This study demonstrates that hepatocellular-specific knockout of autophagy in mice strongly affects the unfolded protein response, another essential cellular homeostatic mechanism. Surprisingly, these alterations were in a specific pattern, leaving the inositol-requiring enzyme-1α pathway unaltered, whereas the protein kinase RNA-like ER kinase and activating transcription factor 6 (ATF6) pathway were respectively induced and reduced. The loss of fasting-induced steatosis in autophagy-deficient mice might in part be explained by the alterations of the ATF6 pathway.
Keywords: nonalcoholic fatty liver, steatosis, autophagy, endoplasmic reticulum stress, unfolded protein response
Abstract
Autophagy and the unfolded protein response (UPR) are key cellular homeostatic mechanisms and are both involved in liver diseases, including nonalcoholic fatty liver disease (NAFLD). Although increasing but conflicting results link these mechanisms to lipid metabolism, their role and potential cross talk herein have been poorly investigated. Therefore, we assessed the effects of hepatocyte-specific autophagy deficiency on liver parenchyma, the UPR, and lipid metabolism. Adult hepatocellular-specific autophagy-deficient mice (Atg7F/FAlb-Cre+) were compared with their autophagy-competent littermates (Atg7+/+Alb-Cre+). Livers were analyzed by electron microscopy, histology, real-time qPCR, and Western blotting. Atg7F/FAlb-Cre+ mice developed hepatomegaly with significant parenchymal injury, as shown by inflammatory infiltrates, hepatocellular apoptosis, pericellular fibrosis, and a pronounced ductular reaction. Surprisingly, the UPR exhibited a pathway-selective pattern upon autophagy deficiency. The activity of the adaptive activating transcription factor 6 (ATF6) pathway was abolished, whereas the proapoptotic protein kinase RNA-like ER kinase pathway was increased compared with Atg7+/+Alb-Cre+ mice. The inositol-requiring enzyme-1α signal was unaltered. Fasting-induced steatosis was absent in Atg7F/FAlb-Cre+ mice. Remarkably, some isolated islands of fat-containing and autophagy-competent cells were observed in these livers. Hepatocellular autophagy is essential for parenchymal integrity in mice. Moreover, in the case of autophagy deficiency, the three different UPR branches are pathway selectively modulated. Attenuation of the ATF6 pathway might explain the observed impairment of fasting-induced steatosis. Finally, autophagy and lipid droplets are directly linked to each other.
NEW & NOTEWORTHY
This study demonstrates that hepatocellular-specific knockout of autophagy in mice strongly affects the unfolded protein response, another essential cellular homeostatic mechanism. Surprisingly, these alterations were in a specific pattern, leaving the inositol-requiring enzyme-1α pathway unaltered, whereas the protein kinase RNA-like ER kinase and activating transcription factor 6 (ATF6) pathway were respectively induced and reduced. The loss of fasting-induced steatosis in autophagy-deficient mice might in part be explained by the alterations of the ATF6 pathway.
autophagy is a cellular process involved in the breakdown of cytoplasmic contents via a lysosomal pathway. It is constitutively active but can be upregulated by several stressors, including metabolic stress (7). Of the three forms of autophagy (macroautophagy, microautophagy, and chaperone-mediated autophagy), macroautophagy (henceforth autophagy) is considered to be the most important one. During autophagy, autophagosomes are formed out of isolation membranes and fuse with lysosomes to enable degradation of their content. Autophagy-related genes (Atg) are responsible for the strict control of the autophagic process, with Atg7 as an essential mediator for the maturation of autophagosomes (40). Although autophagy was initially regarded as a nonselective process, targeted (organelle- or protein-selective) autophagy has also been described (4).
The unfolded protein response (UPR) is another key cellular homeostatic mechanism activated in response to the accumulation of unfolded proteins in the endoplasmic reticulum (ER) (ER stress) (20). ER stress results from perturbation of the normal protein-folding capacity of the ER and induces inflammation and oxidative stress (8). The UPR encompasses three major adaptive mechanisms to restore protein homeostasis, named after the respective ER stress sensor, namely activating transcription factor 6 (ATF6), protein kinase RNA-like ER kinase (PERK), and inositol-requiring enzyme-1α (IRE1α) (20).
Nonalcoholic fatty liver disease (NAFLD), characterized by the accumulation of fat in hepatocytes, has become a leading cause of chronic liver disease in Western countries, with a prevalence of up to 30% (51). When steatosis is accompanied by inflammation and hepatocellular damage, it is called nonalcoholic steatohepatitis (NASH), an entity associated with increased liver- and non-liver-related morbidity and mortality (51). However, the exact mechanisms underlying the development of NASH are incompletely understood (50).
Both autophagy and the UPR are associated with the pathophysiology of NAFLD, among other liver diseases (7, 8). Nevertheless, the exact role of autophagy in NAFLD remains controversial (31), and the role of the individual UPR mediators in NAFLD needs to be investigated (16, 56). The UPR is known to promote autophagic flux; however, there is increasing evidence of an important cross talk between autophagy and the UPR (43). Interestingly, impaired autophagy is linked to increased ER stress in steatosis (17, 55). Despite its known involvement in steatosis (6, 42), ATF6-signaling has not yet been investigated in relation to autophagy.
In this study, we investigated the effects of hepatocellular autophagy on the three different branches of the UPR and found important hepatic injury with a significant pathway-selective modulation in autophagy-deficient mice. The occurrence of lipid droplets was directly linked to autophagy because fasting-induced steatosis was absent in autophagy-deficient mice.
MATERIALS AND METHODS
Animal model.
Hepatocellular autophagy-deficient C57Bl/6J mice were developed by cross breeding homozygous Atg7Flox mice (Atg7F/F) (28) with mice expressing Cre-recombinase under control of the albumin promoter as previously described (33). These mice, hereafter denoted as Atg7F/FAlb-Cre+, were compared with autophagy-competent littermates that lacked the LoxP vector but expressed the Alb-Cre (Atg7+/+Alb-Cre+). To allow in situ detection of the autophagy marker LC3, all mice were cross bred with green fluorescent protein (GFP)-LC3 transgenic mice (36). Genotyping of the offspring was performed by PCR as described (30, 33).
All mice were kept in a 12-h:12-h light/dark cycle with controlled temperature and humidity and had free access to standard chow (Pavan Service, Oud-Turnhout, Belgium) and water ad libitum. Mice were housed in enriched cages and in accordance with international guidelines.
Adult mice (11–12 wk of age) were fasted overnight, after which they were weighed and anesthetized with Nembutal (60 mg/kg ip) (Sanofi, Brussels, Belgium). Whole blood samples were obtained by intracardiac puncture. Next, liver and kidneys were removed, and random liver samples were fixed in 4% neutral buffered formalin for 24 h and dehydrated overnight in 60% isopropanol before paraffin embedding. Alternatively, liver tissue was immediately embedded in Richard-Allan Scientific Neg-50 Frozen Section Medium (6502; Thermo Scientific, Runcorn, UK) using liquid nitrogen and kept at −80°C. Liver tissue of three animals per group was further processed for transmission electron microscopy (TEM), as previously described (34). The remaining liver and kidneys were stored at −80°C until further processing.
All experiments were approved by the Ethical Committee on Animal Experimentation of the University of Antwerp (ECD 2012/40).
Histology and immunohistochemistry.
Sections of paraffin-embedded tissues (5 μm thick) were stained with hematoxylin and eosin (HE), Trichrome-Masson, and Picrosirius red according to standard laboratory techniques. Immunohistochemistry was applied using the following primary antibodies (Table 1): anti-α-smooth muscle actin (α-SMA), anti-pan-cytokeratin, anti-ATG7, anti-LC3B, and anti-SQSTM1/p62. Secondary antibodies were species-appropriate horseradish peroxidase conjugates (Vectastain ABC; Vector Laboratories, Burlingame, CA) or in the case of LC3B the Envision+ system (K4002; DAKO, Carpinteria, CA).
Table 1.
Characteristics of the antibodies used in the study
| Antigen | Antibody Isotype, Clone | Company | Cat. No. |
|---|---|---|---|
| α-SMA | Mouse monoclonal IgG, 1A4 | Sigma-Aldrich | F3777 |
| ATF6 | Mouse monoclonal IgG1, 70B1413 | Abcam | ab11909 |
| ATG7 | Rabbit polyclonal IgG | Sigma-Aldrich | A2856 |
| ATG7 | Rabbit polyclonal IgG | Santa Cruz Biotechnology | sc-33211 |
| β-Actin | Rabbit monoclonal IgG | Cell Signaling | #4970 |
| CHOP | Mouse monoclonal IgG2a, L63F7 | Cell Signaling | 2895 |
| eIF2-α | Rabbit polyclonal IgG | Cell Signaling | 9721 |
| Phospho-eIF2α | Rabbit monoclonal IgG1, 119A11 | Cell Signaling | 3597 |
| GADD34 | Rabbit polyclonal IgG, H193 | Santa Cruz Biotechnology | sc-8327 |
| GAPDH | Rabbit polyclonal IgG | Abcam | ab9485 |
| GRP78 | Rabbit monoclonal IgG, C50B12 | Cell Signaling | 3177 |
| Phospho-IRE1α | Rabbit polyclonal IgG | Novus Biological | nb100-2323 |
| LC3B | Rabbit monoclonal IgG, D11 | Cell Signaling | #3868 |
| SQSTM1/p62 | Rabbit polyclonal IgG | Sigma-Aldrich | P0067 |
| pan-CK | Rabbit polyclonal IgG | DAKO | Z0622 |
| PDIA4 | Rabbit polyclonal IgG | Cell Signaling | 2798 |
| SREBP-1 | Rabbit polyclonal IgG, C-20 | Santa Cruz Biotechnology | Sc-366 |
The specificity, isotype, clone number, and catalog number of the antibodies are indicated if provided by the manufacturer. In case of ATG7, one antibody was used for Western blotting (Sigma-Aldrich), whereas the other antibody was used for immunohistochemistry (Santa Cruz Biotechnology).
Oil-red-O (ORO) and Sudan black B (SBB) staining (O0625 resp. S0395; Sigma-Aldrich, St. Louis, MO) were performed on NEG-50-embedded frozen tissue sections (6 μm thick) according to standard laboratory techniques. Slides were counterstained with Carazzi's hematoxylin.
All samples were blindly assessed by an experienced pathologist (A. Driessen) with a detailed recording of all features. The presence of α-SMA-positive cells is presented as the sum of a semiquantitative score of each acinar liver zone as follows: 0 = no staining; 1 = staining of some sinusoidal lining cells, occupying <1% of a particular zone; 2 = 1–10%; 3 = 10–30%; and 4 = >30% positive sinusoidal lining cells (5). Morphometric measurements of the Picrosirius red- and ORO-positive areas were performed using ImageJ software (version 1.46r; NIH, Bethesda, MD) (53). All images were acquired with Universal Grab 6.1 (DCI Laboratories, Keerbergen, Belgium) using an Olympus BX40 microscope (Tokyo, Japan) and Power HAD Sony 3CCD camera.
Biochemical analysis.
Whole blood samples were centrifuged for 15 min at 3,000 revolution/min. Plasma was snap frozen in liquid nitrogen and stored at −80°C. Samples were analyzed with an automated Vista 1500 System (Siemens Healthcare Diagnostics, Deerfield, CT) for aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), and glucose. Insulin was determined with an ELISA kit (EZRMI-13K; Millipore, Billerica, MA) according to the manufacturer's instructions. When values were below or above the detection limit for a certain parameter, this value was artificially set to this detection limit.
Western blot and ELISA.
Protein samples of the liver were run on a SDS-polyacrylamide gel (Nupage Bis-Tris 4–12%; Life Technologies, Carlsbad, CA) and transferred to a PVDF membrane. Membranes were subsequently blocked in Tris-buffered saline containing 0.05% Tween and 5% nonfat dry milk. Membranes were probed overnight at 4°C with primary antibody solution with the following antibodies (Table 1): anti-ATG7, anti-SQSTM1/p62, anti-ATF6, anti-C/EBP homologous protein (CHOP), anti-eukaryotic initiation factor α (eIF2α), anti-phospho-eIF2α, anti-growth arrest and DNA-damage-inducible 34 (GADD34), anti-glucose-regulated protein, 78 kDa (GRP78), anti-phospho-IRE1α, anti-protein disulfide isomerase family A member 4 (PDIA4), anti-phospho-PERK, anti-β-actin, and anti-GAPDH. In the case of Western blotting for anti-sterol response element-binding protein 1 (SREBP-1), the nuclear fraction was extracted (78833; Thermo Scientific, Waltham, MA) and separately loaded on a gel to detect the cleaved protein (loading control for this fraction was performed by imaging of total protein, not shown). After incubation with appropriate horseradish peroxidase secondary antibodies, chemiluminescent signals were obtained with SuperSignal West Pico (34080; Thermo Scientific) or, in the case of anti-LC3B, SuperSignal West Femto Maximum Sensitivity Substrate (34096; Thermo Scientific) using a Lumi-Imager (Roche, Mannheim, Germany). Each lane was loaded with 20–50 μg of protein, and quantitative densitometry values were normalized against β-actin (LC3B, ATG7, p62) or GAPDH (eIF2α, P-eIF2α). The densitometric analysis was performed using ImageJ. UPR-related antibodies were validated using tunicamycin.
The level of pJNK was assessed via ELISA (DYC1387B-5; R&D Systems Europe, Abingdon, UK) according to manufacturer's instructions.
In vivo caspase-3 activity.
The enzymatic activation of effector caspase-3 was evaluated in liver lysates using the Caspase-3-Glo assay (Promega, Madison, WI) following the manufacturer's instructions. Experiments were performed in quadruplicate.
Real-time PCR.
Total RNA was extracted from all samples using the RNeasy Mini Kit (Qiagen, Westburg BV, The Netherlands) with on-column DNAse treatment. Needle homogenization was performed. The purity and quantity of total RNA were assessed using spectrophotometry (Nanodrop; Thermo Scientific); samples with a 260:280 ratio between 1.8 and 2.0 were accepted.
One microgram of total RNA was converted to single-strand cDNA by reverse transcription (iScript, Bio-Rad, Hercules, CA) with oligo (dT) and random priming. The cDNA was diluted 1/10 and used for real-time quantification using SYBR Green (Sensimix; Bioline Reagents, London, UK) and 250 nM of each primer. A two-step program was run on a LightCyclerR 480 (Roche). Cycling conditions were 95°C for 10 min and 45 cycles of 95°C for 10 s followed by 60°C for 1 min. Melting curve analysis confirmed primer specificities. All reactions were performed in duplicate. Fold change expression was calculated using the ΔΔCT method as instructed by Applied Biosystems (Foster City, CA). The PCR efficiency of each primer pair was calculated using a standard curve of reference cDNA. Amplification efficiency was determined using the formula 10−1/slope. The primer set sequences are listed in Table 2.
Table 2.
Primers used for real-time PCR experiments
| Gene Symbol | Reference Sequence | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Efficiency | R2 |
|---|---|---|---|---|---|
| Atf6 | NM_001081304.1 | GGGCAGGGCCATTCTTGCTGA | AGCCCCCGGGACAAACAGGT | 98.60 | 0.99 |
| Calnexin | NM_001110499.1 | CAACAGGGGAGGTTTATTTTGCT | TCCCACTTTCCATCATATTTGGC | 101.00 | 0.99 |
| Calreticulin | NM_007591.3 | GGTGGCCGCGTCCGTCAATA | AGCAGGAGCGGCACCGAAAG | 95.08 | 0.98 |
| Chop | NM_007837.3 | AGCGCAACATGACAGTGAAG | GTGTAATTCCAGGGGGAGGT | 101.20 | 0.99 |
| Dnajc3 | NM_008929.3 | GCTGAGTGTGGAGTAAATGCG | CGGCTGCGAGTAATTTCTTCC | 103.00 | 0.99 |
| Edem1 | NM_138677.2 | CTTGAGGGACCCCGACGGCT | TCTCAAGCCGCCCCTCCGTT | 104.00 | 0.98 |
| Erdj4 | NM_013760.4 | CGCCCTGTGGCCCTGACTTG | AGCTTTCAGGGGCAAACAGCCA | 98.10 | 0.98 |
| Gapdh | NM_008084.2 | GCCGGCTCAGTGAGACAAG | TGGCACCTTCAGCAACAATG | 95.10 | 0.99 |
| Grp78 | NM_001163434.1 | TGCCGAGCTAAATTACACATTG | CCTTGTGGAGGGATGTACAGA | 107.30 | 0.99 |
| Grp94 | NM_011631.1 | GAGGCGGCTCCTGAGACCGAA | GGACCCTCATGGTGCGTGGC | 101.20 | 0.99 |
| Herpud1 | NM_022331.1 | ACGCCAAGTGTCGTTGTGTGGTC | GCTCGACTGCGCTCAGGGATG | 92.80 | 0.99 |
| Ire1 | NM_023913.2 | GCCCAACGCACATGGCAGGA | TACCCCTGAACGGCGGCTGA | 98.60 | 0.99 |
| Pdia3 | NM_007952.2 | GCAGGCCTAGGGGGTTGGGA | GAGGGCCGGGACCGGAGAAA | 107.10 | 0.99 |
| Pdia4 | NM_009787.2 | ACGAGACCCCGGCGTTCGGA | TGGCACTTTGAGGAGGTGAGCC | 90.60 | 0.99 |
| Xbp1 s | NM_013842.2 | TCTCAAGCCGCCCCTCCGTT | CGGGGTTGCTGGTGTGCCAT | 97.60 | 0.98 |
| Xbp1u | NM_013842.2 | TCTCAAGCCGCCCCTCCGTT | GTGGCTGGCGTGCAAGGGAT | 107.30 | 0.97 |
The references and primer sequences of the analyzed genes are shown. The PCR efficiency of each primer pair was calculated using a standard curve of reference cDNA. Amplification efficiency R2 was determined using the formula 10−1/slope.
Statistics.
All data were analyzed by SPSS (version 22; IBM SPSS Statistics, Armonk, NY). Parametric variables were compared using the Student's t-test, or one-way ANOVA with post hoc Bonferroni correction whenever appropriate. Nonparametric variables were compared using the Mann-Whitney U-test. Figures were created with GraphPad Prism (version 5; GraphPad Software, San Diego, CA). Two-tailed probabilities were calculated; P values <0.05 were considered statistically significant. Data are presented as means ± SE.
RESULTS
Autophagy is defective in livers of Atg7F/FAlb-Cre+ mice.
The excision of the essential autophagy gene Atg7 in the livers of Atg7+/+Alb-Cre+ mice (n = 16) was demonstrated by Western blot showing the elimination of ATG7 and the accumulation of SQSTM1/p62, which is a typical feature of impaired autophagy (data not shown). The opposite was observed in the autophagy-competent mice (Atg7F/FAlb-Cre+, n = 11). Kidneys, used as control for tissue specificity, expressed comparable ATG7 levels in all mice.
Subsequent immunohistochemical analysis of liver sections reconfirmed the accumulation of p62-positive aggregates in Atg7F/FAlb-Cre+ mice, as well as a lack of fine LC3B-positive punctae representative of autophagosomes (data not shown). However, Atg7F/FAlb-Cre+ mice showed large LC3B-positive globular structures, not punctae, predominantly in the pericentral regions. These observations represent the staining of the unconjugated GFP-bound LC3-I protein, which can be incorporated in protein aggregates in an autophagy-independent way (29, 33, 41).
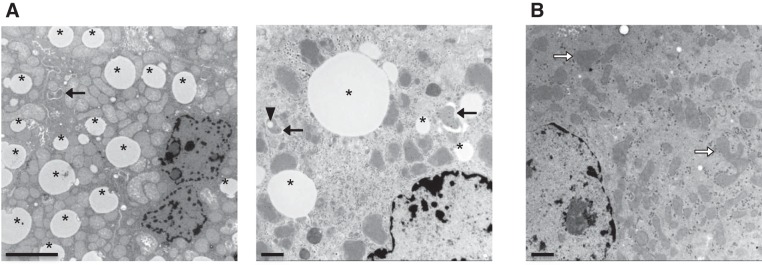
Finally, TEM demonstrated absence of autophagic vacuoles in the vast majority of the hepatocytes of Atg7F/FAlb-Cre+ mice that were still present in the hepatocytes of Atg7+/+Alb-Cre+ mice. Furthermore, we observed accumulation of deformed mitochondria (Fig. 1, A and B). Of note, autophagy was rarely seen in some cells of Atg7F/FAlb-Cre+ mice, as discussed in detail.
Fig. 1.
Transmission electron microscopy (TEM) of the livers of Atg7+/+Alb-Cre+ and Atg7F/FAlb-Cre+ mice. A: early and late autophagic vacuoles (arrows) and lipid droplets (*) are demonstrated in Atg7+/+Alb-Cre+ mice. In 1 of the autophagosomes, there was also the inclusion of a lipid droplet (arrowhead). Scale bars = 5 μm (left), 1 μm (right). B: in livers of Atg7F/FAlb-Cre+ mice, autophagic vacuoles are lacking, and (sometimes deformed) mitochondria (blank arrows) accumulated. Scale bar = 1 μm.
All together, these data validate hepatocyte-specific deficiency of autophagy in the Atg7F/FAlb-Cre+ mice.
Hepatocellular autophagy deficiency causes severe hepatomegaly and pronounced parenchymal damage.
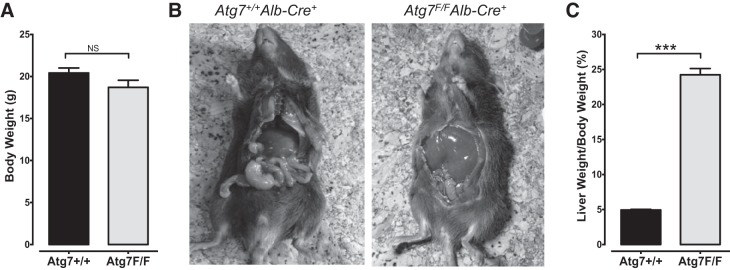
Although body weight of Atg7F/FAlb-Cre+ mice and Atg7+/+Alb-Cre+ controls was comparable (Fig. 2A), Atg7F/FAlb-Cre+ mice displayed severe hepatomegaly, as reflected by a fivefold increase in the liver weight-to-body weight ratio (P < 0.001) compared with Atg7+/+Alb-Cre+ mice (Fig. 2, B and C). Macroscopically, these enlarged livers exhibited an intense red-brownish color and had a more solid consistency compared with the livers of Atg7+/+Alb-Cre+ mice. However, the general wellbeing of Atg7+/+Alb-Cre+ mice did not seem to be hampered by their hepatomegaly. Macroscopic abnormalities (e.g., tumors) could not be observed in the Atg7F/FAlb-Cre+ mice, and spontaneous deaths did not occur.
Fig. 2.
Severe hepatomegaly in Atg7F/FAlb-Cre+ mice but not in Atg7+/+Alb-Cre+ mice. A: body weight of Atg7+/+Alb-Cre+ and Atg7F/FAlb-Cre+ mice was not significantly different (P = 0.098). B: macroscopic view of an Atg7+/+Alb-Cre+ (left) and Atg7F/FAlb-Cre+ (right) mouse illustrating the prominent hepatomegaly in Atg7F/FAlb-Cre+ mice. C: hepatomegaly is also reflected by a significantly higher liver weight-to-body weight ratio in Atg7F/FAlb-Cre+ mice (***P < 0.001).
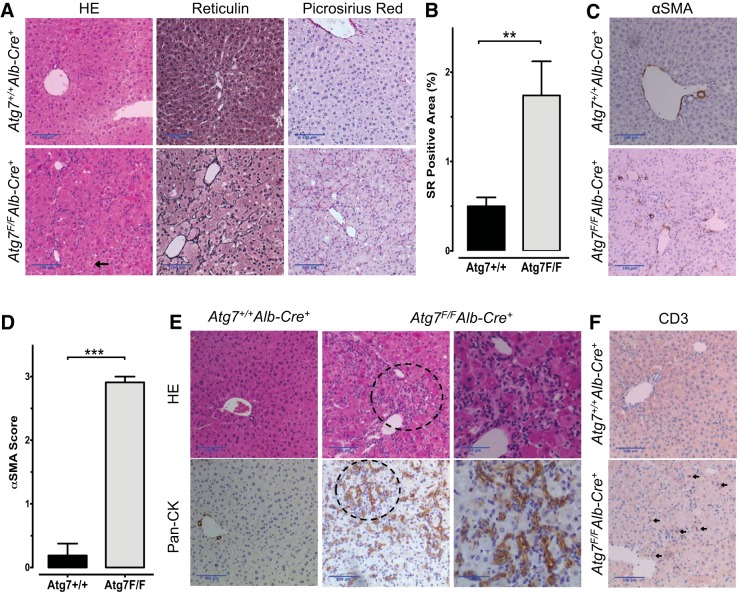
Microscopically, the liver architecture of Atg7F/FAlb-Cre+ mice was severely damaged (Fig. 3A). The hepatocytes were hypertrophic, presented ballooning, and frequently displayed a more dense eosinophilic cytoplasm compatible with a preapoptotic state, which is in line with the sporadic presence of apoptotic bodies. These findings are indicative of severe hepatocellular injury. Reticulin staining demonstrated the disruption of liver trabeculae (Fig. 3A). Moreover, Atg7F/FAlb-Cre+ livers had fine pericellular collagen deposits, as shown by Picrosirius red stain. Morphometrical measurements of the Picrosirius red-positive area showed a significantly higher collagen content in Atg7F/FAlb-Cre+ mice than in Atg7+/+Alb-Cre+ mice (P < 0,01, Fig. 3B) although septa or bridging fibrosis could not be observed. Because activated hepatic stellate cells are considered to be the main source of collagen production (14), an α-SMA staining was performed (Fig. 3C). The α-SMA score was significantly increased in all acinar zones in Atg7F/FAlb-Cre+ mice compared with Atg7+/+Alb-Cre+ mice (Fig. 3D). Furthermore, the cellularity prominently increased in Atg7F/FAlb-Cre+ mice. These cells were organized in a string pattern with sometimes tubular-like structures (Fig. 3E). This pattern is consistent with a pronounced ductular reaction (i.e., the proliferation of hepatic progenitor cells) as confirmed by a pan-cytokeratin stain (27). The increased cellularity was also partly attributable to inflammation with the infiltration of lymphoid cells, as demonstrated by CD3 staining (Fig. 3F).
Fig. 3.
Hepatic microscopic alterations in Atg7F/FAlb-Cre+ mice. A: representative photographs of a hematoxylin and eosin (HE) stain (left), reticulin stain (middle), and Picrosirius red stain (right) of Atg7+/+Alb-Cre+ (top) and Atg7F/FAlb-Cre+ mouse liver sections (bottom), illustrating the severely disturbed architecture of the liver cell plates with hepatocellular hypertrophy, increased fibrosis, thickened trabeculae, and a disrupted arrangement of the trabeculae in Atg7F/FAlb-Cre+ mice. The arrow indicates an apoptotic body. Scale bars = 100 μm. B: morphometric analysis of the Picrosirius red (SR)-positive area. Atg7F/FAlb-Cre+ mice had a significantly larger positive area (Student's t-test, **P < 0.01). C: Atg7F/FAlb-Cre+ mice showed an increased number of α-smooth muscle actin (α-SMA)-positive cells lining the sinusoids, indicative of hepatic stellate cell activation, whereas in Atg7+/+Alb-Cre+ mice solely the perivascular structures are positive. Scale bars = 100 μm. D: α-SMA score. Atg7F/FAlb-Cre+ mice had a significantly higher total score (Mann-Whitney U-test, ***P < 0.001). E: pronounced ductular reaction in Atg7F/FAlb-Cre+ mouse liver with neoductular formation (HE stain, top). The ductular reaction was confirmed by a pan-cytokeratin (CK) stain (bottom), whereas in Atg7+/+Alb-Cre+ mice only bile ducts were stained (bottom, left). Detailed photographs of the 2 encircled regions of the middle panels are shown (right). Scale bars = 100 μm; detailed images, 50 μm. F: Atg7F/FAlb-Cre+ livers showed infiltration of lymphoid cells as demonstrated by a CD3 stain. Arrows indicate CD3-positive cells. Scale bars = 100 μm.
In line with the microscopic findings, transaminases (ALT, AST) and canalicular tests (ALP, GGT) were significantly increased in Atg7F/FAlb-Cre+ mice, both endorsing the hepatocellular injury (Table 3).
Table 3.
Biochemical plasma analyses
|
Atg7+/+Alb-Cre+ |
Atg7F/FAlb-Cre+ |
||||||
|---|---|---|---|---|---|---|---|
| n | Means ± SE | Range, min-max | n | Means ± SE | Range, min-max | P Value | |
| AST, U/l | 15 | 175.1 ± 22.4 | 60.20–360.70 | 10 | 1855.1 ± 279.9 | 770.00–3636.00 | † |
| ALT, U/l | 15 | 20.1 ± 2.6 | 8.00–42.30 | 10 | 737.7 ± 146.2 | 168.30–1682.00 | † |
| ALP, U/l | 15 | 127.6 ± 8.2 | 78.30–203.40 | 10 | 335.7 ± 12.5 | 283.80–383.20 | † |
| GGT, U/l | 15 | 5.6 ± 0.2 | 5.00–6.60 | 10 | 14.4 ± 0.7 | 12.00–18.70 | † |
| Total cholesterol, mg/dl | 15 | 73.5 ± 4.1 | 50.00–97.40 | 10 | 140.8 ± 8.2 | 96.50–171.90 | † |
| HDL cholesterol, mg/dl | 15 | 63.5 ± 5.2 | 6.60–87.80 | 9 | 129.3 ± 7.6 | 88.50–150.00 | † |
| LDL cholesterol, mg/dl | 15 | 6.6 ± 0.8 | 2.90–16.00 | 10 | 12.8 ± 2.1 | 1.00–19.80 | * |
| LDL/HDL ratio | 15 | 0.2 ± 0.2 | 0.03–2.42 | 9 | 0.1 ± 0.0 | 0.01–0.15 | 0.46 |
| Triglycerides, mg/dl | 15 | 67.9 ± 7.7 | 40.20–121.60 | 10 | 15.1 ± 3.8 | 3.00–39.10 | † |
| Glucose, mg/dl | 15 | 106.9 ± 5.7 | 80.70–144.70 | 10 | 87.4 ± 6.4 | 54.80–115.10 | * |
| Insulin, ng/ml | 16 | 0.3 ± 0.0 | 0.20–0.70 | 11 | 0.3 ± 0.0 | 0.20–0.40 | 0.54 |
Applicable values are means ± SE. Main characteristics of autophagy-competent (Atg7+/+Alb-Cre+) and autophagy-deficient (Atg7F/FAlb-Cre+) mice are shown. Sample size (n) varies depending on the plasma volume available for each mouse.
P < 0.05;
P < 0.001.
AST, aspartate transaminase; ALT, alanine transaminase; GGT, γ-glutamyl transferase; ALP, alkaline phosphatase.
Together, autophagy deficiency appears to be detrimental to the liver, as shown by significant parenchymal injury, pericellular fibrosis, and a ductular reaction.
Hepatocellular autophagy deficiency triggers pathway-selective UPR modulation with a proapoptotic pattern.
Because autophagy deficiency could lead to ER stress attributable to the accumulation of misfolded proteins and impairment of ER turnover (43), different UPR pathways were investigated. Tunicamycin-treated cells, which successfully develop ER stress by inhibition of the N-linked glycosylation of proteins (38), served as a positive control in the analyses of UPR (data not shown).
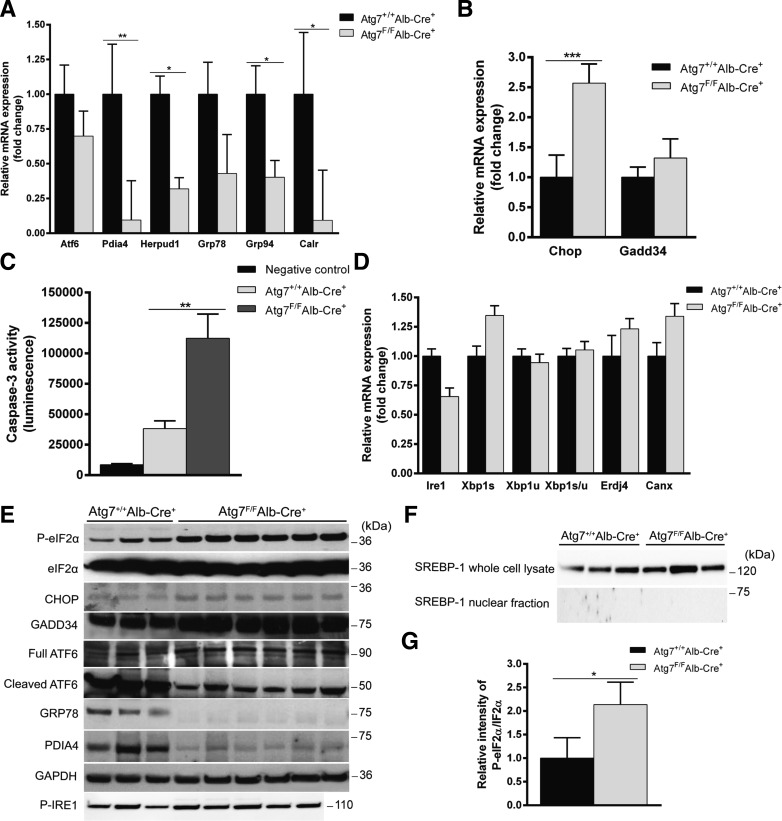
The GRP78 (also known as BiP) acts as a major sensor of unfolded proteins in the ER. Uncoupling of GRP78 of ATF6, PERK, or IRE1α leads to subsequent activation of the respective pathways (20). Both mRNA and protein levels of GRP78 were decreased in Atg7F/FAlb-Cre+ (Fig. 4, A and E).
Fig. 4.
Autophagy deficiency differentially disturbs the unfolded protein response (UPR) pathways. A: RT-qPCR of Atf6 and the chaperones involved in the activating transcription factor 6 (ATF6) pathway. Relative mRNA expression was compared with Atg7+/+Alb-Cre+. Although Atf6 expression itself was unaltered in Atg7F/FAlb-Cre+ mice, its associated chaperones were significantly decreased. (*P < 0.05, **P < 0.01). B: RT-qPCR of the downstream effectors of the protein kinase RNA-like ER kinase (PERK) pathway, Chop and Gadd34. Chop is responsible for the induction of endoplasmic reticulum (ER) stress-induced apoptosis and was significantly upregulated in Atg7F/FAlb-Cre+ mice, whereas Gadd34 was unaltered. (***P < 0.001). C: caspase-3 activity was significantly elevated in Atg7F/FAlb-Cre+ mice. (**P < 0.01). D: RT-qPCR of Ire1 and the chaperones involved in the inositol-requiring enzyme-1α (IRE1α) pathway. Relative mRNA expression was compared with Atg7+/+Alb-Cre+ mice. Neither Ire1a and its major effector XBP1u nor activation of XBP1u to XBP1s was significantly altered in Atg7F/FAlb-Cre+ mice compared with controls. E: Western blot of several chaperones involved in the UPR. GAPDH was used as a loading control. Protein levels changed in line with the mRNA levels. Although the expression of Atf6 was not significantly decreased, its phosphorylation was strongly attenuated in Atg7F/FAlb-Cre+ mice. Antibodies are defined in the text. F: Western blotting for sterol response element-binding protein 1 (SREBP-1) on whole cell and nuclear fractions. G: densitometry analysis of the ratio of phosphorylated eukaryotic initiation factor 2α (eIF2α) to total eIF2α bands normalized to GAPDH and relative to control. (*P < 0.05).
The transcription of the chaperones Pdia4, Herpud1, Grp78, Grp94, and Calreticulin, predominantly regulated by the ATF6 pathway (45), was reduced in Atg7F/FAlb-Cre+ mice, whereas the expression of Atf6 mRNA itself was unaltered (Fig. 4A). Decreased expression of Pdia4 was confirmed at the protein level by Western blot (Fig. 4E). Interestingly, the levels of the active (i.e., the cleaved) form of ATF6 were robustly decreased in Atg7F/FAlb-Cre+ mice compared with Atg7+/+Alb-Cre+ mice (Fig. 4E), further confirming the diminished activation of the cytoprotective ATF6 pathway upon autophagy deficiency. Both ATF6 and the SREBP depend on COPII-transporter vesicles for transport to the Golgi, where cleavage takes place (15, 54). The demonstration of cleaved SREBP-1 in Atg7+/+Alb-Cre+ mice would support the possibility of an interrupted COPII-mediated transportation in Atg7F/FAlb-Cre+ mice. Although the levels of uncleaved SREBP-1 were similar, remnants of cleaved SREBP-1, as these levels rapidly decrease when fasted, could not be detected in the nuclear fraction (Fig. 4, E and F).
In contrast with ATF6, there was a strong activation of the proapoptotic PERK pathway, as shown by increased phosphorylation of eIF2α (Fig. 4, E and G) and increased mRNA and protein levels of CHOP (Fig. 4, B and E). Even though it is known that eIF2α can be phosphorylated by other kinases as well, each related to a specific stimulus (12), direct detection of phosphorylated PERK can be troublesome (48) and was unsuccessful (results not shown). The mRNA and protein levels of Gadd34, providing a negative feedback to limit the translational break mediated by p-eIF2α, were unaltered (Fig. 4, B and E). Because CHOP is a central effector of UPR-mediated apoptosis and increased cell apoptosis was suspected on liver histology (Fig. 3A), hepatic executioner caspase-3 activity was determined and shown to be significantly elevated in Atg7F/FAlb-Cre+ mice (P < 0.01, Fig. 4C).
Finally, the mRNA expression of IRE1α, the phosphorylation of IRE1α, and the IRE1α-mediated splicing of XBP1 mRNA were unaltered in Atg7F/FAlb-Cre+ mice (Fig. 4D). In line with these findings, the other downstream targets of the IRE1α-XBP1 pathways (Erdj4, Calnexin) were also unaltered in Atg7F/FAlb-Cre+ mice compared with their controls (Fig. 4D). Besides XBP1 splicing, IRE1α is also able to activate the “alarm stress” JNK pathway (19). Hence, the activation of JNK was assessed by ELISA and found to be lower in Atg7F/FAlb-Cre+ compared with Atg7+/+Alb-Cre+ mice (0.59 ± 0.07 and 1.38 ± 0.26 ng/mg protein, respectively; P < 0.01), supporting that IRE1α is not activated upon autophagy deficiency.
These results demonstrate that the UPR pathways in the liver were selectively modified by autophagy deficiency, as evidenced by a diminished ATF6 pathway and an enhanced proapoptotic PERK pathway.
Hepatocellular autophagy deficiency leads to absence of fasting-induced steatosis, an improved plasma lipid profile, and reduced glycemia.
In the case of chronic ER stress, loss of ATF6 prevents the development of fatty liver (6). Hence, with consideration of the observed reduction in the ATF6 signaling and given the debated role of autophagy in steatosis (31), the effects on fasting-induced steatosis were investigated.
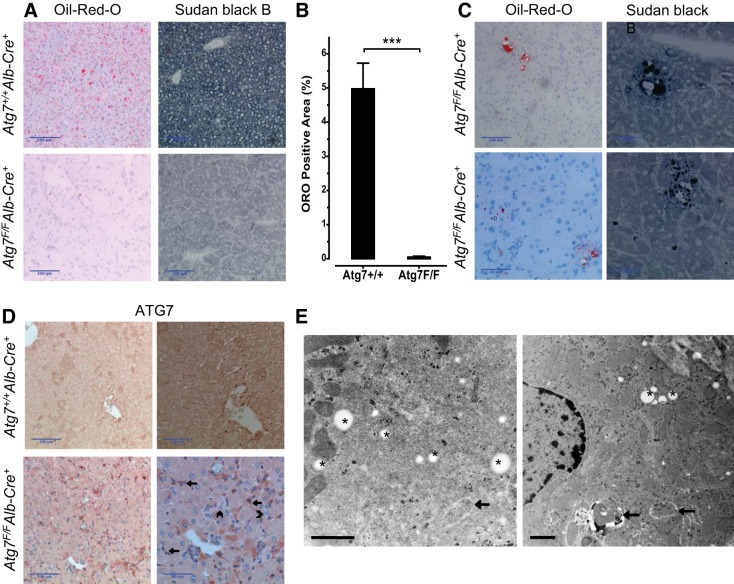
Fasting-induced steatosis is a well-known phenomenon caused by the accumulation of large amounts of triglycerides in hepatocytes after fasting for 6–24 h (18). This form of steatosis was demonstrated in Atg7+/+Alb-Cre+ mice, as shown via ORO staining, whereas Atg7F/FAlb-Cre+ mice had a significantly lower ORO positivity in their liver parenchyma (P < 0.001, Fig. 5, A and B). The absence of the typical fasting-induced steatosis in Atg7F/FAlb-Cre+ mice was subsequently verified with SBB (Fig. 5A). On TEM images, lipid droplets were easily recognized in Atg7+/+Alb-Cre+ livers (Fig. 2A). Occasionally, we also observed lipophagy (i.e., the inclusion of lipid droplets in the autophagosomes) in Atg7+/+Alb-Cre+ mice. In contrast, lipid droplets or autophagic vacuoles were lacking in the vast majority of Atg7F/FAlb-Cre+ hepatocytes (Fig. 2B).
Fig. 5.
Liver fat stains, ATG7 stain, and TEM of Atg7F/FAlb-Cre+ mice. A: Oil-red-O (ORO) and Sudan black B (SBB) stain demonstrated a fasting-induced steatosis in Atg7+/+Alb-Cre+ mice (top), whereas lipids were virtually absent in Atg7F/FAlb-Cre+ mice (bottom). B: morphometric measurement of the ORO-positive area confirmed the virtual absence of ORO-positive lipids in the Atg7F/FAlb-Cre+ mice (Student's t-test, ***P < 0.001). C: representative photographs of ORO- and SBB-positive grouped cells in Atg7F/FAlb-Cre+ mice, which could be focally detected in the liver. These cells are mostly grouped around a larger droplet/vesicle and contain a mixture of larger and smaller lipid droplets seemingly arranged around the nuclei and plasma membranes. D: immunohistochemical staining with ATG7 confirms the knockout of Atg7 in Atg7F/FAlb-Cre+ mice, with absent cytoplasmic staining in the hepatocytes compared with Atg7+/+Alb-Cre+. The darker cells in Atg7F/FAlb-Cre+ mice compared with the surrounding cells appear to correspond to the cells with (condensed cytoplasm) proapoptotic features on the HE stain (see Fig. 3A). Larger magnifications demonstrate the positivity on the nonhepatocytes (arrows), whereas the ductular reaction is negative as well (as these cells also express albumin; arrowheads). Scale bars = 100 μm and 50 μm. E: TEM of the livers of Atg7F/FAlb-Cre+ mice. Some lipid-containing cells could be detected. These cells contained, not only lipid droplets, but also autophagic vacuoles, whereas lipid droplets and/or autophagosomes were not observed in the other hepatocytes. Arrows indicate autophagic vacuoles, asterisks indicate lipid droplets. Scale bars = 1 μm.
Not only was hepatocellular lipid content altered, but also the plasma lipid concentrations were markedly changed. Atg7F/FAlb-Cre+ mice demonstrated a significant increase in total cholesterol, which can be attributed to a doubling of both plasma HDL and LDL cholesterol levels (Table 3). In absolute values, the amount of HDL was substantially higher than that of LDL in Atg7F/FAlb-Cre+ mice. Still, the LDL/HDL ratio was unaltered compared with Atg7+/+Alb-Cre+ mice. Plasma triglyceride levels were significantly lower in Atg7F/FAlb-Cre+ mice (P < 0.001, Table 3). Additionally, fasting glycemia was lower in Atg7F/FAlb-Cre+ mice, whereas fasting insulinemia was unaltered.
In summary, autophagy deficiency hampers fasting-induced steatosis in the liver, increases plasma cholesterol levels with a conserved LDL/HDL ratio, and reduces plasma triglycerides and glycemia.
Lipid-containing and autophagy-competent hepatocytes may occur in Atg7F/FAlb-Cre+ mice.
Remarkably, several atypical ORO-positive areas were observed in the livers of Atg7F/FAlb-Cre+ mice in the absence of fasting-induced steatosis, which could be confirmed in an SBB stain (Fig. 5C). These mixed micro- and macrovesicular fat-positive cell groups did not have a particular zonal distribution and were not observed in Atg7+/+Alb-Cre+ mice. Moreover, the presence of these areas on consecutive sections and on two different fat stainings argued against an artifact.
Because these cells might potentially represent autophagy-competent cells of unknown origin, an additional stain for ATG7 was performed (Fig. 5D), confirming the effective knockout of Atg7 in the hepatocytes of Atg7F/FAlb-Cre+ mice. Notice that the ductular reaction stains as well negative, as it originates out of hepatic progenitor cells (which already expressed albumin), whereas the other nonhepatic cells are positive (Fig. 5D). There were no ATG7-positive cells mimicking the fat-positive cell groups. Cells with a darker appearance tended to correspond to (pro)apoptotic cells upon HE staining and are likely the result of condensation of the cytoplasm. Neither LC3B nor p62 stains revealed cells similar to the fat-positive groups.
Intriguingly, our TEM results also revealed sparse fat-containing hepatocytes in Atg7F/FAlb-Cre+ mice. These cells, not only contained lipid droplets, but also showed signs of autophagy, in contrast to the majority of the hepatocytes in which neither autophagy nor lipid droplets were observed. These TEM findings are in line with the occurrence of autophagy-competent hepatocytes in the livers of Atg7F/FAlb-Cre+ mice and underline the close connections of lipid droplets and autophagy.
DISCUSSION
In this study, we demonstrate the deleterious consequences of hepatocyte-specific autophagy deficiency in relation to the different UPR pathways and the concomitant effects on hepatic and serum lipids.
Hepatocyte-specific knockout of autophagy causes a severe hepatomegaly and hepatic injury as already partially reported in other studies (22, 25, 28, 32, 37, 47, 49). This injury encompasses distortion of the liver architecture, increased apoptosis, general inflammation, activation of hepatic stellate cells, corresponding pericellular fibrosis, and a prominent ductular reaction. The latter was also mentioned in a study with Atg5 knockout mice (37) but was not specifically confirmed using immunohistochemistry. We assume that this ductular reaction can be interpreted as a type 3 ductular reaction (9), i.e., the proliferation of hepatic progenitor cells as an adaptive response to the loss of parenchymal cells and their function. It also implies that the liver is still capable of an adequate compensatory regeneration reaction in the case of hepatocyte-specific autophagy deficiency. However, as albumin is coexpressed in these cells, autophagy will be knocked out already (Fig. 5D), and hence the injured parenchymal cells will not be replaced by autophagy-competent hepatocytes from this source.
Although the ER stress-activated UPR is known to induce autophagy, the knowledge of the impact of autophagy on the UPR is presently limited (43). Our study demonstrates a significant alteration of the hepatic UPR pattern in conditions of autophagy deficiency. Moreover, autophagy deficiency apparently did not lead to a general induction of the UPR but rather had a selective effect on the distinct UPR pathways; the IRE1α pathway was unaltered, the ATF6 pathway was attenuated, and the PERK pathway was strongly activated upon autophagy deficiency. In line with our results, Ni et al. (37) did not observe any changes in the IRE1α pathway, but, in contrast to our findings and those of Yang et al. (55), they did not observe an activation of the PERK pathway either. Because PERK is known to mediate the transcription of many autophagy-related genes and activates autophagic flux (43), its activation may be a compensatory attempt to restore autophagy. The synchronous activation of the PERK-CHOP axis, resulting in enhanced cell apoptosis, is an important but deleterious side effect of this activation. IRE1α is able to stimulate additional cell signaling pathways, among them the JNK pathway. This alarm stress pathway is able to induce apoptosis and may stimulate autophagy as well (19, 52). In line with the unstimulated XBP1 splicing, phosphorylation of JNK was reduced in Atg7F/FAlbCre+ mice as well. Although seemingly contradictory with the results of the PERK pathway and the observed apoptosis, the mechanism of actions in the paradigm of adaptive vs. apoptotic phases of these alternative pathways of the UPR remains not well understood (20). Moreover, the overall findings of the UPR pathways might rather point toward “exhaustion” of the proteins and adaptive capacities of the UPR, thus the transition toward UPR-induced cell death.
To the best of our knowledge, the present study is the first to report effects of autophagy on the ATF6 pathway in mice. ATF6 is a transmembrane protein localized in the ER that has to be transported to the Golgi complex, where it is cleaved to form an active transcription factor, which in turn regulates genes responsible for protein folding, proteasomal degradation of misfolded proteins (ERAD), and genes supporting the XBP1 pathway (20). How exactly autophagy inhibits the ATF6 pathway remains unclear, yet the COPII complex might be involved seeing that our results showed that cleaved ATF6 but not total ATF6 was diminished in the liver of Atg7F/FAlb-Cre+ mice. Levels of SREBP-1 were not supportive for this hypothesis, as cleaved nuclear SREBP-1 becomes already undetectable after 6 h of fasting (21). Accordingly, potential differences in the cleavage of SREBP-1 could not be detected. The COPII complex, not only transports ATF6 to the Golgi complex, but also is involved in the ER-Golgi intermediate compartment and in LC3 lipidation (15). Hence, interruption of the formation of LC3-II to abolish autophagy might interfere with these complexes and prevent ATF6 transport to the Golgi apparatus, thus its activation.
Interestingly, autophagy deficiency, not only had detrimental effects on the liver, but also caused notable effects on the lipid metabolism. Autophagy appears to be necessary for the development of the physiological fasting-induced steatosis, which is no longer present in autophagy-deficient mice. These results are in contrast with augmented steatosis after fasting, as reported by Singh et al. (46). However, even with the observation of lipophagy, our results are in line with earlier observations (25, 32, 33, 44); conflicting results about the exact role of autophagy in lipid metabolism have also been reported for other models of fatty liver (26, 31) and might be explained by minor differences in experimental protocols, the context-dependent behavior of autophagy, and the interaction with other important metabolic body compartments like visceral fat or muscle tissue (26, 31).
The question arises whether the observed effects on the lipid metabolism in autophagy-deficient mice are causally related to the observed UPR pattern. Acute ER stress has been shown to stimulate liver steatosis rather than alleviating it. However, genetic ablation of the different UPR pathways does not prevent steatosis, indicating that the combined action of UPR pathways, rather than a single isolated pathway, is responsible for fat accumulation (42). Autophagy deficiency causes a sustained interruption of cellular homeostasis, pointing to chronic rather than acute ER stress. Ablation of ATF6 in the case of chronic, but not acute, ER stress has been shown to prevent fatty liver (6). This effect of ATF6 in chronic conditions might at least partially explain the observed loss of fasting-induced steatosis.
The importance of autophagy in cellular lipid metabolism is underlined by the finding of fat-containing cells in livers of Atg7F/FAlb-Cre+ mice (Fig. 5C) in a pattern that is clearly distinct from fasting-induced steatosis. These cells were more evident after staining with SBB, which is known to stain a broader spectrum of fats (2), suggesting that these vacuoles may contain another type of fat (i.e., glycolipids or cholesterol). However, glycolipids are unlikely given a negative Periodic Acid-Schiff reaction (data not shown). Intriguingly, TEM revealed the co-occurrence of lipid droplets and autophagic vacuoles in some hepatocytes of Atg7F/FAlb-Cre+ mice, similar to Atg7+/+Alb-Cre+ mice. This finding is indicative of the existence of autophagy-competent hepatocytes in Atg7F/FAlb-Cre+ mice.
Potential explanations for the presence of rare clusters of autophagy-competent cells in Atg7F/FAlb-Cre+ mice include the escape of ablation by Cre recombinase (10) or repopulation of the liver by newly developed autophagy-competent hepatocytes that have not expressed albumin yet and are therefore still autophagy competent (10, 23). The latter explanation is more likely because albumin has been found to be expressed in hepatocytes early during embryogenesis and hepatic progenitor cells already express albumin (24, 35). Despite the occasional presence of fat droplets and autophagosomes on TEM in Atg7F/FAlb-Cre+ mice, these atypical cells are negative for the ATG7 stain, and their fat content does not seem to be identical to that of Atg7+/+Alb-Cre+ hepatocytes. We hypothesize that albumin (thus also Cre recombinase) becomes expressed during the maturation of these albumin-naive prehepatocytes to adult hepatocytes. This gradual change will ultimately lead to the knockout of Atg7. The current and unusual appearance of the lipid droplets in these cells might be a simple consequence of this transformation. Previously formed lipid droplets could be in a weaning state, whereas the absence of autophagy hinders the maintenance or de novo formation of lipid droplets by as yet unclarified mechanisms.
Autophagy deficiency, not only affected the lipid content of the hepatocytes, but also had a clear impact on serum lipids with reduced triglyceride levels and increased levels of HDL and LDL cholesterol. However, the latter was a balanced increase without affecting their relative proportions. Ma et al. (32) also reported that serum triglycerides were decreased in control-fed conditions but not in high-fat diet conditions. However, they did not report on other serum lipids. Additional reports on serum lipids in relation to hepatocyte-specific autophagy deficiency are lacking. Lower triglyceride production (25) and very-low-density lipoprotein production (32) upon autophagy knockout have been reported, which might explain the lower plasma triglycerides but not the higher HDL and LDL levels observed in the present study. Furthermore, neither of these findings explains the absence of hepatocellular fat. The absence of both hepatocellular and serum triglycerides in hepatocellular autophagy deficiency might point to an increased oxidation of fatty acids and/or the inability to incorporate them in triglycerides. Mitochondria play a central role in cellular homeostasis and are removed by nonselective autophagy or selectively by so-called “mitophagy” (11). The observed accumulation of misformed mitochondria implies mitochondrial dysfunction that has previously been linked to impaired steatosis in autophagy-deficient mice via the production of FGF21, leading to increased oxidation outside the liver despite a reduced oxidative phosphorylation (25). Autophagy also prevents the activation of caspase-8 and the mitochondrial death pathway (1), as well as defective mitophagy involved in cell injury (11). Furthermore, in-depth analysis of the mitochondrial dysfunction, given its central role in the pathophysiology of NAFLD (3), related to autophagy deficiency would be of specific interest for further study.
In conclusion, this study shows that the parenchymal integrity of the liver depends on hepatocellular autophagy because autophagy deficiency results in injury, inflammation, and apoptosis. The reciprocal but pathway-selective effects of autophagy on ER stress and the UPR at least partially explain the observed effects. More specifically, the ATF6 pathway might account for the observed impairment of fasting-induced steatosis, in addition to the direct linkage between autophagy and lipid droplets. Hence, autophagy and pathway-selective UPR modulation may become relevant targets in the treatment of NAFLD.
GRANTS
W. Kwanten and S. Francque received funding from the Fund for Scientific Research (FWO) Flanders (11J9513N, 1802154N). H. Van Vlierberghe is senior clinical investigator of the Research Foundation Flanders. Y.-P. Vandewynckel is sponsored by a grant from the Special Research Fund (01D20012), Ghent University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.J.K., W.M., and S.M.F. conception and design of research; W.J.K. and Y.-P.V. performed experiments; W.J.K. and Y.-P.V. analyzed data; W.J.K., Y.-P.V., A.D., and P.B. interpreted results of experiments; W.J.K. prepared figures; W.J.K. drafted manuscript; W.J.K., W.M., B.Y.D.W., P.P.M., V.O.V.H., J.-P.T., H.V.V., and S.M.F. edited and revised manuscript; W.J.K., Y.-P.V., W.M., B.Y.D.W., P.P.M., V.O.V.H., A.D., J.-P.T., P.B., H.V.V., and S.M.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the staff of the laboratory of experimental medicine and pediatrics (LEMP) of the University of Antwerp and Mrs. Eliene Bogaerts of the Ghent University for practical assistance, as well as Mrs. Rita van den Bossche and Dr. Isabel Pintelon (University of Antwerp) for assistance with histology and electron microscopy.
REFERENCES
- 1.Amir M, Zhao E, Fontana L, Rosenberg H, Tanaka K, Gao G, Czaja MJ. Inhibition of hepatocyte autophagy increases tumor necrosis factor-dependent liver injury by promoting caspase-8 activation. Cell Death Differ 20: 878–887, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayliss High O. Lipid Histochemistry (Royal Microscopical Society Microscopy Handbooks). Oxford, UK: Oxford University, 1984. [Google Scholar]
- 3.Begriche K, Massart J, Robin MA, Bonnet F, Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 58: 1497–1507, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Choi AMK, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med 368: 651–662, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Chu CM, Shyu WC, Liaw YF. Comparative studies on expression of alpha-smooth muscle actin in hepatic stellate cells in chronic hepatitis B and C. Dig Dis Sci 53: 1364–1369, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Cinaroglu A, Gao C, Imrie D, Sadler KC. Activating transcription factor 6 plays protective and pathological roles in steatosis due to endoplasmic reticulum stress in zebrafish. Hepatology 54: 495–508, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czaja MJ, Ding WX, Donohue TM, Friedman SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JJ, Perlmutter DH, Randall G, Ray RB, Tsung A, Yin XM. Functions of autophagy in normal and diseased liver. Autophagy 9: 1131–1158, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology 53: 1752–1763, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch 458: 251–259, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Diaz F, Garcia S, Hernandez D, Regev A, Rebelo A, Oca-Cossio J, Moraes CT. Pathophysiology and fate of hepatocytes in a mouse model of mitochondrial hepatopathies. Gut 57: 232–242, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, analysis. Biol Chem 393: 547–564, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2α kinases: Their structures and functions. Cell Mol Life Sci 70: 3493–3511, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espenshade PJ. SREBPs: Sterol-regulated transcription factors. J Cell Sci 119: 973–976, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Friedman SLS. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88: 125–172, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge L, Zhang M, Schekman R. Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment. Elife 3: e04135, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentile CL, Frye M, Pagliassotti MJ. Endoplasmic reticulum stress and the unfolded protein response in nonalcoholic fatty liver disease. Antioxid Redox Signal 15: 505–521, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González-Rodríguez A, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina ME, Vargas-Castrillón J, Lo Iacono O, Corazzari M, Fimia GM, Piacentini M, Muntané J, Boscá L, García-Monzón C, Martín-Sanz P, Valverde ÁM. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis 5: e1179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan HP, Goldstein JL, Brown MS, Liang G. Accelerated fatty acid oxidation in muscle averts fasting-induced hepatic steatosis in SJL/J mice. J Biol Chem 284: 24644–24652, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hetz C, Glimcher LH. Fine-tuning of the unfolded protein response: Assembling the IRE1alpha interactome. Mol Cell 35: 551–561, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hetz C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13: 89–102, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci USA 95: 5987–5992, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, Lee MS, Tanaka K, Komatsu M. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol 193: 275–284, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iverson SV, Comstock KM, Kundert JA, Schmidt EE. Contributions of new hepatocyte lineages to liver growth, maintenance, and regeneration in mice. Hepatology 54: 655–663, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin L, Long L, Green MA, Spear BT. The alpha-fetoprotein enhancer region activates the albumin and alpha-fetoprotein promoters during liver development. Dev Biol 336: 294–300, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim DH, Hur KY, Kim HK, Ko T, Han J, Kim HL, Kim J, Back SH, Komatsu M, Chen H, Chan DC, Konishi M, Itoh N, Choi CS, Lee MS. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med 19: 83–92, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Kim KH, Lee MS. Autophagy—a key player in cellular and body metabolism. Nat Rev Endocrinol 10: 322–337, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Kofman AV, Morgan G, Kirschenbaum A, Osbeck J, Hussain M, Swenson S, Theise ND. Dose- and time-dependent oval cell reaction in acetaminophen-induced murine liver injury. Hepatology 41: 1252–1261, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169: 425–434, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuma A, Matsui M, Mizushima N. LC3, an autophagosome marker, can be incorporated into protein aggregates independent of autophagy: Caution in the interpretation of LC3 localization. Autophagy 3: 323–328, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Kuma A, Mizushima N. Chromosomal mapping of the GFP-LC3 transgene in GFP-LC3 mice. Autophagy 4: 61–62, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Kwanten WJ, Martinet W, Michielsen PP, Francque SM. Role of autophagy in the pathophysiology of nonalcoholic fatty liver disease: A controversial issue. World J Gastroenterol 20: 7325–7338, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma D, Molusky MM, Song J, Hu CR, Fang F, Rui C, Mathew AV, Pennathur S, Liu F, Cheng JX, Guan JL, Lin JD. Autophagy deficiency by hepatic FIP200 deletion uncouples steatosis from liver injury in NAFLD. Mol Endocrinol 27: 1643–1654, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinet W, Schrijvers DM, Timmermans JP, Bult H, De Meyer GRY. Immunohistochemical analysis of macroautophagy: recommendations and limitations. Autophagy 9: 386–402, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinet W, Timmermans JP, De Meyer GRY. Methods to assess autophagy in situ-transmission electron microscopy versus immunohistochemistry. Methods Enzymol 543: 89–114, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell 14: 561–574, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15: 1101–1111, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni HM, Woolbright BL, Williams J, Copple B, Cui W, Luyendyk JP, Jaeschke H, Ding WX. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol 61: 617–625, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oslowski CM, Urano F. Measuring ER Stress and the Unfolded Protein Response Using Mammalian Tissue Culture System. Philadelphia, PA: Elsevier, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26: 149–150, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DCO, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90: 1383–1435, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Rosenfeldt MT, Nixon C, Liu E, Mah LY, Ryan KM. Analysis of macroautophagy by immunohistochemistry. Autophagy 8: 963–969, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GDY, Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell 15: 829–840, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci 40: 141–148, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibata M, Yoshimura K, Furuya N, Koike M, Ueno T, Komatsu M, Arai H, Tanaka K, Kominami E, Uchiyama Y. The MAP1-LC3 conjugation system is involved in lipid droplet formation. Biochem Biophys Res Commun 382: 419–423, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Shoulders MD, Ryno LM, Genereux JC, Moresco JJ, Tu PG, Wu C, Yates JR, Su AI, Kelly JW, Wiseman RL. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep 3: 1279–1292, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature 458: 1131–1135, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, Eishi Y, Hino O, Tanaka K, Mizushima N, Figure S. Autophagy-deficient mice develop multiple liver tumors. Genes Dev 25: 795–800, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teske BF, Baird TD, Wek RC. Methods for analyzing eIF2 kinases and translational control in the unfolded protein response. Methods Enzymol 490: 333–356, 2011. [DOI] [PubMed] [Google Scholar]
- 49.Tian Y, Kuo CF, Sir D, Wang L, Govindarajan S, Petrovic LM, Ou JHJ. Autophagy inhibits oxidative stress and tumor suppressors to exert its dual effect on hepatocarcinogenesis. Cell Death Differ 22: 1025–1034, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 10: 837–858, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34: 274–285, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 30: 678–688, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 72: 2224–2232, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu X, So JS, Park JG, Lee AH. Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin Liver Dis 33: 301–311, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 11: 467–478, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang XQ, Xu CF, Yu CH, Chen WX, Li YM. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 20: 1768–1776, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]