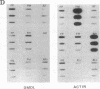
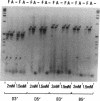
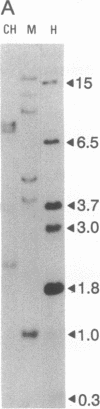
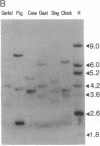
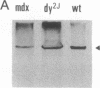
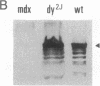
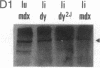
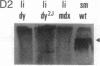
Abstract
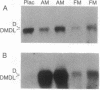
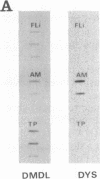
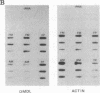
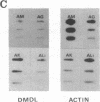
We have previously reported a dystrophin-related locus (DMDL for Duchenne muscular dystrophy-like) on human chromosome 6 that maps close to the dy mutation on mouse chromosome 10. Here we show that this gene is expressed in a wide range of tissues at varying levels. The transcript is particularly abundant in several human fetal tissues, including heart, placenta, and intestine. Studies with antisera raised against a DMDL fusion protein identify a 400,000 Mr protein in all mouse tissues tested, including those of mdx and dy mice. Unlike the dystrophin gene, the DMDL gene transcript is not differentially spliced at the 3' end in either fetal muscle or brain.
Full text
PDF
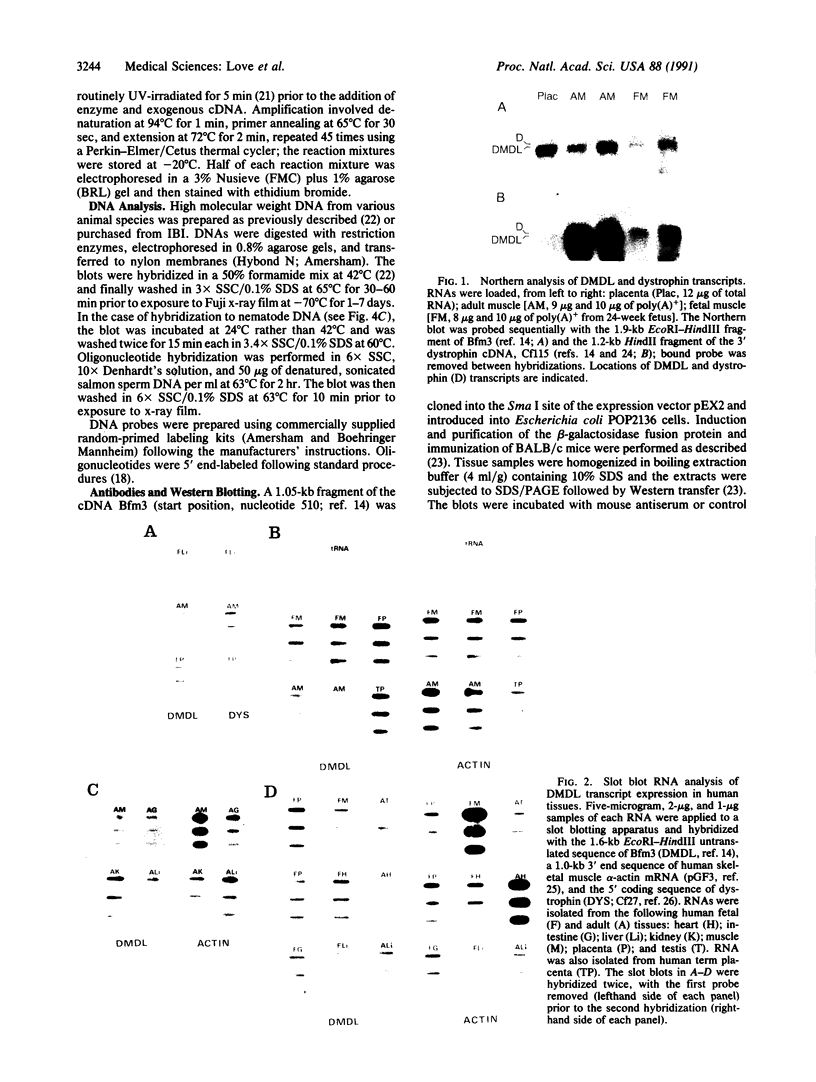
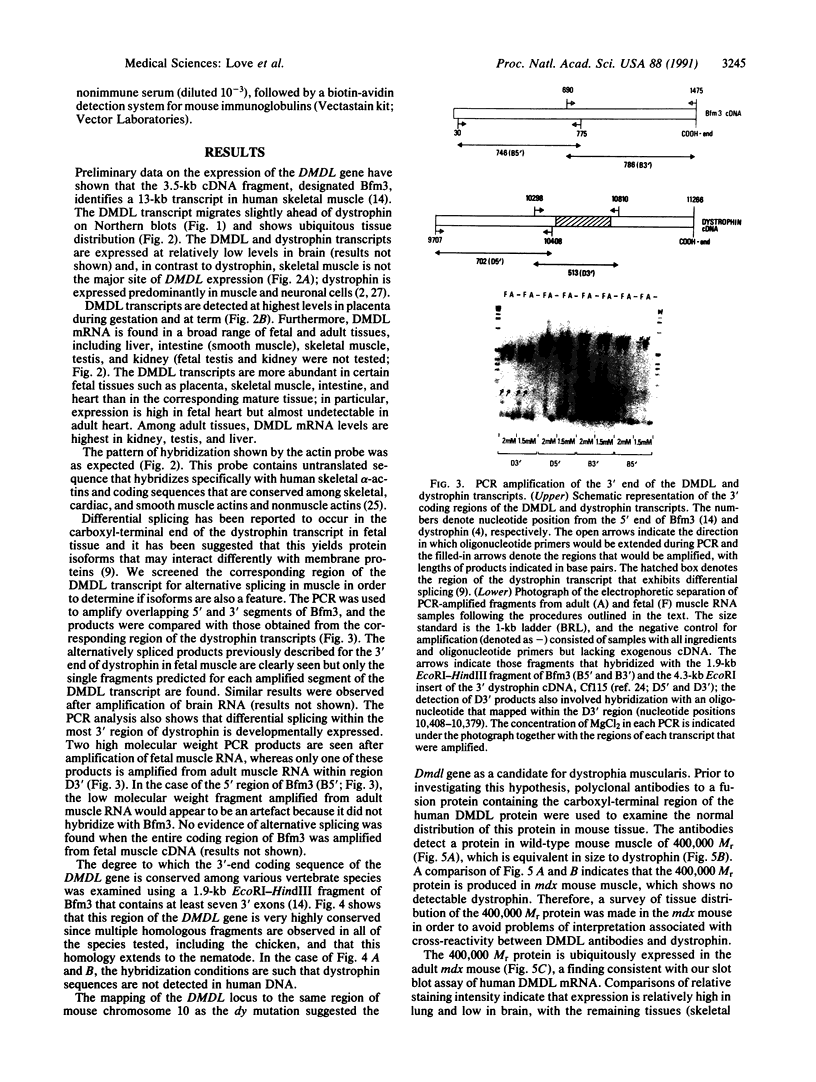
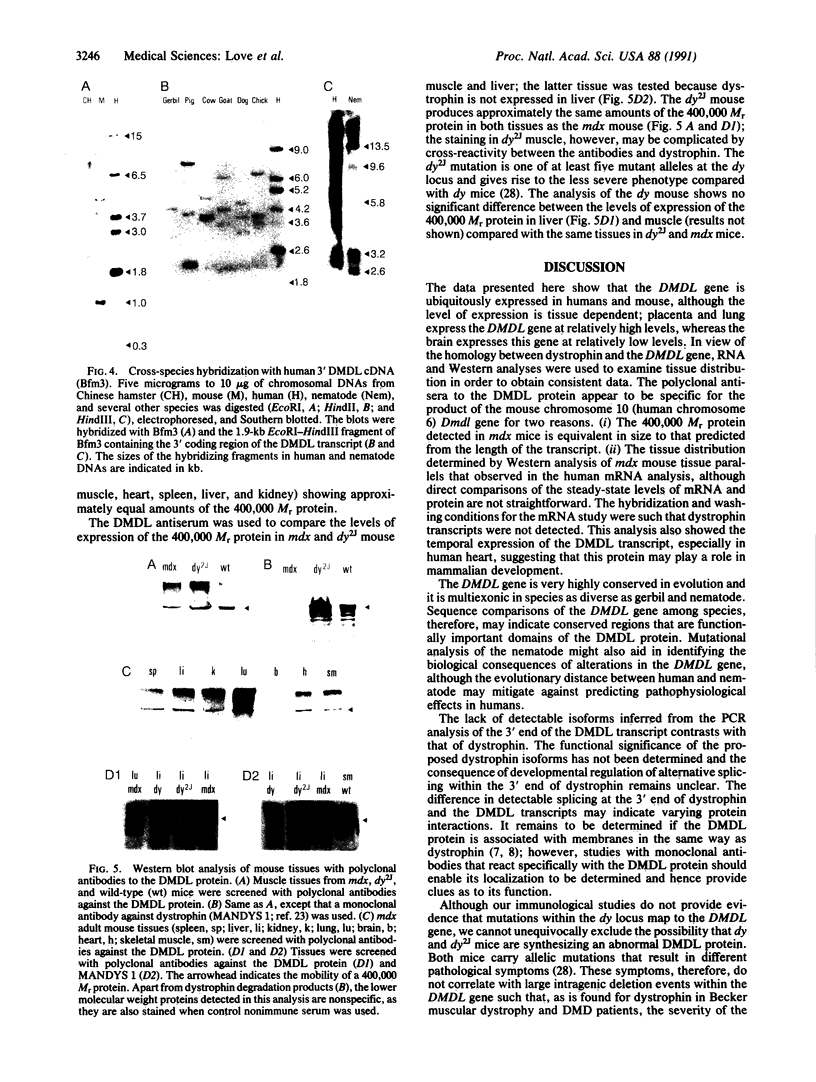

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownson C., Isenberg H., Brown W., Salmons S., Edwards Y. Changes in skeletal muscle gene transcription induced by chronic stimulation. Muscle Nerve. 1988 Nov;11(11):1183–1189. doi: 10.1002/mus.880111113. [DOI] [PubMed] [Google Scholar]
- Buckle V. J., Guenet J. L., Simon-Chazottes D., Love D. R., Davies K. E. Localisation of a dystrophin-related autosomal gene to 6q24 in man, and to mouse chromosome 10 in the region of the dystrophia muscularis (dy) locus. Hum Genet. 1990 Aug;85(3):324–326. doi: 10.1007/BF00206755. [DOI] [PubMed] [Google Scholar]
- Campbell K. P., Kahl S. D. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989 Mar 16;338(6212):259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- Chelly J., Hamard G., Koulakoff A., Kaplan J. C., Kahn A., Berwald-Netter Y. Dystrophin gene transcribed from different promoters in neuronal and glial cells. Nature. 1990 Mar 1;344(6261):64–65. doi: 10.1038/344064a0. [DOI] [PubMed] [Google Scholar]
- Davison M. D., Critchley D. R. alpha-Actinins and the DMD protein contain spectrin-like repeats. Cell. 1988 Jan 29;52(2):159–160. doi: 10.1016/0092-8674(88)90503-x. [DOI] [PubMed] [Google Scholar]
- Den Dunnen J. T., Grootscholten P. M., Bakker E., Blonden L. A., Ginjaar H. B., Wapenaar M. C., van Paassen H. M., van Broeckhoven C., Pearson P. L., van Ommen G. J. Topography of the Duchenne muscular dystrophy (DMD) gene: FIGE and cDNA analysis of 194 cases reveals 115 deletions and 13 duplications. Am J Hum Genet. 1989 Dec;45(6):835–847. [PMC free article] [PubMed] [Google Scholar]
- Ervasti J. M., Ohlendieck K., Kahl S. D., Gaver M. G., Campbell K. P. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990 May 24;345(6273):315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Feener C. A., Koenig M., Kunkel L. M. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature. 1989 Apr 6;338(6215):509–511. doi: 10.1038/338509a0. [DOI] [PubMed] [Google Scholar]
- Forrest S. M., Cross G. S., Flint T., Speer A., Robson K. J., Davies K. E. Further studies of gene deletions that cause Duchenne and Becker muscular dystrophies. Genomics. 1988 Feb;2(2):109–114. doi: 10.1016/0888-7543(88)90091-2. [DOI] [PubMed] [Google Scholar]
- Geisterfer-Lowrance A. A., Kass S., Tanigawa G., Vosberg H. P., McKenna W., Seidman C. E., Seidman J. G. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990 Sep 7;62(5):999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- Gillespie G., Lloyd J., Hopkinson D., Edwards Y. The 3'-untranslated sequence of human skeletal muscle alpha-actin mRNA. J Muscle Res Cell Motil. 1984 Aug;5(4):457–464. doi: 10.1007/BF00818263. [DOI] [PubMed] [Google Scholar]
- Hammonds R. G., Jr Protein sequence of DMD gene is related to actin-binding domain of alpha-actinin. Cell. 1987 Oct 9;51(1):1–1. doi: 10.1016/0092-8674(87)90002-x. [DOI] [PubMed] [Google Scholar]
- Hirzel H. O., Tuchschmid C. R., Schneider J., Krayenbuehl H. P., Schaub M. C. Relationship between myosin isoenzyme composition, hemodynamics, and myocardial structure in various forms of human cardiac hypertrophy. Circ Res. 1985 Nov;57(5):729–740. doi: 10.1161/01.res.57.5.729. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Fischbeck K. H., Brown R. H., Johnson M., Medori R., Loike J. D., Harris J. B., Waterston R., Brooke M., Specht L. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N Engl J Med. 1988 May 26;318(21):1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- Ishiura S., Arahata K., Tsukahara T., Koga R., Anraku H., Yamaguchi M., Kikuchi T., Nonaka I., Sugita H. Antibody against the C-terminal portion of dystrophin crossreacts with the 400 kDa protein in the pia mater of dystrophin-deficient mdx mouse brain. J Biochem. 1990 Apr;107(4):510–513. doi: 10.1093/oxfordjournals.jbchem.a123076. [DOI] [PubMed] [Google Scholar]
- Kenwrick S., Patterson M., Speer A., Fischbeck K., Davies K. Molecular analysis of the Duchenne muscular dystrophy region using pulsed field gel electrophoresis. Cell. 1987 Jan 30;48(2):351–357. doi: 10.1016/0092-8674(87)90438-7. [DOI] [PubMed] [Google Scholar]
- Koenig M., Beggs A. H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C. R., Lindlöf M., Kaariainen H. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989 Oct;45(4):498–506. [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Love D. R., Davies K. E. Duchenne muscular dystrophy: the gene and the protein. Mol Biol Med. 1989 Feb;6(1):7–17. [PubMed] [Google Scholar]
- Love D. R., Flint T. J., Marsden R. F., Bloomfield J. F., Daniels R. J., Forrest S. M., Gabrielli O., Giorgi P., Novelli G., Davies K. E. Characterization of deletions in the dystrophin gene giving mild phenotypes. Am J Med Genet. 1990 Sep;37(1):136–142. doi: 10.1002/ajmg.1320370132. [DOI] [PubMed] [Google Scholar]
- Love D. R., Hill D. F., Dickson G., Spurr N. K., Byth B. C., Marsden R. F., Walsh F. S., Edwards Y. H., Davies K. E. An autosomal transcript in skeletal muscle with homology to dystrophin. Nature. 1989 May 4;339(6219):55–58. doi: 10.1038/339055a0. [DOI] [PubMed] [Google Scholar]
- Mercadier J. J., Bouveret P., Gorza L., Schiaffino S., Clark W. A., Zak R., Swynghedauw B., Schwartz K. Myosin isoenzymes in normal and hypertrophied human ventricular myocardium. Circ Res. 1983 Jul;53(1):52–62. doi: 10.1161/01.res.53.1.52. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Russell E. S., Harman P. J. Dystrophia Muscularis: A HEREDITARY PRIMARY MYOPATHY IN THE HOUSE MOUSE. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1079–1084. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen thi Man, Cartwright A. J., Morris G. E., Love D. R., Bloomfield J. F., Davies K. E. Monoclonal antibodies against defined regions of the muscular dystrophy protein, dystrophin. FEBS Lett. 1990 Mar 26;262(2):237–240. doi: 10.1016/0014-5793(90)80199-s. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Shedding light on PCR contamination. Nature. 1990 Jan 4;343(6253):27–27. doi: 10.1038/343027a0. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Forrest S. M., Cross G. S., Davies K. E. Duchenne and Becker muscular dystrophy mutations: analysis using 2.6 kb of muscle cDNA from the 5' end of the gene. Nucleic Acids Res. 1987 Dec 10;15(23):9761–9769. doi: 10.1093/nar/15.23.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence M. A., Spurr N. K., Field L. L. Report of the committee on the genetic constitution of chromosome 6. Cytogenet Cell Genet. 1989;51(1-4):149–165. doi: 10.1159/000132790. [DOI] [PubMed] [Google Scholar]
- Tanigawa G., Jarcho J. A., Kass S., Solomon S. D., Vosberg H. P., Seidman J. G., Seidman C. E. A molecular basis for familial hypertrophic cardiomyopathy: an alpha/beta cardiac myosin heavy chain hybrid gene. Cell. 1990 Sep 7;62(5):991–998. doi: 10.1016/0092-8674(90)90273-h. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988 Jun 30;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]