Abstract
Reactive oxygen species are implicated in physiological signaling and cell fate decisions. In chronic liver diseases persistent and increased production of oxidative radicals drives a fibrogenic response that is a common feature of disease progression. Despite our understanding the biology of the main prooxidant enzymes, their targets, and antioxidant mechanisms in the liver, there is still lack of knowledge concerning their precise role in the pathogenesis of fibrosis. This review will examine the role of physiological redox signaling in the liver, provide an overview on recent advances in prooxidant and antioxidant pathways that are dysregulated during fibrosis, and highlight possible novel treatment targets.
Keywords: liver fibrosis, oxidative stress, redox signaling, stellate cell activation
during chronic liver diseases of various etiologies, prolonged injury to parenchymal cells triggers a complex cascade of events leading to wound healing with the activation of hepatic stellate cells (HSC), or portal fibroblasts. HSC, Ito cells, or fat-storing cells in physiological states are located in the parasinusoidal space. Their main functions include storing retinoids and producing extracellular matrix (ECM) for the formation of the basement membrane. Portal fibroblasts are considered to be a distinct cell population that express fibulin-2, elastin, and glycophosphatidylinositol-linked glycoprotein (Thy1.1) but do not store vitamin A and are negative for glial fibrillary acidic protein, desmin, or cytoglobin (25). Portal fibroblasts are an important source of TGFβ and ECM during cholestatic liver injury (25, 96).
Increase in matrix stiffness during chronic injury (95) or exposure to soluble factors result in the morphological transition of HSC to myofibroblast-like cells (18, 68, 83). This transdifferentiation is characterized by the loss of retinoids and proliferation and migration of the myofibroblasts with deposition of collagen I in the parenchyma further increasing matrix stiffness. Myofibroblasts express α-smooth muscle actin (α-SMA) and produce fibrogenic cytokines including platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF), transforming growth factor β (TGF-β), and other cytokines. The balance of matrix metalloproteinases (MMPs) and their specific tissue inhibitors (TIMPs) in myofibroblasts is perturbed, with production of pro-MMP-2 and membrane type 1 (MT1)-MMP, which drive the generation of active MMP2 and local degradation of the normal matrix.
Central to the activation process is the increased production of oxidative radicals that modulate key signaling pathways further amplifying the production of ECM. Liver fibrosis can reverse following the resolution of tissue injury, as demonstrated in less advanced disease (50, 77). However, with chronic long-standing injury with a deposition of an increasingly acellular and cross-linked scar tissue, fibrosis becomes irreversible or even progresses after the causative factors are eliminated (59). Reversal of fibrosis hinges on the deactivation of HSC/portal fibroblasts, senescence, or their elimination by apoptosis. Several studies are now focusing on methods to increase fibrosis reversibility and counteract matrix cross-linking (67).
Redox Homeostasis in the Liver
Reactive oxygen species (ROS) are reactive metabolites produced during the reduction of O2 to H2O and are the result of aerobic metabolism. During evolution, ROS served as important signaling molecules to facilitate adaptation to environmental changes such as lack or surplus of nutrients or temperature changes (97). In prokaryotes, for example, there are well-described pathways whereby ROS help in adaptation to stress by inducing activation of transcription factors such as OxyR or SoxR (103). ROS are also known to serve as important second messengers to regulate physiological processes in higher organisms. Key to this concept is the distinction between mild stress that can induce adaptive responses (hormesis) or persistent more significant stress that leads to reduction of antioxidant responses (see Dysregulation of Antioxidant Pathways below) and inefficient adaptation to stress. For instance, in the liver-like cells (oenocytes) of Drosophila or mouse hepatocytes, exposure to low concentrations of ROS increases lifespan by inducing mitochondrial unfolded protein response (UPR; mUPR) genes (84). Evidence also suggests that redox signaling is required for healthy aging (40). Examples of redox targets in the liver include tyrosine phosphatases and protein tyrosine kinases that can be greatly affected by the redox milieu, the specific ROS, their concentration, and availability of antioxidant molecules. Inactivation of protein phosphatases (PTP1B) has been demonstrated to play an important role in maintaining insulin signaling in hepatocytes (35). Insulin can induce the deactivation of protein phosphatase PTEN requiring an H2O2 burst at the plasma membrane (58). In Kupffer cells and neutrophils intact phagocytic NADPH oxidase 2 (NOX2) activities and oxidative burst are essential in protection against bacterial and fungal infections. Deficiency in NOX2 oxidase function because of genetic variants (CYBB, CYBA, NCF1-4) has been recognized as a direct cause of chronic granulomatous disease with recurrent severe infections and liver granulomas. Another example of physiological redox signaling is the maintenance of vascular tone by sinusoidal endothelial cells. Low level of nitric oxide produced by eNOS activity is important in maintaining redox homeostasis; however, during fibrosis endothelial cell dysfunction results in a decreased vasodilative effect that contributes to vascular resistance characteristic of advanced-stage liver disease (42).
Dr. H. Sies in the 1980s originally coined the term “oxidative stress” describing the imbalance of oxidants and antioxidants (30). It is increasingly recognized, however, that the term oxidative stress needs to incorporate several other features, such as detailed analysis of the dynamics of ROS exposure, their degradation kinetics, and their subcellular location. In addition, to study the effects of ROS, experiments have often been conducted using exogenous ROS. One has to be cautious, however, with the interpretation of such studies as, for example, the H2O2 concentrations used were frequently severalfold higher than those observed under pathophysiological states in the tissue (29). Attention should also be paid to the kinetics of reactions in which H2O2 is assumed to play a role. While generally it is accepted that some ROS, e.g., H2O2, can diffuse and elicit signals in a distance from the cell of origin, recent studies are highlighting that cellular compartmentalization of ROS production is a key determinant of whether redox signaling or oxidative damage occur (48, 73). This is particularly true for NADPH oxidases (NOXs) where effective signaling platforms are associated with caveolae/lipid rafts, endosomes, and/or nucleus (94). Redox-active endosomes (redoxosomes) are signaling endosomes that can include NOX complex subunits and contain redox-processing proteins that transmit ROS signals from the interior to redox-sensitive effectors on the endosomal surface, thereby regulating IL-R1 or TNFR1 and NF-κB signaling cascades (73, 88). As these signals are essential in HSC plasticity, an interesting area of future research could be to determine whether redox signaling is required to maintain HSC quiescence. Further studies are required to discover the specific oxidative species, their sources, and concentrations that are essential in maintaining physiological signaling and redox homeostasis in HSC.
Oxidative Stress in Liver Fibrogenesis
The factors that determine the effects of redox radicals in fibrosis include the persistence of noxious stimuli, time course, concentrations, stability of ROS released, and the potency of antioxidant systems. The main radical species in the liver include ROS-like superoxide anion (O2·−), which is formed through one-electron reduction of O2, hydrogen peroxide (H2O2, although it is not a real radical, as it does not have an unpaired electron); and the very toxic hydroxyl radicals (OH·). Generally these radicals are very short lived, and their toxicity is linked to their conversion to a more stable and reactive ROS (28). The reactive nitrosative species include nitric oxide (·NO) and the more stable peroxynitrites.
Subcellular and cellular sources of ROS.
In the liver there are numerous important sources of ROS. In hepatocytes mitochondria are thought to be largest contributors to ROS production, but the role of cytochrome P4502E1 (CYP2E1), NADPH oxidases, and the arachidonic acid and xanthine oxidase systems also play a major role especially during pathological conditions (8, 15, 60). Mitochondrial stress is an important factor in the progression of alcoholic liver disease and nonalcoholic fatty liver disease to steatohepatitis, and it is indirectly involved in fibrosis progression. Mitochondrial ROS production is a result of electron transport from Complexes I and III to oxygen while forming superoxide. Mitochondrial proticity (proton-moving force across the inner membrane) is associated with increased ROS generation that depends on the uncoupling capacity especially by UCP2 and 3 (65). Recent in vivo evidence confirming the role of mitochondrial respiratory function in nonalcoholic steatohepatitis (NASH) came from the studies of Gandhi et al. (31) where the liver specific deletion of Augmenter of liver regeneration (ALR) resulted in defects in mitochondrial fatty acid transport and ATP synthesis, increased oxidative stress, fibrosis, and HCC. Highly oxidized mitochondrial DNA was also recently shown to play a role in the proinflammatory responses via TLR9 activation in NASH (34). Given these results we can postulate that heightened production of mitochondrial redox radicals is central to drive inflammatory and fibrogenic responses in NASH.
Microsomal and to some extent mitochondrial CYP450 2E1 are also important sources of ROS in hepatocytes as they generate high amounts of H2O2 in alcoholic and nonalcoholic steatohepatitis (8, 16, 70). The role of CYP450 2E1 was demonstrated recently in an alcohol binge animal model where both intestinal and hepatic CYP2E1 was critical in nitrosative stress, gut leakiness, steatohepatitis and fibrosis (1). The same group also has shown that CYP2E1-derived radicals promoted aging-related steatosis, apoptosis, and fibrosis (2). In clinical studies CYP2E1 polymorphisms were linked to the severity of alcoholic liver disease (78), with the CYP2E1 c1/c1 genotype and c1 allele being more frequent in cirrhotic patients (33). These findings will have to be corroborated by prospective studies to address whether these data can be used to prognosticate and identify the population at risk.
ROS can be both generated and/or scavenged by peroxisomes (37). Peroxisomal oxidases can generate H2O2 but iNOS was also detected in peroxisomes (61). α-Oxidation of long-chain fatty acids occurs in the peroxisomes but mitochondrial β-oxidation is also supported via the peroxisome proliferator-activated receptor-α (PPARα). It is speculated that increased peroxisomal activity precedes NASH, and disruption of peroxisomes in the PDX5 knockout mice resulted in decreased mitochondrial function and steatosis; however, oxidative stress in this model was not observed (24). On the other hand, peroxisomes also respond to oxidative stress by an increase in catalase activity induced by, e.g., ethanol and thus perform essential scavenger functions (86). PPARs gained a lot of attention in the recent years as lipid sensors that are involved in metabolic, inflammatory processes and cell fate determination. Several studies and extensive reviews highlighted the protective role of PPARγ in liver fibrosis and maintaining HSC quiescence (49, 102). Recently, differential DNA methylation at the PPARγ promoter was identified in the circulating DNA of patients with NASH, correlating with fibrosis (36). Future studies could be done to verify these findings and assess cell-free circulating DNA methylation of PPARγ as a novel biomarker for fibrosis.
The cellular components of the hepatic innate and adaptive immune system are known to be important sources of ROS especially in the context of viral and microbial infection (phagocytic cells, e.g., neutrophils and macrophages) and during chronic processes such as alcoholic liver disease or NASH. Several excellent reviews have addressed the role of macrophages and neutrophils in fibrosis (32, 91, 98). T cell responses are also modulated by redox stress especially by lipid peroxidation and reactive aldehydes such as malondialdehyde (MDA) (5). CD4+ T-cell ablation in MDA-immunized mice significantly improved NASH (90). As CD4+ T-cell-mediated signals are important for an efficient activation of effector CD8+ T cells this can represent a cascade by which redox radicals can activate immune responses in alcoholic steatohepatitis (ASH) and NASH. More recently a link between selective CD4+ T cell loss and increased HCC formation was noted in the methionine choline-deficient diet (MCD)-on Myc NASH model. In this study, CD4+ T cell death was attributed to the release of C18:2 from injured hepatocytes, accumulation of linoleic acid, and mitochondrial redox stress. Blocking ROS with catalase or N-acetylcysteine (NAC) prevented the loss of CD4+ T cells and thereby decreased HCC (64). Cumulatively, these studies may indicate that whereas in early NASH CD4+ T cells play a proinflammatory and fibrogenic role their demise at later stages could correlate to impaired antitumor surveillance.
NADPH oxidases-ROS can be generated directly by stellate cells during their activation process and also by infiltrating macrophages and neutrophils. In these cells, NOXs are major sources of ROS production. The main NOXs that are expressed in the liver are NOX1, NOX2, NOX4, and dual oxidases (DUOXs) in hepatocytes (75). NOX1 and 2 mainly produce superoxide whereas NOX4 directly generates H2O2·−. To form active complexes, multiple subunits have to bind to NOX1 and NOX2, and the interaction between these subunits is an important determinant of enzyme activity. NOX4 is a constitutively active homologue that associates with p22phox and can be found in HSC, hepatocytes, and sinusoidal endothelial cells. Detailed recent reviews have summarized the intricate pathways by which NOXs regulate HSC proliferation, migration, and contraction and cross talks with LPS-TLR4 signaling (75). Briefly, NOXs can be activated by multiple cytokines and mediators including PDGF (4), angiotensin II (7), leptin (22, 45), and alcohol (56) and directly by the phagocytosis of apoptotic bodies from hepatocytes (47); therefore they are considered as central hubs for ROS production (Fig. 1). As to which NOX homologue is activated by each of these mediators in HSC is still under investigation, and the precise molecular mechanism of their action would still require further investigation. Attenuation of fibrosis was seen both in the NOX1 and NOX2 knockout mice in the CCl4 or bile duct ligation (BDL) models (19, 47, 74). Hepatocyte-specific deletion of NOX4 protected against endoplasmic reticulum stress and attenuated fibrosis in two NASH models (9) whereas HSC deleted of NOX4 exhibited reduced activation and production of collagen I. Thus NOXs are nonredundant, key sources of ROS during fibrosis, and targeting nonphagocytic NOXs could become an important antifibrogenic therapeutic strategy (6, 44).
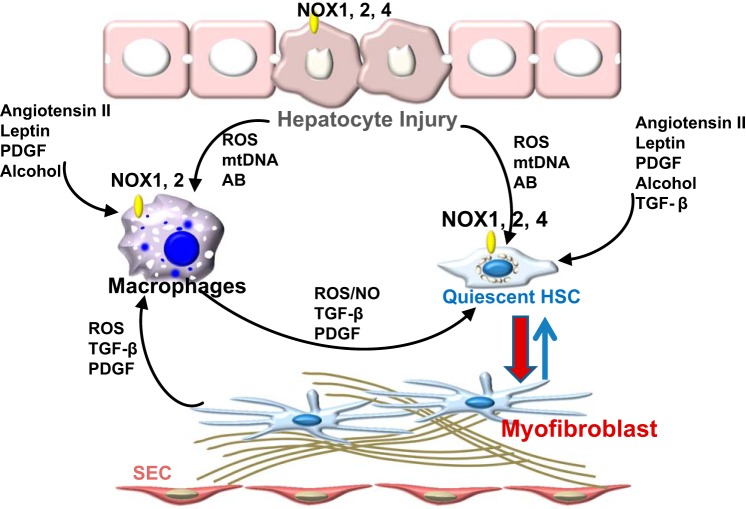
Fig. 1.
Cross talk between liver cells regulating HCS activation via redox pathways. Hepatocyte injury results in generation of ROS [endoplasmic reticulum stress, mitochondria, NADPH oxidases (NOXs)], release of mitochondrial DNA or formation of apoptotic bodies (AB). These trigger activation of liver macrophages and transdifferentiation of quiescent stellate cells to myofibroblasts. Mediators such as angiotensin II, leptin, PDGF, and TGF-β contribute to the activation of NOXs in macrophages and stellate cells, further accelerating the deposition of matrix. SEC, sinusoidal endothelial cells.
As important second messengers, ROS have a major impact on cell fate (13, 66). In this sense they can act as a double edged sword: in hepatocytes pathologically increased ROS in the face of maladaptive UPR or reduced antioxidant defense can lead to proapoptotic signaling (10, 14, 43), whereas in myofibroblasts oxidative radicals can be implicated in proliferation and survival pathways (46). At the center of HSC survival is the activation of NF-κB that can be directly induced by ROS or indirectly by recruited macrophages. Whether myofibroblasts could undergo cell death by a more substantial increase in intracellular oxidative radicals is not known. To address this, more detailed studies should be conducted on cell survival pathways in myofibroblasts. Designing selective myofibroblasts delivery methods by either liposomes or nanotechnology would facilitate targeting and avoiding collateral damage to parenchymal cells.
miRNAs can influence the expression of target genes that are either pro- or antioxidant. An important miRNA-200a target gene is Kelch-like ECH-associated protein 1 (Keap1) that negatively regulates the stability of nuclear factor-erythroid-2-related factor 2 (Nrf2), a known regulator of antioxidants (100). This is in line with the findings demonstrating a feedback loop whereby in hepatocytes oxidative stress-induced miR-200a is involved in p38-mediated phosphorylation of p53 regulating cell death (99). MiR 23b, miR-25, and miR-146a are known to reduce NOX4 in other systems (41); however, their role in liver fibrosis has not been evaluated yet. Upregulation of miR21 downstream of NOX activity suppressed Smad7 thereby favoring the assembly of Smad2/3 and profibrogenic signaling (20). On the other hand, TGFβ and bone morphogenetic protein signaling was shown to regulate miRNA 21 maturation in vascular smooth muscle cells (21). These studies highlight the complexity and the potential reciprocal nature of miRNA redox signaling in fibrosis.
Detection and Quantification of ROS
There are several chemiluminescent methods to measure superoxide or hydrogen peroxide in cells. One of the most common methods using dihydroethydium, however, may not be sensitive because of overlapping fluorescence with 2-OH-ethidium plus interaction with a wide variety of other cellular oxidants including nitric oxide, peroxynitrite, and hypochloride (69). Electron spin resonance and HPLC are more reliable methods; however, these are costly and time consuming. Genetically encoded fluorescent probes have recently been developed that were able to detect subcellular H2O2 (HyPerRed) (27) and have been used to detect NOX4 activity (81). Recent advances in nanotechnology lead to the development of gold nanoprobes that are able to measure the oxidant-dependent degradation of hyaluronic acid (87). Despite these recent advances, however, there is still an urgent need to establish rapid, reproducible, and quantitative methods to detect specific radicals, as currently none of the technologies available provide an accurate and sensitive method to localize and quantify superoxide and H2O2·−. Therefore simultaneous use of several methods is still recommended to facilitate accurate interpretation of results.
Dysregulation of Antioxidant Pathways
To maintain redox homeostasis cells are equipped with powerful antioxidant systems comprised of enzymatic and nonenzymatic molecules. The former group includes superoxide dismutases (SOD), catalase, and enzymes regulating glutathione (GSH) synthesis. Antioxidant enzymes have specific targets and they function as sensors of particular ROS. For instance catalase and peroxiredoxins target H2O2 whereas SODs only target superoxide. SOD1, for example, has been identified as playing an important role in H2O2 production in metabolic signaling (79).
The nonenzymatic antioxidants include coenzyme GSH, Q10, and ROS binding proteins such as thioredoxin (Trx) (63, 101). In physiological situations the amount of H2O2 is under a tight control by these antioxidant systems. During chronic liver injury, however, parenchymal cells are exposed to increasing concentrations of intra- or extracellularly generated ROS. GSH plays a major protective role in liver injury (63). The amount of GSH is tightly linked to oxidative homeostasis and to glucose metabolism. The pentose phosphate pathway and availability of NADPH are important factors for keeping GSH in a reduced form. The transcription factor Nrf2 is a master regulator of a spectrum of genes related to GSH metabolism via the antioxidant responsive element (ARE) on target genes and also plays a role in xenobiotic detoxification and proteome maintenance (63). In response to oxidative stress Nrf2 dissociates from its inhibitory regulator Keap1 and translocates to the nucleus to induce the transcription of antioxidant genes GSH synthetases (Gclc and Gclm), glutathione-S-transferase, GSH peroxidase, GSH reductase, and NAD(P)H quinone oxidoreductase 1 (51). Generally, it is thought that Nrf2 renders protection against liver injury induced by diverse pathogenic stimuli (85). Cell line studies demonstrated that Nrf2 was induced by hepatitis B and C, and knockdown Nrf2 by siRNA lowered cell survival rate (11). Studies on Nrf2 and Keap1 knockout mice showed that Nrf2 was activated by alcohol consumption via CYP2E1 and that it played a protective role (57), and Nrf2 knockout mice were also more sensitive to diet-induced steatosis, inflammation, and fibrosis (17). A study in lung fibrosis demonstrated the importance of NOX4-Nrf2 balance in redox homeostasis in young and aged mice. NOX4 in aging mice had an impaired ability to induce Nrf2 responses, and thus they were more prone to develop fibrosis (38). Data from recent studies highlighted a novel cross talk between Nrf2 and NF-κB/RelA system in hepatocytes that protected the liver from necrosis and fibrosis (54). Notwithstanding there are also recent studies where Nrf2 induction was found to be much less beneficial. In transgenic mice expressing constitutively active Nrf2, no protective effects were found against CCl4-induced liver injury and fibrosis and regeneration was found to be impaired, too. This was likely to be due to the induction of cyclin-dependent kinase inhibitor P15 and proapoptotic protein Bim (55). In a different study persistent Nrf2 activation was shown to actually promote fibrosis and tumor formation in hepatocyte-specific Atg5 knockout mice that are defective in autophagy (71). Thus caution should be exercised when targeting Nrf2 as a therapeutic strategy as prolonged induction actually could result in accelerated fibrosis or development of HCC.
The Forkhead box gene, group O (FOXO) family of transcription factors are important metabolic and antioxidant regulators and their activities are tightly regulated by posttranslational modifications, including phosphorylation or acetylation. FOXO1 is a direct transcriptional regulator of gluconeogenesis by inducing the phosphoenolpyruvate carboxykinase (PEPCK)/glucose 6-phosphate pathway. It is well established that FOXO transcription factors reduce the level of oxidative stress by the induction of enzymes such as catalase and MnSOD. FOXO1 can directly induce MnSOD and inhibit HSC proliferation, and FOXO1 (+/−) mice were found to be more susceptible to BDL-induced fibrosis (3). FOXO proteins could also be directly proapoptotic in activated HSC by downregulating c-FLIP(L/S) and promoting TRAIL-induced apoptosis in LX-2 cells (76). It is important to note that in certain contexts FOXO proteins can have a different, proapoptotic effect on hepatocytes. In the hepatotoxin 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet model inhibition of FOXO3 and Wnt/β-catenin signaling was required for hepatocyte protection against oxidative stress-induced apoptosis (92). Thus different members of the FOXO family may have nonredundant and cell-specific roles in oxidative injury.
Sirtuins are class III histone/protein deacetylases, with Sirt1 and 3 regulating lipid and glucose homeostasis functioning as cellular energy sensors. Sirt1 regulates FOXO3 by increasing its ability to resist oxidative stress and at the same time inducing cell cycle arrest. The complexity of the Sirt/FOXO cross talks has been analyzed by excellent recent reviews (52, 72). Sirt3 in the mitochondria is an important regulator of mitochondrial oxidative stress. A recent study showed that, with age, Sirt3 knockout mice developed fibrosis in multiple organs including heart, liver, kidney, and lungs. Sirt3 deficiency caused induction of TGFβ1 and hyperacetylation of GSK3β negatively regulating its activity to phosphorylate Smad3 and β-catenin (89). Although there are an ever-growing number of studies delineating the importance of dysregulated antioxidant pathways in fibrosis, many questions remain pertaining to the translatability of these findings to human pathology. As many of these regulators have multiple intracellular targets or can have opposite effects on different cells types of the liver as depicted above, caution should be exercised to select appropriate candidates for anti-fibrotic therapy.
Therapeutic Approaches Targeting Pro- or Antioxidant Pathways
As pathological redox signaling is fundamental to fibrosis progression, strategies that reduce redox injury or augment or repair antioxidant responses could result in improvement of liver injury, synthetic function, and portal hypertension. Many studies in the past addressed the use of various antioxidants; however, most of these were disappointing, owing to differences between animal models and human disease, the inability of the agents to reach the relevant cellular locations, and the stage- and cell-specific regulation of oxidant and antioxidant pathways. In many cases trials with antioxidants were conducted without special emphasis to the pharmacokinetic or pharmacodynamic properties of the compounds not taking into account the molecular structure, intestinal uptake, or membrane penetration of the given drug (82). In addition, in several trials the study population was heterogeneous, the studies underpowered, the length of treatment inadequate (62), and the off-target effects not properly tested.
Another issue is that there is a lack of suitable markers to monitor for the effects of antioxidants. Serum alanine aminotransferase or aspartate aminotransferase are often used as surrogates; however, these are nonspecific and may not be in direct correlation with the desired antioxidant effect but rather reflect some other property of the drug tested (e.g., antiapoptotic activity of resveratrol). F2 isoprostanes, 8-hydroxydeoxyguanosine, and protein carbonyls can reflect oxidative damage to lipids, DNA, and proteins, respectively, but these are expensive tests and cannot adequately inform about the overall injury during fibrosis. γ-Glutamyltransferase, on the other hand, can reflect malfunctioning antioxidant system (53) with the caveat that it can also be elevated in other conditions, e.g., hepatocellular carcinoma.
Vitamins E, C, and A and other agents in complementary and alternative medicine have been studied, and, whereas some had protective effects in animal models, none of them have demonstrated clear benefit for patients. Resveratrol, a polyphenol found in a variety of fruits, was shown to exert a wide range of beneficial effects in animal models including decrease in HSC activation, oxidative stress, and portal hypertension (23). In NASH patients high-dose resveratrol had a small effect on improving liver tests, but no histological effects (39). Recently NOXs have emerged as potential targets. NOX1/NOX4 targeting using GKT137831 attenuated fibrosis in the CCl4 (6), BDL models of liver fibrosis (44), in lung fibrosis (93), and in diabetic kidney disease. A phase II trial is underway in diabetic kidney fibrosis (ClinicalTrials.gov NCT02010242). In a recent study Ang-(1–7), which counteracts the effects of angiotensin II, improved BDL-induced-hepatic fibrosis and reduced H2O2 content, protein levels of NOX4, and the NLRP3-inflammasome, while increasing GSH and activity of the Nrf2-antioxidant response element (12). In a different study an agonist of Ang-(1–7), AVE0991, decreased portal pressure without influencing systemic pressure. Although it did not inhibit fibrosis, AVE0991 could be a potential new therapeutic strategy for lowering portal pressure (52). Inhibition of the thromboxane-A2/prostaglandin-endoperoxide receptor by terutroban decreased fibrosis in CCl4-treated rats; however, in BDL rats it did not reduce fibrosis but enhanced eNOS-dependent vasodilatation (80). A recent study showed promising results using redox nanoparticles that were prepared by assembly of redox polymers containing antioxidant nitroxide radicals. Treatment of mice on NASH diets with these nanoparticles reduced liver ROS and improved fibrosis and inflammation (26).
Concluding Remarks
There are several factors that hamper progress in developing successful therapeutics for redox stress. First, to successfully and permanently reduce redox stress in injured livers, further studies are necessary to focus on the key signaling cascades and enzymes that are essential in ROS production. As it is becoming increasingly clear, these enzymes are nonredundant and may play disease and stage-specific roles. Proteomic or metabolomic approaches may help in discovering the central pathways that drive redox-mediated fibrosis progression. Targeting these core ROS-producing enzymes would be important after determining the level of their “safe” inhibition to maintain physiological redox signaling. Second, trials have to be well designed and powered taking into account the possible heterogeneity of the study population. Third, adequate methods need to be developed to serve as surrogate markers of redox stress in the liver. Overcoming these issues would greatly enhance the development of successful therapeutics to combat oxidative stress and liver fibrosis.
GRANTS
This work was supported by National Institutes of Health Grants DK083283 and 01BX002418.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.J.T. prepared figures; drafted manuscript; edited and revised manuscript; approved final version of manuscript.
REFERENCES
- 1.Abdelmegeed MA, Banerjee A, Jang S, Yoo SH, Yun JW, Gonzalez FJ, Keshavarzian A, Song BJ. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic Biol Med 65: 1238–1245, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelmegeed MA, Choi Y, Ha SK, Song BJ. Cytochrome P450-2E1 promotes aging-related hepatic steatosis, apoptosis and fibrosis through increased nitroxidative stress. Free Radic Biol Med 91: 188–202, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi M, Osawa Y, Uchinami H, Kitamura T, Accili D, Brenner DA. The forkhead transcription factor FoxO1 regulates proliferation and transdifferentiation of hepatic stellate cells. Gastroenterology 132: 1434–1446, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, Kawata S. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology 41: 1272–1281, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, Burt AD, Day CP. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut 54: 987–993, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest 112: 1383–1394, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beier JI, McClain CJ. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem 391: 1249–1264, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettaieb A, Jiang JX, Sasaki Y, Chao TI, Kiss Z, Chen X, Tian J, Katsuyama M, Yabe-Nishimura C, Xi Y, Szyndralewiez C, Schroder K, Shah A, Brandes RP, Haj FG, Torok NJ. Hepatocyte nicotinamide adenine dinucleotide phosphate reduced oxidase 4 regulates stress signaling, fibrosis, and insulin sensitivity during development of steatohepatitis in mice. Gastroenterology 149: 468–480.e10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol 59: 583–594, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Burdette D, Olivarez M, Waris G. Activation of transcription factor Nrf2 by hepatitis C virus induces the cell-survival pathway. J Gen Virol 91: 681–690, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Cai SM, Yang RQ, Li Y, Ning ZW, Zhang LL, Zhou GS, Luo W, Li DH, Chen Y, Pan MX, Li X. Angiotensin-(1–7) improve liver fibrosis by regulating the NLRP3 inflammasome via redox balance modulation. Antioxid Redox Signal 24: 795–812, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21: 396–413, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, Fernandez M, Fabregat I. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol 49: 965–976, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Cederbaum AI. Iron and CYP2E1-dependent oxidative stress and toxicity. Alcohol 30: 115–120, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Cederbaum AI. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol 4: 60–73, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhry S, Nazmy MH, Meakin PJ, Dinkova-Kostova AT, Walsh SV, Tsujita T, Dillon JF, Ashford ML, Hayes JD. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med 48: 357–371, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Cong M, Iwaisako K, Jiang C, Kisseleva T. Cell signals influencing hepatic fibrosis. Int J Hepatol 2012: 158547, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui W, Matsuno K, Iwata K, Ibi M, Matsumoto M, Zhang J, Zhu K, Katsuyama M, Torok NJ, Yabe-Nishimura C. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology 54: 949–958, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Dattaroy D, Pourhoseini S, Das S, Alhasson F, Seth RK, Nagarkatti M, Michelotti GA, Diehl AM, Chatterjee S. Micro-RNA 21 inhibition of SMAD7 enhances fibrogenesis via leptin-mediated NADPH oxidase in experimental and human nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 308: G298–G312, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454: 56–61, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Minicis S, Seki E, Oesterreicher C, Schnabl B, Schwabe RF, Brenner DA. Reduced nicotinamide adenine dinucleotide phosphate oxidase mediates fibrotic and inflammatory effects of leptin on hepatic stellate cells. Hepatology 48: 2016–2026, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Di Pascoli M, Divi M, Rodriguez-Vilarrupla A, Rosado E, Gracia-Sancho J, Vilaseca M, Bosch J, Garcia-Pagan JC. Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J Hepatol 58: 904–910, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Dirkx R, Vanhorebeek I, Martens K, Schad A, Grabenbauer M, Fahimi D, Declercq P, Van Veldhoven PP, Baes M. Absence of peroxisomes in mouse hepatocytes causes mitochondrial and ER abnormalities. Hepatology 41: 868–878, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Dranoff JA, Wells RG. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology 51: 1438–1444, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eguchi A, Yoshitomi T, Lazic M, Johnson CD, Vong LB, Wree A, Povero D, Papouchado BG, Nagasaki Y, Feldstein AE. Redox nanoparticles as a novel treatment approach for inflammation and fibrosis associated with nonalcoholic steatohepatitis. Nanomedicine (Lond) 10: 2697–2708, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ermakova YG, Bilan DS, Matlashov ME, Mishina NM, Markvicheva KN, Subach OM, Subach FV, Bogeski I, Hoth M, Enikolopov G, Belousov VV. Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat Commun 5: 5222, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forman HJ. Redox signaling: an evolution from free radicals to aging. Free Radic Biol Med 97: 398–407, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forman HJ. Use and abuse of exogenous H2O2 in studies of signal transduction. Free Radic Biol Med 42: 926–932, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forman HJ, Toyokuni S. Tribute issue: Helmut Sies and oxidative stress: venit, vidit, vicit. Arch Biochem Biophys 595: 2, 2016. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi CR, Chaillet JR, Nalesnik MA, Kumar S, Dangi A, Demetris AJ, Ferrell R, Wu T, Divanovic S, Stankeiwicz T, Shaffer B, Stolz DB, Harvey SA, Wang J, Starzl TE. Liver-specific deletion of augmenter of liver regeneration accelerates development of steatohepatitis and hepatocellular carcinoma in mice. Gastroenterology 148: 379–391 e374, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, Szabo G, Zakhari S. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 300: G516–G525, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Banuelos J, Panduro A, Gordillo-Bastidas D, Gordillo-Bastidas E, Munoz-Valle JF, Gurrola-Diaz CM, Sanchez-Enriquez S, Ruiz-Madrigal B, Bastidas-Ramirez BE. Genetic polymorphisms of genes coding to alcohol-metabolizing enzymes in western Mexicans: association of CYP2E1*c2/CYP2E1*5B allele with cirrhosis and liver function. Alcohol Clin Exp Res 36: 425–431, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest 126: 859–864, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haj FG, Zabolotny JM, Kim YB, Kahn BB, Neel BG. Liver-specific protein-tyrosine phosphatase 1B (PTP1B) re-expression alters glucose homeostasis of PTP1B−/− mice. J Biol Chem 280: 15038–15046, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Hardy T, Zeybel M, Day CP, Dipper C, Masson S, McPherson S, Henderson E, Tiniakos D, White S, French J, Mann DA, Anstee QM, Mann J. Plasma DNA methylation: a potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut 2016. 2016 Mar 21. pii: gutjnl-2016-311526. doi: 10.1136/gutjnl-2016-311526 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartwig S, Knebel B, Goeddeke S, Koellmer C, Jacob S, Nitzgen U, Passlack W, Schiller M, Dicken HD, Haas J, Muller-Wieland D, Lehr S, Kotzka J. So close and yet so far: mitochondria and peroxisomes are one but with specific talents. Arch Physiol Biochem 119: 126–135, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting nox4-nrf2 redox imbalance. Sci Transl Med 6: 231ra247, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heeboll S, Kreuzfeldt M, Hamilton-Dutoit S, Kjaer Poulsen M, Stodkilde-Jorgensen H, Moller HJ, Jessen N, Thorsen K, Kristina Hellberg Y, Bonlokke Pedersen S, Gronbaek H. Placebo-controlled, randomised clinical trial: high-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand J Gastroenterol 51: 456–464, 2016. [DOI] [PubMed] [Google Scholar]
- 40.Hill S, Van Remmen H. Mitochondrial stress signaling in longevity: a new role for mitochondrial function in aging. Redox Biol 2: 936–944, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Im YB, Jee MK, Jung JS, Choi JI, Jang JH, Kang SK. miR23b ameliorates neuropathic pain in spinal cord by silencing NADPH oxidase 4. Antioxid Redox Signal 16: 1046–1060, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Iwakiri Y. Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int 32: 199–213, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol 26, Suppl 1: 173–179, 2011. [DOI] [PubMed] [Google Scholar]
- 44.Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schroder K, Brandes RP, Devaraj S, Torok NJ. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med 53: 289–296, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang JX, Mikami K, Shah VH, Torok NJ. Leptin induces phagocytosis of apoptotic bodies by hepatic stellate cells via a Rho guanosine triphosphatase-dependent mechanism. Hepatology 48: 1497–1505, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang JX, Mikami K, Venugopal S, Li Y, Torok NJ. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J Hepatol 51: 139–148, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang JX, Venugopal S, Serizawa N, Chen X, Scott F, Li Y, Adamson R, Devaraj S, Shah V, Gershwin ME, Friedman SL, Torok NJ. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology 139: 1375–1384, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones DP, Go YM. Redox compartmentalization and cellular stress. Diabetes Obes Metab 12, Suppl 2: 116–125, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kisseleva T, Brenner DA. Inactivation of myofibroblasts during regression of liver fibrosis. Cell Cycle 12: 381–382, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol 22, Suppl 1: S73–S78, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol 244: 57–65, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klotz LO, Sanchez-Ramos C, Prieto-Arroyo I, Urbanek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol 6: 51–72, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koenig G, Seneff S. Gamma-glutamyltransferase: a predictive biomarker of cellular antioxidant inadequacy and disease risk. Dis Markers 2015: 818570, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohler UA, Bohm F, Rolfs F, Egger M, Hornemann T, Pasparakis M, Weber A, Werner S. NF-kappaB/RelA and Nrf2 cooperate to maintain hepatocyte integrity and to prevent development of hepatocellular adenoma. J Hepatol 64: 94–102, 2016. [DOI] [PubMed] [Google Scholar]
- 55.Kohler UA, Kurinna S, Schwitter D, Marti A, Schafer M, Hellerbrand C, Speicher T, Werner S. Activated Nrf2 impairs liver regeneration in mice by activation of genes involved in cell cycle control and apoptosis. Hepatology 60: 670–678, 2014. [DOI] [PubMed] [Google Scholar]
- 56.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest 106: 867–872, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamle J, Marhenke S, Borlak J, von Wasielewski R, Eriksson CJ, Geffers R, Manns MP, Yamamoto M, Vogel A. Nuclear factor-eythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology 134: 1159–1168, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem 277: 20336–20342, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Lee YA, Friedman SL. Reversal, maintenance or progression: what happens to the liver after a virologic cure of hepatitis C? Antiviral Res 107: 23–30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lieber CS. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol 38: 601–628, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Loughran PA, Stolz DB, Vodovotz Y, Watkins SC, Simmons RL, Billiar TR. Monomeric inducible nitric oxide synthase localizes to peroxisomes in hepatocytes. Proc Natl Acad Sci USA 102: 13837–13842, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu SC. Antioxidants in the treatment of chronic liver diseases: why is the efficacy evidence so weak in humans? Hepatology 48: 1359–1361, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Lu SC. Glutathione synthesis. Biochim Biophys Acta 1830: 3143–3153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, Han M, Thornton AM, Zhang H, Egger M, Luo J, Felsher DW, McVicar DW, Weber A, Heikenwalder M, Greten TF. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 531: 253–257, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mailloux RJ, Harper ME. Mitochondrial proticity and ROS signaling: lessons from the uncoupling proteins. Trends Endocrinol Metab 23: 451–458, 2012. [DOI] [PubMed] [Google Scholar]
- 66.Mari M, Colell A, Morales A, von Montfort C, Garcia-Ruiz C, Fernandez-Checa JC. Redox control of liver function in health and disease. Antioxid Redox Signal 12: 1295–1331, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehal WZ, Schuppan D. Antifibrotic therapies in the liver. Semin Liver Dis 35: 184–198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact 193: 225–231, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nauseef WM. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim Biophys Acta 1840: 757–767, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neuman MG, Malnick S, Maor Y, Nanau RM, Melzer E, Ferenci P, Seitz HK, Mueller S, Mell H, Samuel D, Cohen LB, Kharbanda KK, Osna NA, Ganesan M, Thompson KJ, McKillop IH, Bautista A, Bataller R, French SW. Alcoholic liver disease: clinical and translational research. Exp Mol Pathol 99: 596–610, 2015. [DOI] [PubMed] [Google Scholar]
- 71.Ni HM, Woolbright BL, Williams J, Copple B, Cui W, Luyendyk JP, Jaeschke H, Ding WX. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol 61: 617–625, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschop MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 92: 1479–1514, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oakley FD, Abbott D, Li Q, Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal 11: 1313–1333, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paik YH, Iwaisako K, Seki E, Inokuchi S, Schnabl B, Osterreicher CH, Kisseleva T, Brenner DA. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology 53: 1730–1741, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paik YH, Kim J, Aoyama T, De Minicis S, Bataller R, Brenner DA. Role of NADPH oxidases in liver fibrosis. Antioxid Redox Signal 20: 2854–2872, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park SJ, Sohn HY, Yoon J, Park SI. Down-regulation of FoxO-dependent c-FLIP expression mediates TRAIL-induced apoptosis in activated hepatic stellate cells. Cell Signal 21: 1495–1503, 2009. [DOI] [PubMed] [Google Scholar]
- 77.Pinzani M. Liver fibrosis in the post-HCV era. Semin Liver Dis 35: 157–165, 2015. [DOI] [PubMed] [Google Scholar]
- 78.Plemenitas A, Kastelic M, Porcelli S, Serretti A, Rus Makovec M, Kores Plesnicar B, Dolzan V. Genetic variability in CYP2E1 and catalase gene among currently and formerly alcohol-dependent male subjects. Alcohol Alcohol 50: 140–145, 2015. [DOI] [PubMed] [Google Scholar]
- 79.Reddi AR, Culotta VC. SOD1 integrates signals from oxygen and glucose to repress respiration. Cell 152: 224–235, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosado E, Rodriguez-Vilarrupla A, Gracia-Sancho J, Tripathi D, Garcia-Caldero H, Bosch J, Garcia-Pagan JC. Terutroban, a TP-receptor antagonist, reduces portal pressure in cirrhotic rats. Hepatology 58: 1424–1435, 2013. [DOI] [PubMed] [Google Scholar]
- 81.Santos CX, Hafstad AD, Beretta M, Zhang M, Molenaar C, Kopec J, Fotinou D, Murray TV, Cobb AM, Martin D, Zeh Silva M, Anilkumar N, Schroder K, Shanahan CM, Brewer AC, Brandes RP, Blanc E, Parsons M, Belousov V, Cammack R, Hider RC, Steiner RA, Shah AM. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF2alpha-mediated stress signaling. EMBO J 35: 319–334, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt HH, Stocker R, Vollbracht C, Paulsen G, Riley D, Daiber A, Cuadrado A. Antioxidants in translational medicine. Antioxid Redox Signal 23: 1130–1143, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 61: 1066–1079, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen J, Tower J. Aging, MnSOD, and hormesis mechanisms converge on liver mUPR. Cell Cycle 12: 3237–3238, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin SM, Yang JH, Ki SH. Role of the Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev 2013: 763257, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siraki AG, Pourahmad J, Chan TS, Khan S, O'Brien PJ. Endogenous and endobiotic induced reactive oxygen species formation by isolated hepatocytes. Free Radic Biol Med 32: 2–10, 2002. [DOI] [PubMed] [Google Scholar]
- 87.Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules 7: 659–668, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Spencer NY, Engelhardt JF. The basic biology of redoxosomes in cytokine-mediated signal transduction and implications for disease-specific therapies. Biochemistry 53: 1551–1564, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sundaresan NR, Bindu S, Pillai VB, Samant S, Pan Y, Huang JY, Gupta M, Nagalingam RS, Wolfgeher D, Verdin E, Gupta MP. SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating GSK3beta. Mol Cell Biol 36: 678–692, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, Albano E. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 59: 886–897, 2014. [DOI] [PubMed] [Google Scholar]
- 91.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 60: 1090–1096, 2014. [DOI] [PubMed] [Google Scholar]
- 92.Tao GZ, Lehwald N, Jang KY, Baek J, Xu B, Omary MB, Sylvester KG. Wnt/beta-catenin signaling protects mouse liver against oxidative stress-induced apoptosis through the inhibition of forkhead transcription factor FoxO3. J Biol Chem 288: 17214–17224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thannickal VJ. Mechanisms of pulmonary fibrosis: role of activated myofibroblasts and NADPH oxidase. Fibrogenesis Tissue Repair 5: S23, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 11: 1289–1299, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wells RG. Tissue mechanics and fibrosis. Biochim Biophys Acta 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett 559: 107–110, 2004. [DOI] [PubMed] [Google Scholar]
- 97.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300: 650–653, 2003. [DOI] [PubMed] [Google Scholar]
- 98.Woolbright BL, Jaeschke H. Xenobiotic and endobiotic mediated interactions between the cytochrome P450 system and the inflammatory response in the liver. Adv Pharmacol 74: 131–161, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao Y, Yan W, Lu L, Wang Y, Lu W, Cao Y, Cai W. p38/p53/miR-200a-3p feedback loop promotes oxidative stress-mediated liver cell death. Cell Cycle 14: 1548–1558, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Yang JJ, Tao H, Hu W, Liu LP, Shi KH, Deng ZY, Li J. MicroRNA-200a controls Nrf2 activation by target Keap1 in hepatic stellate cell proliferation and fibrosis. Cell Signal 26: 2381–2389, 2014. [DOI] [PubMed] [Google Scholar]
- 101.Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med 30: 29–41, 2009. [DOI] [PubMed] [Google Scholar]
- 102.Zhang F, Lu Y, Zheng S. Peroxisome proliferator-activated receptor-gamma cross-regulation of signaling events implicated in liver fibrogenesis. Cell Signal 24: 596–605, 2012. [DOI] [PubMed] [Google Scholar]
- 103.Zheng M, Storz G. Redox sensing by prokaryotic transcription factors. Biochem Pharmacol 59: 1–6, 2000. [DOI] [PubMed] [Google Scholar]