). MSG significantly increased 3H-triolein recovery in lumen as total and decreased 3H-triolein recovery in L4. MSG had a tendency to increase 3H-triolein recovery rate in L1 (P = 0.095) while decreasing 3H-triolein recovery in stomach (P = 0.068). *P < 0.05. B: 3H-triolein recovery rate in total mucosa and each individual segment were analyzed using t-test with Levene's test for equality of variance. MSG did not significantly affect 3H-triolein recovery in each part of the mucosa and in the total mucosa. However, MSG showed a tendency of decreased 3H-triolein in M1 (P = 0.076).
The luminal recoveries of 14C-CHOL were also measured (Fig. 4A). We found a statistically significant decrease in the recovery of CHOL from the third luminal segment (L3) in animals treated with 2% MSG although this did not result in a statistically significant decrease in total CHOL recovery. MSG was not associated with an accumulation of excess radioactive CHOL in the intestinal mucosa relative to the control animals (Fig. 4B).
Fig. 4.
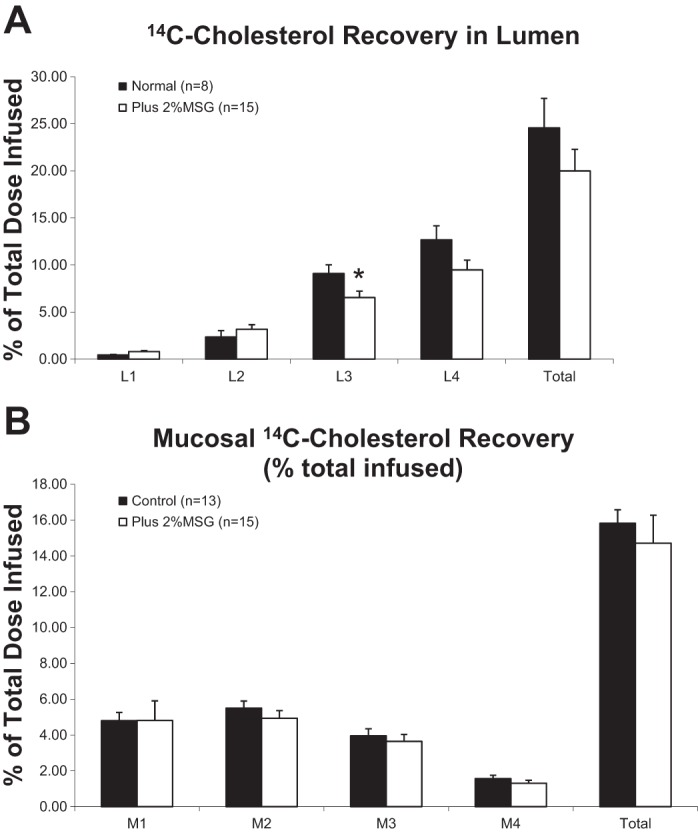
A: 14C-cholesterol recovery rate in the lumen of the different gastrointestinal segments expressed as a percentage of the total infused. L1–L4 represent the 4 equal-length segments from the proximal (L1) to the distal (L4). The luminal recovery of each segment was analyzed using t-test with Levene's test for equality of variance. MSG significantly decreased 14C-cholesterol recovery in the lumen as total and in L3. MSG also showed a tendency to decrease 14C-cholesterol recovery rate in L4 (P = 0.071). *P < 0.05. B: 14C-cholesterol recovery rate in 4 intestinal mucosa segments and the total recovery expressed as a percentage of the infused. Each mucosal segment was analyzed using t-test with Levene's test for equality of variance. MSG did not show an effect on 14C-cholesterol recovery in mucosa.
Portal transport of 3H-TG and 14C-CHOL in control and 2% MSG animals.
Because we cannot entirely account for the suppressed lymphatic transport of 3H-TG and 14C-CHOL both from the recovery of lipids in the intestinal lumen or in the mucosa, one possibility is that there could be increased transport of the labeled lipids by the portal circulation. Although we cannot measure portal blood flow in our model, we utilized the concept that 3-O-methyl glucose is a small molecule, water soluble, and it is transported exclusively by the portal vein. Consequently, by comparing the ratio of 3H-triolein counts in the portal vein to the 14C-3-O-methyl glucose in both the MSG and control animals, we compared the relative portal transport of 3H-triolein in the MSG animals relative to controls. The aim of this experiment was to determine whether the portal transport of lipid is increased in the MSG animals to account for the decreased transport into lymph. Similar to the previous study, both the labeled 3-O-methyl glucose and the lipid emulsion were introduced as a continuous infusion into the duodenum. As shown in Fig. 5A, the transport of 3-O-methyl glucose in portal blood (expressed as a percentage of the dose administered per 50 μl of portal plasma) was superimposable between the MSG animals and the control animals, indicating that the absorption of 3-O-methyl glucose and the portal blood flow was comparable between the MSG animals vs. the controls. In Fig. 5B, the transport of 3H-triolein in portal blood was also expressed as a percentage of the dose administered per 50 μl of portal plasma. It is obvious that the MSG animals had a higher portal transport and expressed a higher percentage of the dose administered per 50 μl of portal plasma than the control animals for all the time points during the study. A sum of the total relative absorption of 3H-triolein to 14C-3-O-methyl glucose over the 6-h infusion period is presented in Table 1 and is averaged and plotted in Fig. 6. MSG-treated animals are transporting significantly more radioactive TG into portal blood than the control animals. Although one may argue that the size of the difference is minor, one should bear in mind that the portal blood flow is extremely rapid, between 14 and 20 ml/min, and such small differences in portal transport can add up to significantly different amounts of 3H-triolein being transported by the MSG animals.
Fig. 5.
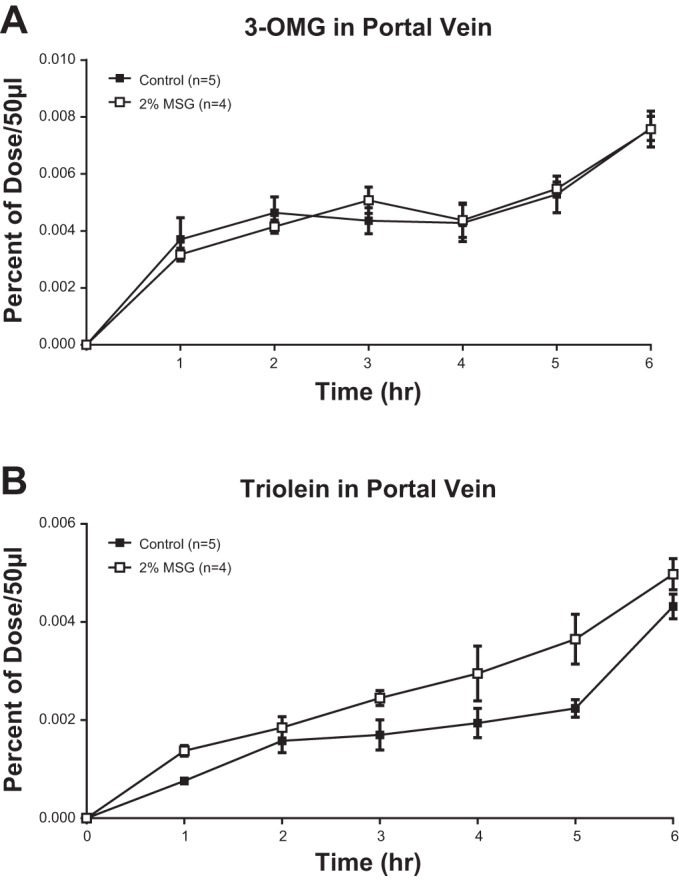
A and B: rats fitted with both mesenteric lymph and portal vein cannulas were infused intraduodenally continuously with a lipid emulsion containing labeled [14C]3-O-methylglucose (OMG) and [3H]-triolein with or without 2% MSG. Timed samples of 50 μl of portal plasma were analyzed and presented as percentages of doses infused per 50 μl of portal plasma (means ± SE shown). A: [14C]3-OMG in portal vein plasma. B: 3H-triolein in portal vein plasma.
Table 1.
Ratio of portal 3H-triolein to 14C-3-OMG over 6 h
| Total Area Under Curve | Control | 2% MSG | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Triolein | 0.0121 | 0.0085 | 0.0109 | 0.0122 | 0.0083 | 0.0181 | 0.0112 | 0.0141 | 0.0158 |
| 3-OMG | 0.0273 | 0.0199 | 0.0261 | 0.0320 | 0.0252 | 0.0233 | 0.0271 | 0.0277 | 0.0261 |
| Ratio of Triolein/3-OMG | 0.4440 | 0.4246 | 0.4165 | 0.3818 | 0.3300 | 0.7785 | 0.4114 | 0.5072 | 0.6034 |
| Average | 0.3994 | 0.5752 | |||||||
| P (t-test) | 0.0457 | ||||||||
Summated ratio of the portal transport of 3H-triolein to 14C-3-O-methyl glucose (OMG) in both control and 2% monosodium glutamate (MSG)-treated animals over the 6-h experiment.
Fig. 6.
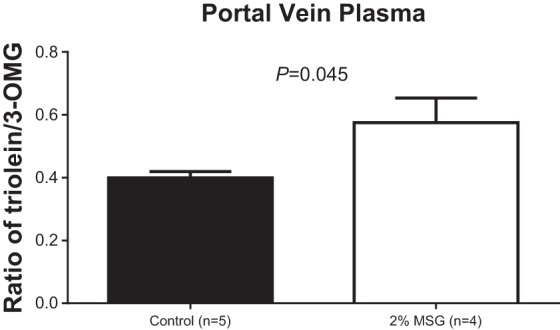
The average of the ratio of 3H-triolein/14C-3-OMG in the control and the 2% MSG animals. Ratios are expressed as means ± SE.
Expression of mucosal transporters and lipid synthetic genes.
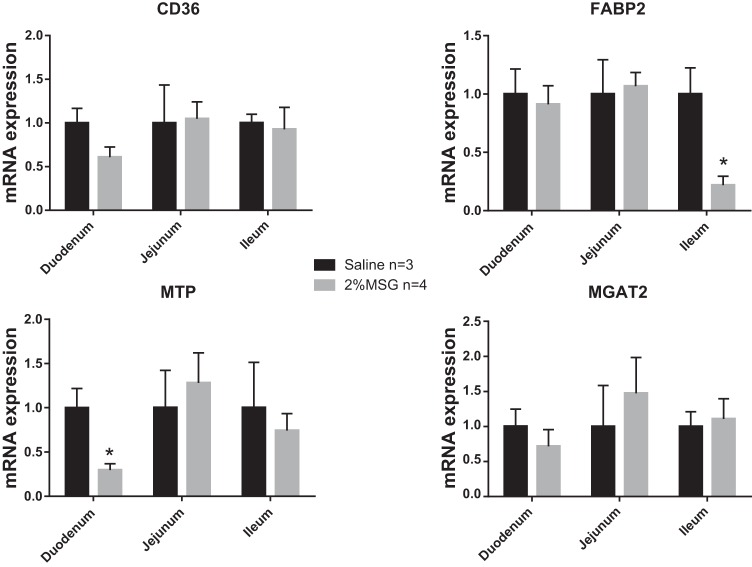
Because we observe a difference in lymphatic accumulation of TG in the MSG-treated rats, we measured the expression of key fatty acid transporters and triglyceride resynthesis machinery in the mucosa. Major steps in this pathway include the transport of fatty acids via fatty acid transporters CD36 and FABP2, the coordinated action of MGAT, followed by the lipidation of the nascent chylomicron particle by MTP. As shown in Fig. 7, there is no change in the expression of CD36, FABP2, and MGAT2, but there is a decrease in the duodenal expression of MTP.
Fig. 7.
RNA was isolated from mucosa of MSG- or saline-treated rats after lipid infusion (n = 3–4 per group). RNA expression was determined by quantitative real-time RT-PCR relative to rat cyclophilin B. Each bar represents the mean ± SE, *P < 0.05.
DISCUSSION
This finding that the treatment of rats with MSG reduces the absorption of both triolein and CHOL via the lymph and increased their absorption via the portal vein is novel and may have relevance to the metabolism of fat when MSG is fed in the diet. Of all macronutrient absorption, lipid is by far the most complex because it involves, not only membrane transporters such as CD36 and FABP, but also the mucosal esterifying enzymes that convert the absorbed 2-MAG and fatty acid to TG for packaging into chylomicrons. In addition, one of the crucial steps in chylomicron formation is the formation of the apolipoprotein B (apoB)-48-containing particles and the subsequent lipidation of these primordial lipid particles to form chylomicron particles (2, 3).
There is one possibility to account for the diminished lymphatic outputs of radioactive TG and CHOL, and that is increased transport of these radioactive lipids via the portal route to the liver. One can argue, however, for another mechanism to explain our observation, that there may be increased metabolism of the fatty acids as fuel for the intestinal epithelial cells in the presence of MSG. However, we do not believe this possibility is likely because this does not apply to CHOL because it is not used as a source of fuel for cells, but lymphatic CHOL output was also significantly reduced by MSG. Furthermore, Windmueller and his colleagues (17, 18) demonstrated that the gut mostly uses glutamine as fuel rather than fatty acids.
Although we cannot measure portal blood flow in our model, we utilized the concept that 3-O-methylglucose is a small molecule and also water soluble that is transported exclusively by the portal vein. Consequently by comparing the ratio of 3H-triolein count in the portal vein to the 14C-O-methyl glucose in both the MSG and the control animals, we can compare the relative portal transport of 3H-triolein in the MSG animals relative to controls. The aim of this study was to determine whether the portal transport of radioactive triolein is increased in the MSG animals to account for the decreased transport in lymph. This approach has been used for the determination of portal transport of other lipophilic molecules such as hexachlorobenzene (7). It is interesting that the MSG animals had a higher portal transport of labeled TG, expressed as a percentage of the dose administered per 50 μl of portal plasma, than the control animals for all the time points during the study. Although the difference observed is not huge, one should note that the portal blood flow is extremely rapid, between 14 and 20 ml/min (15), so small differences in portal transport can add up to significantly different amounts of 3H-triolein being transported to the liver for metabolism by the MSG animals.
Both apoB and MTP are crucial for the efficient synthesis and secretion of chylomicrons (6, 20). Patients with abetalipoproteinemia who have mutations in the MTP gene lose their ability to transfer lipid to apoB-containing TG-rich lipoproteins and thus have decreased lipid absorption, underscoring the importance of MTP in transferring lipid to nascent chylomicrons. We measured the expression of several genes in the chylomicron synthetic pathway, but the only change we observed is in MTP expression. The decrease in MTP gene expression upon MSG feeding could partially explain the change in lipid absorption in these animals away from chylomicron synthesis and secretion toward the portal route normally reserved for medium-chain TGs (10).
Increased intestinal permeability occurs when the epithelial barrier function of the intestine is disrupted, most often by intestinal inflammation as in inflammatory bowel disease (8, 16). Diarrhea is one of many symptoms of increased intestinal permeability. One possible explanation for the altered lipid absorption in rats receiving MSG is that the MSG itself triggers an inflammatory response, leading to disrupted enterocytes lining the small intestine, thus destroying normal absorptive function. We do not observe any markers of altered intestinal permeability in rats receiving MSG, specifically no diarrhea.
These data are exciting because this is the first time that it has been demonstrated that MSG has such a profound effect on the lymphatic transport of TG. An unanswered question is how the absorption of fat via portal vein vs. the lymphatics alters the metabolism of a fatty meal containing MSG. Chylomicrons distribute fatty acids for rapid metabolism by peripheral tissues, and the liver takes up the chylomicron remnants. In contrast, fatty acids transported by the portal blood are taken up by the liver and can be metabolized or further packaged into very-low-density lipoproteins for distribution into the periphery. It has been suggested that medium- and short-chain fatty acids may be metabolically beneficial in some clinical conditions through their direct absorption via portal blood. If MSG treatment results in a similar absorptive repartitioning, this could have a favorable outcome. In addition, for patients with altered gut lipid absorption (i.e., patients with ulcerative colitis and Crohn's disease), this may be a mechanism for increasing nutrient absorption.
In conclusion, the results from this study clearly indicate that MSG enhances the transport of TG and CHOL via the portal circulation. The enhanced portal transport and the reduced lymphatic transport may impact the metabolism of lipids. One probable consequence is that the liver will be handling more of the absorbed TG first before peripheral metabolism. The long-term consequence of this will have to be evaluated in more appropriate chronic feeding studies.
GRANTS
This work was supported by National Institutes of Health grants DK092138 and DK059630 (P. Tso), DK101663 (A. Kohan), as well as a grant from the Ajinomoto Company.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.B.K., D.L., and P.T. analyzed data; A.B.K. and P.T. interpreted results of experiments; A.B.K., M.X., and D.L. prepared figures; A.B.K. drafted manuscript; A.B.K. and P.T. edited and revised manuscript; A.B.K., D.L., and P.T. approved final version of manuscript; Q.Y., M.X., and D.L. performed experiments; P.T. conception and design of research.
REFERENCES
- 1.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med 33: 1349–1352, 1948. [PubMed] [Google Scholar]
- 2.Davidson NO, Kollmer ME, Glickman RM. Apolipoprotein B synthesis in rat small intestine: Regulation by dietary triglyceride and biliary lipid. J Lipid Res 27: 30–39, 1986. [PubMed] [Google Scholar]
- 3.Davidson NO, Magun AM, Brasitus TA, Glickman RM. Intestinal apolipoprotein A-I and B-48 metabolism: Effects of sustained alterations in dietary triglyceride and mucosal cholesterol flux. J Lipid Res 28: 388–402, 1987. [PubMed] [Google Scholar]
- 4.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 5.Giacometti. Free and bound glutamate in natural products. In: Glutamic Acid: Advances in Biochemistry and Physiology. New York, NY: Raven, 1979, p. 25–34. [Google Scholar]
- 6.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res 44: 22–32, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Jandacek RJ, Rider T, Yang Q, Woollett LA, Tso P. Lymphatic and portal vein absorption of organochlorine compounds in rats. Am J Physiol Gastrointest Liver Physiol 296: G226–G234, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji Y, Sakata Y, Tso P. Nutrient-induced inflammation in the intestine. Curr Opin Clin Nutr Metab Care 14: 315–321, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondoh T, Torii K. MSG intake suppresses weight gain, fat deposition, and plasma leptin levels in male Sprague-Dawley rats. Physiol Behav 95: 135–144, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Mansbach CM II, Dowell RF, Pritchett D. Portal transport of absorbed lipids in rats. Am J Physiol Gastrointest Liver Physiol 261: G530–G538, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Mehaia M, Al-Kanhal MA. Taurine and other free amino-acids in milk of camel, goat, cow and man. Milchwissenschaft 47: 351–353, 1992. [Google Scholar]
- 12.Nakamura H, Kawamata Y, Kuwahara T, Smriga M, Sakai R. Long-term ingestion of monosodium L-glutamate did not induce obesity, dyslipidemia or insulin resistance: A two-generation study in mice. J Nutr Sci Vitaminol (Tokyo) 59: 129–135, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura H, Kawamata Y, Kuwahara T, Torii K, Sakai R. Nitrogen in dietary glutamate is utilized exclusively for the synthesis of amino acids in the rat intestine. Am J Physiol Endocrinol Metab 304: E100–E108, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Ren X, Ferreira JG, Yeckel CW, Kondoh T, de Araujo IE. Effects of ad libitum ingestion of monosodium glutamate on weight gain in C57BL6/J mice. Digestion 83, Suppl 1: 32–36, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Sherman IA, Dlugosz JA, Barker F, Sadeghi FM, Pang KS. Dynamics of arterial and portal venous flow interactions in perfused rat liver: An intravital microscopic study. Am J Physiol Gastrointest Liver Physiol 271: G201–G210, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47: 804–811, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Windmueller HG, Wu AL. Biosynthesis of plasma apolipoproteins by rat small intestine without dietary or biliary fat. J Biol Chem 256: 3012–3016, 1981. [PubMed] [Google Scholar]
- 18.Wu AL, Windmueller HG. Relative contributions by liver and intestine to individual plasma apolipoproteins in the rat. J Biol Chem 254: 7316–7322, 1979. [PubMed] [Google Scholar]
- 19.Wu G, Borbolla AG, Knabe DA. The uptake of glutamine and release of arginine, citrulline and proline by the small intestine of developing pigs. J Nutr 124: 2437–2444, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Zamel R, Khan R, Pollex RL, Hegele RA. Abetalipoproteinemia: Two case reports and literature review. Orphanet J Rare Dis 3: 19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]