Abstract
Epidemiological studies support strong links between obesity, diabetes, and pancreatic disorders including pancreatitis and pancreatic adenocarcinoma (PDAC). Type 2 diabetes (T2DM) is associated with insulin resistance, hyperglycemia, and hyperinsulinemia, the latter due to increased insulin secretion by pancreatic beta-cells. We reported that high-fat diet-induced PDAC progression in mice is associated with hyperglycemia, hyperinsulinemia, and activation of pancreatic stellate cells (PaSC). We investigated here the effects of high concentrations of insulin and glucose on mouse and human PaSC growth and fibrosing responses. We found that compared with normal, pancreata from T2DM patients displayed extensive collagen deposition and activated PaSC in islet and peri-islet exocrine pancreas. Mice fed a high-fat diet for up to 12 mo similarly displayed increasing peri-islet fibrosis compared with mice fed control diet. Both quiescent and activated PaSC coexpress insulin (IR; mainly A type) and IGF (IGF-1R) receptors, and both insulin and glucose modulate receptor expression. In cultured PaSC, insulin induced rapid tyrosine autophosphorylation of IR/IGF-1R at specific kinase domain activation loop sites, activated Akt/mTOR/p70S6K signaling, and inactivated FoxO1, a transcription factor that restrains cell growth. Insulin did not promote activation of quiescent PaSC in either 5 mM or 25 mM glucose containing media. However, in activated PaSC, insulin enhanced cell proliferation and augmented production of extracellular matrix proteins, and these effects were abolished by specific inhibition of mTORC1 and mTORC2. In conclusion, our data support the concept that increased local glucose and insulin concentrations associated with obesity and T2DM promote PaSC growth and fibrosing responses.
Keywords: diabetes, obesity, insulin, pancreatic fibrosis, pancreatic stellate cells
in recent years, the incidence of pancreatic adenocarcinoma (PDAC) and chronic pancreatitis, disorders of the exocrine pancreas lacking effective therapies, has been increasing in parallel with the epidemic of obesity and diabetes mellitus (29, 43, 44, 51). Epidemiological studies indicate that chronic pancreatitis and associated type 3c diabetes mellitus markedly increase the risk for PDAC (2, 40). Similarly, obesity (7) and long-standing type 2 diabetes mellitus (T2DM) (5, 51) are well-stablished PDAC risk factors. These data support a closer relationship between disorders of the exocrine and endocrine pancreatic parenchyma than historically perceived, but the basis of these interactions are poorly understood.
T2DM is a complex metabolic disease typically associated with obesity, insulin resistance, and concomitant hyperglycemia. To control blood glucose, pancreatic beta-cells increase insulin secretion, at any rate during the early stages of the disease, resulting in hyperinsulinemia (4). Hyperinsulinemia, hyperglycemia, and dysregulated insulin/insulin-like growth factor I (IGF-1) signaling have been suggested as important factors enhancing tumor progression in many cancers including pancreatic cancer (14, 15, 20, 38). In diabetes-associated PDAC, exocrine pancreatic cells and tumor cells are likely subjected to extremely high concentrations of glucose and insulin. This is due to not only hyperglycemia and hyperinsulinemia, but also the common blood supply between endocrine and exocrine pancreatic cells, with islet-derived insulin acting as a potential growth factor (11). Moreover, progressive pancreatic beta-cell dysfunction in T2DM can lead to inflammation and local elevations in inflammatory cytokines (22), all factors promoting pancreatitis, fibrosis, and tumorigenesis.
Insulin and the highly homologous peptide IGF regulate many cellular functions including cellular growth and proliferation, glucose metabolism, cell differentiation, and migration in normal and cancer cells (45). Insulin and IGF-1 mediate their intracellular effects via activation of their cognate receptors, IR and IGF-1R. Both receptors are transmembrane heterotetrameric proteins, each consisting of two extracellular α-subunits and two transmembrane β-subunits with intrinsic tyrosine kinase activity (48). Because of their high degree of homology, IR and IGF-1R can heterodimerize and form hybrid receptors comprising one α-subunit and one β-subunit each of the IR and IGF-1R. Insulin binds with high affinity to the IR isoforms IR-A and IR-B, with IR-A having a preferential mitogenic role and IR-B a metabolic role (18), as well as with reduced affinity to IGF-1R and hybrid receptors. IGF-1 binds to IGF-1R as well as hybrid receptors (48). Ligand binding to the IR, IGF-1R, or IR/IGF-1R hybrid receptors results in auto/transphosphorylation of their tyrosine kinase domains, recruitment of adaptor-effector complexes, and amplification of signals which propagate to enhance the activities of multiple downstream targets including mitogen-activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase (PI3K)/AKT/mechanistic target of rapamycin (mTOR) signaling pathways, all pivotal to the development and progression of PDAC (10, 31). Importantly, both IR (mainly IR-A) and IGF-1R are overexpressed in most cancers including pancreatic cancer (13, 16, 35), which increases the likelihood of forming hybrid receptors and enhances mitogenic signaling. Based on the critical role of IR and IGF-1R in cancer progression and patient survival, several therapeutic approaches to target these receptors are currently being evaluated in clinical trials (42, 45).
Most research on insulin and IGF signaling in PDAC and other cancer types has focused on the mechanisms underlying cellular growth and survival in cancer cells, while their effects on stromal cells have received less attention. Pancreatic stellate cells (PaSC) are key components of the stroma in PDAC and chronic pancreatitis (3, 34). In the healthy pancreas, PaSC can be found in low numbers in a quiescent state surrounding pancreatic acini and ducts as well as in peri-islet areas (34). During pancreatitis or tumor development, PaSC undergo differentiation into a myofibroblast phenotype, a process termed “activation,” characterized by rapid growth and proliferation, production, and secretion of a variety of extracellular matrix proteins, cytokines, and other factors that regulate matrix physical properties and remodeling, inflammation, and cancer cell growth and invasion (3, 17, 36).
Recent studies in animal models support a role for activated PaSC in the development of islet fibrosis and beta-cell dysfunction during T2DM (24–26, 30, 41). However, the direct effects of hyperinsulinemia and hyperglycemia to alter PaSC biology are largely unknown. In particular, the expression and downstream signaling of IR/IGF-1R in PaSC must be studied to understand the impact of these cells on islet dysregulation during T2DM. We hypothesize that key interactions between PaSC and other cells in the pancreas, such as beta-cells, acinar cells, ductal cells, and cancer cells, underpin the emergence of PDAC-associated diabetes. Here, we examined PaSC activation and islet fibrosis in pancreatic tissues of T2DM patients and mice fed a high-fat diet. We also investigated IR/IGF-1R expression and downstream signaling in mouse and human PaSC, and the effects of high concentrations of insulin and glucose on PaSC growth and fibrosing responses.
MATERIALS AND METHODS
Antibodies and chemicals.
The following antibodies were used for Western blotting and/or immunofluorescence: fibronectin (no. F3648), α-SMA (no. A2547), P4HA2 (no. SAB1100773), β-actin (no. A1978) were purchased from Sigma-Aldrich (St. Louis, MO). Collagen (no. AB765P) was from Millipore (Bedford, MA). Antibodies directed against insulin (no. 4590), IR (no. 3025), IGF-1R (no. 3027), phospho-IGF1Rβ/IRβ (Tyr1135/1136;Tyr1150/1151; no. 3024), phospho-Akt (Ser473; no. 4060), Akt (no. 4691); phospho-mTOR (Ser2448; no. 5536), phospho-p70 S6 Kinase (Thr389; no. 9234), p70S6 Kinase (no. 2708), phospho-p44/42 (Thr202/Tyr204; no. 9101), p44/42 MAPK (no. 9102), phospho-FoxO1 (Thr24; no. 2599), phospho-SAPK/JNK (Thr183/Tyr185; no. 4668), SAPK/JNK (no. 9258), and corresponding HRP-linked secondary antibodies were from Cell Signaling Technology (Danvers, MA). Insulin (no. I9278) and IGF-1 (no. 407252) recombinant proteins were from Sigma-Aldrich and Millipore, respectively. The mTOR inhibitor KU63794 (KU) was purchased from Tocris Bioscience (no. 3725; Minneapolis, MN). SuperSignal West Pico (or Femto) Chemiluminescent Substrate reagent, 4′,6-diamidino-2-phenylindole (DAPI), ProLong Gold antifade mounting medium, and fluorescence-conjugated secondary antibodies were from ThermoFisher Scientific (Waltham, MA).
Tissue digestion for PaSC isolation was performed using Pronase (no. 165921), Collagenase P (no. 1213873), DNase I (no. 10104159001) and bovine serum albumin fraction V (BSA; no. 03116956001), all obtained from Roche Diagnostics (Indianapolis, IN). Density gradients for PaSC separation were prepared using Nycodenz (no. AN1002423; Accurate Chemical & Scientific; Westbury, NY) and Gey's balanced salt solution (GBSS; no. G9779; Sigma-Aldrich). Cell culture DMEM/F12 medium (no. 11330-032), DMEM (no glucose; no. 11966-025), DMEM (25 mM glucose; no. 11965-092) and l-glutamine (no. 25030-081) were from ThermoFisher Scientific; antibiotics/antimycotics (1% Penicillin-Streptomycin; no. 25030-081) and fetal bovine serum (FBS; no. FB11) were from Omega Scientific (Tarzana, CA). All chemicals and kits were used according to the manufacturer's recommendations, unless otherwise indicated.
Human pancreatic tissues.
Formalin-fixed and paraffin-embedded (FFPE) human pancreatic specimens were obtained from cadaveric tissues from organ donors at City of Hope (Duarte, CA). For this study, pancreatic tissue specimens from a total of 6 organ donors were analyzed. All donors were females with ages ranging from 52 to 57 years old. Three of the donors had no history of diabetes and displayed normal pancreas histology, and the other three donors had a previous history of type 2-diabetes and blood glycated hemoglobin (HbA1c) values >8% at the time of death. Table 1 shows more information about the demographics and characteristics of the organ donors. Pancreas donation consent was obtained from donor families for islet transplantation and research. The study was performed in accordance with regulations and IRB protocols approved by the Institutional Review Boards of the City of Hope and the Cedars-Sinai Medical Center.
Table 1.
Clinical and demographics data of the pancreas organ donors
| Sample ID | Pancreas Pathology | Cause of Death | Ethnicity. | Age, yr | Sex | ABO | Height, in. | Weight, lb | BMI | HbA1c, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Hu501 | normal | CVA/stroke | Caucasian | 56 | Female | O(+) | 66 | 161 | 25.9 | NA |
| Hu514 | normal | CVA/stroke | Hispanic | 52 | Female | O(+) | 62 | 171 | 31.5 | 6.4 |
| Hu816 | normal | CVA/stroke | Caucasian | 53 | Female | O(−) | 63 | 217 | 38.4 | NA |
| Hu522 | T2DM | NA | Hispanic | 54 | Female | A(+) | 63 | 158 | 28.0 | 8.1 |
| Hu604 | T2DM | CVA/stroke | Caucasian | 57 | Female | A(−) | 61 | 117 | 22.1 | 10.5 |
| Hu935 | T2DM | Head trauma | Hispanic | 53 | Female | O(+) | 64 | 140 | 24.0 | 11.9 |
Paraffin-embedded pancreatic tissue specimens were obtained from cadaveric tissues from organ donors at City of Hope (Duarte, CA). Indicated are some of the characteristics of the organ donors including whether the donors display a healthy pancreas with no history of diabetes (normal) or whether they had a history of type 2 diabetes (T2DM). All selected donors were females with ages ranging from 52 to 57 yr old. Serial sections were analyzed for collagen deposition by Masson's trichrome staining, and for insulin and α-SMA (a marker of activated stellate cells and myofibroblasts) by immunofluorescence, and representative images are shown in Fig. 1.
ABO, blood group; BMI, body mass index; HbA1c, glycated hemoglobin; NA, not available.
Mouse pancreatic tissues.
Formalin-fixed and paraffin-embedded (FFPE) mouse pancreatic tissues were obtained from mice (C57BL/6 background) fed control (CD) or high-fat, high-calorie diets (HFCD) at the animal core of the University of California, Los Angeles, under the direction of G. Eibl. C57BL/6J male mice at 6 wk of age were randomly placed either on a HFCD or a CD and fed the diets for 3, 6, 9, or 12 mo. All mice were fed ad libitum with free access to clean drinking water throughout the duration of the study, and maintained under regular laboratory conditions with controlled temperature (19–22°C) and 12:12-h light/dark cycle. Food intake and body weight of each animal were measured weekly. After the feeding period, animals were euthanized, and the entire pancreas harvested for histological analysis and other analyses. Animal studies were approved by the Chancellor's Animal Research Committee of the University of California, Los Angeles (CA) in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
The diets were obtained from Dyets and have been described elsewhere (15). The CD was a modified AIN-76A purified rodent diet and contained a total of 3,726 kcal/kg (19% of calories coming from protein, 67% from carbohydrate and 12% from fat). The HFCD had increased caloric content compared with the CD (4,536 kcal/kg vs. 3,726 kcal/kg) with 16% of total calories derived from protein (casein), 43% from carbohydrate (sucrose and cornstarch), and 40% from fat (corn oil). While the amount of casein, sucrose, salts, and vitamins were kept identical in both diets, compared with the CD, the HFCD had a fourfold increase in corn oil. The diets were handled under low-light conditions, and stored at −20°C. The diets were replaced in the mouse cages twice weekly.
Evaluation of collagen deposition and immunofluorescence analysis in human and mouse pancreatic tissues.
Human and mouse pancreas tissues were fixed in 4% buffered formaldehyde, embedded in paraffin, sectioned (4 μm thick), and stained using Masson's trichrome (human tissues) or Sirius red (mouse tissues) approaches to measure the extent of collagen deposition. Whole stained slides were scanned using the Aperio Scanscope AT Turbo slide scanner and digitized images were visualized using the Leica Digital Image Hub (Leica microsystems, Wetzlar, Germany). The total stained collagen area in each specimen was quantified by morphometric analysis in at least 7 digitized, random non-overlapping sections at X200 magnification using the MetaMorph imaging system (Universal Imaging, Downingtown, PA), and expressed as average percentage of total tissue area. At least two specimens per mouse pancreas (head and tail sections) were measured, and 3–4 mice per group were analyzed.
In addition, serial tissue sections from the same specimens were immunostained for insulin and α-SMA to visualize the pancreatic islets and activated pancreatic stellate cells, respectively. Alexa Fluor 488 or Alexa Fluor 594 were used as conjugated secondary antibodies, and 4′6′-diamidino-2-phenylindole (DAPI) as nuclear counterstain. Digitalized images were captured using a Leica TCS SP5 spectral confocal microscope (Leica Microsystems, Buffalo Grove, IL) with the assistance of the Cedars-Sinai Medical Center (Los Angeles, CA) Imaging Core.
Cell culture.
Primary mouse PaSC (mPaSC) were obtained from pancreas tissues from C57BL/6 wild-type mice (Harlan) as previously described (36, 47). Briefly, pancreata from 1–2 mice were excised, minced, and digested in GBSS containing 140 μg/ml pronase, 400 μg/ml collagenase P, and 8.75 units/ml DNase I. The cell suspension was filtered through a 100 μm nylon cell strainer and washed in GBSS supplemented with 0.3% BSA. Then, PaSC were separated by Nycodenz density gradient centrifugation. The Nycodenz gradient was prepared by layering the cell suspension beneath 6 ml of 0.3% BSA/GBSS in a polycarbonate centrifuge tube, and then centrifuged for 20 min at 1,400 g. Quiescent PaSC were collected from a fuzzy band at the interface near the top of the gradient, and expanded in culture up to passage 2. Activated mPaSC were characterized by the presence of α-SMA stress fibers, high production of fibronectin, and reduced expression of GFAP. Animal studies were approved by the Institutional Animal Care and Use Committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Immortalized mouse PaSC (imPaSC) were initially obtained from Dr. Raul Urrutia and used in previous studies by our group (47). Human primary PaSC (hPaSC) were obtained from pancreatic cadaveric tissues from organ donors. Briefly, pancreata were obtained and initially digested at City of Hope (Duarte, CA) as described (39) and further processed at the Cedars-Sinai Medical Center (Los Angeles, CA) to isolate hPaSC following the same procedure indicated above for primary mPaSC. Pancreas donation consent was obtained from donor families for islet transplantation and research. The study was performed in accordance with regulations and IRB protocols approved by the Institutional Review Boards of the Beckman Research Institute of the City of Hope and the Cedars-Sinai Medical Center.
Primary mPaSC and hPaSC, as well as imPaSC, were grown in DMEM/F12 media supplemented with 15% FBS, 2 mM l-glutamine, and antibiotics/antimycotics, in a humidified 5% CO2 atmosphere. For experimental purpose, cells were seeded in 60-mm dishes in DMEM/F12 or DMEM with 5 mM or 25 mM glucose and supplemented with 0.5–10% FBS, and treated with the experimental agents at 60–80% confluency. Additional experimental details are indicated in results.
Western blot analysis.
Cells were homogenized in RIPA buffer containing 50 mmol/l Tris (pH 7.4), 150 mmol/l NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS and a mix of protease and phosphatase inhibitors (Roche Applied Science, Basel, Switzerland). Protein extracts were resolved by SDS-PAGE for immunoblot analysis. The primary and horseradish peroxidase-conjugated specific secondary antibodies used here are indicated in Antibodies and chemicals. Immunoreactive bands were visualized by chemiluminescence (ThermoFisher Scientific) and densitometrically quantified using the PXi Touch Imaging System (Syngene). To estimate protein levels, optical density values in each blot were expressed relative to those of the loading control (β-actin and α-SMA).
RNA analysis by qPCR.
Cellular RNA from quiescent and cultured activated PaSC was extracted using the RNeasy Plus Mini Kit (no. 74034; Qiagen, Valencia, CA). Reverse transcription was performed with the iScript Reverse Transcription Supermix (no. 170–8840; Bio-Rad, Hercules, CA) using 1 μg of total RNA, and the synthesized cDNA samples were used as templates for quantitative real-time PCR (qPCR) analysis. Kits were used according to the manufacturer's instructions. All reactions were performed using the Bio-Rad CFX Connect Real-Time PCR Detection System and the amplifications were done with the iTaq Universal SYBR Green Supermix (Bio-Rad). The following gene-specific oligonucleotide primers were used: IR forward 5′-ATGGGCTTCGGGAGAGGAT-3′ and reverse 5′-GGATGTCCATACCAGGGCAC-3′; IGF-1R forward 5′-GTGGGGGCTCGTGTTTCTC-3′ and reverse 5′-GATCACCGTGCAGTTTTCCA-3; IR-B forward 5′-TGTCCCCAGAAAAACCTCTT-3′ and reverse 5′-GTGACTCCTTGTTCACCACTT-3; IR-AB forward 5′-GGAATGTGGGGATGTCTGTC-3′ and reverse 5′-ATGGTTGGGCAAACTTTCTG-3′; Col1A1 forward 5′-TAGGCCATTGTGTATGCAGC-3′ and reverse 5′-ACATGTTCAGCTTTGTGGACC-3′; Fibronectin forward 5′-CCTTACACGGTTTCCCATTA-3′ and reverse 5′-TTGTCATGGCACCATTTAGA-3′; ACTA2 forward 5′-GTTCAGTGGTGCCTCTGTCA-3′ and reverse 5′-ACTGGGACGACATGGAAAAG-3′; 18S rRNA forward 5′-AGTCCCTGCCCTTTGTACACA-3′ and reverse 5′-CGATCCGAGGGCCTCACTA-3′. Relative transcript levels were calculated using the comparative 2−ΔΔCt method and normalized to the housekeeping gene, 18S rRNA.
MTT assay.
MTT (Thiazolyl Blue Tetrazolium Bromide) assay was used as indicator of cellular metabolic activity and proliferation in PaSC. Cells were seeded in 24-well plates at 1 × 104 cells per well. After the indicated treatments in triplicates, MTT was added to the culture medium to yield a final concentration of 0.5 mg/ml, and cells were then incubated for 3 h at 37°C in CO2 incubator. After removing medium, DMSO was added to dissolve the insoluble formazan. Absorbance was measured at 595 nm using a plate absorbance reader (SpectraMax M3, Molecular Devices, Sunnyvale, CA).
Statistical analyses.
All experiments were performed in triplicate unless otherwise stated. Data are presented as means ± SE. Data were subjected to analysis of variance (ANOVA) followed by Tukey's post hoc test, and two-tailed Student's t-test for comparison between 2 groups. P values <0.05 were considered significant and indicated with an asterisk (*).
RESULTS
Type 2 diabetes is associated with islet fibrosis and activation of pancreatic stellate cells.
Previously, histopathologic analysis of pancreas from experimental animals with T2DM and concomitant hyperglycemia and hyperinsulinemia revealed progressive fibrosis in pancreatic islets, with some islets divided or completely replaced by fibrotic fibers in older animals (24–26). Despite these data in rodents, corresponding similar data from humans are scant. Nevertheless, a recent meta-analysis evaluating pancreatic exocrine insufficiency in patients with type 1 or 2 diabetes reported a high prevalence of moderate to severe pancreatic fibrosis in patients with longstanding diabetes (30). This underscores the need to clarify the involvement of the major early diabetic disturbances in the onset of more advanced pathophysiological mechanisms.
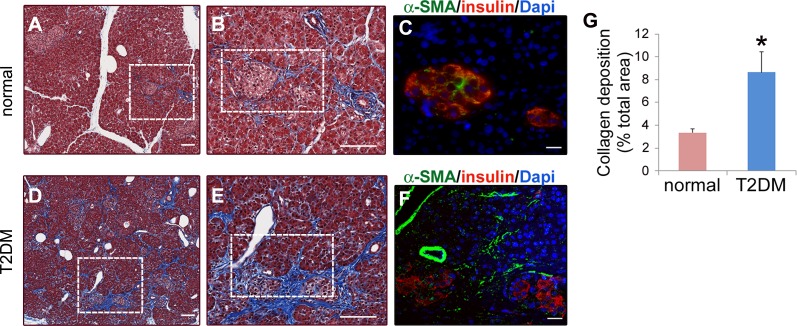
To evaluate the relationship between T2DM and PaSC activation in humans, we performed histopathological analysis of formalin-fixed, paraffin-embedded (FFPE) pancreatic tissue specimens obtained from cadaveric tissues from three organ donors with longstanding T2DM and three nondiabetic (normal) controls without pancreas pathology. As indicated in Table 1, donors were all females, between 52–57 yr of age and of Caucasian or Hispanic ethnicity, with glycated hemoglobin (HbA1c) values >8% in T2DM donors. Serial sections from FFPE tissues were either stained using Masson's trichrome to evaluate the extent of collagen deposition, or analyzed by immunofluorescence for insulin and α-SMA to visualize β cells and activated PaSC, respectively. As illustrated in Fig. 1, A and B, nondiabetic control pancreas appears normal, with bundles of collagen fibers mainly restricted to perivascular regions. In contrast, pancreas sections from T2DM donors displayed extensive collagen deposition, not only in perivascular regions, but also within islets and in a periacinar pattern in the vicinity of affected islets (Fig. 1, D and E). As illustrated in Fig. 1G, pancreatic collagen deposition was significantly increased, by about 3-fold in donors with T2DM compared with controls.
Fig. 1.
Type-2 diabetes patients display extensive collagen deposition and stellate cell activation in pancreatic areas surrounding islets. Paraffin-embedded pancreatic tissue specimens were obtained from cadaveric tissues from organ donors at the City of Hope Medical Institute (Duarte, CA). Serial sections were analyzed for collagen deposition by Masson's trichrome staining (A, B, D, and E), and for insulin and α-SMA by immunofluorescence (C and F). The clinical and demographic data of the organ donors is presented in Table 1. A and D show representative photomicrographs of Masson's trichrome-stained pancreas tissues from organ donors without pancreas pathology (normal; A) and organ donors with type-2 diabetes (T2DM; D). Collagens are visible as the blue stain; scale bar = 100 μm. Dotted boxes in A and D indicate areas with two examples of collagen deposition surrounding islets illustrated at higher magnification in B and E, respectively; Scale bar = 100 μm. C and F: serial pancreatic tissue sections were stained with antibodies against insulin (beta cells in islets; red) and α-SMA (activated stellate cells; green); nuclei were stained with Dapi (blue). C and F illustrate boxed regions in B and E, respectively; scale bar = 20 μm. G: bar graph shows the percentage of collagen-stained area (Masson's trichrome staining) in pancreas sections from normal and T2DM organ donors. Seven randomly selected high-power fields were quantified and averaged to obtain the value for each specimen; n = 3 organ donors for each group. Data in graph are means ± SE; *P < 0.05 compared with normal.
Since PaSC are the main cell type responsible for collagen production in the fibrotic pancreas, we examined whether those peri-islet areas displaying extensive collagen deposition were enriched in activated PaSC. Indeed, we observed that, while α-SMA immunofluorescence was mainly restricted to perivascular regions in normal donors, islets from T2DM donors were fibrotic and displayed abundant α-SMA both within the islets and in surrounding periacinar areas (Fig. 1, C and F). These data suggest that T2DM leads to activation of PaSC and fibrogenesis in both the endocrine and exocrine pancreas.
Long-term feeding with a high-fat, high-calorie diet leads to PaSC activation and increased collagen deposition in mouse pancreas.
Obesity is often linked to T2DM, insulin resistance, and hyperglycemia. Feeding mice a diet high in fat and calories recapitulates many features of human obesity-induced T2DM. Compared with a standard rodent diet (∼12% total energy provided by fat), C57BL/6J mice fed a high-fat diet (>35% of energy by fat) develop sustained hyperglycemia, glucose intolerance, and progressive hyperinsulinemia that persist even after 12 mo on high-fat diet (49). We recently showed that a high-fat, high-calorie diet (HFCD; 40% of total calories derived from corn oil) promotes tumor progression in a mouse model of PDAC driven by a pancreas-specific oncogenic Kras mutation (15), an effect that can be attributed at least in part to elevated IGF-1 mediated signaling (21). Compared with mice fed the control diet, Kras mice fed HFCD for 3 mo developed hyperglycemia and hyperinsulinemia, and had elevated levels of circulating IGF-1. Moreover, these mice exhibited increased numbers of activated PaSC and pancreatic fibrosis. Here, we performed a longitudinal study to determine whether similar association between dysregulated glucose metabolism and pancreatic fibrosis could be observed in wild-type mice. C57BL/6J male mice were randomly allocated to the HFCD (40% of total calories derived from corn oil) or the control diet (CD; 12% total calories from corn oil), and animals were euthanized at 3, 6, 9, and 12 mo.
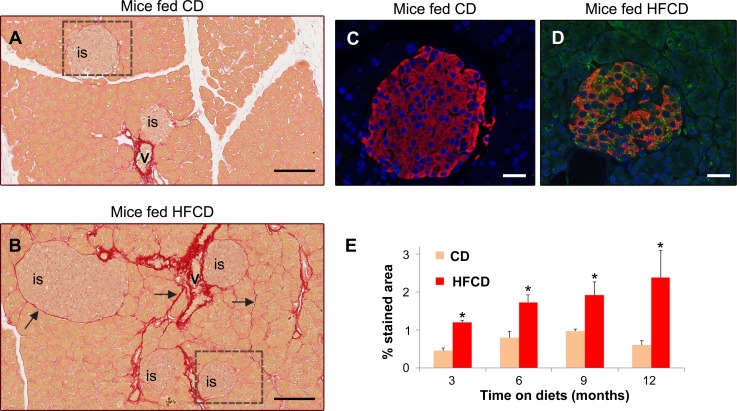
Mice fed HFCD gained significantly more weight than mice fed CD. For example, at 9 mo on diets, mice fed HFCD gained an average of 35 g (SE = 4.5; n = 5) while mice fed CD gained 20.2 g (SE = 3.0; n = 5). As expected, mice on HFCD progressively developed characteristics of “fatty liver” including macro- and microvesicular steatosis, hepatocyte ballooning, and mild fibrosis (not shown). Exocrine pancreas appeared normal under histological examination in mice fed both HFCD and CD, with no signs of acinar cell damage, changes in ductal morphology, or inflammation (not shown). However, we observed islet cell hyperplasia and mild pancreatic fibrosis in mice fed HFCD for 12 mo (Fig. 2). Compared with mice fed CD, which had collagen around vascular elements but not islets, pancreas from mice fed HFCD displayed abundant collagen deposition around islets and adjacent acini (Fig. 2, A and B). Consistent with these data, we identified numerous activated PaSC within islets of HFCD-fed mice (Fig. 2, C and D). The collagen deposition associated with the presence of activated PaSC in the vicinity of islets was progressive, as shown by the time course data (Fig. 2E). In conclusion, we found that both T2DM in humans and high fat-induced obesity in mice, conditions associated with insulin resistance and dysregulated glucose metabolism, are associated with PaSC activation and fibrosis.
Fig. 2.
High-fat diets promote collagen deposition in areas surrounding islets in mouse pancreas. A and B: light-field photomicrographs of Sirius red-stained pancreatic tissue sections of mice fed control chow (CD) or high-fat, high-calorie diet (HFCD) for 12 mo. Collagens are visible as bright red stain. Arrows in B indicate collagen deposition in peri-islet and peri-acinar areas; “is”, islet; “v” blood vessel. The boxed regions are shown at a higher magnification in C and D. Scale bar = 100 μm. C and D: serial pancreatic tissue sections were stained with antibodies against insulin (beta cells in islets; red staining) and α-SMA (a marker of activated stellate cells; green staining); nuclei were stained with Dapi (blue staining). C and D illustrate boxed regions in A and B, respectively; scale bar = 25 μm. E: bar graph shows the percentage of collagen-stained area (Sirius red staining) relative to the total field in pancreas sections from mice fed CD or HFCD for 3, 6, 9, and 12 mo. Seven to ten randomly selected high-power fields were quantified and averaged to obtain the value for each animal; n = 3–4 for each experimental group. Data in graph are means ± SE; *P < 0.05 compared with CD fed mice.
PaSC coexpress insulin (IR) and insulin-like growth factor-1 (IGF-1R) receptors.
As indicated above, we found activated PaSC within islets and in peri-islet areas in pancreas from T2DM patients and mice fed HFCD. Given that endocrine and exocrine cells in the pancreas share a common blood supply, we hypothesized that insulin locally secreted by β cells acts directly on IR receptors in neighboring PaSC, promoting their activation and/or growth. Therefore, we next examined whether PaSC expressed IR and IGF-1R, and whether high concentrations of insulin modulate IR/IGF-1R expression.
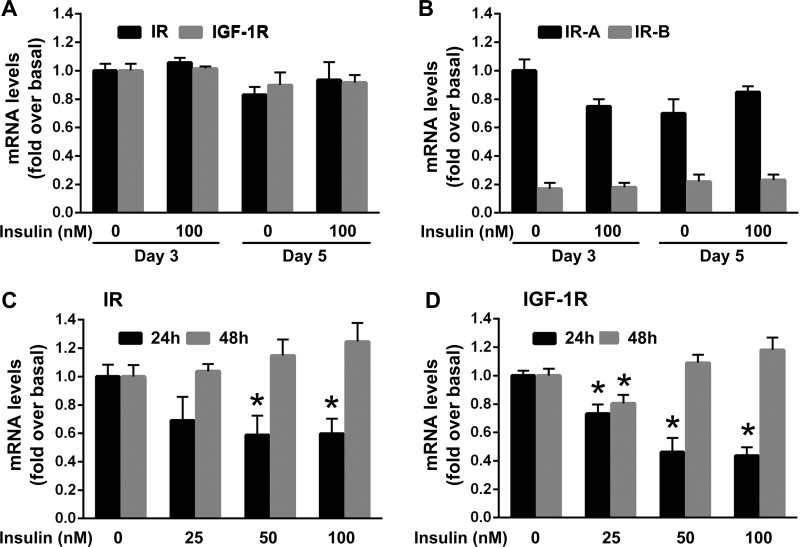
Freshly isolated, quiescent stellate cells obtained from mouse pancreas (mPaSC) were cultured for 48 h (day 3) or 96 h (day 5) in 10% FBS media in the presence or absence of high concentrations of insulin (100 nM). As widely reported (17), after 48 h in culture about 40–60% of cells remained quiescent, while at day 5 all cells were fully activated, as indicated by positive immunoreactivity to the myofibroblast marker α-SMA (not shown). Quiescent and culture-activated primary mPaSC expressed similar mRNA levels of both IR and IGF-1R (Fig. 3A). IR is encoded by a single gene that generates two isoforms (IR-A and IR-B), that differ by the absence or presence of a 36-NT alternate exon 11, by alternate mRNA splicing. While high IR-A/IR-B ratios have been associated with higher proliferative capacity and enhanced glucose uptake in response to insulin/IGF-1, IR-B has a major regulatory role in glucose homeostasis (18, 45). By RNA analysis techniques that could discriminate between the A and B isoforms (see materials and methods), we found that primary mPaSC expressed predominantly the IR-A rather than the IR-B isoform (Fig. 3B), suggesting a mitogenic role for insulin/IGF-1 in these cells.
Fig. 3.
Quiescent and activated mouse PaSC express insulin (IR) and IGF (IGF-1R) receptors. A: freshly isolated, quiescent mouse PaSC (mPaSC) were cultured for 48 h (day 3) or 96 h (day 5) in DMEM/F12 medium (17.5 mM glucose) containing 10% FBS without or with 100 nM insulin. IR and IGF-1R mRNA expression were determined by qPCR. B: quiescent and culture-activated mPaSC (untreated or insulin-treated) preferentially expressed the IR-A isoform compared with the IR-B isoform. Quiescent mPaSC were treated as indicated in A. mRNA expression of the IR isoforms IR-A and IR-B was measured by qPCR. C and D: insulin modulates IR and IGF-1R expression in activated mPaSC. Activated mPaSC were cultured in DMEM/F12 medium with 10% FBS for 48 h. After 4 h serum starvation, cells were treated with insulin (0–100 nM) for up to 48 h in 1% FBS DMEM/F12 medium. Expression levels of IR and IGF-1R were analyzed by qPCR. Data in graphs are means ± SE; data are representative of 3 independent experiments; *P < 0.05 vs. 0 nM insulin.
Short-term treatment with insulin (25–100 nM) dose-dependently and transiently induced downregulation of both IR and IGF-1R expression (Fig. 3, C and D). This effect was likely related with feedback mechanisms that regulate insulin signaling (6, 33). However, after 48 h of insulin stimulation, IR and IGF-1R expression levels were almost entirely restored to their control levels.
Insulin does not induce activation of quiescent PaSC but promotes ECM production in activated primary PaSC.
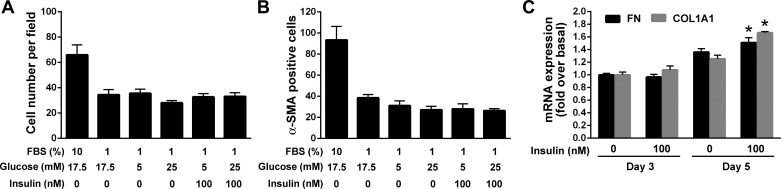
We next examined whether the high concentrations of insulin that may be achieved locally in the pancreas during obesity and T2DM accelerate differentiation of quiescent PaSC into the myofibroblast phenotype. We also tested the separate and/or additive effects of different glucose concentrations on PaSC activation and growth. Freshly isolated, quiescent mPaSC were cultured for 96 h in 1% FBS media containing 5 mM glucose (physiological circulating glucose levels), 17.5 mM glucose, or 25 mM glucose (high glucose concentration), and stimulated with 100 nM insulin. Cells cultured in 10% FBS medium (17.5 mM glucose) were used as positive control for cell activation. Compared with cells cultured in 1% FBS media (either 5 mM or 25 mM glucose), mPaSC cultured in 10% FBS media became activated within 5 days, as indicated by a higher proliferative rate and percentage of cells expressing α-SMA (Fig. 4, A and B). Importantly, insulin treatment did not promote activation of quiescent mPaSC cells, irrespective of the glucose concentration in the culture medium (Fig. 4, A and B). However, insulin stimulation did induce increased collagen and fibronectin expression levels in fully activated primary mPaSC (Fig. 4C). These data suggest that high doses of insulin, either alone or in combination with high glucose levels, are insufficient for significantly accelerating activation of quiescent PaSC in vivo, and other inflammatory/proliferative signals are required. In contrast, insulin stimulation provided to already activated, rather than naïve PaSC, is effective in promoting fibrosing responses.
Fig. 4.
Insulin does not induce activation of quiescent, freshly isolated mouse PaSC but promotes fibrosing responses. Freshly isolated mPaSC were cultured for 5 days in 1% FBS DMEM/F12 media containing 5 mM or 25 mM glucose either alone or with 100 nM insulin. Cells cultured in 10% FBS DMEM/F12 medium (17.5 mM glucose) were used as positive control for cell activation. At day 5, cells were formaldehyde-fixed and stained for α-SMA (a marker of cell activation) and Hoechst 33342 to visualize nuclei. Nuclei (A) and α-SMA positive cells (B) were counted in at least 10 randomly selected fields under low-power magnification (10X). The percentage of α-SMA positive cells was determined by the ratio of α-SMA and Hoechst-positive cells. Results are presented as means ± SE; 2 independent experiments. C: freshly isolated mPaSC were cultured for 48 h (day 3) or 96 h (day 5; fully activated) in 10% serum media with/without 100 nM insulin. Expression levels of collagen (alpha-1 type I; COL1A1) and fibronectin (FN1) mRNA were measured by qPCR. 18S rRNA was used as an internal control. Data in graph represent means ± SE from 3 independent experiments; *P < 0.05 vs. 0 nM insulin.
Insulin induces tyrosine phosphorylation of IR/IGF-1R and activation of Akt/mTOR signaling in activated PaSC.
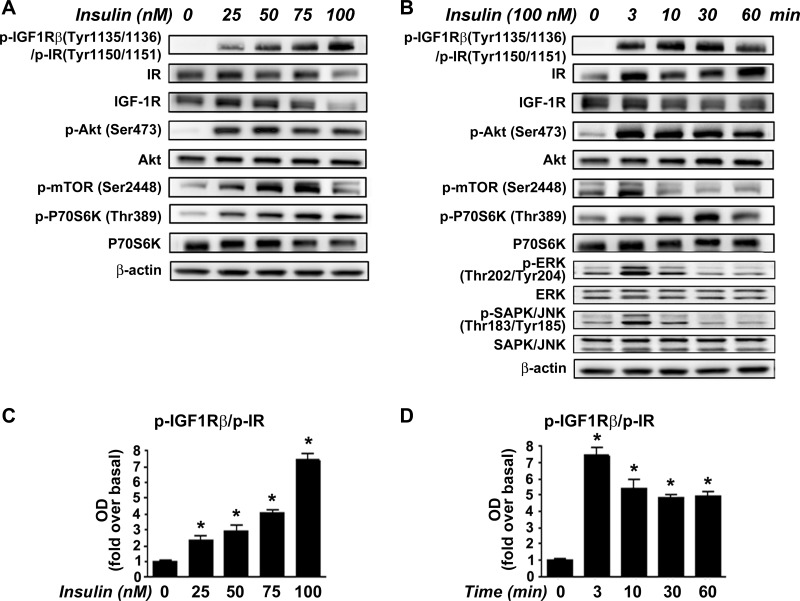
To investigate IR/IGF-1R activation and downstream signaling, we stimulated activated PaSC cultured in 5 mM (physiological concentration) glucose media with 25–100 nM insulin. For these studies, we used mostly immortalized mouse PaSC (imPaSC), and key results were confirmed in primary mPaSC as well as in primary human PaSC (hPaSC) isolated from pancreatic tissues from organ donors. imPaSC display a phenotype similar to primary mPaSC (27, 37). In imPaSC, insulin induced dose-dependent tyrosine phosphorylation of IR/IGF-1R at specific autophosphorylation sites (Fig. 5, A and B), with 100 nM insulin increasing receptor phosphorylation 7.4-fold (Fig. 5, C and D). Insulin effects were observed as early as 3 min after stimulation and persisted for at least 60 min (Fig. 5, B and D). As expected, insulin-induced IR/IGF-1R phosphorylation led to activation of downstream signaling cascades including MAPK/ERK and Akt/mTOR pathways (Fig. 5, A and B). Insulin-mediated activation of MAPK signaling plays important roles in proliferation and cell growth in many cell types. We found a rapid but transiently increased phosphorylation of ERK1/2 and SAPK/JNK in insulin-treated cells. Compared with these effects, insulin activation of Akt/mTOR signaling was robust and sustained. Thus insulin induced Akt phosphorylation at Ser473, a site that promotes Akt activation, at concentrations between 25 and 100 nM (Fig. 5A). As shown in Fig. 5B, stimulation for different times with 100 nM insulin triggered maximal Akt activation at 3 min (6-fold increase over basal; P < 0.05). Akt has been shown to regulate mTOR phosphorylation at Ser2448, a site of importance in the regulation of mTOR function. Accordingly, insulin stimulation rapidly induced phosphorylation of mTOR at Ser2448 and p70 S6 kinase (p70S6K) at Thr389 (Fig. 5, A and B). p70S6K is a mTORC1 downstream kinase required for cell growth, proliferation, and protein translation. Similar results were obtained in primary mPaSC and hPaSC (not shown).
Fig. 5.
Insulin induces rapid tyrosine autophosphorylation of IR/IGF-1R at specific kinase domain activation loop sites, and activation of Akt/mTOR/p70S6K signaling in activated PaSC. imPaSCs were cultured for two days in DMEM containing 5 mM glucose and 10% FBS, starved in serum-free medium for 4 h, and then treated with various concentrations (0–100 nM) of insulin for 30 min (A) or with 100 nM insulin for the times shown (0–60 min) (B). Levels of phosphorylated or total IR, IGF-1R/IR, Akt/mTOR/p70S6K, ERK1/2, and SAPK/JNK were measured in cell lysates by Western blotting. β-Actin was used as loading control. Similar results were obtained using primary mPaSC (not shown). C and D: graphs show protein levels of phosphorylated IGF-1R/IR determined by densitometry. Bars represent means ± SE from 3 independent experiments; *P < 0.05 compared with basal control.
Insulin-induced activation of Akt and MAPK/ERK signaling is enhanced in PaSC cultured in high glucose media.
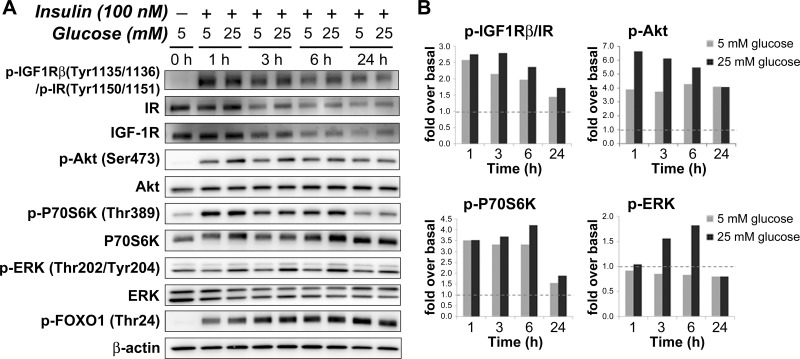
We next examined the effects of combined high insulin and high glucose concentrations on insulin-induced IR/IGF-1R signaling in imPaSC. Cells were maintained in 10% FBS media (5 mM glucose), serum starved for 4 h, and then stimulated with insulin in serum-free media containing 5 mM or 25 mM glucose. Cell lysates were prepared after 1, 3, 6, and 24 h insulin stimulation. As indicated by specific tyrosine phosphorylation, insulin induced IR/IGF-1R activation in cells cultured in both 5 mM and 25 mM glucose media, an effect that persisted for at least 24 h (Fig. 6, A and B). Compared with 5 mM glucose, levels of phosphorylated IR/IGF-1R were slightly higher at various time points in cells treated with 25 mM glucose (Fig. 6, A and B). Consistent with this enhanced receptor activation, significantly higher Akt and ERK1/2 activation levels were induced by insulin stimulation of cells cultured in 25 mM glucose than in 5 mM glucose media. For example, levels of Akt phosphorylation at Ser473 at 3 h increased ∼6-fold over basal in cells treated with high glucose, but only ∼4-fold in cells treated with low glucose concentrations. Similarly, levels of ERK1/2 phosphorylation at Thr202/Tyr204 increased up to ∼1.8-fold over basal in PaSC treated with 25 mM glucose, but remained at basal levels in cells treated with 5 mM glucose (Fig. 6, A and B).
Fig. 6.
Insulin-induced activation of Akt/mTOR/p70S6K and ERK signaling pathways are more pronounced in PaSC cultured in 25 mM glucose compared with 5 mM glucose. imPaSCs were cultured in DMEM media containing 5 mM glucose and 10% FBS for 2 days, starved in serum-free medium for 4 h, and then cultured in 5 mM glucose- or 25 mM glucose-containing media with or without 100 nM insulin for various periods of time (1–24 h). A: cell lysates were analyzed for the indicated targets, and representative immunoblots are shown in A. β-Actin was used as loading control. B: graphs show protein levels of phosphorylated IGF-1R/IR, p70S6K, Akt, and ERK determined by densitometry. As indicated in the graphs, insulin-induced Akt and ERK phosphorylation was more pronounced in cells cultured in media containing 25 mM glucose compared with media containing 5 mM glucose. Data in graphs represent means ± SE from 3 independent experiments.
Since insulin on the whole induced higher activation of Akt than the ERK1/2 pathway, we also looked at the activation state of the forkhead box gene, group O1 (FoxO1), a direct downstream Akt target. FoxO1 is a transcription factor involved in a variety of cellular functions including glucose metabolism, cell proliferation, and cell death (12). In hepatic stellate cells, FoxO1 inhibits differentiation into the activated phenotype and cell proliferation, an effect blocked by Akt-induced phosphorylation at Thr24 and inactivation of FoxO1 (1). In our study, we found marked and persistent insulin-induced phosphorylation of FoxO1 in activated PaSC (Fig. 6A), an effect consistent with increased insulin-induced Akt activation. Also consistent with high glucose-mediated enhancement of Akt Ser473 phosphorylation, there was a modest increase in Akt-mediated FoxO1 phosphorylation by 25 mM glucose that was particularly noticeable at the earliest time points (Fig. 6A). These data further indicate that high concentrations of glucose and insulin, conditions that mimic hyperglycemia and hyperinsulinemia associated with obesity and T2DM progression, induce marked activation of ERK1/2 and Akt/mTOR signaling in cultured activated PaSC.
Effects of high glucose and high insulin on ECM production and PaSC proliferation.
The Akt/mTOR signal transduction pathway modulates cellular growth and protein synthesis by integrating growth factor and nutrient signals and cellular energy status. The mTOR complex 1 (mTORC1) regulates protein translation, e.g., via phosphorylation of the 40S ribosomal protein subunit S6 downstream of p70 S6K (28). Emerging evidence also supports a critical role for mTORC1 in fibroblast reprogramming and plasticity (50). In this context, we reported that activation of quiescent PaSC is linked to upregulation of p70S6K, and that mTORC1 inhibition by rapamycin blocks ECM production in activated PaSC (36). On the other hand, the mTOR complex 2 (mTORC2) regulates cell proliferation by phosphorylating the kinase domains of several protein kinases of the AGC family including Akt at Ser473 (28).
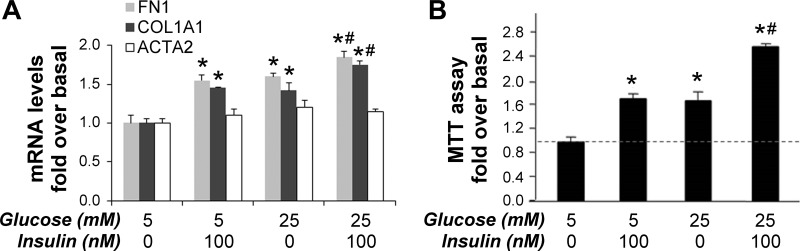
To better understand the biological effects of persistent activation of Akt/mTOR signaling by high concentrations of glucose and insulin, we next examined the influence of these stimuli on regulation of fibrosing responses and PaSC proliferation. imPaSC were cultured for 48 h in media containing 5 mM glucose, serum starved for 4 h, and then stimulated with 100 nM insulin in fresh media containing 5 mM or 25 mM glucose. After 24 h incubation, insulin, 25 mM glucose, and in particular the combination of both increased mRNA levels of the ECM proteins fibronectin and alpha-1 type I collagen (COL1A1), the main component of fibrillar type I collagen in imPaSC (Fig. 7A). In contrast, the combined treatment of insulin and 25 mM glucose did not affect expression levels of α-SMA (Fig. 7A). These data point to a selective promotion of the ECM-secreting activity of PaSC.
Fig. 7.
Insulin stimulates fibrosing responses and cell proliferation in mouse immortalized PaSCs. imPaSC were cultured in 10% serum DMEM media containing 5 mM glucose, starved in serum-free medium for 4 h, and then stimulated for the indicated times with 100 nM insulin in 5 mM or 25 mM glucose-containing media (medium was changed every day with new additions of insulin). A: after 24 h incubation, expression levels of fibronectin (FN1) and α-SMA (ACTA2) were assessed by qPCR. 18S rRNA was used as an internal control. Data in graph represent means ± SE from 3 independent experiments; *P < 0.05 vs. 5 mM glucose without insulin; #P < 0.05 vs. 25 mM glucose without insulin. B: cell proliferation after 72 h stimulation with 100 nM insulin and/or 25 mM glucose was determined by MTT assay. Data in graphs represent means ± SE; n = 3 independent experiments. *P < 0.05 vs. 5 mM glucose without insulin; #P < 0.05 vs. 25 mM glucose without insulin.
Treatment with 100 nM insulin or 25 mM glucose alone induced a similar 1.8-fold increase in cell proliferation compared with that in basal 5 mM glucose, while the combined treatment markedly enhanced imPaSC proliferation, by 2.7-fold (Fig. 7B). Insulin was also effective in increasing cell proliferation in primary mouse and human activated PaSC (not shown), but these cells appeared less sensitive to changes in glucose concentration in the media. The observed differences in sensitivity to glucose between primary and immortalized PaSC could be related to lower proliferation and metabolic rates in primary PaSC (not shown).
The role of mTOR in insulin-induced enhancement of proliferation and fibrosing responses in PaSC.
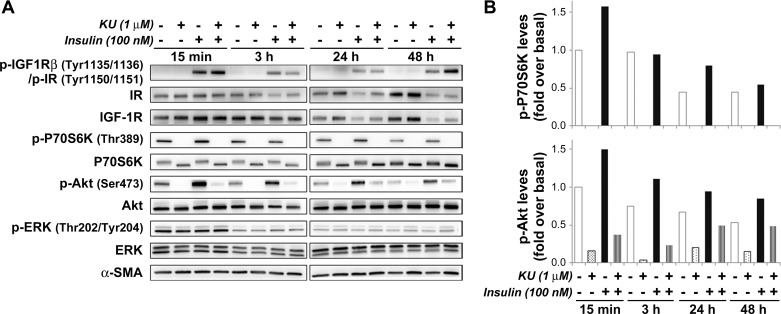
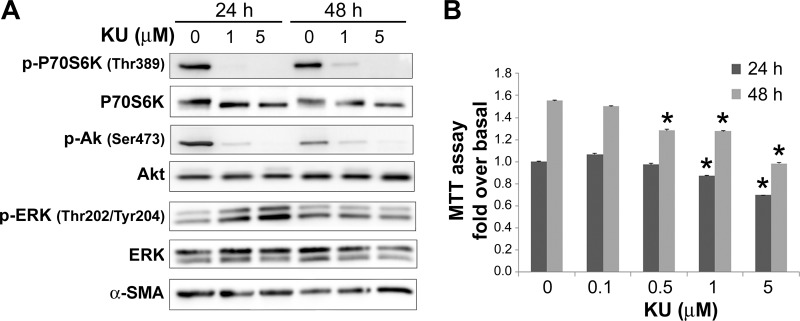
Inhibition of mTORC1- and mTORC2-mediated phosphorylation of substrates has been linked to reduced cell growth and inhibition of protein translation in several cell types. To elucidate the participation of Akt/mTOR signaling pathways on insulin-induced cell proliferation and upregulation of ECM proteins in PaSC, we used the small molecule KU63794 (KU) as a selective dual inhibitor of both mTORC1 and mTORC2 (19, 46). As illustrated in Fig. 8, KU at 1 μM completely inhibited basal and insulin-induced phosphorylation of the mTORC1 substrate p70S6K at Thr389 in imPaSC, and this effect persisted for at least 48 h. As expected, KU significantly diminished insulin-induced phosphorylation of Akt at Ser473, a site regulated by mTORC2, but did not alter ERK1/2 activation (Fig. 8).
Fig. 8.
KU63794 (KU) inhibits insulin-induced Akt/mTOR signaling in PaSCs. imPaSC were cultured in 10% serum DMEM containing 5 mM glucose for 2 days, starved in serum-free medium for 4 h, and then treated for the indicated times in 1% FBS DMEM with or without 100 nM insulin and in the presence or absence of the mTOR inhibitor KU at 1 μM. A: cell lysates were analyzed for the indicated targets, and representative immunoblots are shown. α-SMA was used as loading control. B: the graph shows levels of phosphorylated p706SK Thr389 and Akt Ser473 determined by densitometry. As indicated in the graphs, insulin-induced Akt phosphorylation and, especially, p70S6K phosphorylation were effectively reduced by preincubation with KU. Data in the graphs are representative of 2 independent experiments.
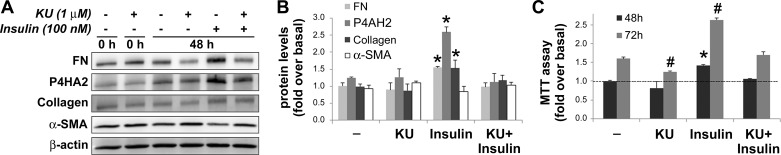
Consistent with its inhibitory effects on Akt/mTOR, KU was effective in blocking insulin-induced upregulation of ECM proteins in imPaSC. As illustrated in Fig. 9, A and B, the increases in protein levels of fibronectin, collagen type I, and the alpha subunit 2 of prolyl 4-hydroxylase (P4HA2; a key endoplasmic reticulum enzyme required for proper collagen folding) elicited by insulin were prevented by preincubation with KU. In contrast, protein levels of the activation marker α-SMA were neither enhanced with insulin nor reduced by KU (Fig. 9, A and B). In addition, KU effectively inhibited insulin-induced imPaSC proliferation (Fig. 9C), and similar effects were observed in primary activated mPaSC (Fig. 10).
Fig. 9.
mTOR inhibition blocks insulin-induced fibrosing responses and cell proliferation in activated PaSC. imPaSC were cultured in 10% serum DMEM containing 5 mM glucose for 2 days, serum starved, preincubated for 30 min with or without KU and then stimulated with or without insulin for the times shown. A: cellular protein lysates were analyzed by Western blot for fibrotic proteins fibronectin (FN) or collagen or the catalytic subunit of prolyl hydroxylase (P4HA2), an important enzyme in collagen biosynthesis. α-SMA was tested as a marker of activation. β-Actin was also analyzed as a loading control. B: multiple experiments of the type shown in A at the 48-h time point were quantitated by densitometry. C: cell proliferation in cells grown as in A was measured by MTT assay. Data in graphs (B and C) represent the means ± SE; n = 3. *P < 0.05 relative to no-insulin control, 48 h. #P < 0.05 relative to no-insulin control, 72 h.
Fig. 10.
The mTOR inhibitor KU63794 (KU) dose-dependently inhibits Akt/mTOR signaling and proliferation in activated PaSC. Culture-activated mPaSCs were cultured for 2 days in 10% FBS DMEM, starved in serum-free medium for 4 h, and then treated in 5% FBS DMEM for 24 or 48 h with KU at the indicated concentrations. KU at 1 and 5 μM effectively blocked Akt/mTOR signaling but not ERK activation (A) and attenuated the cell proliferation response (B) in mPaSC. Data in the graph represent means ± SE from n = 3 independent experiments. *P < 0.05 vs. 0 μM KU.
DISCUSSION
Obesity and diabetes mellitus as well as chronic pancreatitis are established risk factors for PDAC. Type 2 diabetes (T2DM) can increase the risk for PDAC through several potential mechanisms (9), some involving interactions between cell types in the endocrine and the exocrine parenchyma. T2DM is characterized by insulin resistance and concomitant hyperglycemia, as well as periods of hyperinsulinemia due to abnormalities in insulin production and/or action. Insulin is a well-known cellular growth factor and is secreted into islet capillaries at a relatively high concentration. Previous studies have focused on the impact of elevated local and circulating levels of insulin and glucose on pancreatic islet cells and pancreatic acinar cells (8, 24). The effects of high concentrations of insulin and glucose on other cell types in the exocrine parenchyma including pancreatic stellate cells (PaSC) have received less attention. PaSC are key stromal players regulating pancreatic fibrogenesis and inflammation in chronic pancreatitis and PDAC (3). A number of growth factors have been identified as potent inducers of PaSC activation into a highly proliferative myofibroblast phenotype (3); however, few studies have addressed the effects of insulin and glucose levels on the activation state of PaSC and the interaction between islet cells and PaSC.
We hypothesized that high insulin concentrations in the pancreas due to insulin hypersecretion by beta-cells may predispose towards PaSC proliferation and growth, particularly in the hyperglycemic environment associated with T2DM. In the present study, we investigated whether high glucose and insulin/IGF-1 modulated PaSC activation and fibrogenic responses, and examined the operation of insulin/IGF-1 receptor-mediated signaling in these cells.
We found that pancreatic islets and surrounding exocrine parenchyma contain activated PaSC in T2DM patients and in mice fed a high-fat, high-calorie diet for an extended period of time. As expected, PaSC activation in these tissues was associated with local fibrosis as indicated by enhanced collagen deposition in the vicinity of activated PaSC. These data are in agreement with previous studies indicating mild fibrosis and exocrine pancreatopathy in patients with diabetes mellitus (30), as well as with reports describing peri-islet fibrosis in animal models of T2DM (8, 24–26). Taken together, our data provide new evidence indicating that obesity and T2DM create an environment conducive to PaSC activation and fibrogenesis.
This study provides also direct evidence on the functional expression of the IR and IGF-1R and insulin signaling pathway elements in PaSC, and the fluctuating expression levels of these receptors in response to insulin in the absence or presence of elevated glucose. We found that both quiescent and activated PaSC express mainly the type-A IR, a receptor that regulates mitogenesis in response to insulin, and low levels of the type-B receptor that modulates glucose metabolism (18, 44). These cells also express the IGF-1R, suggesting that PaSC may form hybrid receptors and exhibit robust responses to both insulin and IGF in the stroma of chronic pancreatitis and PDAC. These findings have important implications in current PDAC therapy approaches that utilize specific inhibitors of IR/IGF-1R to inhibit cancer cell growth and halt tumor progression. Since PaSC may display pro- or anti-tumor responses, future studies should consider the effects of IR/IGF-1R inhibitors on PaSC and other myofibroblasts in the PDAC stroma.
Insulin signaling is mediated by a complex, highly integrated network that controls many cellular processes. IR/IGF-1R phosphorylation triggers kinase cascades leading to subsequent activation of two major downstream signaling pathways: ERK1/2 and PI3K/Akt/mTOR. ERK1/2 pathway activation is mainly associated with proliferation and differentiation in different cell types, whereas PI3K/Akt/mTOR activation promotes cellular growth as well as protein synthesis. We demonstrated that insulin stimulation of activated PaSC rapidly initiated tyrosine phosphorylation of their cognate receptors IR/IGF-1R at specific autophosphorylation sites, as well as downstream events including ERK1/2 and Akt/mTOR and p70S6K activation. Further, insulin-induced stimulation of signal transduction events was enhanced in the presence of elevated glucose. Akt/mTOR was more acutely regulated by these stimuli than was ERK1/2, so we chose to examine this pathway more extensively in PaSC.
We previously reported that the PI3K inhibitor LY294002 and the mTORC1 inhibitor rapamycin block ECM production in activated PaSC (36). Here we showed that insulin and glucose enhanced cellular proliferation and induced fibrosing responses in immortalized mouse and primary mouse and human PaSC, and these effects were additive in immortalized PaSC. Moreover, these effects were greatly diminished by a selective inhibitor of mTORC1 and mTORC2, supporting a key role for mTOR in regulating the activated, pro-fibrosing phenotype in PaSC.
In our hands, insulin and glucose were effective in stimulating effects on cell proliferation and ECM production in activated PaSC. For example, insulin increased fibronectin-1 and collagen-1A1 expression in culture-activated primary PaSC. However, these stimuli were insufficient to accelerate the activation of quiescent, freshly isolated primary mouse PaSC in our studies. In particular, at low levels (medium with 1% rather than 10%) of serum, insulin or high glucose were unable to promote increased cell proliferation or α-SMA expression. These results differ from a previous report concluding that high glucose concentrations increase protein levels of α-SMA in cultured rat PaSC (32). The high concentrations of glucose used in that study (33.6 mM) and the species differences (rat PaSC vs. mouse PaSC) may account for the discrepant results obtained in our work and that of Nomiyama et al. (32).
Taken together, our data demonstrate that high insulin and high glucose both individually and additively promote the pro-fibrosing myofibroblast phenotype of activated PaSC. These results concurred with the findings of other investigators who reported that hyperglycemia and hyperinsulinemia had additive effects on PaSC proliferation (23) and are important factors promoting a fibrotic response in activated PaSC (52). Our findings suggest that hyperinsulinemia and hyperglycemia by themselves are insufficient to activate PaSC, and other inflammatory signals are required. This would explain why we and others observed only mild fibrosis limited to peri-islet areas and not widespread fibrosis in pancreas tissues from T2DM patients with no history of pancreatitis. Nonetheless, our data support our view that obesity and diabetes, and concomitant hyperglycemia and hyperinsulinemia, primarily act on activated PaSC to increase proliferation and ECM production, which may subsequently contribute to pancreatic fibrogenesis, desmoplasia, and promotion of PDAC development. Additional studies are necessary to explore the gene regulatory networks by which PaSC respond synergistically to high glucose and insulin, and identify agents to interrupt the intra- and intercellular signaling pathways whereby PaSC mediate fibrosis and increased cancer risk.
GRANTS
This research was supported by the National Institutes of Health (NIH; P01 CA163200 to G. Eibl and S. J. Pandol; R01 AA019954 to A. Lugea), the Chinese Natural Science Foundation (81270010 and 81570739 to L. Li), and the China Scholarship Council (to J. Yang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.Y., R.T.W., H.-Y.S., A.L., L.L., and S.J.P. conception and design of research; J.Y., R.T.W., H.-Y.S., A.M., H.-H.C., K.F., A.L., and L.L. performed experiments; J.Y., R.T.W., H.-Y.S., A.L., and L.L. analyzed data; J.Y., R.T.W., H.-Y.S., A.L., and L.L. interpreted results of experiments; J.Y. and A.L. prepared figures; J.Y., R.T.W., H.-Y.S., A.L., L.L., and S.J.P. edited and revised manuscript; J.Y., R.T.W., H.-Y.S., A.M., H.-H.C., G.E., K.F., F.R.K., A.L., L.L., and S.J.P. approved final version of manuscript; R.T.W., A.L., and L.L. drafted manuscript.
REFERENCES
- 1.Adachi M, Osawa Y, Uchinami H, Kitamura T, Accili D, Brenner DA. The forkhead transcription factor FoxO1 regulates proliferation and transdifferentiation of hepatic stellate cells. Gastroenterology 132: 1434–1446, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Andersen DK, Andren-Sandberg A, Duell EJ, Goggins M, Korc M, Petersen GM, Smith JP, Whitcomb DC. Pancreatitis-diabetes-pancreatic cancer: summary of an NIDDK-NCI workshop. Pancreas 42: 1227–1237, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 144: 1210–1219, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashcroft FM, Rorsman P. Diabetes mellitus and the beta cell: the last ten years. Cell 148: 1160–1171, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, Zhang H, Li Z. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer 47: 1928–1937, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 6: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog 51: 53–63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell-Thompson M, Rodriguez-Calvo T, Battaglia M. Abnormalities of the exocrine pancreas in type 1 diabetes. Curr Diab Rep 15: 79, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannata D, Fierz Y, Vijayakumar A, LeRoith D. Type 2 diabetes and cancer: what is the connection? Mt Sinai J Med 77: 197–213, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Chiarini F, Evangelisti C, McCubrey JA, Martelli AM. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci 36: 124–135, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Cohen DH, LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr Relat Cancer 19: F27–F45, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Coomans de Brachene A, Demoulin JB. FOXO transcription factors in cancer development and therapy. Cell Mol Life Sci 73: 1159–1172, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox ME, Gleave ME, Zakikhani M, Bell RH, Piura E, Vickers E, Cunningham M, Larsson O, Fazli L, Pollak M. Insulin receptor expression by human prostate cancers. Prostate 69: 33–40, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y, Andersen DK. Diabetes and pancreatic cancer. Endocr Relat Cancer 19: F9–F26, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Dawson DW, Hertzer K, Moro A, Donald G, Chang HH, Go VL, Pandol SJ, Lugea A, Gukovskaya AS, Li G, Hines OJ, Rozengurt E, Eibl G. High-fat, high-calorie diet promotes early pancreatic neoplasia in the conditional KrasG12D mouse model. Cancer Prev Res (Phila) 6: 1064–1073, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denduluri SK, Idowu O, Wang Z, Liao Z, Yan Z, Mohammed MK, Ye J, Wei Q, Wang J, Zhao L, Luu HH. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis 2: 13–25, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch HJ, Hwang RF, Jaster R, Kleeff J, Kloppel G, Kordes C, Logsdon CD, Masamune A, Michalski CW, Oh J, Phillips PA, Pinzani M, Reiser-Erkan C, Tsukamoto H, Wilson J. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut 61: 172–178, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escribano O, Gomez-Hernandez A, Diaz-Castroverde S, Nevado C, Garcia G, Otero YF, Perdomo L, Beneit N, Benito M. Insulin receptor isoform A confers a higher proliferative capability to pancreatic beta cells enabling glucose availability and IGF-I signaling. Mol Cell Endocrinol 409: 82–91, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, Alessi DR. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). Biochem J 421: 29–42, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong J, Robbins LA, Lugea A, Waldron RT, Jeon CY, Pandol SJ. Diabetes, pancreatic cancer, and metformin therapy. Front Physiol 5: 426, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey AE, Lashinger LM, Hays D, Harrison LM, Lewis K, Fischer SM, Hursting SD. Calorie restriction decreases murine and human pancreatic tumor cell growth, nuclear factor-kappaB activation, and inflammation-related gene expression in an insulin-like growth factor-1-dependent manner. PLos One 9: e94151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasnain SZ, Borg DJ, Harcourt BE, Tong H, Sheng YH, Ng CP, Das I, Wang R, Chen AC, Loudovaris T, Kay TW, Thomas HE, Whitehead JP, Forbes JM, Prins JB, McGuckin MA. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med 20: 1417–1426, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Hong OK, Lee SH, Rhee M, Ko SH, Cho JH, Choi YH, Song KH, Son HY, Yoon KH. Hyperglycemia and hyperinsulinemia have additive effects on activation and proliferation of pancreatic stellate cells: possible explanation of islet-specific fibrosis in type 2 diabetes mellitus. J Cell Biochem 101: 665–675, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Katsuda Y, Ohta T, Miyajima K, Kemmochi Y, Sasase T, Tong B, Shinohara M, Yamada T. Diabetic complications in obese type 2 diabetic rat models. Exp Anim 63: 121–132, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JW, Ko SH, Cho JH, Sun C, Hong OK, Lee SH, Kim JH, Lee KW, Kwon HS, Lee JM, Song KH, Son HY, Yoon KH. Loss of beta-cells with fibrotic islet destruction in type 2 diabetes mellitus. Front Biosci 13: 6022–6033, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Li FF, Chen BJ, Li W, Li L, Zha M, Zhou S, Bachem MG, Sun ZL. Islet stellate cells isolated from fibrotic islet of Goto-Kakizaki rats affect biological behavior of beta-cell. J Diabetes Res 2016: 6924593, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lugea A, Waldron RT, Pandol SJ. Pancreatic adaptive responses in alcohol abuse: role of the unfolded protein response. Pancreatology 15: S1–S5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Majumder S, Chari ST. Chronic pancreatitis. Lancet 387: 1957–1966, 2016. [DOI] [PubMed] [Google Scholar]
- 30.Mohapatra S, Majumder S, Smyrk TC, Zhang L, Matveyenko A, Kudva YC, Chari ST. Diabetes Mellitus Is Associated With an Exocrine Pancreatopathy: Conclusions From a Review of Literature. Pancreas 45: 1104–1110, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morran DC, Wu J, Jamieson NB, Mrowinska A, Kalna G, Karim SA, Au AY, Scarlett CJ, Chang DK, Pajak MZ, Australian Pancreatic Cancer Genome Initiative (APGI), Oien KA, McKay CJ, Carter CR, Gillen G, Champion S, Pimlott SL, Anderson KI, Evans TR, Grimmond SM, Biankin AV, Sansom OJ, Morton JP. Targeting mTOR dependency in pancreatic cancer. Gut 63: 1481–1489, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nomiyama Y, Tashiro M, Yamaguchi T, Watanabe S, Taguchi M, Asaumi H, Nakamura H, Otsuki M. High glucose activates rat pancreatic stellate cells through protein kinase C and p38 mitogen-activated protein kinase pathway. Pancreas 34: 364–372, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Okabayashi Y, Maddux BA, McDonald AR, Logsdon CD, Williams JA, Goldfine ID. Mechanisms of insulin-induced insulin-receptor downregulation. Decrease of receptor biosynthesis and mRNA levels. Diabetes 38: 182–187, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest 117: 50–59, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandini G, Vigneri R, Costantino A, Frasca F, Ippolito A, Fujita-Yamaguchi Y, Siddle K, Goldfine ID, Belfiore A. Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: evidence for a second mechanism of IGF-I signaling. Clin Cancer Res 5: 1935–1944, 1999. [PubMed] [Google Scholar]
- 36.Pandol S, Gukovskaya A, Edderkaoui M, Dawson D, Eibl G, Lugea A. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol 27, Suppl 2: 127–134, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulo JA, Urrutia R, Banks PA, Conwell DL, Steen H. Proteomic analysis of an immortalized mouse pancreatic stellate cell line identifies differentially-expressed proteins in activated vs nonproliferating cell states. J Proteome Res 10: 4835–4844, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem 114: 63–70, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Qi M, McFadden B, Valiente L, Omori K, Bilbao S, Juan J, Rawson J, Oancea AR, Scott S, Nair I, Ferreri K, Mullen Y, Dafoe D, Ei-Shahawy M, Kandeel F, Al-Abdullah IH. Human pancreatic islets isolated from donors with elevated HbA1c levels: islet yield and graft efficacy. Cell Transplant 24: 1879–1886, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol 24: 349–358, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Ryall CL, Viloria K, Lhaf F, Walker AJ, King A, Jones P, Mackintosh D, McNeice R, Kocher H, Flodstrom-Tullberg M, Edling C, Hill NJ. Novel role for matricellular proteins in the regulation of islet beta cell survival: the effect of SPARC on survival, proliferation, and signaling. J Biol Chem 289: 30614–30624, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schanzer JM, Wartha K, Moessner E, Hosse RJ, Moser S, Croasdale R, Trochanowska H, Shao C, Wang P, Shi L, Weinzierl T, Rieder N, Bacac M, Ries CH, Kettenberger H, Schlothauer T, Friess T, Umana P, Klein C. XGFR*, a novel affinity-matured bispecific antibody targeting IGF-1R and EGFR with combined signaling inhibition and enhanced immune activation for the treatment of pancreatic cancer. MAbs 8: 811–827, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Setiawan VW, Pandol SJ, Porcel J, Wilkens LR, Le Marchand L, Pike MC, Monroe KR. Prospective study of alcohol drinking, smoking, and pancreatitis: The Multiethnic Cohort. Pancreas 45: 819–825, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 64: 9–29, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Singh P, Alex JM, Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol 31: 805, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Soares HP, Ni Y, Kisfalvi K, Sinnett-Smith J, Rozengurt E. Different patterns of Akt and ERK feedback activation in response to rapamycin, active-site mTOR inhibitors and metformin in pancreatic cancer cells. PLos One 8: e57289, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su HY, Waldron RT, Gong R, Ramanujan VK, Pandol SJ, Lugea A. The unfolded protein response plays a predominant homeostatic role in response to mitochondrial stress in pancreatic stellate cells. PLos One 11: e0148999, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tatulian SA. Structural dynamics of insulin receptor and transmembrane signaling. Biochemistry 54: 5523–5532, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53, Suppl 3: S215–S219, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Li Y, Zhang H, Huang Y, Zhao P, Tang Y, Qiu X, Ying Y, Li W, Ni S, Zhang M, Liu L, Xu Y, Zhuang Q, Luo Z, Benda C, Song H, Liu B, Lai L, Liu X, Tse HF, Bao X, Chan WY, Esteban MA, Qin B, Pei D. Autophagy and mTORC1 regulate the stochastic phase of somatic cell reprogramming. Nat Cell Biol 17: 715–725, 2015. [DOI] [PubMed] [Google Scholar]
- 51.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 144: 1252–1261, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zechner D, Knapp N, Bobrowski A, Radecke T, Genz B, Vollmar B. Diabetes increases pancreatic fibrosis during chronic inflammation. Exp Biol Med (Maywood) 239: 670–676, 2014. [DOI] [PubMed] [Google Scholar]